Abstract
Purpose of review
Colonization of the host epithelia by pathogenic Escherichia coli is influenced by the ability of the bacteria to interact with host surfaces. Because the initial step of an E. coli infection is to adhere, invade, and persist within host cells, some strategies used by intestinal and extra-intestinal E. coli to infect host cell are presented.
Recent findings
This review highlights recent progress understanding how extra-intestinal pathogenic E. coli strains express specific adhesins/invasins that allow colonization of the urinary tract or the meninges, while intestinal E. coli strains are able to colonize different regions of the intestinal tract using other specialized adhesins/invasins. Finally, evaluation of, different diets and environmental conditions regulating the colonization of these pathogens is discussed.
Summary
Discovery of new interactions between pathogenic E. coli and the host epithelial cells unravels the need of more mechanistic studies that can provide new clues in how to combat these infections.
Keywords: enterohemorrhagic E. coli, enteropathogenic, enterotoxigenic, uropathogenic, enteroaggregative, adherent invasive E. coli
Introduction
Escherichia coli are commonly found as part of the gut flora, where it is the predominant aerobic organism, living in symbiosis with its vertebrate host. However, there are several categories of E. coli strains that have acquired the ability to cause pathogenic processes in the host (1). These E. coli strains can cause intestinal (enteritis, diarrhea, or dysentery), or. extra-intestinal diseases (urinary tract infections, sepsis, or meningitis) (2, 3). To cause infection, pathogenic E. coli interact with the mucosa, by either attaching to the epithelial cells and in some instances, invading the target host cells. Because bacterial adhesion and/or invasion to/into host cells are the first step during infection, it is necessary to understand at a molecular level the mechanisms mediating these initial interactions. This article focus on reviewing recent progress on the understanding of the adhesion/invasion mechanisms used by intestinal and extra-intestinal pathogenic E. coli during colonization of the host cells.
Enterohemorrhagic E. coli (EHEC)
EHEC are a category of pathogenic E. coli that colonize the human large intestine and which can cause bloody diarrhea, or a systemic process known as hemolytic uremic syndrome (4). EHEC strains are characterized by the production of Shiga toxin and the formation of attaching and effacing intestinal lesions (Figure 1). Cattle are a main reservoir for EHEC strains; however several vegetables and fruits can serve as vehicles for EHEC outbreaks (5).
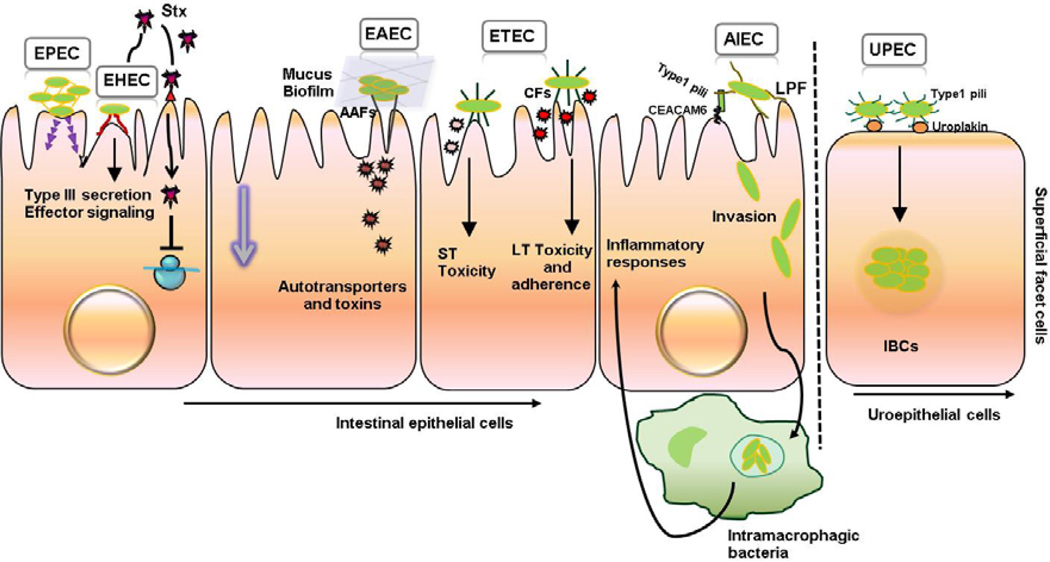
Figure 1.
Pathogenic Escherichia coli colonization of intestinal epithelial cells and uroepithelium. Adherence and/or invasion of intestinal (EPEC, EHEC, EAEC, ETEC, AIEC) and extraintestinal (UPEC) pathogenic Escherichia coli to epithelial cells (See text for details).
EHEC colonization is impacted by nutrient availability and dietary choice. Zumbrun et al found that dietary fiber content affects susceptibility to E. coli O157:H7 infection in mice (6). They treated BALB/c mice with high fiber diet (10% guar gum) or low fiber diet (2% guar gum) for two weeks and then mice were challenge with 109 to 1011 cfu of E. coli O157:H7. The results showed that mice fed with high fiber diet had enhanced levels of butyrate that temporally increased the expression of the Shiga toxin receptor Gb3. Therefore, mice exhibited greater E. coli O157:H7 colonization and reduction in resident Escherichia spp. Sheng et al also showed that cattle fed a hay diet are colonized by EHEC for a longer period of time than grain fed cattle (7). Different diets regulate the colonization of E. coli O157:H7 by altering the composition of gastrointestinal tract microbiota and the study demonstrated that the bacterial SdiA sensor activates genes conferring EHEC acid resistance, increasing efficient colonization of the cattle mucosa (8).
Modulation of host signals in the intestinal epithelia also affects EHEC colonization. Intestinal epithelial cells produced SIGIRR, a negative regulator of interleukin (IL)-1 and TLR signaling, that makes the cells hypo-responsive (9, 10). To address whether hypo-responsiveness affects enteric host defense, Sham et al challenge Sigirr deficient (−/−) mice with the murine pathogen, EHEC-related, Citrobacter rodentium and showed that Sigirr−/− mice are more susceptible to bacterial infection and had a dramatic loss of microbiota (11). The study showed that this host signaling mechanism promotes commensal dependent resistance to EHEC colonization.
Type III secretion system (TTSS) is required for EHEC colonization and attaching and effacing lesion formation. This syringe-like structure used to inject virulence factors into the host cell is exquisitely regulated. Hansen et al revealed that tyrosine phosphorylation in EHEC mediates signaling of virulence properties, including the type III secretion system (12). SspA is a known regulator of the TTSS (13) and a phosphorylated tyrosine residue of this protein positively affects expression and secretion of type III secretion system proteins. Branchu et al also found a new regulator of the TTSS (14) known as the NO-sensor regulator, NsrR. Nitric oxide (NO) reduced EHEC adherence to intestinal epithelial cells, by causing the detachment of the NsrR activator from the type III secretion system-encoding operons (LEE1/4/5), limiting colonization.
Enteropathogenic E. coli (EPEC)
EPEC isolates colonize the small intestine and are one of the leading causes of infantile fatal diarrhea (15). In industrialized countries, there are sporadic diarrheal cases in daycare facilities (16, 17). As for EHEC, EPEC uses the type III secretion system to form attaching and effacing lesions (Figure 1). EPEC strains are subdivided into typical (tEPEC) and atypical EPEC (aEPEC) based on the presence of EPEC adherence factor plasmid associated with the tEPEC localized adherence pattern (18). Some aEPEC form diffuse (DA) or aggregative adherence pattern and Hernandes et al (19) found that the DA pattern is associated with the TTSS system and its suggested that the traslocon serves as the DA adhesin.
Regarding the type III secretion system, recent studies have evaluated the role of the effector protein NleB in pathogenesis. Deletion of nleB1 in C. rodentium caused significant reduction in murine intestinal colonization (20), and NleB has also been shown to modulate the host innate immune system by suppressing tumor necrosis factor (TNF)-mediated NF-κB activation (21). Three studies have investigated the role of NleB1 in EPEC. Gao et al focused on how NleB interfere with NF-κB (22). A proteomic screen was used to identify the host GAPDH as NleB-interacting protein, which results in modification of GAPDH and inhibition of NF-κB-dependent innate immune responses. The other two groups discovered that NleB blocks host death receptor signaling (23, 24), by interacting with two death receptor-signaling proteins, TRADD and FADD. NleB is the first known bacterial virulence factor to target death receptor signaling and it has been suggested that blocking this signaling mechanism may facilitate EPEC and EHEC colonization.
Other studies evaluated ways to reduce EPEC adhesion to host cells. Pan et al expressed synthetic tetrameric-branched peptide that enhanced the expression of Mucin 3 (25). They found that Mucin 3 interacted with EPEC and EHEC and reduced their binding to epithelial cells. In other study, Salcedo et al showed that the combination of gangliosides and sialic acid were able to interfere with EPEC and EHEC adhesion to Caco-2 cells (26).
Enterotoxigenic E. coli (ETEC)
ETEC colonizes the human small intestine and is responsible for neonatal diarrhea in developing countries as well as “travelers’ diarrhea” (27). ETEC adherence to the intestinal mucosa is mainly mediated by diverse adhesive structures known as colonization factors, which in combination with the heat-labile (LT) and/or heat-stable (ST) enterotoxins, causes disruption of fluid homeostasis in the host, resulting in diarrhea (2, 28) (Figure 1).
Guevara et al recently investigated one colonization factors, the CS21 pilus, and its role in adherence and pathogenesis in vivo (29). They found that ETEC CS21 (Longus) adherence to primary intestinal cells was inhibited by anti-LngA sera and the purified LngA protein. In vivo intra-stomach administration of CS21-expressing ETEC strain contributes to 100% lethality of newborn mice, which was reduced in the lngA mutant. A separate study investigated the role of the LT toxin in ETEC colonization (30). The authors observed enhanced adherence to IPEC-J2 cells by various isogenic ETEC constructs carrying different forms of the LT toxin (K88/LT wild type, attenuated toxin form [K88/LTR192G] and expressing just the B subunit [K88/LTB]), in contrast to the attenuated phenotype of the LT-negative construct. LT+ strains blocking binding of wild type ETEC strain to IPEC-J2 cells suggested that LT-driven adherence alters net surface charge on epithelial cells. Another study evaluated the transcriptional pattern of 214 genes at different time points following interaction of prototype ETEC E24377A with epithelial cells (31). The study found a prominent alteration of genes associated with motility, adhesion, toxin production, and global regulatory mechanisms, such as those linked to cAMP receptor protein and c-di-GMP, upon ETEC-host interaction, which suggested that ETEC coordinated its responses to the host environment by sequential activation of different virulence factors.
Among those ETEC strains infecting animals, the most common adhesive fimbriae include K88 or K99 (also called F4 and F5) (28). Recently, Zhou et al have demonstrated that deletion of fliC (encoding the major flagellin protein) and/or the faeG (encoding the F4 major fimbrial subunit) from ETEC strain C83902 significantly reduced its ability to adhere to porcine epithelia IPEC-J2 cells, but also impacting biofilm formation and quorum sensing (32). Interestingly, another study found an alternative way to block the adherence of ETEC K88 to IPEC-J2 by using ETEC anti-adhesives, including casein glycomacropeptide, exopolysaccharide, and vegetable extract (locust bean or wheat bran) (33). Finally, studies with human milk and commercial infant formulas found that the main gangliosides (GM3, GD3, GM1) and free sialic acid (Neu5Ac) are able to impede the adhesion of several pathogenic bacteria, including ETEC (26). Other dietary supplements, such as plantain NSP, also hampered the adherence of ETEC to Caco-2 cells, and has been suggested that blocking M-cell bacterial translocation can subsequently prevent diarrheal episodes (34).
Enteroaggregative E. coli (EAEC)
EAEC are a major cause of acute and persistent diarrhea in the small intestine of children and adults worldwide, including industrialized countries (35). EAEC is also responsible for sporadic cases and several outbreaks (36). At an initial stage of infection, EAEC adhere in a characteristic “stacked-brick” formation to host intestinal mucosa; forming a thick mucoid biofilm. The adherence process is mainly mediated by fimbrial structures called aggregative adherence fimbriae (AAF) (35) (Figure 1). Additionally, several EAEC virulence-related genes have been described but their role in the clinical outcome of infection is not completely defined.
A study recently confirmed a high prevalence, endemicity and heterogeneity of EAEC strains, and found that the plasmid-encoded toxin or AAF/II fimbrial subunit genes were associated significantly with disease (37). However, this study also demonstrated that the pathophysiology of EAEC infections involves a complex and dynamic modulation of several virulence factors. Another study identified an association of the EAEC virulence-encoded aggR gene (virulence regulator), pCDV432 plasmid, and additional virulence gene products, including dispersin and the Air adhesin in 90% of the diarrheagenic isolates that distinguished them from non-diarrheagenic EAEC strains, suggesting heterogeneity among highly pathogenic EAEC strains (38).
Another area of study in EAEC pathogenesis is the contribution of the autotransporter proteins. Munera et al evaluated the role of chromosome-encoded autotransporters in colonization and subsequent induction of diarrheal disease in infant rabbits and found that Shiga toxin-producing EAEC O104:H4 autotransporters, but not its virulence plasmid, are critical for robust colonization and disease (39). EAEC has also been associated with urinary tract infections (40, 41). The autotrasporter Pic has been defined as a gene marker associated with spreading of infection to the urinary tract (40). Finally, comparison of EAEC isolates from HIV-positive and non-HIV diarrheal samples showed that HIV-positive isolates are stronger biofilm producers and more resistant to antibiotics than the non-HIV diarrheal isolates, which confirmed the heterogeneity of the EAEC isolates (42).
Adherent and Invasive E. coli (AIEC)
Inflammatory bowel disease (IBD), particularly Crohn’s disease and ulcerative colitis, are the result of alterations in the intestinal microbiota due to a variety of genetic and environmental factors (43). Interestingly, an increased number of AIEC have been isolated from IBD patients and more frequently found in ileal-Crohn’s disease patients than in healthy controls (44). AIEC strains have the ability to adhere and invade intestinal epithelial cells and survive within macrophages (45). Small et al recently established a chronic infection murine model using prototypical AIEC isolates (46), and demonstrated that AIEC infection stimulates chronic inflammation and fibrosis in mice. This study showed for the first time evidence that an infection with AIEC can cause intestinal symptoms similar to those observed in Crohn’s disease patients.
AIEC adherence depends on the expression of the type 1 pili, long polar fimbriae and the presence of the carcinoembryonic antigen (CEACAM6) as a host cell receptor (47, 48) (Figure 1). Crohn’s disease patients showed abnormal expression of CEACAM6 (48) and as such, Martinez-Medina et al used a model of transgenic mice expressing CEACAMs to assess the effects of a high fat/high sugar western diet on gut microbiota composition, barrier integrity and susceptibility to infection (49). They found that the diet induces changes in gut microbiota composition, altering host homeostasis and promoting AIEC murine gut colonization. With respect to the type 1 pili, a study sequenced the fimH gene (FimH is the adhesin protein located on the tip of the pili) from 45 AIEC strains and 47 non-AIEC E. coli strains. Phylogenetic analysis found that AIEC strains predominantly express FimH with amino acid mutations of a recent evolutionary origin as compared to non-AIEC strains, which represents a feature of pathoadaptive changes in several bacterial pathogens (50). The accumulation of these mutations confers AIEC the ability to adhere to CEACAM-expressing intestinal epithelial cells and to participate in the development of chronic inflammation in a genetically susceptible host. Finally, it is known that the long polar fimbriae help AIEC to interact with intestinal Peyer’s patches and M cells (51). A recent study analyzed the effect of gastrointestinal conditions on AIEC long polar fimbriae expression. The authors found that bile salts strongly enhanced fimbriae expression, causing a higher level of interaction of AIEC with Peyer’s patches and a higher level of translocation through M cell monolayers (52).
Regarding the clinical implications of AIEC recent investigation indicates that colonization of AIEC results in chronic colitis in mice lacking the flagellin receptor TLR5. Transient AIEC colonization drove intestinal inflammation which is associated with altering microbiota composition (53). Proteases and protease inhibitors control microbiota composition, immune response and intestinal function to maintain gut homeostasis. CYLD is a de-ubiquitinase that is significantly downregulated in the intestine of Crohn’s disease patients (54). Decrease CYLD expression results in an enhanced intracellular replication of AIEC (54). Therefore, protection against AIEC during microbiota acquisition might be a strategy to control IBD in genetically susceptible individuals (53).
Uropathogenic E. coli (UPEC)
UPEC are among the most prevalent extra-intestinal bacteria, accounting for 90% of all urinary tract infection (UTI) (2). The most predominant chaperone-usher fimbriae in UPEC strains is the type 1 fimbriae, which is an important determinant for pathogenicity, allowing the interaction of UPEC with urinary tract host epithelia (55, 56). FimH within the type 1 fimbriae bound to α-D-mannosylated uroplakin, facilitating bacterial invasion, colonization and formation of biofilm-like structures called intracellular bacterial communities (IBCs) (57) (Figure 1). A high-throughput insilico analysis and in-vitro binding study discovered that pathoadaptive alleles of FimH, with variant residues outside the binding pocket, affect FimH-mediated acute and chronic pathogenesis of two prototype UPEC strains (56). The study argues that FimH variants, which maintain a high-affinity conformation, were attenuated during chronic bladder infection, implying FimH's ability to switch between conformations is important during pathogenesis.
With respect to the regulatory mechanisms controlling UPEC colonization, Mitra et al found UvrY as a key regulator modulating phase variation during UPEC pathogenesis, down-regulating the expression of type 1 fimbrial structural genes, and influencing biofilm formation, virulence and motility in UPEC strain CFT073 (58). Cpx is another key regulator involved in bacterial adhesion. The deletion of cpxRA impaired the ability of UPEC strain UTI89 to invade and colonize bladder epithelial cells, suggesting that the Cpx system is needed for UPEC persistence in the urinary tract (59). Similarly, mutation in cpxRA and cpxP in CFT073 also greatly reduced virulence tested the zebrafish infection model (59). Finally, natural medicinal plants and secondary metabolites have being study because asiatic acid and ursolic acid decreased expression of P fimbriae and curli fibers, altering cell morphology and adhesion of UPEC to uroepithelial cells (60). Finally, Rafsanjany et al also demonstrated anti-adhesive effects of various medicinal plant extracts against UPEC strains (61).
Conclusion
The recent progress understanding the adhesion/invasion properties of intestinal and extra-intestinal pathogenic E. coli during colonization of host cells reveals that there is a need of further mechanistic studies that can be used for development of specific therapeutic approaches.
Key points.
Recent studies revealed how host signaling responses alter pathogenic E. coli colonization.
Advances in the understanding for the role of pathoadaptive mutations on fimbrial adhesins and their contribution to the pathogenic process.
Development of new murine model of chronic E. coli infection and the study of novel E. coli pathotypes.
Acknowledgments
The laboratory of A.G.T. was supported in part by NIH/NIAID grant AI079154.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References and Recommended reading
- 1.Leimbach A, Hacker J, Dobrindt U. E. coli as an all-rounder: the thin line between commensalism and pathogenicity. Curr Top Microbiol Immunol. 2013;358:3–32. doi: 10.1007/82_2012_303. [DOI] [PubMed] [Google Scholar]
- 2.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nature Rev Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 3. Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev. 2013;4:822–880. doi: 10.1128/CMR.00022-13.. This is a comprehensive review highlighting recent advances in the understanding of pathogenesis of intestinal pathotypes of E. coli.
- 4.Farfan MJ, Torres AG. Molecular mechanisms that mediate colonization of Shiga toxin-producing Escherichia coli strains. Infect Immun. 2012;80:903–913. doi: 10.1128/IAI.05907-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferens WA, Hovde CJ. Escherichia coli O157:H7: animal reservoir and sources of human infection. Foodborne Pathog Dis. 2011;8:465–487. doi: 10.1089/fpd.2010.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zumbrun SD, Melton-Celsa AR, Smith MA, Gilbreath JJ, Merrell DS, O'Brien AD. Dietary choice affects Shiga toxin-producing Escherichia coli (STEC) O157:H7 colonization and disease. Proc Natl Acad Sci U S A. 2013;110:E2126–E2133. doi: 10.1073/pnas.1222014110.. This paper demonstrate that susceptibility to infection and subsequent disease after E. coli O157:H7 consumption may depend, on individual diet and/or commensal flora properties.
- 7.Sheng H, Nguyen YN, Hovde CJ, Sperandio V. SdiA aids enterohemorrhagic Escherichia coli carriage by cattle fed a forage or grain diet. Infect Immun. 2013;81:3472–3478. doi: 10.1128/IAI.00702-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes DT, Terekhova DA, Liou L, Hovde CJ, Sahl JW, Patankar AV, et al. Chemical sensing in mammalian host-bacterial commensal associations. Proc Natl Acad Sci U S A. 2010;107:9831–9836. doi: 10.1073/pnas.1002551107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garlanda C, Riva F, Polentarutti N, Buracchi C, Sironi M, De Bortoli M, et al. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc Natl Acad Sci U S A. 2004;101:3522–3526. doi: 10.1073/pnas.0308680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wald D, Qin J, Zhao Z, Qian Y, Naramura M, Tian L, et al. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003;4:920–927. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 11. Sham HP, Yu EY, Gulen MF, Bhinder G, Stahl M, Chan JM, et al. SIGIRR, a negative regulator of TLR/IL-1R signalling promotes Microbiota dependent resistance to colonization by enteric bacterial pathogens. PLoS Pathog. 2013;9:e1003539. doi: 10.1371/journal.ppat.1003539.. The paper demonstrates that SIGIRR expression by intestinal epithelial cells is a strategy that promotes commensal microbe-based colonization resistance against bacterial pathogens.
- 12.Hansen AM, Chaerkady R, Sharma J, Diaz-Mejia JJ, Tyagi N, Renuse S, et al. The Escherichia coli phosphotyrosine proteome relates to core pathways and virulence. PLoS Pathog. 2013;9:e1003403. doi: 10.1371/journal.ppat.1003403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen AM, Jin DJ. SspA up-regulates gene expression of the LEE pathogenicity island by decreasing H-NS levels in enterohemorrhagic Escherichia coli. BMC Microbiol. 2012;12:231. doi: 10.1186/1471-2180-12-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Branchu P, Matrat S, Vareille M, Garrivier A, Durand A, Crepin S, et al. NsrR, GadE, and GadX interplay in repressing expression of the Escherichia coli O157:H7 LEE pathogenicity island in response to nitric oxide. PLoS Pathog. 2014;10:e1003874. doi: 10.1371/journal.ppat.1003874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochoa TJ, Barletta F, Contreras C, Mercado E. New insights into the epidemiology of enteropathogenic Escherichia coli infection. Trans R Soc Trop Med Hyg. 2008;102:852–856. doi: 10.1016/j.trstmh.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enserink R, Scholts R, Bruijning-Verhagen P, Duizer E, Vennema H, de Boer R, et al. High detection rates of enteropathogens in asymptomatic children attending day care. PLoS ONE. 2014;9:e89496. doi: 10.1371/journal.pone.0089496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yatsuyanagi J, Saito S, Sato H, Miyajima Y, Amano K, Enomoto K. Characterization of enteropathogenic and enteroaggregative Escherichia coli isolated from diarrheal outbreaks. J Clin Microbiol. 2002;40:294–297. doi: 10.1128/JCM.40.1.294-297.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandes RT, Elias WP, Vieira MA, Gomes TA. An overview of atypical enteropathogenic Escherichia coli. FEMS Microbiol Lett. 2009;297:137–149. doi: 10.1111/j.1574-6968.2009.01664.x. [DOI] [PubMed] [Google Scholar]
- 19.Hernandes RT, De la Cruz MA, Yamamoto D, Giron JA, Gomes TA. Dissection of the role of pili and type 2 and 3 secretion systems in adherence and biofilm formation of an atypical enteropathogenic Escherichia coli strain. Infect Immun. 2013;81:3793–3802. doi: 10.1128/IAI.00620-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly M, Hart E, Mundy R, Marches O, Wiles S, Badea L, et al. Essential role of the type III secretion system effector NleB in colonization of mice by Citrobacter rodentium. Infect Immun. 2006;74:2328–2337. doi: 10.1128/IAI.74.4.2328-2337.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newton HJ, Pearson JS, Badea L, Kelly M, Lucas M, Holloway G, et al. The type III effectors NleE and NleB from enteropathogenic E. coli and OspZ from Shigella block nuclear translocation of NF-kappaB p65. PLoS Pathog. 2010;6:e1000898. doi: 10.1371/journal.ppat.1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gao X, Wang X, Pham TH, Feuerbacher LA, Lubos ML, Huang M, et al. NleB, a bacterial effector with glycosyltransferase activity, targets GAPDH function to inhibit NF-kappaB activation. Cell Host Microbe. 2013;13:87–99. doi: 10.1016/j.chom.2012.11.010.. This paper reveals a virulence strategy employed by Attaching and Effacing pathogens to inhibit NF-κB-dependent host innate immune responses.
- 23.Pearson JS, Giogha C, Ong SY, Kennedy CL, Kelly M, Robinson KS, et al. A type III effector antagonizes death receptor signalling during bacterial gut infection. Nature. 2013;501:247–251. doi: 10.1038/nature12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li S, Zhang L, Yao Q, Li L, Dong N, Rong J, et al. Pathogen blocks host death receptor signalling by arginine GlcNAcylation of death domains. Nature. 2013;501:242–246. doi: 10.1038/nature12436.. The paper reveals the mechanism of action of NleB, representing a new model by which bacteria counteract host defenses.
- 25.Pan Q, Tian Y, Li X, Ye J, Liu Y, Song L, et al. Enhanced membrane-tethered mucin 3 (MUC3) expression by a tetrameric branched peptide with a conserved TFLK motif inhibits bacteria adherence. J Biol Chem. 2013;288:5407–5416. doi: 10.1074/jbc.M112.408245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salcedo J, Barbera R, Matencio E, Alegria A, Lagarda MJ. Gangliosides and sialic acid effects upon newborn pathogenic bacteria adhesion: an in vitro study. Food Chem. 2013;136:726–734. doi: 10.1016/j.foodchem.2012.08.078. [DOI] [PubMed] [Google Scholar]
- 27.Beatty ME, Adcock PM, Smith SW, Quinlan K, Kamimoto LA, Rowe SY, et al. Epidemic diarrhea due to enterotoxigenic Escherichia coli. Clin Infect Dis. 2006;42:329–334. doi: 10.1086/499246. [DOI] [PubMed] [Google Scholar]
- 28.Torres AG, Zhou X, Kaper JB. Adherence of diarrheagenic Escherichia coli strains to epithelial cells. Infect Immun. 2005;73:18–29. doi: 10.1128/IAI.73.1.18-29.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guevara CP, Luiz WB, Sierra A, Cruz C, Qadri F, Kaushik RS, et al. Enterotoxigenic Escherichia coli CS21 pilus contributes to adhesion to intestinal cells and to pathogenesis under in vivo conditions. Microbiology. 2013;159:1725–1735. doi: 10.1099/mic.0.065532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fekete PZ, Mateo KS, Zhang W, Moxley RA, Kaushik RS, Francis DH. Both enzymatic and non-enzymatic properties of heat-labile enterotoxin are responsible for LT-enhanced adherence of enterotoxigenic Escherichia coli to porcine IPEC-J2 cells. Vet Microbiol. 2013;164:330–335. doi: 10.1016/j.vetmic.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 31. Kansal R, Rasko DA, Sahl JW, Munson GP, Roy K, Luo Q, et al. Transcriptional modulation of enterotoxigenic Escherichia coli virulence genes in response to epithelial cell interactions. Infect Immun. 2013;81:259–270. doi: 10.1128/IAI.00919-12.. This manuscript demonstrated that pathogen-host interactions are finely coordinated by ETEC during the infectious process.
- 32.Zhou M, Duan Q, Zhu X, Guo Z, Li Y, Hardwidge PR, et al. Both flagella and F4 fimbriae from F4ac+ enterotoxigenic Escherichia coli contribute to attachment to IPEC-J2 cells in vitro. Vet Res. 2013;44:30. doi: 10.1186/1297-9716-44-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez-Ortiz G, Perez JF, Hermes RG, Molist F, Jimenez-Diaz R, Martin-Orue SM. Screening the ability of natural feed ingredients to interfere with the adherence of enterotoxigenic Escherichia coli (ETEC) K88 to the porcine intestinal mucus. Br J Nutr. 2014;111:633–642. doi: 10.1017/S0007114513003024. [DOI] [PubMed] [Google Scholar]
- 34.Roberts CL, Keita AV, Parsons BN, Prorok-Hamon M, Knight P, Winstanley C, et al. Soluble plantain fibre blocks adhesion and M-cell translocation of intestinal pathogens. J Nutr Biochem. 2013;24:97–103. doi: 10.1016/j.jnutbio.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flores J, Okhuysen PC. Enteroaggregative Escherichia coli infection. Curr Opin Gastroenterol. 2009;25:8–11. doi: 10.1097/MOG.0b013e32831dac5e. [DOI] [PubMed] [Google Scholar]
- 36.Weintraub A. Enteroaggregative Escherichia coli : epidemiology, virulence and detection. J Med Microbiol. 2007;56:4–8. doi: 10.1099/jmm.0.46930-0. [DOI] [PubMed] [Google Scholar]
- 37.Lima IF, Boisen N, Quetz Jda S, Havt A, de Carvalho EB, Soares AM, et al. Prevalence of enteroaggregative Escherichia coli and its virulence-related genes in a case-control study among children from north-eastern Brazil. J Med Microbiol. 2013;62:683–693. doi: 10.1099/jmm.0.054262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nuesch-Inderbinen MT, Hofer E, Hachler H, Beutin L, Stephan R. Characteristics of enteroaggregative Escherichia coli isolated from healthy carriers and from patients with diarrhoea. J Med Microbiol. 2013;62:1828–1834. doi: 10.1099/jmm.0.065177-0. [DOI] [PubMed] [Google Scholar]
- 39. Munera D, Ritchie JM, Hatzios SK, Bronson R, Fang G, Schadt EE, et al. Autotransporters but not pAA are critical for rabbit colonization by Shiga toxin-producing Escherichia coli O104:H4. Nat Commun. 2014;5:3080. doi: 10.1038/ncomms4080.. This study revealed that the virulence pAA plasmid in STEC O104:H4 is dispensable for intestinal colonization while the production of autotransporters is critical for development of intestinal pathology.
- 40.Herzog K, Engeler Dusel J, Hugentobler M, Beutin L, Sagesser G, Stephan R, et al. Diarrheagenic enteroaggregative Escherichia coli causing urinary tract infection and bacteremia leading to sepsis. Infection. 2014;42:441–444. doi: 10.1007/s15010-013-0569-x. [DOI] [PubMed] [Google Scholar]
- 41. Boll EJ, Struve C, Boisen N, Olesen B, Stahlhut SG, Krogfelt KA. Role of enteroaggregative Escherichia coli virulence factors in uropathogenesis. Infect Immun. 2013;81:1164–1171. doi: 10.1128/IAI.01376-12.. The study found that EAEC-specific virulence factors increase uropathogenicity and strains carrying these factors might cause a community-acquired urinary tract infections.
- 42.Jafari A, Shafaei E, Oloomi M, Aghasadeghi MR, Bouzari S. Genotypic and Phenotypic Comparison of Enteroaggregative Escherichia coli Isolates from HIV-Positive and non-HIV Diarrheal Samples. Curr HIV Res. 2014 doi: 10.2174/1570162x12666140207161000. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 43.Cieza RJ, Cao AT, Cong Y, Torres AG. Immunomodulation for gastrointestinal infections. Expert Rev Anti Infect Ther. 2012;10:391–400. doi: 10.1586/eri.11.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rolhion N, Darfeuille-Michaud A. Adherent-invasive Escherichia coli in inflammatory bowel disease. Bowel Dis. 2007;13:1277–1283. doi: 10.1002/ibd.20176. [DOI] [PubMed] [Google Scholar]
- 45.Glasser AL, Boudeau J, Barnich N, Perruchot MH, Colombel JF, Darfeuille-Michaud A. Adherent invasive Escherichia coli strains from patients with Crohn's disease survive and replicate within macrophages without inducing host cell death. Infect Immun. 2001;69:5529–5537. doi: 10.1128/IAI.69.9.5529-5537.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Small CL, Reid-Yu SA, McPhee JB, Coombes BK. Persistent infection with Crohn's disease-associated adherent-invasive Escherichia coli leads to chronic inflammation and intestinal fibrosis. Nat Commun. 2013;4:1957. doi: 10.1038/ncomms2957.. This paper describes a murine model in which chronic adherent-invasive E. coli infections result in immunopathology similar to that seen in Crohn's disease patients.
- 47.Glas J, Seiderer J, Fries C, Tillack C, Pfennig S, Weidinger M, et al. CEACAM6 gene variants in inflammatory bowel disease. PLoS One. 2011;6:e19319. doi: 10.1371/journal.pone.0019319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barnich N, Carvalho FA, Glasser AL, Darcha C, Jantscheff P, Allez M, et al. CEACAM6 acts as a receptor for adherent-invasive E. coli supporting ileal mucosa colonization in Crohn disease. J Clin Invest. 2007;117:1566–1574. doi: 10.1172/JCI30504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinez-Medina M, Denizot J, Dreux N, Robin F, Billard E, Bonnet R, et al. Western diet induces dysbiosis with increased E. coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut. 2014;63:116–124. doi: 10.1136/gutjnl-2012-304119. [DOI] [PubMed] [Google Scholar]
- 50. Dreux N, Denizot J, Martinez-Medina M, Mellmann A, Billig M, Kisiela D, et al. Point mutations in FimH adhesin of Crohn's disease-associated adherent-invasive Escherichia coli enhance intestinal inflammatory response. PLoS Pathog. 2013;9:e1003141. doi: 10.1371/journal.ppat.1003141.. This study highlights a mechanism of AIEC virulence evolution that leads to the development of chronic inflammatory bowel disease in a genetically susceptible host.
- 51.Chassaing B, Rolhion N, de Vallee A, Salim SY, Prorok-Hamon M, Neut C, et al. Crohn disease--associated adherent-invasive E. coli bacteria target mouse and human Peyer's patches via long polar fimbriae. J Clin Invest. 2011;121:966–975. doi: 10.1172/JCI44632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chassaing B, Etienne-Mesmin L, Bonnet R, Darfeuille-Michaud A. Bile salts induce long polar fimbriae expression favouring Crohn's disease-associated adherent-invasive Escherichia coli interaction with Peyer's patches. Environ Microbiol. 2013;15:355–371. doi: 10.1111/j.1462-2920.2012.02824.x. [DOI] [PubMed] [Google Scholar]
- 53.Chassaing B, Koren O, Carvalho FA, Ley RE, Gewirtz AT. AIEC pathobiont instigates chronic colitis in susceptible hosts by altering microbiota composition. Gut. 2014;63:1069–1080. doi: 10.1136/gutjnl-2013-304909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cleynen I, Vazeille E, Artieda M, Verspaget HW, Szczypiorska M, Bringer MA, et al. Genetic and microbial factors modulating the ubiquitin proteasome system in inflammatory bowel disease. Gut. 2013 doi: 10.1136/gutjnl-2012-303205. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 55.Sauer FG, Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ. Bacterial pili: molecular mechanisms of pathogenesis. Curr Opin Microbiol. 2000;3:65–72. doi: 10.1016/s1369-5274(99)00053-3. [DOI] [PubMed] [Google Scholar]
- 56. Schwartz DJ, Kalas V, Pinkner JS, Chen SL, Spaulding CN, Dodson KW, et al. Positively selected FimH residues enhance virulence during urinary tract infection by altering FimH conformation. Proc Natl Acad Sci U S A. 2013;110:15530–15537. doi: 10.1073/pnas.1315203110.. This study present evidence indicating that positively selected residues within type 1 fimbriae modulate bacterial fitness during UTI by affecting FimH conformation and function.
- 57.Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 58.Mitra A, Palaniyandi S, Herren CD, Zhu X, Mukhopadhyay S. Pleiotropic roles of uvrY on biofilm formation, motility and virulence in uropathogenic Escherichia coli CFT073. PLoS One. 2013;8:e55492. doi: 10.1371/journal.pone.0055492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Debnath I, Norton JP, Barber AE, Ott EM, Dhakal BK, Kulesus RR, et al. The Cpx stress response system potentiates the fitness and virulence of uropathogenic Escherichia coli. Infect Immun. 2013;81:1450–1459. doi: 10.1128/IAI.01213-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dorota W, Marta K, Dorota TG. Effect of asiatic and ursolic acids on morphology, hydrophobicity, and adhesion of UPECs to uroepithelial cells. Folia Microbiol (Praha) 2013;58:245–252. doi: 10.1007/s12223-012-0205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rafsanjany N, Lechtenberg M, Petereit F, Hensel A. Antiadhesion as a functional concept for protection against uropathogenic Escherichia coli : in vitro studies with traditionally used plants with antiadhesive activity against uropathognic Escherichia coli. J Ethnopharmacol. 2013;145:591–597. doi: 10.1016/j.jep.2012.11.035. [DOI] [PubMed] [Google Scholar]