Abstract
Background
Guidelines recommend Irritable Bowel Syndrome (IBS) diagnosis and management in primary care with minimal investigations; however little evidence exists regarding risk of organic gastrointestinal conditions following diagnosis of IBS and how such risks vary over the long term. This study assesses excess incidence of coeliac disease, inflammatory bowel disease (IBD) and colorectal cancer (CRC) and variation with age and time after IBS diagnosis.
Methods
IBS patients and controls were identified within the UK Clinical Practice Research Dataset. Incidence rates were calculated and stratified by age and time since IBS diagnosis with incident rate ratios generated.
Results
Fifteen years after IBS diagnosis there is a significant cumulative excess incidence of coeliac disease, IBD and CRC in IBS of 3.7% compared to 1.7% in controls. For every 10000 patient years, IBS patients experienced an additional 4 diagnoses of coeliac disease, 13 of IBD and 4 CRCs. In each condition peak excess incidence was in the 6 months following diagnosis. After one year, increased incidence of coeliac disease remained consistent without variation by age. IBD incidence fell slowly, with higher rates in those under 30. CRC incidence was increased only in patients aged 30 to 74 during the first 5 years.
Conclusion
Some IBS patients later receive organic gastrointestinal diagnoses, with the early excess incidence likely detected during diagnostic investigation at the time of IBS diagnosis. More than 5 years after IBS diagnosis there is no increased risk of CRC compared to the general population, but a small excess risk of coeliac disease and IBD persists. Overall, though our findings provide reassurance that non-specialists, especially those in primary care, are unlikely to be missing an organic condition in the majority of their patients. This suggests that current guidelines suggesting avoidance of universal referral for these patients are appropriate.
Introduction
Irritable bowel syndrome (IBS) is a chronic functional condition affecting about 11% of the global population [1]. It is clinically heterogeneous and patients present with various combinations of abdominal pain, altered bowel habit and bloating, but there is no biomarker or unifying structural abnormality to allow definitive diagnosis. Consequently diagnostic criteria have been developed to enable diagnosis based on symptom profile. Current international consensus criteria (Rome III) recommend diagnosing IBS without extensive investigations to exclude other conditions [2]. Alongside these recommendations, there is increasing emphasis on diagnosing and managing IBS within primary care [3], [4].
Despite this, 10–50% [5]–[7] of patients with IBS are referred to secondary care, with many gastroenterologists and generalists still considering IBS a diagnosis of exclusion [8]. Some referrals are due to concern about organic conditions that share similar symptomatology [7], [8], such as cholecystitis, pancreatic insufficiency, endometriosis, lactose intolerance, bile acid malabsorbtion and small bowel bacterial overgrowth. Three conditions of particular interest are coeliac disease, inflammatory bowel disease (IBD) and colorectal cancer (CRC). Currently little is known about the likelihood of a patient having one of these conditions if they have symptoms that suggest IBS [9]. Even less is known about the risk of patients subsequently being diagnosed with these conditions after being diagnosed with IBS. A small meta-analysis suggests prevalence of coeliac disease in patients diagnosed with IBS is around 4 times the population rate [10], but there are no large cohort studies considering this. Incidence rates of IBD and CRC in patients with IBS have previously been calculated, with estimates of IBD incidence between 8 [11] and 16 [12] times that in the general population and no increased incidence of CRC [12]. These studies, however, have short follow up periods and in one the study population were service personnel, unlikely to be a representative sample. Consequently, changes in risk of diagnoses of IBD, coeliac disease and CRC over a long time period after a diagnosis of IBS in a general population has not been studied and variations in risk by age and sex are unknown. A community based study followed 112 patients with IBS for a median of 29 years after diagnosis to assess incidence of organic gastrointestinal disease and found 3.5% were subsequently diagnosed with gastrointestinal cancer between 13 and 30 years later. They did not compare this to diagnoses in the general population to assess any excess risk and there was no incident IBD or coeliac disease [13].
We have therefore conducted a study within contemporary UK practice to determine the risk of diagnosis of coeliac disease, IBD and CRC over 15 years following IBS diagnosis and determined variation in risk according to time since diagnosis, age and sex. To aid better clinical decision making we have determined risk in absolute as well as relative terms.
Methods
Data were taken from the Clinical Practice Research Datalink (CPRD). CPRD is an anonymised longitudinal dataset of over 13 million medical records from over 640 primary care practices across the UK, collected prospectively from routine care since 1987 [14], [15]. Almost two thirds of the practices are linked to the National Health Service Hospital Episode Statistics (HES), providing secondary care data from NHS hospitals in England since 1989 [15], [16]. Only practices with HES linked CPRD records and individuals with records audited to acceptable research quality [14] were included. This project was given ethical approval by the CPRD Independent Scientific Advisory Committee (protocol approval reference 12_047R) and all data was anonymised and de-identified by CPRD before release to us and prior to analysis. For this study we drew data from the July 2012 version of CPRD. All selections and definitions listed below were made using code lists available on request from the corresponding author.
IBS population
People with IBS were identified by a Read code for IBS in their CPRD record for either a clinical or referral episode. Validity of these codes has been established by Huerta et al who contacted the primary care physicians of patients with Read codes consistent with incident IBS in CPRD and found records to be accurate in 77% of cases [17]. The purpose of the study is to examine the correlates of a GP diagnosis of IBS rather than those of the Rome criteria [2], so our inability to validate our code lists against these criteria is of limited importance. This study was only concerned with diagnoses of coeliac disease, IBD or CRC after an initial diagnosis of IBS, so any patients with these diagnoses before an IBS diagnosis were excluded, as were patients aged under 18 or over 75 years at diagnosis. We considered the earliest date associated with an IBS episode to be the date of diagnosis, and this was when the patient entered the study. IBS diagnoses were considered incident when this date was at least one year after the patient began contributing prospective data to CPRD (a previously validated strategy) [18]. 112854 cases of IBS met these criteria. Exit from the study was the earliest of date of death, date of transferring out of the practice or last date of data collection for the CPRD from that practice.
Control population
Individuals were eligible to be controls if they had no recorded diagnosis of IBS and no prescription for peppermint oil or the spasmolytics Meberverine and Alverine (three medications prescribed for treatment of IBS and rarely for other conditions). As the controls, by definition, had no IBS diagnosis they had no diagnosis date to define entry to study and start of follow up. To allow for this and provide a date with which to match them to cases a pseudodiagnosis date was generated. Each potential control was allocated a pseudodiagnosis date randomly during the period they were alive and contributing data to CPRD, and controls were frequency matched [19] by sex, and age at diagnosis/pseudodiagnosis in 3 age bands (18–29, 30–49, and 50–75). The control population was generated five times the size of the case population to provide enough power to detect differences in incidence and conduct the stratified analyses [20]. Exit was defined in the same way as in cases.
Defining other gastrointestinal diagnoses
All coeliac disease [21] and IBD [22] cases were defined as previously described as individuals who had at least one clinical episode with a Read code for coeliac disease or IBD in their CPRD record. CRC [23] cases were identified through either a clinical episode in the CPRD with a CRC Read term code or an episode in the linked HES records with an appropriate ICD-10 code. Incident diagnoses were defined as those where the first episode with an appropriate code occurred after the IBS diagnosis (or pseudodiagnosis) date.
Statistical analysis
All patients with prevalent coeliac disease, IBD or CRC at date of IBS diagnosis (or pseudodiagnosis), or diagnosed on the same day were excluded from the analysis of incidence of that disease. Patients without an IBD clinical code with prescriptions for mesalazine, azathioprine, mecaptopurine and rectal steroids are likely to have these therapies for IBD so were also excluded from IBD incidence analysis. Incidence rates of coeliac disease, IBD and CRC were calculated by dividing the number of cases occurring by the person years at risk and are presented per 10000 person years with 95% confidence intervals (CI) and calculated over whole follow-up and after excluding the 1st year. Nelson-Aalen cumulative hazard plots were generated for each condition separately. Poisson regression was used to estimate rate ratios and 95% CIs for the purposes of comparing the IBS and control cohorts. Likelihood ratio tests were used to check whether interactions exist between the incidence rates of each condition and age, calendar time, sex and smoking, which might suggest subgroups in which particular care should be taken to check for alternative diagnoses. A p value of less than 0.05 was taken to indicate evidence of an interaction. Rates were stratified by current age (in bands 18–29, 30–49, 50–74, 75 and older) and calendar time since diagnosis and compared using rate ratios. As there were few coeliac disease and IBD events in those aged over 75 years, this age group was combined with those aged 50 to 74 years for these analyses. Conversely, there were few CRC events in those aged 18 to 29 years so this group was combined with those aged 30 to 49 years. For the absolute rate differences, the same age groupings were used across the three diseases to allow direct comparison. The IBD group was also stratified into disease type (Crohn's disease, ulcerative colitis and unspecified) to assess any differences.
Results
Cohort demographics
There were 112854 cases of incident IBS aged between 18 and 75 years at diagnosis who contributed 733583 person-years of follow-up. Follow-up was more than five years for 61562 (55%), more than ten years for 27860 (25%) and 9224 cases (8%) had more than 15 years follow-up. To these, 546903 controls were frequency matched contributing 1990165 person-years of follow-up, with 149321 followed up for more than 5 years (27%) and 49368 for more than ten years (9%). The mean age at diagnosis of IBS (or pseudodiagnosis) was 42.9 years in the cases and 42.8 years in the controls (table 1). Figures 1, 2 and 3 shows the cumulative incidence of coeliac disease, IBD and CRC in patients following their IBS diagnosis over 15 years of follow-up compared to controls showing a steep increase in organic diagnoses in the first year or so after IBS diagnosis and then generally proportional risks. Combined, the overall cumulative incidence of being diagnosed with one of these conditions after IBS was 3.7%, compared to 1.7% in controls.
Table 1. Frequency and percentage of patients and controls stratified by age at IBS diagnosis and sex.
| Age at IBS diagnosis | Total | IBS population | Control population | |||
| Cases (%) | Controls (%) | Male (%) | Female (%) | Male (%) | Female (%) | |
| 18–29 | 26954 (23.88) | 126586 (23.15) | 5883 (21.83) | 21071 (78.17) | 27398 (21.64) | 99188 (78.36) |
| 30–49 | 49121 (43.53) | 246339 (45.04) | 14141 (28.79) | 34980 (71.21) | 70635 (28.67) | 175704 (71.33) |
| 50–75 | 36779 (32.59) | 173978 (31.81) | 11478 (31.21) | 25301 (68.79) | 53322 (30.65) | 120656 (69.35) |
| Total | 112854 | 546903 | 31502 (27.91) | 81352 (72.09) | 151355 (27.67) | 395.548 (72.33) |
Figure 1. Cumulative frequency of coeliac disease in patients with IBS following IBS diagnosis compared to controls.
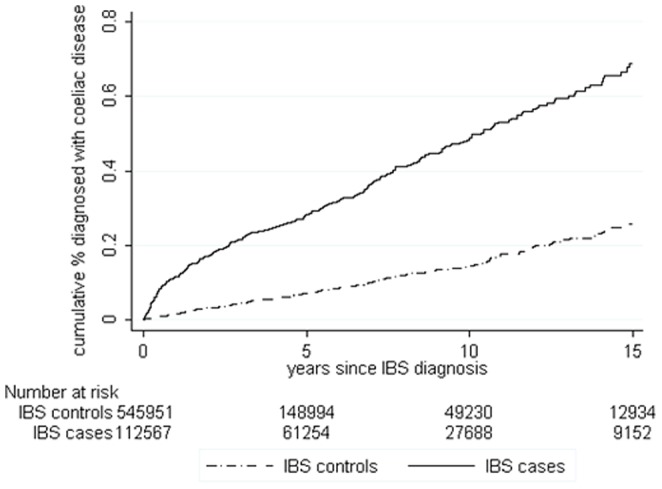
Figure 2. Cumulative frequency of IBD in patients with IBS following IBS diagnosis compared to controls.
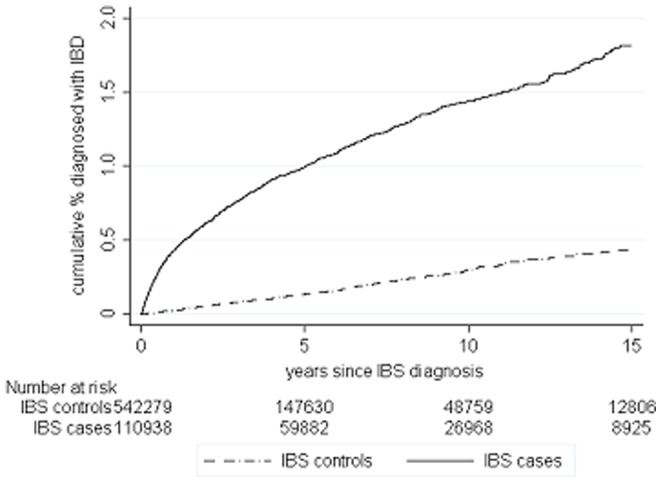
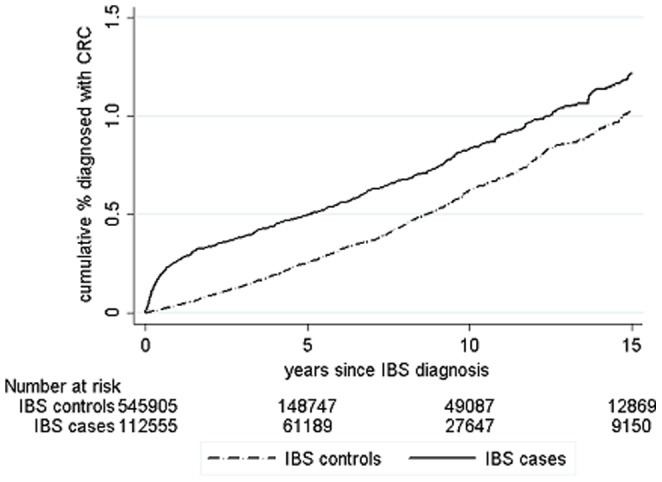
Figure 3. Cumulative frequency of coeliac disease CRC in patients with IBS following IBS diagnosis compared to controls.
Coeliac disease
During follow up 395 IBS cases and 299 controls were diagnosed with coeliac disease. Median time to coeliac disease diagnosis was 2.6 years following IBS diagnosis (IQR 0.7 to 6.8 years) and 3.5 years following pseudodiagnosis (IQR 1.31 to 7.07 years). Mean age at diagnosis of coeliac disease was 49 years in IBS patients and 53 years in controls.
Overall incidence of coeliac disease was 5.26 per 10000 person-years in IBS patients (95% CI 4.77 to 5.81) and 1.49 per 10000 person-years (95% CI 1.33 to 1.67) in controls, with a cumulative incidence of 0.7% of IBS patients after 15 years and 0.25% of controls (figure 1). The cumulative incidence of coeliac disease in IBS patients continues to diverge from that of controls even after 15 years (figure 1).
In IBS patients, an absolute rate difference of 4 extra coeliac disease diagnoses per 10000 person-years was seen over total follow-up compared to controls. The overall incident rate ratio (IRR) was 3.54 (95% CI 3.04 to 4.11), falling to 2.84 when the year after diagnosis was excluded (95% CI 3.38 to 3.38). Table 2 shows how the IRR varies with time since diagnosis and by the age of the patient. In the first 6 months after IBS diagnosis the incidence of coeliac disease was between 9.4 and 26.1 times higher than in controls, but this fell to between 2 and 7 times higher thereafter. This is reflected in the absolute rate difference (table 3) falling from between 12 to 17 additional coeliac disease diagnoses per 10000 person years in the first six months to a consistent excess after one year of between 3 and 4 cases per 10000 person years in those aged under 75 years. There was no significant increase in those aged over 75 years.
Table 2. Incidence rate of coeliac disease after IBS diagnosis and the incidence rate ratio.
| Current age | Time since IBS diagnosis | IBS population | Control population | Rate ratio | 95% CI | ||||||||||
| n † | % ‡ | N †† | Person time (years) | Incidence rate (per 10000 person years) | 95% CI | n † | % ‡ | N †† | Person time (years) | Incidence rate (per 10000 person years) | 95% CI | ||||
| 18–29 | 0–6 months | 20 | 0.07 | 26976 | 12704 | 15.7 | 10.2–24.4 | 3 | 0.00 | 126742 | 49777 | 0.6 | 0.2–1.9 | 26.1 | 7.8–87.9 |
| 6–12 months | 9 | 0.04 | 23812 | 11128 | 8.1 | 4.2–15.5 | 3 | 0.00 | 80991 | 34616 | 0.9 | 0.3–2.7 | 9.3 | 2.5–34.5 | |
| 1–5 years | 23 | 0.11 | 20707 | 49000 | 4.7 | 3.1–7.0 | 7 | 0.01 | 59342 | 104460 | 0.7 | 0.3–1.4 | 7.0 | 3.0–16.3 | |
| 5–10 years | 6 | 0.10 | 6134 | 12943 | 4.6 | 2.1–10.3 | 2 | 0.02 | 9654 | 19058 | 1.0 | 0.3–4.2 | 4.4 | 0.9–21.9 | |
| 10 years to end of follow-up | 0 | 0.00 | 467 | 400 | 0.0 | - | 1 | 0.14 | 736 | 629 | 15.9 | 2.2–110.0 | - | - | |
| 30–49 | 0–6 months | 46 | 0.09 | 50158 | 23983 | 19.1 | 14.4–25.6 | 19 | 0.01 | 251595 | 108719 | 1.7 | 1.1–2.7 | 11.0 | 6.4–18.7 |
| 6–12 months | 15 | 0.03 | 47953 | 22843 | 6.6 | 4.0–10.9 | 17 | 0.01 | 199764 | 90184 | 1.9 | 1.2–3.0 | 3.5 | 1.7–7.0 | |
| 1–5 years | 59 | 0.12 | 50378 | 142459 | 4.1 | 3.2–5.3 | 60 | 0.03 | 178836 | 407334 | 1.5 | 1.1–1.9 | 2.8 | 2.0–4.0 | |
| 5–10 years | 39 | 0.13 | 30147 | 92047 | 4.2 | 3.1–5.8 | 29 | 0.05 | 62419 | 163736 | 1.8 | 1.2–2.5 | 2.4 | 1.5–3.9 | |
| 10 years to end of follow-up | 9 | 0.08 | 11224 | 36409 | 2.5 | 1.3–4.8 | 6 | 0.04 | 15233 | 42967 | 1.4 | 0.6–3.1 | 1.8 | 0.6–5.0 | |
| 50 and older | 0–6 months | 23 | 0.06 | 37514 | 18078 | 12.7 | 8.5–19.1 | 11 | 0.01 | 177546 | 81018 | 1.4 | 0.8–2.5 | 9.4 | 4.6–19.2 |
| 6–12 months | 11 | 0.03 | 36673 | 17671 | 6.2 | 3.4–11.2 | 13 | 0.01 | 157476 | 73446 | 1.8 | 1.0–3.0 | 3.5 | 1.6–7.9 | |
| 1–5 years | 55 | 0.13 | 41393 | 126085 | 4.4 | 3.3–5.7 | 63 | 0.04 | 161835 | 430279 | 1.5 | 1.1–1.9 | 3.0 | 2.1–4.3 | |
| 5–10 years | 44 | 0.13 | 32915 | 111267 | 4.0 | 2.9–5.3 | 38 | 0.04 | 91991 | 277990 | 1.4 | 1.0–1.9 | 2.9 | 1.8–4.5 | |
| 10 years to end of follow-up | 36 | 0.18 | 19968 | 73768 | 4.9 | 3.5–6.8 | 27 | 0.07 | 37207 | 125851 | 2.1 | 1.5–3.1 | 2.3 | 1.4–3.7 | |
Incident rate ratios in bold are statistically significant and have a p-value <0.05.
n is the number of people diagnosed with coeliac disease within the stratum;
% is the proportion that these diagnoses represent within the stratum;
N is the total number of patients contributing time to the stratum. The rates are split by time since IBS was diagnosed and the current age of the patient. This means, for example, that the risk of coeliac disease for someone who is 34 and was diagnosed with IBS 6 years ago can be seen as 2.8 times greater than someone without IBS.
Table 3. Absolute incidence rate difference between IBS cases and controls for each disease stratified by current age and time since IBS diagnosis.
| Current age | Time since IBS diagnosis | Coeliac disease | IBD | CRC | |||
| Absolute rate difference (per 10000 person years) | 95% CI | Absolute rate difference (per 10000 person years) | 95% CI | Absolute rate difference (per 10000 person years) | 95% CI | ||
| 18–29 | 0–6 months | 15.10 | 4.83–16.45 | 66.25 | 51.66–80.84 | 3.14 | 0.06–6.23 |
| 6–12 months | 7.20 | 0.43–9.22 | 38.26 | 26.20–50.33 | 0.90 | −0.86–2.66 | |
| 1–5 years | 4.00 | 1.46–4.71 | 18.51 | 14.35–22.67 | −0.57 | −1.03– −0.12 | |
| 5–10 years | 3.60 | −0.87–6.51 | 12.62 | 5.26–19.99 | - | - | |
| 10 years to end of follow-up | - | - | 9.62 | −4.99–6.91 | - | - | |
| 30–49 | 0–6 months | 17.40 | 9.34–19.38 | 55.21 | 45.45–64.98 | 17.44 | 10.07–22.80 |
| 6–12 months | 4.70 | 1.66–7.98 | 32.39 | 24.61–40.16 | 3.93 | 1.18–6.67 | |
| 1–5 years | 2.60 | 1.34–3.35 | 11.73 | 9.72–13.74 | 0.94 | 0.18–1.69 | |
| 5–10 years | 2.40 | 0.13–2.60 | 4.87 | 2.83–6.91 | 0.05 | −0.79–0.89 | |
| 10 years to end of follow-up | 1.10 | −0.51–3.05 | 5.88 | 2.49–9.27 | −0.26 | −1.96–1.44 | |
| 50–74 | 0–6 months | 11.7 | 6.4–16.9 | 39.52 | 29.88–49.16 | 83.46 | 69.09–97.84 |
| 6–12 months | 4.7 | 0.8–8.5 | 16.93 | 10.09–23.78 | 24.65 | 15.56–33.76 | |
| 1–5 years | 3.1 | 1.8–4.4 | 9.75 | 7.61–11.90 | 2.69 | 0.50–4.89 | |
| 5–10 years | 2.7 | 1.3–4.0 | 6.49 | 4.40–8.59 | 0.37 | −4.85–2.59 | |
| 10 years to end of follow-up | 2.8 | 0.8–4.8 | 3.94 | 1.57–6.31 | −1.09 | −4.14–1.95 | |
| 75 and older | 0–6 months | - | - | - | - | 94.64 | −939.23–496.49 |
| 6–12 months | - | - | - | - | - | - | |
| 1–5 years | −0.8 | −3.9–2.3 | 6.14 | −2.85–15.14 | −4.59 | −17.97–0.78 | |
| 5–10 years | 2.0 | −0.1–4.9 | 3.21 | −1.79–8.21 | 4.58 | −6.34–15.54 | |
| 10 years to end of follow-up | 2.4 | −1.5–6.3 | 5.06 | 0.05–10.10 | −1.76 | −12.01–8.49 | |
Rates in bold are statistically significant with a p-value <0.05 The absolute rate difference indicates how many additional people would be expected to be diagnosed with each condition in a group with IBS, rather than the proportional difference that is presented in the rate ratio measure.
There was no statistically significant difference in the incidence of coeliac disease in men and women and no interaction between coeliac disease incidence and sex or smoking status.
IBD
During follow-up 1184 IBS patients and 569 controls were diagnosed with IBD. The median time to diagnosis of IBD from IBS diagnosis was 1.7 years (IQR 0.49 years to 4.6 years) and 3.1 years (IQR 1.3 to 6.4 years) from pseudodiagnosis in controls. The mean age at IBD diagnosis was 45 years in IBS patients and 52 years in controls.
Overall incidence of IBD was 16.12 per 10000 person-years in IBS patients (95% CI 15.23 to 17.07) and 2.85 per 10000 person-years (95% CI 2.63 to 3.10) in controls. The cumulative incidence of IBD after 15 years was 1.9% of IBS patients and 0.5% of controls and the rates continue to diverge even after 15 years (figure 2).
Over the total follow-up there was an absolute rate difference of 13 extra cases of IBD per 10000 person-years in IBS patients compared to controls. The overall IRR was 5.63 (95% CI 5.11 to 6.24), which fell to 3.98 (95% CI 3.54 to 4.45) after the first year following IBS diagnosis was excluded. In the first 6 months after IBS diagnosis incidence of IBD in IBS was between 16.8 and 24.5 times that seen in the controls depending on age (table 4), an absolute rate difference of between 40 and 66 extra cases of IBD per 10000 person years (table 3). After 5 years, incidence of IBD in IBS patients remained between 2.6 to 5.0 times higher than in controls, with between 3 and 13 additional IBD diagnoses per 10000 person years depending on age.
Table 4. Incidence rate of IBD after IBS diagnosis and the incidence rate ratio.
| Current age | Time since IBS diagnosis | IBS population | Control population | Rate ratio | 95% CI | ||||||||||
| n † | % ‡ | N †† | Person time (years) | Incidence rate (per 10000 person years) | 95% CI | n † | % ‡ | N †† | Person time (years) | Incidence rate (per 10000 person years) | 95% CI | ||||
| 18–29 | 0–6 months | 87 | 0.32 | 26789 | 12596 | 69.1 | 56.0–85.2 | 14 | 0.01 | 126414 | 49652 | 2.8 | 1.7–4.8 | 24.5 | 13.9–43.1 |
| 6–12 months | 45 | 0.17 | 26578 | 11010 | 40.9 | 30.5–54.7 | 9 | 0.01 | 80793 | 34527 | 2.6 | 1.4–5.0 | 15.7 | 7.7–32.1 | |
| 1–5 years | 100 | 0.49 | 20472 | 48266 | 20.7 | 17.0–25.2 | 23 | 0.04 | 59180 | 104129 | 2.2 | 1.5–3.3 | 9.4 | 6.0–14.8 | |
| 5–10 years | 20 | 0.33 | 6027 | 12669 | 15.8 | 10.2–24.5 | 6 | 0.06 | 9607 | 18946 | 3.2 | 1.4–7.1 | 5.0 | 2.0–12.4 | |
| 10 years to end of follow–up | 1 | 0.22 | 458 | 389 | 25.7 | 3.6–182.6 | 1 | 0.14 | 729 | 621 | 16.1 | 2.3–114.3 | 1.6 | 0.1–25.5 | |
| 30–49 | 0–6 months | 137 | 0.28 | 49455 | 23624 | 58.0 | 49.1–68.6 | 30 | 0.01 | 250082 | 108046 | 2.8 | 1.9–4.0 | 20.9 | 14.1–31.0 |
| 6–12 months | 78 | 0.17 | 47191 | 22459 | 34.7 | 27.8–43.4 | 21 | 0.01 | 198503 | 89613 | 2.3 | 1.5–3.6 | 14.8 | 9.2–24.0 | |
| 1–5 years | 195 | 0.39 | 49500 | 139731 | 14.0 | 12.1–16.1 | 90 | 0.05 | 177732 | 404694 | 2.2 | 1.8–2.7 | 6.3 | 4.9–8.1 | |
| 5–10 years | 72 | 0.24 | 29513 | 89988 | 8.0 | 6.4–10.1 | 51 | 0.08 | 62018 | 162675 | 3.1 | 2.4–4.1 | 2.6 | 1.8–3.7 | |
| 10 years to end of follow–up | 30 | 0.27 | 10945 | 35455 | 8.5 | 5.9–12.1 | 11 | 0.07 | 15130 | 42675 | 2.6 | 1.4–4.7 | 3.3 | 1.7–6.6 | |
| 50 and older | 0–6 months | 74 | 0.20 | 37117 | 17689 | 41.8 | 33.3–52.5 | 20 | 0.01 | 177232 | 80144 | 2.5 | 1.6–3.9 | 16.8 | 10.2–27.5 |
| 6–12 months | 34 | 0.09 | 36285 | 17272 | 19.7 | 14.1–27.5 | 22 | 0.01 | 157305 | 72650 | 3.0 | 2.0–4.6 | 6.5 | 3.8-11.1 | |
| 1–5 years | 160 | 0.37 | 43224 | 122965 | 13.0 | 11.1–15.2 | 147 | 0.09 | 168697 | 425404 | 3.5 | 2.9–4.1 | 3.8 | 3.0–4.7 | |
| 5–10 years | 101 | 0.30 | 34179 | 108384 | 9.3 | 7.7–11.3 | 89 | 0.09 | 97062 | 274856 | 3.2 | 2.6–4.0 | 2.9 | 2.2–3.8 | |
| 10 years to end of follow-up | 50 | 0.25 | 19916 | 71811 | 7.0 | 5.3–9.2 | 35 | 0.09 | 39420 | 124469 | 2.8 | 2.0–3.9 | 2.5 | 1.6–3.8 | |
Incident rate ratios in bold are statistically significant and have a p-value <0.05.
n is the number of people diagnosed with IBD within the stratum;
% is the proportion that these diagnoses represent within the stratum;
N is the total number of patients contributing time to the stratum. The rates are split by time since IBS was diagnosed and the current age of the patient. This means, for example, that the risk of IBD for someone who is 34 and was diagnosed with IBS 6 years ago can be seen as 2.6 times greater than someone without IBS.
There was a significant interaction between incidence of IBD and current patient age (p <0.001). Whilst the risk ratios were not statistically significantly different according to age, because the underlying population incidence of IBD is higher in young people, the absolute rate difference in IBS compared to controls is significantly higher in those aged under 30 years after the first year following IBS diagnosis. There was a statistically significant interaction (p<0.001) between IBD incidence and sex. Overall incidence of IBD in men with IBS was 20.3 per 10000 patient years (95% CI 18.4 to 22.4) and 14.6 per 10000 patient years (95% CI 13.6 to 15.6) in women, but there was no statistically significant difference according to sex in controls. Stratification by IBD type showed no significant difference in any of the analyses. There was no interaction between the smoking status of IBS cases and controls and the incidence of IBD in each group.
CRC
During follow up 708 people with IBS and 1148 controls were diagnosed with CRC. The median time from diagnosis of IBS to CRC diagnosis was 1.9 years (IQR 0.4 to 6.6 years), and 4.2 years in controls (IQR 1.8 to 7.9 years). Mean age at diagnosis was 63 years in IBS patients and 67 years in controls.
Overall incidence of CRC was 9.38 per 10000 person-years in IBS patients (95% CI 8.71 to 10.09) and 5.72 per 10000 person-years (95% CI 5.40 to 6.06) in controls. The cumulative incidence of CRC in IBS patients was 1.2% after 15 years and 1% in controls and rates began to converge soon after IBS diagnosis (figure 3).
Over the total follow-up there was an absolute rate difference of 4 extra CRCs per 10000 patient years in IBS patients compared to controls with an overall IRR of 1.64 (95% CI 1.49 to 1.80), which fell to 1.03 (95% CI 0.93 to 1.16) after the first year following IBS diagnosis was excluded, not statistically significantly different from the rate in the controls. In the first 6 months after IBS diagnosis the incidence of CRC was between 4 and 41 times higher than in controls depending on age, falling to between 1.3 and 1.7 times higher after one year (table 5). Patients who were aged under 30 only had an increased incidence of CRC in the first 6 months following IBS diagnosis. After this period and in those aged over 75 years at any time there was no significant increase in incidence of CRC compared to controls (table 3). For those aged 30 to 74, after 5 years there was no statistically significant difference in CRC incidence compared to controls, there is even a trend towards lower rates in patients with IBS.
Table 5. Incidence rate of CRC after IBS diagnosis and the incidence rate ratio.
| Current age | Time since IBS diagnosis | IBS population | Control population | Rate ratio | 95% CI | ||||||||||
| n † | % ‡ | N †† | Person time (years) | Incidence rate (per 10000 person years) | 95% CI | n † | % ‡ | N †† | Person time (years) | Incidence rate (per 10000 person years) | 95% CI | ||||
| 18–49 | 0–6 months | 47 | 0.06 | 77254 | 36747 | 12.8 | 13.3–24.1 | 5 | 0.00 | 378738 | 158672 | 0.3 | 0.2–1.1 | 40.6 | 15.4–98.4 |
| 6–12 months | 11 | 0.02 | 71887 | 34035 | 3.2 | 2.4–8.1 | 4 | 0.00 | 281067 | 124949 | 0.3 | 0.2–1.2 | 10.1 | 3.1–31.4 | |
| 1–5 years | 26 | 0.04 | 71235 | 191965 | 1.4 | 1.2–2.7 | 42 | 0.02 | 238462 | 512415 | 0.8 | 0.6–1.2 | 1.7 | 1.2–3.4 | |
| 5–10 years | 10 | 0.03 | 36389 | 105387 | 0.9 | 0.6–2.0 | 17 | 0.02 | 72164 | 183078 | 0.9 | 0.6–1.7 | 1.0 | 0.5–2.3 | |
| 10 years until end of follow-up | 5 | 0.04 | 11746 | 36993 | 1.4 | 0.6–3.3 | 7 | 0.04 | 16002 | 43691 | 1.6 | 0.8–3.4 | 0.8 | 0.3–2.6 | |
| 50–74 | 0–6 months | 168 | 0.45 | 37387 | 17891 | 93.9 | 80.7–110.0 | 84 | 0.05 | 177109 | 80459 | 10.4 | 8.4–12.9 | 9.0 | 6.9–11.7 |
| 6–12 months | 60 | 0.17 | 36053 | 17254 | 34.8 | 27.0–44.8 | 73 | 0.05 | 155574 | 72141 | 10.1 | 8.0–12.7 | 3.4 | 2.4–4.8 | |
| 1–5 years | 142 | 0.35 | 40362 | 117682 | 12.1 | 10.2414.2 | 380 | 0.24 | 158585 | 405398 | 9.4 | 8.5–10.4 | 1.3 | 1.1–1.6 | |
| 5–10 years | 87 | 0.29 | 30112 | 96815 | 9.0 | 7.3–11.1 | 209 | 0.25 | 84429 | 242501 | 8.6 | 7.5–9.8 | 1.0 | 0.8–1.3 | |
| 10 years to end of follow-up | 52 | 0.32 | 16394 | 60034 | 8.7 | 6.6–11.4 | 98 | 0.31 | 31509 | 100508 | 9.8 | 8.0–11.9 | 0.9 | 0.6–1.2 | |
| 75 and older | 0–6 months | 1 | 0.27 | 369 | 81 | 123.3 | 17.4–880.0 | 1 | 0.06 | 1551 | 349 | 28.7 | 4.0–200.0 | 4.3 | 0.3–68.8 |
| 6–12 months | 0 | 0.00 | 787 | 280 | - | - | 3 | 0.10 | 3011 | 1104 | 27.2 | 8.8–84.9 | - | - | |
| 1–5 years | 19 | 0.53 | 3556 | 7503 | 25.3 | 16.2–39.7 | 70 | 0.61 | 11481 | 23397 | 29.9 | 23.7–37.4 | 0.8 | 0.5–1.4 | |
| 5–10 years | 44 | 0.89 | 4935 | 13762 | 32.0 | 23.8–43.0 | 94 | 0.70 | 13344 | 34311 | 27.4 | 22.4–33.5 | 1.2 | 0.8–1.7 | |
| 10 years to end of follow-up | 31 | 0.78 | 3971 | 13423 | 23.1 | 16.2–32.8 | 61 | 0.75 | 8110 | 24542 | 24.9 | 19.3–32.0 | 0.9 | 0.6–1.4 | |
Incident rate ratios in bold are statistically significant and have a p-value <0.05.
n is the number of people diagnosed with CRC within the stratum;
% is the proportion that these diagnoses represent within the stratum;
N is the total number of patients contributing time to the stratum. The rates are split by time since IBS was diagnosed and the current age of the patient. This means, for example, that the risk of CRC for someone who is 34 and was diagnosed with IBS 6 years ago can be seen as being the same (1.0) someone without IBS.
There was a statistically significant interaction (p = 0.005) between CRC incidence and sex. Overall incidence of CRC in men with IBS was 14.9 per 10000 patient years (95% CI 13.3 to 16.6) and 7.3 per 10000 patient years (95% CI 6.7 to 8.1) in women. In controls, men still had higher rates but the difference was less (7.6 and 5.0 per 10000 patient years respectively). There was no interaction with smoking.
Discussion
During the 15 years following a diagnosis of IBS, cumulative incidence of coeliac disease was 0.7%, 1.8% for IBD and 1.2% for CRC. This means the overall cumulative incidence of IBS patients receiving a diagnosis of at least one of these conditions was 3.7%, an absolute excess of 2.0% compared to people without IBS. In all three conditions, peak excess incidence was in the first 6 months following IBS diagnosis, followed by a marked drop after one year. Median time to each organic diagnosis was also less than three years following IBS diagnosis. This suggests that much of the excess incidence of organic gastrointestinal disease occurs during the diagnostic work-up, in these cases the IBS code does not reflect a final diagnosis but rather it is part of the clinical pathway to obtaining a final organic diagnosis. The most concerning differential diagnosis for physicians and patients is probably CRC [7] and our study has reassuringly shown that incidence is no higher than the general population in young or elderly patients, and for those aged 30 to 74 years the excess incidence is very low after one year and disappears after 5 years following IBS diagnosis. For coeliac disease and IBD the incidence remains higher in IBS patients than in controls at all ages, even after ten years with an IBS diagnosis, but the absolute excess risk although statistically significant, is small in absolute terms.
A major strength of our study is that it is the largest study of its type yet conducted, with power to consider not only the overall incidence rates, but to allow stratification by the individuals' sex, current age and time since IBS diagnosis. Such stratification allows us to more clearly define which of these organic gastroenterological conditions individual patients are at greater risk of. For example, a young male IBS patient is far more likely to have IBD than an elderly female one. A second strength is that the length of follow-up is greater than previous studies. Shorter studies are likely to be biased by the higher rates we have shown in the first 12 months, probably related to the diagnostic work-up for IBS. Our study adds 12 years of follow-up to the findings of Garcia-Rodriguez et al [12], allowing assessment of how incidence of organic disease changes over time since IBS diagnosis. Since most IBS diagnoses are made among those under 50 years such prolonged follow up is of particular importance. A further strength is that all subjects are taken from primary care. Since IBS is mainly diagnosed and managed within primary care, and recommendations suggest that this is optimal practice [2]–[4], our study design allows accurate assessment of the potential for missed organic gastrointestinal disease in patients treated in a typical manner rather than by those with a specialist interest in IBS. Many studies of IBS focus only on patients who attend secondary care who are likely to be systematically different to the majority of IBS patients who never consult secondary care. Studying all IBS patients identified within primary and secondary care removes this bias. Controls were defined as having neither a clinical episode with an IBS Read code attached nor a recorded prescription for peppermint oil, Mebeverine or Alverine. Although people with IBS take many medications, these are the only medications used almost exclusively in IBS. Excluding everyone who had used any medication potentially for IBS would more completely avoid misclassification of controls but would exclude everyone ever prescribed anti-depressants, anxiolytics, laxatives or anti-diarrhoeal agents, potentially selecting a control population with better health than the general population. Studies within the community suggest that around 50-70% of people with symptoms consistent with IBS never seek medical attention [24]. Thus some undiagnosed IBS will exist amongst controls but this should not invalidate our study since its aim is to address the incidence of organic gastrointestinal disease in patients recorded as having IBS within primary care.
Inaccurate coding presents a potential limitation. There is no definitive investigation finding to use as a gold standard for code list validation in IBS. For this study question, however, the lack of truly independent validation is less of a concern because the interest is in organic gastrointestinal diagnoses made in patients labelled as having IBS and consequently treated as such. Previous studies have shown coding of IBS within CPRD to be valid when compared to General Practitioner's opinion. Only 1% of coded incident IBS and 16% of prevalent IBS were not confirmed as IBS by the GP [25]. Similarly, 92% of IBD codes [22], over 90% of CRC codes [23] and a similar proportion of coeliac disease codes [26] have been shown to be valid. There is no reason to suppose that coding errors for events with CRC, IBD or coeliac codes are systematically different between IBS patients and controls, so this should not introduce any bias. These data do not allow assessment of how closely the Rome criteria were employed in establishing the IBS diagnosis or how closely the NICE clinical guidelines were followed. Differences in incidence of organic gastrointestinal disease according to diagnostic work up warrants further study. A further limitation of our study is that we have not considered the impact of confounding by socioeconomic status which might be associated with the risk of coeliac disease or IBD but is not significantly associated with IBS and so we think appreciable confounding unlikely [24], [27].
This is the first large primary care based cohort study to consider the incidence of coeliac disease in patients diagnosed with IBS. We found the cumulative incidence of coeliac disease was 0.7% of the incident IBS cases after 15 years, just under three times that in the controls. Our results are lower than the outcome of a systematic review of coeliac disease in IBS but show a similar increase compared to the general population. The review reported a pooled estimate of coeliac disease prevalence as 4% among 2278 IBS patients, four times that seen in controls [10]. One reason our cumulative incidence rate is lower may relate to as yet undiagnosed coeliac disease in our IBS cases. Also we have measured the cumulative incidence over time rather than prevalence. A study using an earlier version of CPRD in 2000 looked at the rate of diagnosis of IBD and CRC [12]. Then they had just under 3000 IBS cases and included only 5 years following IBS diagnosis. During this period they also saw initially high rates of IBD and CRC fall in the first year. They reported a crude incidence of 26.2 CRCs diagnosed per 10000 patient years [12], (considerably higher than we report over the complete follow-up now available, but similar to the incidence we found in the early follow-up period), and no overall excess compared to controls. They found an overall crude incidence of IBD of 17.8 cases per 10000 person years, 16 times the rate in controls [12] which is similar to our finding. Over their total follow-up they saw no reduction in the rate of IBD, however with increased follow-up we have shown that incidence does decrease over time, but still remains higher than in the general population. A US database study of IBS in over 9000 military personnel looked specifically at the risk of being diagnosed with IBD [11]. The average follow-up was 3.6 years and they found the rate of IBD diagnosis was 8 times higher than in the general population. The slightly lower excess of IBD in this study may reflect differences in medical practice between the USA and UK, but may also reflect a military population being relatively healthier than the population seen in routine UK primary care. Median time from IBS diagnosis to IBD diagnosis was 2.1 years, similar to our findings [11]. A low incidence (1.9%) of organic colonic disease was also found in a study reviewing colonoscopy outcomes in patients having the procedure to confirm IBS [28], with incidence of IBD and CRC not significantly increased compared to healthy controls [28].
The clinical implications here are that a clinical diagnosis of IBS in general practice is highly unlikely to lead to a serious gastrointestinal diagnosis in the following 15 years. Thus patients can be reassured, particularly after the relatively higher risk period immediately after IBS diagnosis has passed. This is especially true for CRC where a trend towards a reduced rate in IBS patients might be explained by IBS patients receiving a colonoscopy during their diagnostic work-up and any lesions being detected at that point. For coeliac disease and IBD, however, the incidence in IBS patients remains above that in the general population throughout. Previous analysis has suggested serological testing for coeliac disease would be cost-effective for all IBS patients at the time of diagnosis [29]. Our results will allow greater accuracy in estimating the parameters of the model underlying this analysis but they do not tell us the value of repeated testing for coeliac disease. As the absolute excess rate of coeliac disease is very small we cannot rule out the possibility that it reflects patients who are seronegative for coeliac disease when diagnosed with IBS seroconverting later on. Similarly for IBD, a recent report shows that over a quarter of patients with endoscopic remission of IBD complain of IBS-like symptoms [30], and this alongside findings that mesalazine may resolve symptoms in some IBS patients [31] could support a suggestion that symptoms compatible with IBS might in some instances reflect clinically undetectable IBD. Hence we cannot rule out the value of repeated investigation for this diagnosis either. Since the overall risk is very low, however, we believe any repeat investigation should be individually assessed.
In summary, our study shows that the vast majority of people who receive an organic diagnosis following a recording of IBS within primary care do so in the first year following their IBS diagnosis, which is clearly part of the diagnostic work up process. Five years after an IBS diagnosis, compared to people without IBS, there remains a small, but nonetheless important and statistically significant, excess risk of organic diagnoses. Overall our findings provide reassurance that non-specialists, especially in primary care who see most people diagnosed with IBS, are unlikely to be missing an organic condition in the majority of their patients. However younger IBS patients, particularly males, have a persistently increased risk of IBD, and patients of all ages still have a slight increased risk of having coeliac disease. This suggests that current guidelines suggesting avoidance of universal referral for these patients are appropriate.
Data Availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. The data used for this study are anonymised patient data routinely collected from UK primary care held by the Clinical Practice Research Datalink (CPRD: http://www.cprd.com/). Data within this database can be accessed with an appropriate license from CPRD and with ethical approval from the Independent Scientific Advisory Committee. Our license does not permit us to make them publicly available to all. We used data from the version collected in July 2012 and have clearly specified the data selected in our methods section. To allow identical data to be obtained by others, via the purchase of a license, we will provide the code lists on request. Licences are available from CPRD: The Clinical Practice Research Datalink Group, The Medicines and Healthcare products Regulatory Agency, 5th Floor, 151 Buckingham Palace Road, Victoria, London SW1W 9SZ, England or (http://www.cprd.com).
Funding Statement
This work was funded by a Medical Research Council (www.mrc.ac.uk) Population Health Scientist Fellowship awarded to CC, grant number G1002360 and project funding from Coeliac UK and CORE to JW (https://www.coeliac.org.uk/campaigns-and-research/current-research/what-is-the-occurrence-and-consequence-of-clinically-diagnosed/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lovell RM, Ford AC (2012) Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 10: 712–721. [DOI] [PubMed] [Google Scholar]
- 2. Longstreth GF, Thompson WG, Chey WD, Houghton L a, Mearin F, et al. (2006) Functional bowel disorders. Gastroenterology 130: 1480–1491. [DOI] [PubMed] [Google Scholar]
- 3. Begtrup LM, Engsbro AL, Kjeldsen J, Larsen P V, de Muckadell OS, et al. (2013) A Positive Diagnostic Strategy is Non-Inferior to a Strategy of Exclusion for Patients with Irritable Bowel Syndrome. Clin Gastroenterol Hepatol 11: 956–962. [DOI] [PubMed] [Google Scholar]
- 4. National Iinstitute for Health and Care Excellence (2008) Irritable bowel syndrome in adults: diagnosis and management of irritable bowel syndrome in primary care. Clinical guideline 61: guidence.nice.org.uk/cg61. [Google Scholar]
- 5. Jones R, Lydeard S (1992) Irritable bowel syndrome in the population. Br Med J 304: 87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Talley NJ, Gabriel SE, Harmsen WS, Zinsmeister AR, Evans RW (1995) Medical costs in community subjects with irritable bowel syndrome. Gastroenterology 109: 1736–1741. [DOI] [PubMed] [Google Scholar]
- 7. Thompson WG, Heaton KW, Smyth GT, Smyth C (2000) Irritable bowel syndrome in general practice: prevalence, characteristics, and referral. Gut 46: 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spiegel BMR, Farid M, Esrailian E, Talley J, Chang L (2010) Is irritable bowel syndrome a diagnosis of exclusion: a survey of primary care providers, gastroenterologists, and IBS experts. Am J Gastroenterol 105: 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cash BD, Schoenfeld P, Chey WD (2002) The utility of diagnostic tests in irritable bowel syndrome patients: a systematic review. Am J Gastroenterol 97: 2812–2819. [DOI] [PubMed] [Google Scholar]
- 10. Ford AC, Chey WD, Talley NJ, Malhotra A, Spiegel BMR, et al. (2009) Yield of diagnostic tests for celiac disease in individuals with symptoms suggestive of irritable bowel syndrome: systematic review and meta-analysis. Arch Intern Med 169: 651–658. [DOI] [PubMed] [Google Scholar]
- 11. Porter CK, Cash BD, Pimentel M, Akinseye A, Riddle MS, et al. (2012) Risk of inflammatory bowel disease following a diagnosis of irritable bowel syndrome. BMC Gastroenterol 12: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. García Rodríguez L a, Ruigómez a, Wallander M a, Johansson S, Olbe L (2000) Detection of colorectal tumor and inflammatory bowel disease during follow-up of patients with initial diagnosis of irritable bowel syndrome. Scand J Gastroenterol 35: 306–311. [DOI] [PubMed] [Google Scholar]
- 13. Owens DM, Nelson DK, Talley NJ (1995) The irritable bowel syndrome: long-term prognosis and the physician-patient interaction. Ann Intern Med 122: 107–112. [DOI] [PubMed] [Google Scholar]
- 14.Clinical Practice Research Dataset (2013). Available: http://www.cprd.com/home/. Accessed 23 May 2013.
- 15.Clinical Practice Research Dataset (2012) CPRD Gold Flat files release notes. July.
- 16.Puri S (2012) Hospital Episode Statistics (HES) data and GOLD documentation.
- 17. Huerta C, García Rodríguez LA, Wallander M-A, Johansson S (2002) Risk of irritable bowel syndrome among asthma patients. Pharmacoepidemiol Drug Saf 11: 31–35. [DOI] [PubMed] [Google Scholar]
- 18. Lewis JD, Bilker WB, Weinstein RB, Strom BL (2005) The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Saf 14: 443–451. [DOI] [PubMed] [Google Scholar]
- 19.Rothman K, Greenlad S, Lash T (2008) Design strategies to improve study accuracy. Modern Epidemiology. Philidelphia, USA: Lippincoll, Williams and Wilkins. pp. 168–182.
- 20. Hennessy S, Bilker WB, Berlin J a, Strom BL (1999) Factors influencing the optimal control-to-case ratio in matched case-control studies. Am J Epidemiol 149: 195–197. [DOI] [PubMed] [Google Scholar]
- 21. Violato M, Gray A, Papanicolas I, Ouellet M (2012) Resource use and costs associated with coeliac disease before and after diagnosis in 3,646 cases: results of a UK primary care database analysis. PLoS One 7: e41308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lewis JD, Brensinger C, Bilker WB, Strom BL (2002) Validity and completeness of the General Practice Research Database for studies of inflammatory bowel disease. Pharmacoepidemiol Drug Saf 11: 211–218. [DOI] [PubMed] [Google Scholar]
- 23. Jick H, Jick S, Derby LE, Vasilakis C, Myers MW, et al. (1997) Calcium-channel blockers and risk of cancer. Lancet 349: 525–528. [DOI] [PubMed] [Google Scholar]
- 25. Ruigómez A, García Rodríguez LA, Johansson S, Wallander M-A, García Rodríguez LA (2003) Is hormone replacement therapy associated with an increased risk of irritable bowel syndrome? Maturitas 44: 133–140. [DOI] [PubMed] [Google Scholar]
- 26. West J, Logan RF, Card TR, Smith C, Hubbard R (2003) Fracture risk in people with celiac disease: a population-based cohort study. Gastroenterology 125: 429–436. [DOI] [PubMed] [Google Scholar]
- 27. Talley NJ, Zinsmeister a R, Melton LJ (1995) Irritable bowel syndrome in a community: symptom subgroups, risk factors, and health care utilization. Am J Epidemiol 142: 76–83. [DOI] [PubMed] [Google Scholar]
- 28. Chey WD, Nojkov B, Rubenstein JH, Dobhan RR, Greenson JK, et al. (2010) The yield of colonoscopy in patients with non-constipated irritable bowel syndrome: results from a prospective, controlled US trial. Am J Gastroenterol 105: 859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mein SM, Ladabaum U (2004) Serological testing for coeliac disease in patients with symptoms of irritable bowel syndrome: a cost-effectiveness analysis. Aliment Pharmacol Ther 19: 1199–1210. [DOI] [PubMed] [Google Scholar]
- 30.Fukuba N, Ishihara S, Sonoyama H, Oka A, Kusunoki R, et al.. (2013) Prevalence of irritable bowel-like symptoms in ulcerative colitis patients with clinical and endoscopic evidence of remission: prospectice multi-centre findings. New insights into Pathophysiol Funct GI Disord Abstr from UEGW.
- 31. Corinaldesi R, Stanghellini V, Cremon C, Gargano L, Cogliandro RF, et al. (2009) Effect of mesalazine on mucosal immune biomarkers in irritable bowel syndrome: a randomized controlled proof-of-concept study. Aliment Pharmacol Ther 30: 245–252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. The data used for this study are anonymised patient data routinely collected from UK primary care held by the Clinical Practice Research Datalink (CPRD: http://www.cprd.com/). Data within this database can be accessed with an appropriate license from CPRD and with ethical approval from the Independent Scientific Advisory Committee. Our license does not permit us to make them publicly available to all. We used data from the version collected in July 2012 and have clearly specified the data selected in our methods section. To allow identical data to be obtained by others, via the purchase of a license, we will provide the code lists on request. Licences are available from CPRD: The Clinical Practice Research Datalink Group, The Medicines and Healthcare products Regulatory Agency, 5th Floor, 151 Buckingham Palace Road, Victoria, London SW1W 9SZ, England or (http://www.cprd.com).


