Abstract
The blood-follicle barrier (BFB) is one of the blood-tissue barriers in mammalian body found in developing follicles in the ovary. The BFB, besides the tight junction (TJ)-permeability barrier of the endothelial cells in the microvessels that surround the developing follicle, is constituted and contributed significantly by the basement membrane of the developing follicle which alters its composition rapidly during follicle development. While the concept of the BFB and its ultrastructure were described more than six decades ago, fewer than 20 reports are found in the literature that were dedicated to investigate the biology, regulation, and function of the BFB either in health or in disease. Furthermore, detailed analysis of the adhesion protein complexes and the regulation of the junction dynamics at the BFB are still missing in the literature. The goal of this short chapter is to provide an update on this important blood-tissue barrier, it is obvious that future investigation is much needed in the field to understand this ultrastructure better in order to treat and better ovarian disorders including ovarian cancer.
INTRODUCTION: THE CONCEPT OF THE BLOOD-FOLLICLE BARRIER (BFB)
Follicular fluid fills the follicular antrum and surrounds the ovum in an ovarian follicle, which in turn provides a unique microenvironment for follicular development and oocyte maturation.1 Based on the morphological studies conducted in the 1950s and 1960s, it is known that ovarian follicles are surrounded by a network of capillaries in the theca interna, the follicular compartment per se is nonvascular, and each follicle is separated from the surrounding microvessels by a unique basement membrane,2–4 illustrating the likely presence of a blood-tissue barrier at the site, which was designated the “blood-liquor barrier” in 1958.5 However, the term BFB was first used in the literature in 1973 when the protein composition and concentration between the follicular fluid and blood serum or plasma were found to be different,6 consistent with a subsequent report using follicular fluids and serum samples from women undergoing IVF.7 This work was further expanded using a proteomic approach, and it was found that human follicular fluid contained several acute inflammatory phase proteins including transferrin, afamin, ceruloplasmin, hemopexin, haptoglobin, and plasma amyloid protein in levels different from the plasma, which also supported the notion that ovulation is similar to an inflammatory response.8 The same study also found some antioxidant enzymes, including superoxide dismutase, glutathione transferase, catalase, paraoxonase, and heat shock protein 27 which can help to protect human follicles from toxic injury mediated by oxidative stress.8 It is believed that proteins in the follicular fluid are similar to the blood plasma, providing important growth factors for follicular development and oocyte maturation.8 In fact, with respect to low-molecular-weight proteins, the components of follicular fluid is similar to the blood plasma.6,9 However, the protein content of human follicular fluid is different from that of the blood plasma due to the selective BFB which serves as a “molecular sieve”.6,10 For instance, in a study using ferritin (MW 500 kDa) and colloidal gold (MW 1000 kDa) as tracers to assess the BFB permeability, it was found that BFB served as a molecular sieve which was permeable to proteins < 500 kDa.11 Subsequently, the BFB was found to be both charge- and size selective in mouse ovaries.12
COMPOSITION OF THE BFB
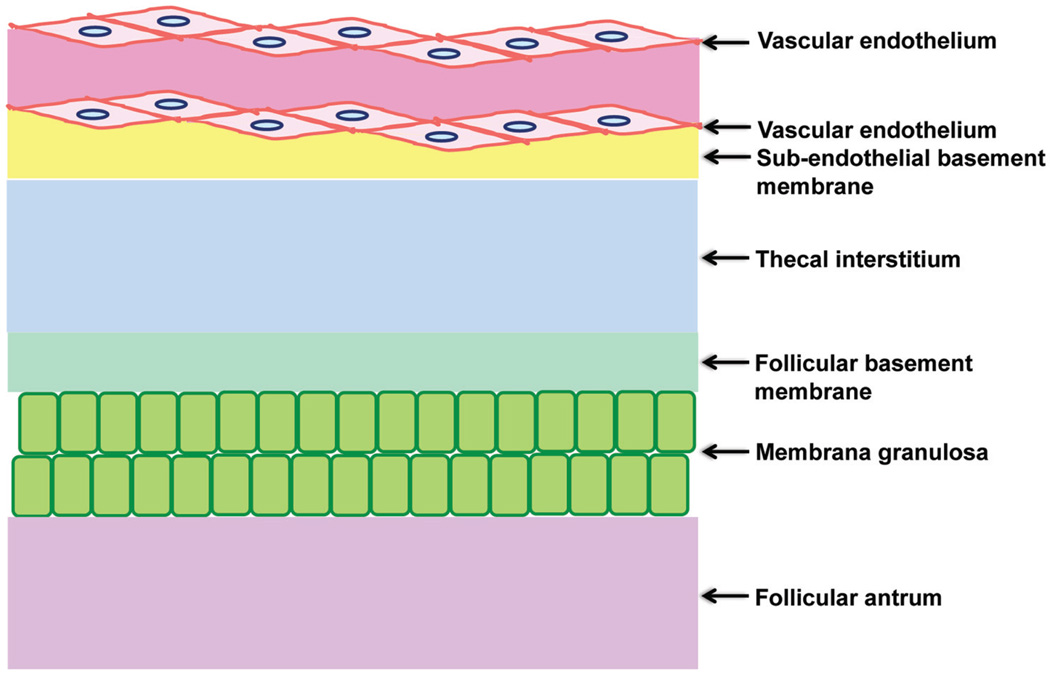
In order to transport from the theca into the follicular antrum, proteins/peptides, sugars, electrolytes, and ions in fluid must pass through the BFB which is composed of the vascular endothelium, sub-endothelial basement membrane, the thecal interstitium, the follicular basement membrane, and the membrana granulosa (see Fig. 1).13
Figure 1.
Schematic drawing illustrating the different components that constitute the blood-follicle barrier.
The Permselectivity of the BFB
A recent study using the ‘in vivo cryotechnique’ (IVCT) to examine mouse ovaries morphologically and immunohistochemically,13 it was shown that the immunostaining of albumin (~69 KDa, referred to as a low molecular size protein) was localized in the blood vessels, the interstitium, and developing follicles, suggesting that proteins with low molecular size can pass through the BFB towards developing follicles. While the immunostaining of mid-sized molecules, such as IgG1 (~150 kDa), IαI (inter-α-trypsin inhibitor, 220 kDa), and fibrinogen (340 KDa), were significantly reduced inside ovarian follicles, suggesting that although proteins with middle molecular size can pass through the blood vessels towards the interstitium, the follicular basement membranes inhibit the permselectivity of these molecules. For high molecular weight proteins, such as IgM (~900 kDa), its immunostaining was mostly restricted to the blood vessels, suggesting that proteins with high molecular size are blocked by the endothelial TJ-barrier of blood vessels. These findings suggest that the BFB is present from early stages of folliculogenesis and it has selective roles during follicular development until ovulation.13
Endothelial Cells of Blood Vessels
The endothelial cells of blood vessels, mostly capillaries, contribute to the BFB which act as a permeability filter regulating the passage of serum proteins from blood vessels to the surrounding tissue, and such passage is tightly controlled by the dynamic nature of the junctional complexes, such as tight and adherens junctions and desmosomes, between endothelial cells.14,15 In the ovary, such as mouse ovaries, the intercellular junctional complexes between endothelial cells are not well characterized, but the blockade of mid-sized proteins by the endothelial TJ-barrier has been shown to be regulated by nitric oxide.16 It is also noteworthy that the BFB was found to be compromised as the result of a loss of ovarian superoxide dismutase activity in diabetic mice that led to ovulation defects, illustrating the critical role of reactive oxygen species to the BFB function.
Subendothelial Basement Membranes
Dependent on the pore size and charge selectivity of the components including Type IV collagen, laminin, and heparan sulfate proteoglycans, the basement membrane of blood vessels such as glomerular basement membrane can served as a molecular sieve.17
Follicular Basement Membranes
Since the permselectivity of the subendothelial basement membranes and the follicular basement membranes is different, it is suggested that this may be due to the presence of unique extracellular matrix components. During folliculogenesis, the follicular basement membrane is a dynamic ultrastructure with its components rapidly change over time.18 In primordial and pre-antral stages, collagen IV alpha 1–6 is present and later on in antral and attretic stages, but only alpha 1 and alpha 2 chains are present in the follicular basement membrane in cows18 and mice.19 On the contrary, perlecan, nidogen 1 and nidogen 2 are the predominant follicular basement membrane components in the pre-antral stage and thereafter in cows.20,21 Laminin alpha 1, beta 2 and gamma 1 are also detected in the follicular basement membrane during follicle development in cows.18 It is possible that such dynamic changes in the follicular basement membrane components contribute changes to the basement membrane permeability during folliculogenesis.
The BFB in Polycystic Ovary and Ovarian Cancer
Polycystic ovary syndrome (PCOS) is one of the most common female hormonal disorders characterized by anovulation, leading to irregular menstruation, infertility and polycystic ovaries.22,23 In PCOS, follicle development is put on hold at an early follicular stages.24 Since BFB regulates the composition of the follicular fluid by determining its component proteins, which in turn regulates follicle development, PCOS can be affected, at least in part, by the permselectivity of the BFB. Previous studies have shown that insulin-like growth factor binding proteins,25 soluble Fas ligand26 and inhibins A and B27 are dysregulated in the follicular fluids in PCOS patients.
Using the IVCT in a mifepristone-induced PCO model, the morphology and the permselectivity of the BFB was examined in mouse ovaries,28 in which the blood vessels were found to be enlarged along with an increase in blood flow, and follicular cysts were formed with thinner membrana granulosa in the blood vessels, the interstitium, and developing follicles.28 The immunostaining of low and high molecular weight proteins, albumin and IgM, respectively, in the ovaries of PCO model mice were similar to that in normal mice. Albumin was detected in the blood vessels, the interstitium, and developing follicles and IgM was mostly retained inside the blood vessels.28 On the contrary, the immunostaining of mid-sized molecules such as IgG, ITI, and fibrinogen had different pattern in the PCO model. The follicular basement membranes blocked the passage of both IgG and ITI from the interstitium to the follicles in PCO model. Moreover, fibrinogen was mostly restricted within the blood vessels and surrounded by the endothelial cells in the PCO mice.28 These findings suggest that the permselectivity of BFB mediated by the endothelial cells of the microvessels and the follicular basement membrane may play important role in the pathogenesis of PCOS.28
Ovarian cancer is the most lethal of all gynecological malignancies.29,30 Approximately 90% of the malignant ovarian tumors are originated from changes in the surface epithelium or surface epithelial inclusion cysts of the ovary.31,32 It is possible that dysregulation of the BFB contributes to: (i) the abnormal changes of the surface epithelium, and/or (ii) the formation of surface epithelial inclusion cysts, of the ovary, which, in turn, leads to cancer progression. This possibility regarding the role of BFB in ovarian cancer must be carefully evaluated in future studies.
THE PERMEABILITY BARRIER FUNCTION OF THE BFB: SELECTIVE TRANSPORT PROCESSES VERSUS MERE FILTRATION
Similar to other blood-tissue barriers, such as the blood-brain barrier and the blood-testis barrier,33–36 the BFB is now known to be more than a “molecular sieve” in the ovary by restricting the transcellular transport of solutes and macromolecules across the various membranes ultrastructures to reach the developing follicles entirely based on their molecular sizes. For instance, IαI (inter-α-trypsin inhibitor, 240 kDa) and pre-α-trypsin inhibitor (125 kDa) were found to be absent from the follicular fluid until an ovulatory stimulus (e.g., during LH surge) was given, and both of these protein were found in the antrum of mature follicles, usually within minutes, and integrally associated with the newly synthesized hyaluronic acid-rich cumulus extracellular matrix.37,38 Subsequent studies have shown that these changes in permeability function at the BFB are also mediated by nitric oxide (NO),16 consistent with earlier findings that NO regulates vascular function and permeability39–41 as well as the Sertoli cell TJ-barrier permeability function.42
CONCLUSION AND FUTURE PERSPECTIVES
As briefly summarized above, the BFB is an important ultrastructure in the ovary by limiting the access of foreign compounds and/or harmful substances (e.g., toxicants, drugs) to the developing follicles. It is intimately related to follicle development and it also regulates the composition of the follicular fluid via subtle changes in its basement membrane composition during folliculogenesis, so that different components (e.g., proteins, peptides, electrolytes, ions, sugars, and others) can be precisely and rapidly recruited to the follicular fluid in response to the needs of developing follicles. A recent study has shown that some therapeutic drugs, such as doxorubicin, an anticancer drug, can permeate the BFB to induce ovarian failure by causing apoptosis of germinal vesicle occytes,43 illustrating much work is needed to understand this blood-tissue barrier so that the reproductive health of women under chemotherapy can be maintained. While the concept of BFB was depicted more than six decades ago and the termed BFB was used almost 40 years ago, yet, less than 20 reports are found in the literature dedicated to study the biology and regulation of the BFB. It is likely that proteins found in the follicular fluid behind the BFB can be putative candidates for diagnostic markers for follicle and/or oocyte maturation and to assess oocyte quality. It is equally possible that follicular fluid proteins can sever as diagnostic, therapeutic and/or prognostic markers for various ovarian diseases including PCOS and ovarian cancers. There are pressing questions remain to be addressed. For instance, what initiates or regulates the formation of follicular fluid? Does this involve LH and/or FSH? What are the changes in cell-cell junctions that facilitate the formation of follicular fluid during folliculogenesis?
ACKNOWLEDGMENTS
This work was supported in part by grants from the National Institutes of Health (NICHD R01 HD056034 to CYC; U54 HD029990 Project 5 to CYC).
REFERENCES
- 1.Rodgers RJ, Irving-Rodgers HF. Formation of the ovarian follicular antrum and follicular fluid. Biol Reprod. 2010;82(6):1021–1029. doi: 10.1095/biolreprod.109.082941. [DOI] [PubMed] [Google Scholar]
- 2.Bjersing L, Cajander S. Ovulation and the mechanism of follicle rupture. I. Light micreoscopic changes in rabbit ovbarian follicles prior to induced ovulation. Cell Tissue Res. 1951;149:287–299. doi: 10.1007/BF00226764. [DOI] [PubMed] [Google Scholar]
- 3.Burr JH, Davis JR. The vascular system of the rabbit ovary and its relationship to ovulation. Anat Rec. 1951;111:273–297. doi: 10.1002/ar.1091110302. [DOI] [PubMed] [Google Scholar]
- 4.Byskov AG. Ultrastructural studies on preovulatory follicles in the mouse ovary. Z Zellforsch Mikrosk Anat. 1969;100:285–299. doi: 10.1007/BF00343884. [DOI] [PubMed] [Google Scholar]
- 5.Zachariae F. Studies on the mechanism of ovulation: permeability of the blood-liquor barrier. Acta Endocrinol. 1958;27:339–342. doi: 10.1530/acta.0.0270339. [DOI] [PubMed] [Google Scholar]
- 6.Shalgi R, Kraicer P, Rimon A, et al. Proteins of human follicular fluid: the blood-follicle barrier. Fertil Steril. 1973;24:429–434. [PubMed] [Google Scholar]
- 7.Schweigert FJ, Gericke B, Wolfram W, et al. Peptide and protein profiles in serum and follicular fluid of women undergoing IVF. Human Reprod. 2006;21:2960–2968. doi: 10.1093/humrep/del257. [DOI] [PubMed] [Google Scholar]
- 8.Angelucci S, Ciavardelli D, Di Giuseppe F, et al. Proteome analysis of human follicular fluid. Biochim Biophys Acta. 2006;1764:1775–1785. doi: 10.1016/j.bbapap.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Gosden RG, Hunter RH, Telfer E, et al. Physiological factors underlying the formation of ovarian follicular fluid. J Reprod Fertil. 1988;82:813–825. doi: 10.1530/jrf.0.0820813. [DOI] [PubMed] [Google Scholar]
- 10.Zachariae F. Studies on the mechanism of ovulation: permeability of the blood-liquor barrier. Acta Endocrinol (Copenh) 1958;27:339–342. doi: 10.1530/acta.0.0270339. [DOI] [PubMed] [Google Scholar]
- 11.Cran DG, Moor RM, Hay MF. Permeability of ovarian follicles to electron-dense macromolecules. Acta Endocrinol (Copenh) 1976;82:631–636. doi: 10.1530/acta.0.0820631. [DOI] [PubMed] [Google Scholar]
- 12.Hess KA, Chen L, Larsen WJ. The ovarian blood follicle barrier is both charge- and size-selective in mice. Biol Reprod. 1998;58:705–711. doi: 10.1095/biolreprod58.3.705. [DOI] [PubMed] [Google Scholar]
- 13.Zhou H, Ohno N, Terada N, et al. Involvement of follicular basement membrane and vascular endothelium in blood follicle barrier formation of mice revealed by ‘in vivo cryotechnique’. Reproduction. 2007;134:307–317. doi: 10.1530/REP-07-0062. [DOI] [PubMed] [Google Scholar]
- 14.Bazzoni G. Endothelial tight junctions: permeable barriers of the vessel wall. Thromb Haemost. 2006;95:36–42. [PubMed] [Google Scholar]
- 15.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Powers RW, Chen L, Russell PT, et al. Gonadotropin-stimulated regulation of blood-follicle barrier is mediated by nitric oxide. Am J Physiol. 1995;269:E290–E298. doi: 10.1152/ajpendo.1995.269.2.E290. [DOI] [PubMed] [Google Scholar]
- 17.Holmquist P, Sjoblad S, Torffvit O. Pore size and charge selectivity of the glomerular membrane at the time of diagnosis of diabetes. Pediatr Nephrol. 2004;19:1361–1366. doi: 10.1007/s00467-004-1610-1. [DOI] [PubMed] [Google Scholar]
- 18.Rodgers RJ, Irving-Rodgers HF, Russell DL. Extracellular matrix of the developing ovarian follicle. Reproduction. 2003;126:415–424. doi: 10.1530/rep.0.1260415. [DOI] [PubMed] [Google Scholar]
- 19.Rodgers HF, Irvine CM, van Wezel IL, et al. Distribution of the alpha1 to alpha6 chains of type IV collagen in bovine follicles. Biol Reprod. 1998;59:1334–1341. doi: 10.1095/biolreprod59.6.1334. [DOI] [PubMed] [Google Scholar]
- 20.Irving-Rodgers HF, Rodgers RJ. Extracellular matrix of the developing ovarian follicle. Semin Reprod Med. 2006;24(4):195–203. doi: 10.1055/s-2006-948549. [DOI] [PubMed] [Google Scholar]
- 21.McArthur ME, Irving-Rodgers HF, Byers S, et al. Identification and immunolocalization of decorin, versican, perlecan, nidogen, and chondroitin sulfate proteoglycans in bovine small-antral ovarian follicles. Biol Reprod. 2000;63:913–924. doi: 10.1095/biolreprod63.3.913. [DOI] [PubMed] [Google Scholar]
- 22.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 23.Legro RS. Polycystic ovary syndrome: the new millenium. Mol Cell Endocrinol. 2001;184:87–93. doi: 10.1016/s0303-7207(01)00640-2. [DOI] [PubMed] [Google Scholar]
- 24.Franks S. Polycystic ovary syndrome: a changing perspective. Clin Endocrinol (Oxf) 1989;31:87–120. doi: 10.1111/j.1365-2265.1989.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 25.San Roman GA, Magoffin DA. Insulin-like growth factor binding proteins in ovarian follicles from women with polycystic ovarian disease: cellular source and levels in follicular fluid. J Clin Endocrinol Metab. 1992;75:1010–1016. doi: 10.1210/jcem.75.4.1383254. [DOI] [PubMed] [Google Scholar]
- 26.Onalan G, Selam B, Baran Y, et al. Serum and follicular fluid levels of soluble Fas, soluble Fas ligand and apoptosis of luteinized granulosa cells in PCOS patients undergoing IVF. Hum Reprod. 2005;20:2391–2395. doi: 10.1093/humrep/dei068. [DOI] [PubMed] [Google Scholar]
- 27.Welt CK, Taylor AE, Fox J, et al. Follicular arrest in polycystic ovary syndrome is associated with deficient inhibin A and B biosynthesis. J Clin Endocrinol Metab. 2005;90:5582–5587. doi: 10.1210/jc.2005-0695. [DOI] [PubMed] [Google Scholar]
- 28.Zhou H, Ohno N, Terada N, et al. Permselectivity of blood follicle barriers in mouse ovaries of the mifepristone-induced polycystic ovary model revealed by in vivo cryotechnique. Reproduction. 2008;136:599–610. doi: 10.1530/REP-08-0022. [DOI] [PubMed] [Google Scholar]
- 29.Siu MKY, Chan HY, Kong DS, et al. p21-activated kinase 4 regulates ovarian cancer cell proliferation, migration, and invasion and contributes to poor prognosis in patients. Proc Natl Acad Sci U S A. 2010;107:18622–18627. doi: 10.1073/pnas.0907481107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siu MKY, Wong OG, Cheung AN. TrkB as a therapeutic target for ovarian cancer. Expert Opin Ther Targets. 2009;13:1169–1178. doi: 10.1517/14728220903196787. [DOI] [PubMed] [Google Scholar]
- 31.Bell DA. Origins and molecular pathology of ovarian cancer. Mod Pathol. 2005;18(Suppl 2):S19–S32. doi: 10.1038/modpathol.3800306. [DOI] [PubMed] [Google Scholar]
- 32.Feeley KM, Wells M. Precursor lesions of ovarian epithelial malignancy. Histopathology. 2001;38:87–95. doi: 10.1046/j.1365-2559.2001.01042.x. [DOI] [PubMed] [Google Scholar]
- 33.Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 35.Miller DS, Bauer B, Hartz AM. Modulation of P-glycoprotein at the blood-brain barrier: Opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008;60:196–209. doi: 10.1124/pr.107.07109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pelletier RM. The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem. 2011;46:49–127. doi: 10.1016/j.proghi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Chen L, Mao SJT, Larsen WJ. Identification of a factor in fetal bovine serum that stabilizes the cumulus extracellular matrix. J Biol Chem. 1992;267:12380–12386. [PubMed] [Google Scholar]
- 38.Chen L, Mao SJT, McLean LR, et al. Proteins of the inter-α-trypsin family stabilize the cumulus extracellular matrix through direct binding with hyaluronic acid. J Biol Chem. 1994;269:28282–28287. [PubMed] [Google Scholar]
- 39.Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol Rev. 1991;432:109–142. [PubMed] [Google Scholar]
- 40.Gu Y, Dee CM, Shen J. Interaction of free radicals, matrix metalloproteinases and caveolin-1 impacts blood-brain barrier permeability. Front Biosci. 2011;3:1216–1231. doi: 10.2741/222. [DOI] [PubMed] [Google Scholar]
- 41.Oberleithner H, Kusche-Vihrog K, Schillers H. Endothelial cells as vascular salt sensors. Kidney Int. 2010;77:490–494. doi: 10.1038/ki.2009.490. [DOI] [PubMed] [Google Scholar]
- 42.Lee NPY, Cheng CY. Regulation of Sertoli cell tight junction dynamics in the rat testis via the nitric oxide synthase/soluble guanylate cyclase/3',5'-cyclic guanosine monophosphate/protein kinase G signaling pathway: an in vitro study. Endocrinology. 2003;144:3114–3129. doi: 10.1210/en.2002-0167. [DOI] [PubMed] [Google Scholar]
- 43.Bar-Joseph H, Ben-Aharon I, Rizel S, et al. Doxorubicin-induced apoptosis in germal vesicle (GV) oocytes. Reprod Toxicol. 2010;30:566–572. doi: 10.1016/j.reprotox.2010.07.003. [DOI] [PubMed] [Google Scholar]