Abstract
The liver has the unique capacity to regenerate in response to a damaging event. Liver regeneration is hereby largely driven by hepatocyte proliferation, which in turn relies on cell cycling. The hepatocyte cell cycle is a complex process that is tightly regulated by several well-established mechanisms. In vitro, isolated hepatocytes do not longer retain this proliferative capacity. However, in vitro cell growth can be boosted by immortalization of hepatocytes. Well-defined immortalization genes can be artificially overexpressed in hepatocytes or the cells can be conditionally immortalized leading to controlled cell proliferation. This paper discusses the current immortalization techniques and provides a state-of-the-art overview of the actually available immortalized hepatocyte-derived cell lines and their applications.
Keywords: hepatocyte, proliferation, senescence, immortalization
1. Introduction
In the field of hepatology, when orthotopic liver transplantation is not possible, human primary hepatocytes represent the ‘gold standard’, in particular for the establishment of bioartificial liver (BAL) support systems [1, 2]. They also serve as an important tool in research and are of particular interest for in vitro pharmaco-toxicology [3, 4]. Consequently, there is a considerable and increasing demand for human primary hepatocytes, yet their use is hampered by inadequate supply, high cost, high variability and low in vitro proliferation capacity. These constraints have prompted a large-scale search for alternative cell sources, such as hepatic cell lines and stem-cell derived hepatocytes [2, 5-9]. In contrast to primary cells, cell lines are readily available, and usually have an unlimited growth potential and high reproducibility [10, 11]. Hepatic cell lines are either derived directly from liver tumor tissue or artificially generated from primary hepatocytes in vitro [5, 6].Several hepatoma-derived cell lines preserve some liver-specific functions, but most of them, with exception of the HepaRG® cells, do not exhibit sufficient functionality to be of pharmaco-toxicological relevance [12-18]. Immortalized hepatocytes are typically derived from healthy primary hepatocytes by using a defined immortalization strategy. Both fetal and adult hepatocytes from different species have already been successfully immortalized, whether or not using a combination of viral oncogenes and the human telomerase reverse transcriptase (hTERT) protein [7, 9, 19-25]. The purpose of this paper is to discuss the different current immortalization strategies and provide an overview of the actually available immortalized hepatic cell lines and their applications. To fully understand these immortalization techniques, the processes of hepatocyte proliferation and senescence are briefly outlined in the preceding part.
2. Hepatocyte proliferation
2.1. Priming phase and commitment to hepatocyte cell cycle progression
Under normal conditions, the adult liver has very little proliferative activity. However, upon partial removal of liver tissue, the remaining intact hepatic lobes start to grow and liver mass is restored within seven to ten days due to the proliferation of mature hepatocytes [26, 27]. Multiple genes involved in cytokine networks become differentially expressed and regulate the initiation of this liver regeneration, a process called the “priming phase” [28-30]. During this step, G0/G1 cell cycle transition and early G1 progression are accomplished and hepatocytes become responsive to mitogenic signals, which leads to deoxyribonucleic acid (DNA) replication [28, 30, 31]. During collagenase perfusion of the liver, a critical step in the isolation procedure of hepatocytes, the messenger ribonucleic acid (mRNA) levels of the proto-oncogenes c-jun and c-fos rapidly increase, suggesting that enzymatic liver dissociation triggers the G0/G1 cell cycle transition of hepatocytes [32, 33]. Indeed, collagenase perfusion of the liver, which is accompanied by release of the cytokine tumor necrosis factor α as well as activation of the intracellular nuclear factor kappa-light-chain-enhancer of activated B cells and mitogen-activated protein kinase (MAPK) pathways, can induce priming of quiescent hepatocytes [28, 32, 34-36]. When the freshly isolated hepatocytes are plated, the sequentially increased expression of other proto-oncogenes, such as junB, junD, c-myc, p53 and c-Ki-ras, indicates that the hepatocytes can proceed to the mid-late G1 phase [28, 32]. However, further progression towards the G1/S cell cycle boundary is only possible after stimulation with appropriate growth factors to overcome the mitogen-dependent mid-late G1 restriction point [32]. This major checkpoint is regulated by the tumor suppressor retinoblastoma protein (pRB) and controls whether the cellular environment supports proliferation [37-39]. The need for mitogenic signals to pursue cell cycling has also been shown in vivo, though intrinsic differences exist between the in vivo and in vitro conditions [31, 40]. In vivo, normal adult hepatocytes return to the G0 state in the absence of growth factor stimulation, but that is not the case in vitro [26, 36, 40]. After attaching to the culture dish, surviving cells remain at the mid-late G1 restriction point, do not proliferate and die early [36, 41].
Several studies designated cyclin D1 as the major intracellular mediator of the mitogenic signals responsible for the regulation of hepatocyte proliferation [32, 40, 42-45]. As such, overexpression of D-type cyclins seems sufficient to overcome the mid-late G1 restriction point and triggers hepatocyte proliferation both, in vivo and in vitro, in the absence of mitogens [43, 45, 46]. Though the latter has been challenged by Wierod et al. [47]. Interestingly, fetal hepatocytes, which express both cyclin D2 and D3, possess a high proliferation rate that is, at least partly, independent of mitogenic pathways and characterized by the constitutive phosphorylation of pRB [48, 49].
Critical growth factors involved in hepatocyte cell cycling include hepatocyte growth factor (HGF), epidermal growth factor (EGF), transforming growth factor (TGF) α, heparin-binding EGF-like growth factor and amphiregulin [29, 50]. Once passed the mid-late G1 restriction point, hepatocytes are irreversibly committed to replicate and no longer require growth factors to complete the first cycle of cell proliferation [40]. From this point onwards, progression through the cell cycle proceeds autonomously and is driven by the sequential formation, activation and destruction of a series of structurally related serine/threonine protein kinase complexes, each composed of a regulatory and a catalytic subunit, cyclin and cyclin-dependent kinase (cdk), respectively [28, 42].
2.2. Hepatocyte cell cycle regulation and control
To date, at least 20 different cdk proteins and 30 cyclins have been identified in mammalian cells. However, only some are involved in cell cycle regulation [28, 51, 52]. Whereas the cdks are expressed throughout the hepatocyte cell cycle, with the notable exception of cdk1, most cyclins display a temporal expression profile, leading to periodic activation of their respective cdk counterparts [36, 42, 53]. Since these individual cyclin/cdk complexes perform unique functions in the cell cycle, their sequential assembly and activation dictates the order in which the cell cycle events occur [28, 51, 54, 55] (Fig.1.). Nevertheless, subsequent progression through the S, G2 and M phases can be impeded by additional cell cycle checkpoints, which are switched on in response to unfavorable conditions [42]. In this context, checkpoints at the G1/S and G2/M boundaries ensure the orderly unfolding of different cell cycle events and inhibit cell cycling in response to DNA damage. Overall, mechanisms associated with activation of the p53/p21 pathway and suppression of the cdc25 family phosphatase activity are initiated, which results in reduced cdk activity and cell cycle arrest [38, 42, 55]. Indeed, in addition to cyclin binding, cdk activity is also regulated by a critical phosphorylation/dephosphorylation equilibrium and counteracted by cell cycle inhibitory proteins, called the cdk inhibitors (cdki) [42, 51, 55] (Fig.1.). Based on their structure and the identity of the cdk targets, two families of cdki have been described, namely the Ink4 family and the Cip/Kip family. The former comprises four distinct proteins, namely p15, p16, p18 and p19/p14, which are specific inhibitors of cdk4/6. The Cip/Kip family proteins, including p21, p27 and p57, bind and inhibit different cdk/cyclin complexes [42, 55].
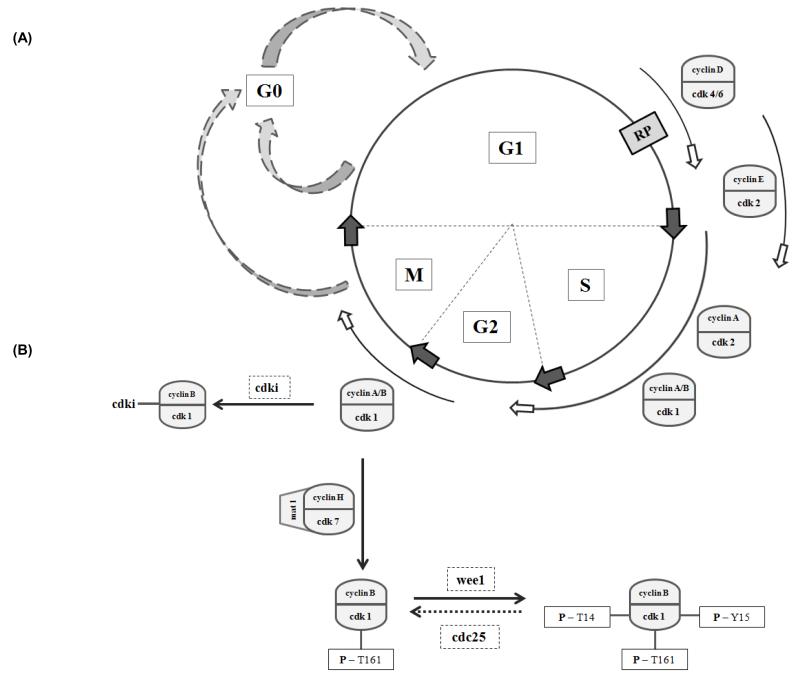
Fig.1. The hepatocyte cell cycle and its regulation.
(A) The hepatocyte cell cycle, as in other eukaryotic cells, is composed of four phases namely the G1, S, G2 and M phase. Under physiological conditions, most hepatocytes in the adult liver escape the active cell cycle and enter a quiescent stage, known as the G0 phase. In this state, hepatocytes do not proliferate, but remain metabolically active. Upon appropriate stimulation, hepatocytes re-enter the cell cycle in the G1 phase [42, 174]. Progression through the mid-late G1 phase is growth factor-dependent. Once beyond the mitogen-dependent restriction point (RP), the cell cycle is completed autonomously, driven by the sequential activation of a series of structurally related serine/threonine protein kinases, the cyclin dependent kinases (cdk) [42]. In contrast with other mammalian cells, hepatocytes possess active cyclin A-cdk1 and cyclin B-cdk1 complexes during the S-phase of their cell cycle, which is suggested to allow rapid and efficient hepatocyte proliferation [175].
(B) The kinase activity of the cdks is tightly regulated by several different mechanisms, including binding to cyclins, binding by cdk inhibitors (cdki) and various phosphorylation/dephosphorylation events.
For example full activation of the cyclin B- cdk1 complex requires its phosphorylation (P) on threonine 161 by the cdk-activating kinase (CAK). Other phosphorylation/dephosphorylation events fine tune kinase activity and thereby facilitate proper mitotic action. Wee1 negatively affects kinase activity by phosphorylating cdk1 on threonine (T) 14 and tyrosine (Y) 15, whereas cdc25 phosphatase restores kinase activity by dephosphorylation of the same amino acids. Furthermore, Cip/Kip cdki can bind to the cyclin B/ckd1 complexes and inhibit their action [39, 42, 51, 55]. Adapted from [28, 51]. CAK, cdk-activating kinase; cdk(i), cyclin dependent kinase (inhibitor); G, gap; M, mitosis; P, phosphorylated; RP, restriction point; S, synthesis; T, threonine; Y, tyrosine.
3. Hepatocyte senescence
Following partial hepatectomy (PH), the remaining hepatocyte population needs to divide on average 1,6 times before the normal liver mass is restored and the regeneration is put back on hold [26, 31, 56]. It has been suggested that TGFβ and activin A, known inhibitors of hepatocyte mitosis, as well as extracellular matrix-driven signals, underlie the termination of hepatocyte growth when the liver regeneration is completed [57-59]. During chronic liver injury, human hepatocytes are repeatedly stimulated to proliferate due to iterative waves of liver destruction and regeneration [60]. This in vivo proliferation capacity was further highlighted by the efficient repopulation of Fah−/−/Rag2−/−/Il2rg−/− mice with human adult hepatocytes for at least four sequential rounds [61]. However, human hepatocytes cannot proliferate indefinitely. Liver cirrhosis is accompanied by a significant rate of hepatocellular senescence and characterized by considerable short hepatocyte telomeres [60]. In humans, telomerase activity of most cell types is repressed early during development. Consequently, telomere DNA in proliferating somatic cells undergoes progressive attrition. Once a critical minimal length is reached, cellular growth is arrested irreversibly, a process known as replicative senescence, which was first described by Hayflick and Moorhead nearly 50 years ago [2, 62-64]. One way to overcome telomere-dependent senescence is by reactivating the telomerase activity with exogenous hTERT [65-67]. In contrast to humans, rodents display substantial telomerase activity in several somatic tissues, including the liver [62, 68-71]. Their telomerase activity increases 24 hours after PH and is enhanced by the preoperative treatment with EGF and HGF, but repressed by MAPK kinase inhibitors [72]. In primary rodent hepatocyte cultures, upregulation of telomerase activity was only notable or further enhanced after addition of growth factors to the culture medium [70, 72]. The high regeneration capacity, characteristic of rodent livers, may be linked to this strong telomerase activity [71]. In this regard, serially transplanted adult mouse hepatocytes have been demonstrated to divide as many as 69 times [31, 73].
However, in vitro, both human and rat adult hepatocytes do not possess spontaneous cell growth and their proliferation capacity remains usually quite limited even when cultured under growth promoting conditions [31, 68, 74-76]. The in vitro premature growth arrest, observed in primary hepatocyte cultures could be related to a telomere-independent senescence mechanism, which remains to be fully elucidated, but is suggested to involve tumor suppressor proteins and cdki [63, 77]. Indeed, several studies support the contribution of cdki p21 and/or p16 to the inhibition of DNA synthesis in primary hepatocyte cultures [78-82]. In this respect, it was demonstrated that the second cell cycle G1 block caused by chronic MAPK pathway activation in mitogen stimulated primary hepatocyte cultures is partly related to p21 induction. Of note, transient MAPK pathway inhibition allows the establishment of multiple replication rounds in these hepatocyte cultures [79].
4. Hepatocyte immortalization strategies
Immortalized hepatocytes are defined as a population of indefinitely dividing parenchymal cells that retain critical liver functions [68]. Since mature hepatocytes normally possess only limited growth potential when stimulated in vitro, immortalization strategies have been developed based mainly on the transduction or transfection of hepatocytes with well-known immortalizing genes. The most frequently used immortalization methods are (i) overexpression of viral oncogenes, (ii) forced expression of hTERT, or (iii) a combination of both [9, 68]. Moreover, some other immortalization genes as well as conditional approaches for hepatocyte immortalization have been described (Fig.2. & Table 1 & Table 2).
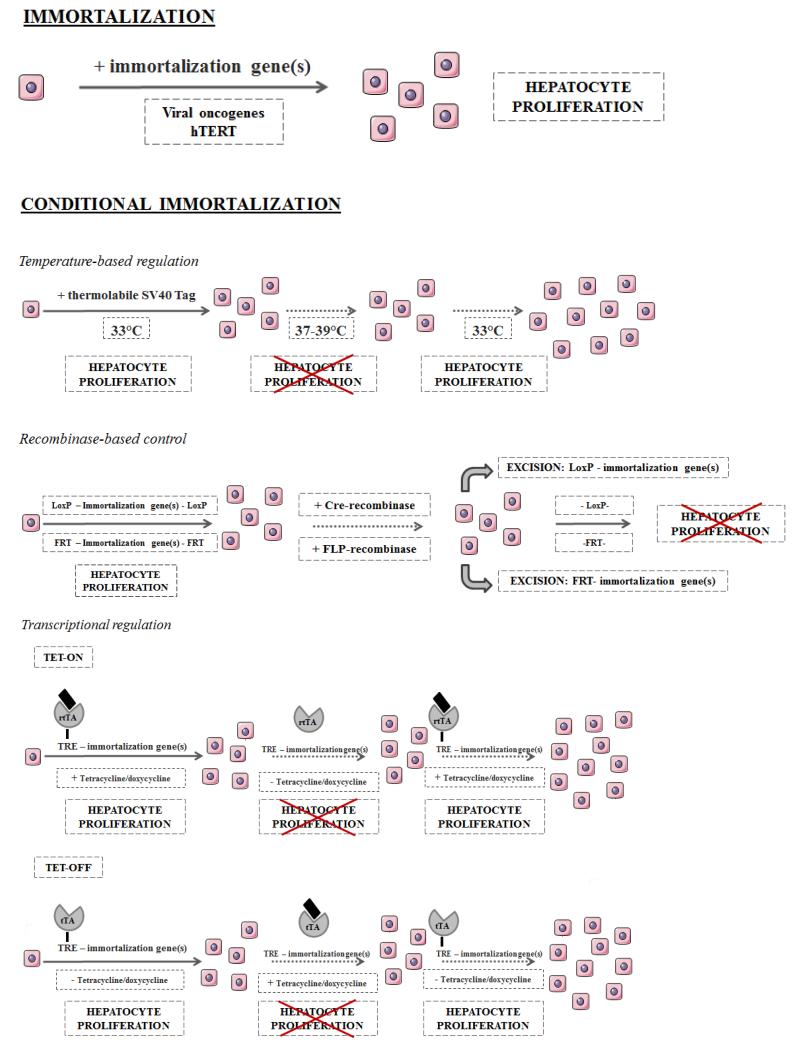
Fig.2. Hepatocyte immortalization strategies.
Several hepatocyte immortalization strategies are available, including transduction or transfection of prototypical immortalization genes. Conditional immortalization by temperature-based regulation, recombinase-based control and transcriptional regulation has been introduced to establish growth-controlled cell lines. Adapted from [10].
rtTA, reverse tetracycline transactivator; TRE, tetracycline responsive element; tTA, tetracycline transactivator.
Table 1. Overview of the functionality and immortalization strategy of in vitro established human and rodent hepatic cell lines.
A1AT, αl-antitrypsin; AFP, α-fetoprotein; AhR, aryl hydrocarbon receptor; ALB, albumin; ALF, acute liver failure; A2M, α2-macroglobulin; APO, apolipoprotein; Arnt, AhR nuclear translocator; ASGP(R), asialoglycoprotein (receptor); BCRP, breast cancer resistance protein; Bmi-1, B lymphoma Mo-MLV insertion region 1 homolog; BSEP, bile salt export pump; CAR, constitutive androstane receptor; C/EBP, Ccaat-enhancer-binding protein; CD, cluster of differentiation; CK, cytokeratin; CLDN, claudin; CYP, cytochrome P450; DMSO, dimethyl sulphoxide; DPP, dipeptidyl peptidase; EH, epoxide hydrolase; EPCAM, epithelial cell adhesion molecule; GGT, γ-glutamyltranspeptidase; G6P, glucose-6-phosphate; GPX, gluthatione peroxidase; GS, glutamine synthetase; GST, gluthatione S-transferase; HBCF, human blood coagulation factor; HGFR, hepatocyte growth factor receptor; HNF, hepatocyte nuclear factor; HPV, human papillomavirus; hTERT, human telomerase reverse transcriptase; IL, interleukin; INF, interferon; MDR, multidrug resistance protein; mRNA, messenger ribonucleic acid; MRP, multidrug resistance-associated protein; NADPH, nicotinamide adenine dinucleotide phosphate; NCAM, neural cell adhesion molecule; NTCP, sodium taurocholate cotransporting polypeptide; OATP, organic anion transporting polypeptide; OCT, organic cation transporter; PEPCK, phosphoenolpyruvate carboxykinase; PT, prothrombin; PXR, pregnane X receptor; Rb, retinoblastoma; SCID, severe combined immunodeficiency; SOD, superoxide dismutase; SV40 Tag, simian virus 40 large T antigen; TAT, tyrosine aminotransferase; TF, transferrin; UGT, uridinediphosphate-glucuronosyltransferase.
| Human hepatic cell lines | |||
|---|---|---|---|
| Cell line | Immortalization strategy | Functionality | Ref |
| Fa2N-4 | Adult hepatocytes |
|
[22, 86, 176] |
| Transfection | |||
| SV40 Tag | |||
| FH-TERT | Fetal hepatocytes |
|
[2] |
| Retroviral vector | |||
| hTERT | |||
| Hc3716- hTERT | Fetal hepatocytes |
|
[108] |
| Retroviral vector | |||
| hTERT | |||
| HepLi5 | Adult hepatocytes |
|
[141] |
| Retroviral vector | |||
| SV40 Tag | |||
| HepLL | Adult hepatocytes |
|
[83] |
| Lipid mediated gene transfer (lipofectamine reagent) | |||
| SV40 Tag | |||
| HepZ | Adult hepatocytes |
|
[112] |
| Lipid mediated gene transfer (lipofectamine reagent) | |||
| Antisense constructions for Rb and p53 + Cotransfection of E2F transcription factors and cyclin D1 | |||
| HHE6E7T-1/2 | Small hepatocytes |
|
[20, 153] |
| Lentiviral and retroviral vectors | |||
| HPV16 E6/E7 + hTERT | |||
| HHL(−5/−7/−16) | Adult hepatocytes |
|
[19] |
| Retroviral vector | |||
| HPV16 E6/E7 | |||
| IHH-A5 | Adult hepatocytes |
|
[135] |
| Lipid mediated gene transfer (lipofectin reagent) | |||
| SV40 Tag | |||
| NeHepLxHT | Neonatal hepatocytes |
|
[9] |
| Retroviral vector | |||
| hTERT | |||
| OUMS-29 | Fetal hepatocytes |
|
[21, 122, 136] |
| Lipid mediated gene transfer (lipofectin reagent) | |||
| SV40 Tag | |||
| PH5CH | Adult hepatocytes |
|
[137] |
| Lipid mediated gene transfer (lipofectin reagent) | |||
| SV40 Tag | |||
| THLE-2/-3 | Adult hepatocytes |
|
[104] |
| Retroviral vector | |||
| SV40 Tag | |||
| TPH1 | Adult hepatocytes |
|
[113] |
| Strontium phosphate precipitation | |||
| HCV core gene | |||
| Conditional immortalization | |||
| 16T-3 | Adult hepatocytes | Reverted 16T-3 cells:
|
[111] |
| Retroviral vector | |||
| hTERT | |||
| Tamoxifen-mediated selfexcision (Cre-LoxP) | |||
| cBAL111 | Fetal hepatocytes |
|
[7] |
| Lentiviral vector | |||
| hTERT | |||
| Transcriptional regulation (Tet-on approach) | |||
| HepCL | Fetal hepatocytes |
|
[124] |
| Retroviral vector | |||
| SV40 Tag | |||
| Temperature-based regulation | |||
| HepLi-4 | Adult hepatocytes | Reverted HepLi-4 cells:
|
[87] |
| Retroviral vector | |||
| SV40 Tag | |||
| Tamoxifen-mediated selfexcision (Cre-LoxP) | |||
| HLTC-7/-11/-15/-17/-19 | Adult hepatocytes |
|
[103] |
| Retroviral vector | |||
| SV40 Tag | |||
| Temperature-based regulation | |||
| IHH10(.3)/12 | Adult hepatocytes |
|
[23] |
| Lentiviral vector | |||
| SV40 Tag + hTERT (IHH10) or SV40 Tag + hTERT + Bmi-1 (IHH12) | |||
| Recombinase- based control (Cre-LoxP) | |||
| NKNT-3 | Adult hepatocytes |
|
[6, 85, 136, 140, 152] |
| Retroviral vector | |||
| SV40 Tag | |||
| Recombinase- based control (Cre-loxP) | |||
| YOCK-13 | Adult hepatocytes |
|
[110] |
| Retroviral vector | |||
| hTERT | |||
| Tamoxifen-mediated self-excision (Cre-LoxP) | |||
| Rodent hepatic cell lines | |||
| Cell line | Immortalization strategy | Functionality | Ref |
| AdPX3/4 | Rat adult hepatocytes |
|
[25] |
| Calcium phosphate precipitation | |||
| E1A & E1B | |||
| C3-II-B-2-3 C4-1-B-2 C8-IV P9 SV40RH1 | Rat adult hepatocytes |
|
[133] |
| Calcium phosphate precipitation | |||
| SV40 DNA | |||
| CWSV | Rat adult hepatocytes |
|
[105, 134, 177] |
| Calcium phosphate precipitation | |||
| SV40 DNA | |||
| RH(1-4/6-10) | Rat adult hepatocytes |
|
[24] |
| Electroporation | |||
| SV40 Tag | |||
| SVHepB4 | Rat adult hepatocytes |
|
[178] |
| Wild type simian virus strain LP | |||
| SV40 Tag | |||
| Conditional immortalization | |||
| Rat adult hepatocytes |
|
[118] | |
| Retroviral vector | |||
| SV40 Tag | |||
| Temperature-based regulation | |||
| Rat adult hepatocytes |
|
[129, 147] | |
| Human artificial mini chromosome | |||
| SV40 Tag | |||
| Recombinase- based control (FLP/FRT) | |||
| BQ1 BV1 WA1 WB6 | Rat adult hepatocytes |
|
[102] |
| Retroviral vector | |||
| SV40 Tag | |||
| Temperature-based regulation | |||
| C8-B | Rat adult hepatocytes |
|
[84] |
| Retroviral vector | |||
| SV40 Tag | |||
| Recombinase- based control (Cre-LoxP) | |||
| H2.35 | Mice adult hepatocytes |
|
[120] |
| Simian virus 40 | |||
| SV40 Tag | |||
| Temperature-based regulation | |||
| L2A2 | Rat adult hepatocytes |
|
[119, 179] |
| Retroviral vector | |||
| SV40 Tag | |||
| Temperature-based regulation | |||
Table 2. Overview of the available immortalization strategies.
HSV-TK, herpes simplex virus thymidine kinase; hTERT, human telomerase reverse transcriptase; n.a., not applicable; SV40 Tag, simian virus 40 large T antigen
| Immortalizing genes | |||
|---|---|---|---|
| Rodent adult hepatocytes | Human adult hepatocytes | Human fetal hepatocytes | |
| Viral oncogenes |
|
|
|
| hTERT | n.a |
|
|
| Viral oncogenes + hTERT | n.a |
|
|
| Conditional immortalization | ||
|---|---|---|
| Advantage | Disadvantage | |
| Temperature-based regulation |
|
|
| Recombinase-based regulation |
|
|
| + negative selection marker (HSV-TK) | Cells that underwent improper recombination can efficiently be eliminated by ganciclovir exposure. | |
| + tamoxifen-mediated self-excision | Elevates the need of a secondary virus-mediated transfer of the recombinase gene. | |
| Transcriptional regulation |
|
|
| Gene transfer | |
|---|---|
| Non-viral | |
| Calcium phosphate precipitation |
|
| Strontium phosphate transfection |
|
| Electroporation |
|
| Lipid-mediated gene transfer |
|
| Viral | |
| Retroviral |
|
| Lentiviral |
|
| Human artificial chromosomes | |
| |
4.1. Immortalization genes
Viral oncogenes
Viral oncogenes include the adenoviral E1A/E1B genes, the simian virus 40 large T antigen (SV40 Tag) and the human papillomavirus 16 (HPV16) E6/E7 genes [68]. All of them have been used to establish hepatocyte-derived cell lines, such as C8-B, HepLL, HHL, AdPX3/4, Fa2N4, HepLi-4 and NKNT-3, suggesting that overexpression of viral oncogenes may be sufficient to overcome the premature in vitro growth arrest of cultured hepatocytes [19, 25, 83-87]. These viral oncogenes typically interfere with cell cycling by inhibiting the p16/pRB and p53 pathways [39, 68]. Hepatic cell lines have also been developed from livers of transgenic rodents overexpressing SV40 Tag [88-90].
While the use of viral oncogenes, e.g. SV40 Tag, has been shown to be sufficient to immortalize rodent cells, overexpression of these oncogenes in human cells most likely only extends lifespan. Immortalization per se requires telomerase reactivation either through mutations or by the use of a second immortalizing gene, hTERT [2, 10, 20, 23, 68, 91, 92]. Furthermore, the use of a combined strategy involving a viral oncogene and hTERT, has also been reported to produce more genetically stable cells [11, 67, 68, 93, 94]. Indeed single use of viral oncogenes has often been demonstrated to induce chromosomal abnormalities [95-101]. Even though karyotype analysis of newly produced hepatic cell lines has not routinely been performed, chromosomal abnormalities have been described in different cell lines even with combined immortalization [20, 21, 102-104]. It is important to mention, however, that activation of an additional oncogene, such as ras is usually needed to observe tumorigenicity [84, 105-107].
Human telomerase reverse transcriptase
The single use of hTERT for immortalization has been suggested to avoid some of the genetic and phenotypic instabilities related to the use of oncogenes and is limited to a number of human cell types, including fetal and neonatal hepatocytes [2, 6, 7, 9, 10, 108, 109]. Unlike adult hepatocytes, these immature cells can still proliferate in vitro and hence do not need cell cycle stimulation for immortalization [2, 6, 7, 9, 49, 109]. However, fetal and neonatal human hepatocytes do not possess indefinite growth potential because inactivation of telomerase causes replicative senescence. Consequently, they require overexpression of hTERT to become immortalized [2, 7, 9, 109]. Contradicting results have been reported when only hTERT was used for immortalization of human adult hepatocytes [20, 110, 111]. As telomerase activity probably does not allow adult hepatocytes to overcome the proposed telomere-independent growth arrest, overexpression of hTERT may be insufficient to drive adult hepatocytes through the cell cycle [5, 7, 66, 68].
Miscellaneous immortalization genes
Specific combinations of immortalization genes, such as SV40 Tag with hTERT and B lymphoma Moloney Murine Leukemia virus (Mo-MLV) insertion region 1 homolog (Bmi-1), have been used to immortalize mature human hepatocytes. Bmi-1, like the viral oncogene HPV16E7, is involved in the inactivation of the p16/pRB pathway. On the other hand, simultaneous transduction with Bmi-1 and hTERT appears insufficient to immortalize the non-dividing hepatocytes [23]. Likewise, a combined HPV16E7/hTERT approach did not promote unlimited growth of human adult hepatocytes [20]. A particular cell line has been produced by co-transfection of human adult hepatocytes with p53 and pRB antisense constructs and plasmids that include E2F and cyclin D1 genes [112]. Furthermore, it seems that the hepatitis C core protein can also specifically immortalize mature human hepatocytes [10, 113, 114]. This core protein is able to induce c-myc and cyclin D1 expression in primary human hepatocytes via activation of the signal transducer and activator of transcription 3 pathway [115].
In general, most of the generated hepatocyte-derived cell lines are not tumorigenic, but display reduced or only limited liver-specific functionality [7, 20, 102]. Taking into account that proliferation and differentiation are mutually exclusive in vitro, it has been shown that overexpression of the cdki, p21 and the use of conditional immortalization strategies can stimulate to some extent differentiation of the cells [6, 23, 84, 85, 102, 111, 116-120]. Other anti-dedifferentiation strategies developed to counteract the loss of functionality in primary hepatocyte cultures, including co-culture systems and overexpression of liver-specific genes have also proven usefull [121, 122].
4.2. Conditional immortalization strategies
Conditional immortalization enables the development of growth-controlled cell lines. At least three strategies have been reported to conditionally immortalize hepatocytes, namely (i) temperature-based regulation, (ii) recombinase-based regulation and (iii) transcriptional regulation. All these methods rely on the observation that hepatocyte proliferation only takes place when immortalizing genes are expressed [10] (Fig.2. & Table 1 & Table 2).
Temperature-based regulation
This method uses a temperature-sensitive SV40 Tag mutant. The immortalizing gene is expressed and active only at the permissive temperature (33°C), leading to the proliferation of hepatocytes. At higher temperatures (37-39°C), the immortalization gene is inactivated and cell cycle progression is no longer stimulated [10]. As no other temperature-labile immortalizing genes have yet been identified, this method is confined to SV40 Tag [10]. Moreover, the use of this strategy is not accompanied by the excision of the immortalization gene from the genome and thus could present a potential risk of tumorigenesis [84, 106, 123]. Nevertheless, some conditionally immortalized hepatic cell lines are based on this principle, and these cell lines can be transplanted efficiently in rat models of acute liver failure and chronic hepatic encephalopathy, usually without occurrence of tumorigenicity [88, 90, 102, 118-120, 124, 125]. However, concerns related to tumorigenicity form an important restriction to clinical appreciation of immortalized human hepatocytes [20]. Importantly, the temperature shift associated with this methodology might induce changes in cellular properties, which can complicate the interpretation of the study outcome. A more sophisticated system based on recombinase regulation is thought to offer a solution for these issues [10, 88, 118, 126, 127].
Recombinase-based control
The site-specific recombinase strategy uses recombinase expression to excise chromosomal DNA segments flanked by two recombination sequences and thereby irreversibly reverts immortalization [10, 128]. Numerous site-specific recombination systems, including the Cre-loxP and the FLP-FRT system, have been used to establish reversible immortalization. These systems have different efficiencies, whereby the Cre-loxP system stands out [123, 128]. In this system, immortalization genes are flanked by two identical DNA sequences, called LoxP sites. The excision of these genes is regulated by Cre recombinase [68, 123]. Proper reversion thus depends on the efficient transfer of the recombinase gene [10]. More recently, a new method based on tamoxifen-mediated self-excision has been established, rendering secondary virus-mediated transfer of the recombinase gene superfluous [87, 110, 111]. Furthermore, the suicide gene herpes simplex virus thymidine kinase (HSV-TK) has been introduced in the recombination construct as negative selection marker. Using this strategy, cells that still express the immortalization gene and HSV-TK gene due to improper recombination can be eliminated by exposure to ganciclovir [23, 123]. Reversible immortalization of numerous hepatocyte-derived cell lines, including C8-B, NKNT-3, IHH and 16T-3 depends on this recombinase-based control approach [23, 84, 85, 110, 111, 129, 130].
Transcriptional regulation
In this method, immortalization reversibility is obtained by transcriptional control of immortalization gene expression and not by recombinase activity. In this way, the risk of chromosomal rearrangement is avoided and repeated cycles of hepatocyte proliferation and growth arrest are allowed [10, 126, 127]. Transcription of immortalizing genes can be controlled by using an artificial promoter/transactivator system, such as the well-known tetracycline system [10]. Two approaches are currently available, the tet-off and the tet-on system, which are composed of a tetracycline-regulated promoter and a tetracycline transactivator (tTA) or reverse tetracycline transactivator (rtTA), respectively. When doxycycline is added to the cell culture medium, it binds to the transactivator. In the tet-on systems, bound rtTA interacts with the tetracycline-regulated promoter and induces the expression of the regulated gene. When using the tet-off method, immortalization genes are expressed in the absence of doxycycline, since only unbound tTA can interact with the gene promoter [126, 131]. The tet-on approach has been successfully used to produce a fetal liver cell line [7]. A drawback of this method, however, is the leaky transgene expression caused by undesired rtTA-tetracycline promoter binding in the absence of doxycycline [126, 131]. A tighter regulation of the transgene expression can be obtained by combining the rtTA system with a tetracycline-controlled transcriptional silencer [131].
4.3. Gene transfer
An effective gene transfer method is of utmost importance for immortalizing hepatocytes [91]. Different nonviral and viral methods have been used to generate immortalized hepatocyte-derived cell lines, namely plasmid transfection, viral transduction and the use of human artificial chromosomes.
Plasmid transfection
Various approaches are available for transfecting plasmids into primary hepatocytes [91, 132]. Due to immortalization, stably transfected cells are selected, allowing simple transfection procedures to be used [132]. Examples of common transfection methods that have been used to immortalize hepatocytes include calcium phosphate precipitation and electroporation [24, 25, 133, 134]. However, both approaches typically display low gene transfer efficiencies and high hepatocyte toxicity [91, 132]. Replacement of calcium by strontium eliminates toxicity but the gene transfer efficiency remains low [91]. Other researchers explored liposomes as gene carriers for hepatocyte immortalization [21, 83, 112, 135-137]. When properly optimized, lipid-mediated gene transfer can achieve high gene transfer efficiencies compared to other transfection approaches [91]. Furthermore, using hepatocyte-specific ligands, more hepatocyte-specific transfections can be achieved [132].
Viral transduction
Transduction with viral particles covers a widely used methodology for gene transfer. Among the available viral vectors, retroviral and lentiviral vectors induce stable integration of the immortalization gene and thus generate sustained transgene expression in the progeny [132, 138]. Furthermore, these vectors do not provoke harmful immune responses and allow integration of large genes [139]. Retroviral vectors, such as the Mo-MLV-derived vectors, have been frequently used to establish human and rodent hepatic cell lines [2, 9, 19, 84, 87, 102-104, 108, 110, 111, 118, 119, 124, 140, 141]. A major flaw in this system is its inability to transduce non-dividing cells, which makes it unsuitable for non-proliferating cells, including hepatocytes [139, 142]. Even when growth factors are added to the cell culture medium to induce hepatocyte mitosis, the efficiency of transduction usually remains limited [132, 139, 142, 143]. Lentiviral vectors derived from the human immunodeficiency virus (HIV) can tackle these issues and transduce both dividing and non-dividing cells by using virus at a relatively high titer [139, 142-144]. Moreover, lentiviral vectors provide high transduction efficiencies without affecting the differentiated hepatic phenotype [139, 143, 145]. Although lentiviral vectors lack hepatocyte specificity, the use of hepatocyte specific promoters can restrict the expression of lentiviral genes to the parenchymal liver cells [144]. Several studies have demonstrated appropiate gene transfer for immortalization of human adult and fetal hepatocytes [7, 20, 23]. Rodent hepatocytes, especially murine hepatocytes are considerably resistant to HIV vector-mediated transduction. This resistance has been related to a block in the immediate-early phase of infection [142]. In addition to the use of higher viral titers, cell culture medium supplied with growth factors, namely EGF and to lesser extent HGF, was found to improve lentiviral transduction efficacy of primary mouse hepatocytes [142, 146]. Similarly, when transducing human adult and fetal hepatocytes, the use of growth factors markedly upregulated the expression of lentiviral genes. Consequently, this transduction approach offers the possibility to reduce the viral load, which as such lowers cost and reduces cellular toxicity [144]. Also the antioxidant, vitamin E proved to significantly enhance lentiviral transduction rates of human and rat adult hepatocytes [142].
Human artificial chromosomes
The generation of a particular rat hepatic cell line was made possible by a more recent gene transfer method, namely generation of human artificial chromosome (HAC) [129, 147]. Although this method generally has lower transfer efficiency than the use of viral vectors, the HACs possess many properties of the ideal gene delivery vector. These include mitotically stable episomal maintenance and incorporation of large genes under control of their regulatory elements, allowing a correct, physiological regulated transgene expression. Furthermore, due to their episomal nature, integration-related complications, such as oncogenesis, should be avoided [138]. Immortalization of human fibroblasts using HAC-mediated episomal expression of hTERT has also been described, potentially offering new perspectives for hepatocyte immortalization [148].
5. Application of immortalized hepatic cell lines
It has repeatedly been postulated that immortalized hepatic cell lines which may offer an unlimited supply of well-characterized, pathogen-free cells could represent an attractive alternative for primary hepatocytes in several clinical applications as well as fundamental and applied research [106, 147, 149]. So far, multiple studies based on immortalized hepatocytes have already been performed.
5.1. Clinical application
Hepatocyte transplantation
The use of different animal models of hepatic impairment made it possible to demonstrate the therapeutic efficiency of transplanted cell lines. In this regard, it was shown that transplantation of conditional immortalized rat hepatocytes could protect portacaval-shunted rats from hyperammonia-induced hepatic encephalopathy [119, 149], improve survival of rat with acute liver failure (ALF) [125], adjust for bilirubin conjugation defect in Gunn rats [150, 151] and correct the global hepatic abnormalities associated with end-stage liver failure in cirrhotic animals [149]. Likewise, several human adult and fetal hepatic cell lines, including HHE6E7T1, NKNT-3, IHH, HepCL, 16T-3 and OUMS-29 were confirmed to promote survival in a pig [111], rat [152] or mice [23, 124, 153] model of ALF. Furthermore, YOCK-13, an insulin-producing human hepatic cell line was reported to control diabetes when transplanted into totally pancreatectomized diabetic pigs [110].
Bioartificial liver systems
For large-scale applications that rely on in vitro hepatic functionality, such as BAL systems, the development of a hepatic cell line that combines both in vitro hepatic function and proliferation capacity would be of great value.
Two human fetal hepatic cell lines, namely HepLi-4 and cBAL111, have already been evaluated as a potential cell source for BAL systems [87, 154]. However, it was revealed that both cell lines possessed insufficient hepatic functionality to be applicable for in vitro applications. The need for in vitro culture conditions that mimic the in vivo situation and promote hepatocyte differentiation in vitro was clearly emphasized [7, 87, 154]. This was further supported by experiments, which showed that cBAL111 cells are able to partly differentiate into functional hepatocytes once transplanted in vivo [7].
Different human adult hepatic cell lines have also been proposed as possible candidates for BAL application, but as for the modified fetal hepatic cell line, OUMS-29/H-11, data on efficacy in animal models of severe liver failure are currently lacking [112, 141, 155-158]. However, the production of ammonia [155] or possible inability to eliminate ammonia [141] are undesirable features for a BAL [158].
Another modified adult hepatic cell line composed of TTNT cells overexpressing IL-1 Ra has already been tested and was not able to improve survival of an ALF rat model [158].
5.2. Fundamental and applied research
Nowadays, human and rodent hepatic cell lines, such as CWSV [159, 160], H2.35 [161, 162], NeHepLxHT [163], OUMS-29 [164] and THLE [165, 166] are still being used for fundamental research. In this regard, a lot of investigations related to hepatotropic viruses have been performed on TPH1 cells [167, 168]. Furthermore, a murine model of HBV viremia based on immortalized human hepatocytes transfected with hepatitis B virus DNA has been created and offers the opportunity for in vivo HBV studies [169]. Several hepatic cell lines have also proven useful as in vitro tool for screening and safety testing of drug candidates. For instance, Hc3716-hTERT cells represented the first model for predicting the side-effects of telomere-targeting drugs in normal cells and it was suggested that the Fa2N4 cell line could be used for routine screening during discovery for pregnane X receptor mediated CYP3A4 induction [108, 170].
6. Conclusions and perspectives
In vitro expansion of human hepatocytes has gained considerable attention, as it might serve many clinical applications and fundamental research purposes. Prominent examples include the establishment of a bioartificial human liver device that can be used to bridge the time until liver transplantation is possible and the creation of a liver-based in vitro tool for screening and safety testing of drug candidates. As freshly isolated and cultured mature hepatocytes inherently have very poor growth potential, efforts have been focused on strategies to immortalize primary hepatocytes while maintaining their liver-specific functions. The currently available methods include transduction or transfection with prototypical immortalization genes and conditional immortalization by temperature-based regulation, recombinase-based control and transcriptional regulation. Although hepatocyte immortalization has been explored for years, it is still in its infancy since no cell lines with high hepatic functionality are yet available. As already postulated more attention should be paid to culture systems that support differentiation of the immortalized hepatocytes [6, 7, 87]. The past decade witnessed the introduction of novel strategies for cell immortalization based on the use of cell cycle regulators to surmount the p16-regulated premature growth arrest observed in several epithelial cells [171, 172]. Similarly, human myogenic cells immortalized by combined overexpression of hTERT, cyclin D1 and a mutant cdk4 isoform were able to overcome a p16-regulated precocious growth arrest without loss of their differentiation potential [173]. Although direct sequestration of p16 could not induce hepatocyte proliferation, it is worthwhile to examine the blocking of p16 control and pRB activity by overexpression of cell cycle regulators [20, 23]. A prerequisite to develop novel hepatocyte immortalization strategies is further fundamental research on the regulation of liver cell growth, especially in vitro. Such efforts should be strongly encouraged as they could lead to the generation of a robust hepatocyte-derived cell line with sustained liver-specific functionality resembling the in vivo situation. It can be anticipated that such a system will not only trigger a lot of interest among clinicians but also in the area of in vitro pharmaco-toxicology.
Key Point Box: Hepatocyte immortalization strategies.
Commonly used immortalization genes include viral oncogenes and hTERT.
Gene transfer is accomplished by viral and nonviral methods.
Conditional immortalization enables the production of growth-controlled cell lines.
Acknowledgements
This work was financially supported by the grants from the University Hospital of Vrije Universiteit Brussel (Willy Gepts Fonds UZ-VUB), the Fund for Scientific Research Flanders (FWO-Vlaanderen), the European Union (FP7/Cosmetics Europe projects HeMiBio and DETECTIVE) and the European Research Council (ERC Starting Grant project CONNECT).
Abbreviations
- BAL
bioartificial liver
- (h)TERT
(human) telomerase reverse transcriptase
- G
gap
- DNA
deoxyribonucleic acid
- mRNA
messenger ribonucleic acid
- MAPK
mitogen-activated protein kinase
- pRB
retinoblastoma protein
- HGF
hepatocyte growth factor
- EGF
epidermal growth factor
- TGF
transforming growth factor
- cdk(s)
cyclin-dependent kinase(s)
- S
synthesis
- M
mitosis
- cdki
cdk inhibitor
- PH
partial hepatectomy
- SV40 Tag
simian virus 40 large T antigen
- HPV16
human papillomavirus type 16
- Mo-MLV
Moloney Murine Leukemia virus
- Bmi-1
B lymphoma Mo-MLV insertion region 1 homolog
- HSV-TK
herpes simplex virus thymidine kinase
- tTA
tetracycline transactivator
- rtTA
reverse tetracycline transactivator
- HIV
human immunodeficiency virus
- HAC
human artificial chromosome
- ALF
acute liver failure
References
- [1].Pan XP, Li LJ. Advances in cell sources of hepatocytes for bioartificial liver. Hepatobiliary Pancreat Dis Int. 2012;11:594–605. doi: 10.1016/s1499-3872(12)60230-6. [DOI] [PubMed] [Google Scholar]
- [2].Wege H, Le HT, Chui MS, Liu L, Wu J, Giri R, et al. Telomerase reconstitution immortalizes human fetal hepatocytes without disrupting their differentiation potential. Gastroenterology. 2003;124:432–444. doi: 10.1053/gast.2003.50064. [DOI] [PubMed] [Google Scholar]
- [3].Gómez-Lechón MJ, Donato MT, Castell JV, Jover R. Human hepatocytes in primary culture: the choice to investigate drug metabolism in man. Curr Drug Metab. 2004;5:443–462. doi: 10.2174/1389200043335414. [DOI] [PubMed] [Google Scholar]
- [4].Hewitt NJ, Lechón MJ, Houston JB, Hallifax D, Brown HS, Maurel P, et al. Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab Rev. 2007;39:159–234. doi: 10.1080/03602530601093489. [DOI] [PubMed] [Google Scholar]
- [5].Allen JW, Bhatia SN. Improving the next generation of bioartificial liver devices. Semin Cell Dev Biol. 2002;13:447–454. doi: 10.1016/s1084952102001337. [DOI] [PubMed] [Google Scholar]
- [6].Chamuleau RA, Deurholt T, Hoekstra R. Which are the right cells to be used in a bioartificial liver? Metab Brain Dis. 2005;20:327–335. doi: 10.1007/s11011-005-7914-4. [DOI] [PubMed] [Google Scholar]
- [7].Deurholt T, van Til NP, Chhatta AA, ten Bloemendaal L, Schwartlander R, Payne C, et al. Novel immortalized human fetal liver cell line, cBAL111, has the potential to differentiate into functional hepatocytes. BMC Biotechnol. 2009;9:89. doi: 10.1186/1472-6750-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hoekstra R, Chamuleau RA. Recent developments on human cell lines for the bioartificial liver. Int J Artif Organs. 2002;25:182–191. doi: 10.1177/039139880202500304. [DOI] [PubMed] [Google Scholar]
- [9].Reid Y, Gaddipati JP, Yadav D, Kantor J. Establishment of a human neonatal hepatocyte cell line. In Vitro Cell Dev Biol Anim. 2009;45:535–542. doi: 10.1007/s11626-009-9219-0. [DOI] [PubMed] [Google Scholar]
- [10].Lipps C, May T, Hauser H, Wirth D. Eternity and functionality - rational access to physiologically relevant cell lines. Biol Chem. 2013;394:1637–1648. doi: 10.1515/hsz-2013-0158. [DOI] [PubMed] [Google Scholar]
- [11].Sinz M, Kim S. Stem cells, immortalized cells and primary cells in ADMET assays. Drug Discovery Today: Technologies. 2006;3:79–85. doi: 10.1016/j.ddtec.2006.03.006. [DOI] [PubMed] [Google Scholar]
- [12].Brandon EF, Raap CD, Meijerman I, Beijnen JH, Schellens JH. An update on in vitro test methods in human hepatic drug biotransformation research: pros and cons. Toxicol Appl Pharmacol. 2003;189:233–246. doi: 10.1016/s0041-008x(03)00128-5. [DOI] [PubMed] [Google Scholar]
- [13].Choi S, Sainz B, Corcoran P, Uprichard S, Jeong H. Characterization of increased drug metabolism activity in dimethyl sulfoxide (DMSO)-treated Huh7 hepatoma cells. Xenobiotica. 2009;39:205–217. doi: 10.1080/00498250802613620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lim PL, Tan W, Latchoumycandane C, Mok WC, Khoo YM, Lee HS, et al. Molecular and functional characterization of drug-metabolizing enzymes and transporter expression in the novel spontaneously immortalized human hepatocyte line HC-04. Toxicol In Vitro. 2007;21:1390–1401. doi: 10.1016/j.tiv.2007.05.003. [DOI] [PubMed] [Google Scholar]
- [15].Szabo M, Veres Z, Baranyai Z, Jakab F, Jemnitz K. Comparison of human hepatoma HepaRG cells with human and rat hepatocytes in uptake transport assays in order to predict a risk of drug induced hepatotoxicity. PLoS One. 2013;8:e59432. doi: 10.1371/journal.pone.0059432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Aninat C, Piton A, Glaise D, Le Charpentier T, Langouët S, Morel F, et al. Expression of cytochromes P450, conjugating enzymes and nuclear receptors in human hepatoma HepaRG cells. Drug Metab Dispos. 2006;34:75–83. doi: 10.1124/dmd.105.006759. [DOI] [PubMed] [Google Scholar]
- [17].Guillouzo A, Corlu A, Aninat C, Glaise D, Morel F, Guguen-Guillouzo C. The human hepatoma HepaRG cells: a highly differentiated model for studies of liver metabolism and toxicity of xenobiotics. Chem Biol Interact. 2007;168:66–73. doi: 10.1016/j.cbi.2006.12.003. [DOI] [PubMed] [Google Scholar]
- [18].Marion MJ, Hantz O, Durantel D. The HepaRG cell line: biological properties and relevance as a tool for cell biology, drug metabolism, and virology studies. Methods Mol Biol. 2010;640:261–272. doi: 10.1007/978-1-60761-688-7_13. [DOI] [PubMed] [Google Scholar]
- [19].Clayton RF, Rinaldi A, Kandyba EE, Edward M, Willberg C, Klenerman P, et al. Liver cell lines for the study of hepatocyte functions and immunological response. Liver Int. 2005;25:389–402. doi: 10.1111/j.1478-3231.2005.01017.x. [DOI] [PubMed] [Google Scholar]
- [20].Tsuruga Y, Kiyono T, Matsushita M, Takahashi T, Kasai H, Matsumoto S, et al. Establishment of immortalized human hepatocytes by introduction of HPV16 E6/E7 and hTERT as cell sources for liver cell-based therapy. Cell Transplant. 2008;17:1083–1094. [PubMed] [Google Scholar]
- [21].Fukaya K, Asahi S, Nagamori S, Sakaguchi M, Gao C, Miyazaki M, et al. Establishment of a human hepatocyte line (OUMS-29) having CYP 1A1 and 1A2 activities from fetal liver tissue by transfection of SV40 LT. In Vitro Cell Dev Biol Anim. 2001;37:266–269. doi: 10.1007/BF02577541. [DOI] [PubMed] [Google Scholar]
- [22].Hariparsad N, Carr BA, Evers R, Chu X. Comparison of immortalized Fa2N-4 cells and human hepatocytes as in vitro models for cytochrome P450 induction. Drug Metab Dispos. 2008;36:1046–1055. doi: 10.1124/dmd.108.020677. [DOI] [PubMed] [Google Scholar]
- [23].Nguyen TH, Mai G, Villiger P, Oberholzer J, Salmon P, Morel P, et al. Treatment of acetaminophen-induced acute liver failure in the mouse with conditionally immortalized human hepatocytes. J Hepatol. 2005;43:1031–1037. doi: 10.1016/j.jhep.2005.05.036. [DOI] [PubMed] [Google Scholar]
- [24].Watanabe N, Odagiri H, Totsuka E, Sasaki M. A new method to immortalize primary cultured rat hepatocytes. Transplant Proc. 2004;36:2457–2461. doi: 10.1016/j.transproceed.2004.08.030. [DOI] [PubMed] [Google Scholar]
- [25].Woodworth CD, Isom HC. Transformation of differentiated rat hepatocytes with adenovirus and adenovirus DNA. J Virol. 1987;61:3570–3579. doi: 10.1128/jvi.61.11.3570-3579.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mangnall D, Bird NC, Majeed AW. The molecular physiology of liver regeneration following partial hepatectomy. Liver Int. 2003;23:124–138. doi: 10.1034/j.1600-0676.2003.00812.x. [DOI] [PubMed] [Google Scholar]
- [27].Malato Y, Naqvi S, Schürmann N, Ng R, Wang B, Zape J, et al. Fate tracing of mature hepatocytes in mouse liver homeostasis and regeneration. J Clin Invest. 2011;121:4850–4860. doi: 10.1172/JCI59261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Corlu A, Loyer P. Regulation of the g1/s transition in hepatocytes: involvement of the cyclin-dependent kinase cdk1 in the DNA replication. Int J Hepatol. 2012;2012:689324. doi: 10.1155/2012/689324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- [30].Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- [31].Fausto N. Liver regeneration. J Hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- [32].Loyer P, Cariou S, Glaise D, Bilodeau M, Baffet G, Guguen-Guillouzo C. Growth factor dependence of progression through G1 and S phases of adult rat hepatocytes in vitro. Evidence of a mitogen restriction point in mid-late G1. J Biol Chem. 1996;271:11484–11492. doi: 10.1074/jbc.271.19.11484. [DOI] [PubMed] [Google Scholar]
- [33].Etienne PL, Baffet G, Desvergne B, Boisnard-Rissel M, Glaise D, Guguen-Guillouzo C. Transient expression of c-fos and constant expression of c-myc in freshly isolated and cultured normal adult rat hepatocytes. Oncogene Res. 1988;3:255–262. [PubMed] [Google Scholar]
- [34].Paine AJ, Andreakos E. Activation of signalling pathways during hepatocyte isolation: relevance to toxicology in vitro. Toxicol In Vitro. 2004;18:187–193. doi: 10.1016/s0887-2333(03)00146-2. [DOI] [PubMed] [Google Scholar]
- [35].Liu ML, Mars WM, Zarnegar R, Michalopoulos GK. Collagenase pretreatment and the mitogenic effects of hepatocyte growth factor and transforming growth factor-alpha in adult rat liver. Hepatology. 1994;19:1521–1527. [PubMed] [Google Scholar]
- [36].Ilyin G, Rescan C, Rialland M, Loyer P, Baffet G, Guguen-Guillouzo C. Growth control and cell cycle progression in cultured hepatocytes. In: Berry M, Edwards A, editors. The Hepatocyte Review. Springer Netherlands; 2000. pp. 263–280. [Google Scholar]
- [37].Mayhew CN, Bosco EE, Fox SR, Okaya T, Tarapore P, Schwemberger SJ, et al. Liver-specific pRB loss results in ectopic cell cycle entry and aberrant ploidy. Cancer Res. 2005;65:4568–4577. doi: 10.1158/0008-5472.CAN-04-4221. [DOI] [PubMed] [Google Scholar]
- [38].Novák B, Tyson JJ. A model for restriction point control of the mammalian cell cycle. J Theor Biol. 2004;230:563–579. doi: 10.1016/j.jtbi.2004.04.039. [DOI] [PubMed] [Google Scholar]
- [39].Schafer KA. The cell cycle: a review. Vet Pathol. 1998;35:461–478. doi: 10.1177/030098589803500601. [DOI] [PubMed] [Google Scholar]
- [40].Talarmin H, Rescan C, Cariou S, Glaise D, Zanninelli G, Bilodeau M, et al. The mitogen-activated protein kinase kinase/extracellular signal-regulated kinase cascade activation is a key signalling pathway involved in the regulation of G(1) phase progression in proliferating hepatocytes. Mol Cell Biol. 1999;19:6003–6011. doi: 10.1128/mcb.19.9.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Corlu A, Ilyin G, Cariou S, Lamy I, Loyer P, Guguen-Guillouzo C. The coculture: a system for studying the regulation of liver differentiation/proliferation activity and its control. Cell Biol Toxicol. 1997;13:235–242. doi: 10.1023/a:1007475122321. [DOI] [PubMed] [Google Scholar]
- [42].Albrecht JH, Mullany LK. Cell Cycle Control in the Liver. In: Arias I, editor. The Liver: Biology and Pathobiology. 5 ed. John Wiley & Sons; Chichester, UK: 2009. pp. 1015–1027. [Google Scholar]
- [43].Nelsen CJ, Rickheim DG, Timchenko NA, Stanley MW, Albrecht JH. Transient expression of cyclin D1 is sufficient to promote hepatocyte replication and liver growth in vivo. Cancer Res. 2001;61:8564–8568. [PubMed] [Google Scholar]
- [44].Rickheim DG, Nelsen CJ, Fassett JT, Timchenko NA, Hansen LK, Albrecht JH. Differential regulation of cyclins D1 and D3 in hepatocyte proliferation. Hepatology. 2002;36:30–38. doi: 10.1053/jhep.2002.33996. [DOI] [PubMed] [Google Scholar]
- [45].Albrecht JH, Hansen LK. Cyclin D1 promotes mitogen-independent cell cycle progression in hepatocytes. Cell Growth Differ. 1999;10:397–404. [PubMed] [Google Scholar]
- [46].Mullany LK, White P, Hanse EA, Nelsen CJ, Goggin MM, Mullany JE, et al. Distinct proliferative and transcriptional effects of the D-type cyclins in vivo. Cell Cycle. 2008;7:2215–2224. doi: 10.4161/cc.7.14.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wierød L, Rosseland CM, Lindeman B, Oksvold MP, Grøsvik H, Skarpen E, et al. CDK2 regulation through PI3K and CDK4 is necessary for cell cycle progression of primary rat hepatocytes. Cell Prolif. 2007;40:475–487. doi: 10.1111/j.1365-2184.2007.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Boylan JM, Gruppuso PA. D-type cyclins and G1 progression during liver development in the rat. Biochem Biophys Res Commun. 2005;330:722–730. doi: 10.1016/j.bbrc.2005.03.042. [DOI] [PubMed] [Google Scholar]
- [49].Curran TR, Bahner RI, Oh W, Gruppuso PA. Mitogen-independent DNA synthesis by fetal rat hepatocytes in primary culture. Exp Cell Res. 1993;209:53–57. doi: 10.1006/excr.1993.1284. [DOI] [PubMed] [Google Scholar]
- [50].Riehle KJ, Dan YY, Campbell JS, Fausto N. New concepts in liver regeneration. J Gastroenterol Hepatol. 2011;26(Suppl 1):203–212. doi: 10.1111/j.1440-1746.2010.06539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci. 2005;30:630–641. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- [52].Malumbres M, Harlow E, Hunt T, Hunter T, Lahti JM, Manning G, et al. Cyclin-dependent kinases: a family portrait. Nat Cell Biol. 2009;11:1275–1276. doi: 10.1038/ncb1109-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Guo Y, Yang K, Harwalkar J, Nye JM, Mason DR, Garrett MD, et al. Phosphorylation of cyclin D1 at Thr 286 during S phase leads to its proteasomal degradation and allows efficient DNA synthesis. Oncogene. 2005;24:2599–2612. doi: 10.1038/sj.onc.1208326. [DOI] [PubMed] [Google Scholar]
- [54].Chauhan A, Lorenzen S, Herzel H, Bernard S. Regulation of mammalian cell cycle progression in the regenerating liver. J Theor Biol. 2011;283:103–112. doi: 10.1016/j.jtbi.2011.05.026. [DOI] [PubMed] [Google Scholar]
- [55].Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36:131–149. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Fausto N, Campbell JS, Riehle KJ. Liver regeneration. J Hepatol. 2012;57:692–694. doi: 10.1016/j.jhep.2012.04.016. [DOI] [PubMed] [Google Scholar]
- [57].Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Oe S, Lemmer ER, Conner EA, Factor VM, Levéen P, Larsson J, et al. Intact signaling by transforming growth factor beta is not required for termination of liver regeneration in mice. Hepatology. 2004;40:1098–1105. doi: 10.1002/hep.20426. [DOI] [PubMed] [Google Scholar]
- [60].Wiemann SU, Satyanarayana A, Tsahuridu M, Tillmann HL, Zender L, Klempnauer J, et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 2002;16:935–942. doi: 10.1096/fj.01-0977com. [DOI] [PubMed] [Google Scholar]
- [61].Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, et al. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/− mice. Nat Biotechnol. 2007;25:903–910. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chiu CP, Harley CB. Replicative senescence and cell immortality: the role of telomeres and telomerase. Proc Soc Exp Biol Med. 1997;214:99–106. doi: 10.3181/00379727-214-44075. [DOI] [PubMed] [Google Scholar]
- [63].Ozturk M, Arslan-Ergul A, Bagislar S, Senturk S, Yuzugullu H. Senescence and immortality in hepatocellular carcinoma. Cancer Lett. 2009;286:103–113. doi: 10.1016/j.canlet.2008.10.048. [DOI] [PubMed] [Google Scholar]
- [64].HAYFLICK L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- [65].Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lee KM, Choi KH, Ouellette MM. Use of exogenous hTERT to immortalize primary human cells. Cytotechnology. 2004;45:33–38. doi: 10.1007/10.1007/s10616-004-5123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zhu J, Wang H, Bishop JM, Blackburn EH. Telomerase extends the lifespan of virus-transformed human cells without net telomere lengthening. Proc Natl Acad Sci U S A. 1999;96:3723–3728. doi: 10.1073/pnas.96.7.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Cascio SM. Novel strategies for immortalization of human hepatocytes. Artif Organs. 2001;25:529–538. doi: 10.1046/j.1525-1594.2001.025007529.x. [DOI] [PubMed] [Google Scholar]
- [69].Gorbunova V, Seluanov A. Coevolution of telomerase activity and body mass in mammals: from mice to beavers. Mech Ageing Dev. 2009;130:3–9. doi: 10.1016/j.mad.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Nozawa K, Kurumiya Y, Yamamoto A, Isobe Y, Suzuki M, Yoshida S. Up-regulation of telomerase in primary cultured rat hepatocytes. J Biochem. 1999;126:361–367. doi: 10.1093/oxfordjournals.jbchem.a022458. [DOI] [PubMed] [Google Scholar]
- [71].Yamaguchi Y, Nozawa K, Savoysky E, Hayakawa N, Nimura Y, Yoshida S. Change in telomerase activity of rat organs during growth and aging. Exp Cell Res. 1998;242:120–127. doi: 10.1006/excr.1998.4102. [DOI] [PubMed] [Google Scholar]
- [72].Inui T, Shinomiya N, Fukasawa M, Kobayashi M, Kuranaga N, Ohkura S, et al. Growth-related signaling regulates activation of telomerase in regenerating hepatocytes. Exp Cell Res. 2002;273:147–156. doi: 10.1006/excr.2001.5446. [DOI] [PubMed] [Google Scholar]
- [73].Overturf K, al-Dhalimy M, Ou CN, Finegold M, Grompe M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol. 1997;151:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- [74].Edwards A, Michalopoulos G. Conditions for growth of hepatocytes in culture. In: Berry M, Edwards A, editors. The Hepatocyte Review. Springer Netherlands; 2000. pp. 73–96. [Google Scholar]
- [75].Block GD, Locker J, Bowen WC, Petersen BE, Katyal S, Strom SC, et al. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J Cell Biol. 1996;132:1133–1149. doi: 10.1083/jcb.132.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Runge DM, Runge D, Dorko K, Pisarov LA, Leckel K, Kostrubsky VE, et al. Epidermal growth factor- and hepatocyte growth factor-receptor activity in serum-free cultures of human hepatocytes. J Hepatol. 1999;30:265–274. doi: 10.1016/s0168-8278(99)80073-7. [DOI] [PubMed] [Google Scholar]
- [77].Ohtani N, Yamakoshi K, Takahashi A, Hara E. The p16INK4a-RB pathway: molecular link between cellular senescence and tumor suppression. J Med Invest. 2004;51:146–153. doi: 10.2152/jmi.51.146. [DOI] [PubMed] [Google Scholar]
- [78].Auer KL, Park JS, Seth P, Coffey RJ, Darlington G, Abo A, et al. Prolonged activation of the mitogen-activated protein kinase pathway promotes DNA synthesis in primary hepatocytes from p21Cip-1/WAF1-null mice, but not in hepatocytes from p16INK4a-null mice. Biochem J. 1998;336(Pt 3):551–560. doi: 10.1042/bj3360551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Frémin C, Bessard A, Ezan F, Gailhouste L, Régeard M, Le Seyec J, et al. Multiple division cycles and long-term survival of hepatocytes are distinctly regulated by extracellular signal-regulated kinases ERK1 and ERK2. Hepatology. 2009;49:930–939. doi: 10.1002/hep.22730. [DOI] [PubMed] [Google Scholar]
- [80].Harashima M, Seki T, Ariga T, Niimi S. Role of p16(INK4a) in the inhibition of DNA synthesis stimulated by HGF or EGF in primary cultured rat hepatocytes. Biomed Res. 2013;34:269–273. doi: 10.2220/biomedres.34.269. [DOI] [PubMed] [Google Scholar]
- [81].Ilyin GP, Glaise D, Gilot D, Baffet G, Guguen-Guillouzo C. Regulation and role of p21 and p27 cyclin-dependent kinase inhibitors during hepatocyte differentiation and growth. Am J Physiol Gastrointest Liver Physiol. 2003;285:G115–127. doi: 10.1152/ajpgi.00309.2002. [DOI] [PubMed] [Google Scholar]
- [82].Tombes RM, Auer KL, Mikkelsen R, Valerie K, Wymann MP, Marshall CJ, et al. The mitogen-activated protein (MAP) kinase cascade can either stimulate or inhibit DNA synthesis in primary cultures of rat hepatocytes depending upon whether its activation is acute/phasic or chronic. Biochem J. 1998;330(Pt 3):1451–1460. doi: 10.1042/bj3301451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Li J, Li LJ, Cao HC, Sheng GP, Yu HY, Xu W, et al. Establishment of highly differentiated immortalized human hepatocyte line with simian virus 40 large tumor antigen for liver based cell therapy. ASAIO J. 2005;51:262–268. doi: 10.1097/01.mat.0000161045.16805.8b. [DOI] [PubMed] [Google Scholar]
- [84].Cai J, Ito M, Westerman KA, Kobayashi N, Leboulch P, Fox IJ. Construction of a non-tumorigenic rat hepatocyte cell line for transplantation: reversal of hepatocyte immortalization by site-specific excision of the SV40 T antigen. J Hepatol. 2000;33:701–708. doi: 10.1016/s0168-8278(00)80299-8. [DOI] [PubMed] [Google Scholar]
- [85].Kobayashi N, Fujiwara T, Westerman KA, Inoue Y, Sakaguchi M, Noguchi H, et al. Prevention of acute liver failure in rats with reversibly immortalized human hepatocytes. Science. 2000;287:1258–1262. doi: 10.1126/science.287.5456.1258. [DOI] [PubMed] [Google Scholar]
- [86].Mills JB, Rose KA, Sadagopan N, Sahi J, de Morais SM. Induction of drug metabolism enzymes and MDR1 using a novel human hepatocyte cell line. J Pharmacol Exp Ther. 2004;309:303–309. doi: 10.1124/jpet.103.061713. [DOI] [PubMed] [Google Scholar]
- [87].Zhao L, Li J, Lv G, Zhang A, Zhou P, Yang Y, et al. Evaluation of a reversibly immortalized human hepatocyte line in bioartificial liver in pigs. African Journal of Biotechnology. 2012;11:4116–4126. [Google Scholar]
- [88].Allen KJ, Reyes R, Demmler K, Mercer JF, Williamson R, Whitehead RH. Conditionally immortalized mouse hepatocytes for use in liver gene therapy. J Gastroenterol Hepatol. 2000;15:1325–1332. [PubMed] [Google Scholar]
- [89].Bulera SJ, Haas MJ, Sattler CA, Li Y, Pitot HC. Cell lines with heterogeneous phenotypes result from a single isolation of albumin-sv40 T-antigen transgenic rat hepatocytes. Hepatology. 1997;25:1192–1203. doi: 10.1002/hep.510250523. [DOI] [PubMed] [Google Scholar]
- [90].Yanai N, Suzuki M, Obinata M. Hepatocyte cell lines established from transgenic mice harboring temperature-sensitive simian virus 40 large T-antigen gene. Exp Cell Res. 1991;197:50–56. doi: 10.1016/0014-4827(91)90478-d. [DOI] [PubMed] [Google Scholar]
- [91].McLean J. Immortalization Strategies for Mammalian Cells. In: Jenkins N, editor. Animal Cell Biotechnology: Methods and Protocols. Humana Press; 1999. pp. 61–72. [Google Scholar]
- [92].Noguchi H, Kobayashi N. Controlled expansion of mammalian cell populations by reversible immortalization. J Biotechnol Biomater. 2013:3. [Google Scholar]
- [93].Gabet AS, Accardi R, Bellopede A, Popp S, Boukamp P, Sylla BS, et al. Impairment of the telomere/telomerase system and genomic instability are associated with keratinocyte immortalization induced by the skin human papillomavirus type 38. FASEB J. 2008;22:622–632. doi: 10.1096/fj.07-8389com. [DOI] [PubMed] [Google Scholar]
- [94].Kyo S, Nakamura M, Kiyono T, Maida Y, Kanaya T, Tanaka M, et al. Successful immortalization of endometrial glandular cells with normal structural and functional characteristics. Am J Pathol. 2003;163:2259–2269. doi: 10.1016/S0002-9440(10)63583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Caporossi D, Bacchetti S. Definition of adenovirus type 5 functions involved in the induction of chromosomal aberrations in human cells. J Gen Virol. 1990;71(Pt 4):801–808. doi: 10.1099/0022-1317-71-4-801. [DOI] [PubMed] [Google Scholar]
- [96].Schramayr S, Caporossi D, Mak I, Jelinek T, Bacchetti S. Chromosomal damage induced by human adenovirus type 12 requires expression of the E1B 55-kilodalton viral protein. J Virol. 1990;64:2090–2095. doi: 10.1128/jvi.64.5.2090-2095.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Chang TH, Ray FA, Thompson DA, Schlegel R. Disregulation of mitotic checkpoints and regulatory proteins following acute expression of SV40 large T antigen in diploid human cells. Oncogene. 1997;14:2383–2393. doi: 10.1038/sj.onc.1201196. [DOI] [PubMed] [Google Scholar]
- [98].Toouli CD, Huschtscha LI, Neumann AA, Noble JR, Colgin LM, Hukku B, et al. Comparison of human mammary epithelial cells immortalized by simian virus 40 T-Antigen or by the telomerase catalytic subunit. Oncogene. 2002;21:128–139. doi: 10.1038/sj.onc.1205014. [DOI] [PubMed] [Google Scholar]
- [99].Stewart N, Bacchetti S. Expression of SV40 large T antigen, but not small t antigen, is required for the induction of chromosomal aberrations in transformed human cells. Virology. 1991;180:49–57. doi: 10.1016/0042-6822(91)90008-y. [DOI] [PubMed] [Google Scholar]
- [100].Ray FA, Peabody DS, Cooper JL, Cram LS, Kraemer PM. SV40 T antigen alone drives karyotype instability that precedes neoplastic transformation of human diploid fibroblasts. J Cell Biochem. 1990;42:13–31. doi: 10.1002/jcb.240420103. [DOI] [PubMed] [Google Scholar]
- [101].Coursen JD, Bennett WP, Gollahon L, Shay JW, Harris CC. Genomic instability and telomerase activity in human bronchial epithelial cells during immortalization by human papillomavirus-16 E6 and E7 genes. Exp Cell Res. 1997;235:245–253. doi: 10.1006/excr.1997.3670. [DOI] [PubMed] [Google Scholar]
- [102].Kim BH, Sung SR, Choi EH, Kim YI, Kim KJ, Dong SH, et al. Dedifferentiation of conditionally immortalized hepatocytes with long-term in vitro passage. Exp Mol Med. 2000;32:29–37. doi: 10.1038/emm.2000.6. [DOI] [PubMed] [Google Scholar]
- [103].Smalley M, Leiper K, Tootle R, McCloskey P, O’Hare MJ, Hodgson H. Immortalization of human hepatocytes by temperature-sensitive SV40 large-T antigen. In Vitro Cell Dev Biol Anim. 2001;37:166–168. doi: 10.1290/1071-2690(2001)037<0166:IOHHBT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- [104].Pfeifer AM, Cole KE, Smoot DT, Weston A, Groopman JD, Shields PG, et al. Simian virus 40 large tumor antigen-immortalized normal human liver epithelial cells express hepatocyte characteristics and metabolize chemical carcinogens. Proc Natl Acad Sci U S A. 1993;90:5123–5127. doi: 10.1073/pnas.90.11.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Woodworth CD, Kreider JW, Mengel L, Miller T, Meng YL, Isom HC. Tumorigenicity of simian virus 40-hepatocyte cell lines: effect of in vitro and in vivo passage on expression of liver-specific genes and oncogenes. Mol Cell Biol. 1988;8:4492–4501. doi: 10.1128/mcb.8.10.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Guha C, Chowdhury N, Chowdhury J. Reversibly immortalized human hepatocytes: an eternal fountain of liver support? Hepatology. 2000;32:440–441. doi: 10.1002/hep.510320240. [DOI] [PubMed] [Google Scholar]
- [107].Isom HC, Woodworth CD, Meng Y, Kreider J, Miller T, Mengel L. Introduction of the ras oncogene transforms a simian virus 40-immortalized hepatocyte cell line without loss of expression of albumin and other liver-specific genes. Cancer Res. 1992;52:940–948. [PubMed] [Google Scholar]
- [108].Waki K, Anno K, Ono T, Ide T, Chayama K, Tahara H. Establishment of functional telomerase immortalized human hepatocytes and a hepatic stellate cell line for telomere-targeting anticancer drug development. Cancer Sci. 2010;101:1678–1685. doi: 10.1111/j.1349-7006.2010.01576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wege H, Chui MS, Le HT, Strom SC, Zern MA. In vitro expansion of human hepatocytes is restricted by telomere-dependent replicative aging. Cell Transplant. 2003;12:897–906. doi: 10.3727/000000003771000138. [DOI] [PubMed] [Google Scholar]
- [110].Okitsu T, Kobayashi N, Jun HS, Shin S, Kim SJ, Han J, et al. Transplantation of reversibly immortalized insulin-secreting human hepatocytes controls diabetes in pancreatectomized pigs. Diabetes. 2004;53:105–112. doi: 10.2337/diabetes.53.1.105. [DOI] [PubMed] [Google Scholar]
- [111].Totsugawa T, Yong C, Rivas-Carrillo JD, Soto-Gutierrez A, Navarro-Alvarez N, Noguchi H, et al. Survival of liver failure pigs by transplantation of reversibly immortalized human hepatocytes with Tamoxifen-mediated self-recombination. J Hepatol. 2007;47:74–82. doi: 10.1016/j.jhep.2007.02.019. [DOI] [PubMed] [Google Scholar]
- [112].Werner A, Duvar S, Müthing J, Büntemeyer H, Kahmann U, Lünsdorf H, et al. Cultivation and characterization of a new immortalized human hepatocyte cell line, HepZ, for use in an artificial liver support system. Ann N Y Acad Sci. 1999;875:364–368. doi: 10.1111/j.1749-6632.1999.tb08518.x. [DOI] [PubMed] [Google Scholar]
- [113].Ray RB, Meyer K, Ray R. Hepatitis C virus core protein promotes immortalization of primary human hepatocytes. Virology. 2000;271:197–204. doi: 10.1006/viro.2000.0295. [DOI] [PubMed] [Google Scholar]
- [114].Basu A, Meyer K, Ray RB, Ray R. Hepatitis C virus core protein is necessary for the maintenance of immortalized human hepatocytes. Virology. 2002;298:53–62. doi: 10.1006/viro.2002.1460. [DOI] [PubMed] [Google Scholar]
- [115].Basu A, Meyer K, Lai KK, Saito K, Di Bisceglie AM, Grosso LE, et al. Microarray analyses and molecular profiling of Stat3 signaling pathway induced by hepatitis C virus core protein in human hepatocytes. Virology. 2006;349:347–358. doi: 10.1016/j.virol.2006.02.023. [DOI] [PubMed] [Google Scholar]
- [116].Kunieda T, Kobayashi N, Sakaguchi M, Okitsu T, Totsugawa T, Watanabe T, et al. Transduction of immortalized human hepatocytes with p21 to enhance differentiated phenotypes. Cell Transplant. 2002;11:421–428. [PubMed] [Google Scholar]
- [117].Kobayashi N, Kunieda T, Sakaguchi M, Okitsu T, Totsugawa T, Maruyama M, et al. Active expression of p21 facilitates differentiation of immortalized human hepatocytes. Transplant Proc. 2003;35:433–434. doi: 10.1016/s0041-1345(02)03784-3. [DOI] [PubMed] [Google Scholar]
- [118].Fox IJ, Chowdhury NR, Gupta S, Kondapalli R, Schilsky ML, Stockert RJ, et al. Conditional immortalization of Gunn rat hepatocytes: an ex vivo model for evaluating methods for bilirubin-UDP-glucuronosyltransferase gene transfer. Hepatology. 1995;21:837–846. [PubMed] [Google Scholar]
- [119].Schumacher IK, Okamoto T, Kim BH, Chowdhury NR, Chowdhury JR, Fox IJ. Transplantation of conditionally immortalized hepatocytes to treat hepatic encephalopathy. Hepatology. 1996;24:337–343. doi: 10.1002/hep.510240209. [DOI] [PubMed] [Google Scholar]
- [120].Zaret KS, DiPersio CM, Jackson DA, Montigny WJ, Weinstat DL. Conditional enhancement of liver-specific gene transcription. Proc Natl Acad Sci U S A. 1988;85:9076–9080. doi: 10.1073/pnas.85.23.9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Watanabe T, Shibata N, Westerman KA, Okitsu T, Allain JE, Sakaguchi M, et al. Establishment of immortalized human hepatic stellate scavenger cells to develop bioartificial livers. Transplantation. 2003;75:1873–1880. doi: 10.1097/01.TP.0000064621.50907.A6. [DOI] [PubMed] [Google Scholar]
- [122].Inoue Y, Miyazaki M, Tsuji T, Sakaguchi M, Fukaya K, Huh NH, et al. Reactivation of liver-specific gene expression in an immortalized human hepatocyte cell line by introduction of the human HNF4alpha2 gene. Int J Mol Med. 2001;8:481–487. doi: 10.3892/ijmm.8.5.481. [DOI] [PubMed] [Google Scholar]
- [123].Paillard F. Reversible cell immortalization with the Cre-lox system. Hum Gene Ther. 1999;10:1597–1598. doi: 10.1089/10430349950017590. [DOI] [PubMed] [Google Scholar]
- [124].Chen Y, Li J, Liu X, Zhao W, Wang Y, Wang X. Transplantation of immortalized human fetal hepatocytes prevents acute liver failure in 90% hepatectomized mice. Transplant Proc. 2010;42:1907–1914. doi: 10.1016/j.transproceed.2010.01.061. [DOI] [PubMed] [Google Scholar]
- [125].Nakamura J, Okamoto T, Schumacher IK, Tabei I, Chowdhury NR, Chowdhury JR, et al. Treatment of surgically induced acute liver failure by transplantation of conditionally immortalized hepatocytes. Transplantation. 1997;63:1541–1547. doi: 10.1097/00007890-199706150-00001. [DOI] [PubMed] [Google Scholar]
- [126].Anastassiadis K, Rostovskaya M, Lubitz S, Weidlich S, Stewart AF. Precise conditional immortalization of mouse cells using tetracycline-regulated SV40 large T-antigen. Genesis. 2010;48:220–232. doi: 10.1002/dvg.20605. [DOI] [PubMed] [Google Scholar]
- [127].May T, Hauser H, Wirth D. Transcriptional control of SV40 T-antigen expression allows a complete reversion of immortalization. Nucleic Acids Res. 2004;32:5529–5538. doi: 10.1093/nar/gkh887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Westerman KA, Leboulch P. Reversible immortalization of mammalian cells mediated by retroviral transfer and site-specific recombination. Proc Natl Acad Sci U S A. 1996;93:8971–8976. doi: 10.1073/pnas.93.17.8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Ito M, Ito R, Yoshihara D, Ikeno M, Kamiya M, Suzuki N, et al. Immortalized hepatocytes using human artificial chromosome. Cell Transplant. 2008;17:165–171. doi: 10.3727/000000008783906883. [DOI] [PubMed] [Google Scholar]
- [130].Kobayashi N, Noguchi H, Fujiwara T, Tanaka N. Establishment of a reversibly immortalized human hepatocyte cell line by using Cre/loxP site-specific recombination. Transplant Proc. 2000;32:1121–1122. doi: 10.1016/s0041-1345(00)01154-4. [DOI] [PubMed] [Google Scholar]
- [131].Jazwa A, Florczyk U, Jozkowicz A, Dulak J. Gene therapy on demand: site specific regulation of gene therapy. Gene. 2013;525:229–238. doi: 10.1016/j.gene.2013.03.093. [DOI] [PubMed] [Google Scholar]
- [132].Wang X, Mani P, Sarkar DP, Roy-Chowdhury N, Roy-Chowdhury J. Ex vivo gene transfer into hepatocytes. Methods Mol Biol. 2009;481:117–140. doi: 10.1007/978-1-59745-201-4_11. [DOI] [PubMed] [Google Scholar]
- [133].Macdonald C, Willett B. The immortalisation of rat hepatocytes by transfection with SV40 sequences. Cytotechnology. 1997;23:161–170. doi: 10.1023/A:1007907416596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Woodworth C, Secott T, Isom HC. Transformation of rat hepatocytes by transfection with simian virus 40 DNA to yield proliferating differentiated cells. Cancer Res. 1986;46:4018–4026. [PubMed] [Google Scholar]
- [135].Schippers IJ, Moshage H, Roelofsen H, Müller M, Heymans HS, Ruiters M, et al. Immortalized human hepatocytes as a tool for the study of hepatocytic (de-)differentiation. Cell Biol Toxicol. 1997;13:375–386. doi: 10.1023/a:1007404028681. [DOI] [PubMed] [Google Scholar]
- [136].Kobayashi N, Noguchi H, Watanabe T, Matsumura T, Totsugawa T, Fujiwara T, et al. Role of immortalized hepatocyte transplantation in acute liver failure. Transplant Proc. 2001;33:645–646. doi: 10.1016/s0041-1345(00)02182-5. [DOI] [PubMed] [Google Scholar]
- [137].Noguchi M, Hirohashi S. Cell lines from non-neoplastic liver and hepatocellular carcinoma tissue from a single patient. In Vitro Cell Dev Biol Anim. 1996;32:135–137. doi: 10.1007/BF02723678. [DOI] [PubMed] [Google Scholar]
- [138].Kazuki Y, Oshimura M. Human artificial chromosomes for gene delivery and the development of animal models. Mol Ther. 2011;19:1591–1601. doi: 10.1038/mt.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Zahler MH, Irani A, Malhi H, Reutens AT, Albanese C, Bouzahzah B, et al. The application of a lentiviral vector for gene transfer in fetal human hepatocytes. J Gene Med. 2000;2:186–193. doi: 10.1002/(SICI)1521-2254(200005/06)2:3<186::AID-JGM100>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- [140].Kobayashi N, Noguchi H, Fujiwara T, Westerman KA, Leboulch P, Tanaka N. Establishment of a highly differentiated immortalized adult human hepatocyte cell line by retroviral gene transfer. Transplant Proc. 2000;32:2368–2369. doi: 10.1016/s0041-1345(00)01702-4. [DOI] [PubMed] [Google Scholar]
- [141].Pan X, Li J, Du W, Yu X, Zhu C, Yu C, et al. Establishment and characterization of immortalized human hepatocyte cell line for applications in bioartificial livers. Biotechnol Lett. 2012;34:2183–2190. doi: 10.1007/s10529-012-1025-1. [DOI] [PubMed] [Google Scholar]
- [142].Nguyen TH, Oberholzer J, Birraux J, Majno P, Morel P, Trono D. Highly efficient lentiviral vector-mediated transduction of nondividing, fully reimplantable primary hepatocytes. Mol Ther. 2002;6:199–209. doi: 10.1006/mthe.2002.0653. [DOI] [PubMed] [Google Scholar]
- [143].Ohashi K, Park F, Kay MA. Hepatocyte transplantation: clinical and experimental application. J Mol Med (Berl) 2001;79:617–630. doi: 10.1007/s001090100260. [DOI] [PubMed] [Google Scholar]
- [144].Selden C, Mellor N, Rees M, Laurson J, Kirwan M, Escors D, et al. Growth factors improve gene expression after lentiviral transduction in human adult and fetal hepatocytes. J Gene Med. 2007;9:67–76. doi: 10.1002/jgm.1000. [DOI] [PubMed] [Google Scholar]
- [145].Zamule SM, Strom SC, Omiecinski CJ. Preservation of hepatic phenotype in lentiviral-transduced primary human hepatocytes. Chem Biol Interact. 2008;173:179–186. doi: 10.1016/j.cbi.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Rothe M, Rittelmeyer I, Iken M, Rüdrich U, Schambach A, Glage S, et al. Epidermal growth factor improves lentivirus vector gene transfer into primary mouse hepatocytes. Gene Ther. 2012;19:425–434. doi: 10.1038/gt.2011.117. [DOI] [PubMed] [Google Scholar]
- [147].Ito M, Ikeno M, Nagata H, Yamamoto T, Hiroguchi A, Fox IJ, et al. Treatment of nonalbumin rats by transplantation of immortalized hepatocytes using artificial human chromosome. Transplant Proc. 2009;41:422–424. doi: 10.1016/j.transproceed.2008.10.023. [DOI] [PubMed] [Google Scholar]
- [148].Shitara S, Kakeda M, Nagata K, Hiratsuka M, Sano A, Osawa K, et al. Telomerase-mediated life-span extension of human primary fibroblasts by human artificial chromosome (HAC) vector. Biochem Biophys Res Commun. 2008;369:807–811. doi: 10.1016/j.bbrc.2008.02.119. [DOI] [PubMed] [Google Scholar]
- [149].Cai J, Ito M, Nagata H, Westerman KA, Lafleur D, Chowdhury JR, et al. Treatment of liver failure in rats with end-stage cirrhosis by transplantation of immortalized hepatocytes. Hepatology. 2002;36:386–394. doi: 10.1053/jhep.2002.34614. [DOI] [PubMed] [Google Scholar]
- [150].Tada K, Roy-Chowdhury N, Prasad V, Kim BH, Manchikalapudi P, Fox IJ, et al. Long-term amelioration of bilirubin glucuronidation defect in Gunn rats by transplanting genetically modified immortalized autologous hepatocytes. Cell Transplant. 1998;7:607–616. doi: 10.1177/096368979800700611. [DOI] [PubMed] [Google Scholar]
- [151].Kim BH, Han YS, Dong SH, Kim HJ, Chang YW, Lee JI, et al. Temporary amelioration of bilirubin conjugation defect in Gunn rats by transplanting conditionally immortalized hepatocytes. J Gastroenterol Hepatol. 2002;17:690–696. doi: 10.1046/j.1440-1746.2002.02744.x. [DOI] [PubMed] [Google Scholar]
- [152].Kobayashi N, Noguchi H, Fujiwara T, Tanaka N. Xenotransplantation of immortalized human hepatocytes for experimental acute liver failure in rats. Transplant Proc. 2000;32:1123–1124. doi: 10.1016/s0041-1345(00)01155-6. [DOI] [PubMed] [Google Scholar]
- [153].Tsuruga Y, Kiyono T, Matsushita M, Takahashi T, Kasai N, Matsumoto S, et al. Effect of intrasplenic transplantation of immortalized human hepatocytes in the treatment of acetaminophen-induced acute liver failure SCID mice. Transplant Proc. 2008;40:617–619. doi: 10.1016/j.transproceed.2008.02.007. [DOI] [PubMed] [Google Scholar]
- [154].Poyck PP, van Wijk AC, van der Hoeven TV, de Waart DR, Chamuleau RA, van Gulik TM, et al. Evaluation of a new immortalized human fetal liver cell line (cBAL111) for application in bioartificial liver. J Hepatol. 2008;48:266–275. doi: 10.1016/j.jhep.2007.09.018. [DOI] [PubMed] [Google Scholar]
- [155].Werner A, Duvar S, Müthing J, Büntemeyer H, Lünsdorf H, Strauss M, et al. Cultivation of immortalized human hepatocytes HepZ on macroporous CultiSpher G microcarriers. Biotechnol Bioeng. 2000;68:59–70. doi: 10.1002/(sici)1097-0290(20000405)68:1<59::aid-bit7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- [156].Yu CB, Lv GL, Pan XP, Chen YS, Cao HC, Zhang YM, et al. In vitro large-scale cultivation and evaluation of microencapsulated immortalized human hepatocytes (HepLL) in roller bottles. Int J Artif Organs. 2009;32:272–281. doi: 10.1177/039139880903200504. [DOI] [PubMed] [Google Scholar]
- [157].Akiyama I, Tomiyama K, Sakaguchi M, Takaishi M, Mori M, Hosokawa M, et al. Expression of CYP3A4 by an immortalized human hepatocyte line in a three-dimensional culture using a radial-flow bioreactor. Int J Mol Med. 2004;14:663–668. [PubMed] [Google Scholar]
- [158].Nibourg GA, Chamuleau RA, van Gulik TM, Hoekstra R. Proliferative human cell sources applied as biocomponent in bioartificial livers: a review. Expert Opin Biol Ther. 2012;12:905–921. doi: 10.1517/14712598.2012.685714. [DOI] [PubMed] [Google Scholar]
- [159].Buzzelli MD, Nagarajan M, Radtka JF, Shumate ML, Navaratnarajah M, Lang CH, et al. Nuclear factor-kappaB mediates the inhibitory effects of tumor necrosis factor-alpha on growth hormone-inducible gene expression in liver. Endocrinology. 2008;149:6378–6388. doi: 10.1210/en.2007-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Ahmed TA, Buzzelli MD, Lang CH, Capen JB, Shumate ML, Navaratnarajah M, et al. Interleukin-6 inhibits growth hormone-mediated gene expression in hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1793–1803. doi: 10.1152/ajpgi.00547.2006. [DOI] [PubMed] [Google Scholar]
- [161].Bai J, Li J, Mao Q. Construction of a single lentiviral vector containing tetracycline-inducible Alb-uPA for transduction of uPA expression in murine hepatocytes. PLoS One. 2013;8:e61412. doi: 10.1371/journal.pone.0061412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Samavati L, Lee I, Mathes I, Lottspeich F, Hüttemann M. Tumor necrosis factor alpha inhibits oxidative phosphorylation through tyrosine phosphorylation at subunit I of cytochrome c oxidase. J Biol Chem. 2008;283:21134–21144. doi: 10.1074/jbc.M801954200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Gao Y, Theng SS, Zhuo J, Teo WB, Ren J, Lee CG. FAT10, an ubiquitin-like protein, confers malignant properties in non-tumorigenic and tumorigenic cells. Carcinogenesis. 2014;35:923–934. doi: 10.1093/carcin/bgt407. [DOI] [PubMed] [Google Scholar]
- [164].Tomimaru Y, Xu CQ, Nambotin SB, Yan T, Wands JR, Kim M. Loss of exon 4 in a human T-cell factor-4 isoform promotes hepatic tumourigenicity. Liver Int. 2013;33:1536–1548. doi: 10.1111/liv.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Rand AA, Rooney JP, Butt CM, Meyer JN, Mabury SA. Cellular toxicity associated with exposure to perfluorinated carboxylates (PFCAs) and their metabolic precursors. Chem Res Toxicol. 2014;27:42–50. doi: 10.1021/tx400317p. [DOI] [PubMed] [Google Scholar]
- [166].Krajka-Kuźniak V, Paluszczak J, Baer-Dubowska W. Xanthohumol induces phase II enzymes via Nrf2 in human hepatocytes in vitro. Toxicol In Vitro. 2013;27:149–156. doi: 10.1016/j.tiv.2012.10.008. [DOI] [PubMed] [Google Scholar]
- [167].Raychoudhuri A, Shrivastava S, Steele R, Dash S, Kanda T, Ray R, et al. Hepatitis C virus infection impairs IRF-7 translocation and Alpha interferon synthesis in immortalized human hepatocytes. J Virol. 2010;84:10991–10998. doi: 10.1128/JVI.00900-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Raychoudhuri A, Shrivastava S, Steele R, Kim H, Ray R, Ray RB. ISG56 and IFITM1 proteins inhibit hepatitis C virus replication. J Virol. 2011;85:12881–12889. doi: 10.1128/JVI.05633-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Brown JJ, Parashar B, Moshage H, Tanaka KE, Engelhardt D, Rabbani E, et al. A long-term hepatitis B viremia model generated by transplanting nontumorigenic immortalized human hepatocytes in Rag-2-deficient mice. Hepatology. 2000;31:173–181. doi: 10.1002/hep.510310126. [DOI] [PubMed] [Google Scholar]
- [170].McGinnity DF, Zhang G, Kenny JR, Hamilton GA, Otmani S, Stams KR, et al. Evaluation of multiple in vitro systems for assessment of CYP3A4 induction in drug discovery: human hepatocytes, pregnane X receptor reporter gene, and Fa2N-4 and HepaRG cells. Drug Metab Dispos. 2009;37:1259–1268. doi: 10.1124/dmd.109.026526. [DOI] [PubMed] [Google Scholar]
- [171].Ramirez RD, Herbert BS, Vaughan MB, Zou Y, Gandia K, Morales CP, et al. Bypass of telomere-dependent replicative senescence (M1) upon overexpression of Cdk4 in normal human epithelial cells. Oncogene. 2003;22:433–444. doi: 10.1038/sj.onc.1206046. [DOI] [PubMed] [Google Scholar]
- [172].Ramirez RD, Morales CP, Herbert BS, Rohde JM, Passons C, Shay JW, et al. Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. Genes Dev. 2001;15:398–403. doi: 10.1101/gad.859201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Shiomi K, Kiyono T, Okamura K, Uezumi M, Goto Y, Yasumoto S, et al. CDK4 and cyclin D1 allow human myogenic cells to recapture growth property without compromising differentiation potential. Gene Ther. 2011;18:857–866. doi: 10.1038/gt.2011.44. [DOI] [PubMed] [Google Scholar]
- [174].Cooper G. The Eukaryotic Cell Cycle. In: Cooper G, editor. The Cell: A Molecular Approach. 2 ed. Sinauer Associates; Sunderland (MA): 2000. [Google Scholar]
- [175].Garnier D, Loyer P, Ribault C, Guguen-Guillouzo C, Corlu A. Cyclin-dependent kinase 1 plays a critical role in DNA replication control during rat liver regeneration. Hepatology. 2009;50:1946–1956. doi: 10.1002/hep.23225. [DOI] [PubMed] [Google Scholar]
- [176].LeCluyse E, Sinz M, Hewitt N, Ferguson S, Jasminder S. Cytochrome P450 Induction. In: Lu C, Li A, editors. Enzyme Inhibition in Drug Discovery and Development: The Good and the Bad. Wiley; 2010. pp. 265–314. [Google Scholar]
- [177].Woodworth CD, Isom HC. Regulation of albumin gene expression in a series of rat hepatocyte cell lines immortalized by simian virus 40 and maintained in chemically defined medium. Mol Cell Biol. 1987;7:3740–3748. doi: 10.1128/mcb.7.10.3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Bayad J, Bagrel D, Sabolovic N, Magdalou J, Siest G. Expression and regulation of drug metabolizing enzymes in an immortalized rat hepatocyte cell line. Biochem Pharmacol. 1991;42:1345–1351. doi: 10.1016/0006-2952(91)90444-a. [DOI] [PubMed] [Google Scholar]
- [179].Kim BH, Sung SR, Park JK, Kim YI, Kim KJ, Dong SH, et al. Survival of conditionally immortalized hepatocytes in the spleen of syngeneic rats. J Gastroenterol Hepatol. 2001;16:52–60. doi: 10.1046/j.1440-1746.2001.02393.x. [DOI] [PubMed] [Google Scholar]