Abstract
Simvastatin is among the most commonly used prescription medications for cholesterol reduction. A single coding single-nucleotide polymorphism, rs4149056T>C, in SLCO1B1 increases systemic exposure to simvastatin and the risk of muscle toxicity. We summarize evidence from the literature supporting this association and provide therapeutic recommendations for simvastatin based on SLCO1B1 genotype. This article is an update to the 2012 Clinical Pharmacogenetics Implementation Consortium guideline for SLCO1B1 and simvastatin-induced myopathy.
This update to the 2012 guideline1 provides information that enables the interpretation of SLCO1B1 genotype tests so that the results can be used to guide dosing of simvastatin. Detailed guidelines for the use of simvastatin are beyond the scope of this article. Although polymorphisms in SLCO1B1 affect multiple statins, the strength of the evidence is highest for simvastatin; therefore, we focus our recommendations accordingly.
Focused Literature Review and Update
A systematic literature review was conducted, focusing on SLCO1B1 gene polymorphisms and statin-related end points in humans (details in the Supplementary Material online).
All Clinical Pharmacogenetics Implementation Consortium guidelines are updated periodically. The dosing recommendations provided in the 2012 Clinical Pharmacogenetics Implementation Consortium guideline for SLCO1B1 and simvastatin-induced myopathy have not changed and are included here. However, this updated guideline also provides a brief review regarding SLCO1B1 genotype and risk of myopathy for other statins. Furthermore, the accompanying supplementary material has been updated, including the addition of resources to facilitate incorporation of SLCO1B1 pharmacogenetics into an electronic health record with clinical decision support.
Drug: Simvastatin
Background
In 2012, simvastatin was the most commonly prescribed generic statin formulation in the United States (http://www.pharmacytimes.com/publications/issue/2013/July2013/Top-200-Drugs-of-2012).
The most common statin-related adverse drug reaction (ADR) is skeletal muscle toxicity.2 Statin-related muscle problems include myalgias (pain without evidence of muscle degradation), myopathy (pain with evidence of muscle degradation), and rhabdomyolysis (severe muscle damage with acute kidney injury). Frequency varies by definition; but, overall, statin-related myalgias are common, occurring in 1–5% of exposed subjects. In patients without arthritis, National Health and Nutrition Examination Survey data suggest a “number needed to harm” is 17 (ref. 3).
Statins have a wide therapeutic index,4 and severe ADRs are therefore relatively uncommon. Nonetheless, even though the myopathy rate is low, the high prevalence of the clinical indication (hypercholesterolemia and cardiovascular disease) creates a situation in which the absolute number of ADRs is substantial. Furthermore, in the absence of severe myopathy, many patients still opt to discontinue statin therapy due to intermediate toxicity, and statin nonadherence thus has the potential to increase the burden of cardiovascular disease on our healthcare infrastructure. It is with this perspective, a goal toward optimizing adherence, that we present the current guideline.
Gene: SLCO1B1
Background
The SLCO1B1 gene locus occupies 109 kb on chromosome 12 (Chr 12p12.2). Although many single-nucleotide polymorphisms (SNPs) have been identified in SLCO1B1, only a few are known to have functional effects.5,6 The common c.521T>C variant, rs4149056, produces a p.V174A substitution and is contained within SLCO1B1*5, *15, and *17 haplotypes. The minor C allele at this locus has been associated with decreased transport function in vitro7,8 and decreased clearance for a number of drugs in vivo.9,10,11 Additional SLCO1B1 variants have been identified and are discussed in further detail in the supplementary material (Supplementary Material online; Supplementary Tables S1 and S2 online); however, the dosing recommendations in this guideline are specific for variant alleles in which there are clear data linking SLCO1B1 genotype to simvastatin-induced myopathy (SLCO1B1*5, *15, and *17).
SLCO1B1 (alternative names include OATP1B1, OATP-C) is the protein product of the SLCO1B1 gene and facilitates the hepatic uptake of statins, as well as numerous endogenous compounds (e.g., bilirubin and 17-β-glucuronosyl estradiol).6,12 Changes in the function of this transporter (as occur during drug–drug interactions) can markedly increase the severity of statin-related muscle damage. For example, cyclosporine, a strong inhibitor of CYP3A4 and SLCO1B1, increases the area under the curve (AUC) for simvastatin acid by three- to eightfold.6
Beyond acquired inhibition, genetic variability in SLCO1B1 also affects plasma concentration of statins. The overall pharmacokinetic profiles appear to be affected more for simvastatin than for any other drug in the class.13,14,15 Other transporters potentially influencing the distribution and tissue uptake of statins include SLCO1B3, SLCO2B1, SLCO1A2, and sodium-dependent taurocholate cotransporting polypeptide.6,12
Genetic test interpretation
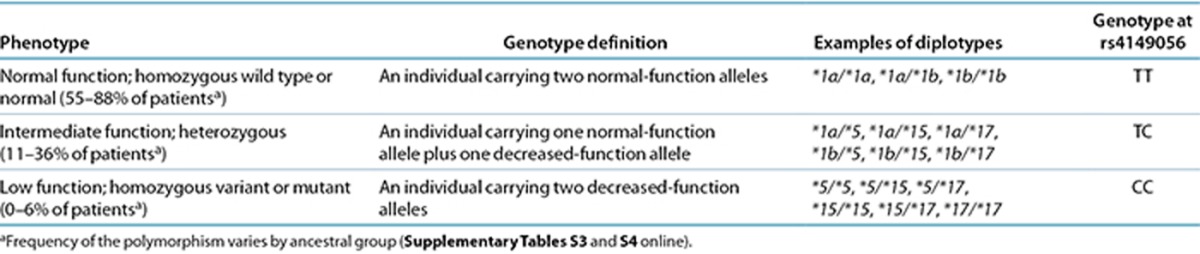
The assignment of the likely SLCO1B1 phenotype, based on * allele diplotypes, has been summarized in Table 1, based on rs4149056. SLCO1B1 alleles are often named using * allele nomenclature, representing various SNPs alone or in combination (http://www.pharmgkb.org/gene/PA134865839#tabview=tab4&subtab=33) (Supplementary Table S1 online) that are associated with low SLCO1B1 protein expression or function (Supplementary Table S2 online). The minor C allele at rs4149056 is contained within SLCO1B1*5 (rs4149056 alone) as well as the *15 and *17 haplotypes and is associated with lower plasma clearance of simvastatin. The magnitude of this effect is similar for the *5, *15, and *17 haplotypes.11 SLCO1B1 alleles have been extensively studied in multiple geographically, racially, and ethnically diverse groups. In general, rs4149056 is present at a minor allele frequency of between 5 and 20% in most populations (see Supplementary Tables S3 and S4 online). Although all SLCO1B1 genetic tests interrogate rs4149056, other variants in this gene are also likely to be important, but their interpretation and application to statin prescribing is less clear. One of the limitations inherent in any genotype-only test is that very rare or de novo variants, which might have functional importance, will not generally be included.
Table 1. Assignment of likely SLCO1B1 phenotype based on genotype.
Available genetic test options
Commercially available clinical testing options are presented within the Supplementary Material online and are freely available at http://www.PharmGKB.org. The rs4149056 SNP can be genotyped alone (e.g., PCR-based single-SNP assay) or multiplexed on a variety of array-based platforms. See the Supplementary Material online for details. This variant is also among the pharmacogenetic content available for participants in direct-to-consumer genomic offerings (e.g., 23andme.com).
Incidental findings
Genetic variability in SLCO1B1 influences the hepatic uptake of other drugs (e.g., methotrexate)5,16 as well as important endogenous compounds (e.g., bilirubin)17 (Supplementary Material online).
Linking genetic variability to variability in drug-related phenotypes
There is substantial evidence linking SLCO1B1 genotype with phenotypic variability (see Supplementary Table S5 online). Application of a grading system to evidence linking genotypic variability indicates a high quality of evidence in the majority of cases. For simvastatin, the evidence linking myopathy to rs4149056 in SLCO1B1 is of high quality, and this association has been reproduced in randomized trials and clinical practice–based cohorts. Conversely, the association of rs4149056 with myopathy has been less compelling for other statins. We therefore focus this guideline on simvastatin.
SLCO1B1 polymorphisms clearly impact the pharmacokinetics of simvastatin and, to a lesser degree, the pharmacokinetics of other statins13 (Supplementary Table S6 online). Pasanen et al.11 determined that homozygous carriers of the C allele at rs4149056 (CC genotype) had much greater exposure to the active simvastatin acid (AUC0–12) than subjects homozygous for the ancestral T allele. In single-dose studies (Supplementary Figure S1 online), the observed plasma AUCs of active simvastatin acid, pitavastatin, atorvastatin, pravastatin, and rosuvastatin have been 221%, 162–191%, 144%, 57–130%, and 62–117% higher in rs4149056 CC homozygotes than in rs4149056 TT homozygotes (Supplementary Figure S1 online).
In 2008, the SEARCH Collaborative (Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine) Group conducted a case–control study of simvastatin-induced myopathy using archived DNA from a randomized trial including more than 12,000 subjects who had received either low-dose simvastatin (20 mg daily) or high-dose simvastatin (80 mg daily) post–myocardial infarction.18 During the course of the trial, 49 subjects in the high-dose arm developed myopathy (creatine kinase (CK) > 10-fold the upper limit of normal with pain), compared with two subjects in the low-dose arm. An additional 49 subjects in the same treatment arm developed “incipient” myopathy (CK > 3-fold the upper limit of normal, and five times the patient's baseline level). Of these combined incipient and definite myopathy cases, 85 underwent whole-genome scanning (with a platform containing 317,000 SNPs), and the results were compared with genome-wide data from 90 nonmyopathic controls (frequency matched from within the same treatment arm). A single-nucleotide variant survived statistical correction for multiple testing: a base substitution in the SLCO1B1 gene. After genomic resequencing, the putative causative allele, rs4149056, was retested for association in a subset of definite myopathy cases from the original cohort, revealing an odds ratio (OR) for myopathy of 4.5 per copy of the minor C allele.18 Approximately 25% of the general population are carriers of this allele, and 1 of 100 patients taking high-dose simvastatin have an ADR.
The association between rs4149056 and statin-induced muscle toxicity has since been replicated in both a second independent trial and a practice-based longitudinal cohort. The Heart Protection Study enrolled more than 20,000 subjects with known vascular disease or vascular risk factors, and randomized each study participant to either 40 mg simvastatin daily or placebo.19,20 Twenty-four cases of myopathy (10 definite + 14 incipient) were identified from among the 10,269 participants receiving 40 mg simvastatin, and 21 of these cases later underwent retrospective genotyping for rs4149056.18 In this validation cohort, the relative risk was 2.6 per copy of the minor C allele.18 Although both the SEARCH trial and the Heart Protection Study represent randomized controlled treatment trials, the effect size for rs4149056 was lower in the Heart Protection Study (i.e., at the 40-mg dose) than in SEARCH (i.e., at the 80-mg dose), underscoring the importance of dose.
Practice-based data suggest that the association between rs4149056 and muscle toxicity is stronger for simvastatin than for other drugs within the class. The C allele at rs4149056 has recently been shown to influence the rate of medication adherence for simvastatin.21 In the STRENGTH study, 509 hypercholesterolemic patients were randomized to simvastatin, atorvastatin, or pravastatin, and followed for 16 weeks. The primary end point of the study was a composite of study drug discontinuation for any adverse effect, myalgia or muscle cramping, and/or elevated serum CK levels > 3-fold the upper limit of normal. The overall effect size was highest for simvastatin (OR: 2.8; 95% confidence interval (CI): 1.3–6.0) and rather modest for atorvastatin (OR: 1.6; 95% CI: 0.7–3.7). No significant association was observed for pravastatin (OR: 1.0; 95% CI: 0.4–2.6). As with the STRENGTH study, other groups have reported a modest association between rs4149056 and atorvastatin intolerance based on adverse muscle symptoms,22 but the association between rs4149056 and laboratory-confirmed myopathy has been less compelling for atorvastatin.23
From the medical records of nearly 9,000 patients followed in an academic lipid clinic, Brunham and colleagues identified 25 laboratory-confirmed myopathy cases (frequency 0.26%).23 All 25 cases, along with drug-exposed controls (frequency matched 2:1), were genotyped for rs4149056, revealing an OR for myopathy of 2.3 per copy of the minor C allele at rs4149056. The odds ratio was the highest, 3.2 (95% CI: 0.83–11.96), for subjects with the CC genotype exposed to simvastatin. No such relationship was observed using a similar number of atorvastatin cases (OR: 1.06; 95% CI: 0.22–4.80).23 These observations are consistent with reported differences in clearance (Supplementary Figure S1 online). To date, there is little evidence that rs4149056 genotype alters symptomatic intolerance or myopathy for pravastatin21 or rosuvastatin.22 Hence, the observed clinical association between rs4149056 and myopathy may not be a class effect; it likely depends on the relative importance of SLCO1B1 to the clearance of the statin.
Therapeutic recommendations
In 2011 (updated in 2013), the US Food and Drug Administration added warnings to the simvastatin product label to direct providers away from initiating at the 80-mg simvastatin dose. The agency's recommendations are further summarized in Supplementary Table S7 online. The American College of Cardiology and the American Heart Association recently issued updated clinical practice recommendations for the treatment of elevated blood cholesterol to reduce atherosclerotic cardiovascular disease, including guidelines for which patients should receive which statin at which intensity.24 Based on these new guidelines, it is anticipated that increasing numbers of patients will receive statin therapy; however, it is unclear as to what extent the incidence of statin-induced myopathy will also be affected. At lower simvastatin doses (e.g., 40 mg daily), it is our position that SLCO1B1 genotype (if available) could be used to warn providers about modest increases in myopathy risk for patients with a C allele at rs4149056. In these circumstances, we recommend a lower dose of simvastatin or use an alternative statin (e.g., pravastatin or rosuvastatin), and we also highlight the potential utility of routine CK surveillance (Table 2). If patients with a C allele at rs4149056 do not achieve optimal LDL cholesterol-lowering efficacy with a lower dose (e.g., 20 mg) of simvastatin, we recommend that the prescribing physician consider an alternate statin based on (i) potency differences (i.e., use a lower dose of a higher potency statin such as atorvastatin, rosuvastatin, or pitavastatin); (ii) drug–drug interactions (e.g., boceprevir, clarithromycin, cyclosporine, strong CYP3A4 inhibitors); and (iii) relevant comorbidities (e.g., trauma, significant renal impairment, post–solid organ transplant, thyroid disease).
Table 2. Dosing recommendations for simvastatin based on SLCO1B1 phenotype.

At the time of this writing, there are no data available regarding SLCO1B1 genotype effects on simvastatin response or myopathy in pediatric patients, and no data to show that the rs4149056 SNP in SLCO1B1 affects simvastatin's disposition differently in children compared with adults.
Use of clinical decision support tools within electronic health records can assist clinicians in using genetic information to optimize drug therapy.25,26,27 Clinical implementation resources include workflow diagrams (Supplementary Figures S2 and S3 online), tables that translate genotype test results into an interpreted phenotype, example text for documentation in the electronic health record and point-of-care alerts, and cross-references for drug and gene names to widely used terminologies and standardized nomenclature systems (Supplementary Tables S8–S12 online).
Recommendations for incidental findings
Not applicable.
Other considerations
Other factors influencing simvastatin-induced myopathy. Other factors known to influence a patient's risk for developing statin-induced muscle toxicity include increased statin dose, advanced age, small body mass index, female gender, metabolic comorbidities (e.g., hypothyroidism), intense physical exercise, and Asian or African ancestry.2,28,29,30,31 Since polypharmacy is common in the elderly, the association with age is partly attributable to drug–drug interactions as well as to increases in chronic renal or hepatic disease.32
Statin dose is the strongest independent predictor of myopathy risk. Myotoxicity is roughly sixfold higher in patients on high-dose than on lower-dose statin therapy.33 Among the statins, a growing body of evidence suggests that the influence of dose may be the greatest for simvastatin.18 The molecular mechanism of statin-induced myopathy is unclear.
Role of SLCO1B1 genotype and other statin-induced myopathies. The relationship between rs4149056 and simvastatin-related muscle toxicity has been clearly established, whereas the relationship between this polymorphism and the safety of other statins is less clear. Previous studies evaluating atorvastatin safety in individuals with the rs4149056 polymorphism yielded conflicting results. A small study by Puccetti and colleagues evaluating 46 individuals with muscle intolerance compared with matched controls demonstrated a statistically significant association between rs4149056 and atorvastatin-related muscle symptoms (OR: 2.7; 95% CI: 1.3–4.9; P < 0.001) (ref. 22). The STRENGTH trial also demonstrated a nonsignificant increase in muscular adverse events occurring in SLCO1B1*5 carriers taking atorvastatin.21 In addition, two small studies and a meta-analysis failed to detect a statistically significant association between the rs4149056 variant and myopathy in individuals taking 10–80 mg of atorvastatin.23,34,35 Of note, these studies were either underpowered or it was unclear whether power was met; so, the possibility of an actual association being present but undetected represents an important limitation of these three studies. Therefore, although there may be an increased risk in rs4149056 C-allele carriers using atorvastatin, the current data are inconclusive.
We were unable to identify any safety studies evaluating fluvastatin or pitavastatin use in individuals with the rs4149056 C allele, and the STRENGTH trial is the only safety study evaluating pravastatin use in this population. In the STRENGTH trial, there was no significant increase in muscular adverse events in SLCO1B1*5 carriers taking pravastatin, although a relatively low maximum pravastatin dose was used in the study compared with the maximum simvastatin and atorvastatin doses evaluated.21 With regard to rosuvastatin, in a subgroup analysis of 8,782 JUPITER trial participants, 417 subjects being treated with 20 mg daily experienced myalgia vs. 369 subjects on placebo (hazard ratio (HR): 1.13 (0.98–1.30), P = 0.09), but there was no statistically significant association between the incidence of myalgia for rosuvastatin vs. placebo in the presence of the rs4149056 C allele in this adequately powered analysis (HR: 0.95; 95% CI: 0.79–1.15).36 Based on the results of this analysis, rosuvastatin use for an approximate dose of 20 mg daily has not been associated with an increased risk of myalgia compared with placebo in carriers of the minor allele of rs4149056 and could represent a reasonably safe alternative to simvastatin therapy in rs4149056 C-allele carriers.
Drug–drug interactions. In the context of statin monotherapy, myopathy rates are low.37 The frequency of this ADR increases with coadministration of medications altering the pharmacokinetics of statins (e.g., coadministration with gemfibrozil). See the Supplementary Material online for more discussion.
Potential Benefits and Risks for the Patient
Based on the highly prevalent use of simvastatin, a potential benefit of preemptive SLCO1B1 testing is a significant reduction in the incidence of simvastatin-induced myopathies and rhabdomyolysis, by identifying those at significant risk and recommending a lower simvastatin dose or an alternative statin as appropriate. In addition, genotyping may promote statin adherence and lower low-density lipoprotein cholesterol levels.
A possible risk could be an error in genotyping. Since genotypes are lifelong test results, any such error could stay in the medical record for the life of the patient. An error in genotyping could result in a decrease in simvastatin dose that was not otherwise necessary and could result in inadequate lipid-lowering therapy. However, since there are many effective, generic statins now available, it is often possible to switch statins rather than use a reduced simvastatin dose in high-risk patients, which further minimizes the potential for risk from a reduced simvastatin dose, especially in those who are homozygous for rs4149056C. Another potential risk to pre-emptive genotyping is that a patient's knowledge of a genetic profile associated with a high risk of adverse events may lead the patient to associate unrelated adverse events (i.e., nonspecific myalgias/arthralgias) to his/her statin therapy. If a patient then prematurely discontinues his/her statin and/or the physician reduces the dose, this may result in higher low-density lipoprotein cholesterol and cardiovascular risk.
Caveats: Appropriate use and/or Potential Misuse of Genetic Tests
For the 40-mg simvastatin dose, the relative risk of myopathy is 2.6 per copy of the C allele at rs4149056. The risk is higher for the 80-mg simvastatin dose (myopathy OR 4.5 for the TC genotype, ~20.0 for the CC genotype). Nonetheless, simvastatin-related muscle toxicity can still occur in the absence of rs4149056. Thus, a TT genotype does not imply the absence of another potentially deleterious variant in SLCO1B1 or elsewhere. Further, because rs4149056C can also be inherited in combination with other SLCO1B1 gene variants known to have protective effects, it should not be presumed that the C allele at rs4149056 confers risk with 100% certainty.
Acknowledgments
The authors acknowledge the critical input of members of the Clinical Pharmacogenetics Implementation Consortium (CPIC) of the Pharmacogenomics Research Network, particularly Mary V. Relling (St. Jude Children's Research Hospital). This work was funded by the National Institutes of Health (NIH)/National Institute of General Medical Science (PAAR4Kids (UO1 GM92666) and PharmGKB (R24 GM61374)). The following NIH grant support is acknowledged: U01 HL0105918, U01HL069757 (R.M.K), U19HL065962 (D.M.R.), U01HG006378 (D.M.R.), U01GM074492 and U01HG007269 (R.M.C.-D.), R01 HL118049 (D.V.), and R24 GM61374 (L.G. and T.E.K.). M.W. is supported by the Swedish Research Council (Medicine 523-2008-5568 and 521-2011-2440), the Swedish Heart and Lung Foundation, and the Clinical Research Support (ALF) at Uppsala University. M.N. has grants from the Sigrid Jusélius Foundation (Helsinki, Finland), the Helsinki University Central Hospital (Helsinki, Finland), and the European Research Council (Brussels, Belgium). D.V. is also supported by the Department of Defense (FA8650-13-2-6375).
CPIC guidelines reflect expert consensus based on clinical evidence and peer-reviewed literature available at the time they are written, and are intended only to assist clinicians in decision making, as well as to identify questions for further research. New evidence may have emerged since the time a guideline was submitted for publication. Guidelines are limited in scope and are not applicable to interventions or diseases that are not specifically identified. Guidelines do not account for all individual variations among patients and cannot be considered inclusive of all proper methods of care or exclusive of other treatments. It remains the responsibility of the health-care provider to determine the best course of treatment for the patient. Adherence to any guideline is voluntary, with the ultimate determination regarding its application being solely made by the clinician and the patient. CPIC assumes no responsibility for any injury to people or damage to property related to any use of CPIC's guidelines, or for any errors or omissions.
The funding organizations played no role in the writing of these guidelines. D.V. is a consultant for RenaissanceRx and has funding through the Department of Defense supporting an ongoing clinical trial of genotype-guided statin therapy (NCT01894230). The other authors declared no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/cpt
Supplementary Material
References
- Wilke R.A., et al. Clinical Pharmacogenomics Implementation Consortium (CPIC) The Clinical Pharmacogenomics Implementation Consortium: CPIC guideline for SLCO1B1 and simvastatin-induced myopathy. Clin. Pharmacol. Ther. 2012;92:112–117. doi: 10.1038/clpt.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke R.A., et al. Identifying genetic risk factors for serious adverse drug reactions: current progress and challenges. Nat. Rev. Drug Discov. 2007;6:904–916. doi: 10.1038/nrd2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner C., Rippberger M.J., Smith J.K., Leveille S.G., Davis R.B., Mittleman M.A. Statin use and musculoskeletal pain among adults with and without arthritis. Am. J. Med. 2012;125:176–182. doi: 10.1016/j.amjmed.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke R.A., Dolan M.E. Genetics and variable drug response. JAMA. 2011;306:306–307. doi: 10.1001/jama.2011.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey L.B., et al. Rare versus common variants in pharmacogenetics: SLCO1B1 variation and methotrexate disposition. Genome Res. 2012;22:1–8. doi: 10.1101/gr.129668.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi M., Pasanen M.K., Neuvonen P.J. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol. Rev. 2011;63:157–181. doi: 10.1124/pr.110.002857. [DOI] [PubMed] [Google Scholar]
- Kameyama Y., Yamashita K., Kobayashi K., Hosokawa M., Chiba K. Functional characterization of SLCO1B1 (OATP-C) variants, SLCO1B1*5, SLCO1B1*15 and SLCO1B1*15+C1007G, by using transient expression systems of HeLa and HEK293 cells. Pharmacogenet. Genomics. 2005;15:513–522. doi: 10.1097/01.fpc.0000170913.73780.5f. [DOI] [PubMed] [Google Scholar]
- Tirona R.G., Leake B.F., Merino G., Kim R.B. Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J. Biol. Chem. 2001;276:35669–35675. doi: 10.1074/jbc.M103792200. [DOI] [PubMed] [Google Scholar]
- Niemi M., et al. High plasma pravastatin concentrations are associated with single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide-C (OATP-C, SLCO1B1) Pharmacogenetics. 2004;14:429–440. doi: 10.1097/01.fpc.0000114750.08559.32. [DOI] [PubMed] [Google Scholar]
- Pasanen M.K., Fredrikson H., Neuvonen P.J., Niemi M. Different effects of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and rosuvastatin. Clin. Pharmacol. Ther. 2007;82:726–733. doi: 10.1038/sj.clpt.6100220. [DOI] [PubMed] [Google Scholar]
- Pasanen M.K., Neuvonen M., Neuvonen P.J., Niemi M. SLCO1B1 polymorphism markedly affects the pharmacokinetics of simvastatin acid. Pharmacogenet. Genomics. 2006;16:873–879. doi: 10.1097/01.fpc.0000230416.82349.90. [DOI] [PubMed] [Google Scholar]
- Ho R.H., et al. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology. 2006;130:1793–1806. doi: 10.1053/j.gastro.2006.02.034. [DOI] [PubMed] [Google Scholar]
- Niemi M. Transporter pharmacogenetics and statin toxicity. Clin. Pharmacol. Ther. 2010;87:130–133. doi: 10.1038/clpt.2009.197. [DOI] [PubMed] [Google Scholar]
- Kalliokoski A., Niemi M. Impact of OATP transporters on pharmacokinetics. Br. J. Pharmacol. 2009;158:693–705. doi: 10.1111/j.1476-5381.2009.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGorter M.K., et al. Clinical and pharmacogenetic predictors of circulating atorvastatin and rosuvastatin concentrations in routine clinical care. Circ. Cardiovasc. Genet. 2013;6:400–408. doi: 10.1161/CIRCGENETICS.113.000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey L.B., et al. Genome-wide study of methotrexate clearance replicates SLCO1B1. Blood. 2013;121:898–904. doi: 10.1182/blood-2012-08-452839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Steeg E., et al. Complete OATP1B1 and OATP1B3 deficiency causes human Rotor syndrome by interrupting conjugated bilirubin reuptake into the liver. J. Clin. Invest. 2012;122:519–528. doi: 10.1172/JCI59526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link E., et al. SLCO1B1 variants and statin-induced myopathy--a genomewide study. N. Engl. J. Med. 2008;359:789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- Heart Protection Study Collaborative Group. Bulbulia R., et al. Effects on 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20,536 high-risk individuals: a randomised controlled trial. Lancet. 2011;378:2013–2020. doi: 10.1016/S0140-6736(11)61125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial Lancet 3607–22.2002. 12114036 [Google Scholar]
- Voora D., et al. The SLCO1B1*5 genetic variant is associated with statin-induced side effects. J. Am. Coll. Cardiol. 2009;54:1609–1616. doi: 10.1016/j.jacc.2009.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puccetti L., Ciani F., Auteri A. Genetic involvement in statins induced myopathy. Preliminary data from an observational case-control study. Atherosclerosis. 2010;211:28–29. doi: 10.1016/j.atherosclerosis.2010.02.026. [DOI] [PubMed] [Google Scholar]
- Brunham L.R., et al. Differential effect of the rs4149056 variant in SLCO1B1 on myopathy associated with simvastatin and atorvastatin. Pharmacogenomics J. 2012;12:233–237. doi: 10.1038/tpj.2010.92. [DOI] [PubMed] [Google Scholar]
- Stone N.J., et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Shuldiner A.R., et al. Pharmacogenomics Research Network Translational Pharmacogenetics Program Group The Pharmacogenomics Research Network Translational Pharmacogenetics Program: overcoming challenges of real-world implementation. Clin. Pharmacol. Ther. 2013;94:207–210. doi: 10.1038/clpt.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke R.A., et al. The emerging role of electronic medical records in pharmacogenomics. Clin. Pharmacol. Ther. 2011;89:379–386. doi: 10.1038/clpt.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullo I.J., Jarvik G.P., Manolio T.A., Williams M.S., Roden D.M. Leveraging the electronic health record to implement genomic medicine. Genet. Med. 2013;15:270–271. doi: 10.1038/gim.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lemos J.A., et al. Investigators Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- Chung J.Y., et al. Effect of OATP1B1 (SLCO1B1) variant alleles on the pharmacokinetics of pitavastatin in healthy volunteers. Clin. Pharmacol. Ther. 2005;78:342–350. doi: 10.1016/j.clpt.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Lee E., et al. Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin. Pharmacol. Ther. 2005;78:330–341. doi: 10.1016/j.clpt.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Hippisley-Cox J., Coupland C. Individualising the risks of statins in men and women in England and Wales: population-based cohort study. Heart. 2010;96:939–947. doi: 10.1136/hrt.2010.199034. [DOI] [PubMed] [Google Scholar]
- Thompson P.D., Clarkson P., Karas R.H. Statin-associated myopathy. JAMA. 2003;289:1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- McClure D.L., Valuck R.J., Glanz M., Murphy J.R., Hokanson J.E. Statin and statin-fibrate use was significantly associated with increased myositis risk in a managed care population. J. Clin. Epidemiol. 2007;60:812–818. doi: 10.1016/j.jclinepi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Santos P.C., et al. SLCO1B1 haplotypes are not associated with atorvastatin-induced myalgia in Brazilian patients with familial hypercholesterolemia. Eur. J. Clin. Pharmacol. 2012;68:273–279. doi: 10.1007/s00228-011-1125-1. [DOI] [PubMed] [Google Scholar]
- Carr D.F., et al. SLCO1B1 genetic variant associated with statin-induced myopathy: a proof-of-concept study using the clinical practice research datalink. Clin. Pharmacol. Ther. 2013;94:695–701. doi: 10.1038/clpt.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danik J.S., Chasman D.I., MacFadyen J.G., Nyberg F., Barratt B.J., Ridker P.M. Lack of association between SLCO1B1 polymorphisms and clinical myalgia following rosuvastatin therapy. Am. Heart J. 2013;165:1008–1014. doi: 10.1016/j.ahj.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Graham D.J., et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292:2585–2590. doi: 10.1001/jama.292.21.2585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


