Abstract
A 41-year-old man with no familial history of gastric cancer was diagnosed as with intramucosal early gastric cancer. Two months after the first endoscopic submucosal dissection for signet-ring cell carcinoma (SRCC), the appearance of previously unrecognized multiple erosions of SRCC was noticed. Pathological examination after a total gastrectomy and Roux-en-Y reconstruction with D2 lymph node dissection were performed. Postoperative pathological examination revealed 90 and more lesions, which tempted the attending pathologist to refer to genetic tests for the predisposition though the patient had no familial history of gastric cancer. There were no mutations in all the exons of CDH1 with conventional DNA sequencing, but multiplex ligation-dependent probe amplification, and reverse transcription-polymerase chain reaction analyses disclosed a large genomic deletion (c.1566-?_1711+?del), leading to the mRNA with loss of the exon 11. Among family members, his son was found to be a carrier of this change, while his parents were negative for the familial CDH1 mutation, implying that this change is a de novo event in the proband. The present report is the first description of a de novo large genomic deletion of CDH1 gene associated with early-onset diffuse gastric cancer. When the clinician finds a relatively-young patient who has multiple SRCCs, CDH1 germline mutation should be considered, even for patients with no familial history.
Keywords: Endoscopic submucosal dissection, Signet-ring cell carcinoma, Stomach neoplasms, CDH1, Mutation
Introduction
Endoscopic submucosal dissection (ESD) is the preferred treatment method for locally dissecting early gastric cancer (EGC) with a negligible risk of lymph node metastasis [1]. Currently nearly 100 % curability has been achieved for EGC with radical surgery, thus ESD is also expected to be performed at a comparable success rate. Metachronous recurrence of EGC after ESD is an important problem, but recurrence can be detected with adequate follow-up endogastroduodenoscopy (EGD), and most patients can receive early endoscopic treatment again [2]. In Japan, the previous consensus has recommended curative ESD that are indicated only for small intramucosal differentiated-type EGC [1]. Recently, the criteria have been expanded to other pathological types such as the undifferentiated-type under regulated conditions [3–5].
In Japan, gastric cancer is a major cause of cancer death, and Helicobacter pylori infection is considered to be a major cause of gastric cancer [6]. Although epidemiological studies have shown that there are patients who have a significant familial history of gastric cancer, the pathogenesis of familial gastric cancer (FGC) has not been clearly explained. CDH1 germline mutations are associated with the development of autosomal cancer syndrome, namely hereditary diffuse gastric cancer (HDGC); about 25–30 % of families fulfilling the clinical criteria for HDGC established by the International Gastric Cancer Linkage Consortium (IGCLC) have constitutional alterations of the CDH1 gene [7]. The CDH1 gene maps to chromosome 16q22.1 and consists of 16 exons that encode E-cadherin, which is a major component of adherens junctions. Carrying the abnormal CDH1 gene confers more than an 80 % lifetime risk of developing gastric cancer.
In 2012, the first conclusive case of a de novo CDH1 germline mutation (c.1792 C>T (R598X)) in a woman whose daughter was diagnosed with early-onset diffuse gastric cancer was reported [8]. On the other hand, CDH1 germline mutations can also be identified in sporadic early-onset gastric cancer in less than 4 % of patients who are 35 years of age at the time of diagnosis, presenting as de novo mutations [9]. Although the incidence of gastric cancer is relatively high in Japan, the detection rate of CDH1 germline mutations in Japanese patients with FGC is low compared to that in European patients [10].
We report the first case of early-onset diffuse gastric cancer associated with a de novo large genomic deletion of CDH1 gene, and the pathological feature including gross appearance of the surgical specimen of the stomach was pathognomonic.
Case report
An asymptomatic 41-year-old man was admitted to our hospital with EGC revealed by cancer screening EGD. There were no abnormalities in the physical examination or laboratory data, and no medical history of malignant tumors. He smoked 20–60 cigarettes a day for 22 years. An enzyme immunoassay to qualitatively detect serum IgG antibodies to Helicobacter pylori was negative. A protruding lesion, 5-mm in size, with erosion was observed on the greater curvature of the antrum and no other lesions were observed endoscopically. Biopsy from the erosion indicated signet-ring cell carcinoma (SRCC). ESD was performed on the lesion in accordance with clinical trial (JCOG1009/1010) [5], resulting in en bloc resection. Precise pathological examination of 3- to 4-mm slices from the entire specimen revealed a lesion, 13 × 13 mm in size, which was a SRCC limited in the mucosal layer (Fig. 1a, b). Lymphovascular involvement was not observed and horizontal/vertical margins were negative. According to the above-mentioned pathological analysis, the definitive diagnosis was f Stage IA SRCC (L, Gre, 0-IIb, pT1a, cN0, cH0, cP0, cM0) and resection was thought to be curative [1].
Fig. 1.
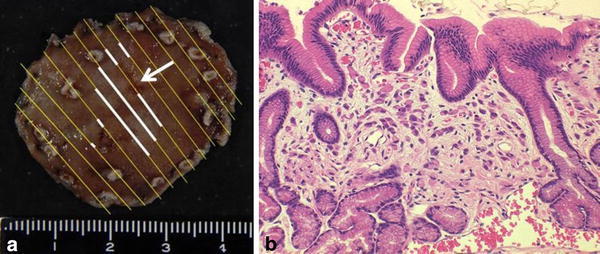
a Lesion resected with ESD. Resected specimen was 40 × 38 mm. The lesion was step-sectioned at 3- to 4-mm intervals, and then examined pathologically. Although SRCCs at the biopsy site disappeared (as an arrow indicates), the entire specimen revealed a lesion 13 × 13 mm in size (as white lines indicate). The resection was curative and the horizontal margin was 6.5 mm. b The specimen showed localized neoplastic cells with signet-ring features limited to the mucosal layer. Signet-ring cells are easily detected on Haematoxylin and eosin (H&E) sections
Follow-up EGD performed 2 months later revealed no local recurrences at the ESD site, but three new erosions appeared on the gastric angle and antrum (Fig. 2a, b). Biopsies from these erosions again indicated multiple lesions of SRCC. Apparently they were likely to be intramucosal, but there was a high incidence of metachronous multiple lesions. We considered the possibility of HDGC that we could not control multiple SRCCs by repeated ESD or distal gastrectomy similar to a previous report [11]. After the explanation about the risk for recurrence or metachronous lesions in any part of the stomach, next month, a total gastrectomy and Roux-en-Y reconstruction with D2 lymph node dissection were carried out as the patient wished. Postoperative pathological examination revealed that 90 and more lesions, each less than 5-mm in size, showing type 0-IIb SRCCs in the mucosal layer, and they were distributed in the whole stomach (Fig. 3). The lesions may be missed by a cursory look. The reason for the discrepancy between preoperative endoscopic findings and final pathological diagnosis would be the difficulty in locating endoscopically SRCC foci in the superficial lamina propria, just beneath normal-looking surface epithelium or gastric pits. The tumor cells had a low proliferative index and reduced expression of E-cadherin with immunohistochemistry (Fig. 4c). We suspected the involvement of a genetic abnormality of CDH1, although he had no familial history of gastric cancer and did not meet clinical criteria for HDGC defined by the IGCLC [7].
Fig. 2.
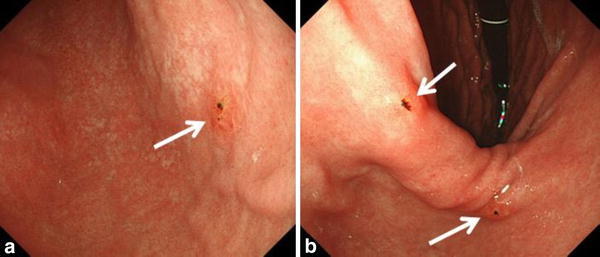
EGD view 2 months after ESD. EGC is visible as the arrows indicate. a The posterior wall of the lower gastric body, near the gastric angle. b The lesser curvature of the gastric angle and the distal antrum
Fig. 3.

Surgical specimen. Postoperative pathological examination revealed 90 and more lesions. White circles indicate infiltrating SRCCs
Fig. 4.
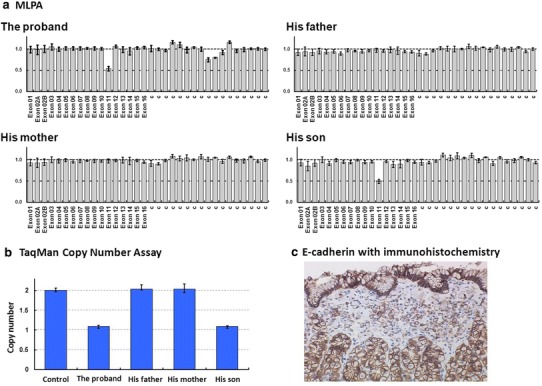
a, b Germline copy number change in the CDH1 gene of a patient. Since there were no point mutation of CDH1, the DNAs were tested for large genomic deletions in the CDH1 gene using the SALSA P083-B1 CDH1 multiplex ligation-dependent probe amplification (MLPA) kit (MRC-Holland, Amsterdam, The Netherlands). The reactions were performed according to the manufacturer’s instruction. The results are presented as the ratios to the normal copy numbers (two in this case). Seventeen columns in the right side of the graph are control DNAs. Probe ratios below 0.7 and above 1.3 were regarded as indicative of a decrease and increase, respectively, in the gene dosage. As shown in the figure, unambiguous decreases in exon 11 were noticed in the proband and his son. c E-cadherin with immunohistochemistry. E-cadherin expression was suppressed in signet-ring cells, while normal glands were uniformly and intensely positive
After informed consent was obtained, DNA was extracted from the peripheral blood of the patient. First, a polymerase chain reaction (PCR)-direct sequencing analysis was performed for all the exons of CDH1, but no mutations were identified. Next, a multiplex ligation dependent probe amplification (MLPA) analysis was performed as previously described [12] (Fig. 4a). This revealed that the exon 11 of the CDH1 transcript was heterozygously deleted in this case; thus, a heterozygous c.1566-?_1711+?del germline mutation existed in the proband. For validation, CDH1 copy numbers in exons 11 were measured using TaqMan copy number assays according to the manufacturer’s instructions, and the copy number was 1 (the normal copy number is 2) (Fig. 4b).
A program of genetic counseling and DNA testing was provided to other members of his family (Fig. 5). We could not contact his brother. Predictive genetics testing was performed on his parents and his son. Among family members, his 15-year-old son was a carrier of the mutation, and his 71-year-old father and 65-year-old mother were negative for the familial CDH1 mutation. These results lead to the conclusion that a large genomic deletion of CDH1 gene identified in this proband and in his son probably occurred de novo in the proband. The proband’s 13-year-old daughter has not yet been tested.
Fig. 5.
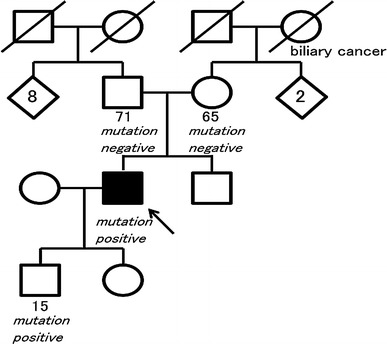
Pedigree of family with germline CDH1 alteration. Squares indicate males; circles indicate females. Solid symbols indicate the gastric cancer patient. Symbols with a slash indicate deceased individuals. An arrow points to the proband. The numbers below the symbols indicate age at the time family members were analyzed
Discussion
E-cadherin is a calcium-dependent cell membrane protein involved in cell–cell adhesion and confers cell polarity [13]. CDH1 mutations are associated with a risk of early-onset diffuse gastric cancer, in addition to increased risk of lobular breast cancer and signet-ring colon cancer [7]. There was a report of six patients from one large kindred who underwent total gastrectomy for CDH1 mutation without any evidence of gastric cancer from detailed preoperative imaging studies, including high magnification endoscopy with methylene blue chromoendoscopy and multiple random biopsies. Each patient was found to have multiple sites of invasive diffuse SRCC without lymph node metastases [14]. The guideline recommends advising CDH1 mutation positive patients with normal gastric biopsies to undergo prophylactic gastrectomy once the genetic testing results are known and once individuals are older than 20 years. Rare cases of clinically significant diffuse gastric cancer have been reported in affected families before the age of 18, but the overall risk of diffuse gastric cancer before the age of 20 is low [15]. In the present case, the proband’s children should receive surveillance EGD in the future.
To date, over 100 kindreds with CDH1 germline mutations have been identified, and few cases of a concluded de novo CDH1 germline mutation have been reported [8]. However, we think there are many unidentified patients who were not tested or in whom mutations could not be identified with conventional DNA sequencing. Importantly, 4 % of these mutation positive families exhibited large germline deletions of CDH1 that were not detectable with conventional DNA sequencing [16]. Analyses of large genomic deletions with MLPA are recommended in cases where simple DNA sequencing is unrevealing [16, 17]. We propose that screening for germline large rearrangements of CDH1 should be included in CDH1 genetic testing for FGC in young patients with diffuse gastric cancer [12].
The present report is the first description of a de novo large genomic deletion of the CDH1 gene associated with the production of a truncated CDH1 protein. The c.1566-?_1711+?del germline mutation observed in this case has not been reported previously either as a somatic or a germline mutation, so we think this is novel and further characterization of its RNA would be interesting. Importantly, it was verified that this mutation passes the gene on to the proband’s offspring without change.
The ESD technique has rapidly permeated worldwide for treating EGC, but obviously it is not suitable for multiple occurrences such as in CDH1 mutation positive patients. As this instructive case shows, unusual morphologic feature may suggest prepared endoscopists should suspect genetic predisposition. In such a case, genetic counseling should also be provided. A prudent follow-up is necessary not only for the proband but also for the proband’s family. When a clinician finds the relatively-young patient who has multiple SRCCs, unusual conditions such as CDH1 germline mutation should be considered, even if patients have no familial histories of gastric cancer.
Acknowledgments
This work was supported by grants from the Ministry of Health, Labour and Welfare (10103838), Ministry of Education, Culture, Sports, Science and Technology (S-001), National Cancer Center Research and Development Fund, Princess Takamatsu Cancer Research Fund, and the Smoking Research Foundation.
Conflict of interest
None.
References
- 1.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 2.Nakajima T, Oda I, Gotoda T, Hamanaka H, Eguchi T, Yokoi C, et al. Metachronous gastric cancers after endoscopic resection: how effective is annual endoscopic surveillance? Gastric Cancer. 2006;9:93–98. doi: 10.1007/s10120-006-0372-9. [DOI] [PubMed] [Google Scholar]
- 3.Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148–152. doi: 10.1007/s10120-009-0515-x. [DOI] [PubMed] [Google Scholar]
- 4.Okada K, Fujisaki J, Yoshida T, Ishikawa H, Suganuma T, Kasuga A, et al. Long-term outcomes of endoscopic submucosal dissection for undifferentiated-type early gastric cancer. Endoscopy. 2012;44:122–127. doi: 10.1055/s-0031-1291486. [DOI] [PubMed] [Google Scholar]
- 5.Takizawa K, Takashima A, Kimura A, Mizusawa J, Hasuike N, Ono H, et al. A Phase II clinical trial of endoscopic submucosal dissection for early gastric cancer of undifferentiated type: Japan clinical oncology group study JCOG1009/1010. Jpn J Clin Oncol. 2013;43:87–91. doi: 10.1093/jjco/hys189. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki H, Iwasaki E, Hibi T. Helicobacter pylori and gastric cancer. Gastric Cancer. 2009;12:79–87. doi: 10.1007/s10120-009-0507-x. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald RC, Hardwick R, Huntsman D, Carneiro F, Guilford P, Blair V, et al. International gastric cancer linkage consortium. J Med Genet. 2010;47:436–444. doi: 10.1136/jmg.2009.074237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah MA, Salo-Mullen E, Stadler Z, Ruggeri JM, Mirander M, Pristyazhnyuk Y, et al. De novo CDH1 mutation in a family presenting with early-onset diffuse gastric cancer. Clin Genet. 2012;82:283–287. doi: 10.1111/j.1399-0004.2011.01744.x. [DOI] [PubMed] [Google Scholar]
- 9.Corso G, Pedrazzani C, Pinheiro H, Fernandes E, Marrelli D, Rinnovati A, et al. E-cadherin genetic screening and clinico-pathologic characteristics of early onset gastric cancer. Eur J Cancer. 2011;47:631–639. doi: 10.1016/j.ejca.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Shinmura K, Kohno T, Takahashi M, Sasaki A, Ochiai A, Guilford P, et al. Familial gastric cancer: clinicopathological characteristics, RER phenotype and germline p53 and E-cadherin mutations. Carcinogenesis. 1999;20:1127–1131. doi: 10.1093/carcin/20.6.1127. [DOI] [PubMed] [Google Scholar]
- 11.Tokunaga M, Ohyama S, Kuraoka K, Hiki N, Fukunaga T, Tsuchida T, et al. Twenty-two metachronous multiple signet-ring cell carcinomas treated with repeated gastrectomies and repeated endoscopic mucosal resections: report of a case. Surg Today. 2009;39:430–433. doi: 10.1007/s00595-008-3862-z. [DOI] [PubMed] [Google Scholar]
- 12.Yamada H, Shinmura K, Ito H, Kasami M, Sasaki N, Shima H, et al. Germline alterations in the CDH1 gene in familial gastric cancer in the Japanese population. Cancer Sci. 2011;102:1782–1788. doi: 10.1111/j.1349-7006.2011.02038.x. [DOI] [PubMed] [Google Scholar]
- 13.Humar B, Guilford P. Hereditary diffuse gastric cancer: a manifestation of lost cell polarity. Cancer Sci. 2009;100:1151–1157. doi: 10.1111/j.1349-7006.2009.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norton JA, Ham CM, Van Dam J, Jeffrey RB, Longacre TA, Huntsman DG, et al. CDH1 truncating mutations in the E-cadherin gene: an indication for total gastrectomy to treat hereditary diffuse gastric cancer. Ann Surg. 2007;245:873–879. doi: 10.1097/01.sla.0000254370.29893.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blair V, Martin I, Shaw D, Winship I, Kerr D, Arnold J, et al. Hereditary diffuse gastric cancer: diagnosis and management. Clin Gastroenterol Hepatol. 2006;4:262–275. doi: 10.1016/j.cgh.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Oliveira C, Senz J, Kaurah P, Pinheiro H, Sanges R, Haegert A, et al. Germline CDH1 deletions in hereditary diffuse gastric cancer families. Hum Mol Genet. 2009;18:1545–1555. doi: 10.1093/hmg/ddp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S, Chung JW, Jeong TD, Park YS, Lee JH, Ahn JY, et al. Searching for E-cadherin gene mutations in early onset diffuse gastric cancer and hereditary diffuse gastric cancer in Korean patients. Fam Cancer 2012 (Epub ahead of print). [DOI] [PubMed]


