Summary
Rationale
There are several adult studies using computed tomography (CT-scan) to examine lung aeration changes during or after a recruitment maneuver (RM) in ventilated patients with acute lung injury (ALI). However, there are no published data on the lung aeration changes during or after a RM in ventilated pediatric patients with ALI.
Objective
To describe CT-scan lung aeration changes and gas exchange after lung recruitment in pediatric ALI and assess the safety of transporting patients in the acute phase of ALI to the CT-scanner.
Methods
We present a case series completed in a subset of six patients enrolled in our previously published study of efficacy and safety of lung recruitment in pediatric patients with ALI.
Intervention
RM using incremental positive end-expiratory pressure.
Results
There was a variable increase in aerated and poorly aerated lung after the RM ranging from 3% to 72% (median 20%; interquartile range 6, 47; P = 0.03). All patients had improvement in the ratio of partial pressure of arterial oxygen over fraction of inspired oxygen (PaO2/FiO2) after the RM (median 14%; interquartile range: 8, 72; P = 0.03). There was a decrease in the partial pressure of arterial carbon dioxide (PaCO2) in four of six subjects after the RM (median −5%; interquartile range: −9, 2; P = 0.5). One subject had transient hypercapnia (41% increase in PaCO2) during the RM and this correlated with the smallest increase (3%) in aerated and poorly aerated lung. All patients tolerated the RM without hemodynamic compromise, barotrauma, hypoxemia, or dysrhythmias.
Conclusions
Lung recruitment results in improved lung aeration as detected by lung tomography. This is accompanied by improvements in oxygenation and ventilation. However, the clinical significance of these findings is uncertain. Transporting patients in early ALI to the CT-scanner seems safe and feasible.
Keywords: ARDS, respiratory distress syndrome, ALI, recruitment maneuvers
INTRODUCTION
Acute lung injury (ALI) is characterized by lung alveolar collapse, decreased compliance, and hypoxemia due to increased intrapulmonary shunt.1 Lung recruitment refers to the dynamic process of opening previously collapsed lung units by increasing transpulmonary pressure.2 Recruitment maneuvers (RMs) can be applied by increasing volume or pressure over time.3 RMs improve lung aeration as detected by magnetic resonance imaging (MRI) in anesthetized children with healthy lungs.4 There are several adult studies using computed tomography (CT)-scan to examine lung aeration changes during or following RMs in patients with ALI.5–9 However, there are no published data on the lung aeration changes during or after a recruitment maneuver (RM) in ventilated pediatric patients with ALI. The aims of this study were (a) to describe lung aeration changes after a RM in ventilated pediatric patients with ALI, and compare these changes with changes in gas exchange, and (b) to assess the safety of obtaining CT-scans in the acute phase of ALI.
We hypothesized that a RM would improve lung aeration in ventilated pediatric patients with ALI and obtaining CT-scans in the acute phase of ALI would be safe.
This case series was completed in a subset of six patients enrolled in our previously published study of efficacy and safety of lung recruitment in pediatric patients with ALI.3
MATERIALS AND METHODS
The Institutional Review Board at Children’s Hospital and Research Center Oakland (CHRCO) as well as the Committee on Human Research at the University of California San Francisco (UCSF) Medical Center approved the study. A data safety monitoring board (DSMB) was established to assure the continuing safety of research participants.
Study Design and Subjects
This case series was completed in a subset of six patients enrolled in our previously published study of efficacy and safety of lung recruitment in pediatric patients with ALI.3 The parent study assessed the safety and efficacy of a RM, the open lung tool (OLT), in pediatric patients with ALI. The OLT software available on the Servo-I ventilator (Servo-I, Maquet Critical Care, Solna, Sweden) was used to display real-time dynamic compliance (Cdyn) during the application of a RM.10 The original OLT algorithm was not designed to be used in children. Therefore, a modified OLT program was used during the application of our RM. Twenty-one ventilated pediatric patients with ALI underwent a RM using incremental positive end-expiratory pressure (PEEP). Main results from our prior study showed oxygenation, measured by the ratio of partial pressure of arterial oxygen over fraction of inspired oxygen (PaO2/FiO2 ratio), increased 53% immediately after the RM and persisted with an increase of 80% over the baseline at 4 hr and 40% at 12 hr after the RM. The RM was well tolerated except for significant increase in PaCO2 in three patients. There were no serious adverse events related to the RM.
All patients in the Pediatric Intensive Care Unit (PICU) at CHRCO were prospectively screened for the present study. Informed consent was obtained for all participants in the study. For feasibility in this pilot study, we decided to enroll only six patients—2 infants, 2 toddlers, and 2 teenagers—within 72 hr of meeting the American-European Consensus Conference criteria definitions of ALI.11 Patients were excluded if they had recent pulmonary resection surgery (<7 days), hemodynamic instability proihibiting transport out of the PICU, ETT air leak >25% of inspiratory tidal volume, pneumothorax -without a chest tube in place-, bronchopleural fistula, increased intracranial pressure (ICP > 20), severe head injury, acidosis (start arterial pH < 7.25), cyanotic congenital heart disease, clinician team deemed patient unacceptable candidate or family not willing to consent.
All patients were mechanically ventilated using the Servo-I ventilator (Servo-I, Maquet Critical Care, Solna, Sweden). We used a lung protective ventilation strategy to limit peak pressures to less than 35 cmH2O and tidal volume to 6–8 ml/kg of ideal body weight. PEEP was adjusted to keep an oxygen saturation of 88–93% on FiO2 less than 0.6. The RM used for this study was the same as that used for the parent investigation.3
Recruitment Maneuver
Patients were transported to the CT-scan room connected to the Servo-I ventilator and ventilator settings were not changed in an attempt to prevent derecruitment. The RM was performed in the CT-scan room by a respiratory therapist with attending and/or fellow investigator supervision. All patients were sedated with midazolam and muscle-relaxed with vecuronium prior to the RM. Vital signs were continuously monitored during the maneuver.
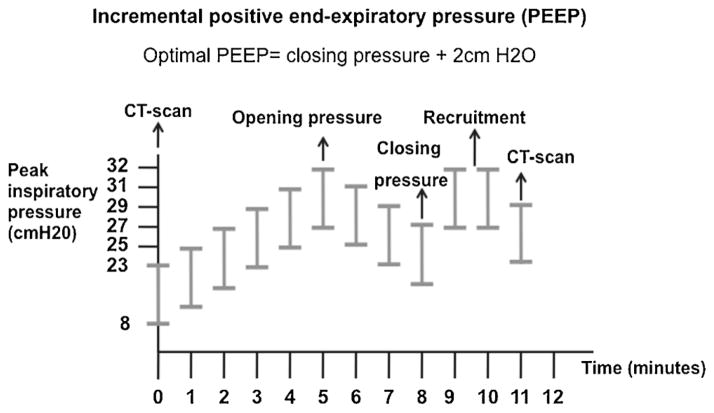
The RM was divided in two distinct parts (Fig. 1). The first part of the maneuver consisted of finding the critical opening pressure. Critical opening pressure was defined as the PEEP yielding highest Cdyn. The RM was performed in Assist-Control/Pressure Control mode. The pressure above PEEP was set at 15 cmH2O to assure a tidal volume of at least 4 ml/kg and FiO2 at 100%. Inspiratory/expiratory ratio was set at 1:1. Ventilator rate stayed unchanged if the patient’s respiratory rate before the maneuver was less than 30 breaths per minute (bpm) or ventilator rate set at 30 bpm if the patient’s respiratory rate before starting the maneuver was greater than 30 bpm. PEEP was set at 8 cmH2O and increased by 2 cmH2O every 1-min until a drop in Cdyn or peak pressure reached 45 cmH2O, whichever occurred first.
Fig. 1.
Study protocol.
The second part of the RM consisted of a decremental PEEP titration to find the critical closing pressure. We defined critical closing pressure as the PEEP yielding highest Cdyn during the decremental PEEP trial. We defined optimal PEEP as the critical closing pressure plus 2 cmH2O. Starting at critical opening pressure, we reduced PEEP by 2 cmH20 every 1-min until a drop in Cdyn was identified. After finishing the decremental PEEP titration, we re-recruited the lung for 2 min at opening pressure and adjusted the ventilator with the same parameters used at the beginning of the RM but setting optimal PEEP determined during the maneuver. One RM was completed per patient in all the study subjects. After the RM, FiO2 was adjusted to keep SpO2 88–93%. Arterial blood gases (ABG) were collected before and immediately after the RM.
As in the parent study, the RM was terminated immediately if the patient developed hypotension, hypoxemia, bradycardia, if the first ABG after patient placed on initial RM settings showed severe acidosis or if the end-tidal CO2 (ETCO2) increased significantly from the pre-maneuver value-increase in ETCO2 more than 20 torr if arterial pH 7.25–7.35, more than 30 torr if arterial pH 7.36–7.45, and more than 40 torr if art pH > 7.46. If the RM was stopped secondary to an unacceptably high increase in ETCO2, an ABG was drawn immediately to document partial pressure of arterial carbon dioxide (PaCO2).
Computed Tomography Scan
We used a Brilliance 40 CT-scanner (Brilliance 40, Phillips, Eindhoven, The Netherlands) with standard resolution/filter and 1.25 mm cuts. The matrix was 512 × 512 and exposures at 120 Kw and 20–100 mA, based on patient size. Radiation dose depended on the size of the patient and the CT-scan technique. No contrast material was used. In order to limit radiation exposure, patients underwent limited end-expiratory lung CT-scans—apex, hilum and bases—before and immediately after the RM. We decided to do end-expiratory slices—functional residual capacity—because some regions recruited during inspiration undergo collapse at the end of expiration.12 The first CT-scan was done upon transitioning to the initial RM settings on a PEEP of 8 cmH2O. The second CT-scan was done immediately after lung recruitment with “optimal PEEP” as determined by the decremental PEEP trial.
For CT-scan analysis, we arbitrarily defined two lung compartments: a nonaerated lung compartment between −100 and +100 Hounsefield Units (HU) and an aerated and poorly aerated lung compartment between −101 and −900 HU. A pediatric radiologist and one of the investigators (JB) independently read all CT-scans. Both investigators were not blinded to subject identity and temporal relationship of CT scans (pre- or post-RM). For each CT slice, the area (in square millimeters) of aerated and poorly lung was measured. The change (%) in aerated and poorly aerated lung for each pair (pre-and post-RM) of CT slices was calculated for each of the three different lung levels. Inter-rater reliability testing indicated very good correlation in measurements of aerated and poorly aerated lung regions between the two study investigators (r = 0.9; P ≤ 0.01) and no significant difference in measurement of aerated and poorly aerated lung regions between the two study investigators (P ≥ 0.05).
Statistical Analysis
Descriptive statistics were computed for each of the variables at pre- and post-RM. Prior to combining the measurements of the two study investigators, we ran the Wilcoxon test for matched data to compare CT-scan aerated and poorly aerated lung measurements. We further computed the change from pre- to post-RM for each variable. A significance level of 0.05 was used for all statistical tests. Data were analyzed using SAS version 9.2 (SAS Institute, Cary, NC, 1999).
RESULTS
Six patients were entered in the study between December 2007 and March 2009. The RM was performed once in each patient and within 72 hr of meeting ALI criteria.
Patient demographics and clinical characteristics are shown in Table 1. Three patients had pulmonary ALI and three patients had extrapulmonary ALI.
TABLE 1.
Characteristics of Study Subjects
| Subject | Sex/age (years) |
Race/ Ethnicity |
Diagnosis | PRISM III | A/PA lung regions Δ% (Pre, Post in mm2) |
PIP Δ% (Pre, Post in cmH2O) |
PaO2/FiO2 Δ% (Pre, Post) |
OI Δ% (Pre, Post) |
PaCO2 Δ% (Pre, Post in mmHg) |
Ventilator-free days |
Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/0.5 | NA/L | Pneumonia | 3 | 9 (5944, 6480) | −12 (36, 32) | 72 (103, 177) | −26 (15, 11) | −5 (79, 75) | 19 | S |
| 2 | F/0.1 | NA/L | Cardiopulmonary bypass surgery | 4 | 6 (4208, 4465) | −15 (27, 23) | 14 (58, 66) | −12 (28, 24) | 2 (47, 48) | 22 | S |
| 3 | F/15 | W/non-L | Septic shock | 15 | 31 (7765, 10172) | −18 (40, 33) | 99 (98, 195) | −39 (19, 12) | −9 (55, 50) | 12 | S |
| 4 | M/1.2 | NA/L | Septic shock | 15 | 72 (6578, 11343) | −20 (36, 29) | 8 (304, 327) | 30 (5, 6) | −9 (35, 32) | 23 | S |
| 5 | M/14 | W/non-L | Pneumonia | 11 | 47 (7227, 10629) | 6 (31, 33) | 7 (90, 96) | 17 (18, 21) | −6 (53, 50) | 0 | D |
| 6 | M/2.5 | NA/L | Near-drowning | 42 | 3 (4886, 4968) | 13 (41, 36) | 15 (65, 75) | −3 (25, 24) | 41 (70, 99) | 17 | S |
Sex: male (M), female (F); Race/Ethnicity: native American (NA); latino (L), non-latino (non-L); white (W); PRISM III: pediatric risk of mortality III; A/PA lung regions: aerated and poorly aerated lung regions; PIP: peak inspiratory pressure; PaO2/FiO2: ratio of partial pressure of arterial oxygen over fraction of inspired oxygen; OI: oxygenation index; PaCO2: partial pressure of arterial carbon dioxide; Δ%: Post-RM − Pre-RM/Pre-RM; (pre, post): pre-recruitment maneuver, post-recruitment maneuver; Outcome at intensive care unit discharge: death (D), survive (S).
Effect of Lung Recruitment on Lung Aeration
There was variable increase in aerated and poorly aerated lung after the RM ranging from 3% to 72% (Table 1; Figs. 2–4; median 20%; interquartile range 6, 47; P = 0.03). Improved lung aeration was accompanied by a decrease in peak inspiratory pressure after the RM (median −14%; interquartile range −18, −12; P =0.06).
Fig. 2.
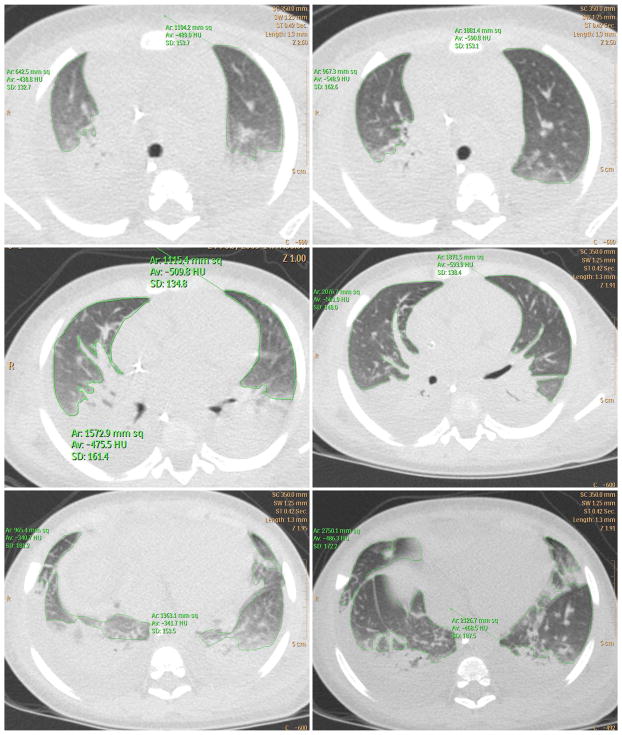
Computed tomography scan of subjects 1, 2, 3, and 5 shows variable increase in lung aeration following lung recruitment. The region of aerated and poorly aerated lung has been marked by tracing a continuous green line. Pre-RM, pre-recruitment maneuver; post-RM, post-recruitment maneuver; upper row, apical section; middle row, hilum section; lower row, basal section. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/ppul]
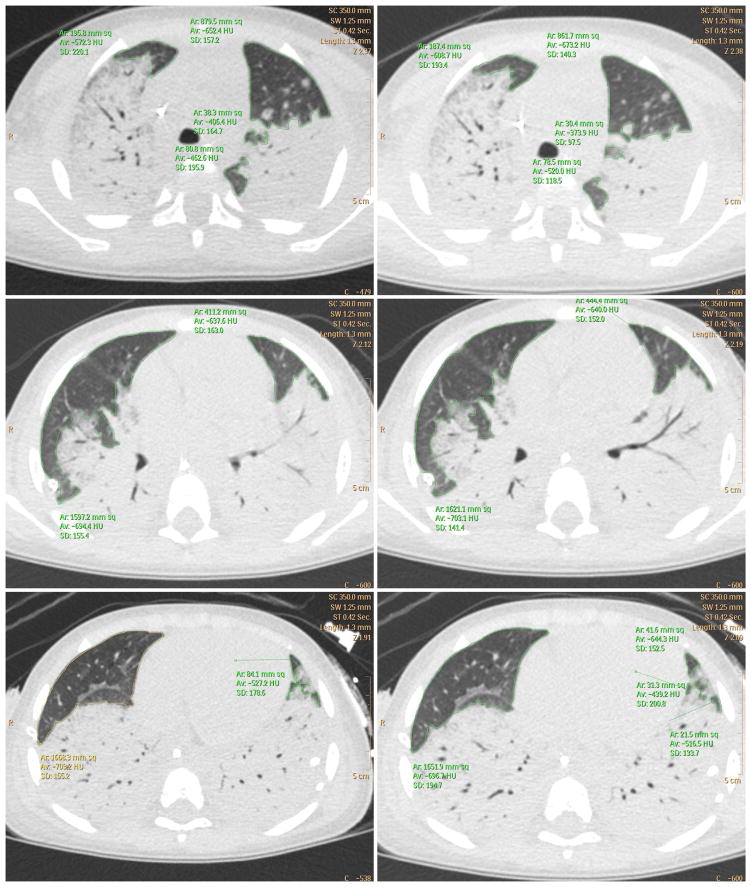
Fig. 4.
Computed tomography scan of subject 4 shows highest (72%) increase in lung aeration following lung recruitment. The region of aerated and poorly aerated lung has been marked by tracing a continuous green line. Left column: pre-recruitment maneuver. Right column post-recruitment maneuver. Upper row: apical section. Middle row: hilum section. Lower row: basal section. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/ppul]
Effect of Lung Recruitment on Oxygenation
As in the parent study, all patients had improvement in the PaO2/FiO2 ratio after the RM (Table 1; median 14%; interquartile range 8, 72; P = 0.03). The smallest improvement was 7% and the greatest improvement 99%. Oxygenation improvement was also reflected by a decrease in the oxygenation index (OI) after the RM (median −9%; interquartile range −27, 17; P = 0.5).
Effect of Lung Recruitment on Ventilation
There was a decrease in PaCO2 in four of six subjects after the RM (Table 1; median −5%; interquartile range −9, 2; P = 0.5). One subject had transient hypercapnia (41% increase in PaCO2) during the RM and this correlated with the smallest (3%) increase in lung aeration following lung recruitment (Fig. 3).
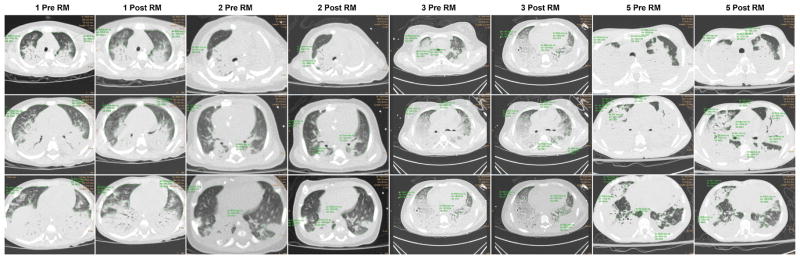
Fig. 3.
Computed tomography scan of subject 6 shows minimal (3%) increase in lung aeration following lung recruitment. The region of aerated and poorly aerated lung has been marked by tracing a continuous green line. Left column: pre-recruitment maneuver. Right column: post-recruitment maneuver. Upper row: apical section. Middle row: hilum section. Lower row: basal section. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/ppul]
Safety
All patients tolerated the procedure of traveling to and from the CT-scanner while remained connected to the Servo-I ventilator, getting on and off CT-scan gurney as well as having the lung CT-scan both before and after the RM. As in the parent study,3 the RM itself was well tolerated without any instances of hemodynamic compromise (hypotension and/or bradycardia), barotrauma, hypoxemia, or arrhythmias. There was one case of transient hypercapnia that occurred during the RM that was entirely resolved by the end of the maneuver. This subject completed the RM.
DISCUSSION
Our previously published study showed that the modified OLT RM might safely improve oxygenation and ventilation, with these benefits potentially lasting up to 12 hr, in pediatric patients with ALI. It was unclear from this study if the oxygenation improvement post-RM was due to lung recruitment and/or other factors such as cardiac output or blood flow redistribution within the injured lung. In this feasibility study, lung recruitment using the modified OLT RM resulted in improved lung aeration as detected by lung tomography. This was accompanied by improvements in oxygenation and ventilation. Transporting patients in early ALI to the CT-scanner was safe and feasible.
Recruitment maneuvers improve lung aeration as detected by magnetic resonance imaging in anesthetized children with healthy lungs.4 Despite the small number of patients, our data are similar to other adult studies that have shown that RMs improve oxygenation by recruiting collapsed alveoli in ALI patients.5 We were able to increase lung aeration in all subjects, although the percentage increase in lung aeration varied widely. Gattinoni et al., also found a widely variable percentage increase in lung aeration in adult patients with ALI.6
In ALI patients, PEEP-induced alveolar recruitment can be associated with some degree of lung hyperinflation.9,13 Recruitment of collapsed alveoli should improve oxygenation and ventilation. When recruitment is the explanation for the increased oxygenation, carbon dioxide exchange is not compromised and may even improve, reflecting increased alveolar ventilation.14 In this pilot study, all subjects improved oxygenation, as measured by the PaO2/FiO2 ratio, however not all subjects improved ventilation (as measured by PaCO2). In four of six subjects ventilation and oxygenation improved with increase in lung aeration. Accordingly, the increase in lung aeration in these subjects most likely represents alveolar recruitment. In the other two subjects, the oxygenation improvement may be partially accounted for by redirected blood flow within the injured lung.14 It is quite possible that part of the increase in lung aeration in these two subjects could reflect some degree of alveolar hyperinflation. Unfortunately, our study was limited to a qualitative assessment of lung inflation.
Serious hemodynamic and barotrauma effects from RM are rare in adult patients with ALI.15 Duff et al., reported 14% RMs had to be stopped secondary to agitation or bradycardia in ventilated PICU patients.16 The authors reported no barotrauma or significant hemodynamic effects from RMs. In our study all patients tolerated the RM without hemodynamic compromise, barotrauma, hypoxemia, or dysrhythmias. Our patients were sedated and muscle relaxed before the maneuver, which may have contributed to better tolerance.
This study was subject to several limitations. The small sample size and highly heterogeneous subject population of this single-center feasibility study, limit the generalizability of the results.
The two investigators in charge of reading the study CT-scans were not blinded to subject identity and temporal relationship of CT scans (pre- or post-RM). The CHRCO IRB specifically requested we limit radiation exposure of pediatric subjects. In an effort to limit radiation exposure, the attending radiologist (RC), specifically created an imaging algorithm for the study. This research algorithm consisted of limited tomograms (three slices per CT-scan, total of six slices per patient) to avoid unacceptable radiation exposure of pediatric subjects. Because the research algorithm differed from our routine whole lung clinical algorithm the attending radiologist co-investigator (RC) implemented the three-tomogram algorithm for each patient directly. For safety purposes, the primary investigator of the project (JB) escorted the study patients and administered the modified RM while the patient was in the CT scanner himself. Therefore, in an effort to decrease potential bias introduced by the study investigators being unblinded, the investigators each analyzed the data independently, at separate times, in separate locations and not in consultation with each other. Further the two investigators evaluating the CT scans represented two different pediatric subspecialties.
We did not quantify lung hyperinflation and were not able to differentiate normally aerated from poorly aerated lung regions because of software limitations. The software we used to analyze lung CT compartments does not distinguish voxel aeration. Two basic approaches are available to quantify lung compartments. In the first, the density area, as it is seen morphologically (i.e., consolidation or ground glass), is estimated (visually or electronically) as a percentage of the total lung area studied. The second, which requires additional, specialized software, is based on the CT number frequency distribution of the lung in which a normally aerated voxel may be distinguished from a hyperinflated, a poorly aerated, and a nonaerated voxel.17 The software available to us dictated that we use the first approach for this study; therefore, we were not able to differentiate voxel aeration. Using this method, contiguous areas of hyperinflated and collapsed alveoli may morphologically look like poorly aerated or normally aerated alveoli; in this situation, estimation of lung aeration may be inaccurate.
Two basic CT-scan protocols are described in the literature. The more traditional approach, representative sampling, uses one to three axial images to infer the whole lung behavior. The second protocol uses whole lung CT-scan. The limits of representative sampling are greater the more inhomogeneous the lung impairment. Another limitation is the impossibility to scan exactly the same anatomical structures in different ventilator settings. CT-scanning the whole lung is ideal but exposes the pediatric subject to an unacceptable amount of radiation, particularly in a research setting where participation is voluntary. If CT-scan is to be more widely applied to the ALI patients, clinicians and researchers must gain consensus on whether or not to use the whole lung or segmental tomogram lung CT protocol.
CT-scan is not part of routine imaging in ALI and is best reserved for solving clinical dilemmas.17 Adult studies show that CT yields additional information in 66% of patients with ALI and has direct influence on treatment in 22% of patients.18 Adult data strongly suggest that despite the difficulties of transporting critically ill patients to the CT room, the rate of detection of unsuspected thoracic changes fully justifies the use of CT-scan in ALI patients when the bedside X-ray examination does not explain the clinical findings.12 Whereas the change in respiratory indices before and after the RM did alter clinical management of the patients enrolled in this study, the information obtained from the limited lung CT-scans did not influence treatment in this pilot study. Further studies are needed to evaluate the therapeutic implications of routine CT-scan in pediatric ALI.
Imaging procedures are an important source of exposure to ionizing radiation in the United States and can result in high cumulative effective doses of radiation.19 The risk of cancer attributable to a given dose of radiation from CT studies is greater in children than adults, both because they are inherently more radiosensitive and because they have more remaining years of life during which a radiation-induced cancer could develop.20 The typical effective radiation dose from a chest CT (7 mSv) is equivalent to 350 posterior-anterior chest radiographs.21 By using a low-dose, three CT slice sampling protocol as opposed to CT slices covering the whole chest; we were able to decrease radiation exposure by a factor of 70. In fact, subjects in our study were exposed to an average amount of 0.19 mSy of effective radiation (0.10 mSv). This radiation amount is equivalent to 5 posterior–anterior chest radiographs or 12 days of exposure to natural background radiation. The future of lung imaging might be modalities that do not expose the patient to radiation such as MRI or electrical impedance tomography (EIT). MRI is a radiation-free noninvasive technique that has been successfully used to detect atelectasis in anesthetized children.4 However, current MRI technology does not allow for continuous monitoring at the bedside. EIT is a noninvasive, radiation-free real-time bedside imaging method that has shown good correlation with CT-scan for estimating recruitable alveolar collapse.22
In Conclusions, the modified lung RM used in this study may improve lung aeration as well as oxygenation in pediatric patients with early ALI. In four of six subjects ventilation and oxygenation improved with increase in lung aeration. Assessing lung aeration using CT-scan in early ALI seems safe but does not alter management plans and exposes the patient to radiation whose cumulative effect may increase the risk of cancer at later stages in life.
Implementation on a wider scale, quantitative assessment of lung hyperinflation, evaluation of repeated RMs, and evaluation of the RM in later phase ALI are required to further validate our results.
Acknowledgments
We thank the staff at Oakland Children’s Hospital and Research Center Oakland for their participation in this project. Supported by an institutional departmental fund from Charlotte Coleman Frey Foundation and the Children’s Hospital Research Center Oakland Clinical Translational Science Institute. This publication was made possible by grant number UL1RR024131-01 from the National Center for Research Resources.
Funding source: Charlotte Coleman Frey Foundation fund and National Center for Research Resources grant UL1RR024131-01.
Footnotes
Conflict of interest: None.
The present work was performed at the Pediatric Critical Care Department, Children’s Hospital and Research Center Oakland, Oakland, California.
References
- 1.Piantadosi CA, Schwartz DA. The acute respiratory distress syndrome. Ann Intern Med. 2004;141:460–470. doi: 10.7326/0003-4819-141-6-200409210-00012. [DOI] [PubMed] [Google Scholar]
- 2.Papadakos PJ, Lachmann B. The open lung concept of mechanical ventilation: the role of recruitment and stabilization. Crit Care Clin. 2007;23:241–250. ix–x. doi: 10.1016/j.ccc.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Boriosi JP, Sapru A, Hanson JH, Asselin J, Gildengorin G, Newman V, Sabato K, Flori HR. Efficacy and safety of lung recruitment in pediatric patients with acute lung injury. Pediatr Crit Care Med. 2011;12:431–436. doi: 10.1097/PCC.0b013e3181fe329d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tusman G, Bohm SH, Tempra A, Melkun F, Garcia E, Turchetto E, Mulder PG, Lachmann B. Effects of recruitment maneuver on atelectasis in anesthetized children. Anesthesiology. 2003;98:14–22. doi: 10.1097/00000542-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Bugedo G, Bruhn A, Hernandez G, Rojas G, Varela C, Tapia JC, Castillo L. Lung computed tomography during a lung recruitment maneuver in patients with acute lung injury. Intensive Care Med. 2003;29:218–225. doi: 10.1007/s00134-002-1618-6. [DOI] [PubMed] [Google Scholar]
- 6.Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354:1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 7.Puybasset L, Cluzel P, Gusman P, Grenier P, Preteux F, Rouby JJ. Regional distribution of gas and tissue in acute respiratory distress syndrome. I. Consequences for lung morphology. CT Scan ARDS Study Group. Intensive Care Med. 2000;26:857–869. doi: 10.1007/s001340051274. [DOI] [PubMed] [Google Scholar]
- 8.Malbouisson LM, Muller JC, Constantin JM, Lu Q, Puybasset L, Rouby JJ. Computed tomography assessment of positive end-expiratory pressure-induced alveolar recruitment in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1444–1450. doi: 10.1164/ajrccm.163.6.2005001. [DOI] [PubMed] [Google Scholar]
- 9.Vieira S, Puybasset L, Richecoeur J, Qin L, Cluzel P, Gusman P, Coriat P, Rouby JJ. A lung computed tomographic assessment of positive end-expiratory pressure-induced lung overdistention. Am J Respir Crit Care Med. 1998;158:1571–1577. doi: 10.1164/ajrccm.158.5.9802101. [DOI] [PubMed] [Google Scholar]
- 10.Maquet Critical Care AB. SE-171 95 SOLNA S. Lung recruitment pocket guide. 2005:1–19. [Google Scholar]
- 11.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R. Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee Intensive Care Med. 1994;20:225–232. doi: 10.1007/BF01704707. [DOI] [PubMed] [Google Scholar]
- 12.Gattinoni L, Chiumello D, Cressoni M, Valenza F. Pulmonary computed tomography and adult respiratory distress syndrome. Swiss Med Wkly. 2005;135:169–174. doi: 10.4414/smw.2005.10936. [DOI] [PubMed] [Google Scholar]
- 13.Dambrosio M, Roupie E, Mollet JJ, Anglade MC, Vasile N, Lemaire F, Brochard L. Effects of positive end-expiratory pressure and different tidal volumes on alveolar recruitment and hyperinflation. Anesthesiology. 1997;87:495–503. doi: 10.1097/00000542-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Marini JJ, Gattinoni L. Ventilator management of acute respiratory distress syndrome: A consensus of two. Crit Care Med. 2004;32:250–255. doi: 10.1097/01.CCM.0000104946.66723.A8. [DOI] [PubMed] [Google Scholar]
- 15.Fan E, Wilcox ME, Brower RG, Stewart TE, Mehta S, Lapinsky SE, Meade MO, Ferguson ND. Recruitment maneuvers for acute lung injury: a systematic review. Am J Respir Crit Care Med. 2008;178:1156–1163. doi: 10.1164/rccm.200802-335OC. [DOI] [PubMed] [Google Scholar]
- 16.Duff JP, Rosychuk RJ, Joffe AR. The safety and efficacy of sustained inflations as a lung recruitment maneuver in pediatric intensive care unit patients. Intensive Care Med. 2007;33:1778–1786. doi: 10.1007/s00134-007-0764-2. [DOI] [PubMed] [Google Scholar]
- 17.Gattinoni L, Caironi P, Pelosi P, Goodman LR. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med. 2001;164:1701–1711. doi: 10.1164/ajrccm.164.9.2103121. [DOI] [PubMed] [Google Scholar]
- 18.Tagliabue M, Casella TC, Zincone GE, Fumagalli R, Salvini E. CT and chest radiography in the evaluation of adult respiratory distress syndrome. Acta Radiol. 1994;35:230–234. [PubMed] [Google Scholar]
- 19.Fazel R, Krumholz HM, Wang Y, Ross JS, Chen J, Ting HH, Shah ND, Nasir K, Einstein AJ, Nallamothu BK. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361:849–857. doi: 10.1056/NEJMoa0901249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenner DJ, Hall EJ. Computed tomography – an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 21.Mettler FA, Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248:254–263. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 22.Costa EL, Borges JB, Melo A, Suarez-Sipmann F, Toufen C, Jr, Bohm SH, Amato MB. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensive Care Med. 2009;35:1132–1137. doi: 10.1007/s00134-009-1447-y. [DOI] [PubMed] [Google Scholar]