Abstract
We have shown that ex vivo pre-conditioning of bone marrow-derived dendritic cells (DC) with low molecular weight hyaluronan (LMW HA) induces antitumor immunity against colorectal carcinoma (CRC) in mice. In the present study we investigated the effects of LMW HA priming on human-tumor-pulsed monocytes-derived dendritic cells (DC/TL) obtained from healthy donors and patients with CRC. LMW HA treatment resulted in an improved maturation state of DC/TL and an enhanced mixed leucocyte reaction activity in vivo. Importantly, pre-conditioning of DC/TL with LMW HA increased their ability to migrate and reduced their attraction to human tumor derived supernatants. These effects were associated with increased CCR7 expression levels in DC. Indeed, a significant increase in migratory response toward CCL21 was observed in LMW HA primed tumor-pulsed monocyte-derived dendritic cells (DC/TL/LMW HA) when compared to LWM HA untreated cells (DC/TL). Moreover, LMW HA priming modulated other mechanisms implicated in DC migration toward lymph nodes such as the metalloproteinase activity. Furthermore, it also resulted in a significant reduction in DC migratory capacity toward tumor supernatant and IL8 in vitro. Consistently, LMW HA dramatically enhanced in vivo DC recruitment to tumor-regional lymph nodes and reduced DC migration toward tumor tissue. This study shows that LMW HA –a poorly immunogenic molecule- represents a promising candidate to improve human DC maturation protocols in the context of DC-based vaccines development, due to its ability to enhance their immunogenic properties as well as their migratory capacity toward lymph nodes instead of tumors.
Introduction
Colorectal carcinoma (CRC) is a one of the leading causes of cancer-related death worldwide. Unfortunately, there is no curative treatment for patients who are not amenable of surgical resection [1]. Thus, new therapeutic strategies are needed for advanced CRC patients and those based on mounting immune responses against tumors might play a key role [2], [3].
Dendritic cells (DC) are professional antigen presenting cells that have the capacity to generate innate and adaptive immune responses, and are essential to induce immunity against cancer [4]. DC migrate from peripheral blood to different organs and tissues wherein they capture antigens and process them to form MHC-II-peptide complexes. This non-activated (immature) DC can present self-antigens to T cells, which leads to immune tolerance either through T cell deletion or through the differentiation of regulatory or suppressor T cells [5]. By contrast, activated (mature) antigen-loaded DC, which express some specific molecules such as CD40, CD80 and CD86, can launch the differentiation of antigen-specific T cells into effect tor T cells with unique functions and cytokine profiles [6]. The use of mature DC to prime responses to tumor associated antigens (TAA) provides a promising approach for cancer immunotherapy, but clinically relevant responses have been rather poor until now [7].
Issues regarding the optimal dose and route of administration for DC vaccination used in cancer therapy remain to be addressed. In fact, only a small proportion of DC intradermally injected reaches the draining lymph nodes [8], [9]. We have previously demonstrated both in vivo and in vitro that DC pre-conditioning with low molecular weight hyaluronan (LMW HA) is able to enhance DC migration toward regional lymph nodes in mice [10]. This effect was shown to be independent of two of the HA receptors, CD44 and TLR4, and to be likely mediated, at least partially, by an increased CCR7 expression [11]. CCR7 is a key molecule which interacts with the chemokines CCL19 and CCL21 and it was found to be crucial for guiding DC migration from peripheral tissues to draining lymph nodes [12]–[14].
A number of cytokines and factors have been used as culture medium supplement in order to increase such migratory capacity in DC, including PGE2 and the TLR3 agonist Poly (I:C) [15]. However, these compounds might also induce the expression of IDO (indoleamine-2,3-dioxygenase) and thereby would eventually suppress immune responses or generate tolerogenic DC [15], [16]. In addition, it has been observed that poly (I:C) and LPS can affect the maturation process of peripheral blood monocytes by inducing the so-called suppressors of cytokine signaling (SOCS) activation [17], [18].
Tumors induce immunosuppression by secreting different cytokines and immunosuppressive molecules which finally interfere with approaches aimed at generating anti-cancer immunity [19]. Among them, IL-8 is a chemokine produced in large amounts by the majority of tumors [20]. IL-8 has been implicated in the resistance to antiangiogenic therapies [21] and in the failure of DC-based immunotherapy protocols [22]. In fact, DC can produce and respond to IL-8, a molecule shown to inhibit anti-tumor immune responses when it was peri/intra-tumoraly injected [23].
Hyaluronic acid (HA) is a lineal, large and ubiquitous glycosaminoglycan with a simple chemical structure found mainly in tissues undergoing cell proliferation, regeneration and repair [24]. HA functions are well known to be size-dependent and the LMW HA form has been shown to induce the expression of pro-inflammatory genes such as IL-8, IL-12, TNF-α and inducible NO synthase in many types of cells including DC [25], [26]. In addition, LMW HA or its small fragments were shown to stimulate T cell responses by activating and up-regulating co-stimulatory molecules on DC in a CD44-independent and TLR4-dependent manner [27], [28]. Moreover, it has also been demonstrated that LMW HA can act as an adjuvant promoting antigen-specific T cell responses in vivo through TLR2 stimulation [29].
The aim of this work was to evaluate the effects of LMW HA pre-conditioning on tumor-pulsed human DC obtained from both healthy donors (HD) and CRC patients. We found that LMW HA induced DC/TL maturation state able to enhance lymphocyte proliferation, and most importantly increasing their migratory capacity and avoiding tumor tissue attraction. These results provide the rationale for the potential use of LMW HA as an immunostimulatory molecule for DC-based vaccines protocols in the treatment of cancer patients.
Materials and Methods
Reagents
Pharmaceutical endotoxin-free LMW HA of definite size (1–3×105 Da) from CPN spol.s.r.o (Czech Republic) was supplied by Farmatrade (Buenos Aires, Argentina). A stock solution of 5 mg/ml LMW HA was prepared, and the presence of endotoxins was determined by Limulus amebocyte lysate (LAL) assays with a sensitivity limit of 0.05–0.1 endotoxin units (EU) per ml (Sigma–Aldrich). GM-CSF was obtained from PrepoTech or Bioprofarma; CCL21, from PeproTech, and IL-4 and Poly (I:C), from Invitrogen.
Blood and tumor tissue
CRC patients and HD were recruited in the study after signing informed consent forms. Peripheral blood sample and colorectal carcinoma tissue were obtained at the time of surgical resection at the Austral University Hospital. Informed consent was obtained from all patients in accordance with our IRB.
Generation of dendritic cells
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Paque gradient. Cells were plated into 6-well plates for 2 h. Then, adhered cells were subsequently cultured for up to 7 days in RPMI 1640 medium (Invitrogen), containing 10% fetal calf serum (Invitrogen), 2 mM L-glutamine, 100 U/mL penicillin, 100 µg/ml streptomycin, human recombinant GM-CSF (Bioprofarma, Growgen, 350 ng/ml), human recombinant IL-4 (IL-4 R&D Systems 500 UI/ml) and 2-βMercaptoethanol (0.05 M). In HA stimulation experiments, cells were treated from day 3 with LMW HA (50 µg/ml). At day 7, DC were pulsed with autologous tumor lysates (200 µg/106 cells/ml) for 12–18 h, with or without LMW HA (50 µg/ml). Poly (I:C) (10 µg/ml) treatment was used for DC activation and as another control condition. Cells were then centrifuged and used for experiments.
Tumor lysates
Tumor tissues were frozen at −80°C until use. Then, tumor samples were dispersed with needle and scalpel and disrupted by 5 freeze-thaw cycles. For large debris removal, tumor lysates were centrifuged at 300 rpm for 10 min. The supernatant was collected and passed through a 0.22 µm Millipore Express, sterile, low binding proteins filter unit. The protein concentration of the lysate was determined by Bradford assay. The resulting tumor lysates were aliquoted and stored at −80°C until use.
Flow cytometry
Staining and flow cytometric analyses of generated DC were carried out using standard procedures. Briefly, cells were stained with different conjugated antibodies as follows: anti-CD11c (B-ly6), anti-MHC-II (G46-6), anti-CD40 (5C3), anti-CD80 (L307.4), anti-CD86 (2331), and their respective isotypes controls (BD Biosciences, San Diego, CA, USA) on ice for 30 min, washed thoroughly with PBS-1% BSA. Cells were then fixed with 1% paraformaldehyde and subjected to flow cytometry (FACSCalibur, BectonDickinson-BD, USA). Data were analyzed using Cyflogic software.
Mixed lymphocyte reaction
Tumor-free female Nu/Nu nude mice were intraperitoneally injected with 1.5×106 DC/TL pre-treated or not with LMW HA, and with allogeneic healthy human-derived CFSE-labeled PBLs (5×106). Forty eight hours later, cells were obtained by intraperitoneal lavages and incubated with mouse anti-human CD3 antibody for 30 min. They were subsequently incubated with biotinylated anti-mouse antibody and avidin-Texas Red for 45 min. Samples were analyzed using a FACSCalibur Flow Cytometer (Becton Dickinson). For FACS analysis lymphocytes were gated based on CD3+ marks and the number of T cell divisions was measured as proportional to the dilution of CFSE intensity.
Lymph node and tumor conditioned medium generation
Tumoral tissues were obtained from Nu/Nu nude mice carrying human s.c. implanted CRC fresh fragments. Mice were sacrificed; lymph node and tumor tissue were removed and minced into pieces smaller than 1 mm3. To obtain lymph node (LNCM) and tumor conditioned medium (TCM), the minced fragments were transferred into a 24 well tissue culture plate (6 fragments/well) with 500 µL of DMEM (plus 2 µmol/L glutamine, 100 U/mL penicillin and 100 mg/mL streptomycin) with 0.05% BSA but without FBS. The supernatant was collected 24 h later and stored at –80°C until use.
In vitro migration assays
For in vitro migration assays, micro-chemotaxis Boyden Chamber units were used. DC/TL (1,5–2,5×104) treated or not with LMW HA (50 µg/ml) or Poly (I:C) (10 µg/ml) were placed into the upper chamber of the transwell unit. In some assays DC/TL treated or not with LMW HA were preincubated for 24 h with an IL-8 neutralizing antibody (20 µg/ml). Chemoattractant medium containing 100 ng/ml of CCL21, LNCM, TCM, human recombinant IL-8 (20 ng/ml) or DMEM as negative control was placed in the lower chamber of the transwell unit. In blocking assays neutralizing IL-8 or isotype antibody (20 µg/ml) was added to TCM. The system was incubated for 4 h at 37°C in a 5% CO2 humidified atmosphere. Cells which migrated through the membrane pores were stained with DAPI and counted using UV microscopy with a 10x objective lens: 5 fields per well were analyzed and the mean number of cells/field ± SEM were calculated. Results are shown as DC/TL and DC/TL/LMW HA migration index. Poly (I:C) was used as a positive control maturation stimulus.
Zymography
Metalloproteinase activity was determined by zymography. Supernatant of culture from DC/TL treated or not with LMW HA was run on a 10% SDS PAGE containing 0.1% gelatin (Sigma-Aldrich). The gel was stained with Coomassie Brilliant Blue R-250 for 30 min at room temperature. Gelatinase activity was visualized by negative staining; gel images were obtained with a digital camera (Canon EOS 5D), and were subjected to densitometric analysis using Scion Image software (Scion Corporation, Frederick, MD). Relative MMP-2 activity was obtained by normalizing values to untreated samples (DC/TL). HT1080 cell line supernatant was used as positive control of MMP-2 and MMP-9 activity [30].
qPCR
Total RNA from DC/TL treated or not with LMW HA was extracted by Tri Reagent (Sigma-Aldrich Co., St. Louis MO, USA). Two micrograms of RNA were reverse transcribed with 200 U of SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) and 500 ng of Oligo (dT) primers. cDNAs were subjected to real time PCR. Each 25 µl reaction volume contained 1 unit Taq DNA polymerase (Invitrogen), 1× PCR reaction buffer (20 mM Tris-HCl, pH 8.4, and 50 mM KCl), 1.5 mM Mg2Cl, 200 µM of dNTPs and 0.4 µM of each specific primer: TLR2 forward 5′-GGGTTGAAGCACTGGACAAT-3′ and reverse 5′-CTGCCCTTGCAGATACCATT-3′; TLR4 forward 5′-TGAGCAGTCGTGCTGGTATC-3′ and reverse 5′-CAGGGCTTTTCTGAGTCGTC-3′; CD44 forward 5′-GCGCAGATCGATTTGAATTAA-3′ and reverse 5′-GTGCCCTTCTATGAACCCAT-3′; IL-8 forward 5′-GGTGCAGTTTTGCCAAGGAG-3′ and reverse 5′-TTCCTTGGGGTCCAGACAGA-3′; CXCR1 forward 5′-TTTTCCGCCAGGCTTACCAT-3′ and reverse 5′- AACACCATCCGCCATTTTGC-3′; CXCR2 forward 5′- TAAGTGGAGCCCCGTGGGG-3′ and reverse 5′- TGGGCTCAGGGGCAGGATG-3′. PCR conditions were: 90 seconds at 94°C and then 40 cycles of 30 seconds at 94°C, 30 seconds at 60°C and 30 seconds at 72°C. Values were normalized to levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH; used as housekeeping) transcript (forward 5′-CATCTCTGCCCCCTCTGCTG-3′ and reverse 5′-GCCTGCTTCACCACCTTCTTG-3′). cDNA was quantified using the OligoGreen Single Stranded Quantification kit (Invitrogen) according to the manufacturer’s instructions. Data were processed by the ΔΔCt method. The relative amount of the PCR product amplified from untreated cells was set as 1. A non-template control (NTC) was run in every assay, and all determinations were performed as triplicates in three separated experiments.
Tumor tissue cytokine array
Tumor conditioned medium was obtained as previously described and detection of human chemokines was performed using Human Chemokine Antibody Array C1 (RayBio C-Series) according to the manufacturer's instructions. The chemokines analyzed were: BLC, CCL28, CCL23, CTACK, CXCL16, EN78, Eotaxin1, Eotaxin2, Eotaxin3, Fractalkine, GCP2, GRO, GROα, HCC4, I-309, I-TAC, IL-8, IP-10, XCL1, MCP-1, MCP-2, MCP-3, MCP-4, MDC, MIG, MIP-1α, MIP-1β, MIP-1δ, MIP-3α, MIP-3β, MPIF1, NAP2, PARC, RANTES, SDF-1α, SDF-1β, TARC, TECK.
In vivo migration assays
DC/TL treated or not with LMW HA and stained with CM-Dil were inoculated subcutaneously into the back of nude mice, between tumor and nearest lymph node, after 72 h of s.c. tumor implantation. At 48 h mice were sacrificed and tumor and ipsilateral inguinal lymph nodes were removed, dissected and dissociated by enzymatic digestion with D-collagenase (Cabiochem). Red blood cells were removed using a lysis solution (0.15 M NH4Cl, 1 mM KHCO3, 0.1 Na2-EDTA). The remnant cells were subsequently fixed in 1% paraformaldehyde and subjected to flow cytometric analysis (FACSCalibur, Becton–Dickinson, BD). Data were processed using Cyflogic software.
Ethics statement
Human cells and tumor tissues were obtained from healthy donors/patients after written informed consent and protocol were approved by the “Institutional Evaluation Committee” (CIE) from School of Biomedical Sciences, Austral University (Protocol No. 12-019). Animals were maintained at our Animal Resource Facilities (School of Biomedical Sciences, Austral University) in accordance with the experimental ethical committee and the NIH guidelines on the ethical use of animals. The “Animal Care Committee” from School of Biomedical Sciences, Austral University, approved the experimental protocol. All surgery was performed under isoflurane anesthesia, and all efforts were made to minimize suffering.
Statistical analysis
Paired t test or Mann–Whitney (InStat, GraphPad Software) were used for statistical analyses. Differences with p values ≤0.05 were considered as statistically significant.
Results
Patient characteristics and dendritic cells generation
Fifteen HD and 25 CRC patients were recruited in this study. Nineteen patients had liver metastasis and 39% had received previous chemotherapy. DC were generated from PBMC. DC were co-cultured with autologous tumor lysates (TL) at day 6 for 18 h. In HA condition, LMW HA (50 µg/ml) was added to the culture medium (see Materials and Methods). At day 6, cultured DC displayed a relatively immature phenotype but became more mature when they were pulsed with autologous TL. When CD11+ DC/TL were gated we evidenced that LMW HA pre-incubation induced a statistically significant up-regulation of MHC-II and CD86 expression in HD DC (p≤0.05) (table 1).
Table 1. Phenotipic analyses of LMW HA pre-treated DC.
| Healthy donors (HD) | CRC Patients | |||||||
| DC | DC/TL | DC/TL/LMW HA | DC/TL/Poly I:C | DC | DC/TL | DC/TL/LMW HA | DC/TL/Poly I:C | |
| MHC II | 220 | 224δ | 238* | 217 | 135 | 174 | 192 | 180 |
| CD86 | 265 | 225δ | 238* | 218 | 109 | 171 | 170 | 148 |
| CD83 | 164 | 157 | 160 | 157 | 101 | 93 | 112 | 133 |
| CD40 | 120 | 113 | 108 | 123 | 397 | 384 | 375 | 381 |
PBMC-derived DC from healthy donors (HD) and colorectal carcinoma (CRC) patients were stained with mAbs anti-CD11c, MHC-II, CD86, CD83 and CD40. CD11c+ cells were gated and the co-expression of several markers was analyzed. Data are expressed as geometric mean fluorescence.
*vs HD DC/LT,
vs CRC patients DC/TL;
p≤0,05.
Dendritic cells are potent stimulators in mixed lymphocyte reaction after LMW HA stimulation
We then asked whether LMW HA treatment would impact on DC antigen presentation. To this end we performed an in vivo MLR assay using allogeneic CFSE-labeled PBL from HD and LMW HA primed/non-primed DC/TL which were injected inside nude mice peritoneal cavity as previously described [23]. As a result, a 72% and 80% increase in PBL proliferation activity was observed in the LMW HA primed DC/TL (DC/TL/LMW HA) condition when compared to LMW HA non-primed DC/TL (DC/TL) one in HD or CRC patients, respectively (p≤0,05) (Fig. 1, b).
Figure 1. LMW HA treatment of DC enhances human T-cell proliferation in vivo.
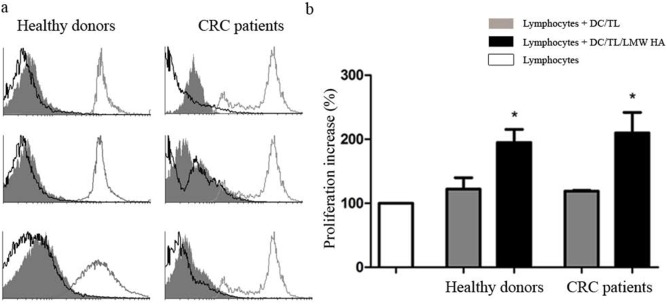
Nude mice received intraperitoneal injections of human-derived CFSE-labeled PBLs (5×106) and allogeneic mature DC/TL, or DC/TL/LMW HA (1,5×106). Proliferation was monitored 48 h later by FACS-gated lymphocytes from peritoneal lavages by quantifying fluorescent dye signal which is inversely correlated to their proliferation rate. Fluorescence intensity in the input undivided lymphocytes was over 95% (gray line in the histogram). a) Three histograms representative of both HD and CRC patients are shown. Gray line: undivided lymphocytes; gray shadow: Lymphocytes + DC/TL; black line: Lymphocytes + DC/TL/LMW HA. b) Bars represent percentage of lymphocyte proliferation increase ± SEM respect to undivided lymphocytes (HD n: 5 and CRC patients n: 5). White bar: lymphocyte alone; gray bar: DC/TL; black bar: DC/TL/LMW HA. *DC/TL vs DC/TL/LMW HA; Paired t test; p≤0.05.
LMW HA treatment increases migratory response of DC toward CCL21 and induces their CCR7 expression
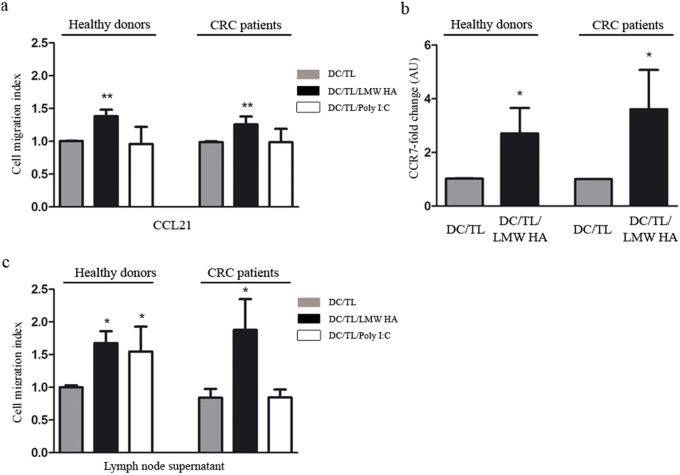
The process of DC migration is insufficiently understood [31]. Once DC capture tumor antigens, DC acquire the ability to cross through the endothelium and to migrate through the extracellular matrix and thereby entering the lymph node inner cortex [32]–[34]. Indeed, it is well known that both lymph nodes and tumors secrete several cytokines which might attract DC [22]. To further understand mechanisms involved in LMW HA effects on DC we analyzed the CCL21/CCR7 axis and found an increased ability to migrate toward CCL21 in DC/TL/LMW HA in vitro when compared to DC/TL condition, in both HD or CRC patients (38% and 25%, respectively; p≤0,01) (Fig. 2, a). In addition, treatment with LMW HA upregulated the expression levels of CCR7 in DC (HD DC fold change 2,7; and CRC patients DC fold change 3,6; p≤0,05) (Fig. 2, b).
Figure 2. LMW HA treatment increases migratory response of DC toward CCL21 inducing their CCR7 expression and increases their migratory response toward lymph node supernatant.
a) Migratory ability of DC treated or not with LMW HA toward CCR7 ligand CCL21 (200 ng/ml) was evaluated in a Boyden chamber system. DC were loaded into the upper well of the chamber and CCL21 was added to the lower chamber. Data are expressed as DC/TL and DC/TL/LMW HA migration index. Poly (I:C) was used as a positive control of maturation stimulus. b) CCR7 expression was examined by qPCR. Black bars represent CCR7 fold-change expression in comparison with DC/TL. c) Similar chemotaxis assays were set up placing lymph node supernatant from tumor bearing nude mice in the lower chamber as chemoattractant. Bars represent DC migration index from both HD and CRC patients. Gray bar: DC/TL; black bar: DC/TL/LMW HA; white bar: DC/TL/Poly (I:C). DC/TL vs DC/TL/LMW HA. Mann Whitney t test; * p≤0.05; **p≤0.01.
LMW HA treatment increase DC migratory capacity toward lymph node supernatant
Migration of DC to lymph node allows their interaction with naïve T cells, a critical step in the induction of immunity against cancer and a challenge for DC-based vaccine protocols [10]. Therefore, we decided to mimic in vitro the conditions faced by DC when they are inoculated in vivo by using lymph node conditioned medium (LNCM) as a chemoattractant. Interestingly, a potent migration of DC toward LNCM was found when they were pre-incubated with LMW HA in comparison with DC/TL condition. In contrast, poly I:C induced migration of DC only when they derive from HD (Fig. 2, c).
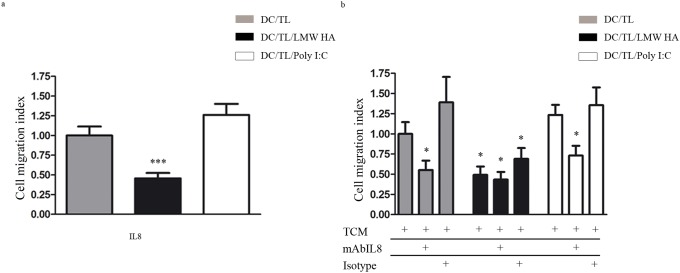
LMW HA priming of DC reduces their migratory response to IL-8 and inhibited their attraction to tumor conditioned medium
It was recently described that DC IL-8 pre-exposure abrogated DC tumor attraction [23]. We found that LMW HA treatment up-regulated IL-8 expression in DC/TL (Fig. 3, a). Thus, to study whether LMW HA pre-conditioned DC might decrease their response to tumor-derived signals we decided to analyze the IL-8/CXCR axis. After the confirmation of IL-8 expression in tumor conditioned medium (TCM) by a cytokine array assay performed to explore changes in the profile of chemokines secreted by tumors (Fig. 3, b) we observed that only LMW HA pre-treatment decreased by 50% the fraction of DC that migrated toward human recombinant IL-8 (Fig. 4, a). It is worth noting that a reduce migration of DC towards TCM was only observed in DC/TL/LMW HA experimental group when compared to DC/TL control (Fig. 4, b). Moreover, when IL-8 was blocked with an anti-IL-8 mAb a 40% reduction in DC migratory response to TCM was observed in DC/TL or DC/TL/Poly (I:C) conditions, while no changes were observed on DC/TL/LMW HA group (Fig. 4, b). These in vitro results suggest the possibility that IL-8 is important for DC attraction toward tumors and that LMW HA pre-treatment may ameliorate this effect.
Figure 3. IL-8 expression is upregulated in LMW HA treated DC and is present in tumor supernatant.
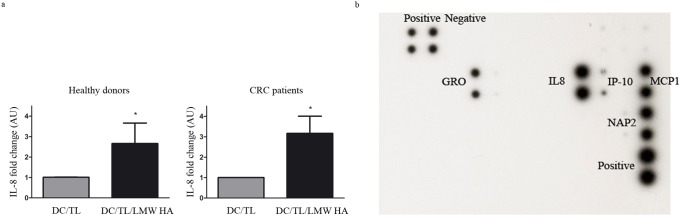
a) IL-8 expression was evaluated by qPCR. b) Human chemokine antibody array (38 proteins) was performed from tumor culture supernatant. The signal intensity for each antigen-specific antibody spot is proportional to the relative concentration of the antigen in the sample. Gray bars: DC/LT; black bars: DC/TL/LMW HA. Mann Whitney t test; *p≤0,05. * vs. DC/TL.
Figure 4. LMW HA induces resistance in human DC to IL-8 chemoattraction.
a) Chemotaxis assays were set up placing IL-8 in the lower chamber and LMW HA treated or untreated DC were placed in the upper chamber. b) A similar migration assay was set up placing TCM with addition of a specific antibody against IL-8 (mAbIL-8) or isotype antibody (20 µg/ml) in the bottom chamber as chemoattractant. Graphs represent data from 4 independent experiments similarly performed expressed as migration index respect to DC/TL migration toward TCM; five fields were counted for each quadruplicate well. Gray bars: DC/LT; black bars: DC/TL/LMW HA; white bars: DC/TL/Poly (I:C). Mann Whitney t test; *p≤0,05; ***p≤0,001. * vs. DC/TL.
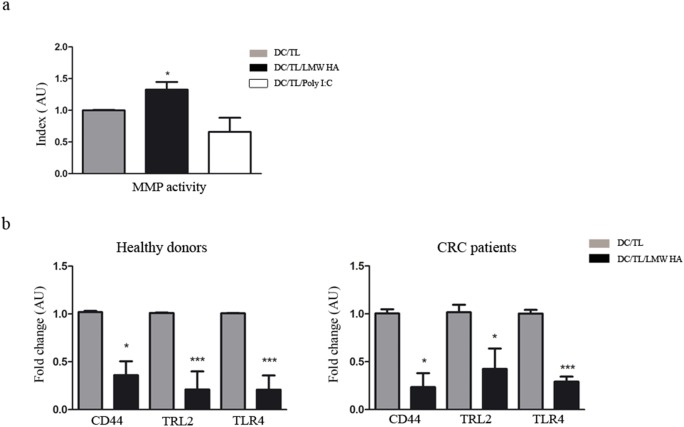
Pre-treatment with LMW HA induces metalloproteinase activity and modulates the expression of HA receptors in DC
Considering that metalloproteinases (MMPs) participate in DC migration through the ECM [35] we further evaluated if LMW HA might affect DC/TL MMP’s activity by zymography. Indeed, pre-incubation of DC with LMW HA resulted in a 30% increase in the gelatinolytic activity when compared to control (Fig. 5, a). HA is a component of the ECM with the capacity to induce cell signaling mechanisms on cells through several receptors such as CD44 [36], TLR-2 [29], and TLR-4 [37]. These receptors have been implicated in DC maturation as well in adhesion and migration processes. To further study the effects of LMW HA on DC/TL we analyzed its impact on HA receptors expression by qRT-PCR. Interestingly, a down-regulation of the HA receptors CD44, TLR-2, and TLR-4 was found in DC/TL/LMW HA derived from HD and CRC patients when compared to DC/TL control group (Fig. 5, b).
Figure 5. DC pre-treated with LMW HA induces MMP activity and downregulates HA receptors expression.
a) MMP activity was analyzed by zymography and quantified by densitometry. Relative MMP activity was obtained by normalizing values to untreated samples (DC/TL). Bars represent MMP activity from HD. b) qPCR of HA receptors was performed. The results are expressed as fold change related to DC/TL. Gray bar: DC/TL; black bar: DC/TL/LMW HA. DC/TL vs DC/TL/LMW HA. Mann Whitney t test; *p≤0,05; ***p≤0,001.
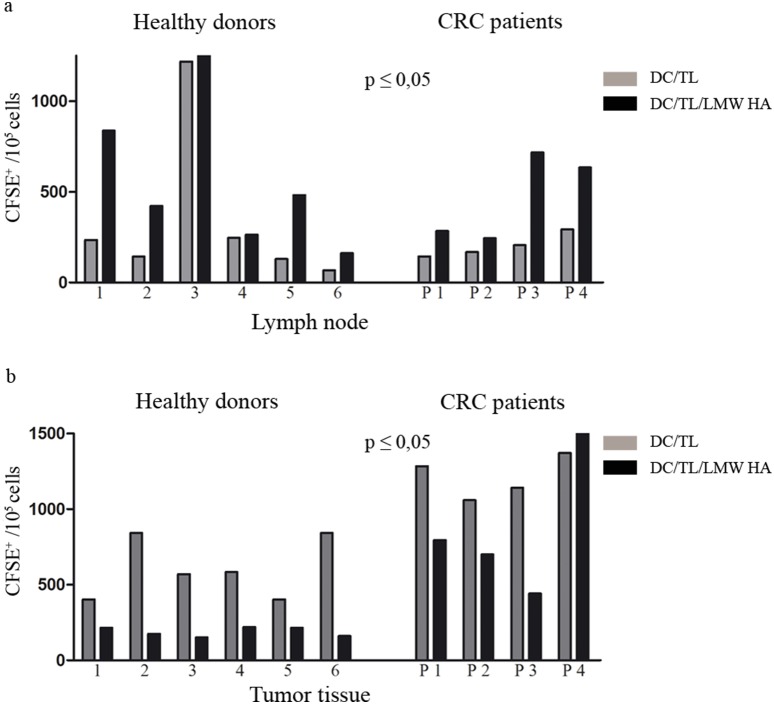
DC pre-incubated with LMW HA show increased in vivo migration capacity towards lymph node
We have previously reported that bone marrow-derived DC/TL pre-incubated with LMW HA show a higher capability to migrate toward regional lymph node once they were inoculated subcutaneously in a murine model of CRC [11]. Based on that, we aimed at analyzing this feature in PBMC-derived human DC obtained from HD and CRC patients. For this purpose, we chose a nude mouse xenograft model based on generating tumors from human CRC samples. DC/TL treated or not with LMW HA and stained with CMDil were subcutaneously inoculated in mice in a region between the tumor and regional lymph node. As shown in Fig. 6 and when compared to DC/TL, DC/TL/LMW HA from HD and CRC patients migrated more efficiently toward regional lymph nodes but were less attracted to tumors (lymph node: HD 460±253 vs. 703±229 cells, CRC 202±32 vs. 469±120 cells; tumor: HD 608±81 vs. 193±12 cells; CRC 1162±65 vs. 646±105 cells; DC/TL vs. DC/TL/LMW HA, respectively).
Figure 6. Pre-incubation of DC with LMW HA increases their migration toward lymph node and reduces their tumor attraction in vivo.
DC/TL were or not LMW HA treated, and after CM DiL labeling were inoculated s.c. in human-tumor bearing nude mice. Twenty four hours later lymph nodes (a) and tumors (b) were surgically removed and a cell suspension was obtained from these tissues. Number of fluorescent DC was counted by flow cytometry. The upper panel (a) shows DC/TL migration toward lymph nodes. The lower panel shows migration toward tumor tissue (b). Bars represent the number of migrated DC per individual HD (1–6) and CRC patients (P 1–4). Gray bar: DC/TL; black bar: DC/TL/LMW HA. * DC/TL vs DC/TL/LMW HA. Paired t test; *p≤0,05.
Discussion
A number of strategies for cancer immunotherapy are currently under preclinical and clinical evaluation [38]. Activation and stimulation of DC migration are key steps for the induction of a potent and specific antitumor T cell response. To date, several DC-based cancer vaccination strategies have been employed in the clinic with limited success [39]–[41]; although in 2010 the FDA approved the first antigen presenting cell therapeutic cancer vaccine to prolong survival of patients with advanced hormone-refractory prostate cancer [42]. Different “cocktails” containing cytokines, growth factors and other compounds have been used for maturation and activation of DC including IL-1β, IL-6, TNF, IFN-α, IFN-γ, PGE2 and Poly(I:C) [43], [44]. Our previous results showed that pre-incubation of DC with LMW HA was able to induce an efficient antitumoral effect in a CRC mouse model which was found to be mediated by the stimulation of DC maturation and activation, as well as by the induction of a potent migratory capacity towards lymphoid areas in vitro and in vivo [11]. In the present study we demonstrated that LMW HA represents a potent stimulator of migration toward lymph node for human DC obtained from both HD and CRC patients, likely partially involving CCR7/CCL21 axis and inducing resistance to IL-8, a tumor-derived DC chemoattractant. In addition, LMW HA treatment enhanced MLR-stimulating capacity of DC in an in vivo assay. Thus, this work adds new data regarding the ability of LMW HA to improve migratory capacity of DC that could be relevant in future design of potential cancer vaccines protocols.
Different studies showed that oligosaccharides of HA, but not high molecular weight (HMW) HA, can be used to stimulate immune responses involving DC activation [27], [29], [45], [46]. Nevertheless, this is the first report addressing LMW HA effects on DC derived from patients with cancer and on their migratory response to lymph node signals. As expected, DC/TL from HD showed higher expression levels of maturation markers than those obtained from CRC patients. LMW HA was able to induce stimulatory effects on DC promoting an up-regulation of MHC-II and the co-stimulatory molecule CD86 when obtained from HD samples but not in those from CRC patients. This result could be, at least in part, due to the immunosuppressive state of cancer patients [47], [48]. However, both groups of DC have the capacity to induce alloreactivity of human lymphocytes in a MLR model. In addition, LMW HA treatment was found to induce a down-regulation of TLR-2 and TLR-4 in both HD volunteers and CRC patients. These receptors belong to a large family of membrane proteins with cytoplasmic signaling domains and extracellular domains capable of recognizing pathogen-associated molecular patterns (PAMPs). Segal et al. have followed the expression of these molecules during the differentiation process of monocytes to activated dendritic cells. Down-regulation of these receptors might suggest a mature DC phenotype as it was demonstrated in LPS stimulated DC [49]. Therefore, it seems that LMW HA has a stimulatory effect on DC pulsed with tumor lysate in both HD and CRC patients.
Clinical application of DC-based cancer vaccines has been limited due to their weak efficacy. One limitation might be related to the failure of DC to reach secondary lymphoid organs. It is known that only a small percentage of inoculated DC migrate toward the lymph nodes where they could activate naïve T cells [8], [50], [51]. Once DC are inoculated they undergo a kind of conflicting force attraction between tumoral- and lymphoid-derived chemokines. This contrary stimulus and the specific repertoire receptors expressed by DC determine whether they would migrate to the lymph node (for antigenic presentation and lymphocyte activation) or to tumors. It has been shown that different DC maturation stimuli such as IL1β, TNF-α, PGE2 or poly (I:C) induce the expression of CCR7 which enhances DC migration response toward lymph node. However, those molecules might exert a negative effect on DC in different ways [52]. For example, it was shown that PGE2 and Poly (I:C) can induce a tolerogenic state on DC [16]. Moreover, some studies reported that LPS and poly (I:C) can induce inhibitory effects on DC by activation SOCS signals [17], [18]. We have herein shown in vitro evidences that LMW HA pre-conditioning enhances DC migration to CCL21 and inhibits their attraction toward IL-8. Despite it is likely that many molecules involved in DC migration do not cross-react across species differences, we demonstrated in vitro and in vivo that DC/TL/LMW HA increase their migration toward LNCM from tumor bearing mice and lymph node, respectively. In addition their attraction to TCM and tumor tissue was also diminished. These phenomena seem to be at least partially mediated by upregulation of CCR7 expression levels, which is the main receptor of CCL19 and CCL21. This cytokine is expressed by lymphatic endothelial and lymph node stromal cells, and was shown essential to attract DC toward them [12]–[14].
Another mechanism that might affect DC recruitment toward the lymph node is the capacity to digest extracellular matrix by MMPs, thus facilitating migration through connective tissues and crossing basement membranes [35]. MMPs also act cleaving cell surface receptors or their ligands, promoting migration by releasing or activating ligands for receptors that control cell motility, or suppressing migration by inactivating chemokines [53]. In line with this, Ratzinger et al. demonstrated that MMP-2 and MMP-9 are involved in the emigration of DC from epidermal skin explants as well as dermal DC from the dermis [35]. In addition, it was previously shown in DC derived from healthy volunteers [27] as well as in macrophages [54] and in tumor cell lines [55], that HA-oligosaccharide but not HMW HA up-regulates the activity of these MMPs through TLR-4 activation and by promoting CD44-EGFR interaction enhancing Akt signaling and cell migration events [56]. We herein showed that LMW HA was able to induce MMP activity in DC derived from healthy volunteers.
It is well established that HA size is critical for HA function. In fact, only HA fragments induce inflammatory cytokines in different cell types [57]. For this reason we used LMW HA of defined size (1−3×105 Da) in our experiments. LMW HA can act through several known receptors such as CD44, TLR2 and TLR4 which are expressed on immune cells such as monocytes and DC [37], [58]. CD44 is the major cell surface receptor for HA and has an important role in cell adhesion and migration [59]. We found that LMW HA treatment inhibited the expression of this receptor in DC/TL. Nevertheless, CD44 down-regulation not only did not impair cell migration but also might instead help DC detachment from ECM and migration through interstitial tissue. Indeed, we have found that bone marrow derived DC from both wild type and CD44-deficient mice have a comparable capacity to migrate to CCL21 in vitro [11]. Moreover, it was also shown that CD44 deficient mice exhibited an improvement of epidermal LC migration to LNs after epicutaneous sensitization with protein antigen and, additionally, they showed stronger predominant Th responses after immunization [60].
Another key finding of this study was that LMW HA induced the expression of IL-8 in DC/TL. Enhanced IL-8 expression in response to HA fragments has previously been described in several cellular types such as endothelial [37], epithelial [61] and cancer cells [62]. IL-8 is a pro-inflammatory cytokine present at large amount in cancer microenvironment and, noteworthy, involved in cell migration process. In fact, transcription of both IL-8 and MMP-2 has been reported to be regulated through NFκβ [63] which in turn is activated by HA [62]. Luca et al. evidenced that IL-8 affects DC migratory capacity by increasing MMP-2 expression [64]. In addition, IL-8 acts in an autocrine and paracrine fashion on melanoma cells causing their enhanced migratory capacity [65]. Furthermore, it was described that pre-exposure to IL-8 results in desensitization of the DC to the chemotactic effects of IL-8. This effect is relevant taking into account the large production of this molecule by tumor cell and their critical role in DC intratumoral retention [22]. It is important to emphasize that IL-8 was found not being able to modify neither DC maturation nor CXCR1 and CXCR2 expression [22], [23]. Altogether, these effects could explain, at least in part, both the higher DC migration into LNs and the lower attraction to tumor tissue without impairment in their immunologic status.
Finally, this study showed for the first time that LMW HA -a highly conserved and poorly immunogenic molecule- represents a promising candidate to be used in DC maturation protocols in order to increase their activation status as well as to improve their migratory capacity toward lymph nodes and inhibited their recruitment into tumors.
Acknowledgments
We would like to thank Soledad Arregui and Guillermo Gastón for expert technical assistance; Silvina Montal, Carina Chwat and Franco Nievas for help us to obtain tumor tissue. This work was supported by grants from Austral University (to MR: #I01-12; FP I02-12; MG T13-12) and from Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) grants. To LA: PICT-2007/00082; MG: PICTO 2008/00115; MG and GM: PICT 2010/2818; GM and LA: PICT 2012/1407. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This work was supported by grants from Austral University (to MR: #I01-12; FP I02-12; MG T13-12) and from Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) grants. To LA: PICT-2007/00082; MG: PICTO 2008/00115; MG and GM: PICT 2010/2818; GM: PICT 2012/1407. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60: 277–300. [DOI] [PubMed] [Google Scholar]
- 2. Berzofsky JA, Terabe M, Oh S, Belyakov IM, Ahlers JD, et al. (2004) Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J Clin Invest 113: 1515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gilboa E (2004) The promise of cancer vaccines. Nat Rev Cancer 4: 401–411. [DOI] [PubMed] [Google Scholar]
- 4. Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392: 245–252. [DOI] [PubMed] [Google Scholar]
- 5. Segura E, Albiston AL, Wicks IP, Chai SY, Villadangos JA (2009) Different cross-presentation pathways in steady-state and inflammatory dendritic cells. Proc Natl Acad Sci U S A 106: 20377–20381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alvarez D, Vollmann EH, von Andrian UH (2008) Mechanisms and consequences of dendritic cell migration. Immunity 29: 325–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silk KM, Silk JD, Ichiryu N, Davies TJ, Nolan KF, et al. (2011) Cross-presentation of tumour antigens by human induced pluripotent stem cell-derived CD141(+)XCR1(+) dendritic cells. Gene Ther. [DOI] [PubMed]
- 8. Morse MA, Coleman RE, Akabani G, Niehaus N, Coleman D, et al. (1999) Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res 59: 56–58. [PubMed] [Google Scholar]
- 9. De Vries IJ, Krooshoop DJ, Scharenborg NM, Lesterhuis WJ, Diepstra JH, et al. (2003) Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res 63: 12–17. [PubMed] [Google Scholar]
- 10. MartIn-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, et al. (2003) Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med 198: 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alaniz L, Rizzo M, Garcia MG, Piccioni F, Aquino JB, et al. (2011) Low molecular weight hyaluronan preconditioning of tumor-pulsed dendritic cells increases their migratory ability and induces immunity against murine colorectal carcinoma. Cancer Immunol Immunother 60: 1383–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, et al. (1998) Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med 188: 373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahamed J, Haribabu B, Ali H (2001) Cutting edge: Differential regulation of chemoattractant receptor-induced degranulation and chemokine production by receptor phosphorylation. J Immunol 167: 3559–3563. [DOI] [PubMed] [Google Scholar]
- 14. Allavena P, Sica A, Vecchi A, Locati M, Sozzani S, et al. (2000) The chemokine receptor switch paradigm and dendritic cell migration: its significance in tumor tissues. Immunol Rev 177: 141–149. [DOI] [PubMed] [Google Scholar]
- 15. Scandella E, Men Y, Gillessen S, Forster R, Groettrup M (2002) Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood 100: 1354–1361. [DOI] [PubMed] [Google Scholar]
- 16. Moller I, Michel K, Frech N, Burger M, Pfeifer D, et al. (2008) Dendritic cell maturation with poly(I:C)-based versus PGE2-based cytokine combinations results in differential functional characteristics relevant to clinical application. J Immunother 31: 506–519. [DOI] [PubMed] [Google Scholar]
- 17. Yoshimura A, Naka T, Kubo M (2007) SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol 7: 454–465. [DOI] [PubMed] [Google Scholar]
- 18. Bartz H, Avalos NM, Baetz A, Heeg K, Dalpke AH (2006) Involvement of suppressors of cytokine signaling in toll-like receptor-mediated block of dendritic cell differentiation. Blood 108: 4102–4108. [DOI] [PubMed] [Google Scholar]
- 19. Hurwitz AA, Watkins SK (2012) Immune suppression in the tumor microenvironment: a role for dendritic cell-mediated tolerization of T cells. Cancer Immunol Immunother 61: 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xie K (2001) Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev 12: 375–391. [DOI] [PubMed] [Google Scholar]
- 21. Huang D, Ding Y, Zhou M, Rini BI, Petillo D, et al. (2010) Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res 70: 1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Feijoo E, Alfaro C, Mazzolini G, Serra P, Penuelas I, et al. (2005) Dendritic cells delivered inside human carcinomas are sequestered by interleukin-8. Int J Cancer 116: 275–281. [DOI] [PubMed] [Google Scholar]
- 23. Alfaro C, Suarez N, Martinez-Forero I, Palazon A, Rouzaut A, et al. (2011) Carcinoma-derived interleukin-8 disorients dendritic cell migration without impairing T-cell stimulation. PLoS One 6: e17922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Toole BP (2004) Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 4: 528–539. [DOI] [PubMed] [Google Scholar]
- 25. McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, et al. (1996) Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest 98: 2403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McKee CM, Lowenstein CJ, Horton MR, Wu J, Bao C, et al. (1997) Hyaluronan fragments induce nitric-oxide synthase in murine macrophages through a nuclear factor kappaB-dependent mechanism. J Biol Chem 272: 8013–8018. [DOI] [PubMed] [Google Scholar]
- 27. Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, et al. (2002) Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med 195: 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Termeer CC, Hennies J, Voith U, Ahrens T, Weiss JM, et al. (2000) Oligosaccharides of hyaluronan are potent activators of dendritic cells. J Immunol 165: 1863–1870. [DOI] [PubMed] [Google Scholar]
- 29. Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, et al. (2006) Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol 177: 1272–1281. [DOI] [PubMed] [Google Scholar]
- 30. Rodriguez-Salvador J, Armas-Pineda C, Perezpena-Diazconti M, Chico-Ponce de Leon F, Sosa-Sainz G, et al. (2005) Effect of sodium butyrate on pro-matrix metalloproteinase-9 and -2 differential secretion in pediatric tumors and cell lines. J Exp Clin Cancer Res 24: 463–473. [PubMed] [Google Scholar]
- 31. Adema GJ, de Vries IJ, Punt CJ, Figdor CG (2005) Migration of dendritic cell based cancer vaccines: in vivo veritas? Curr Opin Immunol 17: 170–174. [DOI] [PubMed] [Google Scholar]
- 32. Verdijk P, Aarntzen EH, Punt CJ, de Vries IJ, Figdor CG (2008) Maximizing dendritic cell migration in cancer immunotherapy. Expert Opin Biol Ther 8: 865–874. [DOI] [PubMed] [Google Scholar]
- 33. Sozzani S, Allavena P, Vecchi A, Mantovani A (2000) Chemokines and dendritic cell traffic. J Clin Immunol 20: 151–160. [DOI] [PubMed] [Google Scholar]
- 34. Bianchi G, D’Amico G, Varone L, Sozzani S, Mantovani A, et al. (2000) In vitro studies on the trafficking of dendritic cells through endothelial cells and extra-cellular matrix. Dev Immunol 7: 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ratzinger G, Stoitzner P, Ebner S, Lutz MB, Layton GT, et al. (2002) Matrix metalloproteinases 9 and 2 are necessary for the migration of Langerhans cells and dermal dendritic cells from human and murine skin. J Immunol 168: 4361–4371. [DOI] [PubMed] [Google Scholar]
- 36. Lesley J, Hascall VC, Tammi M, Hyman R (2000) Hyaluronan binding by cell surface CD44. J Biol Chem 275: 26967–26975. [DOI] [PubMed] [Google Scholar]
- 37. Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, et al. (2004) Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem 279: 17079–17084. [DOI] [PubMed] [Google Scholar]
- 38. Dalerba P, Maccalli C, Casati C, Castelli C, Parmiani G (2003) Immunology and immunotherapy of colorectal cancer. Crit Rev Oncol Hematol 46: 33–57. [DOI] [PubMed] [Google Scholar]
- 39. Steinman RM, Banchereau J (2007) Taking dendritic cells into medicine. Nature 449: 419–426. [DOI] [PubMed] [Google Scholar]
- 40. Dauer M, Schnurr M, Eigler A (2008) Dendritic cell-based cancer vaccination: quo vadis? Expert Rev Vaccines 7: 1041–1053. [DOI] [PubMed] [Google Scholar]
- 41. Pascual V, Allantaz F, Patel P, Palucka AK, Chaussabel D, et al. (2008) How the study of children with rheumatic diseases identified interferon-alpha and interleukin-1 as novel therapeutic targets. Immunol Rev 223: 39–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, et al. (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363: 411–422. [DOI] [PubMed] [Google Scholar]
- 43. Giermasz AS, Urban JA, Nakamura Y, Watchmaker P, Cumberland RL, et al. (2009) Type-1 polarized dendritic cells primed for high IL-12 production show enhanced activity as cancer vaccines. Cancer Immunol Immunother 58: 1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Banchereau J, Schuler-Thurner B, Palucka AK, Schuler G (2001) Dendritic cells as vectors for therapy. Cell 106: 271–274. [DOI] [PubMed] [Google Scholar]
- 45. Do Y, Nagarkatti PS, Nagarkatti M (2004) Role of CD44 and hyaluronic acid (HA) in activation of alloreactive and antigen-specific T cells by bone marrow-derived dendritic cells. J Immunother 27: 1–12. [DOI] [PubMed] [Google Scholar]
- 46. Mummert ME (2005) Immunologic roles of hyaluronan. Immunol Res 31: 189–206. [DOI] [PubMed] [Google Scholar]
- 47. Munoz C, Carlet J, Fitting C, Misset B, Bleriot JP, et al. (1991) Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest 88: 1747–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lang S, Lauffer L, Clausen C, Lohr I, Schmitt B, et al. (2003) Impaired monocyte function in cancer patients: restoration with a cyclooxygenase-2 inhibitor. FASEB J 17: 286–288. [DOI] [PubMed] [Google Scholar]
- 49. Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, et al. (2001) Regulation of Toll-like receptors in human monocytes and dendritic cells. J Immunol 166: 249–255. [DOI] [PubMed] [Google Scholar]
- 50. Lesterhuis WJ, de Vries IJ, Schreibelt G, Lambeck AJ, Aarntzen EH, et al. (2011) Route of administration modulates the induction of dendritic cell vaccine-induced antigen-specific T cells in advanced melanoma patients. Clin Cancer Res 17: 5725–5735. [DOI] [PubMed] [Google Scholar]
- 51. de Vries IJ, Lesterhuis WJ, Barentsz JO, Verdijk P, van Krieken JH, et al. (2005) Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol 23: 1407–1413. [DOI] [PubMed] [Google Scholar]
- 52. van Helden SF, Krooshoop DJ, Broers KC, Raymakers RA, Figdor CG, et al. (2006) A critical role for prostaglandin E2 in podosome dissolution and induction of high-speed migration during dendritic cell maturation. J Immunol 177: 1567–1574. [DOI] [PubMed] [Google Scholar]
- 53. Parks WC, Wilson CL, Lopez-Boado YS (2004) Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 4: 617–629. [DOI] [PubMed] [Google Scholar]
- 54. Noble PW, McKee CM, Cowman M, Shin HS (1996) Hyaluronan fragments activate an NF-kappa B/I-kappa B alpha autoregulatory loop in murine macrophages. J Exp Med 183: 2373–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fieber C, Baumann P, Vallon R, Termeer C, Simon JC, et al. (2004) Hyaluronan-oligosaccharide-induced transcription of metalloproteases. J Cell Sci 117: 359–367. [DOI] [PubMed] [Google Scholar]
- 56. Kim Y, Lee YS, Choe J, Lee H, Kim YM, et al. (2008) CD44-epidermal growth factor receptor interaction mediates hyaluronic acid-promoted cell motility by activating protein kinase C signaling involving Akt, Rac1, Phox, reactive oxygen species, focal adhesion kinase, and MMP-2. J Biol Chem 283: 22513–22528. [DOI] [PubMed] [Google Scholar]
- 57. Alaniz L, Garcia M, Rizzo M, Piccioni F, Mazzolini G (2009) Altered hyaluronan biosynthesis and cancer progression: an immunological perspective. Mini Rev Med Chem 9: 1538–1546. [DOI] [PubMed] [Google Scholar]
- 58. Yamawaki H, Hirohata S, Miyoshi T, Takahashi K, Ogawa H, et al. (2009) Hyaluronan receptors involved in cytokine induction in monocytes. Glycobiology 19: 83–92. [DOI] [PubMed] [Google Scholar]
- 59. Isacke CM, Yarwood H (2002) The hyaluronan receptor, CD44. Int J Biochem Cell Biol 34: 718–721. [DOI] [PubMed] [Google Scholar]
- 60. Miaw SC, Chen JS, Hsieh PC, Liu CY, Chung MH, et al. (2010) CD44-deficient mice do not exhibit impairment of epidermal Langerhans cell migration to lymph nodes after epicutaneous sensitization with protein. J Invest Dermatol 130: 2674–2677. [DOI] [PubMed] [Google Scholar]
- 61. Mascarenhas MM, Day RM, Ochoa CD, Choi WI, Yu L, et al. (2004) Low molecular weight hyaluronan from stretched lung enhances interleukin-8 expression. Am J Respir Cell Mol Biol 30: 51–60. [DOI] [PubMed] [Google Scholar]
- 62. Voelcker V, Gebhardt C, Averbeck M, Saalbach A, Wolf V, et al. (2008) Hyaluronan fragments induce cytokine and metalloprotease upregulation in human melanoma cells in part by signalling via TLR4. Exp Dermatol 17: 100–107. [DOI] [PubMed] [Google Scholar]
- 63. Kim H, Koh G (2000) Lipopolysaccharide activates matrix metalloproteinase-2 in endothelial cells through an NF-kappaB-dependent pathway. Biochem Biophys Res Commun 269: 401–405. [DOI] [PubMed] [Google Scholar]
- 64. Luca M, Huang S, Gershenwald JE, Singh RK, Reich R, et al. (1997) Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. Am J Pathol 151: 1105–1113. [PMC free article] [PubMed] [Google Scholar]
- 65. Gebhardt C, Averbeck M, Viertel A, Kauer F, Saalbach A, et al. (2007) Ultraviolet-B irradiation enhances melanoma cell motility via induction of autocrine interleukin 8 secretion. Exp Dermatol 16: 636–643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.