Abstract
Background and Objectives
Thrombomodulin (TM), an integral membrane glycoprotein expressed on the lumenal surface of vascular endothelial cells, promotes anti-coagulant and anti-inflammatory properties. Release of functional TM from the endothelium surface into plasma has also been reported. Much is still unknown however about how endothelial TM is regulated by physiologic hemodynamic forces (and particularly cyclic strain) intrinsic to endothelial-mediated vascular homeostasis.
Methods
This study employed human aortic endothelial cells (HAECs) to investigate the effects of equibiaxial cyclic strain (7.5%, 60 cycles/min, 24 hrs), and to a lesser extent, laminar shear stress (10 dynes/cm2, 24 hrs), on TM expression and release. Time-, dose- and frequency-dependency studies were performed.
Results
Our initial studies demonstrated that cyclic strain strongly downregulated TM expression in a p38- and receptor tyrosine kinase-dependent manner. This was in contrast to the upregulatory effect of shear stress. Moreover, both forces significantly upregulated TM release over a 48 hr period. With continuing focus on the cyclic strain-induced TM release, we noted both dose (0–7.5%) and frequency (0.5–2.0 Hz) dependency, with no attenuation of strain-induced TM release observed following inhibition of MAP kinases (p38, ERK-1/2), receptor tyrosine kinase, or eNOS. The concerted impact of cyclic strain and inflammatory mediators on TM release from HAECs was also investigated. In this respect, both TNFα (100 ng/ml) and ox-LDL (10–50 µg/ml) appeared to potentiate strain-induced TM release. Finally, inhibition of neither MMPs (GM6001) nor rhomboids (3,4-dichloroisocoumarin) had any effect on strain-induced TM release. However, significantly elevated levels (2.1 fold) of TM were observed in isolated microparticle fractions following 7.5% strain for 24 hrs.
Conclusions
A preliminary in vitro investigation into the effects of cyclic strain on TM in HAECs is presented. Physiologic cyclic strain was observed to downregulate TM expression, whilst upregulating in a time-, dose- and frequency-dependent manner the release of TM.
Introduction
Thrombomodulin (TM), a multi-domain type-1 membrane glycoprotein constitutively expressed on the lumenal surface of vascular endothelial cells, binds circulating thrombin to elicit the concomitant activation of protein C (amongst various other homeostatic actions). As such, TM is a central determinant of vascular endothelial thromboresistance by promoting anti-coagulant and anti-inflammatory properties within the vessel wall [1]. Shedding or release of soluble TM (sTM) into circulating blood has also been widely reported [2]–[5].
Given the importance of TM to vascular homeostasis, a clearer understanding of how it is regulated within the vascular endothelium by physiological hemodynamic forces is of significant interest. Blood flow-associated hemodynamic forces, namely cyclic strain (stretch) and laminar shear stress, within specific physiological limits, typically work in concert to exert a beneficial influence on endothelial-dependent regulation of vessel homeostasis [6]. In this regard, endothelial cells employ well characterised mechanosensor mechanisms to enable them to sense and respond to their hemodynamic environment, thus facilitating either acute or chronic remodeling of blood vessel architecture to complement circulatory conditions [7], [8]. Moreover, dysregulation (e.g. attenuation, hyper-elevation) of either of these forces can contribute to endothelial activation that may lead to vessel remodeling and vascular diseases (e.g. atherosclerosis, hypertension, stroke, vein graft thrombosis, ventilator-induced lung injury, retinopathy) [9]–[13].
Some regulatory links between endothelial TM expression and hemodynamic forces have previously been demonstrated. Shear-dependent up-regulation of TM expression has been reported in human retinal microvascular endothelial cells [14], human umbilical vein endothelial cells (HUVECs) [15], human abdominal aortic endothelial cells (HAAECs) [16], and even in a mouse transverse aortic constriction model of flow-dependent remodeling [17], observations basically consistent with the atheroprotective nature of laminar shear. However, the effect on endothelial TM expression of physiologic cyclic strain, the repetitive outward stretching of the vessel wall in synchronization with the cardiac cycle, has received considerably less attention in the literature. Sperry et al. [18] have previously demonstrated strain-dependent reduction in endothelial TM expression within rabbit autologous vein grafts. Interestingly, these in vivo observations contrast with those of Chen et al. [19] who demonstrated a sustained increase in TM protein expression following 21% cyclic strain of HUVECs, the pathological levels of strain applied in this study unfortunately rendering these observations somewhat difficult to interpret.
With the exception of the aforementioned ex vivo study by Sperry and co-workers, to our knowledge there are no existing in vitro studies investigating the influence of physiologic cyclic strain on TM expression in vascular endothelial cells, or indeed the influence of either physiologic cyclic strain or shear stress on endothelial TM release. This paper now addresses this knowledge deficit using human aortic endothelial cell (HAEC) culture models. Particular emphasis is placed on how physiologic levels of cyclic strain, the lesser studied force with respect to TM regulation, may influence the expression and release of endothelial TM.
Materials and Methods
Materials
Unless otherwise stated, all reagents were purchased from Sigma-Aldrich (Dublin, IRL). All primers were purchased from Eurofins MWG Operon (London, UK).
Cell culture
Primary-derived human aortic endothelial cells (HAECs) were obtained from Promocell GmBH (Heidelberg, Germany - Cat No. C-12271) and routinely grown in Promocell Endothelial Cell Growth Media MV (Cat No. C-22020) supplemented with 5% fetal calf serum, 0.4% endothelial cell growth supplement/bovine hypothalamic extract, heparin (90 µg/mL), hydrocortisone (1 µg/ml), epidermal growth factor (10 ng/mL), and antibiotics (100 U/mL penicillin, 100 µg/mL streptomycin). All cells (passages 5–12) were grown and maintained in a humidified atmosphere of 5% CO2/95% air at 37°C.
Hemodynamic force studies
For cyclic strain (CS) studies, the earlier method of Sweeney et al. was employed with minor modifications [20]. HAECs were seeded into 6-well ProNectin-coated Bioflex plates (Dunn Labortechnik GmBH - Asbach, Germany) at a density of approximately 5×105 cells/well. At confluency, a Flexercell Tension Plus FX-4000T system (Flexcell International Corp. – NC, USA) was subsequently used to apply a physiological level of equibiaxial cyclic strain to each plate (0–7.5% strain, 60 cycles/min, 0–48 hr, cardiac waveform). Cells were also seeded into standard 6-well plates and exposed to physiological levels of laminar shear stress (LSS) (10 dynes/cm2, 0–48 hr) on an orbital rotator as previously described [21], [22].
Following experiments, both cells and conditioned media were routinely harvested for analysis. For cell lysate preparation, cells were washed thrice in PBS before being scraped into radioimmunoprecipitation assay (RIPA) lysis buffer (64 mM HEPES pH 7.5, 192 mM NaCL, 1.28% w/v Triton X-100, 0.64% w/v sodium deoxycholate, 0.128% w/v sodium dodecyl sulfate, 0.5 M sodium fluoride, 0.5 M EDTA, 0.1 M sodium phosphate, 10 mM sodium orthovanadate, and 1× protease/phosphatase inhibitor cocktail) and transferred into a pre-chilled micro-centrifuge tube. Continuous lysate rotation was applied for 1 hr at 4°C, prior to lysate clarification by centrifugation at 10,000×g for 20 min at 4°C to sediment any triton-insoluble material. Clarified lysates were quantified by BCA assay [23]. Conditioned media samples were collected from each Bioflex well (1 ml/well, 2 ml/well for microparticle fractionation studies) and centrifuged at 700×g for 15 min to remove crude cellular debris. Media samples were routinely assayed by ELISA prior to freezing. All samples were ultimately stored at −80°C.
Microparticle fractionation and flow cytometry
For the isolation of microparticles present in HAEC-conditioned media, the methods of Lacroix et al. were employed with minor modifications [24], [25]. Following experiments, conditioned media was harvested and initially centrifuged at 300×g for 5 min and subsequently at 2500×g for 10 min to facilitate removal of cellular debris. Media was then centrifuged at 20,000×g for 90 min, after which the supernatant was set aside and the pellet resuspended in 1 ml of phosphate buffered saline (PBS). The washed pellet was subjected to a second spin at 20,000×g for 90 min to yield a final microparticle pellet that was resuspended in lysis buffer (100 mM Tris HCl, pH 8.1, 0.5% Triton X-100, protease inhibitor mix). Microparticle lysates were then stored at −80°C until required for TM ELISA. All centrifugation steps proceeded at 4°C.
For analysis of the effect of cyclic strain on endothelial MP production, we employed flow cytometry of non-lysed MP fractions (isolated as described above) following cyclic strain for 24 hrs at 0 or 7.5%. Briefly, MP fractions were resuspended in 400 µl of FACS buffer (filtered PBS containing 2% fetal bovine serum and 0.1% sodium azide). PE-conjugated anti-VE-cadherin mouse IgG (Catalog 560410, BD Biosciences, Oxford, UK) and FITC-conjugated recombinant annexin V (Catalog 31490013, ImmunoTools, Germany) were added to MP fractions (20 µl and 5 µl, respectively) and left to incubate as per manufacturer guidelines (60 min for VE-cadherin and 15 min for annexin V). Following incubation, the MPs were pelleted and resuspended in a final volume of 250 µl of fresh FACS buffer before being read by flow cytometry for 60 seconds at a fixed flow rate to assess MP levels. The flow cytometer (FACS Aria) was pre-calibrated using a 2% suspension of standard 0.1 µm polystyrene-latex beads (Catalog LB-1, Sigma-Aldrich) with further optimisation performed using a suspension of MPs isolated from control HAECs. Both single- and double-stained MP control samples were employed for compensation purposes. Final values were normalised per 1×105 cells. All FACS data analysis employed FlowJo software.
Real-time PCR
Following experiments, endothelial cells were harvested for extraction of total RNA according to the method of Chomczynski and Sacchi [26] using TRIzol reagent (Life Technologies, Dublin, IRL). cDNA was generated from 1 µg of total RNA using a high capacity cDNA reverse transcription kit (Life Technologies). Quantitation of the final cDNA was determined using a NanoDrop 3300 Spectrophotometer (Thermo Scientific, DE, USA). Amplification of target cDNA sequences using gene-specific primers was subsequently performed and analyzed as previously described [27]. Ribosomal subunit S18 was routinely used for normalization purposes. Primer pairs (shown below) were screened for correct product size (1% agarose gel electrophoresis) and underwent melt-curve analysis for primer-dimers. TM (107 bp): Forward 5′-ACCTTCCTCAATGCCAGTCAG-3′; Reverse 5′-GCCGTCGCCGTTCAGTAG-3′; S18 (250 bp): Forward 5′-CAGCCACCCGAGATTGAGCA-3′; Reverse 5′- TAGTAGCGACGGGCGGTGTG-3′.
Western immunoblotting
Western blotting was employed to confirm TM protein expression and for semi-quantitative comparison of cellular TM levels between control and strained samples. Following experiments, endothelial cell lysates were harvested, resolved by 10% SDS-PAGE under reducing conditions, and electroblotted as previously described [27]. Membranes were blocked for 60 min in tris-buffered saline (TBS: 10 mM Tris pH 8.0, 150 mM NaCl) containing 5% w/v skim milk before being incubated overnight in primary antisera with gentle agitation at 4°C. Primary antisera were prepared in TBS (+5% skim milk): 10 µg/ml anti-TM mouse polyclonal IgG (Abcam, Cambridge, UK) and 1 µg/mL anti-GAPDH rabbit monoclonal IgG (Santa Cruz Biotechnology, CA, USA). Membranes were then washed thrice in TBS containing 0.1% Tween (TBST) before being incubated for 2 hrs in secondary antisera with gentle agitation at room temperature. Secondary antisera (Amersham Pharmacia Biotech, Buckinghamshire, UK) were prepared in TBST (+5% milk): 1/3000 HRP-conjugated goat anti-mouse IgG (TM) and 1/3000 HRP-conjugated goat anti-rabbit IgG (GAPDH). Membranes were developed using a Luminata Western HRP kit (Millipore, Cork, IRL) followed by chemiluminescent imaging using a G-Box gel-documentation system (Syngene, UK). Scanning densitometry of Western blots was performed uysing NIH ImageJ software.
Enzyme-linked immunosorbent assay (ELISA)
A Thrombomodulin/BDCA-3 DuoSet ELISA Kit (R&D Systems, MN, USA) was employed as per manufacturer instructions (with minor modifications) to accurately measure absolute TM levels in HAEC lysates and conditioned media. Briefly, F96 maxisorp Nunc-Immuno 96-well plates (Bio-Sciences Ltd., Dun Laoghaoire, IRL) were coated with 50 µl/well of the capture antibody and incubated overnight at room temperature. The plate was then blocked by adding 150 µl of Reagent Diluent to each well and incubated for 1 hr at room temperature. HAEC total protein lysates were routinely pre-diluted 1/20 in PBS containing 25% FCS (within the linear range for the ELISA – Figure S1A), whilst conditioned media samples were undiluted. All samples and TM standards were subsequently added to the ELISA plate in duplicate at 50 µl/well. Assays proceeded for 2 hr at room temperature. The standard curve ranged from 31.25 to 2000 pg/ml of recombinant human TM (Figure S1B). Following sample incubation, 50 µl of the detection antibody was added to each well and then incubated for a further 2 hr at room temperature. Post-incubation, 50 µl of streptavidin-HRP was dispensed to each well and incubated for 20 min at room temperature in the dark. 50 µl of substrate solution was then added to each well and incubated for a further 20 min at room temperature in the dark. Reactions were terminated with the addition of 25 µl of stop solution to each well and the plate luminescence subsequently read at both 570 nm and 450 nm (wave correction was used to subtract the readings at 570 nm from 450 nm to correct for optical imperfections in the plate). For normalization purposes, TM levels in HAEC lysates were routinely presented as pg/µg of total protein, whilst TM levels in conditioned media were routinely presented as pg/105 cells.
Statistical analysis
Results are expressed as mean±s.e.m. Experimental points were performed in triplicate with a minimum of three independent experiments (n = 3). Statistical comparisons between control and experimental groups was by ANOVA in conjunction with a Dunnett's post-hoc test for multiple comparisons (*). A Student's t-test was also employed for pairwise comparisons (α, δ). A value of P≤0.05 was considered significant.
Results
Cyclic strain and shear stress modulate TM expression and release in HAECs
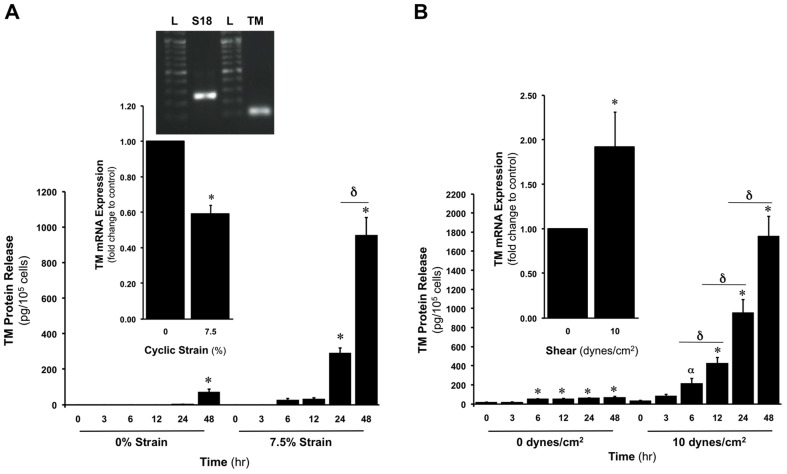
The effect of CS (0 or 7.5%, 24 hr) on TM expression in HAECs was initially monitored. Relative to unstrained cells, we observed a significant reduction in TM mRNA (41%) in response to 7.5% strain (Figure 1A inset). The time-dependent release of TM from HAECs at 7.5% CS was also monitored over 48 hr, with significant levels of TM release apparent between 12–24 hr following strain onset (Figure 1). Importantly, no significant increase in apoptosis or loss in cell viability was observed in response to elevated strains (up to 12.5%), as monitored by flow cytometry using an Alexa Fluor 488 Annexin V-PI/Dead Cell Apoptosis Kit (Bio-Sciences)[28] and trypan blue exclusion assay, respectively (data not shown).
Figure 1. Effect of CS and LSS on TM expression and release in HAECs.
(A) Time-dependent effect of CS (0 or 7.5%, 0–48 hr) on TM release. Inset shows effect of CS (0 or 7.5%, 24 hr) on TM mRNA levels. Agarose gel inset confirms predicted S18 and TM cDNA fragment sizes (250 and 107 bp, respectively) for designed primers. Gel is representative. Key: L, ladder. (B) Time-dependent effect of LSS (0 or 10 dynes/cm2, 0–48 hr) on TM release. Inset shows effect of LSS (0 or 10 dynes/cm2, 24 hr) on TM mRNA levels. *P≤0.05 versus unstrained or unsheared control. α P = 0.0008 versus 0 hr sheared control. δ P≤0.05.
We next monitored the effect of LSS (0 or 10 dynes/cm2, 24 hr) on TM expression in HAECs. Relative to unsheared cells, we observed a significant increase in TM mRNA (92%) in response to 10 dynes/cm2 LSS (Figure 1B inset). The time-dependent release of TM from HAECs at 10 dynes/cm2 was also monitored over 48 hr. We observed that significant levels of TM release commenced between 6–12 hr following shear onset, with levels of released TM after 48 hr approximately 2-fold higher compared to CS-induced release (Figure 1B).
Considering the noticeable scarcity of published information on TM regulation by CS, it was therefore decided to focus exclusively on this stimulus for all subsequent experiments.
CS downregulates TM protein expression
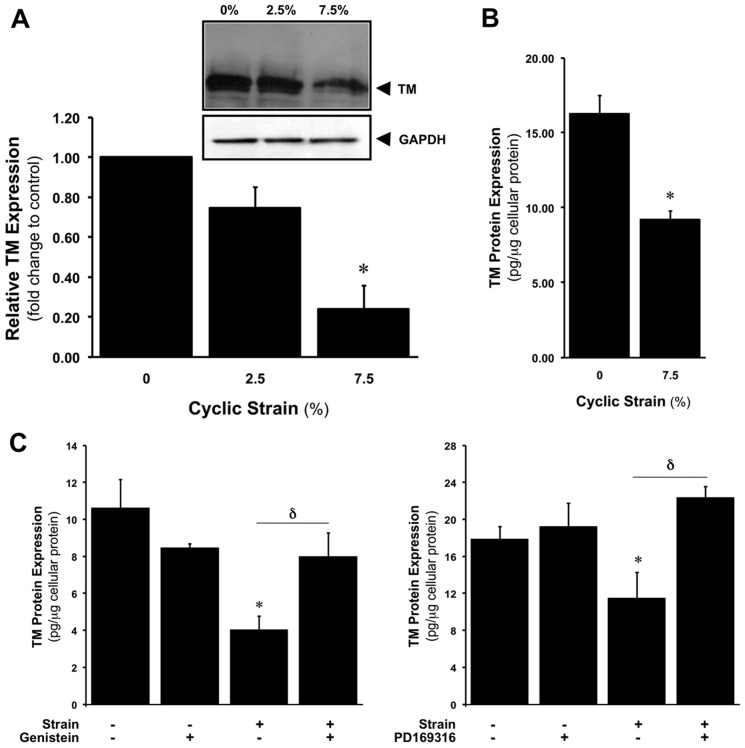
Following strain experiments, total protein lysates were harvested and analysed for TM levels. Using Western blotting, CS (0–7.5%, 24 hr) was shown to reduce TM cellular protein levels in HAECs in a dose-dependent manner (Figure 2A). CS-dependent reduction in protein expression (∼43.5% at 7.5% CS for 24 hr) was also confirmed by ELISA (Figure 2B). In separate experiments, blockade of either receptor tyrosine kinase or p38 MAP kinase activation using genistein and PD169316, respectively, prevented the strain-dependent reduction in TM protein levels (Figure 2C).
Figure 2. CS-dependent downregulation of TM protein expression in HAECs.
Effect of CS (0–7.5%, 24 hr) on TM protein levels as monitored by (A) Western blotting and (B) ELISA. Histogram below gels represents the densitometric fold change in relative TM protein expression. *P≤0.05 versus 0% CS. All gels are representative. (C) Effect of 2 µM genistein and 10 µM PD169316 on CS-dependent downregulation of TM protein expression. *P≤0.05 versus untreated static control. δ P≤0.05.
CS upregulates TM protein release
Following strain experiments, conditioned media was also harvested and analysed for TM levels by ELISA. In this regard, we observed that CS (0–7.5%, 24 hr) increased the release of TM into the media in a dose-dependent manner (Figure 3A), with maximal release occurring by 7.5% CS. In separate experiments, blockade of either receptor tyrosine kinase, MAP kinase (p38, ERK-1/2), or eNOS activation using genistein, PD169316/PD98059, and L-NG-nitroarginine methyl ester (L-NAME), respectively, did not attenuate the strain-dependent upregulation in TM release (Figure 3B).
Figure 3. CS-dependent upregulation of TM protein release in HAECs.
(A) Effect of CS (0–7.5%, 24 hr) on TM protein release. *P≤0.05 versus static control. δ P≤0.05. (B) Effect of 10 µM PD169316, 10 µM PD98059, 2 µM genistein and 1 mM L-NAME on CS-dependent upregulation of TM protein release. *P≤0.05 versus untreated static control. δ P≤0.05.
CS modulates TM expression and release in a frequency-dependent manner
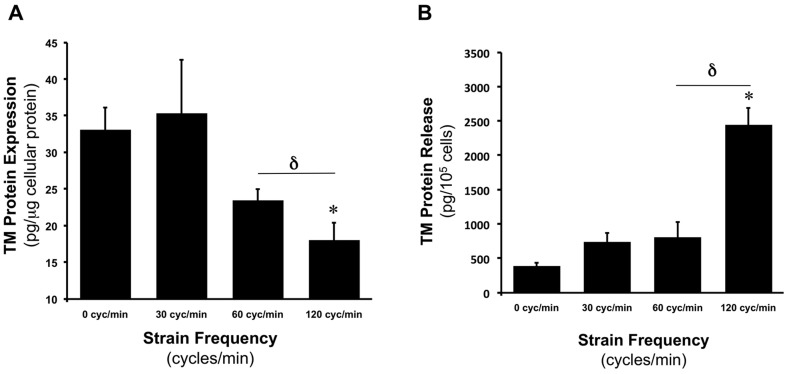
In another series of experiments, HAECs were subjected to a constant CS amplitude of 7.5% (24 hr), whilst frequency was varied (0.5, 1, and 2 Hz – corresponding to 30, 60 and 120 cycles per min). Following strain experiments, cell lysates and conditioned media were harvested for analysis of TM levels by ELISA. Relative to 0% (0 Hz) controls, increasing CS frequency progressively decreased TM protein expression, with lowest TM levels observed at 2 Hz (Figure 4A). Analysis of conditioned media however, revealed a profound increase in TM release (6.3-fold) when strain frequency was increased from 0 to 2 Hz (Figure 4B).
Figure 4. Frequency-dependent effects of CS on TM expression and release in HAECs.
Effect of CS frequency (0% at 0 cycles/min versus 7.5% at 30–120 cycles/min for 24 hr) on (A) TM cellular protein levels and (B) TM release. *P≤0.05 versus static control. δ P≤0.05.
CS-induced TM release is enhanced in the presence of inflammatory mediators
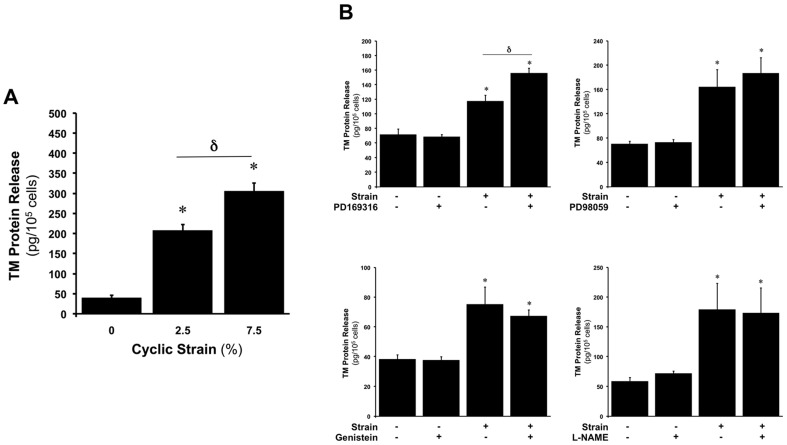
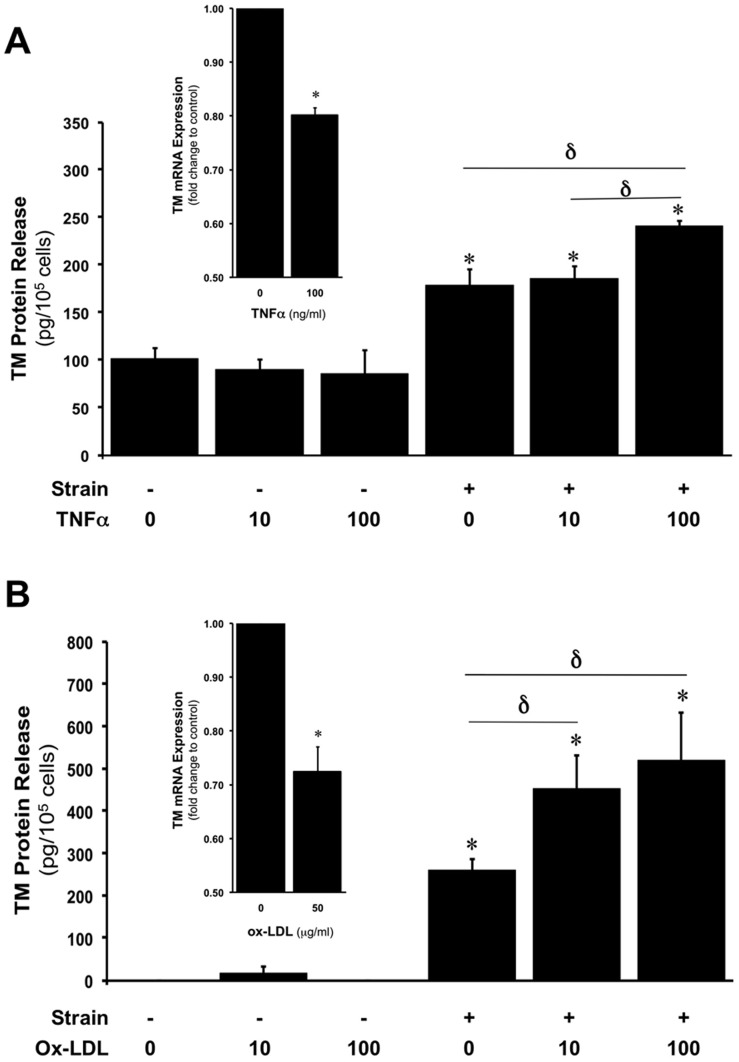
HAECs were subjected to CS (0 or 7.5%, 24 hr) in the absence and presence of tumour necrosis factor-α (TNFα, 0–100 ng/ml) and oxidised low density lipoprotein (ox-LDL, 0–50 µg/ml), concentration ranges consistent with those previously employed for these agents in other in vitro cell studies [28], [29]. In static HAECs, neither reagent was seen to significantly induce TM release. By contrast, in strained HAECs, elevated concentrations of either TNFα (100 ng/ml, Figure 5A) or ox-LDL (10–50 µg/ml, Figure 5B) led to significantly higher levels of TM release relative to strained untreated HAECs. Additionally, static HAEC treatment for 24 hr with TNFα (100 ng/ml) and ox-LDL (50 µg/ml) downregulated TM mRNA levels by 20% and 28%, respectively (Figure 5A,B insets). By contrast, elevated glucose (15–30 mM) had no significant effect on TM expression levels.
Figure 5. Effects of inflammatory mediators on CS-induced TM release in HAECs.
(A) Effect of CS (0 or 7.5%, 24 hr) on TM release from HAECs in the presence of 0, 10, and 100 ng/ml of TNFα. (B) Effect of CS (0 or 7.5%, 24 hr) on TM release from HAECs in the presence of 0, 10, and 50 µg/ml of ox-LDL. *P≤0.05 versus untreated 0% CS. δ P≤0.05. Histogram insets in (A) and (B) show effect of upper concentrations of TNFα and ox-LDL, respectively, on TM mRNA levels. *P≤0.05 versus untreated controls.
CS-induced TM release from HAECs is protease-independent and microparticle-dependent
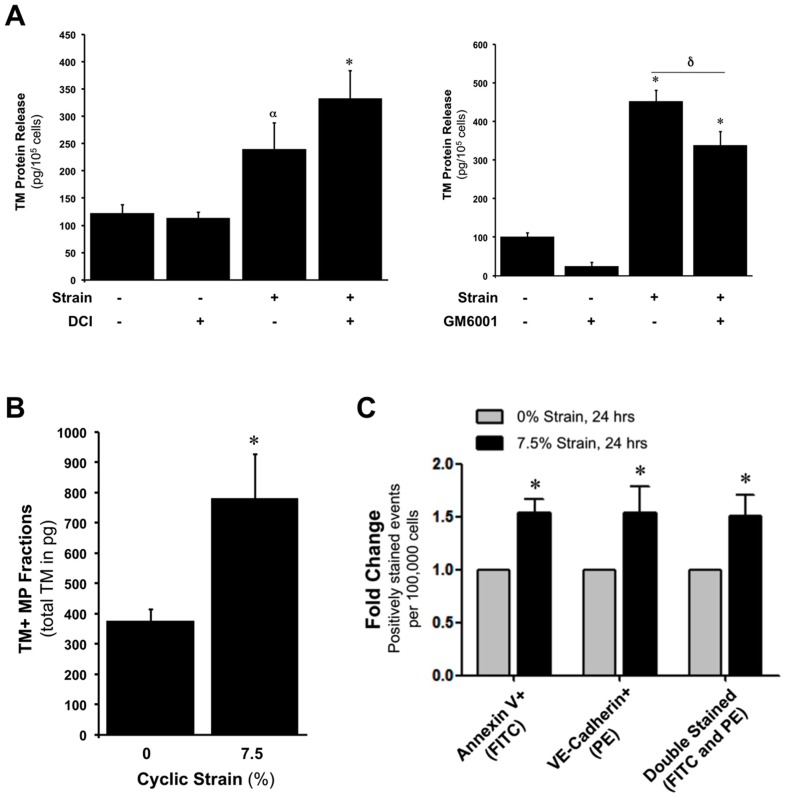
HAECs were subjected to CS (0 or 7.5%, 24 hr) in the absence and presence of 10 mM GM6001 or 10 mM 3,2-dichloroisocoumarin (DCI), selective inhibitors of matrix metalloproteinases (MMPs) and rhomboids [30], [31], respectively, proteases which have been previously implicated in the release of TM from cells [32], [33]. In this respect, neither inhibitor was found to significantly block this effect (following correction for any baseline inhibitor effects)(Figure 6A). In a final series of experiments, HAECs were again subjected to CS (0 or 7.5%, 24 hr) and the conditioned media harvested for extraction of microparticles by high speed centrifugation. CS was found to upregulate the release of annexin V+/VE-cadherin+ MPs by 1.5 fold (Figure 6C). Lysed microparticle fractions were subsequently analysed for TM levels by ELISA. Microparticle extracts from both static and strained HAECs were found to be positive for TM, with strained fractions consistently exhibiting up to 2.1 fold more TM than static fractions (Figure 6B).
Figure 6. Proteolytic and non-proteolytic mechanisms of TM release from HAECs.
(A) Effect of protease inhibitors, DCI (rhomboids) and GM6001 (MMPs), on TM release from HAECs under static and strain (0 or 7.5%, 24 hr) conditions. (B) Confirmation of TM presence in HAEC microparticle (MP) fractions harvested from conditioned media following CS treatment (0 or 7.5% strain, 24 hr). (C) Confirmation of CS-dependent increase in annexin V+/VE-Cadherin+ HAEC MP release following CS treatment (0 or 7.5% strain, 24 hr). *P≤0.05 versus untreated 0% CS. α P = 0.045 versus untreated 0% CS. δ P≤0.05.
Discussion
TM is a pivotal determinant of endothelial thromboresistance and vessel homeostasis. TM-mediated binding of thrombin leading to protein C activation has beneficial anti-coagulant, anti-fibrinolytic, and anti-inflammatory outcomes for the vessel wall. In the current paper we employed in vitro HAEC culture models to investigate the regulatory influence of physiologic hemodynamic forces, namely, equibiaxial cyclic strain and laminar shear stress, on endothelial TM expression and release, with particular emphasis placed on the former stimulus. Our initial investigations clearly demonstrated that cyclic strain could significantly downregulate TM expression (mRNA and protein), an event which was found to be sensitive to inhibition of both p38 MAPK and receptor tyrosine kinase signaling, and which contrasts sharply with the observed upregulatory effect of shear stress on TM expression. These observations are consistent with previous reports into the apparently opposite effects of cyclic strain [18] and shear stress [14]–[17] on vascular endothelial TM expression, and highlight the likelihood that basal levels of endothelial TM expression under physiological conditions in vivo most likely represent a careful balance between a myriad of potentially opposing stimuli [1]. As such, downregulatory stimuli (e.g. cyclic strain, cAMP, c-reactive protein, phorbolesters) would likely balance out the effects of upregulatory stimuli (e.g. shear stress, thrombin, VEGF), helping to establish a steady state TM expression level under normal physiological conditions. By way of a possible transcriptional explanation for the opposite effects of strain and shear on TM expression, the 5′untranslated region of the TM promoter displays a CACCC motif, which is known to facilitate positive gene regulation by Krüppel-like factor 2 (KLF2), a transcription factor that is activated by shear stress, but not by cyclic strain [34], [35]. The cyclic strain-dependent activation of NF-κB [36], an established negative regulator of TM expression [37] is also of relevance. Finally, it can be noted that our results differ from those of Chen et al. who demonstrate that 18 hr exposure of HUVECs to 21% (but not 15%) cyclic strain leads to induction of TM expression (>2-fold), an increase which the authors attribute to NO-mediated stabilization of TM protein via S-nitrosylation, and not to transcriptional activation [19]. Differences in cell type (HAEC versus HUVEC), applied strain level (physiological versus pathological), and strain system (FX4000T versus FX2000) may all account for the observed disparity between these observations.
Interestingly, there have been no in vitro studies thus far comprehensively documenting the influence of either hemodynamic force on endothelial TM release. In this respect, our next investigations demonstrated time-dependent release of TM from HAECs in response to physiologic levels of cyclic strain and shear stress. With continuing focus on the former stimulus, our studies subsequently demonstrated that physiologic cyclic strain could induce TM release from HAECs in both a dose- and frequency-dependent manner. Moreover, we noted that the cyclic strain-induced release of TM was not effected by inhibition of either MAP kinase (p38, ERK1/2), receptor tyrosine kinase, or endothelial nitric oxide synthase (eNOS) activation. The release or shedding of TM from cell surfaces has received much attention in the literature [1]. As circulating TM levels in plasma are typically in the ng/ml range in healthy humans, this would suggest that the stimulated release of TM by physiologic hemodynamic forces may contribute to normal vessel homeostasis. In this respect, it is worth noting that circulating TM has been shown to bind thrombin and exhibit anti-coagulant ability [38].
The potent increase in TM release (∼3-fold) that accompanied a doubling of the strain frequency (from 1 to 2 Hz) is also quite noteworthy. The observed frequency-dependent induction of TM release may involve a cytoskeletal mechanism, given the pivotal role of the actin cytoskeleton in cellular secretory and microvesiculation processes [39], [40]. In support of this, recent work by Hsu et al. has shown that endothelial stress fibre alignment and turnover are highly sensitive to strain frequency [41]. The increase in strain frequency is also analogous to an elevation in normal heart rate (i.e. from 60–120 beats per minute), and in this respect it can be noted that increased levels of circulating TM have been associated with physical exercise [42], [43], which is characterised by elevated shearing and heart rate.
As endothelial activation in vivo may involve the combined influence of both hemodynamic and humoral stimuli, we next decided to examine the concerted impact of cyclic strain and inflammatory mediators, namely TNFα and ox-LDL, on TM release from HAECs (in associated studies, the effect of elevated glucose levels, ranging from normal to diabetic, was also examined – Figure S2). Our investigations initially confirmed downregulation of TM mRNA levels in response to TNFα treatment of HAECs. This is consistent with other studies [44], [45] and likely reflects the proinflammatory nature of TNFα. We also noted that TNFα treatment alone (0–100 ng/ml) had no significant effect on TM release from unstrained HAECs. This is consistent with similar observations by Boehme et al. who reported that TNFα treatment alone had no effect on TM release from HUVECs [46]. Interestingly however, these authors (and others) noted that endothelial TM release was significantly increased following the concerted action of TNFα and neutrophils, likely arising from cytokine activation of neutrophil elastase and cathepsin G, which may cause enzymatic release of TM from endothelial cells [46]–[48]. We further noted that 100 ng/ml TNFα combined with 7.5% strain appeared to potentiate TM release from HAECs. The reason for this is as yet unknown. In this respect, it is noteworthy that the ROS-generating ability of cyclic strain in vascular endothelial cells has previously been found to be significantly potentiated by co-treatment with TNFα [49], leading us to speculate that a ROS-dependent mechanism may account for our observations. This appears unlikely however given the potent ROS-induction that would have accompanied the treatment of endothelial cells with TNFα alone [28]. Finally, the possibility that TNFα treatment may be enhancing TM internalization via endocytosis, thereby reducing surface levels of TM available for release either by enzymatic or microparticle means, is also a potential mechanistic consideration under static and strain conditions [50], [51]. Whilst beyond the scope of the present study, these mechanistic concepts surrounding TM release merit further investigation.
In related experiments, it was noted that ox-LDL could downregulate TM mRNA levels in HAECs, again consistent with earlier studies [29]. As with TNFα, it was also noted that ox-LDL treatment alone (0–50 µg/ml) had no significant effect on TM release from unstrained HAECs, whilst appearing to potentiate TM release under 7.5% strain at the elevated ox-LDL concentrations (10–50 µg/ml). It has previously been demonstrated that the concerted action of cyclic strain and ox-LDL can synergistically increase the expression of lectin-like ox-LDL receptor-1 (LOX-1), albeit in chondrocytes and vascular smooth muscle cells [52], [53], with consequences for cell signalling and functions. Whilst undetermined at this time, this phenomenon may also be relevant to the concerted action of ox-LDL and cyclic strain on TM release from HAECs. It is also noteworthy that ox-LDL can attenuate agonist-stimulated nitric oxide (NO) release from HUVECs [54]. Given the regulatory cross-talk that exists between thrombomodulin and NO [55], blockade of strain-dependent eNOS activation and NO release by ox-LDL may constitute another possible mechanism for its potentiation of TM release. Finally, it can be noted that clinical studies have demonstrated a direct correlation between circulating ox-LDL and sTM levels [56], [57].
In a final series of experiments, we sought to better understand the mechanism of TM release from HAECs in response to cyclic strain. A considerable volume of research has previously focused on the proteolytic release of TM from cells into tissue fluids such as plasma, urine, vitreous fluid, and synovial fluid [1]. In this regard, our investigations did not indicate a significant role for either MMPs or rhomboids (RHBDL2-like intramembrane serine proteases) in the strain-induced release of TM from HAECs, despite previous studies linking these enzymes to sTM shedding in endothelial and non-endothelial cells, respectively [32], [33]. In addition to proteolytic release however, a few studies point to the microvesicular pathway (microparticles and/or exosomes) as a mechanism for TM shedding from activated cells [58], prompting us to investigate this possibility in our HAEC cyclic strain model. Following preparation of microparticle fractions from HAEC-conditioned media by high speed centrifugation [24], [25], analysis revealed significantly higher quantities of TM in microparticle fractions from strained cells, suggesting that physiologic cyclic strain can stimulate release of TM(+) microparticles from HAECs. Although speculative at present, strain-induced TM(+) endothelial microparticles could exhibit anti-coagulant and anti-inflammatory effects to help fine-tune vascular homeostasis. This is consistent with the general view that endothelial microparticles function as important conveyors of biological information capable of biomolecule dissemination and exchange with other vascular cells. Whilst frequently viewed as being indicative of endothelial activation and apoptosis, and of reflecting potentially deletarious consequences for vascular homeostasis and disease progression, more recent studies have demonstrated the plasticity of endothelial microparticles with respect to phenotype and function. Indeed, several studies have now confirmed the ability of endothelial microparticles to promote cytoprotective effects and endothelial repair, as well as exhibiting anti-coagulant and anti-inflammatory actions [59]. Finally, in light of our observations, it is also noteworthy that both cyclic strain and shear stress have very recently been demonstrated to regulate microparticle generation in cultured endothelial cells [60], [61].
In summary, an investigation into the effects of hemodynamic forces, and specifically cyclic strain, on TM regulation in HAECs is presented. Physiologic cyclic strain was observed to downregulate TM expression (mRNA and protein), whilst upregulating in a time-, dose- and frequency-dependent manner the release of TM into media. Inflammatory mediators, TNFα and ox-LDL, were observed to potentiate strain-induced TM release. Moreover, evidence is also presented in support of a microvesicular mechanism for TM release by cyclic strain. To our knowledge, these are the first in vitro studies to comprehensively assess the regulatory influence of physiologic cyclic strain on vascular endothelial TM dynamics. It should be noted however that these are preliminary investigations using vascular cell cultures and model systems (e.g. Flexercell) that, whilst well established within the literature, only offer a partial approximation of in vivo physiology. As such, follow up studies using more advanced ex vivo and in vivo models of hemodynamic loading will ultimately be necessary to reinforce the physiological conclusions made in our study.
Supporting Information
Performance characteristics for the human thrombomodulin/BDCA-3 DuoSet ELISA. (A) Linear range of the ELISA monitored over a broad range of HAEC lysate concentrations (Note: cell lysates were routinely assayed in the 1∶20 dilution range). (B) ELISA standard curve (0–2000 pg/ml).
(TIF)
Effects of elevated glucose on CS-induced TM release in HAECs. Effect of CS (0 or 7.5%, 24 hr) on TM release from HAECs in the presence of 5, 15, and 30 mM glucose. *P≤0.05 versus 5 mM 0% CS. δ P≤0.05 versus 5 mM 7.5% CS.
(TIF)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by the National Development Plan/Higher Education Authority of Ireland Programme for Research in Third Level Institutes - HEA/PRTLI Cycle 4; T3/Targeted Therapeutics & Theranostics (PMC), and the National Development Plan/Higher Education Authority of Ireland Programme for Research in Third Level Institutes - HEA/PRTLI Cycle 5; BioAT - BioAnalysis and Therapeutics (PMC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Martin FA, Murphy RP, Cummins PM (2013) Thrombomodulin and the vascular endothelium: Insights into functional, regulatory, and therapeutic aspects. Am J Physiol Heart Circ Physiol 304: H1585–H1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zycinska K, Wardyn KA, Zielonka TM, Krupa R, Lukas W (2009) Clinical implications of serum thrombomodulin in PR3-ANCA-associated vasculitis. Eur J Med Res 14: 268–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lin SM, Wang YM, Lin HC, Lee KY, Huang CD, et al. (2008) Serum thrombomodulin level relates to the clinical course of disseminated intravascular coagulation, multiorgan dysfunction syndrome, and mortality in patients with sepsis. Crit Care Med 36: 683–689. [DOI] [PubMed] [Google Scholar]
- 4. Rousseau A, Favier R, Van Dreden P (2009) Elevated circulating soluble thrombomodulin activity, tissue factor activity and circulating procoagulant phospholipids: new and useful markers for pre-eclampsia? Eur J Obstet Gynecol Reprod Biol 146: 46–49. [DOI] [PubMed] [Google Scholar]
- 5. Dharmasaroja P, Dharmasaroja PA, Sobhon P (2012) Increased plasma soluble thrombomodulin levels in cardioembolic stroke. Clin Appl Thromb Hemost 18: 289–293. [DOI] [PubMed] [Google Scholar]
- 6. Ando J, Yamamoto K (2011) Effects of shear stress and stretch on endothelial function. Antioxid Redox Signal 15: 1389–1403. [DOI] [PubMed] [Google Scholar]
- 7. Wang JH, Thampatty BP (2006) An introductory review of cell mechanobiology. Biomechan Model Mechanobiol 5: 1–16. [DOI] [PubMed] [Google Scholar]
- 8. Hahn C, Schwartz MA (2009) Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol 10: 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tineli RA, Viaro F, Dalio MB, Reis GS, Basseto S, et al. (2007) Mechanical forces and human saphenous veins: coronary artery bypass graft implications. Rev Bras Cir Cardiovasc 22: 87–95. [DOI] [PubMed] [Google Scholar]
- 10. Suzuma I, Hata Y, Clermont A, Pokras F, Rook SL, et al. (2001) Cyclic stretch and hypertension induce retinal expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2: potential mechanisms for exacerbation of diabetic retinopathy by hypertension. Diabetes 50: 444–454. [DOI] [PubMed] [Google Scholar]
- 11. Kario K (2007) Vascular damage in exaggerated morning surge in blood pressure. Hypertension 49: 771–772. [DOI] [PubMed] [Google Scholar]
- 12. Groen HC, Gijsen FJ, van der Lugt A, Ferguson MS, Hatsukami TS, et al. (2008) High shear stress influences plaque vulnerability. Neth Heart J 16: 280–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thacher TN, Silacci P, Stergiopulos N, da Silva RF (2010) Autonomous effects of shear stress and cyclic circumferential stretch regarding endothelial dysfunction and oxidative stress: an ex vivo arterial model. J Vasc Res 47: 336–345. [DOI] [PubMed] [Google Scholar]
- 14. Ishibazawa A, Nagaoka T, Takahashi T, Yamamoto K, Kamiya A, et al. (2011) Effects of shear stress on the gene expressions of endothelial nitric oxide synthase, endothelin-1, and thrombomodulin in human retinal microvascular endothelial cells. Invest Ophthalmol Vis Sci 52: 8496–8504. [DOI] [PubMed] [Google Scholar]
- 15. Takada Y, Shinkai F, Kondo S, Yamamoto S, Tsuboi H, et al. (1994) Fluid shear stress increases the expression of thrombomodulin by cultured human endothelial cells. Biochem Biophys Res Commun 205: 1345–1352. [DOI] [PubMed] [Google Scholar]
- 16. Rossi J, Rouleau L, Tardif JC, Leask RL (2010) Effect of simvastatin on Kruppel-like factor-2, endothelial nitric oxide synthase and thrombomodulin expression in endothelial cells under shear stress. Life Sci 87: 92–99. [DOI] [PubMed] [Google Scholar]
- 17. Li YH, Hsieh CY, Wang DL, Chung HC, Liu SL, et al. (2007) Remodeling of carotid arteries is associated with increased expression of thrombomodulin in a mouse transverse aortic constriction model. Thromb Haemost 97: 658–664. [PubMed] [Google Scholar]
- 18. Sperry JL, Deming CB, Bian C, Walinsky PL, Kass DA, et al. (2003) Wall tension is a potent negative regulator of in vivo thrombomodulin expression. Circ Res 92: 41–47. [DOI] [PubMed] [Google Scholar]
- 19. Chen SC, Cheng JJ, Wu SE, Shen HC, Shyu KG, et al. (2008) Cyclic strain-induced thrombomodulin expression in endothelial cells is mediated by nitric oxide, but not hydrogen peroxide. Acta Cardiol Sin 24: 144–150. [Google Scholar]
- 20. von Offenberg Sweeney N, Cummins PM, Birney YA, Redmond EM, Cahill PA (2004) Cyclic strain-induced endothelial MMP-2: role in vascular smooth muscle cell migration. Biochem Biophys Res Commun 320: 325–333. [DOI] [PubMed] [Google Scholar]
- 21. Hendrickson RJ, Cahill PA, Sitzmann JV, Redmond EM (1999) Ethanol enhances basal and flow-stimulated nitric oxide synthase activity in vitro by activating an inhibitory guanine nucleotide binding protein. J Pharmacol Exp Ther 289: 1293–1300. [PubMed] [Google Scholar]
- 22. Fitzpatrick PA, Guinan AF, Walsh TG, Murphy RP, Killeen MT, et al. (2009) Down-regulation of neprilysin (EC3.4.24.11) expression in vascular endothelial cells by laminar shear stress involves NADPH oxidase-dependent ROS production. Int J Biochem Cell Biol 41: 2287–2294. [DOI] [PubMed] [Google Scholar]
- 23. Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, et al. (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76–85. [DOI] [PubMed] [Google Scholar]
- 24. Lacroix R, Sabatier F, Mialhe A, Basire A, Pannell R, et al. (2007) Activation of plasminogen into plasmin at the surface of endothelial microparticles: a mechanism that modulates angiogenic properties of endothelial progenitor cells in vitro . Blood 110: 2432–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lacroix R, Plawinski L, Robert S, Doeuvre L, Sabatier F, et al. (2012) Leukocyte- and endothelial-derived microparticles: a circulating source for fibrinolysis. Haematologica 97: 1864–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159. [DOI] [PubMed] [Google Scholar]
- 27. Guinan AF, Rochfort KD, Fitzpatrick PA, Walsh TG, Pierotti AR, et al. (2013) Shear stress is a positive regulator of thimet oligopeptidase (EC3.4.24.15) in vascular endothelial cells: consequences for MHC1 levels. Cardiovasc Res 99: 545–554. [DOI] [PubMed] [Google Scholar]
- 28. Rochfort KD, Collins LE, Murphy RP, Cummins PM (2014) Downregulation of blood-brain barrier phenotype by proinflammatory cytokines involves NADPH oxidase-dependent ROS generation: consequences for interendothelial adherens and tight junctions. PloS One 9: e101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ishii H, Tezuka T, Ishikawa H, Takada K, Oida K, et al. (2003) Oxidized phospholipids in oxidized low-density lipoprotein down-regulate thrombomodulin transcription in vascular endothelial cells through a decrease in the binding of RARbeta-RXRalpha heterodimers and Sp1 and Sp3 to their binding sequences in the TM promoter. Blood 101: 4765–4774. [DOI] [PubMed] [Google Scholar]
- 30. Wolf EV, Zeißler A, Vosyka O, Zeiler E, Sieber S, et al. (2013) A new class of rhomboid protease inhibitors discovered by activity-based fluorescence polarization. PLoS One 8: e72307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li C, Cantor WJ, Nili N, Robinson R, Fenkell L, et al. (2002) Arterial repair after stenting and the effects of GM6001, a matrix metalloproteinase inhibitor. J Am Coll Cardiol 39: 1852–1858. [DOI] [PubMed] [Google Scholar]
- 32. Lohi O, Urban S, Freeman M (2004) Diverse substrate recognition mechanisms for rhomboids; thrombomodulin is cleaved by mammalian rhomboids. Curr Biol 14: 236–241. [DOI] [PubMed] [Google Scholar]
- 33. Wu HL, Lin CI, Huang YL, Chen PS, Kuo CH, et al. (2008) Lysophosphatidic acid stimulates thrombomodulin lectin-like domain shedding in human endothelial cells. Biochem Biophys Res Commun 367: 162–168. [DOI] [PubMed] [Google Scholar]
- 34. Dekker RJ, van Thienen JV, Rohlena J, de Jager SC, Elderkamp YW, et al. (2005) Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol 167: 609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, et al. (2002) Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Krüppel-like factor (KLF2). Blood 100: 1689–1698. [DOI] [PubMed] [Google Scholar]
- 36. Wang DS, Proffit D, Tsao PS (2001) Mechanotransduction of endothelial oxidative stress induced by cyclic strain. Endothelium 8: 283–291. [DOI] [PubMed] [Google Scholar]
- 37. Wu CT, Chang YH, Lin P, Chen WC, Chen MF (2014) Thrombomodulin expression regulates tumorigenesis in bladder cancer. BMC Cancer 14: 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hosaka Y, Takahashi Y, Ishii H (1998) Thrombomodulin in human plasma contributes to inhibit fibrinolysis through acceleration of thrombin-dependent activation of plasma procarboxypeptidase B. Thromb Haemost 79: 371–377. [PubMed] [Google Scholar]
- 39. Latham SL, Chaponnier C, Dugina V, Couraud PO, Grau GE, et al. (2013) Cooperation between β- and γ-cytoplasmic actins in the mechanical regulation of endothelial microparticle formation. FASEB J 27: 672–683. [DOI] [PubMed] [Google Scholar]
- 40. Vischer UM, Barth H, Wollheim CB (2000) Regulated von Willebrand factor secretion is associated with agonist-specific patterns of cytoskeletal remodeling in cultured endothelial cells. Arterioscler Thromb Vasc Biol 20: 883–891. [DOI] [PubMed] [Google Scholar]
- 41. Hsu HJ, Lee CF, Kaunas R (2009) A dynamic stochastic model of frequency-dependent stress fiber alignment induced by cyclic stretch. PLoS One 4: e4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weiss C, Welsch B, Albert M, Friedmann B, Strobel G, et al. (1998) Coagulation and thrombomodulin in response to exercise of different type and duration. Med Sci Sports Exerc 30: 1205–1210. [DOI] [PubMed] [Google Scholar]
- 43. Bartzeliotou AI, Margeli AP, Tsironi M, Skenderi K, Bacoula C, et al. (2007) Circulating levels of adhesion molecules and markers of endothelial activation in acute inflammation induced by prolonged brisk exercise. Clin Biochem 40: 765–770. [DOI] [PubMed] [Google Scholar]
- 44. Nan B, Lin P, Lumsden AB, Yao Q, Chen C (2005) Effects of TNF-alpha and curcumin on the expression of thrombomodulin and endothelial protein C receptor in human endothelial cells. Thromb Res 115: 417–426. [DOI] [PubMed] [Google Scholar]
- 45. Lin PY, Shen HC, Chen CJ, Wu SE, Kao HL, et al. (2010) The inhibition in tumor necrosis factor-alpha-induced attenuation in endothelial thrombomodulin expression by carvedilol is mediated by nuclear factor-kappaB and reactive oxygen species. J Thromb Thrombolysis 29: 52–59. [DOI] [PubMed] [Google Scholar]
- 46. Boehme MW, Deng Y, Raeth U, Bierhaus A, Ziegler R, et al. (1996) Release of thrombomodulin from endothelial cells by concerted action of TNF-alpha and neutrophils: in vivo and in vitro studies. Immunology 87: 134–140. [PMC free article] [PubMed] [Google Scholar]
- 47. Redl H, Schlag G, Schiesser A, Davies J (1995) Thrombomodulin release in baboon sepsis: its dependence on the dose of Escherichia coli and the presence of tumor necrosis factor. J Infect Dis 171: 1522–1527. [DOI] [PubMed] [Google Scholar]
- 48. MacGregor IR, Perrie AM, Donnelly SC, Haslett C (1997) Modulation of human endothelial thrombomodulin by neutrophils and their release products. Am J Respir Crit Care Med 155: 47–52. [DOI] [PubMed] [Google Scholar]
- 49. Matsushita H, Lee KH, Tsao PS (2001) Cyclic strain induces reactive oxygen species production via an endothelial NAD(P)H oxidase. J Cell Biochem Suppl 36 99–106. [DOI] [PubMed] [Google Scholar]
- 50. Chu M, Bird CH, Teasdale M, Bird PI (1998) Turnover of thrombomodulin at the cell surface occurs at a similar rate to receptors that are not actively internalized. Thromb Haemost 80: 119–127. [PubMed] [Google Scholar]
- 51. Teasdale MS, Bird CH, Bird P (1994) Internalization of the anticoagulant thrombomodulin is constitutive and does not require a signal in the cytoplasmic domain. Immunol Cell Biol 72: 480–488. [DOI] [PubMed] [Google Scholar]
- 52. Akagi M, Nishimura S, Yoshida K, Kakinuma T, Sawamura T, et al. (2006) Cyclic tensile stretch load and oxidized low density lipoprotein synergistically induce lectin-like oxidized ldl receptor-1 in cultured bovine chondrocytes, resulting in decreased cell viability and proteoglycan synthesis. J Orthop Res 24: 1782–1790. [DOI] [PubMed] [Google Scholar]
- 53. Zhang Z, Zhang M, Li Y, Liu S, Ping S, et al. (2013) Simvastatin inhibits the additive activation of ERK1/2 and proliferation of rat vascular smooth muscle cells induced by combined mechanical stress and oxLDL through LOX-1 pathway. Cell Signal 25: 332–340. [DOI] [PubMed] [Google Scholar]
- 54. Jay MT, Chirico S, Siow RC, Bruckdorfer KR, Jacobs M, et al. (1997) Modulation of vascular tone by low density lipoproteins: effects on L-arginine transport and nitric oxide synthesis. Exp Physiol 82: 349–360. [DOI] [PubMed] [Google Scholar]
- 55. David-Dufilho M, Millanvoye-Van Brussel E, Topal G, Walch L, Brunet A, et al. (2005) Endothelial thrombomodulin induces Ca2+ signals and nitric oxide synthesis through epidermal growth factor receptor kinase and calmodulin kinase II. J Biol Chem 280: 35999–36006. [DOI] [PubMed] [Google Scholar]
- 56. Hong SC, Zhao SP, Liu Q, Wu ZH (2008) Effect of the anti-oxidant probucol on soluble thrombomodulin (sTM) in hypercholesterolemic rabbits. Int J Cardiol 123: 180–182. [DOI] [PubMed] [Google Scholar]
- 57. Porreca E, Di Febbo C, Moretta V, Angelini A, Guglielmi MD, et al. (2004) Circulating leptin is associated with oxidized LDL in postmenopausal women. Atherosclerosis 175: 139–143. [DOI] [PubMed] [Google Scholar]
- 58. Satta N, Freyssinet JM, Toti F (1997) The significance of human monocyte thrombomodulin during membrane vesiculation and after stimulation by lipopolysaccharide. Br J Haematol 96: 534–542. [DOI] [PubMed] [Google Scholar]
- 59. Dignat-George F, Boulanger CM (2011) The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol 31: 27–33. [DOI] [PubMed] [Google Scholar]
- 60. Vion AC, Ramkhelawon B, Loyer X, Chironi G, Devue C, et al. (2013) Shear stress regulates endothelial microparticle release. Circ Res 112: 1323–1333. [DOI] [PubMed] [Google Scholar]
- 61. Vion AC, Birukova AA, Boulanger CM, Birukov KG (2013) Mechanical forces stimulate endothelial microparticle generation via caspase-dependent apoptosis-independent mechanism. Pulm Circ 3: 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Performance characteristics for the human thrombomodulin/BDCA-3 DuoSet ELISA. (A) Linear range of the ELISA monitored over a broad range of HAEC lysate concentrations (Note: cell lysates were routinely assayed in the 1∶20 dilution range). (B) ELISA standard curve (0–2000 pg/ml).
(TIF)
Effects of elevated glucose on CS-induced TM release in HAECs. Effect of CS (0 or 7.5%, 24 hr) on TM release from HAECs in the presence of 5, 15, and 30 mM glucose. *P≤0.05 versus 5 mM 0% CS. δ P≤0.05 versus 5 mM 7.5% CS.
(TIF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.