Abstract
Background
Observational studies suggest that B vitamin supplementation reduces cardiovascular risk in adults, but this association remains controversial. This study aimed to summarize the evidence from randomized controlled trials (RCTs) investigating B vitamin supplementation for the primary or secondary prevention of major adverse cardiovascular outcomes and to perform a cumulative meta-analysis to determine the evidence base.
Methodology and Principal Findings
In April 2013, we searched PubMed, Embase, and the Cochrane Library to identify relevant RCTs. We included RCTs investigating the effect of B vitamin supplementation on cardiovascular outcome. Relative risk (RR) was used to measure the effect using a random-effect model. Statistical heterogeneity scores were assessed using the Q statistic. We included data on 57,952 individuals from 24 RCTs: 12 primary prevention trials and 12 secondary prevention trials. In 23 of these trials, 10,917 major adverse cardiovascular events (MACE) occurred; in 20 trials, 7,203 deaths occurred; in 15 trials, 3,422 cardiac deaths occurred; in 19 trials, 3,623 myocardial infarctions (MI) occurred; and in 18 trials, 2,465 strokes occurred. B vitamin supplementation had little or no effect on the incidence of MACE (RR, 0.98; 95% confidence interval [CI]: 0.93–1.03; P = 0.37), total mortality (RR, 1.01; 95% CI: 0.97–1.05; P = 0.77), cardiac death (RR, 0.96; 95% CI: 0.90–1.02; P = 0.21), MI (RR, 0.99; 95% CI: 0.93–1.06; P = 0.82), or stroke (RR, 0.94; 95% CI: 0.85–1.03; P = 0.18).
Conclusion/Significance
B vitamin supplementation, when used for primary or secondary prevention, is not associated with a reduction in MACE, total mortality, cardiac death, MI, or stroke.
Introduction
The potential role of B vitamins (folate, B6, and B12) in reducing cardiovascular risk has been supported by observational studies [1]–[5]. Although the mechanism of action is unclear, B vitamins may affect cardiovascular outcome by lowering homocysteine concentrations, which correlate strongly with the risk of coronary disease [1]–[5] and stroke [6]–[8]. A meta-analysis [9] of observational studies suggested that lowering the plasma homocysteine level by 25% reduced the risk of coronary heart disease by 11% and the risk of stroke by 19%. Daily supplementation with folic acid has been shown to lower the plasma homocysteine level by approximately 25% and adding vitamin B12 further lowers the level by approximately 7%, indicating that B vitamins supplements lower homocysteine levels significantly [10]–[11]. Despite its ability to lower homocysteine levels, meta-analyses of randomized controlled trials (RCTs) [12]–[19] indicate that, although folic acid supplementation may reduce the risk of stroke in specific subsets of patients, it is not associated with a reduction in a composite of all-cause death, nonfatal acute myocardial infarction (MI), acute hospitalization for unstable angina pectoris, and nonfatal thromboembolic stroke (MACE), MI, cardiac death, or total mortality. The association between B vitamin supplementation and reduction of cardiovascular risk has not been confirmed by a RCT [20]–[31]. Furthermore, previous meta-analyses have not investigated the potential interaction of supplementation with both vitamin B6 and B12 on cardiovascular risk [12]–[17], [19].
The effect of B vitamin supplementation on primary and secondary prevention of adverse cardiovascular outcomes has been studied in numerous RCTs [20]–[43]. In this study, we performed a meta-analysis of these RCTs to evaluate the effect of B vitamin supplementation on cardiovascular risk in specific subpopulations and attempt to determine the role of folic acid supplementation interaction with vitamin B6 and B12 in reducing cardiovascular risk. Furthermore, we used cumulative meta-analysis to determine the evidence base for routine B vitamin supplementation in clinical practice.
Methods
Data Sources, Search Strategy, and Selection Criteria
This review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Statement, 2009 (Checklist S1) [44]. RCTs evaluating the effect of B vitamin supplementation on cardiovascular risk written in the English language were included in our study, regardless of the publication status (published, in press, or in progress) and the effect of B vitamin supplementation on MACE, total mortality, cardiac death, MI, and stroke were examined. Relevant trials were identified using the following procedure:
(1) Electronic searches: we searched PubMed, Embase, and the Cochrane Central Register of Controlled Trials electronic databases for articles published through April 2013, using “B vitamins” AND “randomized controlled trials” AND “clinical trials” AND “human” AND “English” as the search terms.
(2) Other sources: we searched ongoing RCTs in the metaRegister of Controlled Trials, which lists trials that are registered as completed but not yet published. Furthermore, we reviewed bibliographies of publications for potentially relevant trials. Medical subject headings, methods, patient population, interventions, and outcome variables of these studies were used to identify relevant trials.
The literature search, data extraction, and quality assessment were undertaken by 2 investigators (CZ and PJM) independently with a standardized approach. Any inconsistencies between these investigators were identified by the primary investigator (YHZ) and resolved by consensus. We restricted our study to RCTs, which are less likely than observational studies to be subject to confounding variables or bias. A study was eligible for inclusion in our meta-analysis if the following criteria were met: (1) the study was a RCT; (2) the trial evaluated the effects of B vitamin supplementation compared with placebo; (3) the study's follow-up continued for at least 6 months; and (4) the trial reported at least 1 of the following outcomes: MACE, total mortality, cardiac death, MI, or stroke.
Data Collection and Quality Assessment
All data from included trials was extracted independently by 2 investigators (CZ and PJM) using a standardized protocol. Each data set was reviewed by a third investigator (YHZ), and any discrepancies between the 2 investigator's data were resolved by discussion. The data collected from each study included first author or study group name, publication year, study design, type of blinding, number of patients, percentage of men, mean age, background fortification, current disease status, baseline total homocysteine level, baseline folate level, intervention regimens, controls, and the duration of follow-up. The outcomes investigated included MACE, total mortality, cardiac death, MI, and stroke. Study quality was assessed using the Jadad score [45], which is based on the 5 following subscales: randomization (1 or 0), concealment of the treatment allocation (1 or 0), blinding (1 or 0), completeness of follow-up (1 or 0), and the use of intention-to-treat analysis (1 or 0). A score system ranging from 1 to 5 has been developed for quality assessment.
Statistical Analysis
We assigned the results of each RCT as dichotomous frequency data. Individual study relative risks (RR) and 95% confidence intervals (CI) were calculated from event numbers extracted from each trial before data pooling. The overall RR and 95% CI of MACE, total mortality, cardiac death, MI, and stroke were also calculated. Both fixed-effect and random-effect models were used to evaluate the pooled RR for B vitamin supplementation compared with placebo. Although both models yielded similar findings, results from the random-effect model, which assume that the true underlying effect varies among included trials, are presented here [46]–[47]. Heterogeneity of the treatment effect between studies was evaluated using the Q statistic, and P values <0.10 were considered statistically significant [48]–[49]. In the cumulative meta-analysis, outcome data for MACE, total mortality, cardiac death, MI, and stroke from all available trials were included sequentially according to the year in which they first became available.
We explored potential sources of heterogeneity in estimates of the treatment effect on MACE with univariate meta-regression [50] (for number of participants, mean age, percentage of men, baseline homocysteine level, baseline folate level, dose of folic acid, dose of vitamin B6, dose of vitamin B12, net decrease in homocysteine level, and duration of follow-up). Subsequently, subgroup analyses were conducted for MACE on the basis of number of participants, mean age, percentage of men, number of trial centers, baseline homocysteine level, baseline folate level, intervention regimens, dose of folic acid, dose of vitamin B6, dose of vitamin B12, net decrease in homocysteine level, background fortification, control, disease prevention, renal status, duration of follow-up, and Jadad score [45]. Interaction tests [51] were performed to compare differences between estimates of the 2 subsets, which were based on Student t distribution rather than on normal distribution because the number of included studies was small. We also performed a sensitivity analysis by removing each individual trial from the meta-analysis [52]. Several methods were used to check for potential publication bias. Visual inspection of funnel plots for MACE, total mortality, cardiac death, MI, and stroke were conducted. The Egger (53) and Begg test (54) were used to statistically assess publication bias for MACE, total mortality, cardiac death, MI, and stroke. All reported P values were two-sided, and P values of <0.05 were considered statistically significant for all included studies. Statistical analyses were performed using STATA software (version 10.0 StataCorp, Texas, USA).
Results
Search of the Published Literature
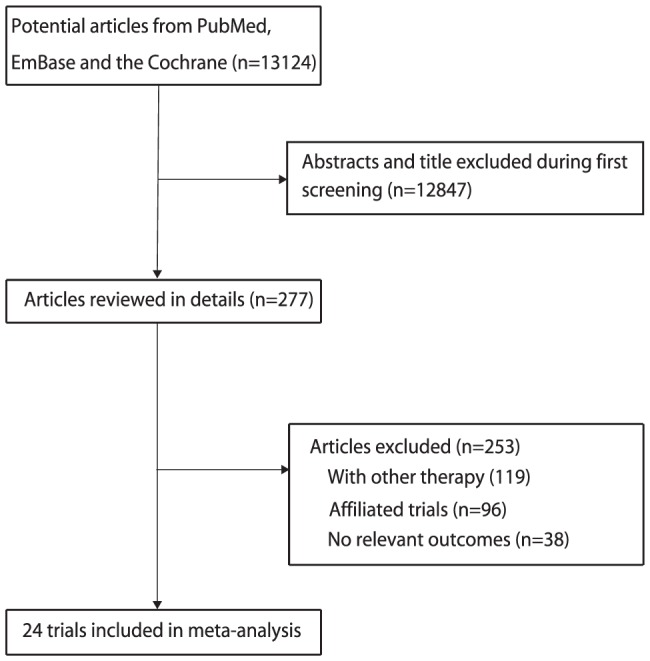
We identified 13,124 articles during our initial electronic search, of which 12,847 were excluded during an initial review of the titles and abstracts. We retrieved the full text for the remaining 277 articles, and 24 RCTs [20]–[43] met the inclusion criteria of our meta-analysis ( Figure 1 ). B vitamin supplementation for primary prevention of major cardiovascular outcome was studied in 12 trials [32]–[43], and the remaining 12 trials studied its use for secondary prevention [20]–[31].
Figure 1. Flow diagram of the literature search and trials selection process.
Characteristics of the Included Studies
Tables 1 and S1 summarizes the baseline characteristics of the 57,952 individuals included in the trials investigated. The follow-up period for participants ranged from 0.7 to 7.3 years, the number of individuals included in each trial ranged from 81 to 12064, and the net change in total homocysteine level ranged from 1.3 to 26.0 µmol/L. In the intervention groups, the dose of folic acid ranged from 0.5 to 40 mg/day, the dose of vitamin B6 ranged from 3 to 250 mg/day, and the dose of vitamin B12 ranged from 6 to 2000 µg/day. In the meta-analysis, the trial outcome was MACE in 23 trials [20]–[36], [38]–[43], total mortality in 20 trials [21]–[31], [33], [35], [37]–[43], cardiac death in 15 trials [21]–[23], [25]–[27], [29]–[31], [34]–[36], [39], [42], [43], MI in 19 trials [21]–[31], [33], [35], [37]–[39], [41]–[43], and stroke in 18 trials [22], [23], [25]–[31], [33]–[35], [37]–[39], [41]–[43]. We restricted the inclusion criteria to RCTs with a minimum follow-up of 6 months to ensure a reliable conclusion.
Table 1. Design and characteristic of trials included in our meta-analysis*.
| Source | Publication year | No. of patients | Mean age, y | Percentage male (%) | Background fortification | Disease status | Primary/Secondary prevention | Baseline folate status (nmol/L) | Baseline homocysteine (µmol/L) | Net decrease in homocysteine (µmol/L) | Intervention | Follow-up (year) | Jadad score |
| Baker F(20) | 2002 | 1882 | NG | NG | No | CHD | Secondary | NG | 11.2 | −1.5 | 5.0 mg folic acid; placebo | 1.7 | 1 |
| The Swiss Heart Study(21) | 2002 | 553 | 63 | 80 | No | Coronary angioplasty | Secondary | NG | 11.2 | −2.9 | 1.0 mg folic acid, 10 mg vitamin B6, and 0.4 mg vitamin B12; Placebo | 1.0 | 3 |
| M Righetti(32) | 2003 | 81 | 64 | 56 | No | ESRD | Primary | 6.06 | 50.3 | −26.0 | 25 mg folic acid; 5 mg folic acid; untreated | 1.0 | 1 |
| VISP Trial Investigators(22) | 2004 | 3680 | 66 | 63 | Yes | Ischemic stroke | Secondary | NG | 12.3 | −2.1 | 25 mg of vitamin B6, 0.4 mg of vitamin B12, and 2.5 mg of folic acid; 200 µg of vitamin B6, 6 µg of vitamin B12, and 20 µg of folic acid | 2.0 | 5 |
| A Liem(23) | 2004 | 283 | 59 | 69 | No | CHD | Secondary | NG | NG | NG | 5 mg folic acid; placebo | 1.0 | 2 |
| EM Wrone(33) | 2004 | 510 | 60 | 50 | Yes | ESRD | Primary | 47.07 | 32.9 | −3.6 | 15 mg folic acid, 12.5 mg vitamin B6, 6 µg vitamin B12; 5 mg folic acid, 12.5 mg vitamin B6, 6 µg vitamin B12; 1 mg folic acid, 12.5 mg vitamin B6, 6 µg vitamin B12 | 2.0 | 4 |
| H Lange(24) | 2004 | 636 | 61 | 77 | No | Coronary Stenting | Secondary | NG | 12.6 | −3.6 | 1.2 mg of folic acid, 48 mg of vitamin B6,and 60 µg of vitamin B12; placebo | 0.7 | 3 |
| A Liem(25) | 2005 | 593 | 65 | 78 | No | CHD | Secondary | 16 | 12.1 | −1.3 | 0.5 mg folic acid; usual care | 3.2 | 3 |
| NORVIT Trial Investigators(26) | 2006 | 3749 | 63 | 74 | No | MI | Secondary | 10.95 | 13.1 | −2.3 | 0.8 mg of folic acid, 0.4 mg of vitamin B12, and 40 mg of vitamin B6; placebo | 3.3 | 5 |
| (HOPE) 2 Investigators(27) | 2006 | 5522 | 69 | 72 | Parial | DM or vascular disease | Secondary | 28.0 | 12.2 | −3.3 | 2.5 mg of folic acid, 50 mg of vitamin B6, and 1 mg of vitamin B12; placebo | 5.0 | 4 |
| ASFAST Study Group(34) | 2006 | 315 | 56 | 68 | Yes | ESRD | Primary | NG | 27.0 | −4.7 | 15 mg folic acid; placebo | 3.6 | 4 |
| M Righetti(35) | 2006 | 114 | 64 | 55 | No | Hemodialysis | Primary | 22.32 | 31.7 | −15.1 | 5 mg folic acid plus vitamin B1 250 mg, vitamin B6 250 mg, vitamin B12 500 µg; untreated | 2.4 | 2 |
| ACA Vianna(36) | 2007 | 186 | 48 | 59 | No | ESRD | Primary | 9.99 | 24.6 | −10.5 | Folic acid 10 mg 3 times a week; placebo | 2.0 | 2 |
| Polyp Prevention Study Group(37) | 2007 | 1021 | 57 | 64 | Yes | Colorectal adenomas | Primary | 23.70 | 9.8 | NG | 1 mg/d of folic acid daily; placebo | 7.0 | 4 |
| Veterans Affairs Site Investigators(38) | 2007 | 2056 | 66 | 98 | Yes | CKD or ESRD and high tHcy | Primary | 35.34 | 22.4 | −5.1 | 40 mg of folic acid, 100 mg of vitamin B6, and 2 mg of vitaminB12; placebo | 3.2 | 4 |
| WAFACS Study Group(39) | 2008 | 5442 | 63 | 0 | No | Health professionals | Primary | NG | NG | NG | 2.5 mg of folic acid, 50 mg of vitamin B6, and 1 mg of vitamin B12; placebo | 7.3 | 3 |
| WENBIT Study Group(28) | 2008 | 3096 | 62 | 80 | No | Coronary angiography | Secondary | NG | 11.1 | −2.8 | Folic acid, 0.8 mg, plus vitamin B12, 0.4 mg, plus vitamin B6, 40 mg; folic acid plus vitamin B12; vitaminB6 alone; placebo | 3.1 | 4 |
| BVAIT Research Group(40) | 2009 | 506 | 61 | 61 | Yes | Initial tHcy>8.5 umol/L | Primary | 21.41 | 9.6 | −2.1 | 5 mg folic acid, 0.4 mg vitamin B12 plus 50 mg vitamin B6; placebo | 3.1 | 3 |
| DIVINe Study Group(41) | 2010 | 238 | 60 | 75 | Yes | Diabetic nephropathy | Primary | 35.12 | 15.6 | −4.8 | 2.5 mg folic acid, 25 mg vitamin B6, and 1 mg vitamin B12; placebo | 3.0 | 4 |
| J Heinz(42) | 2010 | 650 | 61 | 58 | No | ESRD | Primary | 14.1 | 29.0 | −8.6 | 2.5 mg folic acid, 25 µg vitamin B12, and 10 mg vitamin B6; 0.1 mg folic acid, 2 µg vitamin B12, and 0.5 mg vitamin B6 | 2.1 | 5 |
| SEARCH Collaborative Group(29) | 2010 | 12064 | 64 | 83 | No | MI | Secondary | 16.76 | 13.5 | −3.8 | 2 mg folic acid plus 1 mg vitamin B12 daily; placebo | 6.7 | 4 |
| SU.FOL.OM3 Collaborative Group(30) | 2010 | 2501 | 61 | 79 | No | MI, angina, or ischaemic stroke | Secondary | 15.29 | 12.8 | −2.7 | 5-methyltetrahydrofolate (560 µg), vitamin B-6 (3 mg), and vitamin B-12 (20 µg); placebo | 4.7 | 5 |
| FAVORIT Study Group(43) | 2011 | 4110 | 52 | 63 | Yes | Kidney transplant recipients | Primary | NG | 16.4 | −4.1 | 5.0 mg folic acid, 50 mg vitamin B6, and 1.0 mg vitamin B12; 1.4 mg vitamin B6 and 0.002 mg vitamin B12 | 4.0 | 4 |
| VITATOPS Study Group(31) | 2012 | 8164 | 63 | 64 | Partial | TIA or stroke | Secondary | NG | 14.3 | −3.8 | 2 mg folic acid, 25 mg vitamin B6, and 0·5 mg vitamin B12; placebo | 3.4 | 5 |
*CHD: coronary heart disease; ESRD: End-stage renal disease; MI: myocardial infarction; DM: diabetes mellitus; CKD: chronic kidney disease; TIA: transient ischaemic attack; NG: not give.
Risk of Bias in Individual Trials
The quality of the trials was assessed using the Jadad score (45) and are summarized in Table 1 . We considered a score ≥4 to indicate a high-quality study. Five trials [22], [26], [30], [31], [42] had a Jadad score of 5, 9 trials [27]–[29], [33], [34], [37], [38], [41], [43] scored 4, 5 trials [21], [24], [35], [39], [40] scored 3, 3 trials [23], [35], [36] scored 2, and the remaining 2 trials [20], [32] scored 1.
Effects of B Vitamins on Major Cardiovascular Outcomes
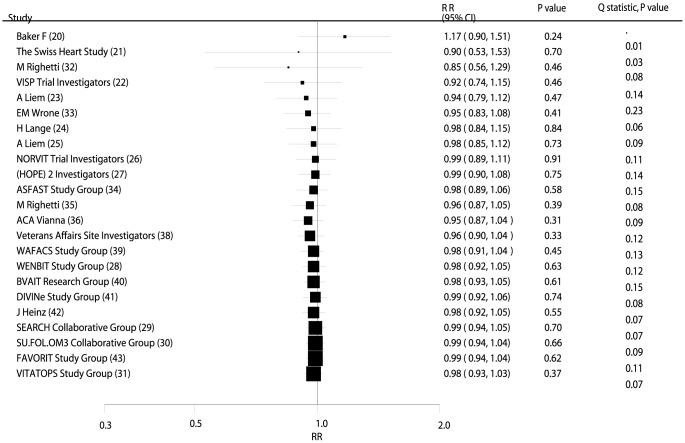
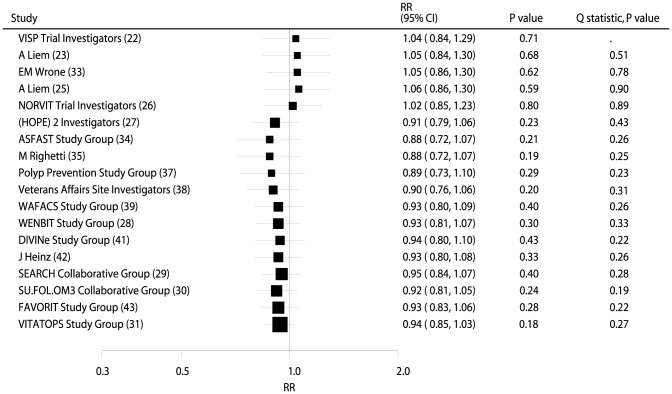
Data from 56,925 individuals was used to assess the effect of B vitamin supplementation on MACE and included 10,917 MACE. Overall, B vitamin supplementation reduced the risk of MACE by 2%, but this was not statistically significant (RR, 0.98; 95% CI: 0.93–1.03; P = 0.37, Figure 2 and Figure S1). Heterogeneity was observed in the magnitude of the effect across the trials (P = 0.07). However, after sequential exclusion of each trial from all pooled analysis, the conclusion was not affected by the exclusion of any specific trial.
Figure 2. Cumulative meta-analysis of the B vitamins supplementation for major adverse cardiovascular event.
RR, relative risk; CI, confidence interval.
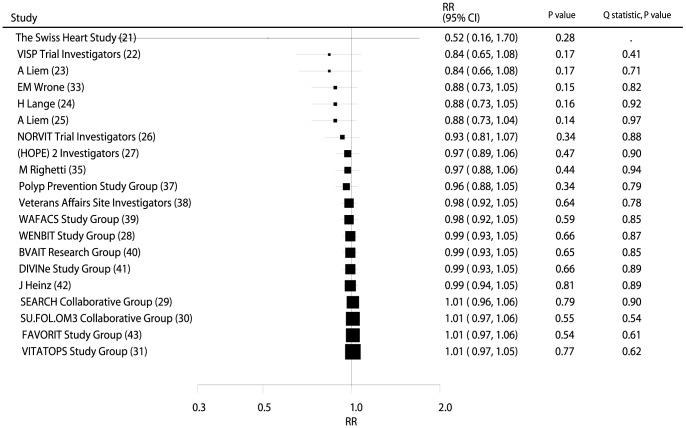
Data from 55,482 individuals was used to assess the effect of B vitamin supplementation on total mortality and included 7,203 deaths. No significant difference in the number of deaths was observed between participants receiving B vitamins compared with those receiving placebo (RR, 1.01; 95% CI: 0.97–1.05; P = 0.77, without evidence of heterogeneity; Figure 3 and Figure S2).
Figure 3. Cumulative meta-analysis of the B vitamins supplementation for total mortality.
RR, relative risk; CI, confidence interval.
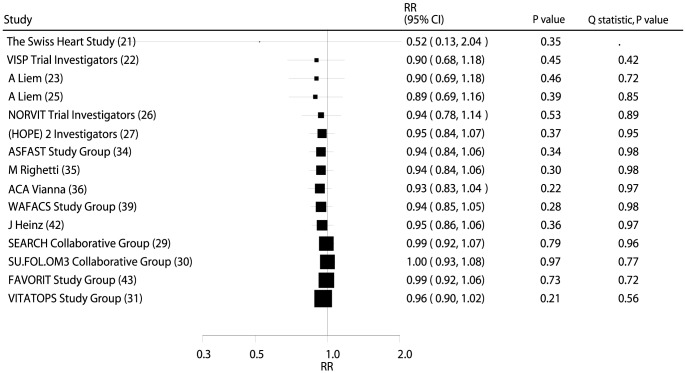
Data from 47,926 individuals was used to assess the effect of B vitamin supplementation on cardiac death and included 3,422 cardiac deaths. B vitamin supplementation caused a 4% reduction in cardiac death; however, this was not a significant change (RR, 0.96; 95% CI: 0.90–1.02; P = 0.21, without evidence of heterogeneity; Figure 4 and Figure S3).
Figure 4. Cumulative meta-analysis of the B vitamins supplementation for cardiac death.
RR, relative risk; CI, confidence interval.
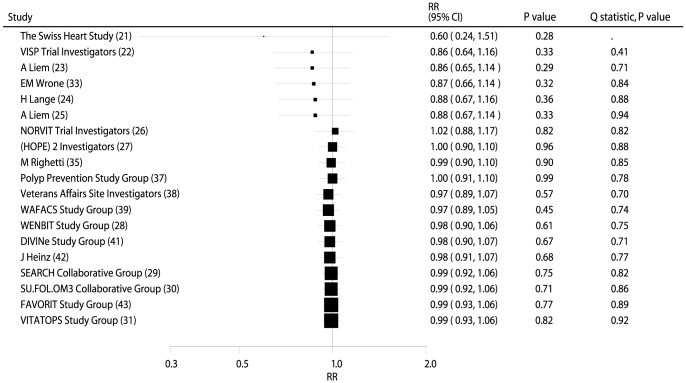
Data from 54,976 individuals was used to assess the effect of B vitamin supplementation on MI, and included 3,623 MIs. There were no significant differences between participants receiving B vitamins compared with placebo for MI (RR, 0.99; 95% CI: 0.93–1.06; P = 0.82, without evidence of heterogeneity, Figure 5 and Figure S4).
Figure 5. Cumulative meta-analysis of the B vitamins supplementation for myocardial infarction.
RR, relative risk; CI, confidence interval.
Data from 54,102 individuals and 2,465 stroke events was used to assess the effect of B vitamin supplementation on stroke. B vitamin supplementation reduced incident stroke by 6%, but this was not a significant reduction (RR, 0.94; 95% CI: 0.85–1.03; P = 0.18; with no statistical heterogeneity, Figure 6 and Figure S5). Sensitivity analysis was conducted for the incidence of stroke. However, after sequential exclusion of each trial from all pooled analysis, the conclusion was not affected by the exclusion of any specific trial.
Figure 6. Cumulative meta-analysis of the B vitamins supplementation for stroke.
RR, relative risk; CI, confidence interval.
Cumulative Meta-Analysis
On cumulative meta-analysis for MACE ( Figure 2 ), the original nonsignificant result for a B vitamin effect persisted, and the effect was slight and borderline nonsignificant. Similarly, the nonsignificant result persisted when cumulative meta-analyses for total mortality ( Figure 3 ), cardiac death ( Figure 4 ), MI ( Figure 5 ), and stroke ( Figure 6 ) were conducted.
Meta-Regression and Subgroup Analyses
Heterogeneity testing for the analysis showed a P<0.10 for MACE. We concluded that heterogeneity was statistically significant in the overall analysis. We, therefore, conducted a meta-regression analysis [50] for MACE that included the number of participants, mean age, percentage of men, baseline homocysteine level, baseline folate level, dose of folic acid, dose of vitamin B6, dose of vitamin B12, net decrease in homocysteine level, and duration of follow-up. Overall, we detected a net decrease in homocysteine (P = 0.043) that contributed to the association between B vitamin supplementation and MACE (Figure S6). However, the number of participants (P = 0.435), mean age (P = 0.763), percentage of men (P = 0.866), baseline homocysteine level (P = 0.094), baseline folate level (P = 0.797), dose of folic acid (P = 0.840), dose of vitamin B6 (P = 0.933), dose of vitamin B12 (P = 0.614), and duration of follow-up (P = 0.186) were not significant factors contributing to the association between B vitamin supplementation and MACE (Figure S6).
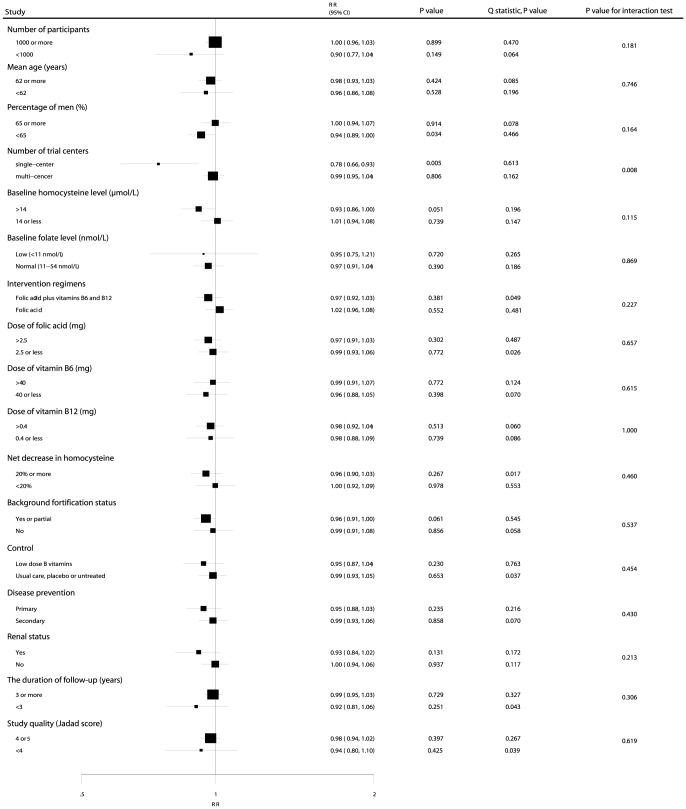
Subgroup analyses were conducted for MACE to minimize heterogeneity among the included trials and to evaluate the effect of B vitamin supplementation in specific subpopulations. B vitamin supplementation significantly reduced the risk of MACE if the percentage of men in the study was <65% (RR, 0.94; 95% CI: 0.89–1.00; P = 0.034; Figure 7 ) and the study was a single-center trial (RR, 0.78; 95% CI: 0.66–0.93; P = 0.005; Figure 7 ). Furthermore, we noted that B vitamin supplementation was associated with a nonsignificant reduction in MACE when the baseline homocysteine level was>14 µmol/L (RR, 0.93; 95% CI: 0.86–1.00; P = 0.051; Figure 7 ) or dietary grain fortification had been taken to boost the folate level (RR, 0.96; 95% CI: 0.91–1.00; P = 0.061; Figure 7 ). No other significant differences were identified in the predefined factors between those who took B vitamin supplements and those who took placebo ( Figure 7 ). Furthermore, there was no significant difference in the effect of B vitamins on MACE between the 2 subgroups by factors that could affect the treatment effect except center of trials (P = 0.008; Figure 7 ).
Figure 7. Subgroup analysis for the effect of B vitamins supplementation on major cardiovascular events.
RR, relative risk; CI, confidence interval.
Publication Bias
Review of funnel plots did not rule out the potential for publication bias for MACE, total mortality, cardiac death, MI, and stroke (Figure S7). However, the Egger [53] and Begg tests [54] showed no evidence of publication bias for MACE (P value for Egger, 0.342; P value for Begg, 0.267), total mortality (P value for Egger, 0.312; P value for Begg, 0.183), cardiac death (P value for Egger, 0.631; P value for Begg, 0.621), MI (P value for Egger, 0.816; P value for Begg, 0.944), and stroke (P value for Egger, 0.992; P value for Begg, 1.000).
Discussion
Several observational studies [1]–[5] have suggested that B vitamin supplementation may improve major cardiovascular outcomes. However, this effect has not been confirmed to date. In the present study, we included RCTs and explored all possible correlations between B vitamin supplementation and the outcomes of MACE, total mortality, cardiac death, MI, and stroke. Furthermore, we conducted a cumulative meta-analysis to explore the value of routine B vitamin supplementation in clinical practice. This large quantitative study included 57,952 individuals from 24 trials with a broad range of baseline characteristics. Our results suggest that B vitamin supplementation has no significant effect on MACE, total mortality, cardiac death, MI, or stroke. Furthermore, in a cumulative meta-analysis, this B vitamin effect persisted and remained nonsignificant.
We reviewed previous meta-analyses and found that the hypothesized protective effect of B vitamins comes from observational studies [9], which, we suggest, may overestimate the effect on major cardiovascular outcomes. Several systematic reviews and meta-analyses of RCTs [12]–[17] have evaluated the impact of folic acid supplementation on major cardiovascular outcomes and have found no evidence to support a significant association. In a meta-analysis, Wang et al. [19] indicated that B vitamins significantly reduced the risk of stroke, but subgroup analysis showed that this effect was based on trials that included only participants without a previous stroke history. Lee et al. [55] suggested that folic acid supplementation administered as primary prevention significantly reduced the risk of stroke at>3 years of follow-up and that the homocysteine-lowering effect was>20%. However, this meta-analysis included trials with a male: female ratio of >2. Gao et al. [56] found that B vitamin supplementation given to reduce homocysteine levels influenced the risk of stroke events, especially in subgroups with a follow-up of>3 years (RR, 0.92; 95% CI: 0.84–1.00), without a history of grain fortification (RR, 0.91; 95% CI: 0.83–1.00), or without chronic kidney disease (RR, 0.93; 95% CI: 0.86–1.01), but these differences were not significant. In the present study, all pooled RR estimate points for stroke were <1 (evidence accumulated up to 2006) with a potential trend toward moving leftward in the cumulative meta-analysis of B vitamin supplementation. We suggest a potential protective effect of B vitamins on stroke events. However, this trend is not obvious and requires validation.
There was no significant difference between B vitamin supplementation and placebo for the RR of major cardiovascular outcomes. However, several trials included in our study reported inconsistent results. Schnyder et al. [21] indicated that homocysteine-lowering therapy with B complex vitamins reduced the risk of MACE in patients after percutaneous coronary intervention. However, Lange et al. [24] indicated that B vitamin complex supplementation after coronary stenting increases the risk of in-stent restenosis and the need for target-vessel revascularization. The possible reasons for these inconsistencies are as follows: (1) a history of B vitamin supplementation may mask treatment effect; (2) patients in our meta-analysis had variable disease status, which also affects major cardiovascular outcomes; (3) elevated homocysteine levels impair vascular function, but the effect of homocysteine on cardiovascular outcomes may not be directly related to coronary disease pathogenesis, which is largely attributable to plaque formation and rupture [57]; and (4) high doses of B vitamin may adversely affect vascular remodeling and myocardial repair and may increase complications and death in patients with cardiovascular disease [58]. Therefore, although B vitamin supplementation may have direct effects on major cardiovascular outcomes, these effects may be counterbalanced by other effects.
Cumulative meta-analyses suggest that a nonsignificant response persisted for MACE, total mortality, cardiac death, MI, and stroke. However, the proposed nonsignificant protective effect of B vitamin supplementation for MI has been refuted by the NORVIT Trial Investigators [26], who specifically included individuals with MI, which may have contributed to higher recurrence and suggests a nonsignificant harmful effect for B vitamin supplementation. However, the proposed nonsignificant harmful effect of B vitamin supplementation for stroke was refuted by the HOPE 2 Trial [27]. This trial suggested that B vitamin supplementation reduced the risk of stroke by 25% (95% CI: 0.59–0.97), but this may be due to a considerably lower number of stroke events than coronary events. In addition, the CIs of the estimated risk reduction were wide. Furthermore, the results were not adjusted for the multiplicity of potential outcomes.
In our study, subgroup analysis suggested that B vitamin supplementation reduced the risk of MACE in specific subgroups. The main findings were as follows. First, although no significant difference was observed in MACE for the B vitamin supplementation group compared with the placebo group, in populations with a baseline total homocysteine level >14 µmol/L and those with a net decrease in homocysteine level of >20% there seemed to be a slight, but nonsignificant, benefit. In this study, baseline homocysteine levels were only available for whole populations, not individuals. Second, folate fortification of the grain supply may influence the risk of MACE, because it reduces the prevalence of low folate levels and high total homocysteine levels, so the mean difference in total homocysteine levels between groups narrows. Fortification reduces the number of participants with a high total homocysteine level, which is the population most likely to benefit from supplementation [59]. In this study, we found that B vitamin supplementation reduced the risk of MACE if participants had a background of grain fortification. Those with background fortification received higher doses of vitamin B12, which may be more effective at preventing MACE. Third, the protective effect of B vitamins was more evident if the percentage of men in the study was <65%. Among men, there is a higher rate of smoking and drinking, both of which increase the risk of MACE. Therefore, the effect of B vitamins on the risk of MACE in studies with a higher percentage of male subjects may be reduced or balanced by a higher rate of smoking and drinking. Fourth, there was a significant difference between single-center and multi-center trials in the risk for MACE. This difference could be due to chance because only 6 trials were single-center, and they had a low sample size and low rate of cardiovascular disease occurrence. Fifth, no significant differences were observed in MACE between participants with a low folate level and those with a normal folate level. Folate level data were not available for several trials, so this conclusion was similar to those of a previous meta-analysis [60]. Sixth, the B vitamin dose showed no significant effect because all doses investigated were in the mega-dose range, and there may have been differences in the control groups in different studies. Seventh, B vitamin supplementation seems to offer a benefit for primary prevention of MACE but not for secondary prevention. Participants with pre-existing cardiovascular disease have higher recurrence and mortality rates. Finally, B vitamins seem to benefit participants with kidney disease but not those without kidney disease; patients with kidney disease may have higher homocysteine concentrations and a higher rate of cardiovascular disease.
The limitations of our study are as follows: (1) different types and doses of supplements might result in bias; (2) the use of background B vitamin supplementation might have impaired our ability to identify a treatment effect; (3) differences in diagnosis and reporting may have contributed to the differences in major cardiovascular outcomes in some trials; (4) stratified analyses based on background therapy in patients with previous disease were unavailable; (5) data on hemoglobin levels were not available, so we could not evaluate the potential confounding role of hemoglobin when evaluating the effect of vitamins B on the risk of major cardiovascular outcomes; (6) several trials with low Jadad score were included in our study, which could bias the results; and (7) since inherent assumptions are made for any meta-analysis, the analysis used pooled data, and individual patient data were unavailable; this restricted us from performing a more detailed analysis and obtaining more comprehensive results.
In conclusion, we found that B vitamin supplementation has little or no effect on major cardiovascular outcomes across various patient populations. We believe the use of B vitamin supplementation as a structured intervention in everyday clinical practice is not justified. A meta-analysis of individual patient data might be more appropriate to determine how the duration and dose of supplementation influence treatment outcomes.
Supporting Information
Effect of B vitamins supplementation on the risk of major cardiovascular events.
(EPS)
Effect of B vitamins supplementation on the risk of total mortality.
(EPS)
Effect of B vitamins supplementation on the risk of cardiac death.
(EPS)
Effect of B vitamins supplementation on the risk of myocardial infarction.
(EPS)
Effect of B vitamins supplementation on the risk of stroke.
(EPS)
Meta-regression of number of participants, mean age, percentage male, baseline homocyteine, baseline folate status, dose of folic acid, dose of vitamin B6, dose of vitamin B12, net decrease in homocysteine, and the duration of the follow-up periods.
(EPS)
Funnel plots for major cardiovascular events, total mortality, cardiac death, myocardial infarction, and stroke.
(EPS)
Additional characteristic of trials included in our meta-analysis.
(DOC)
PRISMA Checklist.
(DOC)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by Disciplines Group Construction Project of Pudong Health Bureau of Shanghai (PWZxkq2011-01) and Shanghai key speciality of traditional Chinese medicine (ZYXK2012010). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Folsom AR, Nieto FJ, McGovern PG, Tsai MY, Malinow MR, et al. (1998) Prospective study of coronary heart disease incidence in relation to fasting total homocysteine, related geneitic polymorphisms, and B vitamins: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 98: 204–210. [DOI] [PubMed] [Google Scholar]
- 2. Verhoef P, Kok FJ, Kruyssen DA, Schouten EG, Witteman JC, et al. (1997) Plasma total homocysteine, B vitamins, and risk of atherosclerosis. Arterioscler Thromb Vasc Biol 17: 985–995. [DOI] [PubMed] [Google Scholar]
- 3. Schwartz SM, Siscovick DS, Malinow MR, Rosendaal FR, Beverly RK, et al. (1997) Myocardial infarction in young women in relation to plasma total homocysteine, folate, and a common variant in the methylenetetrahydrofolate reductase gene. Circulation 96: 412–7. [DOI] [PubMed] [Google Scholar]
- 4. Chambers JC, Obeid OA, Refsum H, Ueland P, Hackett D, et al. (2000) Plasma homocysteine concentrations and risk of coronary heart disease in UK Indian, Asian and European men. Lancet 355: 523–527. [DOI] [PubMed] [Google Scholar]
- 5. Robinson K, Mayer EL, Miller DP, Green R, van Lente F, et al. (1995) Hyperhomocysteinemia and low pyridoxal phosphate. Common and independent reversible risk factors for coronary artery disease. Circulation 92: 2825–2830. [DOI] [PubMed] [Google Scholar]
- 6. Perry IJ, Refsum H, Morris RW, Ebrahim SB, Ueland PM, et al. (1995) Prospective study of serum total homocysteine concentration and risk of stroke in middle-aged British men. Lancet 346: 1395–1398. [DOI] [PubMed] [Google Scholar]
- 7. Bots ML, Launer LJ, Lindemans J, Hoes AW, Hofman A, et al. (1999) Homocysteine and short-term risk of myocardial infarction and stroke in the elderly: the Rotterdam Study. Arch Intern Med 159: 38–44. [DOI] [PubMed] [Google Scholar]
- 8. Evers S, Koch HG, Grotemeyer KH, Lange B, Deufel T, et al. (1997) Features, symptoms, and neurophysiological findings in stroke associated with hyperhomocysteinemia. Arch Neurol 54: 1276–1282. [DOI] [PubMed] [Google Scholar]
- 9. The Homocysteine Studies Collaboration (2002) Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA 288: 2015–2022. [DOI] [PubMed] [Google Scholar]
- 10. Homocysteine Lowering Trialists' Collaboration (1998) Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. BMJ 316: 894–898. [PMC free article] [PubMed] [Google Scholar]
- 11. Homocysteine Lowering Trialists' Collaboration (2005) Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr 82: 806–812. [DOI] [PubMed] [Google Scholar]
- 12. Bazzano LA, Reynolds K, Holder KN, He J (2006) Effect of folic acid supplementation on risk of cardiovascular diseases a meta-analysis of randomized controlled trials. JAMA 296: 2720–2726. [DOI] [PubMed] [Google Scholar]
- 13. Zhou Y-H, Tang J-Y, Wu M-J, Lu J, Wei X, et al. (2011) Effect of folic acid supplementation on cardiovascular outcomes: a systematic review and meta-analysis. PLoS ONE 6: e25142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jardine MJ, Kang A, Zoungas S, Navaneethan SD, Ninomiya T, et al. (2012) The effect of folic acid based homocysteine lowering on cardiovascular events in people with kidney disease: systematic review and meta-analysis. BMJ 344: e3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pan Y, Guo LL, Cai LL, Zhu XJ, Shu JL, et al. (2012) Homocysteine-lowering therapy does not lead to reduction in cardiovascular outcomes in chronic kidney disease patients: a meta-analysis of randomised, controlled trials. Br J Nutr 108: 400–7. [DOI] [PubMed] [Google Scholar]
- 16. Clarke R, Halsey J, Lewington S, Lonn E, Armitage J, et al. (2010) Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: Meta-analysis of 8 randomized trials involving 37 485 individuals. Arch Intern Med 170: 1622–31. [DOI] [PubMed] [Google Scholar]
- 17. Miller ER, Juraschek S, Pastor-Barriuso R, Bazzano LA, Appel LJ, et al. (2010) Meta-analysis of folic acid supplementation trials on risk of cardiovasculardisease and risk interaction with baseline homocysteine levels. Am J Cardiol 106: 517–27. [DOI] [PubMed] [Google Scholar]
- 18. C Zhang, FL Chi, TH Xie, YH Zhou (2013) Effect of B-vitamin Supplementation on Stroke: a Meta-Analysis of Randomized Controlled Trials. PLoS One 8: e81577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang X, Qin X, Demirtas H, Li J, Mao G, et al. (2007) Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet 369: 1876–82. [DOI] [PubMed] [Google Scholar]
- 20.Baker F, Picton D, Blackwood S, Hunt J, Erskine M, et al. (2002) Blinded comparison of folic acid and placebo in patients with ischemic heart disease: an outcome trial [abstract]. Circulation (suppl 2): 741S.
- 21. Schnyder G, Roffi M, Flammer Y, Pin R, Hess OM (2002) Effect of homocysteine-lowering therapy with folic acid, vitamin B12, and vitamin B6 on clinical outcome after percutaneous coronary intervention: the Swiss Heart Study: a randomized controlled trial. JAMA 288: 973–979. [DOI] [PubMed] [Google Scholar]
- 22. Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, et al. (2004) Lowering Homocysteine in Patients With Ischemic Stroke to Prevent Recurrent Stroke, Myocardial Infarction, and Death The Vitamin Intervention for Stroke Prevention (VISP) Randomized Controlled Trial. JAMA 291: 565–575. [DOI] [PubMed] [Google Scholar]
- 23. Liem AH, van Boven AJ, Veeger NJ, Withagen AJ, Robles de Medina RM, et al. (2004) Folic Acid on Risk Diminishment After Acute Myocarial Infarction Study Group. Efficacy of folic acid when added to statin therapy in patients with hypercholesterolemia following acute myocardial infarction: a randomised pilot trial. Int J Cardiol 93: 175–179. [DOI] [PubMed] [Google Scholar]
- 24. Lange H, Suryapranata H, De Luca G, Börner C, Dille J, et al. (2004) Folate therapy and in-stent restenosis after coronary stenting. N Engl J Med 350: 2673–2681. [DOI] [PubMed] [Google Scholar]
- 25. Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, Jukema JW, van Veldhuisen DJ (2005) Secondary prevention with folic acid: results of the Goes extension study. Heart 91: 1213–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. The NORVIT Trial Investigators (2006) Homocysteine Lowering and Cardiovascular Events after Acute Myocardial Infarction. N Engl J Med 354: 1578–88. [DOI] [PubMed] [Google Scholar]
- 27. The Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators (2006) Homocysteine Lowering with Folic Acid and B Vitamins in Vascular Disease. N Engl J Med 354: 1567–77. [DOI] [PubMed] [Google Scholar]
- 28. Ebbing M, Bleie Ø, Ueland PM, Nordrehaug JE, Nilsen DW, et al. (2008) Mortality and Cardiovascular Events in Patients Treated With Homocysteine-Lowering B Vitamins After Coronary Angiography A Randomized Controlled Trial. JAMA 300: 795–804. [DOI] [PubMed] [Google Scholar]
- 29. Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group (2010) Effects of Homocysteine-Lowering With Folic Acid Plus Vitamin B12 vs Placebo on Mortality and Major Morbidity in Myocardial Infarction Survivors A Randomized Trial. JAMA 303: 2486–2494. [DOI] [PubMed] [Google Scholar]
- 30. The SU.FOL.OM3 Collaborative Group (2010) Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled Trial. BMJ 341: c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. The VITATOPS Trial Study Group (2010) B vitamins in patients with recent transient ischaemic attack or stroke in the VITAmins TO Prevent Stroke (VITATOPS) trial: a randomised, double-blind, parallel, placebo-controlled trial. Lancet Neurol 9: 855–65. [DOI] [PubMed] [Google Scholar]
- 32. Righetti M, Ferrario GM, Milani S, Serbelloni P, La Rosa L, et al. (2003) Effects of folic acid treatment on homocysteine levels and vascular disease in hemodialysis patients. Med Sci Monit 9: PI19–PI24. [PubMed] [Google Scholar]
- 33. Wrone EM, Hornberger JM, Zehnder JL, McCann LM, Coplon NS, et al. (2004) Randomized trial of folic acid for prevention of cardiovascular events in endstage renal disease. J AmSoc Nephrol 15: 420–426. [DOI] [PubMed] [Google Scholar]
- 34. Zoungas S, McGrath BP, Branley P, Kerr PG, Muske C, et al. (2006) Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. J Am Coll Cardiol 47: 1108–1116. [DOI] [PubMed] [Google Scholar]
- 35. Righetti M, Serbelloni P, Milani S, Ferrario G (2006) Homocysteine- lowering vitamin B treatment decreases cardiovascular events in hemodialysis patients. Blood Purif 24: 379–386. [DOI] [PubMed] [Google Scholar]
- 36. Vianna ACA, Mocelin AJ, Matsuo T, Morais-Filho D, Largura A, et al. (2007) Uremic hyperhomocysteinemia: A randomized trial of folate treatment for the prevention of cardiovascular events. Hemodialysis International 11: 210–216. [DOI] [PubMed] [Google Scholar]
- 37. The Polyp Prevention Study Group (2007) Folic Acid for the Prevention of Colorectal Adenomas A Randomized Clinical Trial. JAMA 297: 2351–2359. [DOI] [PubMed] [Google Scholar]
- 38. The Veterans Affairs Site Investigators (2007) Effect of Homocysteine Lowering on Mortality and Vascular Disease in Advanced Chronic Kidney Disease and End-stage Renal Disease A Randomized Controlled Trial. JAMA 298: 1163–1170. [DOI] [PubMed] [Google Scholar]
- 39. Albert CM, Cook NR, Gaziano JM, Zaharris E, MacFadyen J, et al. (2008) Effect of Folic Acid and B-Vitamins on Risk of Cardiovascular Events and Total Mortality among Women at High Risk for Cardiovascular Disease: A Randomized Trial. JAMA 299: 2027–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. The BVAIT Research Group (2009) High-Dose B Vitamin Supplementation and Progression of Subclinical Atherosclerosis A Randomized Controlled Trial. Stroke 40: 730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. House AA, Eliasziw M, Cattran DC, Churchill DN, Oliver MJ, et al. (2010) Effect of B-Vitamin Therapy on Progression of Diabetic Nephropathy A Randomized Controlled Trial. JAMA 303: 1603–1609. [DOI] [PubMed] [Google Scholar]
- 42. Heinz J, Kropf S, Domröse U, Westphal S, Borucki K, et al. (2010) B Vitamins and the Risk of Total Mortality and Cardiovascular Disease in End-Stage Renal Disease: Results of a Randomized Controlled Trial. Circulation 121: 1432–1438. [DOI] [PubMed] [Google Scholar]
- 43. The FAVORIT Study Group (2011) Homocysteine-Lowering and Cardiovascular Disease Outcomes in Kidney Transplant Recipients Primary Results From the Folic Acid for Vascular Outcome Reduction in Transplantation Trial. Circulation 123: 1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) PRISMA Group (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Plos Medicine 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jadad AR, Moore RA, Carroll D (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 46. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 47. Ades AE, Lu G, Higgins JP (2005) The interpretation of random-effects metaanalysis in decision models. Med Decis Making 25: 646–54. [DOI] [PubMed] [Google Scholar]
- 48.Deeks JJ, Higgins JPT, Altman DG (2008) Analyzing data and undertaking meta-analyses. In: Higgins J, Green S, eds.Cochrane Handbook for Systematic Reviews of Interventions 5.0.1. Oxford, UK: The Cochrane Collaboration: chap 9.
- 49. Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thompson SG, Higgins JP (2002) How should meta-regression analyses be undertaken and interpreted? Stat Med 21: 1559–73. [DOI] [PubMed] [Google Scholar]
- 51. Altman DG, Bland JM (2003) Interaction revisited: the difference between two estimates. BMJ 326: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tobias A (1999) Assessing the influence of a single study in meta-analysis. Stata Tech Bull 47: 15–17. [Google Scholar]
- 53. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 55. Lee M, Hong KS, Chang SC, Saver JL (2010) Efficacy of Homocysteine-Lowering Therapy With Folic Acid in Stroke Prevention: A Meta-Analysis. Stroke 41: 1205–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ji Y, Tan S, Xu Y, Chandra A, Shi C, et al. (2013) Vitamin B supplementation, homocysteine levels, and the risk of cerebrovascular disease: A meta-analysis. Neurology 81: 1–10. [DOI] [PubMed] [Google Scholar]
- 57. Eikelboom JW, Lonn E, Genest J Jr, Hankey G, Yusuf S (1999) Homocysteine and cardiovascular disease: a critical review of the epidemiologic evidence. Ann Intern Med 131: 363–375. [DOI] [PubMed] [Google Scholar]
- 58. Loscalzo J (2006) Homocysteine trials: clear outcomes for complex reasons. N Engl J Med 354: 1629–1632. [DOI] [PubMed] [Google Scholar]
- 59. Stampfer MJ, Malinow MR, Willett WC, Newcomer LM, Upson B, et al. (1992) A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA 268: 877–81. [PubMed] [Google Scholar]
- 60. Holmes MV, Newcombe P, Hubacek JA, Sofat R, Ricketts SL, et al. (2011) Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: a meta-analysis of genetic studies and randomised trials. Lancet 378: 584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of B vitamins supplementation on the risk of major cardiovascular events.
(EPS)
Effect of B vitamins supplementation on the risk of total mortality.
(EPS)
Effect of B vitamins supplementation on the risk of cardiac death.
(EPS)
Effect of B vitamins supplementation on the risk of myocardial infarction.
(EPS)
Effect of B vitamins supplementation on the risk of stroke.
(EPS)
Meta-regression of number of participants, mean age, percentage male, baseline homocyteine, baseline folate status, dose of folic acid, dose of vitamin B6, dose of vitamin B12, net decrease in homocysteine, and the duration of the follow-up periods.
(EPS)
Funnel plots for major cardiovascular events, total mortality, cardiac death, myocardial infarction, and stroke.
(EPS)
Additional characteristic of trials included in our meta-analysis.
(DOC)
PRISMA Checklist.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.