Abstract
Obstructive sleep apnea (OSA) is accompanied by neurocognitive impairment, likely mediated by injury to various brain regions. We evaluated brain morphological changes in patients with OSA and their relationship to neuropsychological and oximetric data. Sixteen patients affected by moderate-severe OSA (age: 55.8±6.7 years, 13 males) and fourteen control subjects (age: 57.6±5.1 years, 9 males) underwent 3.0 Tesla brain magnetic resonance imaging (MRI) and neuropsychological testing evaluating short and long-term memory, executive functions, language, attention, praxia and non-verbal learning. Volumetric segmentation of cortical and subcortical structures and voxel-based morphometry (VBM) were performed. Patients and controls differed significantly in Rey Auditory- Verbal Learning test (immediate and delayed recall), Stroop test and Digit span backward scores. Volumes of cortical gray matter (GM), right hippocampus, right and left caudate were smaller in patients compared to controls, with also brain parenchymal fraction (a normalized measure of cerebral atrophy) approaching statistical significance. Differences remained significant after controlling for comorbidities (hypertension, diabetes, smoking, hypercholesterolemia). VBM analysis showed regions of decreased GM volume in right and left hippocampus and within more lateral temporal areas in patients with OSA. Our findings indicate that the significant cognitive impairment seen in patients with moderate-severe OSA is associated with brain tissue damage in regions involved in several cognitive tasks. We conclude that OSA can increase brain susceptibility to the effects of aging and other clinical and pathological occurrences.
Keywords: sleep apnea, neurocognitive, magnetic resonance imaging, voxelbased morphometry, segmentation, lung
1. INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by repetitive episodes of complete (apnea) or partial (hypopnea) obstruction of the upper airway during sleep. These episodes result in decreased arterial oxygen saturation and transient arousals with marked disruption of normal sleep architecture (American Academy of Sleep Medicine, 1999). Excessive daytime sleepiness (EDS), fatigue and neuropsychological impairments are common clinical features in individuals with OSA. OSA is accompanied by impairment in several cognitive domains, including attention and vigilance decrements, memory gaps, and abnormalities in executive functions (Kim et al., 1997; Ferini-Strambi et al., 2003; Saunamaki and Jehkonen, 2007), although the reported presence and degree of such impairment shows great variability between studies (Décary et al., 2000; Aloia et al., 2004). These functional alterations are likely related to structural tissue damage and metabolic stress occurring in different brain tissue compartments and neural structures. Previous structural neuroimaging studies in patients with OSA have reported inconsistent findings. Although routine magnetic resonance imaging (MRI) often fails to demonstrate obvious cerebral damage (Davies et al., 2001), a higher prevalence of silent cerebrovascular lesions has been recently reported in patients with moderatesevere OSA (Nishibayashi et al., 2008). More sensitive and quantitative MRI techniques can reveal structural alterations in specific brain regions implicated in cognitive functions. Such alterations were detected both in white matter (WM) using diffusion tensor imaging (Macey et al., 2008) and in several gray matter (GM) regions using voxel-based morphometry (VBM) (Macey et al., 2002; Morrell et al., 2003; Yaouhi et al., 2009; Joo et al., 2010). However, other researchers have not confirmed the VBM findings in OSA patients (O’Donoghue et al., 2005), probably because more stringent and conservative statistical methods were applied. VBM is used in GM volume studies to detect regional group differences in tissue volume, density or concentration, and to investigate correlations between regional GM measures and clinical or neuropsychological variables (Ashburner and Friston, 2000; Good et al., 2001). We have undertaken a similar approach and, in addition, we performed a hypothesis-driven segmentation of cortical and subcortical structures with the following objectives: i) to evaluate brain structural changes and neurocognitive profile in a group of moderate-to-severe OSA individuals compared to a control population; ii) to assess the relationship between MRI outcome variables and neuropsychological tests, as well as nocturnal respiratory data.
2. MATERIALS AND METHODS
2.1 Subjects
The study cohort comprised thirty subjects, sixteen affected by OSA and fourteen normal controls. Sixteen newly diagnosed right-handed patients affected by moderate-to-severe OSA (mean apnea-hypopnea index, AHI, [standard deviation, SD]: 52.5 [26] events/hour) were enrolled (13 males and 3 females; mean age [SD]: 55.8 [6.7] years) (table 1). All patients were untreated for OSA. Twelve of them (75%) had severe OSA (AHI of 31.6 to 106.3/h, mean [SD]: 63.3 [20.3]/h), while the remaining four (25%) had moderate OSA (AHI of 15.8 to 25.6/h, mean [SD]: 20.2 [4.1]/h). Fourteen right-handed control subjects (9 males and 5 females; mean age [SD]: 57.6 [5.2] years) who did not suffer from OSA based on thorough history and physical examination (including an interview with the bed partner, when available) were also recruited. Patients and controls were assessed by a detailed clinical interview, physical examination and the administration of questionnaires for the evaluation of daytime sleepiness (Epworth sleepiness scale – ESS [Johns, 1991]). Exclusion criteria were history of head injury, cerebral ischemia, encephalitis, mental disorder, major cardiovascular disorder, alcohol or illicit drug abuse, score below 28/30 on the Mini Mental State Examination (Folstein et al., 1975), body mass index (BMI) > 40 Kg/m2, claustrophobia and body metallic implants or devices. The study was approved by the local institutional Ethics Committee and all subjects gave their informed consent prior to their participation.
Table 1.
Demographic and clinical characteristics of study participants. Means ± standard deviations are reported; P-values refer to 2-tailed Student’s T-test.
| Characteristic | Patients (n=16) |
Controls (n=14) |
P-value | |
|---|---|---|---|---|
| Age (years) | 55.8 ± 6.7 | 57.6 ± 5.2 | 0.42 | |
| Gender | 13 M, 3 F | 9 M, 5 F | 0.31 a | |
| Education (years) | 12.3 ± 4.1 | 14.1 ± 4.6 | 0.26 | |
| Body mass index (BMI) | 31.7 ± 4.4 | 25.5 ± 2.4 | <0.01 | |
| Epworth sleepiness scale (ESS) | 8.5 ± 4.5 | 2.6 ± 1.6 | <0.01 | |
| Apnea-hypopnea index (AHI) | 52.5 ± 26.0 | - | - | |
| Oxygen desaturation index (ODI) | 51.0 ± 23.3 | - | - | |
| Oxygen saturation (mean) (%) | 92.0 ± 3.1 | - | - | |
| Desaturation time (%) | <90% | 21.9 ± 20.7 | - | - |
| <80% | 3.7 ± 8.2 | - | - | |
| <70% | 0.5 ± 1.8 | - | - | |
| Hypertension | 10 (62.5%) | 5 (35.7%) | 0.27 a | |
| Diabetes (Type 2) | 1 (6.2%) | 1 (7.1%) | 1 a | |
| Hypercholesterolemia | 4 (25%) | 1 (7.1%) | 0.34 a | |
| History of smoking | 6 (37.5%) | 3 (21.4%) | 0.44 a | |
| Intake of cardiovascular medications | 9 (56.2%) | 4 (28.6%) | 0.16 a | |
P-value refers to Fisher’s Exact Test
2.2 Nocturnal cardiorespiratory monitoring
OSA patients underwent a complete cardiorespiratory monitoring run using a handheld device (Embletta, Flaga-Iceland). Thoraco-abdominal respiratory movements were recorded by strain gauges. Nasal airflow was measured by a nasal cannula, oral airflow by a thermal sensor, snoring by a microphone, one-lead electrocardiogram by thoracic electrodes, and oxygen saturation by a finger pulse oximeter. In addition all patients underwent respiratory function tests (spirometry and blood gas analysis) to exclude chronic obstructive pulmonary disease. In accordance with the American Academy of Sleep Medicine guidelines, obstructive apnea was defined as a reduction in airflow > 90% lasting at least 10 seconds and associated with continued or increased inspiratory effort; hypopnea was defined as a reduction in airflow ≥ 30% lasting at least 10 seconds and accompanied by a 4% or greater oxygen desaturation.
2.3 Neuropsychological evaluation
The Mini-Mental State Examination (Folstein et al., 1975) and an extended version of the Mental Deterioration Battery (Caltagirone et al., 1979; Carlesimo et al., 1996) were carried out in all participants within 48 hours of MRI examination. Administration of the battery required approximately 90 minutes and scores were corrected for age and education level. Short and long-term memory, executive functions, language, attention, non-verbal learning and praxia (i.e. the ability to perform complex motor sequences and exercises) were evaluated:
Short and long-term memory
-
-
Rey Auditory-Verbal Learning (RAVL) test: a list of 15-words is read to the subject five times. Measures include immediate recall (the sum of the words recalled in the five trials) and a 15-min delayed recall (the number of words recalled 15 min after the last word presentation).
-
-
Digit span forward and backward tests: subjects are asked to listen to and repeat sequences of single digits; the number of digits in each sequence is gradually increased. In the backward part of the test, subjects repeat the sequences in reverse order (Orsini et al., 1987).
-
-
Visual memory test: subjects are required to view a simple figure for 3 seconds and then to recognize it in a multiple-choice condition. Score ranges from 0 to 22.
-
-
Rey-Osterreith Complex Figure recall: the subject is asked to reproduce a bidimensional complex figure from memory without forewarning, 15 min after copy. Score ranges from 0 to 36 (Osterrieth, 1944).
Constructional Praxia
-
-
Copying drawings with and without landmarks: this task requires the reproduction of a geometrical figure both by freehand and by joining landmarks already traced on the sheet.
-
-
Rey-Osterreith Complex Figure copy: the subject is asked to copy a bi-dimensional complex figure. Score ranges from 0 to 36 (Osterrieth, 1944).
General Intelligence
-
-
Raven’s Advanced Progressive Matrices (36 items): a set of 3 subtests (labeled A, Ab and B) to evaluate non-verbal intelligence, visual processing speed, cognitive speed and flexibility. It consists in choosing from a set of distractors the item logically missing in a given visual/spatial set (Raven, 1947).
Language
-
-
Semantic verbal fluency task: subjects have to produce as many words as they can that fall into 3 semantic categories, in a time limit of 1 min per sub-test.
Executive functions
-
-
Stroop Color/Word test: participants are required to name the color ink that a colorword (e.g. RED) is presented in, both in congruent (e.g. when the word RED is printed in red link) and in incongruent conditions (e.g. when the word BLUE is printed in red link). In the latter condition an increase in the number of errors and the time taken to respond is observed (“Stroop interference effect”) (Stroop, 1935). The test is considered the “paradigmatic measure of selective attention” (Carter et al., 1995).
-
-
Phonological verbal fluency task: subjects have to produce as many words as they can beginning with a given letter (A, F, S), in a time limit of 1 min per sub-test.
-
-
Digit span backward test (see above).
2.4 Brain MR Imaging
High-resolution MRI scans of the whole brain were performed using a 3.0 Tesla system (3T Allegra, Siemens Medical Solutions, Erlangen, Germany). The following sequences were acquired:
Axial 3D T1-weighted magnetization-prepared rapid-acquisition gradient echo (MPRAGE) (FOV 20.8×25.6 cm2, matrix 208×256, in plane resolution 1×1 mm2, 176 slices, 1 mm slice thickness).
Sagittal 3D TSE T2-weighted (T2) (FOV 22.0×25.6 cm2, matrix 220×256, in plane resolution 1×1 mm2, 176 slices, 1 mm slice thickness).
Sagittal 3D TSE T2 Fluid-attenuated inversion recovery (FLAIR) (FOV 20.8×25.6 cm2, matrix 208×256, in plane resolution 1×1 mm2, 144 slices, 1.3 mm slice thickness).
2.5 Image Analysis
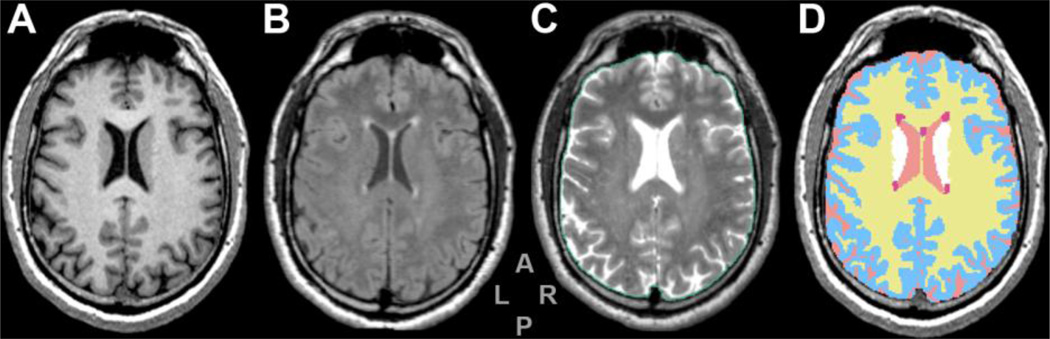
2.5.1 Brain tissue class segmentation (Fig. 1)
Figure 1. Series from 3D-Slicer segmentation module.
Figure shows a same-level slice from the MPRAGE (A), FLAIR (B) and T2 (C) series and the segmentation output of 3D-Slicer module (D) which was obtained using MPRAGE and FLAIR as inputs. Colors in panel D: yellow (WM), pale blue (GM), pink (CSF), red (WMH) and white (putamen). The ICV is outlined in green (panel C) and includes cortical CSF.
This figure is intended for color reproduction on the Web and in print.
MR image pre-processing included correction of magnetic field and radio-frequencyrelated signal inhomogeneities (Sled et al., 1998) and linear affine registration of FLAIR and T2 series to the MPRAGE series (Jenkinson and Smith, 2001). Brain parenchyma classification into GM, normal appearing WM, abnormal T2-hyperintense WM (WMH) and cerebrospinal fluid (CSF) was performed using a semi-automated segmentation pipeline previously described (Moscufo et al., 2009). Two segmentation modules from 3D-Slicer (www.slicer.org) (Pohl et al., 2004) and FreeSurfer (www.surfer.nmr.mgh.harvard.edu) (Fischl et al., 2002) were used on the MPRAGE and FLAIR series. In order to maximize exclusion of false WMH, outputs from above were merged and only those WMH areas identified in both modules were selected. The brain parenchyma segmentation maps were reviewed and the WMHs were manually edited by an expert when appropriate. Volumes in milliliters (mL) were calculated for each subject by multiplying the number of voxels in each tissue class by the nominal volume of a single voxel (i.e., 0.001 mL). To account for subjects’ head size differences, all volumes were normalized and expressed as percent of the intracranial cavity volume (ICV). The ICV was outlined on the T2 series using an in-house semi-automated method and included cortical CSF. The brain parenchymal fraction (BPF), an indicator of brain atrophy, was determined as follows: [WM+GM]/ICV.
2.5.2 Anatomical Brain Parcellation
Parcellation and volumetry of cortical and subcortical brain structures was performed with the FreeSurfer image analysis suite. This image processing includes several steps that have been previously described (Segonne et al., 2004; Fischl et al., 2002, 2004). FreeSurfer morphometric procedures have been demonstrated to show good test-retest reliability across scanner manufacturers and across field strengths (Han et al., 2006). The volume of cortical GM and subcortical structures (hippocampus, amygdala, cerebellum, caudate, putamen, thalamus) were obtained with this technique. Visual quality control of the final segmentation output was performed for each subject.
2.5.3 VBM analysis
Automated comparison of the GM volume between the groups on a voxel-by-voxel basis was performed using MRI images that were spatially normalized into stereotactic space (Ashburner and Friston, 2000). All T1 images were normalized and segmented into GM, WM and CSF, using respectively NewSegment and Dartel modules included in SPM8 (Wellcome Department of Cognitive Neurology, London, UK).
2.6 Statistical analysis
Statistical analysis was performed using the SPSS statistical software package (version 13.0, SPSS, Chicago, IL). Mean differences in clinical, neuropsychological and volumetric MRI variables between patients and controls were compared by means of the two-tailed T-test; non-continuous data comparisons between groups were analyzed using the Fisher’s Exact Test.
For VBM between-group comparisons two ANOVA models, including respectively GM and WM modulated and smoothed (10 mm FWHM kernel) maps, were employed. In each model controls and patients with OSA were entered as independent groups. Additional multiple regression analyses, including GM maps from all subjects, were performed to investigate correlations between scores obtained on individual cognitive tests, regional MRI volumes, and nocturnal respiratory data. Only those tests where patients reported significantly different scores than controls were considered for correlation analysis. As in cross-sectional group analyses, demographic characteristics, comorbidities and ICV were entered as nuisance variables together with education level. The threshold for statistical significance was p<0.05.
3. RESULTS
3.1 Subjects characteristics (Table 1)
Subjects with OSA and controls had similar age and comparable representation of the two genders. Presence of more men than women in the study cohort reflects the higher prevalence of OSA among men in the general population (Young et al., 1993). Patients and controls differed significantly for BMI and ESS scores (p<0.01). Despite a higher prevalence of comorbidities (hypertension, diabetes, hypercholesterolemia, history of smoking and medication intake) in the OSA group, the differences with the control group were not significant (p>0.05).
3.2 Neuropsychological results (Table 2)
Table 2.
Scores in neurocognitive tests in OSA patients and controls. Means ± standard deviations [range] are reported; P-values refer to 2-tailed Student’s T-test.
| Test | Patients (n=16) |
Controls (n=14) |
P-value | |
|---|---|---|---|---|
| Mini Mental State Examination | 29.5 ± 0.8 [28–30] | 29.6 ± 0.6 [28–30] | 0.60 | |
| Rey Auditory-Verbal Learning test | immediate recall | 40.9 ± 5.4 [32.2–48.0] | 45.9 ± 6.4 [33.0–55.7] | 0.026 |
| delayed recall | 7.7 ± 2.3 [3.3–11.2] | 9.7 ± 1.4 [7.2–12.5] | 0.011 | |
| Digit span | forward | 5.6 ± 0.6 [5–7] | 5.9 ± 0.4 [5–6] | 0.23 |
| backward | 3.8 ± 0.9 [2–5] | 4.4 ± 0.6 [3–5] | 0.049 | |
| Visual memory | 20.4 ± 1.2 [18.4–22.0] | 19.7 ± 1.7 [17.1–22.0] | 0.22 | |
| Copy drawings | without landmarks | 9.9 ± 2.1 [6.5–12.0] | 10.1 ± 1.2 [7.5–12.0] | 0.72 |
| with landmarks | 66 ± 8 3.4 [59.2–70.0] | 66.4 ± 4.1 [58.3–70.0] | 0.76 | |
| Rey-Osterreith figure | copy | 30.4 ± 6.1 [19.5–36.0] | 33.9 ± 3.0 [26.7–36.0] | 0.06 |
| recall | 14.7 ± 5.7 [5.0–28.8] | 17.3 ± 4.0 [9.6–25.0] | 0.17 | |
| Raven’s Advanced Progressive Matrices | 29.7 ± 3.9 [20.3–36.0] | 31.7 ± 2.6 [28.3–36] | 0.11 | |
| Word fluency | semantic | 39 ± 7 0.5 [39.0–40.0] | 39.9 ± 0.3 [39.0–40.0] | 0.11 |
| phonological | 26.7 ± 8.8 [14.2–44.2] | 28.5 ± 6.6 [17.9–38.0] | 0.56 | |
| Stroop test | interference time | 40.3 ± 13.1 [25.0–60.0] | 33.9 ± 5.0 [24.0–41.0] | 0.10 |
| ratio | 1.12 ± 0.31 [0.74–1.76] | 0.89 ± 0.20 [0.60–1.19] | 0.021 | |
Compared to the controls, subjects with OSA reported a lower performance at the RAVL test, both for immediate (p=0.026) and delayed recall (p=0.011), at the Stroop test (p=0.021), and at the Digit span backward test (p=0.049). No other significant differences between the two groups were detected.
3.3 MRI results
3.3.1 Global brain atrophy (Table 3)
Table 3.
Volumes of brain structures (% of ICV) in OSA patients and controls. Means ± standard deviations are reported; P-values refer to 2-tailed Student’s T-test.
| Structure | Patients (n=16) |
Controls (n=14) |
P-value | |
|---|---|---|---|---|
| Brain parenchymal fraction (BPF) a | 83.7 ± 2.6 | 85.3 ± 2.3 | 0.068 | |
| Total gray matter (GM) a | 41.5 ± 3.4 | 43.5 ± 2.9 | 0.09 | |
| Total white matter (WM) a | 42.1 ± 1.4 | 41.8 ± 1.1 | 0.46 | |
| White matter hyperintensities (WMH) a | 0.167 ± 0.402 | 0.109 ± 0.101 | 0.60 | |
| Cortical GM volume b | Left | 14.7 ± 2.8 | 16.8 ± 1.1 | 0.015 |
| Right | 14.5 ± 3.1 | 16.8 ± 1.0 | 0.012 | |
| Total | 29.2 ± 5.9 | 33.6 ± 2.1 | 0.012 | |
| Hippocampus b | Left | 0.288 ± 0.054 | 0.290 ± 0.031 | 0.94 |
| Right | 0.279 ± 0.040 | 0.312 ± 0.033 | 0.024 | |
| Amygdala b | Left | 0.115 ± 0.016 | 0.119 ± 0.013 | 0.53 |
| Right | 0.123 ± 0.015 | 0.128 ± 0.016 | 0.38 | |
| Caudate b | Left | 0.210 ± 0.046 | 0.246 ± 0.040 | 0.030 |
| Right | 0.218 ± 0.036 | 0.244 ± 0.030 | 0.044 | |
| Putamen b | Left | 0.338 ± 0.054 | 0.343 ± 0.042 | 0.77 |
| Right | 0.318 ± 0.045 | 0.327 ± 0.034 | 0.56 | |
| Thalamus b | Left | 0.445 ± 0.052 | 0.478 ± 0.044 | 0.07 |
| Right | 0.444 ± 0.055 | 0.466 ± 0.054 | 0.28 | |
| Cerebellum b | 7.27 ± 1.78 | 7.83 ± 2.03 | 0.43 | |
Volume obtained from 3D-Slicer brain segmentation
Volume obtained from FreeSurfer brain segmentation
OSA subjects had reduced cortical GM volume compared to controls (p=0.012). This difference remained significant after controlling for age, gender, ESS and comorbidities, but not after controlling for BMI. BPF was lower in OSA patients compared to controls, with a difference approaching the statistical significance (p=0.068). No significant difference was found between patients and controls in global WM volumes and global burden of WMH (p>0.40).
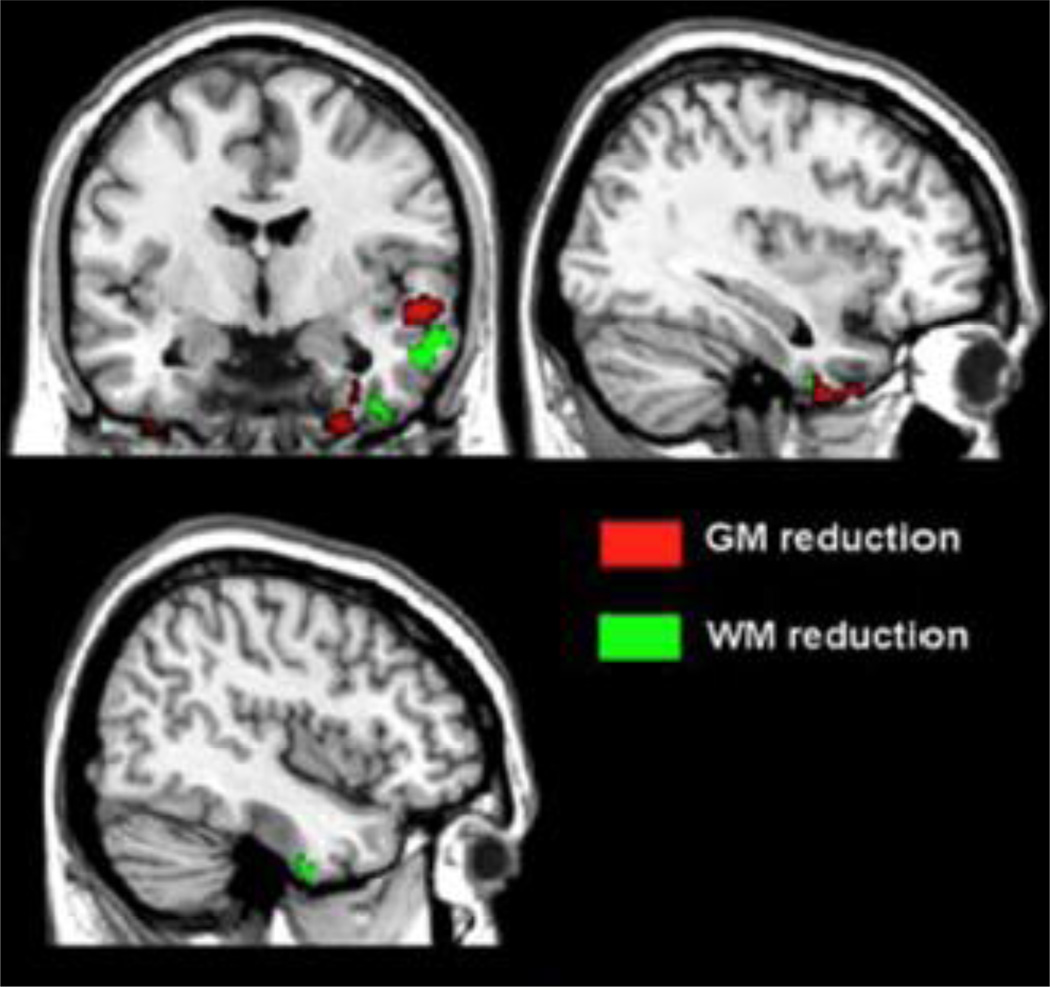
3.3.2 VBM analysis (Fig. 2)
Figure 2. VBM analysis showing regions of GM and WM reduction in patients with OSA compared to controls.
Figure shows significant changes of GM volume in right hippocampus (MNI: 30; −5; −48) and changes of WM volume in an anatomically contiguous region, across groups (p Family-wise Error corrected < 0.05).
This figure is intended for color reproduction on the Web and in print.
Patients with OSA compared to control subjects showed a region of decreased GM volume in the right hippocampus (PFWE cluster level corr. <0.05; MNI Coordinates [x,y,z]= 30; −5; −48). At uncorrected level, a reduction in GM volume was also present in the left hippocampus, and in some lateral temporal areas of both hemispheres. Moreover, patients with OSA compared to healthy controls showed two regions of reduced WM volume within the right temporal lobe (p uncorr. < 0.001). Interestingly, one of them was localized nearby the hippocampal region where GM atrophy was also detected (see above). Multiple regression analysis revealed a direct association between scores obtained at the RAVL test and GM volume in the left orbitofrontal cortex (OFC) (MNI Coordinates [x,y,z]= −6; 41; −24; p uncorr. <0.001).
3.3.3 Regional atrophy through structural segmentation (Table 3)
No relevant errors were detected in any of the automated FreeSurfer segmentation outputs upon visual quality control. Volumes were calculated from these maps and used in the analysis. We found that the right hippocampus was smaller in OSA patients compared to controls (p=0.024), with the significance remaining also after controlling for age, BMI, gender, ESS and comorbidities (a minor exception was represented by hypertension, p=0.058). The volumes of caudate bilaterally in the OSA group were smaller than controls (right caudate, p=0.044; left caudate p=0.030). However, the differences were no longer significant after controlling for BMI, gender and hypertension (both sides) as well as after controlling for age and smoking (right side). No significant differences were found between patients and controls in the volumes of cerebellum, amygdala, putamen and thalamus.
3.4 Correlations between neuropsychological, clinical and MRI variables
For the whole cohort, total hippocampal volume was correlated with the score in RAVL test (delayed recall) (r=0.388, p=0.034) and inversely correlated with the interference time recorded in the Stroop Color/Word test (r=−0.372, p=0.043). BPF was associated with scores in RAVL test (delayed recall) (r=0.387, p=0.034), in Word fluency semantic (r=0.465, p=0.010), in Rey-Osterreith Complex Figure copy (r=0.423, p=0.020) and delayed recall (r=0.592, p=0.001). ESS was inversely related to scores in RAVL test (immediate recall) (r=−0.41, p=0.024) and in Digit span forward (r=−0.37, p=0.046). In patients group, but not in controls, we found marked negative correlations between age and MRI findings, in particular total hippocampal volume (r=−0.589, p=0.016), left hippocampus volume (r=−0.620, p=0.010), total amygdala volume (r=−0.511, p=0.043) and BPF (r=−0.539, p=0.031). No significant correlations between nocturnal respiratory data, neuropsychological and MRI findings were found.
4. DISCUSSION
In the present study, we found multiple structural changes in OSA patients using different quantitative approaches of MRI analysis. VBM showed a significant GM reduction in right and left hippocampal volumes in OSA patients compared to controls. This finding was confirmed for the right hippocampus also by the volumetric analysis. It can be argued that VBM is a more sensitive technique to show mild parenchymal damage not yet resulting in a loss of volume detectable by automated parcellation and volumetry of subcortical structures. The significant decrease in cortical gray matter volume, affecting equally the right and the left hemispheres, reflects on the BPF, a normalized measure of cerebral atrophy; no difference was found in white matter volume. Finally, a significantly smaller caudate volume was found bilaterally in OSA patients. Our neurocognitive results reveal a significant impairment in OSA subjects, which is independent from cardiovascular comorbidities. In particular the most impaired cognitive domains were executive functions and verbal memory.
A strength of the present study is represented by the comparison of neuropsychological, neuroimaging and polygraphic findings. In our sample, a verbal memory test score (RAVL test delayed recall) was significantly correlated with total hippocampal volume. Hippocampal volume was also inversely associated to the interference time recorded in the Stroop Color/Word test. On the contrary, we did not find significant relationships between neuropsychological deficits and polygraphic variables. Also no correlation between severity of OSA and reduction in cortical volume and BPF was observed. Finally, we found a significant negative correlation between EDS (evaluated by means of the ESS score) and scores obtained at verbal memory tests.
The presence and the extent of neurobehavioural changes in individuals affected by sleep apnea is still a matter of debate. Some researchers argue that EDS is the main cause of the neuropsychological deficits in patients with OSA and that the comorbidities usually observed in these patients (cardiovascular diseases, obesity, physical inactivity) are more important than sleep apnea per se in affecting neurocognitive functions (Lim and Veasey, 2010). Moreover there is a large heterogeneity in neuropsychological tests across different studies in OSA, making difficult a direct comparison of results. To address this latter issue Décary et al. (2000) proposed a standardized neuropsychological test battery for the evaluation of OSA patients. Our cohort showed more extensive neurocognitive impairment compared to previous studies (Redline et al., 1997; Salorio et al., 2002; Yaouhi et al., 2009). Yaouhi et al. (2009), who demonstrated only a minor cognitive impairment in their cohort, studied less severe OSA patients than ours (mean AHI [SD]: 38.3 [14.3] vs 52.5 [26.0]/h). Other researchers who did not report appreciable cognitive deficits included only patients with mild to moderate OSA (Redline et al., 1997). Similarly, Salorio et al. (2002), who identified cognitive deficits only on tasks requiring greater integration of executive control and long-term memory abilities, included a significant number of patients with mild OSA in their sample. Other neuropsychological studies on severe OSA subjects with respiratory index similar to those of our patients reported more diffuse deficits, particularly in terms of executive functioning, attention, learning and memory (Ferini-Strambi et al., 2003; Lim et al., 2007). The finding of impairment in memory and executive functions is essentially in agreement with the conclusions of previous reviews (Beebe and Gozal, 2002; Aloia et al., 2004; Saunamaki and Jehkonen, 2007). It is also in agreement with evidence from animal studies suggesting that both intermittent hypoxia (Kalaria et al., 2004) and sleep fragmentation (Tung et al., 2005) (the two essential features of OSA syndrome) can independently lead to neuronal loss in the hippocampus and pre-frontal cortex, areas that are closely associated with memory processes and executive functions. The severity of OSA syndrome in our sample could also explain the finding of global brain tissue damage, which has been rarely reported in previous cross-sectional studies in the form of reduction of the ratio of total gray-to-white matter volumes (Macey et al., 2002). This outcome is likely a consequence of apnea events and of the subsequent chronic intermittent hypoxemia. Cortical atrophy may also be induced by factors other than hypoxic events, for example cardiovascular comorbidities (mainly arterial hypertension), which are known to affect brain tissue both globally (Enzinger et al., 2005; Ropele et al., 2010) and focally (den Heijer et al., 2005). This explanation however appears less probable in our cohort because patients and controls were matched for cardiovascular disease and there was no significant difference in white matter lesion burden between the two groups. A likely role of apnea events in affecting cortical volume seems to be supported also by the observation that the significant difference in gray matter volume between OSA and controls is independent of cardiovascular comorbidities. It is important noting that white matter is also susceptible to hypoxia and while we have not detected significant change we cannot rule out the presence of damage in this compartment. The presence of a degree of hippocampal atrophy in our patients appears to be consistent with the neuropsychological results and also with previous reports showing a decreased hippocampal volume as the most consistent finding provided by structural neuroimaging studies in OSA (Zimmerman and Aloia, 2006). The significantly smaller caudate volume that we found bilaterally in OSA patients may partially contribute to explain the impairment in executive functions, since this structure (especially the head of the caudate) is thought to be involved in the socalled “pre-frontal circuit” (Saint-Cyr et al., 1988; Eslinger and Grattan, 1993). Furthermore, this MRI finding seems to confirm a recent report of VBM analysis in patients with severe OSA (Joo et al., 2010), showing several cortical and subcortical regions of reduced GM concentration (including bilateral caudate nuclei) in severe OSA patients compared to healthy controls. Functional neuroimaging studies in OSA have supported the hypothesis of an involvement of the pre-frontal circuit in the impairment in executive functions. Absence of dorsolateral prefrontal activation (Thomas et al., 2005) or, alternatively, increased bilateral activation of prefrontal regions (Archbold et al., 2009) have been reported during working memory tasks in patients with untreated OSA.
Two previous studies (Gale and Hopkins, 2004; Yaouhi et al., 2009) correlated results in different neurobehavioural tests and MRI findings in OSA patients. Yaouhi and colleagues did not reveal significant correlations in their sample (however they found correlations between neuropsychological/clinical data and brain metabolism evaluated by resting-state positron emission tomography). Gale and colleagues, who detected hippocampal atrophy in 36% of their OSA patients, reported positive correlations between non-verbal memory and information processing tests and both right and left hippocampus. Interestingly, in our sample, hippocampal volume was also associated with the score in an executive functions test. This outcome provides an additional evidence for an association between alterations in the orbitofrontal cortex (that we could detect by VBM) and in limbic areas and executive functioning impairment (Wagner et al., 2008; Keller et al., 2009). In the present study we could not find significant correlations between neuropsychological and respiratory data. This result is consistent with those from previous studies (Sauter et al., 2000; Adams et al., 2001) showing that the link between neuropsychological and sleep data (including nocturnal respiratory indexes) is rarely strong and consistent. Moreover, it is well known that the characterization of disease severity should not be based only on the AHI, but also on clinical features (mainly the disease duration) which are often not easily measurable. Previous studies showed that impairment in attention, vigilance and memory function is mostly related to EDS, while hypoxemia correlates more with deficits in executive functions (Bédard et al., 1991; Décary et al., 2000; Engleman et al., 2000; Brown, 2005). In our sample we found a negative correlation between EDS and verbal memory tests, while no significant correlation between oximetric data and executive functions tests was reported. This lack of correlation could be due to the fact that our sample was moderately hypoxemic (the mean percentage of time spent under 90% and under 80% of desaturation was 21.9% and 3.7%, respectively). However, this degree of desaturation could be sufficient to cause the reduction in hippocampal volume and in cortical gray matter that we observed, since these areas are known to be particularly vulnerable to hypoxia (Gale and Hopkins, 2004; Konaka et al., 2007).
The chronic intermittent hypoxemia observed in OSA patients might be seen as a factor which expedites the effects of other conditions, particularly aging, that are known to cause brain atrophy or damage. Consistent with that, our patients showed negative correlations between age and several brain structure volumes (total and left hippocampus, amygdala and BPF) while none of them were significant in the control group.
5. CONCLUSIONS
Our findings confirm that moderate-severe OSA can be accompanied by significant cognitive impairment. The brain image analysis performed using different techniques highlighted the presence of tissue damage in regions involved in several cognitive domains. Such damage appeared to be independent of differences in age, gender, use of tobacco and cardiovascular comorbidities. Therefore the presence of OSA could be seen as a factor which expedites the process of brain aging by increasing the susceptibility of specific cerebral structures to clinical and pathological occurrences.
ACKNOWLEDGEMENTS
This study was supported by NIH grants R01 HL 090897, R01 HL085188, K24 HL 093218, 1 P01 HL 095491, R01 AG022092-01, PO1 AG004390, and AHA 0840159N and the American Sleep Medicine Foundation.
Dr. Guttmann receives research support from Teva Neuroscience, the Maurice M. Pechet Foundation, the National Multiple Sclerosis Society (NMSS) (RG3574A1 and Pediatric MS Center of Excellence Award), the National Institutes of Health (NIH) (R01 NS036524-05, R01 NS055083-01A1, 1R01NS05270,* *UL1 RR025758-01, 2P41RR013218 Supplement, 2U01AI063623-06), and has received support from the NMSS (RG 3798-A-2) and NIH (P41 RR013218). Dr. Guttmann also has received a honorarium for participation in an advisory board meeting from Johnson & Johnson Pharmaceutical Services, LLC and is an inventor on U.S. patents number 6,080,164; 6,684,098 B2; and 10/966,588.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
Dr. Torelli has no conflict of interest.
Dr. Moscufo has no conflict of interest.
Dr. Garreffa has no conflict of interest.
Dr. Placidi has no conflict of interest.
Dr. Romigi has no conflict of interest.
Dr. Zannino has no conflict of interest.
Dr. Bozzali has no conflict of interest.
Dr. Fasano has no conflict of interest.
Dr. Giulietti has no conflict of interest.
Dr. Djonlagic has no conflict of interest.
Dr. Malhotra has received consulting and/or research income from Philips, Pfizer, Merck, Cephalon, Itamar, Sleep Group Solutions, Sleep HealthCenters, Apnex, Sepracor, Ethicon, Medtronic.
Prof. Marciani has no conflict of interest.
Contributor Information
Federico Torelli, Email: ftorelli@bwh.harvard.edu.
Nicola Moscufo, Email: moscufo@bwh.harvard.edu.
Girolamo Garreffa, Email: ggarreff@tin.it.
Fabio Placidi, Email: fbplacidi@libero.it.
Andrea Romigi, Email: a_romigi@inwind.it.
Silvana Zannino, Email: silv.zannino@tiscali.it.
Marco Bozzali, Email: m.bozzali@hsantalucia.it.
Fabrizio Fasano, Email: f.fasano@hsantalucia.it.
Giovanni Giulietti, Email: giulietti.giovanni@gmail.com.
Ina Djonlagic, Email: idjonlag@bidmc.harvard.edu.
Atul Malhotra, Email: AMALHOTRA1@PARTNERS.ORG.
Maria Grazia Marciani, Email: marciani@uniroma2.it.
Charles RG Guttmann, Email: guttmann@bwh.harvard.edu.
REFERENCES
- Adams N, Strauss M, Schluchter M, Redline S. Relation of measures of sleep-disordered breathing to neuropsychological functioning. Am. J. Respir. Crit. Care Med. 2001;163(7):1626–1631. doi: 10.1164/ajrccm.163.7.2004014. [DOI] [PubMed] [Google Scholar]
- Aloia MS, Arnedt JT, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J. Int. Neuropsychol. Soc. 2004;10(5):772–785. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- Archbold KH, Borghesani PR, Mahurin RK, Kapur VK, Landis CA. Neural activation patterns working memory tasks and OSA disease severity: preliminary findings. J. Clin. Sleep Med. 2009;5(1):21–27. [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. NeuroImage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bédard MA, Montplaisir J, Richer F, Rouleau I, Malo J. Obstructive sleep apnea syndrome: pathogenesis of neuropsychological deficits. J. Clin. Exp. Neuropsychol. 1991;13(6):950–964. doi: 10.1080/01688639108405110. [DOI] [PubMed] [Google Scholar]
- Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J. Sleep Res. 2002;11(1):1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- Brown WD. The psychosocial aspects of obstructive sleep apnea. Semin. Respir. Crit. Care Med. 2005;26(1):33–43. doi: 10.1055/s-2005-864199. [DOI] [PubMed] [Google Scholar]
- Caltagirone C, Gainotti G, Masullo C, Miceli G. Validity of some neuropsychological tests in the assessment of mental deterioration. Acta Psychiatr. Scand. 1979;60:50–56. doi: 10.1111/j.1600-0447.1979.tb00264.x. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Caltagirone C, Gainotti C. The mental deterioration battery: normative data, diagnostic reliability and qualitative analysis of cognitive impairment. The Group of the standardization of the Mental Deterioration Battery. Eur. Neurol. 1996;36(6):378–384. doi: 10.1159/000117297. [DOI] [PubMed] [Google Scholar]
- Carter CS, Mintun M, Cohen JD. Interference and facilitation effects during selective attention: an H2 15O PET study of Stroop task performance. NeuroImage. 1995;2:264–272. doi: 10.1006/nimg.1995.1034. [DOI] [PubMed] [Google Scholar]
- Davies CW, Crosby JH, Mullins RL, Traill ZC, Anslow P, Davies RJ, et al. Case control study of cerebrovascular damage defined by magnetic resonance imaging in patients with OSA and normal matched control subjects. Sleep. 2001;24(6):715–720. doi: 10.1093/sleep/24.6.715. [DOI] [PubMed] [Google Scholar]
- Décary A, Rouleau I, Montplaisir J. Cognitive deficits associated with sleep apnea syndrome: a proposed neuropsychological test battery. Sleep. 2000;23(3):369–381. [PubMed] [Google Scholar]
- den Heijer T, Launer LJ, Prins ND, van Dijk EJ, Vermeer SE, Hofman A, et al. Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology. 2005;64(2):263–267. doi: 10.1212/01.WNL.0000149641.55751.2E. [DOI] [PubMed] [Google Scholar]
- Engleman HM, Kingshott RN, Martin SE, Douglas NJ. Cognitive function in the sleep apnea/hypopnea syndrome (SAHS) Sleep. 2000;23(Suppl 4):S102–S108. [PubMed] [Google Scholar]
- Enzinger C, Fazekas F, Matthews PM, Ropele S, Schmidt H, Smith S, et al. Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology. 2005;64(10):1704–1711. doi: 10.1212/01.WNL.0000161871.83614.BB. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Grattan LM. Frontal lobe and frontal-striatal substrates for different forms of human cognitive flexibility. Neuropsychologia. 1993;31(1):17–28. doi: 10.1016/0028-3932(93)90077-d. [DOI] [PubMed] [Google Scholar]
- Ferini-Strambi L, Baietto C, Di Gioia MR, Castaldi P, Castronovo C, Zucconi M, et al. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP) Brain Res. Bull. 2003;61(1):87–92. doi: 10.1016/s0361-9230(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Ségonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004;23(Suppl 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "ini-mental state" A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gale SD, Hopkins RO. Effects of hypoxia on the brain: neuroimaging and neuropsychological findings following carbon monoxide poisoning and obstructive sleep apnea. J. Int. Neuropsychol. Soc. 2004;10(1):60–71. doi: 10.1017/S1355617704101082. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32(1):180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Joo EY, Tae WS, Lee MJ, Kang JW, Park HS, Lee JY, et al. Reduced brain gray matter concentration in patients with obstructive sleep apnea syndrome. Sleep. 2010;33(2):235–241. doi: 10.1093/sleep/33.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaria RN, Spoors L, Laude EA, Emery CJ, Thwaites-Bee D, Fairlie J, et al. Hypoxia of sleep apnoea: cardiopulmonary and cerebral changes after intermittent hypoxia in rats. Respir. Physiol. Neurobiol. 2004;140(1):53–62. doi: 10.1016/j.resp.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Keller SS, Baker G, Downes JJ, Roberts N. Quantitative MRI of the prefrontal cortex and executive function in patients with temporal lobe epilepsy. Epilepsy Behav. 2009;15(2):186–195. doi: 10.1016/j.yebeh.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Kim HC, Young T, Matthews CG, Weber SM, Woodward AR, Palta M. Sleep-disordered breathing and neuropsychological deficits. A population-based study. Am. J. Respir. Crit. Care Med. 1997;156(6):1813–1819. doi: 10.1164/ajrccm.156.6.9610026. [DOI] [PubMed] [Google Scholar]
- Konaka K, Miyashita K, Naritomi H. Changes in diffusion-weighted magnetic resonance imaging findings in the acute and subacute phases of anoxic encephalopathy. J. Stroke Cerebrovasc. Dis. 2007;16(2):82–83. doi: 10.1016/j.jstrokecerebrovasdis.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Lim DC, Veasey SC. Neural injury in sleep apnea. Curr. Neurol. Neurosci. Rep. 2010;10(1):47–52. doi: 10.1007/s11910-009-0078-6. [DOI] [PubMed] [Google Scholar]
- Lim W, Bardwell WA, Loredo JS, Kim EJ, Ancoli-Israel S, Morgan EE, et al. Neuropsychological effects of 2-week continuous positive airway pressure treatment and supplemental oxygen in patients with obstructive sleep apnea: a randomized placebo-controlled study. J. Clin. Sleep Med. 2007;3(4):380–386. [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Henderson LA, Macey KE, Alger JR, Frysinger RC, Woo MA, et al. Brain morphology associated with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2002;166(10):1382–1387. doi: 10.1164/rccm.200201-050OC. [DOI] [PubMed] [Google Scholar]
- Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31(7):967–977. [PMC free article] [PubMed] [Google Scholar]
- Morrell MJ, McRobbie DW, Quest RA, Cummin AR, Ghiassi R, Corfield DR. Changes in brain morphology associated with obstructive sleep apnea. Sleep Med. 2003;4(5):451–454. doi: 10.1016/s1389-9457(03)00159-x. [DOI] [PubMed] [Google Scholar]
- Moscufo N, Guttmann CR, Meier D, Csapo I, Hildenbrand PG, Healy BC, et al. Brain regional lesion burden and impaired mobility in the elderly. Neurobiol. Aging. 2009 May 8; doi: 10.1016/j.neurobiolaging.2009.04.010. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibayashi M, Miyamoto M, Miyamoto T, Suzuki K, Hirata K. Correlation between severity of obstructive sleep apnea and prevalence of silent cerebrovascular lesions. J. Clin. Sleep Med. 2008;4(3):242–247. [PMC free article] [PubMed] [Google Scholar]
- O'Donoghue FJ, Briellmann RS, Rochford PD, Abbott DF, Pell GS, Chan CH, et al. Cerebral structural changes in severe obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2005;171(10):1185–1190. doi: 10.1164/rccm.200406-738OC. [DOI] [PubMed] [Google Scholar]
- Orsini A, Grossi D, Capitani E, Laiacona M, Pagagno C, Vallar G. Verbal and spatial immediate memory span: Normative data from 1355 adults and 1112 children. Ital. J. Neurol. Sci. 1987;8:539–548. doi: 10.1007/BF02333660. [DOI] [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d’une figure complexe. Arch. Psychol. 1944;30:206–356. [Google Scholar]
- Pohl KM, Bouix S, Kikinis R, Grimson WEL. Anatomical guided segmentation with non-stationary tissue class distributions in an expectation-maximization framework; IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Arlington; 2004. pp. 81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JC. Guide to using coloured progressive matrices: Set A, Ab, B. London: Board and Book Forms; 1947. [Google Scholar]
- Redline S, Strauss ME, Adams N, Winters M, Roebuck T, Spry K, et al. Neuropsychological function in mild sleep-disordered breathing. Sleep. 1997;20(2):160–167. doi: 10.1093/sleep/20.2.160. [DOI] [PubMed] [Google Scholar]
- Ropele S, Enzinger C, Söllinger M, Langkammer C, Wallner-Blazek M, Schmidt R, et al. The impact of sex and vascular risk factors on brain tissue changes with aging: Magnetization Transfer Imaging results of the Austrian Stroke Prevention Study. Am. J. Neuroradiol. 2010 Mar 11; doi: 10.3174/ajnr.A2042. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Cyr JA, Taylor AE, Lang AE. Procedural learning and neostriatal dysfunction in man. Brain. 1988;111(Pt 4):941–959. doi: 10.1093/brain/111.4.941. [DOI] [PubMed] [Google Scholar]
- Salorio CF, White DA, Piccirillo J, Duntley SP, Uhles ML. Learning, memory, and executive control in individuals with obstructive sleep apnea syndrome. J. Clin. Exp. Neuropsychol. 2002;24(1):93–100. doi: 10.1076/jcen.24.1.93.973. [DOI] [PubMed] [Google Scholar]
- Saunamaki T, Jehkonen M. A review of executive functions in obstructive sleep apnea syndrome. Acta Neurol. Scand. 2007;115:1–11. doi: 10.1111/j.1600-0404.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- Sauter C, Asenbaum S, Popovic R, Bauer H, Lamm C, Klösch G, et al. Excessive daytime sleepiness in patients suffering from different levels of obstructive sleep apnoea syndrome. J. Sleep Res. 2000;9(3):293–301. doi: 10.1046/j.1365-2869.2000.00211.x. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935;18:643–662. [Google Scholar]
- Thomas RJ, Rosen BR, Stern CE, Weiss JW, Kwong KK. Functional imaging of working memory in obstructive sleep-disordered breathing. J. Appl. Physiol. 2005;98(6):2226–2234. doi: 10.1152/japplphysiol.01225.2004. [DOI] [PubMed] [Google Scholar]
- Tung A, Takase L, Fornal C, Jacobs B. Effects of sleep deprivation and recovery sleep upon cell proliferation in adult rat dentate gyrus. Neuroscience. 2005;134(3):721–723. doi: 10.1016/j.neuroscience.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Wagner G, Koch K, Schachtzabel C, Reichenbach JR, Sauer H, Schlösser Md RG. Enhanced rostral anterior cingulate cortex activation during cognitive control is related to orbitofrontal volume reduction in unipolar depression. J. Psychiatry Neurosci. 2008;33(3):199–208. [PMC free article] [PubMed] [Google Scholar]
- Yaouhi K, Bertran F, Clochon P, Mézenge F, Denise P, Foret J, et al. A combined neuropsychological and brain imaging study of obstructive sleep apnea. J. Sleep Res. 2009;18(1):36–48. doi: 10.1111/j.1365-2869.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, Aloia MS. A review of neuroimaging in obstructive sleep apnea. J. Clin. Sleep Med. 2006;2(4):461–471. [PubMed] [Google Scholar]