Abstract
Recent work showed that arterial compliance may be unexpectedly elevated in obese children, attributable to accelerated growth and maturation. We hypothesize that children with obesity or type 2 diabetes may reach peak arterial maturation earlier in life and then experience an earlier, and potentially more rapid decline in arterial compliance, leading toward earlier cardiovascular disease development.
Keywords: arterial compliance, arterial stiffness, vascular health, adolescents, maturation
INTRODUCTION
Cardiovascular disease has been the leading cause of death in the United States for the past 80 years and results in 40% of all-cause mortality in developed countries (6). One of the most important contributors to cardiovascular disease risk is obesity. The Munster Heart Study followed 1 million adults over 14 years and showed that body mass index (BMI) was strongly and positively associated with risk of all-cause and cardiovascular mortality (38). The prevalence of obesity during childhood has risen in the last 30 years to its current level of ~18% in the United States, with even higher rates in some racial/ethnic minority populations (32). This trend is concerning because it portents a future increase in cardiovascular disease and potential early death in people who become obese early in life and maintain an unhealthy lifestyle into adulthood. In the Bogalusa Heart Study of 5-10 year old obese children, 58% of the population had one risk factor for cardiovascular disease and 25% had two or more (38). Thus, although age-adjusted cardiovascular mortality in the United States has declined since 1960, the rise in childhood obesity may begin to counter that trend. Studying the causes and consequences of childhood obesity and cardiovascular disease risk, and implementing effective prevention strategies are therefore strategic goals to assure healthier futures for today's youth.
Assessing vascular structure and function in children is important due to increasingly sedentary and obesogenic lifestyles adopted in the past 30 years; we expect that obese children with low habitual physical activity are at increased risk for cardiovascular disease and at an earlier age than in prior generations. Thus, it is important to study components of cardiovascular structure and function in youth in order to describe patterns of development, the impact of obesity, diabetes and/or metabolic syndrome, and to identify the early markers of potential dysfunction or disease. We recently reported the effects of obesity and type 2 diabetes on arterial compliance in children and adolescents (29-31). In contrast to most studies in adults and some studies in children, we found that obesity and type 2 diabetes were associated with a paradoxical increase in arterial compliance (29-31). This finding was attributed in part to the earlier maturation and increased body size that occurs in obese children. We hypothesize, however, that our findings represent a type of short-term adaptation in children with obesity or type 2 diabetes that is not sustainable over the long term. Recent evidence supports that the process of arterial stiffening may occur more rapidly in obese adolescents (8). In this review we discuss the studies of arterial compliance in children that have been published to date and how our data and that of others supports the novel hypothesis that children with obesity or type 2 diabetes may reach peak arterial maturation earlier in life and then experience an earlier, and potentially more rapid decline in arterial compliance. Such a scenario could contribute to increased cardiovascular disease risk early in life in people who remain obese during childhood and young adulthood.
Arterial compliance is the amount of arterial expansion and recoil that occurs with cardiac pulsation and relaxation, and is linked to both structural and functional properties of the artery (19). As an index of cardiovascular health, changes in arterial compliance precede and predict future cardiovascular events. Several non-invasive techniques have been developed that can be used to measure arterial compliance in children and adults. Alterations in arterial stiffness are measured as changes in pulse wave velocity or the arterial pulse waveform (19). It has been demonstrated that obese adults have lower arterial compliance compared to normal weight controls (1) although this may not be the same response in children, as described in later sections of this review. Increased brachial systolic and pulse pressure resulting from decreased arterial compliance is associated with increased risk of cardiovascular events in older adults (23). Low arterial compliance has also been associated with microvascular disease and contributes to the development of end-organ damage, stroke, and renal failure in adulthood (27). Indices of arterial compliance such as aortic pulse wave velocity and central systolic augmentation index are strongly associated with cardiovascular disease independent of conventional risk factors (23). Additionally, the presence of conventional risk factors (hypertension, elevated low-density lipoprotein cholesterol, low high-density lipoprotein cholesterol) predicts the development of decreased arterial compliance in adulthood (27). Collectively, the majority of data available for studies of adults has demonstrated that arterial compliance is an important measure of vascular health that is reduced during aging and further reduced in the presence of obesity, T2D, and hypertension (3, 23). Relatively less information is available about arterial compliance in children, especially the impact of health status and lifestyle factors.
ARTERIAL COMPLIANCE IN ADULTS
In cross-sectional studies of healthy adults arterial compliance has been reported to be inversely associated with age of the study participants. For example, peak values for large (also referred to as C1) and small (also known as C2) arterial compliance in a cross-sectional study of healthy, normal weight men and women were observed at ages 25-30 years old and then declined about 1% per year to age 70 years (14). This decline with normal aging is attributable to structural changes in the vascular walls, which demonstrate reduced elastic properties in older people (14). The presence of obesity, hypertension, and hyperlipidemia may accelerate the age-related decline in arterial compliance in adults since each of those conditions is independently associated with decreased arterial compliance in age- and sex-matched cohort studies (1, 4, 39). For example, Acree, et al. (1) reported that large (C1) and small (C2) arterial compliance were 17% and 13% lower, respectively, in older adults (mean age 61 years old) who were obese than people who were normal weight, even after controlling for differences in body surface area and the presence of hypertension and hyperlipidemia. Likewise, adults with T2D also have decreased arterial compliance compared to non-diabetic controls (2, 5). In their study, Aoun and colleagues (2) compared 122 adults with T2D to 122 age- and sex-matched non-diabetic controls and found that the T2D group had 9% higher aortic pulsewave velocity. Pulsewave velocity is calculated as the time required for a pulse pressure waveform to travel a measured distance through the arterial tree. Since pulsewaves travel faster through stiffer, less elastic vessels, the results reported by Aoun and colleagues (2), acquired from measured pulsewaves at the carotid and femoral arteries for an estimate of central arterial (aortic) properties, demonstrated that adults with T2D had reduced arterial compliance compared to people without diabetes. However, in that study the carotid augmentation index, another measure of compliance that is calculated as the difference in waveform magnitude between the peak aortic pressure and the first systolic peak, divided by the pulse pressure and expressed as a ratio, was not different between groups. The authors interpreted their findings as evidence that the negative effects of diabetes on arterial wall properties in their cohort was more evident in the large, central arteries than in the smaller, peripheral arteries. Predictor variables that were positively related to central arterial stiffness included age, mean arterial pressure, fasting glucose, and smoking, while kidney function was a negative predictor (2). In contrast, Brooks et al. (5) found that augmentation index was 6% higher in middle-aged men with T2D compared to healthy controls, demonstrating that diabetes was associated with less compliant arteries. Notably, though, this difference was only evident in men, but not women, with T2D. Collectively, most of the data from studies of adults support that one or more indices of arterial compliance are reduced in the presence of obesity and/or T2D, although the mechanisms responsible are not yet clear and some variations among reports are not yet explained.
ARTERIAL COMPLIANCE IN CHILDREN: EFFECT OF OBESITY
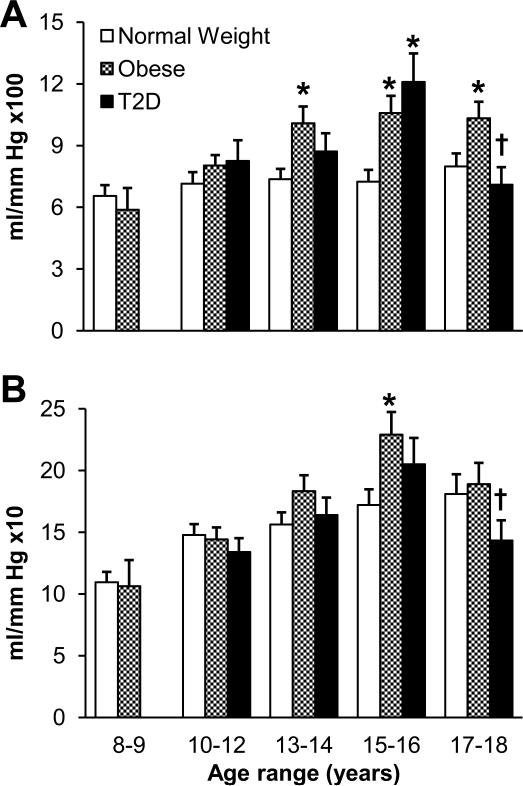
The reported effect of childhood obesity on arterial compliance has not been consistent within the literature. We recently showed that arterial compliance was increased in obese children compared to their normal peers (29). We performed diastolic pulsewave contour analysis in 61 normal weight (BMI range = 25-75th percentile for age and sex based on United States Centers for Disease Control growth curves) and 62 obese (BMI ≥ 95th percentile) children. Small artery compliance was 30% higher and large artery compliance was 18% higher in obese compared to normal weight participants (Figure 1). Multivariate regression analysis revealed that the higher arterial compliance values in obese youth were attributable to their higher fat mass and higher lean mass; both fat and lean mass were significant, independent predictors of arterial compliance in this young cohort (29). Although fasting insulin was nearly 4-fold higher in the obese children and insulin acts as a vasodilator, the variation in insulin concentration did not explain the differences in arterial compliance. The obese children had normal fasting glucose and were normotensive so glycemia and blood pressure were also not predictive of the differences in arterial compliance. As depicted in Figure 1, small and large artery compliance increased with age, particularly in the obese children. These data agree with previous evidence that arterial compliance increases during childhood and early adulthood (14). However, in our study obese children had more advanced pubertal development (Tanner stage) for chronological age, a common finding in pediatric clinics. Tanner stage was a positive predictor for large, though not small, artery compliance. Our interpretation of these data was that the evidence of advanced development and the larger body size (both lean and fat mass) in obese adolescents may contribute to a leftward shift in the curve describing the relationship between age and arterial compliance (Figure 2). Such a scenario may represent an appropriate response to the child's size and developmental age during adolescence, but based on the findings that compliance declines with age and obesity in adulthood (1, 2, 4, 5, 14, 39), we predict that obese children may experience earlier attainment of peak arterial compliance, in both the small and large arteries (29), followed by earlier decline compared to age-matched normal weight young adults (Figure 2). To address this prediction will require a longitudinal cohort study design with repeat measures of arterial compliance and several of the descriptive characteristics that may explain differences among subgroups and the changes in arterial compliance over time.
Figure 1.
Arterial compliance in children and the effect of obesity and type 2 diabetes. A) Small artery elasticity index measured using diastolic pulsewave contour analysis in children classified as healthy normal weight (N = 61), obese (N = 63), or obese with type 2 diabetes (T2DM, N = 34). B) Large artery elasticity index measured with the same method in the same children as in panel A. * P<0.05 versus Normal Weight group. † P<0.05 for comparison between 15-16y and 17-18y age groups with T2D. There were no children in the 8-9y age range with T2DM. [Adapted from (29). Copyright © 2012 John Wiley and Sons. Used with permission; Adapted from (31). Copyright © 2013 John Wiley and Sons. Used with permission.]
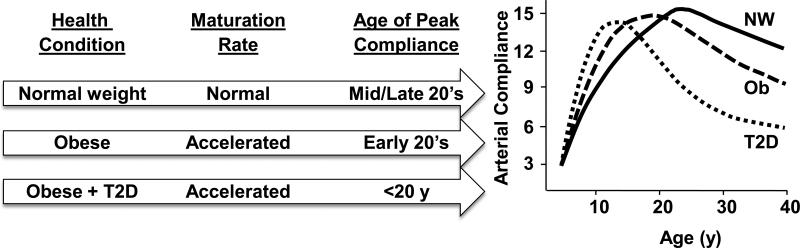
Figure 2.
Hypothesized impact of childhood obesity or type 2 diabetes on arterial compliance from adolescence into adulthood. The curve for people classified as normal weight (NW) is a composite from data on children and adults measured in our center using diastolic pulsewave contour analysis (7, 14, 29). Curves for people with obesity (Ob), or obesity plus type 2 diabetes (T2DM) are based on our measurements on children 8-18 years old (29); the curves for those two groups into adulthood (age >18 years) are hypothesized projections based on the adult literature that has reported lower arterial compliance in adults with obesity or diabetes [e.g., (1, 2, 4, 5, 39)]. Data shown depict small artery elastic index (y-axis units are in ml/mmHg x100) but similar age-related changes were obtained for large artery elastic index. Our data demonstrate higher values of arterial compliance in children who are obese or obese T2D compared to normal weight peers (29, 31). We propose that this is attributable, in part, to earlier physical maturation and attainment of adult stature. However, during early adulthood the negative effects of obesity, which are likely accelerated by the presence of T2DM, are expected to result in earlier decline in vascular function. As described in the text, some studies have reported that obesity may be associated with lower arterial compliance even during childhood (25, 28) or early adulthood (33), a finding that is different from what is depicted in this figure.
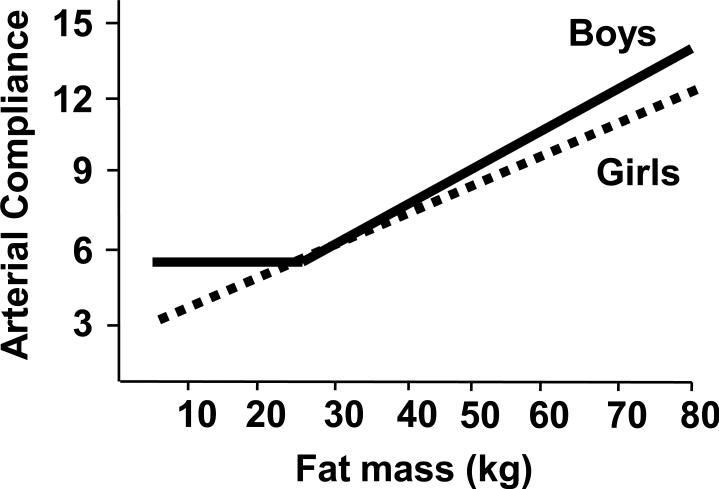
An additional finding from our studies was that the association between body composition and arterial compliance differed between boys and girls (Figure 3). As a group boys had higher compliance values than girls, but this difference was attributable primarily to differences between the boys and girls in the normal body weight range. Within the subgroup of normal weight children, the sex-difference in arterial compliance was best explained by the higher lean body mass in boys. Fat mass was a positive predictor of small and large artery compliance in normal weight girls but was not associated with arterial compliance in normal weight boys. In contrast, within the subgroups of obese boys and girls, there was no difference between sexes for lean mass, fat mass, or arterial compliance, and the relationship between fat mass and arterial compliance was similar for boys and girls. Thus, the sex-differences in body composition, particularly body fat, and arterial compliance that were present in normal weight children were not present in obese children. As shown in the summary in Figure 3, arterial compliance was positively correlated with total body fat, measured by dual energy X-ray absorptiometry (DXA), across the range of measured values in girls. However, that relationship was only present for boys with more than 24kg of fat mass; below the 24kg threshold, fat mass and arterial compliance were not significantly associated in boys. The mechanism that explains this relationship is not yet clear but is it possible that the vascularity of adipose tissue or the secretion of vasoactive compounds from adipose tissue must reach a higher level in boys than girls before arterial compliance is affected.
Figure 3.
Differential association between adiposity and arterial compliance in boys and girls. The figure shows results of regression analyses performed with the data on small artery elastic index (y-axis units are in ml/mmHg ×100) from normal weight and obese children described in Figure 1; there was a similar relationship between fat mass and large artery elastic index. Total body fat mass was positively associated with both the small and large arterial elasticity indices across the range of body fat for girls. A similar association was evident in boys with > 24kg fat mass, but not for boys below that threshold. [Adapted from (30). Copyright © 2012 the authors. Used with permission.]
Other studies have also reported that obese children have increased arterial compliance. Chalmers and colleagues (7) used diastolic pulsewave contour analysis and showed that large artery compliance was 38% higher and small artery compliance was 20% higher in obese versus normal weight children (mean age: 13 years old) even after controlling for height, blood pressure, sex, and Tanner stage. In agreement, Dangardt and colleagues (9) reported that carotid-radial pulse wave velocity was 11% lower (indicating increased arterial compliance) in obese adolescent girls than in girls within the normal range for BMI. In a large cohort of pre-pubertal children, ages ~9-12 years old, in the United Kingdom, Donald and colleagues (11) reported that BMI was negatively correlated with pulse wave velocity and positively correlated with brachial artery distensibility coefficient (a measure of arterial elasticity) which could contribute to higher arterial compliance in children with elevated BMI. They also reported that flow-mediated brachial artery dilation (a measure of vascular function in response to vessel occlusion and resumption of blood flow) was also positively correlated with BMI. Flow mediated dilation is typically reduced in the presence of obesity or diabetes in adults and some studies of children (34). Although the three separate measures of vascular function reported by Donald and colleagues (11) were not well-correlated with one-another, they each suggest that children with higher BMI display changes in arterial structure and/or function that are not typical for studies of obese adults. Whether this reflects adaptive processes or early pathology in obese children is not yet clear. Donald et al. (11) reported that brachial artery diameter was a significant predictor for each of the vascular outcomes in their study. Thus, while the studies cited (7, 9, 11) were not designed to identify the underlying mechanism that explains why obese children may have the unexpected vascular differences observed, they suggest a potential role for vessel size that should be considered in future studies.
In contrast to the studies described above, there are reports that central arterial compliance is reduced in obese children. For example, Tounian and colleagues (28) showed that severely obese children, median age ~12 years old, had 7% lower central compliance and 14% lower vessel distensibility, measured at the carotid artery with ultrasound techniques, compared to a normal weight control group. Urbina and colleagues (33) performed carotid ultrasonographic assessments on 446 children and young adults (age range 10-24 years old, mean age 17.8 years) and found that obese participants had ~20% stiffer arteries than their normal weight peers. In that study, carotid stiffness was positively associated with age and blood pressure. Similarly, Sakuragi and colleagues found that BMI and total body fat measured by DXA were positively associated with pulse wave velocity measured by applanation tonometry of the carotid and femoral arteries in 573 Japanese children 9-10 years old (25). It is not yet clear what factors, whether methodological, or characteristics of the study populations, account for the differences among the studies that have measured the effect of obesity on arterial compliance. Some possibilities are considered in a later section.
ARTERIAL COMPLIANCE IN CHILDREN WITH T2DM
To our knowledge there have only been three published studies that described the effect of T2D on arterial compliance in children. We found that children with T2D (who were all overweight or obese) had, like obese children without T2D, higher arterial compliance compared to normal weight controls (31). In our sample of 10-18 year olds with T2D (average duration of diagnosis 1.9 ± 1.7 years) small artery compliance was 24% higher than age-matched normal weight youth but not different from obese peers (Figure 1). The difference in small artery compliance between the T2D and normal weight groups was most evident in children 15-16 years old. Small artery compliance in the T2D group was positively associated with total lean body mass, systolic blood pressure, and fasting glucose. In contrast, large artery compliance in the T2D group was not different from either normal weight or obese children. A notable finding was that large artery compliance in the T2D group increased with age from ages 10-16 years and then declined significantly in 17-18 year olds; small artery compliance followed a similar, albeit not-statistically significant pattern (Figure 1). While a larger follow-up study that includes young adults is required for confirmation, our data support the hypothesis that children with T2D follow a pattern of elevated arterial compliance at younger ages that is similar to obese children but start to show signs of declining compliance earlier than expected for normal weight or obese peers (Figure 2).
In contrast to our findings, Gungor and colleagues (16) reported that pulse wave velocity, and therefore arterial stiffness, measured with ultrasonography at the carotid artery in children with T2D (average duration of 1.7 years) was 55% greater than normal weight and 32% greater than obese comparison groups of similar age (~15 years old). Urbina and colleagues (33) also found that carotid artery stiffness was increased in children and young adults (age range 10-24 years old) with T2D. Beta-stiffness and Young's elastic modulus values were 12-20% higher in the T2D group compared to a normal weight group but not different compared to an obese group with the same average BMI (33).
INTERPRETATIONS AND POTENTIAL MECHANISMS
It is not yet clear why there is a lack of consensus on the effects of obesity and T2D on arterial compliance in children and adolescents. There are differences among studies in the ages and clinical features of the study participants, methodology used to measure vascular parameters, and approaches used to analyze that data that may contribute to the different outcomes. There is also the challenge of drawing conclusions from the relatively few studies published so far.
Variations in the age, maturation, race/ethnicity, and/or clinical history of the participants may to contribute to differences among studies. In our studies, for example, arterial compliance varied with age, in addition to the effects of obesity and diabetes (Figure 1). The value of presenting age-related pattern of arterial compliance is that it reveals potential differences among groups that may be missed if analysis is limited to aggregate statistics. Notably, we found that arterial compliance had a stronger association with chronological age than Tanner maturation score. Our data suggest that for youth with T2D, arterial compliance may be increased in younger participants but decreased in older cohorts (Figure 2). In the study by Urbina and colleagues (33), the average age of the participants was 18 years old and people up to 24 years old were included. It is therefore possible that the reduced arterial compliance in the T2D group in that study was attributable to more pronounced effects of T2D in the older participants or that the carotid artery is already dilated resulting in less smooth muscle relaxation and lower distensibility in older children and young adults. Analyses of participants with T2D may also be challenging if the duration of diabetes or medication use is not known or not presented. In the study by Gungor, et al. (16), some of the participants were on multiple medications; it is possible that those participants had a higher level of cardiometabolic dysfunction that contributed to their reduced arterial compliance at the time of measurement. By comparison, participants with T2D in our investigation were only using lifestyle approaches or metformin to manage their blood glucose and were not using other medications, such as insulin, thiazolidinediones, or lipid-lowering agents, with known cardiovascular or metabolic effects (31). In addition to the effects of age, our data analyses revealed that variations in body composition and sexual maturation predict differences in arterial compliance and that data analyses may need to account for interactions between the effects of body size and sex (29, 30).
Methodological differences may also contribute to the different outcomes reported. There are several methodological approaches that have been used to measure arterial compliance in children and adults. All approaches have strengths and limitations and provide different insights about vascular function, as reviewed elsewhere (34); this variation may complicate comparisons among studies that use different methods. Our studies (29, 31) and others that have recently reported that arterial compliance was increased in obese children used techniques with measurements made at the radial artery (7, 9). In the studies in which arterial compliance was reduced in children with obesity or T2D, the measurements were made at the carotid and/or femoral artery and provided a measure of central compliance (16, 25, 28, 33). Whether this reveals that obesity exerts differential effects in different parts of the vascular tree in children, or is a reflection of the strengths and limitations of each method is not yet clear. Peripheral vessels such as the radial artery may be more dilated and compliant than central vessels in obese children but comparison studies are required to confirm this possibility.
Among the potentially important co-variates that have been understudied so far are diet, physical activity, and physical fitness. Of the studies in children in which arterial compliance was compared in normal weight and obese children, physical activity was measured using accelerometry in only two reports (7, 25). In both of those studies, however, physical activity was not a significant predictor of arterial compliance after controlling for variables such as age, sex, and blood pressure. None of the studies examining obesity in children reported data on dietary history and only one reported a measure of aerobic fitness (25). In the latter study, aerobic fitness was measured in children 8-12 years old using a 20-meter shuttle run and was found to be a positive predictor of arterial compliance. Since physically active lifestyle is associated with better vascular function, such as higher arterial compliance in adults (26), aerobic fitness and physical activity require more attention as modifying variables in studies of children, particularly those designed to assess the specific effects of obesity, diabetes, or metabolic syndrome.
The implications of our findings, as depicted in Figure 2, are that childhood obesity may result in earlier attainment of peak values for arterial compliance but may also drive an earlier decline during adulthood. T2D may further accelerate that decline. A potential explanation for why obese or T2D children do not have more pronounced reduction in arterial compliance at earlier ages is the relatively lower development of atherosclerotic changes. Obese adults have more atherosclerosis compared to obese children (10, 13, 17), which attenuates vascular smooth muscle relaxation. The increased luminal pressure in atherosclerotic vessels in older obese people due to atherosclerosis and decreased elastin in the vascular wall is expected to result in decreased arterial compliance (26). However, a premature reduction in vascular function as depicted in Figure 2 is consistent with the earlier development of cardiovascular disease risk in people who were obese during childhood (38). As already noted, some (7, 9, 11, 29), though not all (25, 28, 33), studies suggest that during childhood there is an ability to accommodate the negative impact of obesity on the vascular system (i.e. arterial compliance is not reduced and may be increased), although new evidence demonstrates that the consequences of obesity are likely emerge in children who remain obese (8). Dangardt and colleagues (8) recently published a five year follow-up to their initial study in which they reported that obese children had lower arterial stiffness at age ~13.8 years old compared to a group of normal weight children. In the follow-up, when the participants were ~18-19 years old, arterial stiffness was higher in the obese group compared to the normal weight group. This reversal occurred because over the 5-year interim pulsewave velocity increased 25% in the obese group but only 3% in the normal weight group. Further, there were parallel changes in diastolic blood pressure and changes in arterial stiffness were associated with changes in arterial vessel diameter. These data suggest that obese children have a capacity to increase arterial compliance as an adaptive response but under the pressure of ongoing obesity this adaptation is temporary, and eventually the established pattern of reduced arterial compliance that has been observed in obese adults will become apparent in obese youth. Thus, the data collected cross-sectionally (25, 28, 29, 31, 33) and the longitudinal data of Dangardt, et al. (8) highlight the need to monitor cardiovascular health of obese children and develop strategies to avoid development of elevated risk during the transition to adulthood.
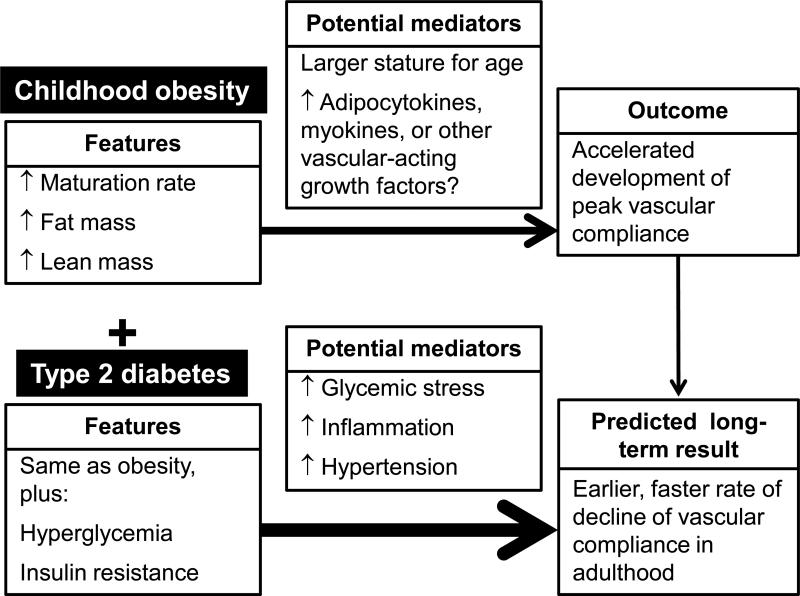
The mechanisms that account for how early maturation and excess adiposity result in higher arterial compliance in obese children are not yet clear. In the studies by Donald et al. (11) and Dangardt, et al. (8) arterial diameter was reported to play an important role in the compliance of the vessel. Therefore, in the obese child it is possible that the vascular system accommodates an increase in blood volume through systemic vasodilation. In our studies we found that fat mass and lean mass were each positively associated with arterial compliance in obese children (29, 30). Thus, it is possible that differences in arterial structure and function in obese children can be attributed, in part, to their larger stature and larger vascular network, which is attained at an earlier age because of faster maturation (Figure 4). The overall vascular tone is mediated through several mechanisms. First, the endothelium provides vascular control through the actions of nitric oxide and endothelin. Nitric oxide (NO) released from the endothelium via sheer stress results in the relaxation of the adjacent smooth muscle thereby resulting in vasodilation. Opposing the action of NO is endothelin, which causes smooth muscle contraction and vasoconstriction. The autonomic nervous system also plays a role maintaining vascular tone. Neurohormonal mechanisms, such as the renin-angiotensin-aldosterone system, also affect vascular tone (15). Additionally, since it is established that adipocytes and skeletal muscle communicate with other tissues by secreting several hormones and cytokines, it is possible that compounds released from either fat depots or lean tissues act on the vasculature to increase arterial compliance (Figure 4). Compounds that stimulate the production of nitric oxide synthase (eNOS) in vascular endothelial cells could lead to increased nitric oxide release, greater smooth muscle relaxation, and increased arterial compliance (37). At least two hormones, insulin and visfatin, that are elevated in obesity are known to increase eNOS (20, 24). In adults, both eNOS protein and mRNA content are higher in the subcutaneous fat of obese individuals relative to that of normal weight controls (12). Although infusion of insulin promotes eNOS activity and vasodilatation (36), insulin resistance is associated with lower eNOS mRNA expression and activity in adults (18). In our analyses, fasting insulin and calculated insulin resistance indices were not correlated with vascular compliance (29). Our interpretation was that insulin per se is unlikely to be a primary mediator of the differences in arterial compliance in children, but other vasoactive compounds, including those produced by adipose tissue, may play a role.
Figure 4.
Features of obesity and type 2 diabetes in childhood and the potential mediators that contribute to higher arterial compliance in obese youth.
Ongoing exposure to obesity, particularly if accompanied by T2D is expected to result in premature reduction in arterial compliance and increased cardiovascular disease risk, even in youth. For adolescents with T2D we observed higher fasting glucose, insulin resistance, hypertriglyceridemia, inflammation, and systolic blood pressure compared to normal weight or obese peers (31). These features have all been shown in adults to be associated with cardiovascular disease risk and are therefore expected to contribute to a decline of arterial compliance at an earlier age than in obese youth (Figure 4). In particular, glycemic variability in people with diabetes is likely to cause oxidative and osmotic stress on the vasculature (22).
FUTURE DIRECTIONS
Since there have been few investigations on arterial compliance in youth, additional confirmation studies are needed, especially those that include a wide age range of participants, compare outcomes from different methods of assessing vascular function, and account for potential modifying variables like sex, body composition, aerobic fitness, and lifestyle factors like diet and physical activity. Longitudinal studies, like those presented by Dangardt et al. (8) would be particularly useful to follow trends in the development of arterial compliance from childhood into adulthood since the majority of current data are based on cross-sectional comparisons. Recent trends for obesity in children suggest that the number of obese adults will continue to increase for the foreseeable future, which could contribute to higher cardiovascular disease risk. Obviously, individual-, family-, and community-based interventions to curtail the rise in obesity could have major impact but require significant resources to be successful. Interventions that emphasize physical activity are essential. Although the effect of exercise training on arterial compliance in obese or T2D children has, to our knowledge, not been reported, other components of vascular function such as endothelial-dependent flow-mediated dilation have been reported to improve in response to exercise training in obese children (21, 35). These strategies could help counter the effects of obesity, independent of weight loss.
SUMMARY
The presence of obesity and T2D in children and adolescents may be associated with increased arterial compliance that appears to be attributable to earlier maturation and increased fat and lean tissue mass. Based on our data (29, 31) and that of others (7-9, 11) we hypothesize that obese and T2D children may attain their peak potential for arterial compliance earlier in life than normal weight children but may also experience earlier decline in vascular health, and therefore increased cardiovascular risk, in adulthood (Figure 2). Additional confirmation of this proposal, along with lifestyle interventions that address the cardiometabolic disease risk of obese and T2D youth are the next step.
Acknowledgments
Funding support:
Funding for the authors’ work was provided by the Endocrine Fellows Foundation, Marilyn Fishman Grant for Diabetes Research, the Lawson Wilkins Pediatric Endocrine Society Clinical Scholars Award, the University of Oklahoma Health Sciences Center Department of Pediatric Diabetes and Endocrinology, and NIH Grant Number P20 RR 024215 from the COBRE Program of the National Center for Research Resources.
REFERENCES
- 1.Acree LS, Montgomery PS, Gardner AW. The influence of obesity on arterial compliance in adult men and women. Vasc. Med. 2007;12:183–8. doi: 10.1177/1358863X07079323. [DOI] [PubMed] [Google Scholar]
- 2.Aoun S, Blacher J, Safar ME, Mourad JJ. Diabetes mellitus and renal failure: effects on large artery stiffness. J. Hum. Hypertens. 2001;15:693–700. doi: 10.1038/sj.jhh.1001253. [DOI] [PubMed] [Google Scholar]
- 3.Arnett DK, Evans GW, Riley WA. Arterial stiffness: a new cardiovascular risk factor? Am. J. Epidemiol. 1994;140:669–82. doi: 10.1093/oxfordjournals.aje.a117315. [DOI] [PubMed] [Google Scholar]
- 4.Arnett DK, Glasser SP, McVeigh G, et al. Blood pressure and arterial compliance in young adults: the Minnesota Children's Blood Pressure Study. Am. J. Hypertens. 2001;14:200–5. doi: 10.1016/s0895-7061(00)01262-0. [DOI] [PubMed] [Google Scholar]
- 5.Brooks BA, Molyneaux LM, Yue DK. Augmentation of central arterial pressure in type 2 diabetes. Diabet. Med. 2001;18:374–80. doi: 10.1046/j.1464-5491.2001.00479.x. [DOI] [PubMed] [Google Scholar]
- 6.Centers_for_Disease_Control_and_Prevention Prevalence of heart disease-United States, 2005. MMWR. 2007;56:113–8. [PubMed] [Google Scholar]
- 7.Chalmers LJ, Copeland KC, Hester C, Fields DA, Gardner AW. Paradoxical increase in arterial compliance in overweight pubertal children. Angiology. 2011;62:565–70. doi: 10.1177/0003319711399117. [DOI] [PubMed] [Google Scholar]
- 8.Dangardt F, Chen Y, Berggren K, Osika W, Friberg P. Increased rate of arterial stiffening with obesity in adolescents: A five-year follow-up study. PLoS One. 2013;8:e57454. doi: 10.1371/journal.pone.0057454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dangardt F, Osika W, Volkmann R, Gan LM, Friberg P. Obese children show increased intimal wall thickness and decreased pulse wave velocity. Clin Physiol Funct Imaging. 2008;28:287–93. doi: 10.1111/j.1475-097X.2008.00806.x. [DOI] [PubMed] [Google Scholar]
- 10.Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: the Muscatine Study. Circulation. 2001;104:2815–9. doi: 10.1161/hc4601.099486. [DOI] [PubMed] [Google Scholar]
- 11.Donald AE, Charakida M, Falaschetti E, et al. Determinants of vascular phenotype in a large childhood population: the Avon Longitudinal Study of Parents and Children (ALSPAC). Eur. Heart J. 2010;31:1502–10. doi: 10.1093/eurheartj/ehq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elizalde M, Ryden M, van Harmelen V, et al. Expression of nitric oxide synthases in subcutaneous adipose tissue of nonobese and obese humans. J. Lipid Res. 2000;41:1244–51. [PubMed] [Google Scholar]
- 13.Freedman DS, Dietz WH, Tang R, et al. The relation of obesity throughout life to carotid intima-media thickness in adulthood: the Bogalusa Heart Study. Int. J. Obes. Relat. Metab. Disord. 2004;28:156–66. doi: 10.1038/sj.ijo.0802515. [DOI] [PubMed] [Google Scholar]
- 14.Gardner AW, Parker DE. Association between arterial compliance and age in participants 9 to 77 years old. Angiology. 2010;61:37–41. doi: 10.1177/0003319709339588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golan DE, Tashijan AH, Armstrong EJ, Armstrong AW. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. 3rd Edition Lippincott Williams & Wilkins; Philadelphia, PA: 2012. [Google Scholar]
- 16.Gungor N, Thompson T, Sutton-Tyrrell K, Janosky J, Arslanian S. Early signs of cardiovascular disease in youth with obesity and type 2 diabetes. Diabetes Care. 2005;28:1219–21. doi: 10.2337/diacare.28.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juonala M, Viikari JS, Kahonen M, et al. Childhood levels of serum apolipoproteins B and A-I predict carotid intima-media thickness and brachial endothelial function in adulthood: the cardiovascular risk in young Finns study. J. Am. Coll. Cardiol. 2008;52:293–9. doi: 10.1016/j.jacc.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 18.Kearney MT, Duncan ER, Kahn M, Wheatcroft SB. Insulin resistance and endothelial cell dysfunction: studies in mammalian models. Exp. Physiol. 2008;93:158–63. doi: 10.1113/expphysiol.2007.039172. [DOI] [PubMed] [Google Scholar]
- 19.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur. Heart J. 2006;27:2588–605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 20.Lovren F, Pan Y, Shukla PC, et al. Visfatin activates eNOS via Akt and MAP kinases and improves endothelial cell function and angiogenesis in vitro and in vivo: translational implications for atherosclerosis. Am. J. Physiol. Endocrinol. Metab. 2009;296:E1440–9. doi: 10.1152/ajpendo.90780.2008. [DOI] [PubMed] [Google Scholar]
- 21.Meyer AA, Kundt G, Lenschow U, Schuff-Werner P, Kienast W. Improvement of early vascular changes and cardiovascular risk factors in obese children after a six-month exercise program. J. Am. Coll. Cardiol. 2006;48:1865–70. doi: 10.1016/j.jacc.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 22.Monnier L, Mas E, Ginet C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–7. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 23.O'Rourke MF, Hashimoto J. Arterial stiffness: a modifiable cardiovascular risk factor? J Cardiopulm Rehabil Prev. 2008;28:225–37. doi: 10.1097/01.HCR.0000327179.21498.38. [DOI] [PubMed] [Google Scholar]
- 24.Ritchie SA, Kohlhaas CF, Boyd AR, et al. Insulin-stimulated phosphorylation of endothelial nitric oxide synthase at serine-615 contributes to nitric oxide synthesis. Biochem. J. 2010;426:85–90. doi: 10.1042/BJ20091580. [DOI] [PubMed] [Google Scholar]
- 25.Sakuragi S, Abhayaratna K, Gravenmaker KJ, et al. Influence of adiposity and physical activity on arterial stiffness in healthy children: the lifestyle of our kids study. Hypertension. 2009;53:611–6. doi: 10.1161/HYPERTENSIONAHA.108.123364. [DOI] [PubMed] [Google Scholar]
- 26.Seals DR, DeSouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J. Appl. Physiol. 2008;105:1323–32. doi: 10.1152/japplphysiol.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Short KR, Blackett PR, Gardner AW, Copeland KC. Vascular health in children and adolescents: effects of obesity and diabetes. Vasc Hlth Risk Mgmt. 2009;5:973–90. doi: 10.2147/vhrm.s7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tounian P, Aggoun Y, Dubern B, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–4. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- 29.Tryggestad JB, Thompson DM, Copeland KC, Short KR. Obese children have higher arterial elasticity without a difference in endothelial function: the role of body composition. Obesity. 2012;20:165–71. doi: 10.1038/oby.2011.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tryggestad JB, Thompson DM, Copeland KC, Short KR. Sex differences in vascular compliance in normal-weight but not obese boys and girls: the effect of body composition. Int J Pediatr. 2012;2012:607895. doi: 10.1155/2012/607895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tryggestad JB, Thompson DM, Copeland KC, Short KR. Arterial compliance is increased in children with type 2 diabetes compared with normal weight peers but not obese peers. Pediatr Diabetes. 2013;14:259–66. doi: 10.1111/pedi.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.U.S._Preventive_Services_Task_Force Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. Pediatrics. 2010;125:361–7. doi: 10.1542/peds.2009-2037. [DOI] [PubMed] [Google Scholar]
- 33.Urbina EM, Kimball TR, McCoy CE, Khoury PR, Daniels SR, Dolan LM. Youth with obesity and obesity-related type 2 diabetes mellitus demonstrate abnormalities in carotid structure and function. Circulation. 2009;119:2913–9. doi: 10.1161/CIRCULATIONAHA.108.830380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urbina EM, Williams RV, Alpert BS, et al. Noninvasive assessment of subclinical athersclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension. 2009;54:919–50. doi: 10.1161/HYPERTENSIONAHA.109.192639. [DOI] [PubMed] [Google Scholar]
- 35.Watts K, Beye P, Siafarikas A, et al. Exercise training normalizes vascular dysfunction and improves central adiposity in obese adolescents. J. Am. Coll. Cardiol. 2004;43:1823–7. doi: 10.1016/j.jacc.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 36.Westerbacka J, Vehkavaara S, Bergholm R, Wilkinson I, Cockcroft J, Yki-Jarvinen H. Marked resistance of the ability of insulin to decrease arterial stiffness characterizes human obesity. Diabetes. 1999;48:821–7. doi: 10.2337/diabetes.48.4.821. [DOI] [PubMed] [Google Scholar]
- 37.Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR. Nitric oxide regulates local arterial distensibility in vivo. Circulation. 2002;105:213–7. doi: 10.1161/hc0202.101970. [DOI] [PubMed] [Google Scholar]
- 38.Zalesin KC, Franklin BA, Miller WM, Peterson ED, McCullough PA. Impact of obesity on cardiovascular disease. Endocrinol. Metab. Clin. North Am. 2008;37:663–84. doi: 10.1016/j.ecl.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Zebekakis PE, Nawrot T, Thijs L, et al. Obesity is associated with increased arterial stiffness from adolescence until old age. J. Hypertens. 2005;23:1839–46. doi: 10.1097/01.hjh.0000179511.93889.e9. [DOI] [PubMed] [Google Scholar]