Introduction
The extracellular matrix (ECM), present between cells, is the authentic substrate for most cells in living organisms. Interplay between the ECM and cells plays a critical role in regulating cell differentiation, proliferation, apoptosis, and matrix synthesis. A substantial characteristic of hyaline cartilage is that a few chondrocytes, which are the sole cells in this tissue, are surrounded by the abundant ECM. Therefore, the ECM is considered to be a key regulator of cartilage development and regeneration.
The limited potential of articular cartilage to self-repair leads to the demand for surgical procedures to treat symptomatic cartilaginous lesions. However, no previous procedures have successfully provided long-lasting hyaline cartilage repair for such lesions. On the other hand, cartilage tissue engineering or autologous chondrocyte implantation (ACI) is expected to be an ideal procedure for cartilaginous lesions [1, 2]. Tissue engineering techniques basically comprises three key factors including scaffolds, cells, and signals. Among these factors, a number of studies have emphasized the importance of selecting suitable biomaterials as scaffolds for cell adhesion, proliferation, and ECM production [2–9]. For the reason mentioned in the first paragraph, scaffolds for cartilage regeneration are especially required to provide the functional role of tissue-specific ECM.
Scaffold criteria for cartilage tissue engineering
Scaffolds for articular cartilage regeneration should have the potential to both withstand a mechanically stressed environment, and to support chondrogenesis while maintaining the chondrocyte phenotype. To meet those requirements, the following criteria must be considered in developing cartilage tissue engineering scaffolds: (1) biocompatibility with no or minimal inflammation; (2) noncytotoxity; (3) biodegradability; (4) three-dimensional (3-D) structure; (5) adequate void space for cell infiltration, proliferation, ECM production, and nutrient diffusion; (6) high cellular adhesivity; (7) appropriate mechanical strength in living joints; and 8) versatility for implanting into a variety of lesion shapes and sizes [10].
Types and design of scaffold biomaterials for cartilage tissue engineering
Scaffold biomaterials include both naturally occurring and synthetic materials. Unfortunately, each type of material has characteristic disadvantages. The major limitations of naturally occurring materials are due to the immunogenicity and the lot-to-lot variability in molecular structure associated with animal sourcing. In contrast, synthetic materials have a greater potential for toxicity and chronic inflammation than occurs with natural materials.
Additionally, scaffold biomaterials for cartilage regeneration can be classified into four categories as follows: protein-based materials (fibrin, gelatin, collagen, etc.), carbohydrate-based materials (agarose, polylactic acid, polyglycolic acid, hyaluronan, alginate, chitosan, etc.), synthetic/artificial polymer-based materials, and combined materials [10]. Regarding available shapes of the scaffolds, there are three main categories of shapes used in cartilage tissue engineering, including membranes/sheets, gels/foams, and 3-D fabricated matrices.
Current procedures for cartilage regeneration are based on three main approaches: (1) cultured cell implantation, (2) engineered tissue implantation, or (3) guided tissue regeneration. To achieve cartilage regeneration successfully, the selection of biomaterials and shapes for determining scaffold designs must be based on the targeted approach intended to be used by surgeons.
Strategic scaffold designs based on approach for cartilage regeneration
Scaffolds for cultured cell implantation
Since the first clinical report by Brittberg et al. [1], ACI has been the most widely performed cartilage tissue regeneration technique. In the first report, the authors cultured the isolated chondrocytes in a suspension medium. The cultured cells were then implanted into the chondral lesion of the knee without any specific carrier materials. The defect filled with suspension containing chondrocytes was covered with a periosteal flap harvested from the proximal tibia. Their results suggest that ACI restores joint function by forming predominantly hyaline-like cartilage containing type II collagen.
Among the scaffolds used for cartilage tissue engineering, hydrogels are considered to be a suitable scaffold and cell carrier for cultured cell implantation. These materials promote chondrocyte adhesion in a manner that mimics the cartilage ECM and maintains the chondrocyte phenotype in a way that is unachievable in a monolayer culture system [11–15]. Additionally, their viscoelasticity effectively transduces mechanical signals to embedded chondrocytes for control of cellular activities. In terms of carrier functions, hydrogels can easily adopt a variety of shapes and sizes of cartilaginous lesions. However, most of the hydrogels clinically used require periosteal coverage over the lesion to prevent the leakage of implanted cells, leading to open surgery.
Towards a less invasive procedure, several authors applied a 3-D hyaluronan-based scaffold (Hyalograft C) to perform arthroscopic ACI for the treatment of cartilaginous lesions in the knee [16, 17]. This method, which does not need periosteal coverage, has potential for reducing patient morbidity, operation time, and treatment cost. Techniques based on the usage of such hydrogels will allow less invasive surgery for cartilage regeneration.
Scaffolds for engineered tissue implantation
In articular cartilage tissue engineering, we must consider that the articular surface is subject to excessive mechanical stress. The severe mechanical conditions result in dedifferentiation of the implanted chondrocytes, degeneration of the regenerated tissues, and roughness of the tissue surface. To overcome these considerations, a reasonable approach is to transplant mechanically and histologically mature engineered cartilage into cartilaginous lesions. This approach probably necessitates a long-term culture period in a bioreactor system, which provides appropriate nutrition and mechanical stimulation for the seeded cells. Consequently, scaffolds have to maintain the initial shape against applied mechanical stress and accelerate chondrogenesis during the culture period.
To meet these requirements, Yamane et al. [18] have developed a novel 3-D scaffold fabricated from chitosan-based hyaluronic acid (HA) hybrid polymer fibers. The authors demonstrated that these hybrid polymer fibers have superior adhesivity of chondrocytes and the capability to maintain chondrocyte phenotype. These fibers also maintained the initial shape of the 3-D scaffold during cultivation and enhanced cartilage tissue regeneration in vitro. Regarding the pore size of the fabrication, Yamane et al. [19] suggested that a relatively large size of 400 um significantly increased the ECM synthesis by cultured chondrocytes.
Based on the data obtained from these in vitro studies, Kasahara et al. [20] and Iwasaki et al. [21] created two different types of scaffolds fabricated from the novel hybrid polymer fibers. One was a cushion-type scaffold consisting of two sheets (Fig. 1a), the other was a cylinder-type scaffold (Fig. 1b). To prepare tissue-engineered constructs, a chondrocyte suspension containing 3 × 105 and 7 × 105 cells isolated from 8 week-old Japanese white rabbits was seeded onto the cushion-type (cushion group) and the cylinder-type (cylinder group) scaffolds, respectively. After 1-week static culture, each sample was dynamically cultured in a disposable, high aspect ratio vessel bioreactor (HARV, 50 ml; Synthecon, Inc., Houston, Tx, USA) for a further 4 or 7 weeks. Table 1 summarizes biochemical and biomechanical assessments of engineered cartilage at each time point [20]. Macroscopic (Fig. 2) and histological (Fig. 3) findings at 8 weeks after cultivation showed hyaline-like cartilage regeneration with abundant GAG and type II collagen products [20]. Regarding the mechanical property of engineered cartilage, the cushion-type scaffold significantly increased the Young’s modulus from 5 to 8 weeks after cultivation. Although the modulus of the cylinder group was significantly inferior to that of the cushion group, the value was comparable to that of normal cartilage in rabbits. These results suggested that the developed 3-D scaffolds regenerated mature tissue close to articular hyaline cartilage.
Fig. 1.
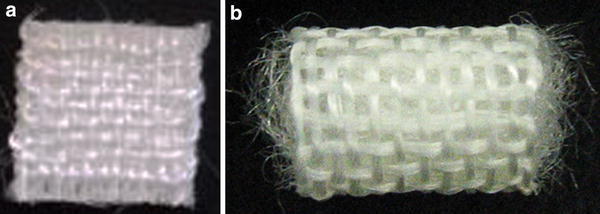
Three-dimensional (3-D) scaffolds fabricated from chitosan-based hyaluronic acid (HA) hybrid polymer fibers. a Cushion-type (8 × 8 mm wide and 1 mm thick); b cylinder type (10 mm high and 6 mm diameter. The pore size of both scaffolds is 400 μm [21]. Reproduced with permission of Iwasaki N, Kasahara Y, Yamane S, Igarashi T, Minami A, Nishimura S-I. Chitosan-based hyaluronic acid hybrid polymer fibers as a scaffold biomaterial for cartilage tissue engineering. Polymers 2011;3:100–13
Table 1.
Biochemical and biomechanical assessments of engineered cartilage in both materials [20]
| Cushion-type | Cylinder-type | |||
|---|---|---|---|---|
| 5 weeks | 8 weeks | 5 weeks | 8 weeks | |
| Total amount of DNA (μg) | 53.8 ± 1.4 | 95.5 ± 2.1* | 97.9 ± 3.2 | 132.3 ± 6.6† |
| Total amount of protein (μg) | 1108.4 ± 49.3 | 2178.9 ± 114.5* | 1655.9 ± 82.9 | 2677.5 ± 356.0† |
| Protein/DNA ratio | 20.9 ± 1.7 | 22.9 ± 2.5 | 17.0 ± 1.0 | 20.1 ± 1.9 |
| Young’s modulus (MPa) | 4.9 ± 1.1 | 12.2 ± 2.4*,** | 2.8 ± 0.5 | 3.2 ± 0.7 |
Mean ± standard error. N = 5 in each group
Reproduced with permission of Kasahara Y, Iwasaki N, Yamane S, Igarashi T, Majima T, Nonaka S, Harada K, Nishimura S, Minami A. Development of mature cartilage constructs using novel three-dimensional porous scaffolds for enhanced repair of osteochondral defects. J Biomed Mater Res A 2008;86:127–36
* p < 0.01 vs. 5 weeks, † p < 0.05 vs. 5 weeks, ** p < 0.001 vs. Cylinder-type scaffold at 8 weeks
Fig. 2.
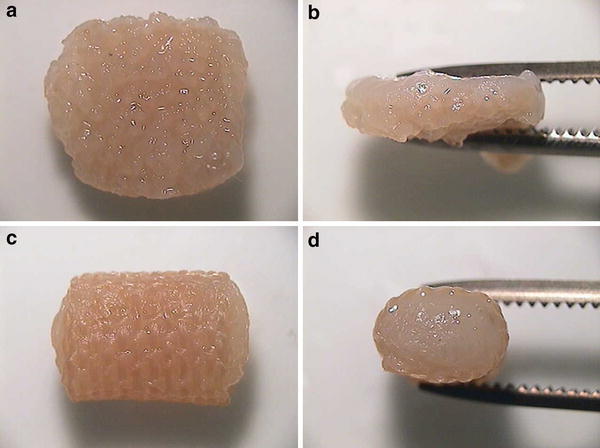
In vitro macroscopic appearance of engineered cartilage in each scaffold at 8 weeks after cultivation. a, b Cushion-type; c, d cylinder-type [20]. Reproduced with permission of Kasahara Y, Iwasaki N, Yamane S, Igarashi T, Majima T, Nonaka S, Harada K, Nishimura S, Minami A. Development of mature cartilage constructs using novel three-dimensional porous scaffolds for enhanced repair of osteochondral defects. J Biomed Mater Res A 2008;86:127–36
Fig. 3.
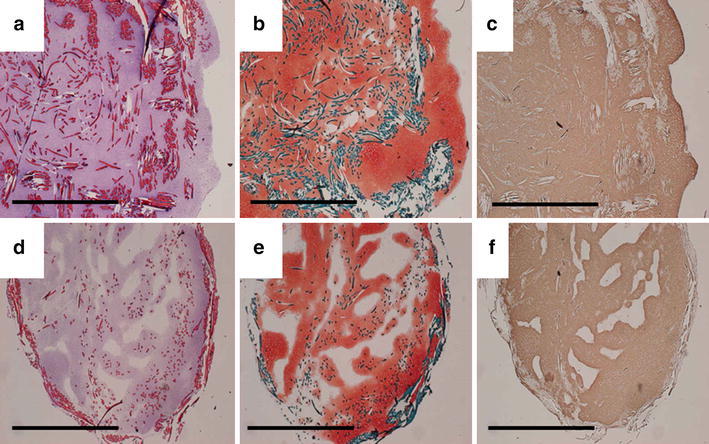
In vitro histological and immunohistochemical findings of engineered cartilage in each scaffold at 8 weeks after cultivation. a–c Cushion-type; d–f cylinder-type. Hematoxylin and eosin staining reveals that cultured cells are distributed throughout the entire scaffolds (a, d). Safranin-O staining indicates the production of a proteoglycan-rich cartilaginous matrix (b, e). Immunohistochemical staining with antitype II collagen shows the abundant production of type II collagen (c, f). Scale bars indicate 5 mm [20]. Reproduced with permission of Kasahara Y, Iwasaki N, Yamane S, Igarashi T, Majima T, Nonaka S, Harada K, Nishimura S, Minami A. Development of mature cartilage constructs using novel three-dimensional porous scaffolds for enhanced repair of osteochondral defects. J Biomed Mater Res A 2008;86:127–36
Then, the mature tissue-engineered cartilage using each scaffold was press-fit implanted into the osteochondral defects (5 mm in diameter and 2 mm deep) on the patellar groove of mature Japanese white rabbits (2.6–3.1 kg weight). For postoperative evaluations, animals were euthanized at 12 weeks postoperatively and the femoral condyles were harvested. The macroscopic appearance of osteochondral defects showed a repair with cartilage-like tissue in both scaffold groups over the no treatment group (Fig. 4) [20, 21]. Histological findings also demonstrated that the defects in both scaffold groups were repaired with hyaline-like cartilage or a combination of hyaline-like cartilage and fibrocartilage (Fig. 5) [20]. The histological scores according to the criteria of Wayne [22] and the Young’s modulus as a mechanical property of both scaffold groups significantly improved, compared to those of the no treatment group (Table 2) [20]. There were no statistically significant differences in the Young’s modulus between the reparative tissue of both scaffold groups and normal cartilage.
Fig. 4.
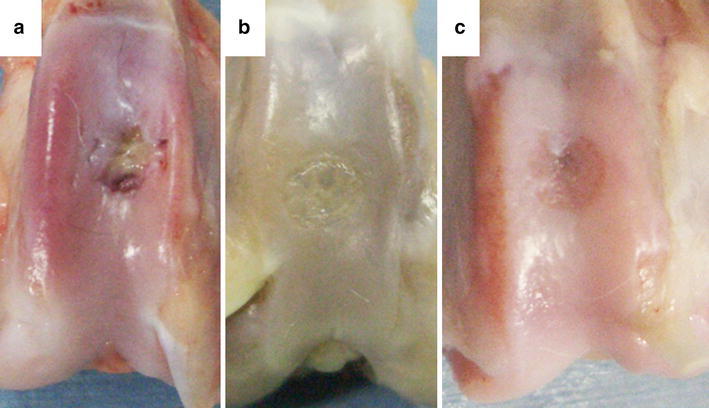
Macroscopic appearance of osteochondral defects at 12 weeks after operation. The obtained findings demonstrate a repair with cartilage-like tissue in the cushion-type (b) and the cylinder-type (c) treatment groups over the no treatment group (a) [21]. Reproduced with permission of Iwasaki N, Kasahara Y, Yamane S, Igarashi T, Minami A, Nishimura S-I. Chitosan-based hyaluronic acid hybrid polymer fibers as a scaffold biomaterial for cartilage tissue engineering. Polymers 2011;3:100–13
Fig. 5.
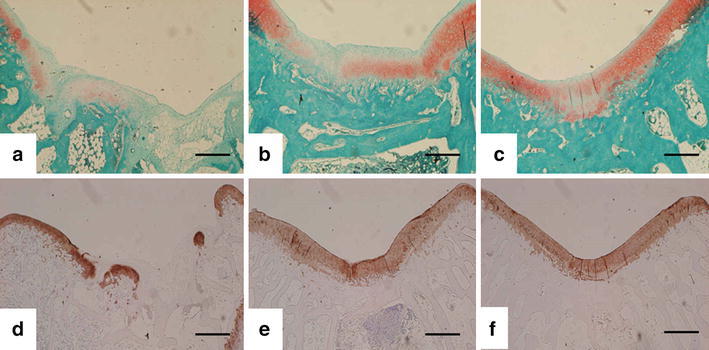
Histological and immunohistochemical findings of reparative tissues in each scaffold at 12 weeks after operation. a, d No treatment group; b, e cushion-type group; c, f cylinder-type group. Safranin-O staining (a–c) and immunohistochemical staining with antitype II collagen (d–f) indicate a repair with hyaline-like cartilage in both the cushion-type and the cylinder-type treatment groups. Scale bars indicate 1 mm [20]. Reproduced with permission of Kasahara Y, Iwasaki N, Yamane S, Igarashi T, Majima T, Nonaka S, Harada K, Nishimura S, Minami A. Development of mature cartilage constructs using novel three-dimensional porous scaffolds for enhanced repair of osteochondral defects. J Biomed Mater Res A 2008;86:127–36
Table 2.
Quantitative assessments of reparative tissue at 12 weeks postoperatively [20]
| No treatment | Cushion-type | Cylinder-type | Normal cartilage | |
|---|---|---|---|---|
| Histological score | 5.3 ± 0.7 | 10.1 ± 1.4* | 9.3 ± 1.6** | / |
| Young’s modulus (MPa) | 10.4 ± 3.8 | 1.9 ± 0.6 | 1.7 ± 0.6 | 3.2 ± 0.6 |
Mean ± standard error. N = 7 in each group
Reproduced with permission of Kasahara Y, Iwasaki N, Yamane S, Igarashi T, Majima T, Nonaka S, Harada K, Nishimura S, Minami A. Development of mature cartilage constructs using novel three-dimensional porous scaffolds for enhanced repair of osteochondral defects. J Biomed Mater Res A 2008;86:127–36
* p < 0.001 vs. no treatment, ** p < 0.005 vs. no treatment
The authors succeeded in regenerating mature hyaline-like cartilage using the novel fabricated 3-D scaffold in vitro. The implantation of the engineered tissue plays functional roles in repairing osteochondral defects in living joints.
Scaffolds for guided tissue regeneration
To date, a number of studies have demonstrated favorable clinical outcomes of ACI procedures for the treatment of cartilaginous lesions [1, 23]. However, prospective randomized trials have not clearly suggested dominance of this procedure over other operations for such lesions [24, 25].
A possible reason for the unsatisfactory outcomes is the adverse effects resulting from the invasive procedures involved in the current ACI technique, which is based on a two-stage operation, the first stage to harvest cartilage tissue to isolate chondrocytes, and the second stage to implant cultured chondrocytes into the cartilaginous lesion. These processes may result in donor site morbidity, dedifferentiation of cultured chondrocytes, and cost ineffectiveness. Our technique for engineered tissue implantation mentioned above also faces these limitations. On the other hand, guided tissue regeneration is a cell-free approach involving the implantation of naturally occurring or synthetic scaffolds into the cartilage defects to complement natural biological repair [10]. This approach shows great potential for overcoming the above limitations of the current ACI technique.
To perform guided tissue regeneration, Igarashi et al. [26] developed an in situ forming gel based on alginate as an injectable material. This naturally occurring material was ultrapurified by an original technique to reduce drastically its endotoxity. The previous study has demonstrated that the ultrapurified alginate gel (UPAL gel) has favorable biological effects on in vitro proliferation and chondrogenesis of bone marrow stromal cells (BMSCs) [26]. In addition, the previous in vivo studies using rabbits and canines have shown that implantation of autologous BMSCs using the UPAL gel enhanced cartilage repair in cartilaginous lesions [26, 27]. Furthermore, the results obtained from these studies indicate that the acellular UPAL gel implantation technique has the potential to repair osteochondral defects by effectively recruiting host BMSCs.
Stromal cell-derived factor-1 (SDF-1)/pre-B cell growth-stimulating factor, belonging to the CXC subfamily of chemokines, is a critical chemokine for stem cells homing to bone marrow in the repair of injured tissues. Unlike other chemokines, SDF-1 is unique in that it binds only to its receptor, CXCR4. Regarding musculoskeletal tissues, Shimode et al. [28] demonstrated that a local upregulation of SDF-1 after ligament injuries enhanced the homing of BMSCs to the injured site in a rat experimental model. Consequently, the author hypothesized that the local administration of UPAL gel containing SDF-1 could enhance the repair of osteochondral defects by recruiting host BMSCs to the defect site.
The in vivo study using a rabbit model demonstrated the positive chemotactic effect of SDF-1 on the homing of host cells to osteochondral defects [29]. Additionally, the acellular implantation of UPAL gel containing SDF-1 enhanced the reparative process of cartilaginous lesions histologically (Fig. 6) and biomechanically (Table 3) [29]. In conclusion, the author successfully achieved hyaline-like cartilage repair by local administration of SDF-1 using UPAL gel without cultured cells. The obtained results show potential for tissue guided regeneration using the novel hydrogel in the treatment of cartilaginous lesions. Further studies will clarify the clinical indications for this technique, including cartilage defect and patient factors.
Fig. 6.
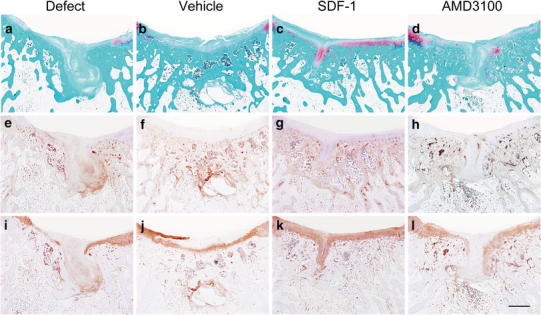
Histological findings of reparative tissues at 16 weeks after operation. a–d Safranin-O staining; e–h immunohistochemical staining with anti-type I collagen antibody; i–l immunohistochemical staining with anti-type II collagen antibody. Defect, no treatment; Vehicle, UPAL gel containing 10 μg/ml bovine serum albumin; SDF-1, UPAL gel containing 10 μg/ml SDF-1 (Miltenyi Biotec Inc., Auburn, CA, USA); and AMD3100, UPAL gel containing 250 μg/ml AMD3100, an antagonist of CXCR4, (Sigma-Aldrich, Saint Louis, MO, USA). Scale bar: 1 mm. The SDF-1 group indicates a repair with the hyaline-like cartilage and normal subchondral bone structures [29]. Reproduced with permission of Sukegawa A, Iwasaki N, Kasahara Y, Onodera T, Igarashi T, Minami A. Repair of rabbit osteochondral defects by an acellular technique with an ultrapurified alginate gel containing stromal cell-derived factor-1. Tissue Eng Part A 2012;18:934–45
Table 3.
Compressive modulus of reparative tissue in experimental groups [29]
| 4 weeks (MPa) | 8 weeks (MPa) | |
|---|---|---|
| No treatment | 0.66 ± 0.08* | 0.59 ± 0.11* |
| Vehicle | 0.50 ± 0.16* | 1.82 ± 0.28**,†† |
| SDF-1 | 0.89 ± 0.05* | 2.34 ± 0.38† |
| AMD3100 | 0.60 ± 0.93* | 1.89 ± 0.18**,†† |
| Normal cartilage | 2.89 ± 0.25 | |
Mean ± standard error. N = 5 in each group at each time point. Vehicle, UPAL gel without cells; SDF-1, UPAL gel containing SDF-1; AMD3100, UPAL gel containing SDF-1 antagonist
Reproduced with permission of Sukegawa A, Iwasaki N, Kasahara Y, Onodera T, Igarashi T, Minami A. Repair of rabbit osteochondral defects by an acellular technique with an ultrapurified alginate gel containing stromal cell-derived factor-1. Tissue Eng Part A 2012;18:934–45
* p < 0.001 vs. normal cartilage, ** p < 0.05 vs. normal cartilage, † p < 0.001 vs. no treatment at the same time, †† p < 0.01 vs. no treatment at the same time
Future trends
Cartilage tissue engineering or ACI has been clinically applied to cartilaginous lesions in the treatment of a variety of disorders and trauma. However, as mentioned above, the expected efficacy of this procedure has not been achieved in prospective randomized trials [24, 25]. To improve the clinical outcomes, we need to develop novel scaffolds for cartilage regeneration. Especially, future studies on biomaterials for cartilage tissue engineering will focus on the following considerations.
First, acellular scaffold-based techniques combined with chemokines or bone marrow stimulating techniques must be established for a one-step surgery. Second, arthroscopic techniques using in situ forming materials without periosteal coverage are required to achieve a minimally invasive surgery for cartilage regeneration. The attainment of these goals will eliminate morbidity, lessen the high cost and complicated logistics related to cell culture, and lead to improved clinical outcomes for cartilage regenerative medicine. Third, scaffolds which can maintain well-differentiated chondrocytes should be developed to regenerate mature hyaline-like cartilage. To accomplish this, the fundamental biomaterials for scaffolds require functions to eliminate dedifferentiated chondrocytes from the culture system and enhance the chondrogenesis of stem cells, such as BMSCs or iPS cells. Finally, scaffolds for engineering entire osteochondral units should be created. In clinical fields, most articular cartilaginous lesions are associated with subchondral bone defects. Therefore, an ideal treatment strategy is needed for not only cartilage repair, but also osteochondral repair.
Conclusions
Currently, the clinical application of cartilage tissue engineering or ACI to patients with osteochondral defects has surpassed 20 years. Although this innovative technique must be an ideal one for the treatment of cartilaginous lesions, previous clinical studies have not clearly demonstrated significant superiority of its postoperative outcomes over other operations [24, 25]. To achieve better outcomes of current cartilage tissue engineering or ACI techniques, there is still room for improvement in the clinically available scaffolds. The final goals of this treatment must be to enhance hyaline-like cartilage repair and to establish minimally invasive techniques. The application of novel developed scaffolds will play a crucial role in achieving these goals.
Acknowledgments
The authors thank Dr. Tomohiro Onodera (Department of Orthopaedic Surgery, Hokkaido University Graduate School of Medicine), Dr. Yasuhiko Kasahara (Department of Orthopaedic Surgery, Hokkaido University Graduate School of Medicine), Dr. Tatsuya Igarashi (Department of Orthopaedic Surgery, Kushiro Rosai Hospital), Dr. Shintarou Yamane (Hokushin Higashi Hospital), Dr. Atsushi Sukegawa (Department of Orthopaedic Surgery, Ebetsu City Hospital), and Mr. Mark Hamilton (Tokai University, Sapporo, Japan) for their enthusiastic assistance in manuscript preparation.
Conflict of interest
The works presented here were partly supported by the Hokkaido Bureau of Economy, Trade and Industry, Northern Advancement Center for Science and Technology in Hokkaido, a Grant-in-Aid for New Energy and Industrial Technology Development Organization (03A04002a), Grants-in Aid for Scientific Research (B-15390447 and B-22390285) from Japan Society for the Promotion of Science, ‘Development of a Novel Injectable Material for the Treatment of Joint Disorders’ Science and Technology Incubation Program in Advanced Regions, Japan Science and Technology Agency, a research grant from Suhara Memorial Foundation, and research grants from Mochida Pharmaceutical CO., LTD.
References
- 1.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 2.Vacanti CA, Langer R, Vacanti JP. Synthetic polymers seeded with chondrocytes provide a template for new cartilage formation. Plast Reconstr Surg. 1991;88:753–759. doi: 10.1097/00006534-199111000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Aigner J, Tegeler J, Hutzler P, Campoccia D, Pavesio A, Hammer C, Kastenbauer E, Naumann A. Cartilage tissue engineering with novel nonwoven structured biomaterial based on hyaluronic acid benzyl ester. J Biomed Mater Res. 1998;42:172–181. doi: 10.1002/(SICI)1097-4636(199811)42:2<172::AID-JBM2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 4.Ishaug-Riley SL, Okun LE, Prado G, Applegate MA, Ratcliffe A. Human articular chondrocyte adhesion and proliferation on synthetic biodegradable polymer films. Biomaterials. 1999;20:2245–2256. doi: 10.1016/S0142-9612(99)00155-6. [DOI] [PubMed] [Google Scholar]
- 5.Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20:1133–1142. doi: 10.1016/S0142-9612(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 6.Nehrer S, Breina HA, Ramappa A, Shortkroff S, Young G, Minas T, Sledge CB, Yannas IV, Spector M. Canine chondrocytes seeded in type I and type II collagen implants investigated in vitro. J Biomed Mater Res. 1997;38:95–104. doi: 10.1002/(SICI)1097-4636(199722)38:2<95::AID-JBM3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 7.Suh JKF, Matthew HWT. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21:2589–2598. doi: 10.1016/S0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 8.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–2543. doi: 10.1016/S0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 9.LeBaron RG, Athanasiou KA. Ex vivo synthesis of articular cartilage. Biomaterials. 2000;21:2575–2587. doi: 10.1016/S0142-9612(00)00125-3. [DOI] [PubMed] [Google Scholar]
- 10.Dhollander AAM, Sánchez VRG, Almqvist KF, Verdonk R, Verbruggen G, Verdonk PCM. The use of scaffolds in the treatment of osteochondral lesions in the knee: current concepts and future trends. J Knee Surg. 2012;25:179–186. doi: 10.1055/s-0032-1322596. [DOI] [PubMed] [Google Scholar]
- 11.Cushing MC, Anseth KS. Materials science. Hydrogel cell cultures. Science. 2007;316:1133–1134. doi: 10.1126/science.1140171. [DOI] [PubMed] [Google Scholar]
- 12.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 13.Yamaoka H, Asato H, Ogasawara T, Nishizawa S, Takahashi T, Nakatsuka T, Koshima I, Nakamura K, Kawaguchi H, Chung UI, Takato T, Hoshi K. Cartilage tissue engineering using human articular chondrocytes embedded in different hydrogel materials. J Biomed Mater Res A. 2006;78:1–11. doi: 10.1002/jbm.a.30655. [DOI] [PubMed] [Google Scholar]
- 14.Passaretti D, Silverman RP, Huang W, Kirchhoff CH, Ashiku S, Randolph MA, Yaremchuk MJ. Cultured chondrocytes produce injectable tissue-engineered cartilage in hydrogel polymer. Tissue Eng. 2001;7:805–815. doi: 10.1089/107632701753337744. [DOI] [PubMed] [Google Scholar]
- 15.Spiller KL, Maher SA, Lowman AM. Hydrogels for the repair of articular cartilage defects. Tissue Eng. 2011;17:281–299. doi: 10.1089/ten.teb.2011.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcacci M, Zaffagnini S, Kon E, Visani A, Iacono F, Loreti I. Arthroscopic autologous chondrocyte transplantation: technical note. Knee Surg Sports Traumatol Arthrosc. 2002;10:154–159. doi: 10.1007/s00167-001-0275-6. [DOI] [PubMed] [Google Scholar]
- 17.Solchaga LA, Dennis JE, Goldberg VM, Caplan AI. Hyaluronic acid-based polymers as cell carriers for tissue-engineered repair of bone and cartilage. J Orthop Res. 1999;17:205–213. doi: 10.1002/jor.1100170209. [DOI] [PubMed] [Google Scholar]
- 18.Yamane S, Iwasaki N, Majima T, Funakoshi T, Masuko T, Harada K, Minami A, Monde K, Nishimura S. Feasibility of chitosan-based hyaluronic acid hybrid biomaterial for a novel scaffold in cartilage tissue engineering. Biomaterials. 2005;26:611–619. doi: 10.1016/j.biomaterials.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Yamane S, Iwasaki N, Kasahara Y, Harada K, Majima T, Monde K, Nishimura S, Minami A. Effect of pore size on in vitro cartilage formation using chitosan-based hyaluronic acid hybrid polymer fibers. J Biomed Mater Res A. 2007;81:586–593. doi: 10.1002/jbm.a.31095. [DOI] [PubMed] [Google Scholar]
- 20.Kasahara Y, Iwasaki N, Yamane S, Igarashi T, Majima T, Nonaka S, Harada K, Nishimura S, Minami A. Development of mature cartilage constructs using novel three-dimensional porous scaffolds for enhanced repair of osteochondral defects. J Biomed Mater Res A. 2008;86:127–136. doi: 10.1002/jbm.a.31259. [DOI] [PubMed] [Google Scholar]
- 21.Iwasaki N, Kasahara Y, Yamane S, Igarashi T, Minami A, Nishimura S-I. Chitosan-based hyaluronic acid hybrid polymer fibers as a scaffold biomaterial for cartilage tissue engineering. Polymers. 2011;3:100–113. doi: 10.3390/polym3010100. [DOI] [Google Scholar]
- 22.Wayne JS, McDowell CL, Shields KJ, Tuan RS. In vivo response of polylactic acid alginate scaffolds and bone marrow-derived cells for cartilage tissue engineering. Tissue Eng. 2005;11:953–963. doi: 10.1089/ten.2005.11.953. [DOI] [PubMed] [Google Scholar]
- 23.Tohyama H, Yasuda K, Minami A, Majima T, Iwasaki N, Muneta T, Sekiya I, Yagishita K, Takahashi S, Kurokouchi K, Uchio Y, Iwasa J, Deie M, Adachi N, Sugawara K, Ochi M. Atelocollagen-associated autologous chondrocyte implantation for the repair of chondral defects of the knee: a prospective multicenter clinical trial in Japan. J Orthop Sci. 2009;14:579–588. doi: 10.1007/s00776-009-1384-1. [DOI] [PubMed] [Google Scholar]
- 24.Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85:185–192. doi: 10.2106/00004623-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Jt Surg Am. 2010;92:2220–2233. doi: 10.2106/JBJS.J.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Igarashi T, Iwasaki N, Kasahara Y, Minami A. A cellular implantation system using an injectable ultra-purified alginate gel for repair of osteochondral defects in a rabbit model. J Biomed Mater Res A. 2010;94:844–855. doi: 10.1002/jbm.a.32762. [DOI] [PubMed] [Google Scholar]
- 27.Igarashi T, Iwasaki N, Kawamura T, Kasahara Y, Tsukuda Y, Ohzawa N, Ito M, Izumisawa Y, Minami A. Repair of articular cartilage defects with a novel injectable in situ forming material in a canine model. J Biomed Mater Res A. 2012;100:180–187. doi: 10.1002/jbm.a.33248. [DOI] [PubMed] [Google Scholar]
- 28.Shimode K, Iwasaki N, Majima T, Funakoshi T, Sawaguchi N, Onodera T, Minami A. Local upregulation of stromal cell-derived factor-1 after ligament injuries enhances homing rate of bone marrow stromal cells in rats. Tissue Eng Part A. 2009;15:2277–2284. doi: 10.1089/ten.tea.2008.0224. [DOI] [PubMed] [Google Scholar]
- 29.Sukegawa A, Iwasaki N, Kasahara Y, Onodera T, Igarashi T, Minami A. Repair of rabbit osteochondral defects by an acellular technique with an ultrapurified alginate gel containing stromal cell-derived factor-1. Tissue Eng Part A. 2012;18:934–945. doi: 10.1089/ten.tea.2011.0380. [DOI] [PubMed] [Google Scholar]


