Abstract
Background
Although persons with kidney disease, including those dependent on dialysis, often present clinically with signs and symptoms consistent with frailty, there is limited information about sociodemographic and clinical risk factors that may be associated.
Methods
745 patients undergoing hemodialysis (HD) 2009–2011 in 7 Atlanta dialysis clinics and 7 San Francisco Bay Area dialysis clinics were assessed using the validated Fried frailty index (recent unintentional weight loss, reported exhaustion, low grip strength, slow walk speed, low physical activity) that defines frailty as the presence of 3 or more criteria. Study coordinators interviewed participants; measured grip strength, walk speed and body composition; and reviewed records for clinical and laboratory parameters. Logistic regression models were used to estimate the association of patient characteristics with frailty.
Results
In adjusted analyses, peripheral vascular disease (PVD) and cardiac diseases including dysrhythmia, atrial fibrillation, tachycardia, pericarditis, and cardiac arrest were associated with higher odds for frailty, while black race and higher serum albumin concentration were associated with lower odds for frailty.
Conclusions
In multivariable analyses, HD patients’ risk for frailty, as assessed by the presence of 3 or more criteria that comprise the Fried frailty index, was increased in association with PVD and cardiac conditions such as dysrhythmia and atrial fibrillation, and was decreased for those with higher serum albumin concentration and for blacks compared with whites. Among patients who met the Fried definition of frailty, 78% scored as frail on walk speed and 56% scored as frail on grip strength, the 2 physical performance measures.
Key Indexing Terms: Frailty, Hemodialysis, Risk factors, United States Renal Data System
Frailty is a construct that connotes a state of low physiological reserve and vulnerability to stressors, conveying increased likelihood of adverse health outcomes. Thus, individuals identified as frail are more likely to incur need for assistance with activities of daily living, to fall, to require institutionalization, and to die.1,2 Although older adults have been the primary focus of research on frailty, there is increasing evidence that frailty is also associated with adverse health outcomes in chronic disease populations that are not exclusively geriatric.3–8 This evidence includes a small number of studies of patients with chronic kidney disease (CKD)3,4 and dialysis dependent end-stage renal disease (ESRD).6–8
The frailty index developed and validated by Fried and colleagues is widely used to identify frailty.9 Fried et al characterized frailty as a syndrome marked by recent unintentional weight loss, poor endurance and energy (exhaustion), muscle weakness, slowed gait speed, and low physical activity, with the presence of 3 or more of these elements providing an operational definition of frailty.1 Gyamiani et al observed that individuals with kidney disease are especially likely to present with signs and symptoms consistent with components of the frailty syndrome,10 but empirical investigation remains limited. Studies that have explored frailty in ESRD patients undergoing dialysis have lacked physical performance assessments or have had limited power to detect subgroup differences due to small sample size.6–8 In this study we applied the Fried measurement protocol to assess frailty in a large cohort of ESRD patients undergoing conventional hemodialysis (HD) in outpatient dialysis clinics, which is the ESRD therapy used by approximately 400,000 treated ESRD patients in the U.S.11
PATIENTS AND METHODS
Study Population
ACTIVE/ADIPOSE (A Cohort Study to Investigate the Value of Exercise in ESRD/Analyses Designed to Investigate the Paradox of Obesity and Survival in ESRD) is a multi-center study of prevalent patients on HD coordinated by the United States Renal Data System (USRDS).12 The data collection sites are 7 outpatient dialysis clinics in the Atlanta, Georgia metropolitan area and 7 outpatient dialysis clinics in the San Francisco Bay Area, California, at which 771 patients were enrolled and participated in baseline assessments during 2009–2011. Study participants included adults (≥18 years), English- or Spanish-speaking, on HD for at least 3 months, and capable of providing informed consent. Exclusion criteria included current treatment by peritoneal dialysis or home HD, evidence of active malignancy, and imminent geographic relocation; vulnerable populations (pregnant women, prisoners, persons with significant mental illness) were also excluded. Amputees and patients with prior or pending transplantation were eligible. Among eligible patients undergoing HD at the study clinics during the 2-year enrollment period, 85% provided informed consent and were enrolled. Reasons most frequently given by those who declined to participate were that they were “not interested,” “too busy,” or “enrolled in another study.”
Institutional review boards at Emory University and the University of California San Francisco approved the study, and all participants provided written informed consent. This report focuses on 745 participants who were evaluable for frailty, i.e. had information for 3 or more non-missing components of the Fried index, as specified in the Fried et al methodology.1 Only 26 (3.4%) study participants lacked sufficient information for determining frailty status based on the Fried et al measures, and their sociodemographic characteristics did not differ significantly from the other 745 patients.
Measures and Data Collection
Indicators specified by Fried et al were used to assess the 5 criteria that comprise the frailty index1: (1) weight loss in the past 12 months; (2) poor endurance and energy; (3) weakness, defined by grip strength; (4) slowness, defined by timed walk speed; and (5) low physical activity level (Table 1). Data sources included a brief interview with participants, performance measures of grip strength and walk speed, measured height and waist circumference, and current clinical and laboratory data abstracted from medical records. The maximal grip strength in kilograms was identified from 3 trials in both hands. Walk speed was the fastest time in seconds from 2 trials to walk 15 feet at the participant’s usual pace. Consistent with previous studies, participants unable to walk were classified in the slowest quintile for that indicator.9 Physical performance was assessed pre-HD on the midweek treatment day, and consistency of measurement procedures among study coordinators was monitored by the investigators. Study coordinators rescheduled the physical performance assessments as needed to accommodate participants who were tired, ill, or otherwise declined to complete the physical assessments on an originally scheduled day.
TABLE 1.
Fried frailty index: criteria and indicators1
| Frailty criteria | Indicators |
|---|---|
| Weight loss | Loss of >10 pounds in past 12 months, unintentional |
| Exhaustion | Response of “a moderate amount of the time (3–4 days)” or “most of the time” to either of two CES-D scale items: “I felt that everything I did was an effort”; “I could not get going” during the past week |
| Weakness | Maximal grip strength in kg using Jamar hand-held dynamometer. Lowest 20%, stratified by gender and BMI quartiles. |
| Slowness | Time in seconds to walk 15 feet at usual pace. Slowest 20%, stratified by gender and standing height. |
| Low physical activity level | Weighted score of kilocalories expended per week in physical activities “you have done in the past 2 weeks” reported on short version of Minnesota Leisure Time Activity questionnaire. Lowest 20% for each gender. |
| Frailty | Presence of 3 or more of the above criteria |
BMI = body mass index; CES-D = Center for Epidemiologic Studies-Depression
Demographic variables including age, sex, race, Medicaid coverage (socioeconomic indicator), primary ESRD diagnosis, and length of time since ESRD treatment start (i.e. “vintage”) were ascertained from patient report and USRDS Standard Analysis Files (SAFs). Race was patient-reported; for the small number of participants who declined to specify their race, race information was taken from the USRDS Medical Evidence SAF. Age was categorized as ≥65 vs. <65. ESRD therapy duration was categorized as 1 year or less vs. more than 1 year because of the high morbidity and mortality known to be associated with patients’ first year of therapy. Patients reported the highest education level that they had attained and their smoking history. Comorbidities and laboratory data were abstracted from clinic medical records. Body mass index (BMI) was calculated as kg/m2, using measured height and pre-HD weight recorded on the day that physical performance assessments were made.
Statistical Analyses
Sociodemographic and clinical characteristics of the study cohort were described using summary statistics (% and mean [S.D.]). The association of sociodemographic and clinical characteristics with “frail” status was estimated in univariable and multivariable logistic regression models that included all variables for which the summary statistics indicated significant differences between frail and non-frail participants, as well as gender, educational status, COPD, BMI, serum phosphate, and ESRD vintage ≤1 year. The interactions of age and vintage with all other variables were examined. In a sensitivity analysis, the race reference group was defined as white non-Hispanic, and white Hispanics, blacks, and others were then compared with this reference group.
Statistical analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC, USA). Multivariable models included participants with data for all covariates; no missing data were imputed.
RESULTS
Patient Characteristics
The ACTIVE-ADIPOSE cohort was similar to the US prevalent center HD population in sex distribution (59.5% male vs. 55.7% male in the overall center HD population) and in the proportion of patients with diabetes and hypertension as primary causes of ESRD (71.1% vs. 74.0% in the overall center HD population).11 Higher proportions of black patients and patients of other races were represented, as would be expected from the selected study sites, and the overall age of the study population was younger (28.2% aged 65+ vs. 44.7% of the overall center HD population aged 65+). Most of the cohort (82%) had been receiving ESRD therapy for more than 1 year. The mean time since ESRD treatment start was 5.0 + 5.1 years, with median vintage = 3.3 years. Participants’ sociodemographic and clinical characteristics are shown in Table 2, for the cohort as a whole and separately for patients classified as frail and patients not classified as frail.
TABLE 2.
Characteristics: All study participants with frailty classification, and by frailty status
| Characteristics | All participants (n=745) | Non-Frail (n=642) | Frail (n=103) |
|---|---|---|---|
| Race, % | |||
| White | 23.6 | 22.0 | 34.0a |
| Black | 61.9 | 63.7 | 50.5 |
| Other | 14.5 | 14.3 | 15.5 |
| Male, % | 59.5 | 59.8 | 57.3 |
| Age ≥ 65, % | 28.2 | 25.4 | 45.6c |
| Age, mean (S.D.) | 57.1 (14.1) | 56.0 (13.9) | 64.1 (13.2)c |
| At least high school education, % | 76.1 | 77.2 | 68.9 |
| Living alone, % | 26.8 | 26.7 | 27.2 |
| Medicaid at treatment start, % | 29.4 | 28.8 | 33.3 |
| Current smoker, % | 18.2 | 19.0 | 13.6 |
| Diabetes, % | 50.8 | 48.8 | 63.1b |
| COPD, % | 8.1 | 7.5 | 11.7 |
| CHF, % | 28.7 | 26.9 | 39.8b |
| CAD/MI, % | 27.1 | 25.4 | 37.9b |
| CVA/TIA, % | 10.4 | 9.1 | 18.5b |
| PVD, % | 9.7 | 7.5 | 23.3c |
| Other cardiac diseases, % | 26.0 | 23.0 | 44.7c |
| Cancer, % | 7.7 | 7.5 | 8.7 |
| BMI, kg/m2, mean (S.D.) | 28.2 (6.9) | 28.1 (6.8) | 28.8 (7.5) |
| Waist circumference, cm, mean (S.D.) | 101.1 (16.8) | 100.9 (16.8) | 103.8 (17.1) |
| Hours/HD treatment, mean (S.D.) | 3.5 (0.5) | 3.5 (0.5) | 3.5 (0.5) |
| HD sessions/week, mean (S.D.) | 3.0 (0.2) | 3.0 (0.2) | 3.0 (0.2) |
| Vascular access type, % | |||
| Arteriovenous fistula | 62.4 | 63.5 | 55.3 |
| Arteriovenous graft | 17.3 | 17.1 | 18.5 |
| Central venous catheter | 20.1 | 19.1 | 26.2 |
| Hemoglobin, g/dL, mean (S.D.) | 11.5 (1.3) | 11.6 (1.3) | 11.3 (1.3) |
| Serum albumin, g/dL, mean (S.D.) | 3.9 (0.4) | 4.0 (0.4) | 3.7 (0.5)c |
| Serum bicarbonate, mEq/L, mean (S.D.) | 22.9 (3.5) | 22.8 (3.5) | 23.7 (3.6)a |
| Serum phosphate, mg/dL, mean (S.D.) | 5.3 (1.6) | 5.4 (1.7) | 5.1 (1.4) |
| LDL-C, mg/dL, mean (S.D.) | 73.9 (33.6) | 75.2 (33.8) | 65.1 (30.5) |
| HDL-C, mg/dL, mean (S.D.) | 45.1 (15.2) | 45.4 (15.1) | 42.6 (1.8) |
| Serum potassium, mEq/L, mean (S.D.)d | 4.6 (0.6) | 4.6 (0.5) | 4.3 (0.7) |
| Kt/V, mean (S.D.) | 1.6 (0.3) | 1.6 (0.4) | 1.6 (0.3) |
| ESRD therapy ≤1 year, % | 17.9 | 17.1 | 22.3 |
P < 0.05.
P < 0.01.
P < 0.001.
Values available for subset.
BMI indicates body mass index; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; ESRD, end-stage renal disease; HD, hemodialysis; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; other cardiac disease, cardiac dysrhythmia, atrial fibrillation, tachycardia, pericarditis, cardiac arrest; other race, Asian, Native Hawaiian, other Pacific Islander, Native American, other; PVD, peripheral vascular disease; TIA, transient ischemic attack.
Association of Patient Characteristics with Frailty
In unadjusted associations of sociodemographic and clinical variables with frailty, participants classified as frail were less likely to be black. Participants classified as frail were more likely to be age 65 or older and to have diabetes and cardiovascular conditions, including congestive heart failure (CHF), coronary artery disease (CAD) or myocardial infarction (MI), cerebrovascular accident (CVA) or transient ischemia attack (TIA), peripheral vascular disease [PVD], and other cardiac disease (i.e. cardiac dysrhythmia, atrial fibrillation, tachycardia, pericarditis, cardiac arrest). Frail participants had lower hemoglobin and serum albumin concentrations and higher serum bicarbonate levels (Table 3).
TABLE 3.
Unadjusted and multivariable-adjusted associations of patient characteristics with frailty: ACTIVE-ADIPOSE hemodialysis cohort
| Classified as Frail | ||
|---|---|---|
| Characteristics | Unadjusted OR (95% CI) | a Adjusted OR (95% CI) |
| Race | ||
| White (reference) | 1.00 | 1.00 |
| Black | 0.51 (0.32, 0.82)b | 0.58 (0.34, 0.99)b |
| Other | 0.70 (0.37, 1.34) | 0.63 (0.30, 1.33) |
| Gender | ||
| Male (reference) | 1.00 | 1.00 |
| Female | 1.11 (0.73, 1.69) | 0.88 (0.54, 1.45) |
| Age ≥ 65 | 2.47 (1.61, 3.78)d | 1.61 (0.97, 2.68) |
| At least high school education | 0.65 (0.42, 1.03) | 0.67 (0.39, 1.13) |
| Diabetes | 1.79 (1.17, 2.75)c | 1.39 (0.83, 2.35) |
| COPD | 1.62 (0.83, 3.17) | 0.86 (0.38, 1.91) |
| CHF | 1.80 (1.17, 2.76)c | 1.21 (0.72, 2.03) |
| CAD/MI | 1.79 (1.16, 2.78)c | 0.81 (0.46, 1.43) |
| CVA/TIA | 2.27 (1.29, 3.99)c | 1.78 (0.89, 3.54) |
| PVD | 3.74 (2.17, 6.44)d | 2.28 (1.17, 4.44)b |
| Other cardiac diseases | 2.70 (1.76, 4.15)d | 1.90 (1.09, 3.31)b |
| BMI | ||
| <18.5 | 1.51 (0.41, 5.57) | 1.25 (0.27, 5.78) |
| [18.5, 25] (reference) | 1.00 | 1.00 |
| [25, 30] | 0.93 (0.54, 1.59) | 0.79 (0.43, 1.46) |
| ≥ 30 | 1.18 (0.72, 1.94) | 1.02 (0.57, 1.81) |
| Hemoglobin | 0.83 (0.71, 0.98)b | 0.92 (0.76, 1.11) |
| Serum albumin | 0.14 (0.08, 0.24)d | 0.18 (0.10, 0.34)d |
| Serum bicarbonate | 1.07 (1.01, 1.14)b | 1.01 (0.94, 1.09) |
| Serum phosphate | 0.89 (0.78, 1.02) | 0.98 (0.83, 1.15) |
| ESRD therapy ≤1 year | 1.39 (0.84, 2.31) | 1.08 (0.60, 1.96) |
N= 727 participants with information for all covariates.
P < 0.05.
P < 0.01.
P < 0.001.
BMI indicates body mass index; CAD, coronary artery disease; CHF, congestive heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; MI, myocardial infarction; other cardiac disease, cardiac dysrhythmia, atrial fibrillation, tachycardia, pericarditis, cardiac arrest; OR, odds ratio; PVD, peripheral vascular disease; TIA, transient ischemic attack.
Race, PVD, other cardiac disease, and serum albumin concentration were independently associated with frailty in the multivariable analysis. Black patients were less likely than white patients to be classified as frail (odds ratio [OR], 0.58 [95% CI, 0.34–0.99], P = 0.04). Patients with PVD (OR, 2.28 [95% CI, 1.17–4.44], P = 0.02), and other cardiac disease (OR, 1.90 [95% CI, 1.09–3.31], P = 0.02) were more likely to be classified as frail. Higher serum albumin concentration was associated with lower likelihood of frailty classification (OR, 0.18 [95% CI, 0.10–0.34], P < 0.001).
There was no significant interaction between age and race (P = 0.43) nor between age and any other predictor variable in the model, and model results were similar when age and years of ESRD therapy (i.e. vintage) were included as continuous variables. In a sensitivity analysis that restricted the race reference group to non-Hispanic whites, results for blacks and participants of other races were virtually unchanged, and results for Hispanic whites did not differ from those for non-Hispanic whites.
DISCUSSION
Among patients undergoing HD, this study showed increased frailty risk associated with PVD and other cardiac diseases. Severe leg pain, exacerbated by walking, is characteristic of PVD. An association between PVD and frailty was reported for the small HD sample (N=146) studied by McAdams-DeMarco et al as well.8 In our study, patients with cardiac diseases such as dysrhythmia and atrial fibrillation were approximately 2 times more likely to be classified as frail compared with patients who did not have these conditions. These associations underscore the importance of early disease detection and initiation of therapeutic measures for PVD and cardiac comorbidity.13,14
The significance of several variables that were associated with frailty status in the univariable analyses, including older age, was attenuated in adjusted analyses that controlled for the effects of diabetes, cardiovascular comorbidities, and serum albumin. No association between female sex and frailty was evident, consistent with the recent study conducted by McAdams-DeMarco et al8 in which performance-based measures of muscle strength and gait speed were obtained as in the study we conducted. However, when patient-reported physical functioning was substituted for grip and walk performance measures in the earlier studies by Johansen et al and Bao et al, women were significantly more likely than men to be classified as frail.6,7 As we and others have noted, a patient-reported health status measure (physical functioning) is a different metric from performance-based assessment of muscle strength and gait speed and may produce markedly different estimates of frailty.15,16
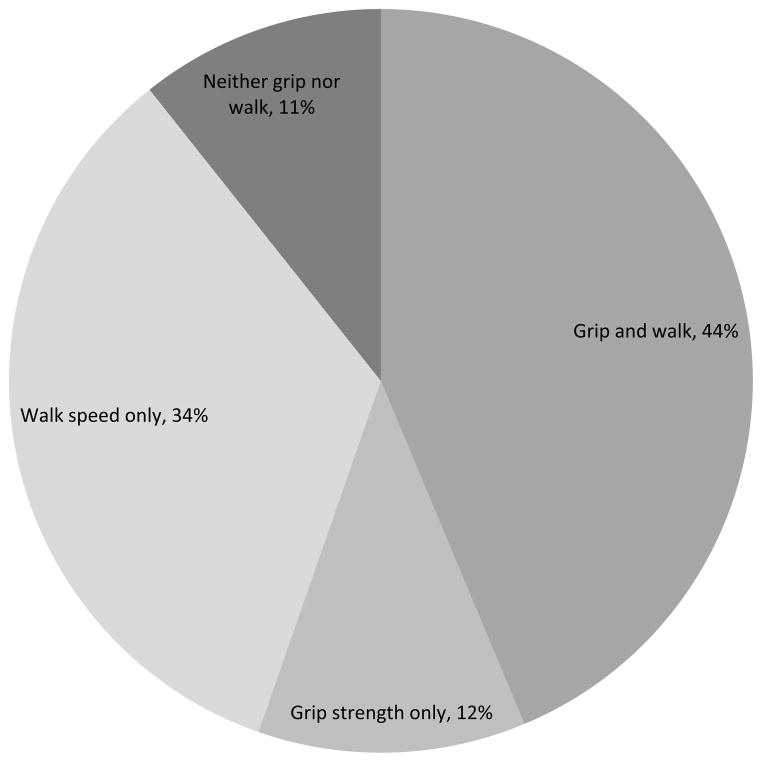
Among the 103 study participants who were defined as frail, Figure 1 shows the proportions who scored as frail on the two measured physical performance criteria, walk speed and grip strength. Of the participants defined as frail, 78% were classified as frail in walk speed and 56% were classified as frail in grip strength; 44% were classified as frail by both measures, and only 11% did not score as frail on either measure. The significance of gait (walk) speed for predicting frailty in older adults has been demonstrated in work by Studenski and colleagues,17,18 and gait speed may be an especially useful screening tool to identify individuals who are at high risk of health and functional decline. It is also of interest that self-reported “exhaustion,” described by Fried et al. as an indicator of poor endurance, characterized 66% of the study participants who were defined as frail.
FIGURE 1.
Among the 103 participants defined as frail, % who were classified as frail by the measured physical performance criteria
We observed pronounced black/white differences in grip strength, an indicator of muscle weakness, which may reflect race differences in serum creatinine concentration and muscle mass.19 Dietary intake was not assessed in the ACTIVE-ADIPOSE study, but higher serum albumin concentration was associated with lower risk for frailty. Based on age-, gender- and diabetes-matched analyses, blacks on HD may have dietary intakes higher in energy and fat compared with whites on HD.20 It has also been suggested that in black women the central distribution of body fat may have a weaker effect on atherogenic risk factors such as levels of cholesterol, triglycerides and sex hormone-binding globulin and degree of peripheral insulin resistance.21
Strengths of our research include carefully collected data on frailty/strength parameters that are not typically collected in dialysis patients. The study population was large, and multiple patient characteristics and treatment-related factors were considered. The ACTIVE-ADIPOSE study successfully enrolled and evaluated over 700 patients accrued over two years at 14 clinics. Most of the patients who were approached agreed to participate. We applied frailty criteria and indicators as defined and validated by Fried and colleagues.
We also acknowledge several limitations of this study. First, the ACTIVE-ADIPOSE cohort shares many similarities with the general ESRD population, but the fact that participants were limited to 7 outpatient clinics in the Atlanta area and 7 outpatient clinics in the San Francisco Bay Area yielded a subset that was not highly representative of the national ESRD population, especially with respect to age composition. Also, it is possible that the study findings are biased by the geographic location of study participants. Second, there may also have been differences between patients who consented and those who declined to participate, although 85% of eligible patients did provide informed consent and were enrolled and the reasons given for declining to participate did not appear to differ systematically by patient sociodemographic characteristics. Third, we observed no association between frailty status and patients’ length of time that ESRD therapy had been received, but no information was available about participants’ frailty status prior to entry into the study. Other potential confounders, such as pre-HD serum potassium, must also be acknowledged. Finally, although age was controlled in the analyses, we acknowledge that black participants were younger on average than whites (mean [median] age = 55.5 [56.3] years among blacks and 59.5 [59.5] years among whites). However, race differences were not evident on most of the clinical variables, and African-American patients were significantly less likely than whites to have an arteriovenous fistula and had a lower average Kt/V. While both groups were younger than the overall prevalent HD population, and estimation of frailty prevalence in any population reflects the demographic and clinical characteristics of that population,9 the precise estimate of frailty would be unlikely to influence the associations of risk factors with frailty that we observed in this large cohort.
Investigation of frailty is clinically important because frailty predicts adverse outcomes,1–8 and a fundamental characteristic of frailty is the potential to treat components of the syndrome by intervening in the frailty process.22 Reducing the likelihood that individuals will experience falls, loss of functional independence, and institutionalization would have profound quality of life benefits as well as being cost-effective. A physical performance measure such as walk speed that requires little time to perform may be especially useful in the outpatient clinical setting as a screening tool to identify individuals who are at risk for frailty.18
CONCLUSION
In multivariable analyses of data from a large prevalent cohort, HD patients’ risk for frailty, as assessed by the presence of 3 or more criteria that comprise the Fried frailty index, was increased in association with PVD and cardiac conditions such as dysrhythmia and atrial fibrillation, and was decreased for participants with higher serum albumin concentration and for blacks compared with whites. Among patients who met the Fried definition of frailty, 78% scored as frail on walk speed and 56% scored as frail on grip strength, the 2 physical performance measures.
Acknowledgments
Supported by contract HHSN267200715004C, ADB No. N01-DK-7-5004 (N.G.K.) from the National Institutes of Health. The interpretation and reporting of the data presented here are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
The authors acknowledge with thanks the many helpful recommendations received from the reviewers.
Footnotes
Conflicts of Interest
The authors have no financial or other conflicts of interest to disclose.
References
- 1.Fried LP, Tangen CM, Walston J, et al. for the Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Bio Sci Med Sci. 2007;62(7):731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilhelm-Leen ER, Hall YN, Tamura MK, et al. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med. 2009;122:664–671. doi: 10.1016/j.amjmed.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roshanravan B, Khatri M, Robinson-Cohen C, et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60(6):912–921. doi: 10.1053/j.ajkd.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Önen NR, Agbebi A, Shacham E, et al. Frailty among HIV-infected persons in an urban outpatient care setting. J Infect. 2009;59:346–352. doi: 10.1016/j.jinf.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Johansen KL, Chertow GM, Jin C, et al. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 7.Bao Y, Dalrymple L, Chertow GM, et al. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172(14):1071–1077. doi: 10.1001/archinternmed.2012.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61:896–901. doi: 10.1111/jgs.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saum K-U, Müller H, Stegmaier C, et al. Development and evaluation of a modification of the Fried frailty criteria using population-independent cutpoints. J Am Geriatr Soc. 2012;60:2110–2115. doi: 10.1111/j.1532-5415.2012.04192.x. [DOI] [PubMed] [Google Scholar]
- 10.Gyamiani G, Basu A, Geraci S, et al. Depression, screening and quality of life in chronic kidney disease. Am J Med Sci. 2011;342(3):186–191. doi: 10.1097/MAJ.0b013e3182113d9e. [DOI] [PubMed] [Google Scholar]
- 11.US Renal Data System. USRDS 2012 Annual Data Report. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda: 2012. p. 390. Table D11. [Google Scholar]
- 12.US Renal Data System. USRDS 2011 Annual Data Report. Chapter 9 National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda: 2011. [Google Scholar]
- 13.Paraskevas KI, Koupidis SA, Tzovaras AA, et al. Screening for peripheral artery disease in dialysis patients: an opportunity for early disease detection and timely initiation of appropriate therapeutic measures. Int Urol Nephrol. 2011;43:142–145. doi: 10.1007/s11255-010-9892-7. [DOI] [PubMed] [Google Scholar]
- 14.Szczech LA. Atrial fibrillation: the beat is faster than the answers. Kidney Int. 2012;81:432–433. doi: 10.1038/ki.2011.430. [DOI] [PubMed] [Google Scholar]
- 15.Kutner NG, Zhang R. Frailty in dialysis-dependent ESRD patients. JAMA Int Med. 2013;173 (1):78–79. doi: 10.1001/2013.jamainternmed.750. [DOI] [PubMed] [Google Scholar]
- 16.Painter P, Kuskowski M. A closer look at frailty in ESRD: getting the measure right. Hemo Int. 2013;17:41–49. doi: 10.1111/j.1542-4758.2012.00719.x. [DOI] [PubMed] [Google Scholar]
- 17.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;30 (5):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viccaro LJ, Perera S, Studenski S. Is timed up and go better than gait speed in predicting health, function, and falls in older adults? J Am Geriatr Soc. 2011;59:887–892. doi: 10.1111/j.1532-5415.2011.03336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu J, Johansen KL, Hsu C-y, et al. Higher serum creatinine concentrations in black patients with chronic kidney disease: beyond nutritional status and body composition. Clin J Am Soc Nephrol. 2008;3:992–997. doi: 10.2215/CJN.00090108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noori N, Kovesdy CP, Dukkipati R, et al. Racial and ethnic differences in mortality of hemodialysis patients: role of dietary and nutritional status and inflammation. Am J Nephrol. 2011;33(2):157–167. doi: 10.1159/000323972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens J, Guatman SP, Keil JE. Body mass index and fat patterning as correlates of lipids and hypertension in an elderly, biracial population. J Gerontol. 1993;48:M249–M254. doi: 10.1093/geronj/48.6.m249. [DOI] [PubMed] [Google Scholar]
- 22.Michel J-P, Newton JL, Kirkwood TBL. Medical challenges of improving the quality of a longer life. JAMA. 2008;299:688–690. doi: 10.1001/jama.299.6.688. [DOI] [PubMed] [Google Scholar]