Abstract
Pulmonary infections in critically ill patients are common and are associated with high morbidity and mortality. Piperacillin–tazobactam is a frequently used therapy in critically ill patients with pulmonary infection. Antibiotic concentrations in the lung reflect target‐site antibiotic concentrations in patients with pneumonia. The aim of this study was to assess the plasma and intrapulmonary pharmacokinetics (PK) of piperacillin–tazobactam in critically ill patients administered standard piperacillin–tazobactam regimens. A population PK model was developed to describe plasma and intrapulmonary piperacillin and tazobactam concentrations. The probability of piperacillin exposures reaching pharmacodynamic end points and the impact of pulmonary permeability on piperacillin and tazobactam pulmonary penetration was explored. The median piperacillin and tazobactam pulmonary penetration ratios were 49.3 and 121.2%, respectively. Pulmonary piperacillin and tazobactam concentrations were unpredictable and negatively correlated with pulmonary permeability. Current piperacillin–tazobactam regimens may be insufficient to treat pneumonia caused by piperacillin–tazobactam–susceptible organisms in some critically ill patients.
Clinical Pharmacology & Therapeutics (2014); 96 4, 438–448. doi:10.1038/clpt.2014.131
Pulmonary infection in critically ill patients results in an unacceptably high mortality and morbidity, which increases the length of hospital stay and associated health‐care costs. 1 , 2 Approximately 16% of patients admitted to intensive care units present with a pulmonary infection. 3 In addition, the lung is the primary site of infection in more than 60% of nosocomial infections occurring within intensive care units. 3 Attributable mortality from ventilator‐associated pneumonia (VAP) is estimated to be 13% but may be as high as 69% in certain subgroups. 4 Pulmonary infections in critically ill patients are caused by a wide range of organisms, including difficult‐to‐treat organisms such as Pseudomonas aeruginosa. 5 The use of appropriately targeted antimicrobial chemotherapy is associated with improved clinical outcomes. 6 However, clinical outcomes in patients who are infected with a susceptible organism and who are receiving an appropriate antimicrobial agent remain suboptimal. This is partly due to marked pharmacokinetic (PK) variability occurring in critically ill patients. 7 The PK of critically ill patients may be affected by physiological changes associated with illness, which typically result in a higher proportion of patients receiving suboptimal drug exposure when a fixed regimen is used. 7 , 8 , 9 In addition, many currently licensed drug regimens are informed by studies performed in non–critically ill patients, and may not necessarily be appropriate outside that context.
Piperacillin–tazobactam is a combination of an extended‐spectrum β‐lactam antibiotic (piperacillin) and a β‐lactamase inhibitor (tazobactam). Piperacillin–tazobactam has a broad spectrum of action that includes Gram‐positive, Gram‐negative, and anaerobic bacteria. 10 Consequently, piperacillin–tazobactam is a common choice for both directed and empirical treatment of critically ill patients. 11 The pharmacodynamic index that best links piperacillin concentrations with its antimicrobial effect is the fraction of the dosing interval that unbound piperacillin concentrations are above the minimum inhibitory concentration (MIC). 12 Near‐maximal antimicrobial effect is generally observed when free piperacillin concentrations exceed the MIC for at least 50% of the dosing interval (50% fT<MIC). 13 However, 100% fT<MIC may be more appropriate for critically ill patients. 14 The global increase in the incidence of antimicrobial resistance has focused attention on antimicrobial drug regimens that are safe and effective, and that also minimize the probability of the emergence of antimicrobial resistance. 15 We recently used a hollow fiber infection model of piperacillin–tazobactam vs. Pseudomonas aeruginosa to demonstrate that a trough (C min) total piperacillin concentration–to‐MIC ratio of between 3 and 10 prevents the emergence of antimicrobial resistance. 16 Identification of piperacillin–tazobactam regimens that enable the attainment of pharmacodynamic targets for both efficacy and suppression of emergence of antimicrobial resistance may lead to improved clinical outcomes and increase the clinical longevity of this commonly used agent.
Adequate antibiotic concentrations at the site of infection are required for effective antimicrobial activity. 17 For pulmonary infection, the epithelial lining fluid (ELF) represents a compartment that is both clinically relevant and accessible for measurement of drug concentrations. 18 , 19 In general, clinical β–lactam exposure–response relationships within ELF are poorly defined. 18 An understanding of drug penetration into ELF and drug exposure–response relationships within that compartment is an important consideration when bridging from preclinical to clinical studies. 20 The pulmonary penetration ratio, or partition coefficient, relates drug exposure in ELF to drug exposure in plasma. Healthy volunteer data suggest that the area under the concentration–time curve (AUC) in ELF is ≈25% and ≈50% of the plasma piperacillin and tazobactam AUCs, respectively. 21 Although there is a general paucity of information regarding ELF penetration of antimicrobial agents in critically ill patients, two studies suggest that piperacillin and tazobactam ELF concentrations are ≈50% and 65–90% of their respective paired plasma concentrations. 22 , 23
The primary aim of this clinical study was to quantify the pulmonary penetration of piperacillin and tazobactam in critically ill patients. We also investigated factors that may influence the penetration of drug into the lung. A PK pharmacokinetic model was used to describe the observed plasma and ELF concentrations of piperacillin and tazobactam in critically ill patients ( Supplementary Table S1 online). Monte Carlo simulation was used to explore the impact of pharmacokinetic variability on plasma and ELF piperacillin exposures to achieve the desired pharmacodynamic target. In addition, the influence of pulmonary permeability on pulmonary piperacillin and tazobactam concentrations was investigated.
RESULTS
Pharmacokinetic study
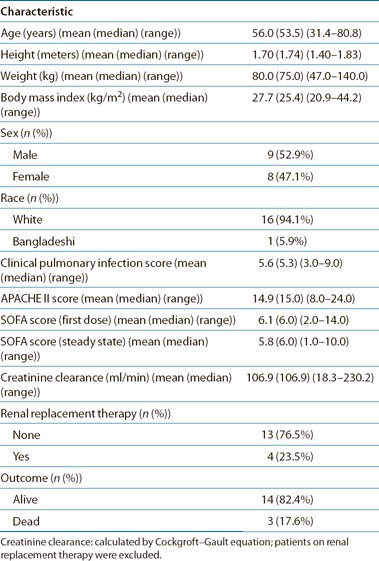
Between June 2012 and July 2013, 18 critically ill patients with a mean age of 56 years and a mean APACHE II score of 15 were enrolled ( Table 1 ). One patient who was infected with a novel coronavirus was excluded from all analyses because of issues related to biosafety. First–dose pharmacokinetics (PK) were assessed in four patients. Steady–state PK were assessed in 17 patients who had received a mean of 8.8 doses (range: 2–16). Four patients were on renal replacement therapy and received piperacillin–tazobactam every 12 h. The remaining 13 patients received piperacillin–tazobactam every 8 h. Five patients received piperacillin–tazobactam over 5 min, whereas the remaining 12 patients received piperacillin–tazobactam over 30 min. In total, 128 plasma and 31 ELF samples were obtained for pharmacokinetic analyses. Three piperacillin plasma samples, 3 piperacillin ELF samples, and 14 tazobactam plasma samples were below the limit of assay quantification.
Table 1.
Table showing the patients' underlying demographics, severity of disease, and outcome
Three nondirected bronchial lavage (NBL) specimens (two from a single patient) were not collected because of a clinical requirement for a high fraction of inspired oxygen that precluded sampling. A single patient had a drop in oxygen saturation from 95 to 88% that required a temporary increase in the fraction of inspired oxygen. A change in oxygen saturation was not observed in any other patient. No other changes in respiratory or cardiovascular parameters were observed in the 4 h after collection of the other NBL samples.
Population PK analysis
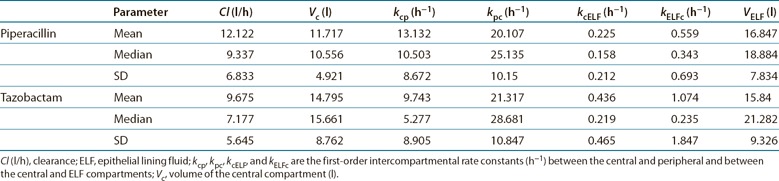
The fit of the mathematical model to the observed data was acceptable. A linear regression of the predicted‐vs.‐observed plasma piperacillin and tazobactam concentrations revealed the following relationship: observed piperacillin concentration = 0.884 × predicted piperacillin concentration + 2.01; r 2 = 0.901. Similarly, the observed tazobactam concentration = 0.880 × predicted tazobactam concentration + 0.165; r 2 = 0.839. A linear regression of the predicted and observed piperacillin and tazobactam concentrations in the ELF was given by observed piperacillin concentration = 0.790 × predicted piperacillin concentration − 1.65; r 2 = 0.812; and observed tazobactam concentration = 0.827 × predicted tazobactam concentration + 1.21; r 2 = 0.878. For plasma piperacillin and tazobactam concentrations, the mean weighted biases were −0.00999 and 0.0214, respectively, and the bias‐adjusted mean weighted precision values were 25.5 and 1.22, respectively. For piperacillin and tazobactam concentrations in ELF, the mean weighted biases were −0.057 and 0.169, and the bias‐adjusted mean weighted precision values were 0.124 and 7.23, respectively. The parameter estimates from the population analysis are summarized in Table 2 . The intercompartmental piperacillin clearances between the central and peripheral and central and ELF compartments were 153.87 and 2.64 l/h, respectively. The intercompartmental tazobactam clearances between the central and peripheral and central and ELF compartments were 144.15 and 6.45 l/h, respectively. The volumes of the peripheral compartments were 7.65 and 6.78 l for piperacillin and tazobactam, respectively.
Table 2.
Piperacillin and tazobactam population pharmacokinetic parameter estimates obtained by Pmetrics
External validation of the population PK analysis
A plot of observed piperacillin and tazobactam concentrations from a previously published study overlaid by the predicted 5th, 25th, 50th, 75th, and 95th percentile drug concentrations from this study (simulated using the population PK model) revealed a high degree of concordance ( Figure 1 ). 22
Figure 1.
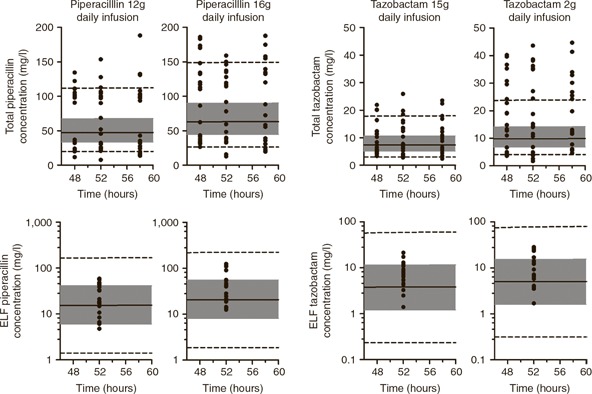
External validation of the piperacillin and tazobactam population model. The panels in the top row show total plasma drug concentrations, and the lower panels show ELF drug concentrations. Each panel shows the median drug concentration (solid black line), the interquartile range (shaded gray area), and the 5th and 95th percentiles (dotted black lines). Overlying data points represent observed data from Boselli et al. 22 ELF, epithelial lining fluid.
Plasma piperacillin/tazobactam concentration and pulmonary penetration
Simulated concentration–time profiles, showing the median and 5th, 25th, 75th, and 95th percentile drug concentrations in both plasma and ELF after administration of five simulated doses of 4 g of piperacillin and 0.5 g of tazobactam, each as a 30–min infusion every 8 h, are shown in Figure 2 . The median AUCELF/AUCunbound plasma penetration ratio was 49.3% (range: 2.0–515.9%) for piperacillin and 121.2% (range: 11.0–391.3%) for tazobactam.
Figure 2.
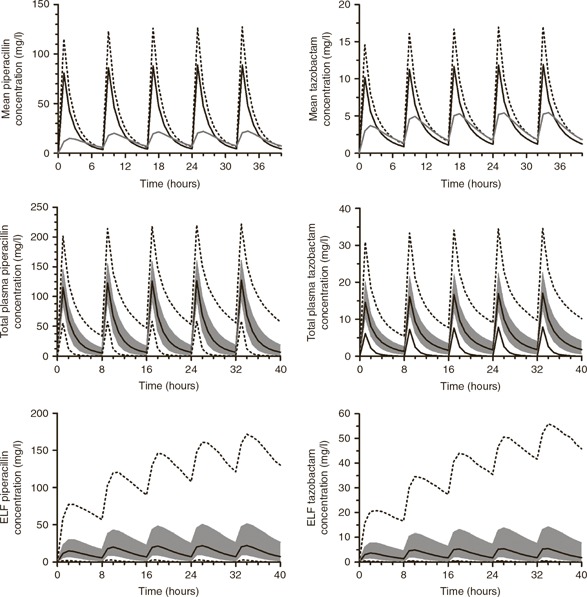
concentration–time profiles for piperacillin (left) and tazobactam (right). The top panel compares total drug concentration (dotted line), unbound drug concentration (solid black line), and ELF drug concentration (solid gray line). The middle panels show unbound plasma concentration, and the lower two panels show ELF concentrations with median drug concentration (solid black line), the interquartile range (shaded gray area), and the 5th and 95th percentiles (dotted black lines). ELF, epithelial lining fluid.
Simulated plasma and ELF exposures for each individual patient (using the Bayesian posterior parameter estimates) enabled an assessment of the drug penetration from plasma to ELF and of the interrelationship between the two coadministered drugs. There was no statistically significant correlation between ELF piperacillin exposure (AUCELF) and unbound plasma piperacillin exposure (AUCunbound plasma) (r = 0.369, P = 0.159; Figure 3 ). Similarly, there was no statistically significant correlation between ELF tazobactam exposure (AUCELF) and unbound plasma tazobactam exposure (AUCunbound plasma) (r = 0.306, P = 0.248; Figure 3 ). Unbound tazobactam exposure in the plasma of critically ill patients was statistically significantly positively correlated with unbound piperacillin plasma exposures (AUCunbound plasma) (r = 0.864; P < 0.0001; Figure 4 ). There was also a statistically significant positive correlation between tazobactam and piperacillin ELF exposures (AUCELF) (r = 0.604; P = 0.013; Figure 4 ).
Figure 3.
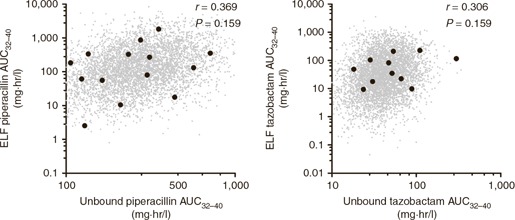
Relationship between unbound plasma and ELF drug concentrations for piperacillin (left) and tazobactam (right) for each of the observed trial patients (black dots) and for 5,000 simulated patients (small gray dots). AUC, area under the concentration–time curve; ELF, epithelial lining fluid.
Figure 4.
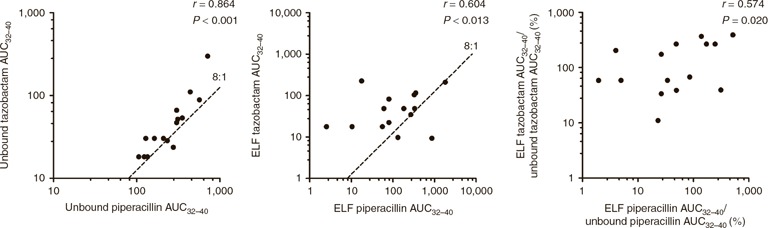
Comparison of the piperacillin and tazobactam exposures in the plasma (left) and ELF (center), as well as the plasma: ELF ratio (right). The dotted lines illustrate the 8:1 ratio of piperacillin to tazobactam in the administered piperacillin 4.0 g/tazobactam 0.5 g preparation. AUC, area under the concentration–time curve; ELF, epithelial lining fluid.
Mean pulmonary permeability, as estimated by the ratio of urea‐corrected total protein in ELF to plasma total protein concentration, was 0.1226 (median = 0.0795; SD = 0.1155). A statistically significant negative correlation was observed between the piperacillin penetration ratio (AUCELF/AUCunbound plasma) and pulmonary permeability (r = −0.593; P = 0.016; Figure 5 ). By contrast, no statistically significant correlation was seen between the tazobactam penetration ratio (AUCELF/AUCunbound plasma) and pulmonary permeability (r = −0.064; P = 0.815; Figure 5 ).
Figure 5.
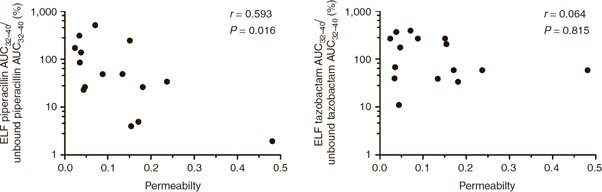
Comparison of the plasma: ELF ratio for piperacillin (left) and tazobactam (right) exposure with pulmonary permeability. AUC, area under the concentration–time curve; ELF, epithelial lining fluid.
Probability of target attainment analysis
Monte Carlo simulation was used to estimate the probability of achieving predefined pharmacodynamic targets. The results of the probability of target attainment analysis for piperacillin are shown in Figure 6 . The administration of 4 g piperacillin three times daily, as a 30‐min infusion to treat an organism with a MIC of 1 mg/l, resulted in 96, 77, and 64% of patients achieving a pharmacodynamic target of 50% fT>MIC, 100% fT>MIC, and C min/MIC > 3.4, respectively. The treatment of an organism with a MIC of 16 mg/l (i.e., the current recommended breakpoint for Pseudomonas aeruginosa) 24 , 25 resulted in 54, 20, and 6% patients achieving a pharmacodynamic target of 50% fT>MIC, 100% fT>MIC, and C min/MIC > 3.4, respectively.
Figure 6.
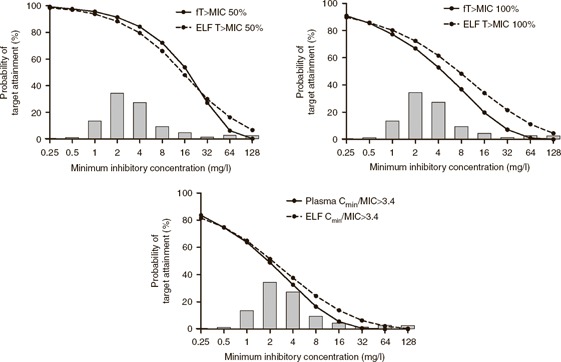
Results of the Monte Carlo simulation with the probability of target attainments, for unbound (solid line) and ELF (dashed line) piperacillin, against a range of MICs. The pharmacodynamic targets are the fraction of patients whose drug concentration was about the MIC for 50% (left panel) or 100% (middle panel) of the dosing interval and the fraction of patients whose trough piperacillin concentration to MIC ratio was ≥3.4. Histogram shows MIC distribution for organisms causing hospital‐acquired and ventilator‐associated pneumonia. 26 ELF, epithelial lining fluid; MIC, minimum inhibitory concentration.
The predicted target attainments in plasma and ELF, for each MIC, were similar. For example, the target attainment rates using an end point of unbound piperacillin concentrations that were 50% fT>MIC were 96 and 54% for MICs of 1 and 16 mg/l, respectively. In comparison, the use of the same pharmacodynamic target in ELF (i.e., 50% T>MIC) resulted in target attainment rates of 94 and 48% for MICs of 1 and 16 mg/l, respectively. For the most susceptible organisms (i.e., having MICs in the range 0.25–1 mg/l), both the unbound plasma and ELF concentrations were above the MIC for 50% of the dosing interval in >90% of simulated patients.
From the frequency distribution of piperacillin–tazobactam susceptibilities of isolates causing hospital‐acquired and VAP, the overall response rate of critically ill patients with VAP can be estimated ( Figure 6 ). 26 If piperacillin were administered empirically (i.e., the MIC is not known), 80% of critically ill patients with VAP would achieve plasma 50% fT>MIC, and 77% of patients would achieve ELF 50% T>MIC. By contrast, if piperacillin were administered in critically ill patients with VAP caused by a susceptible organism (i.e., MIC ≤ 16 mg/l), 86% of patients would achieve plasma 50% fT>MIC, and 82% of patients would achieve ELF 50% T>MIC. For suppression of emergence of antimicrobial resistance following empirical administration of piperacillin, 38% of critically ill patients with VAP would achieve plasma C min/MIC > 3.4, and 41% of patients would achieve ELF C min/MIC > 3.4. If piperacillin were administered to critically ill patients with VAP caused by a susceptible organism, 42% of patients would achieve plasma C min/MIC > 3.4, and 45% of patients would achieve ELF C min/MIC > 3.4.
DISCUSSION
Inspection of the concentration–time profiles for both piperacillin and tazobactam illustrates marked PK variability in critically ill patients ( Figure 2 ). The PK variability is notably more evident in the lung as compared with plasma for both compounds. The estimates of clearance and volume of the central compartment from the population PK model are consistent with previously published values. 9 , 27 , 28 The validity and generalizability of our results are further suggested by the concordance of simulated concentrations from the population PK model with data from a previously published group of critically ill patients (see Figure 1 ). 22
β‐Lactam antibiotics penetrate the lung by passive diffusion. 18 , 29 Diffusion into tissues is dependent on the concentration gradient across biological membranes, the surface area of the membrane, and a diffusion coefficent. 30 The diffusion coefficient is principally influenced by the physicochemical characteristics of the drug (e.g., the degree of lipophilicity) and the extent of protein binding. 31 , 32 There was a positive correlation between plasma and ELF exposures for both piperacillin and tazobactam. However, these relationships did not reach statistical significance, which is unexpected and perhaps due to the modest number of patients in the study. In this study we used the ratio of total protein in ELF to plasma as a surrogate measure of lung permeability. 33 We expected to see an increase in the diffusion of drug with an increasing pulmonary protein penetration. However, we observed a statistically significant negative correlation between the piperacillin pulmonary penetration ratio (AUCELF/AUCunbound plasma) and pulmonary permeability ( Figure 5 ). As pulmonary permeability increased, there was a reduction in the relative proportion of piperacillin penetrating the lung. For tazobactam, there was no statistically significant correlation between the pulmonary penetration ratio (AUCELF/AUCunbound plasma) and pulmonary permeability. The relationship between pulmonary permeability and pulmonary drug concentration has not previously been investigated for β‐lactam antibiotics. Pulmonary vancomycin penetration has been shown to be higher in patients with increased pulmonary permeability as measured by ELF albumin concentration. 34 Lung penetration of β‐lactam antibiotics has been shown to be dependent on plasma albumin concentration. 31 , 35 , 36 Increased tissue penetration occurs when higher unbound plasma β‐lactam antibiotic concentrations are observed in patients with hypoalbuminemia. This phenomenon primarily affects highly protein bound agents such as ertapenem and flucloxacillin. 31 , 35 , 36
There are a number of potential explanations for the relationship between piperacillin lung penetration and permeability. Methodologically, this is a small study with extreme PK variability in both the observed plasma and ELF drug concentrations. Multiplication of the measured pulmonary sample concentration by a dilution factor, derived from a comparison of urea concentrations in plasma and pulmonary samples, 37 , 38 may contribute to the greater variability observed in the pulmonary drug concentrations as compared with plasma concentrations. A possible biological explanation includes dilution of intrapulmonary piperacillin due to larger ELF volumes that are associated with increasing pulmonary permeability. Alternatively, β‐lactam antibiotics are substrates for organic anion transporters in other organs, such as the kidney. 39 Disruption of an active transport system may occur in the injured lungs of critically ill patients, which exhibit increased permeability to protein. Another explanation may be that an increase in pulmonary protein permeability preferentially affects diffusion of piperacillin and tazobactam into and out of the lung. Therefore, in lungs with low protein permeability, piperacillin diffuses into the lung faster than it diffuses out. The reverse occurs in lungs with higher protein permeability. Validation of the negative correlation of pulmonary piperacillin penetration and pulmonary permeability is required in a similar clinical cohort.
Measurement of antimicrobial agents in ELF may not truly reflect drug concentration within other pulmonary subcompartments. Microdialysis techniques may provide a better estimate of pulmonary drug concentrations than bronchoalveolar lavage for quantifying drug concentrations in ELF. 40 However, due to practical difficulties with pulmonary microdialysis, ELF sampling remains the most commonly utilized technique in both preclinical and clinical studies. 17 , 41 In this study we used NBL; NBL is a safe and effective way of sampling the lung and quantifying antimicrobial drug concentrations in the ELF of critically ill patients. 42 NBL is less invasive than bronchoscopy and bronchoalveolar lavage, enabling multiple NBL samples to be collected throughout the dosing interval. The collection of two NBL samples from each patient, rather than one bronchoalveolar lavage sample, provides a more robust estimate of the concentration–time profile in the ELF of individual patients. Only one minor adverse event was reported following NBL.
For β–lactam antibiotics, the pharmacodynamic index that best links drug exposure with the antibacterial effect is the fraction of the dosing interval that the free drug concentrations are above the MIC. 12 For piperacillin, an unbound piperacillin concentration above the MIC for at least 50% of the dosing interval is generally associated with near maximal efficacy (50% fT>MIC). 13 We recently demonstrated that a trough unbound piperacillin concentration to MIC ratio of >3.4 is required to suppress the emergence of antimicrobial resistance (C min/MIC > 3.4). 16 From the target attainment analysis ( Figure 6 ), the empirical administration of 4 g piperacillin three times daily, as a 30–min infusion (i.e., the MIC is not known), results in an 80% probability of attainment of a pharmacodynamic target of plasma 50% fT>MIC or a 77% probability of attainment of a pharmacodynamic target of 50% ELF T>MIC. The probabilities of achieving the same pharmacodynamic targets increase to 86 and 82% for plasma and ELF, respectively, when the MIC is known and organisms with MICs beyond the breakpoint (i.e.,>16 mg/l) are excluded. Therefore, 14–18% of patients with a “susceptible” organism will have suboptimal drug exposure. Furthermore, ~60% of patients will not achieve the plasma or ELF pharmacodynamic targets associated with suppression of antimicrobial resistance (C min/MIC > 3.4). This analysis identifies two important issues. First, 4 g piperacillin three times daily, as a 30–min infusion, is inadequate for effective treatment and suppression of emergence of antimicrobial resistance in an unacceptably high proportion of critically ill patients, and especially in those with pneumonia resulting from infection with a less susceptible organism. Second, the probabilities of achieving each of the pharmacodynamic targets (e.g., fT>MIC and C min/MIC) in plasma and ELF are similar. Plasma piperacillin concentrations do not precisely predict ELF piperacillin concentrations. Consequently, some individuals with “sufficient” plasma piperacillin exposure will have inadequate ELF piperacillin exposures and vice versa. ELF rather than plasma exposure has been shown to predict outcome for other antimicrobial agents. 17 The causative organisms were not isolated in our 17 patients, which makes exploration of the relationship between piperacillin plasma and ELF PK, as well as pharmacodynamic (fT>MIC, C min/MIC) and clinical outcomes, impossible. Further appropriately powered clinical studies are required to examine whether piperacillin exposure in plasma or ELF better predicts clinical outcomes.
The addition of tazobactam to piperacillin extends the activity of the β‐lactam to β‐lactamase‐producing strains of organisms such as Enterobacteriaceae, Staphylococcus aureus, Haemophilus influenzae, and Moraxella catarrhalis. 43 The current regimen of piperacillin–tazobactam, at a fixed 8:1 ratio, is supported by in vitro studies. 44 , 45 , 46 However, the pharmacodynamic index that best links β‐lactamase inhibitor exposure with effect is poorly defined. Both (i) the fraction of the dosing interval that the β‐lactamase inhibitor concentration is above a threshold (T>threshold) and (ii) the area under the β‐lactamase inhibitor concentration–time curve have been suggested as the relevant pharmacodynamic indexes. 47 , 48 , 49 The required concentration of β‐lactamase inhibitor is dependent on the amount and type of β‐lactamase. 50 Tazobactam penetrates the lung of most critically ill patients, but there is marked variability. Therefore, a subset of patients may have insufficient pulmonary tazobactam concentrations to adequately inhibit some β‐lactamases that cause hydrolysis of piperacillin and clinical failure despite adequate piperacillin exposure. Increasing the tazobactam dosage (while maintaining the piperacillin dosage) may overcome β‐lactamase production, as has been demonstrated in an in vivo meningitis model. 51 Because plasma tazobactam exposure does not reflect tazobactam exposure in ELF, the identification of patients with poor pulmonary tazobactam penetration is difficult and appears to require direct sampling from the lung.
In conclusion, the primary aim of this study was to develop and validate a mathematical model to describe piperacillin and tazobactam concentrations in plasma and the lung of critically ill patients. In addition, we showed an unexpected relationship of increased pulmonary permeability being associated with a reduction in pulmonary piperacillin penetration. We also demonstrated that predicting pulmonary piperacillin and tazobactam exposures on the basis of plasma drug exposures may be unreliable. Appropriately powered clinical trials are required to further define the relationship between plasma and pulmonary drug exposures and to establish the impact of pulmonary, rather than plasma, drug exposure on clinical outcome. In addition, preclinical and clinical studies are required to investigate mechanisms of lung penetration in patients with pneumonia. Biomarkers related to pulmonary permeability or drug penetration could be incorporated as covariates into mathematical models to improve predictions of pulmonary drug exposures. New regimens of piperacillin–tazobactam may be required that optimize drug concentrations in the lung, at the site of infection. It is likely that a single regimen is not suitable for all individuals. If ELF exposure is shown to predict clinical outcome with greater accuracy than plasma exposure, and covariates for pulmonary drug penetration cannot be identified, then direct measurement of drug concentrations in the pulmonary compartment and adjustment of individual regimens may be required.
METHODS
PK study. This was a prospective, open–label, single–arm PK study. The study was conducted in accordance with the Declaration of Helsinki and was approved in the United Kingdom by both the local research ethics committee and the Medicine and Healthcare Products Regulatory Agency (EudraCT number: 2011–004470–28). Intubated patients who received piperacillin–tazobactam for suspected or documented pulmonary infection at the University Hospital of South Manchester National Health Service Trust, Manchester, UK were eligible for inclusion. Piperacillin–tazobactam 4 g/0.5 g (Stragen, Reigate, UK) was administered over less than 30 min every 8 h except in patients with a creatinine clearance of <20 ml/min or those on renal replacement therapy, who were administered the drug every 12 h. Written informed consent was obtained from the next of kin of all patients participating in the study. In addition, retrospective informed consent was obtained from all patients who survived and regained capacity to give consent. Demographic data (including age, sex, race, height, and weight), disease severity (by APACHE II and SOFA scores), underlying renal function, presence of renal replacement therapy, and clinical outcome were recorded.
Sampling was performed after administration of the first dose of piperacillin–tazobactam, if possible. All patients underwent sampling at steady state. The mean half‐life of piperacillin is ≈0.75 h, so patients were assumed to be at steady state by the second dose. 9 A previously published, optimally designed sampling schedule was used to inform the timings for collection of the plasma samples. 9 Plasma samples were collected at .5, 1.5, 2.5, 3.75, 5, and 6 h after initiation of the infusion for the first dose; immediately before the dose; and at .25, .75, 2, 3.5, and 4.5 h after initiation of the infusion at steady state. All plasma samples were collected in lithium heparin‐containing tubes. Immediately after collection, all plasma samples were centrifuged at 1,400g for 12 min. Samples were stored at −80 °C in 0.4‐ml aliquots before analysis.
NBL samples were assumed to be equivalent to bronchoalveolar lavage samples and were used for recovery of intrapulmonary samples. 42 Two intrapulmonary samples were collected from each patient during the steady–state dosing interval. Samples were collected at .75 and 2 h or at .75 and 3.5 h after initiation of the infusion. Patients (i) requiring > 80% inspired oxygen; (ii) requiring > 12 cmH2o positive end expiratory pressure; (iii) in whom endotracheal suction led to a severe and prolonged desaturation; (iv) with severe bronchospasm; (v) with uncontrolled or persistently raised intracranial pressure; or (vi) with severe disseminated intravascular coagulation did not have NBL samples collected. Briefly, suitable patients were preoxygenated with 100% oxygen for 2 min before sampling. A suction catheter was introduced into the bronchial tree until wedged, and 20 ml of sterile normal (0.9%) saline was instilled over 5–10 s and then immediately aspirated. Typically, 10 ml of normal saline was recovered. Patients were monitored for 4 h after the NBL for signs of cardiorespiratory compromise. Immediately after collection, all NBL samples were filtered through a 48–µm filter and centrifuged at 2,000g for 10 min. Samples were stored at −80 °C in 0.5–ml aliquots before analysis.
Piperacillin, tazobactam, urea, and protein assays. Piperacillin and tazobactam concentrations in plasma and lavage fluid were measured using a validated liquid chromatography–tandem mass spectrometry method with an Agilent 6420 Triple Quad Mass spectrometer (Agilent Technologies UK, Cheshire, UK). Twenty microliters of extracted sample was injected onto a Synergi 4u Hydro RP 80A 100 × 2.0 mm column (Phenomenex, Cheshire, UK). The standard curves for piperacillin and tazobactam, encompassing the concentration ranges of 0.02–10.0 and 0.02–5.0 mg/l, respectively, for plasma and 0.02–10.0 mg/l for lavage fluid were constructed in plasma and blank lavage fluid, respectively. The standard curves were made from stock solutions of 1 mg/l of piperacillin and tazobactam, respectively. The internal standard was caffeine in water at 0.1 mg/l (Sigma Aldrich, Dorset, UK). The between–day coefficients of variation were <17.4% for piperacillin and <15.5% for tazobactam. The lower limit of detection for piperacillin and tazobactam in plasma and lavage fluid was 0.02 mg/l.
Urea concentrations in plasma and lavage fluid were performed using a colorimetric technique (QuantiChromTM Urea Assay Kit DIUR–500, Gentaur BVBA–Bioxys, Kampenhout, Belgium). The standard curve for the urea assay is linear over a concentration range of 0–100 mg/dl. Plasma samples were diluted at 1:5 before measuring urea concentration. Drug concentrations in NBL samples were assumed to reflect ELF drug concentrations after correcting for the dilution introduced by lavage sampling, using the urea dilution method. 37 , 38 Urea concentration was assumed to be the same in plasma and ELF. Therefore, comparison of urea concentrations in the plasma and lavage fluid enables an estimation of the dilution caused by instillation of lavage fluid to the lung. The concentrations of piperacillin and tazobactam in ELF were estimated using the following formula:
where [Drug]ELF and [Drug]lavage are the concentrations of either piperacillin or tazobactam in ELF and lavage fluid, respectively. [Urea]plasma and [Urea]lavage are the concentrations of urea in the plasma and lavage fluid, respectively.
Pulmonary permeability was assumed to be proportional to the ratio of the mean total protein concentrations in plasma and ELF. 33 Total protein was quantified in plasma using the total protein assay on an Abbott Architect C16000 (Abbott Laboratories, North Chicago, IL). This colorimetric assay uses biuret reagent to detect the presence of peptide bonds. The limit of detection for total protein was 0.5 g/dl. The limit of quantification was 0.76 g/dl. The imprecision of the total protein assay is ≤3% of the total coefficient of variation. Protein in ELF was quantified using a UPro assay on an Abbott Architect c8000 (Abbott Laboratories). This assay uses a turbidimetric procedure in which benzethonium chloride is used as the protein denaturing agent. The limit of quantification and detection for the UPro assay is 6.75 mg/dl. The imprecision of the assay is ≤7.8% of the total coefficient of variation. ELF protein concentration was corrected for dilution using the urea dilution method described above.
Population PK analysis. All data were analyzed using a population PK methodology with the nonparametric adaptive grid (NPAG) program Pmetrics 1.1.3. 52 For both piperacillin and tazobactam, a three‐compartment structural mathematical model was assumed to be most appropriate for population analysis.
The differential equations for the three‐compartment structural mathematical model used are shown above. X 1, X 2, and X 3 are the amounts of piperacillin (in mg) in the central, peripheral, and ELF compartments, respectively. R(1) represents the infusion of piperacillin. Cl (l/h) is the clearance, and V c is the volume of the central compartment (L). k cp, k pc, k cELF, and k ELFc are the first‐order intercompartmental rate constants between the central and peripheral and central and ELF compartments, respectively. Covariates were not included in the structural model.
Elimination and movement of drug to and from the central compartment to the peripheral or ELF compartments was a first‐order process. The PK data were weighted by the inverse of the measured assay variance for both piperacillin and tazobactam. Samples with drug concentrations below the limit of assay quantification were excluded from analysis. A polynomial describing the assay variance was derived from regression of the measured mean drug concentrations and the SD for samples with known high and low piperacillin and tazobactam concentrations. The means, medians, and SDs of the population parameters were estimated. Bayesian posterior estimates for each parameter were also obtained for each patient (using the “population of one” utility in NPAG). Scatter plots of observed vs. predicted piperacillin concentrations were examined for the population as a whole and for individual patients. The fit of the structural model to the data was assessed using (i) the log‐likelihood value; (ii) the coefficients of determination (r 2), and the slope and y‐intercept from regression of the observed‐predicted plots before and after the Bayesian step; and (iii) the Akaike information criterion.
Intercompartmental clearance and the volume of the peripheral compartment was estimated algebraically using the equation below.
where Q is the intercompartmental clearance (in l/h); V c and V p are the volumes of the central and periperpheral compartments, respectively; and k cp and k pc are the intercompartmental rate constants (h−1).
External validation of the population PK analysis. All simulations were performed in ADPAT 5 (ref. 53). Observed data from a previous pharmacokinetic study were used as a validation data set. 22 In this study, by Boselli et al., 22 40 patients were administered a 30‐min intravenous loading dose of piperacillin–tazobactam 4/0.5 g followed by a daily continuous infusion of either 12/1.5 or 16/2 g. Three plasma samples (at least 4 h apart) and one NBL sample were collected after at least 48 h of piperacillin–tazobactam. Monte Carlo simulations were performed for 5,000 subjects using the regimens utilized by Boselli et al. 22 The parameter estimates (estimates of, e.g., clearance and volume) from the population PK analysis outlined in our study (rather than the Boselli study) were utilized. The median and 5th, 25th, 75th, and 95th percentile piperacillin and tazobactam concentrations in plasma and ELF from the simulation were plotted. The observed piperacillin and tazobactam concentrations in plasma and ELF, from Boselli et al. 22 , were overlaid on the simulated data. A visual inspection was made of the ability of the population PK model to predict the validation data.
Simulations to estimate piperacillin–tazobactam exposure in plasma and ELF. Monte Carlo simulation was performed using a 5,000–subject simulation. The mean parameter vector and the full covariance matrix from the population PK analysis was embedded in subroutine PRIOR of the ADAPT5 program. 53 , 54 Normal and log–normal parameter distributions were explored in the simulations. The ability to recapitulate the original parameter values and their dispersions was used to select which parameter distribution was selected. For piperacillin and tazobactam, the median and 5th–percentile, 25th–percentile, 75th–percentile, and 95th–percentile total, unbound, and ELF concentrations for the population were identified every hour. Again, a regimen of piperacillin–tazobactam 4/0.5 g, administered over 30 min, every 8 h, was used for the simulations. The unbound plasma and ELF AUCs for both drugs were calculated for the fifth dose (32–40 h after initiation of therapy).
Simulations for each of the 17 patients were performed using the Bayesian posterior (individual) parameter estimates (i.e., clearance, volume of the central compartment, and intercompartmental rate constants). A regimen of piperacillin–tazobactam 4/0.5 g, administered over 30 min, every 8 h, was used for the simulations, except for those for the three patients administered piperacillin–tazobactam 4/0.5 g, administered over 30 min, every 12 h, due to renal impairment. For both piperacillin and tazobactam, the AUC was estimated for plasma, for the unbound plasma fraction, and for the total ELF. Protein binding for both piperacillin and tazobactam was assumed to be 30%. 55 The AUCs were calculated at steady state (five doses/32 h after initiation of therapy for patients with an estimated glomerular filtration rate ≥ 20 ml/min or four doses/36 h after initiation of therapy for patients with an estimated glomerular filtration rate < 20 ml/min). The correlations of total ELF to unbound plasma exposure for both piperacillin and tazobactam were assessed. Similarly, the correlations of pulmonary permeability to pulmonary piperacillin and tazobactam penetration ratios were assessed. All correlations were analyzed using the Spearman rank test (GraphPad Prism version 5 for Windows, GraphPad Software, San Diego, CA, http://www.graphpad.com).
Finally a 5,000‐subject simulation was performed using 4 g piperacillin, administered over 30 min, every 8 h. The fraction of simulated subjects who achieved six predefined pharmacodynamic targets for a range of MICs from 0.5 to 128 mg/l was determined. The pharmacodynamic targets, assumed to be relevant in critically ill patients, were an unbound plasma or ELF piperacillin concentration above the MIC for 50% of the dosing interval (50% fT<MIC), 100% of the dosing interval (100% fT<MIC), or a trough piperacillin concentration to MIC ratio of ≥3.4. The cumulative response of patients, with a range of MICs defined as susceptible to piperacillin and achieving each of the pharmacodynamic targets, was estimated using a published MIC distribution for organisms causing hospital‐acquired and ventilator‐associated pneumonia. 26
ACKNOWLEDGMENTS
The authors acknowledge the support of the National Institute for Health Research Greater Manchester Comprehensive Local Research Network and the University Hospital of South Manchester National Health Service Foundation Trust.
This article is independent research supported by the National Institute for Health Research Clinical Research Facility at the University Hospital of South Manchester National Health Service (NHS) Foundation Trust. The views expressed in this article are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health.
AUTHOR CONTRIBUTIONS
T.W.F., B.I., I.M., A.M.B., and W.W.H. wrote the manuscript. T.W.F., K.M., A.M.B., and W.W.H. designed the research. T.W.F., K.M., B.I., I.M., S.W., J.G., and A.M.B. performed the research. T.W.F. and W.W.H. analyzed the data.
CONFLICT OF INTEREST
T.W.F. is an MRC Clinical Training Fellow supported by the North West England Medical Research Council Fellowship Scheme in Clinical Pharmacology and Therapeutics, which is funded by the Medical Research Council (grant number G1000417/94909), ICON, GlaxoSmithKline, AstraZeneca, and the Medical Evaluation Unit. W.W.H. is supported by a Clinician Scientist Fellowship from the National Institute of Health Research. The other authors declared no conflict of interest.
Study Highlights

Supporting information
Supplementary Table S1
References
- 1. Vincent, J.L. et al; Sepsis Occurrence in Acutely Ill Patients Investigators . Sepsis in European intensive care units: results of the SOAP study. Crit. Care Med. 34, 344–353 (2006). [DOI] [PubMed] [Google Scholar]
- 2. Martin, G.S. , Mannino, D.M. , Eaton, S. & Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348, 1546–1554 (2003). [DOI] [PubMed] [Google Scholar]
- 3. Vincent, J.L. et al; EPIC II Group of Investigators . International study of the prevalence and outcomes of infection in intensive care units. JAMA 302, 2323–2329 (2009). [DOI] [PubMed] [Google Scholar]
- 4. Melsen, W.G. et al Attributable mortality of ventilator–associated pneumonia: a meta–analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 13, 665–671 (2013). [DOI] [PubMed] [Google Scholar]
- 5. Kollef, M.H. , Shorr, A. , Tabak, Y.P. , Gupta, V. , Liu, L.Z. & Johannes, R.S. Epidemiology and outcomes of health–care–associated pneumonia: results from a large US database of culture–positive pneumonia. Chest 128, 3854–3862 (2005). [DOI] [PubMed] [Google Scholar]
- 6. Kollef, M.H. , Sherman, G. , Ward, S. & Fraser, V.J. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 115, 462–474 (1999). [DOI] [PubMed] [Google Scholar]
- 7. Patel, N. , Scheetz, M.H. , Drusano, G.L. & Lodise, T.P. Identification of optimal renal dosage adjustments for traditional and extended–infusion piperacillin–tazobactam dosing regimens in hospitalized patients. Antimicrob. Agents Chemother. 54, 460–465 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Udy, A.A. , Roberts, J.A. & Lipman, J. Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med. 39, 2070–2082 (2013). [DOI] [PubMed] [Google Scholar]
- 9. Felton, T.W. et al Population pharmacokinetics of extended–infusion piperacillin–tazobactam in hospitalized patients with nosocomial infections. Antimicrob. Agents Chemother. 56, 4087–4094 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayashi, Y. , Roberts, J.A. , Paterson, D.L. & Lipman, J. Pharmacokinetic evaluation of piperacillin–tazobactam. Expert Opin. Drug Metab. Toxicol. 6, 1017–1031 (2010). [DOI] [PubMed] [Google Scholar]
- 11. Malacarne, P. , Rossi, C. & Bertolini, G. ; GiViTI Group . Antibiotic usage in intensive care units: a pharmaco–epidemiological multicentre study. J. Antimicrob. Chemother. 54, 221–224 (2004). [DOI] [PubMed] [Google Scholar]
- 12. Craig, W.A. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26, 1–10; quiz 11 (1998). [DOI] [PubMed] [Google Scholar]
- 13. Drusano, G.L. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug'. Nat. Rev. Microbiol. 2, 289–300 (2004). [DOI] [PubMed] [Google Scholar]
- 14. McKinnon, P.S. , Paladino, J.A. & Schentag, J.J. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int. J. Antimicrob. Agents 31, 345–351 (2008). [DOI] [PubMed] [Google Scholar]
- 15. Boucher, H.W. et al Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48, 1–12 (2009). [DOI] [PubMed] [Google Scholar]
- 16. Felton, T.W. et al Impact of bolus dosing versus continuous infusion of piperacillin and tazobactam on the development of antimicrobial resistance in Pseudomonas aeruginosa . Antimicrob. Agents Chemother. 57, 5811–5819 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ambrose, P.G. , Bhavnani, S.M. , Ellis–Grosse, E.J. & Drusano, G.L. Pharmacokinetic–pharmacodynamic considerations in the design of hospital–acquired or ventilator–associated bacterial pneumonia studies: look before you leap! Clin. Infect. Dis. 51 (suppl. 1), S103–S110 (2010). [DOI] [PubMed] [Google Scholar]
- 18. Rodvold, K.A. , George, J.M. & Yoo, L. Penetration of anti–infective agents into pulmonary epithelial lining fluid: focus on antibacterial agents. Clin. Pharmacokinet. 50, 637–664 (2011). [DOI] [PubMed] [Google Scholar]
- 19. Drusano, G.L. Infection site concentrations: their therapeutic importance and the macrolide and macrolide–like class of antibiotics. Pharmacotherapy 25, 150S–158S (2005). [DOI] [PubMed] [Google Scholar]
- 20. Rodvold, K.A. et al Identifying exposure targets for treatment of staphylococcal pneumonia with ceftobiprole. Antimicrob. Agents Chemother. 53, 3294–3301 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chandorkar, G. , Huntington, J.A. , Gotfried, M.H. , Rodvold, K.A. & Umeh, O. Intrapulmonary penetration of ceftolozane/tazobactam and piperacillin/tazobactam in healthy adult subjects. J. Antimicrob. Chemother. 67, 2463–2469 (2012). [DOI] [PubMed] [Google Scholar]
- 22. Boselli, E. et al Alveolar concentrations of piperacillin/tazobactam administered in continuous infusion to patients with ventilator–associated pneumonia. Crit. Care Med. 36, 1500–1506 (2008). [DOI] [PubMed] [Google Scholar]
- 23. Boselli, E. et al Steady–state plasma and intrapulmonary concentrations of piperacillin£tazobactam 4 g/0.5 g administered to critically ill patients with severe nosocomial pneumonia. Intensive Care Med. 30, 976–979 (2004). [DOI] [PubMed] [Google Scholar]
- 24. EUCAST: European Committee on Antimicrobial Susceptibility Testing. Piperacillin–Tazobactam / Pseudomonas aeruginosa International MIC Distribution – Reference Database 2014–06–27 <http://mic.eucast.org/Eucast2/regShow.jsp?Id=6919#histogram_data> (2014).
- 25. Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing; Twenty‐First Informational Supplement. CLSI Document M100‐S23. Clinical and Laboratory Standards Institute, Wayne, PA, (2013). [Google Scholar]
- 26. Masterton, R.G. et al Guidelines for the management of hospital–acquired pneumonia in the UK: report of the working party on hospital–acquired pneumonia of the British Society for Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 62, 5–34 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lodise, T.P. Jr , Lomaestro, B. , Rodvold, K.A. , Danziger, L.H. & Drusano, G.L. Pharmacodynamic profiling of piperacillin in the presence of tazobactam in patients through the use of population pharmacokinetic models and Monte Carlo simulation. Antimicrob. Agents Chemother. 48, 4718–4724 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roberts, J.A. , Kirkpatrick, C.M. , Roberts, M.S. , Dalley, A.J. & Lipman, J. First–dose and steady–state population pharmacokinetics and pharmacodynamics of piperacillin by continuous or intermittent dosing in critically ill patients with sepsis. Int. J. Antimicrob. Agents 35, 156–163 (2010). [DOI] [PubMed] [Google Scholar]
- 29. Honeybourne, D. Antibiotic penetration into lung tissues. Thorax 49, 104–106 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barza, M. & Cuchural, G. General principles of antibiotic tissue penetration. J. Antimicrob. Chemother. 15 (suppl. A), 59–75 (1985). [DOI] [PubMed] [Google Scholar]
- 31. Ulldemolins, M. , Roberts, J.A. , Wallis, S.C. , Rello, J. & Lipman, J. Flucloxacillin dosing in critically ill patients with hypoalbuminaemia: special emphasis on unbound pharmacokinetics. J. Antimicrob. Chemother. 65, 1771–1778 (2010). [DOI] [PubMed] [Google Scholar]
- 32. Pennington, J.E. Penetration of antibiotics into respiratory secretions. Rev. Infect. Dis. 3, 67–73 (1981). [DOI] [PubMed] [Google Scholar]
- 33. Holter, J.F. , Weiland, J.E. , Pacht, E.R. , Gadek, J.E. & Davis, W.B. Protein permeability in the adult respiratory distress syndrome. Loss of size selectivity of the alveolar epithelium. J. Clin. Invest. 78, 1513–1522 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lamer, C. et al Analysis of vancomycin entry into pulmonary lining fluid by bronchoalveolar lavage in critically ill patients. Antimicrob. Agents Chemother. 37, 281–286 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brink, A.J. , Richards, G.A. , Schillack, V. , Kiem, S. & Schentag, J. Pharmacokinetics of once–daily dosing of ertapenem in critically ill patients with severe sepsis. Int. J. Antimicrob. Agents 33, 432–436 (2009). [DOI] [PubMed] [Google Scholar]
- 36. Burkhardt, O. et al Ertapenem in critically ill patients with early–onset ventilator–associated pneumonia: pharmacokinetics with special consideration of free–drug concentration. J. Antimicrob. Chemother. 59, 277–284 (2007). [DOI] [PubMed] [Google Scholar]
- 37. Yamazaki, K. , Ogura, S. , Ishizaka, A. , Oh–hara, T. & Nishimura, M. Bronchoscopic microsampling method for measuring drug concentration in epithelial lining fluid. Am. J. Respir. Crit. Care Med. 168, 1304–1307 (2003). [DOI] [PubMed] [Google Scholar]
- 38. Dargaville, P.A. , South, M. , Vervaart, P. & Dougall, P.N.M.C. Validity of markers of dilution in small volume lung lavage. Am. J. Respir. Crit. Care Med. 160, 778–784 (1999). [DOI] [PubMed] [Google Scholar]
- 39. Burckhardt, G. & Burckhardt, B.C. In vitro and in vivo evidence of the importance of organic anion transporters (OATs) in drug therapy. Handb. Exp. Pharmacol. 201, 29–104 (2011). [DOI] [PubMed] [Google Scholar]
- 40. Kiem, S. & Schentag, J.J. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob. Agents Chemother. 52, 24–36 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dhanani, J. et al Antimicrobial chemotherapy and lung microdialysis: a review. Int. J. Antimicrob. Agents 36, 491–500 (2010). [DOI] [PubMed] [Google Scholar]
- 42. Boselli, E. et al Reliability of mini–bronchoalveolar lavage for the measurement of epithelial lining fluid concentrations of tobramycin in critically ill patients. Intensive Care Med. 33, 1519–1523 (2007). [DOI] [PubMed] [Google Scholar]
- 43. Drawz, S.M. & Bonomo, R.A. Three decades of beta–lactamase inhibitors. Clin. Microbiol. Rev. 23, 160–201 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jones, R.N. & Barry, A.L. Studies to optimize the in vitro testing of piperacillin combined with tazobactam (YTR 830). Diagn. Microbiol. Infect. Dis. 12, 495–510 (1989). [DOI] [PubMed] [Google Scholar]
- 45. Aronoff, S.C. , Jacobs, M.R. , Johenning, S. & Yamabe, S. Comparative activities of the beta–lactamase inhibitors YTR 830, sodium clavulanate, and sulbactam combined with amoxicillin or ampicillin. Antimicrob. Agents Chemother. 26, 580–582 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jacobs, M.R. , Aronoff, S.C. , Johenning, S. , Shlaes, D.M. & Yamabe, S. Comparative activities of the beta–lactamase inhibitors YTR 830, clavulanate, and sulbactam combined with ampicillin and broad–spectrum penicillins against defined beta–lactamase–producing aerobic gram–negative bacilli. Antimicrob. Agents Chemother. 29, 980–985 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vanscoy, B. et al Pharmacological basis of ß–lactamase inhibitor therapeutics: tazobactam in combination with Ceftolozane. Antimicrob. Agents Chemother. 57, 5924–5930 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Craig, W.A. & Andes, D.R. In vivo activities of ceftolozane, a new cephalosporin, with and without tazobactam against Pseudomonas aeruginosa and Enterobacteriaceae, including strains with extended–spectrum ß–lactamases, in the thighs of neutropenic mice. Antimicrob. Agents Chemother. 57, 1577–1582 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Strayer, A.H. , Gilbert, D.H. , Pivarnik, P. , Medeiros, A.A. , Zinner, S.H. & Dudley, M.N. Pharmacodynamics of piperacillin alone and in combination with tazobactam against piperacillin–resistant and –susceptible organisms in an in vitro model of infection. Antimicrob. Agents Chemother. 38, 2351–2356 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lister, P.D. , Prevan, A.M. & Sanders, C.C. Importance of beta–lactamase inhibitor pharmacokinetics in the pharmacodynamics of inhibitor–drug combinations: studies with piperacillin–tazobactam and piperacillin–sulbactam. Antimicrob. Agents Chemother. 41, 721–727 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Leleu, G. , Kitzis, M.D. , Vallois, J.M. , Gutmann, L. & Decazes, J.M. Different ratios of the piperacillin–tazobactam combination for treatment of experimental meningitis due to Klebsiella pneumoniae producing the TEM–3 extended–spectrum beta–lactamase. Antimicrob. Agents Chemother. 38, 195–199 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Neely, M.N. , van Guilder, M.G. , Yamada, W.M. , Schumitzky, A. & Jelliffe, R.W. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther. Drug Monit. 34, 467–476 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. D'Argenio, D.Z. , Schumitzky, A. & Xiaoning, W. ADAPT 5 User's Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software <http://bmsr.usc.edu/files/2013/02/ADAPT5-User-Guide.pdf>. (2009).
- 54. D'Argenio, D.Z. & Schumitzky, A. A program package for simulation and parameter estimation in pharmacokinetic systems. Comput. Programs Biomed. 9, 115–134 (1979). [DOI] [PubMed] [Google Scholar]
- 55. Sörgel, F. & Kinzig, M. The chemistry, pharmacokinetics and tissue distribution of piperacillin/tazobactam. J. Antimicrob. Chemother. 31 (suppl. A), 39–60 (1993). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1


