Abstract
Background & Aims
Heat shock protein 90 (hsp90) is an emerging therapeutic target in chronic liver diseases. Hsp90 plays an important role in liver immune cell activation; however its role in alcoholic liver disease (ALD) remains elusive. Here we hypothesize that hsp90 is crucial in alcohol induced steatosis and pro-inflammatory cytokine production. To test this hypothesis, we employed a pharmacological inhibitor of hsp90, 17-DMAG [17-Dimethylamino-ethylamino-17-demethoxygeldanamycin] in an in vivo mouse model of acute and chronic alcoholic liver injury.
Methods
C57BL/6 mice were given either a single dose of ethanol via oral gavage (acute) or chronically fed alcohol for 2 weeks followed by oral gavage (chronic-binge). 17-DMAG was administered during or at the end of feeding. Liver injury parameters, inflammatory cytokines and lipid metabolism genes were analyzed.
Results
Our results reveal increased expression of hsp90 in human and mouse alcoholic livers. In vivo inhibition of hsp90, using 17-DMAG, not only prevents but also alleviates alcoholic liver injury, determined by lower serum ALT, AST and reduced hepatic triglycerides. Mechanistic analysis shows that 17-DMAG decreases alcohol mediated oxidative stress, reduces serum endotoxin, decreases inflammatory cells, and diminishes sensitization of liver macrophages to LPS, resulting in down-regulation of CD14, NFκB inhibition, and decreased pro-inflammatory cytokine production. Hsp90 inhibition decreases fatty acid synthesis genes via reduced nuclear SREBP-1 and favors fatty acid oxidation genes via PPARα.
Conclusion
Inhibition of hsp90 decreases alcohol induced steatosis and pro-inflammatory cytokines and inhibits alcoholic liver injury. Hsp90 is relevant in human alcoholic cirrhosis and promising therapeutic target in ALD.
Keywords: Ethanol, steatohepatitis, 17-DMAG, cellular stress, hsp90, HSF1, alcoholic liver disease
Introduction
Alcoholic liver disease (ALD) is an increasing health concern worldwide [1]. The clinical progression of liver injury in ALD encompasses a range of disorders including early alcoholic fatty liver, steatohepatitis, cirrhosis, and in few percent cases hepatocellular cancer [2]. While simple fatty liver induced by alcohol consumption can be reversed after withdrawal, more severe forms of liver injury can develop in 35% of heavy alcohol drinkers [1, 3]. Therapeutic strategies including alcohol abstinence, corticosteroids, biologics such as anti-TNFα, nutritional therapy and ultimately liver transplantation have been used, with limited or no success due to infectious complications [4, 5]. Thus, an urgent need to explore novel therapies for ALD is apparent. Recent attempts have reported manipulations of cytokine, IL-22 [6] and the use of IL-1 receptor antagonist [7] in mouse models of ALD.
Stress induced heat shock proteins (hsps) are ubiquitious and highly conserved proteins, induced by a wide variety of physiological and environmental insults such as toxic chemicals, heat, hydrogen peroxide and alcohol. Originally identified for their cytoprotective function, these proteins are now recognized to play an important causative role in chronic diseases such as cancer, neurodegeneration, athesclerosis and diabetes [8]. Targeting hsp90 to induce tumor cell apoptosis is currently in Phase I/II and III clinical trials for cancer therapy [9]. Hsp90 has also been reported as an attractive therapeutic target in hepatocellular carcinoma [10], is important in fibrosis [11] and facilitates hepatitis C virus replication during alcohol exposure [12, 13]. Similar to other stress signals, chronic alcohol induces hsp90 in the liver [14]. Here we explore the pathophysiological role of hsp90 in ALD.
Alcoholic liver disease is multifactorial and involves development of fatty liver or steatosis, macrophage sensitization and pro-inflammatory cytokine production and oxidative stress [15]. We showed that chronic alcohol activates transcription factor HSF1 and induces hsp90 in human monocytes and murine macrophages in vitro [14]. Furthermore, we reported that inhibition of hsp90 in vivo prevented lipopolysaccharide mediated macrophage activation in the liver [16]. However, the significance and function of hsp90 in the alcoholic liver remains unexplored. Here we hypothesize that chronic alcohol induces hsp90 in liver and contributes to hepatic injury via regulation of signaling molecules important in fatty acid metabolism and pro-inflammatory cytokine production by alcohol. To test this hypothesis, we administered 17-DMAG, a water-soluble hsp90 specific inhibitor in a mouse model of alcohol induced liver injury. In both, acute and chronic models of alcoholic liver injury, we report that 17-DMAG treatment ameliorates alcohol-mediated steatosis and prevents alcohol-induced sensitization of liver macrophages (LMs) resulting in reduction of pro-inflammatory cytokine production. Our novel in vivo findings suggest that hsp90 is a potential therapeutic target for treatment and management of ALD.
Materials and Methods
Human cirrhotic and normal healthy liver samples
The Liver Tissue Cell Distribution System (LTCDS, the Division of Pediatric Gastroenterology and Nutrition, University of Minnesota, Minneapolis, MN) provided 10 normal human liver and 10 alcoholic cirrhotic human liver from patients who received transplantation, described in Table 1 and details in the Supplementary information. Normal liver tissue was the non-involved surrounding tissue, obtained from patients undergoing partial hepatectomy for liver cancer.
Table 1.
Biochemical profile of alcoholic cirrhosis patients included in the study
| Controls (n=10) | Alcoholic cirrhosis group (n=10) |
|
|---|---|---|
| Age (years) | 53.80 ± 14.66 (28 –78) | 55.20 ± 9.55 (38 –65) |
| Gender - M/F (%male) | 5/5 (50%) | 9/1 (90%) |
| AST (UI/L) | 33.20 ± 17.03 (13 – 63) | 105.56 ± 148.08 (31 – 492) |
| Bilirubin (mg/dL) | 0.3 – 1.9 | 10.45 ± 7.49 (2.5 – 26) |
| Alkaline Phosphatase (UI/L) | 44 – 147 | 205.77 ± 109.63 (74– 386) |
Animal models of alcoholic liver injury
All animals received proper care in agreement with animal protocols approved by the Institutional Animal Use and Care Committee of the UMMS. To determine the in vivo efficacy of 17-DMAG we employed an acute and chronic-binge alcoholic liver injury model. The detailed experimental designs are described in Supplementary information.
Other Methods
The following methods are described in the supplementary information, including isolation of liver cell types, serum biochemical assays and cytokines, electrophoretic mobility shift assay (EMSA), real-time polymerase chain reaction (PCR) and western blotting analysis, cell-culture reagents and stimulations, transfections and LC-MS/MS analysis.
Statistical Analysis
Statistical significance was determined using the t-test [for cell lines] or nonparametric ANOVA followed by Kruskal-Wallis test [for animal studies]. Data are presented as mean ± standard error, and were considered statistically significant at p< 0.05.
Results
Hsp90 is elevated in human and experimental murine ALD
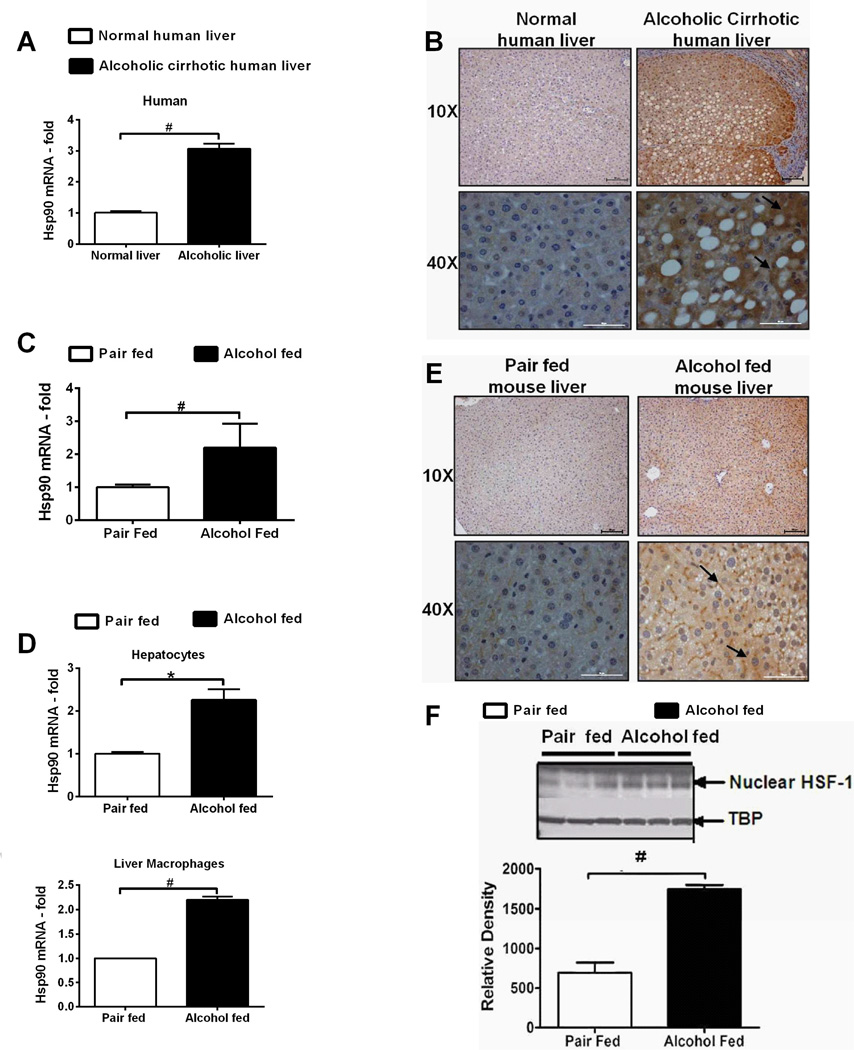
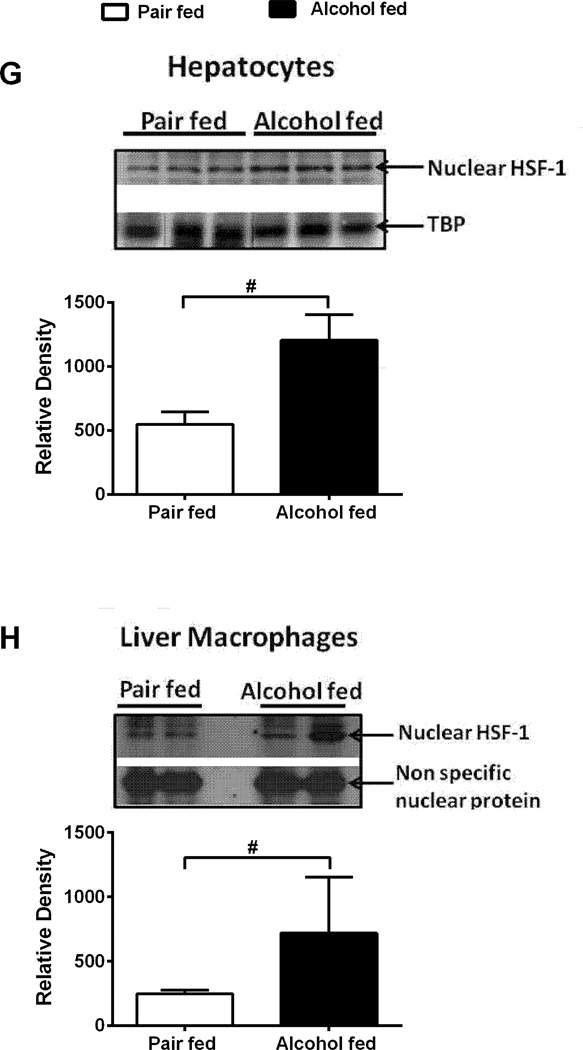
The potential role of hsp90 in pathogenesis of ALD is still unclear. To investigate the clinical significance of hsp90 in ALD, we first assessed hsp90 expression in human alcoholic liver. Hsp90 mRNA (Fig 1A) and protein (Suppl Fig 1A) was increased in livers of human alcoholic cirrhosis patients. Immunohistochemistry using an anti-hsp90α (cytoplasmic, inducible form: referred as hsp90 throughout) antibody, revealed increased diffuse staining in parenchymal nodules and no staining in fibrous septa in cirrhotic compared to normal human livers (Fig 1B). Next, hsp90 expression was examined in a clinically relevant chronic-binge alcoholic liver injury model (NIAAA-Gao model), that mimics acute-on-chronic liver injury observed in alcoholic hepatitis patients. Hsp90 mRNA (Fig 1C) was increased in alcoholic mouse whole liver as well as in isolated hepatocytes and LMs (Fig 1D). Immunohistochemistry (Fig 1E) and western blotting (Suppl Fig 1B) showed up-regulation of hsp90 in alcoholic livers. Expression of hsp90 is regulated by the transcription factor, HSF1 [17]. Increased nuclear HSF1 in whole livers (Fig 1F), hepatocytes (Fig 1G), LMs (Fig 1H) and DNA binding (Suppl Fig 1C) was observed in alcoholic mouse liver. These results provide evidence that cytoplasmic inducible hsp90 is increased in human and mouse ALD likely via HSF1.
Figure 1. Hsp90 is elevated in human and experimental ALD.
Hsp90 mRNA in human alcoholic cirrhosis (A) and in chronic alcohol fed mice liver (C) was analyzed by real time PCR. Immunohistochemistry of hsp90 in human (B) and murine (E) alcoholic liver, depicted in black arrows indicate diffuse cytoplasmic localization of hsp90. Hsp90 mRNA (D) in primary hepatocytes and LMs was analyzed by real time PCR. Nuclear extracts from alcohol fed mouse livers (F), primary hepatocytes (G) and LMs (H) were immunoblotted for HSF1. TATA-box Binding Protein (TBP) is shown as internal loading control for liver and hepatocytes. A non specific nuclear protein band in nuclear extracts was used to confirm equal loading for LMs. LMs were pooled from 3 mice per sample (n=6, per group). A representative gel picture is shown. Bars represent mean ± SE, (n=6, mice), (n=10, human). *p<0.005, #p<0.001.
Pharmacologic inhibition of Hsp90 alleviates acute and chronic alcohol induced liver injury
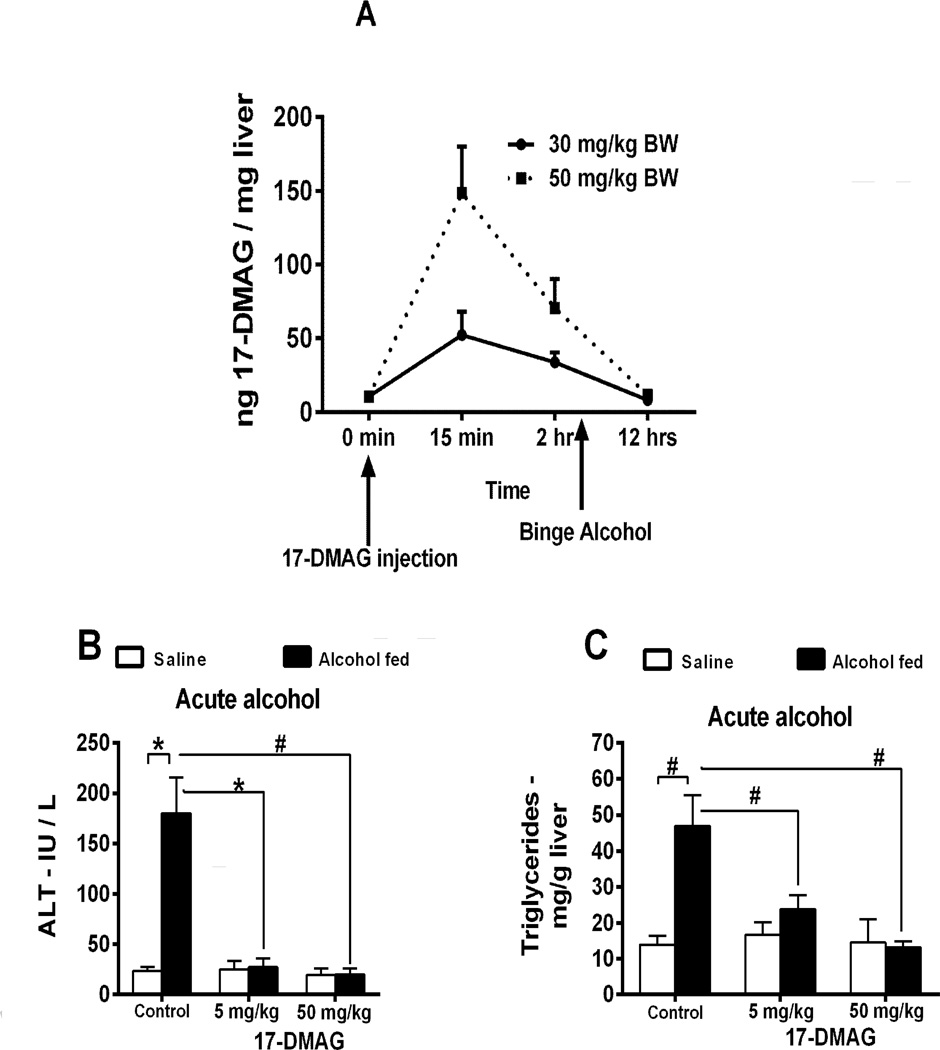
Hsp90 inhibitors are currently in clinical trials for cancer [9] and reported in liver diseases [10, 12, 13]. Here, we test the efficacy of 17-DMAG in ALD. First, we performed LC-MS/MS analysis of liver tissue lysates to confirm bioavailability of 17-DMAG. Our results show nanogram quantities of 17-DMAG in the liver within 15 minutes of i.p. injection, which is metabolized and cleared after 12 hours (Fig 2A). Administration of hsp90 inhibitors induces nuclear translocation of HSF1, and promotes transcription of heat shock protein 70 (hsp70) [17]. Nuclear HSF1 (Suppl Fig 2A) and hsp70 mRNA (Suppl Fig 2B) was observed in 17-DMAG treated alcoholic liver. Further, alcohol exposed macrophages (Suppl Fig 2C) and hepatocytes (Suppl Fig 2D) exhibited increased hsp70 promoter driven reporter activity after treatment with 17-DMAG confirming hsp90 inhibition. These results show that 17-DMAG effectively inhibits hsp90 in alcoholic liver macrophages and hepatocytes, confirming its bioavailability.
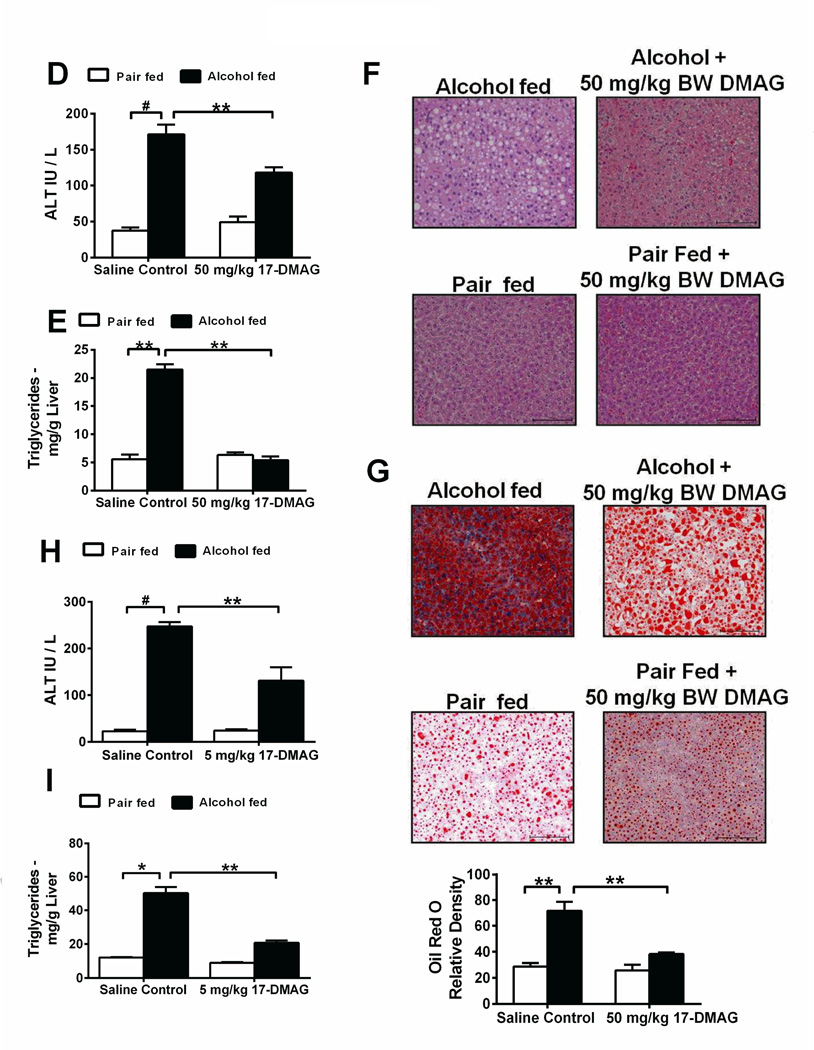
Figure 2. Hsp90 inhibition using 17-DMAG alleviates acute and chronic alcohol induced liver injury.
Bioavailability of 17-DMAG (A) was assayed by LC-MS/MS in liver. Serum ALT (B) and liver triglycerides (C) analyzed in acute alcohol and 17-DMAG administered mice. Serum ALT (D), liver triglycerides (E), histology (F) and Oil Red O staining (G) were investigated in mice fed chronic-binge alcohol and treated with 17-DMAG at the end of the feeding. Bar graph (G) shows relative density of lipids measured using ImageJ software. 17-DMAG administered every alternate day during alcohol feeding and serum ALT (H) and liver triglycerides (I) analyzed. Bars represent mean ± SE, (n=6). **p<0.05, *p<0.0005, #p<0.00005.
Next, we tested 17-DMAG in a model of acute alcoholic liver injury. Low dose (5 mg/kg BW) of 17-DMAG administered either 30 minutes before every alcohol gavage or high dose (50 mg/kg BW) injected once 30 mins after final gavage significantly reduced serum ALT (Fig 2B) and liver triglycerides (Fig 2C) compared to saline control mice. To determine the effect of hsp90 inhibition in chronic liver injury, we used NIAAA chronic-binge model of ALD, and treated mice with a single dose of 17-DMAG, at 30 and 50 mg/kg BW at the end of the 2 week chronic-binge alcohol feeding. Significant reduction in serum ALT (Fig 2D; Suppl Fig 2E) and AST (Suppl Fig 2F) without an effect on liver/body weight ratio (Suppl Fig 2G) was observed. 17-DMAG treatment decreased steatosis as indicated by reduced triglycerides (Fig 2E; Suppl Fig 2H) and confirmed by histology (Fig 2F) and Oil-Red O staining (Fig 2G). Finally, to determine the effect of hsp90 inhibition on prevention of ALD, C57BL/6 mice received i.p. injections of 17-DMAG at 2.5 and 5 mg/kg BW, every other day during alcohol feeding. Serum ALT (Fig 2H) and AST (Suppl Fig 2I) levels were significantly reduced at both concentrations, without alteration in liver/body weight ratio (Suppl Fig 2J). Liver triglycerides were significantly lower after 17-DMAG treatment in a dose dependent manner (Fig 2I), consistent with histology (Suppl Fig 2K) and Oilred O stained livers (Suppl Fig 2L), suggesting that inhibition of hsp90 can prevent the onset of liver steatosis and injury. These results provide compelling evidence that treatment with an hsp90 inhibitor prevents acute and chronic alcoholic liver injury.
17-DMAG treatment inhibits oxidative stress and decreases pro-inflammatory cytokine production
Induction of oxidative stress and sensitization to endotoxin resulting in pro-inflammatory cytokine production are important features of ALD [18]. 17-DMAG treatment significantly decreased alcohol mediated oxidative stress measured by TBARS and 4-HNE, and prevented alcohol mediated decrease in hepatic glutathione (GSH) content (Table 2). Interestingly, chronic alcohol induced metabolizing enzymes CYP2E1 in liver microsomal fractions (Suppl Fig 3A) and ADH1 expression was not altered by 17-DMAG (Suppl Fig 3B).
Table 2.
17-DMAG reduces alcohol mediated oxidative stress in the liver
| Whole Liver | Pair fed | Alcohol fed | Alcohol + 17-DMAG – 50 mg/kg BW |
|---|---|---|---|
| TBARS | 3.53 ± 0.34 | 5.43 ± 1.07a | 4.04 ± 0.74c |
| 4-HNE | 0.43 ± 0.15 | 1.39 ± 0.69a | 0.66 ± 0.15c |
| GSH | 100.00 ± 10.00 | 65.37 ± 11.11a | 99.79 ± 8.13c |
TBARS, 4-HNE and GSH were assessed in liver whole cell lysate after 17-DMAG treatment as described in Supplemental Methods.
p<0.05, pair fed vs alcohol fed
p<0.05, alcohol fed vs alcohol + 50 mg/ kg BW 17-DMAG,
Nonparametric ANOVA followed by Kruskal-Wallis test. Values shown are mean ± SE, n=6.
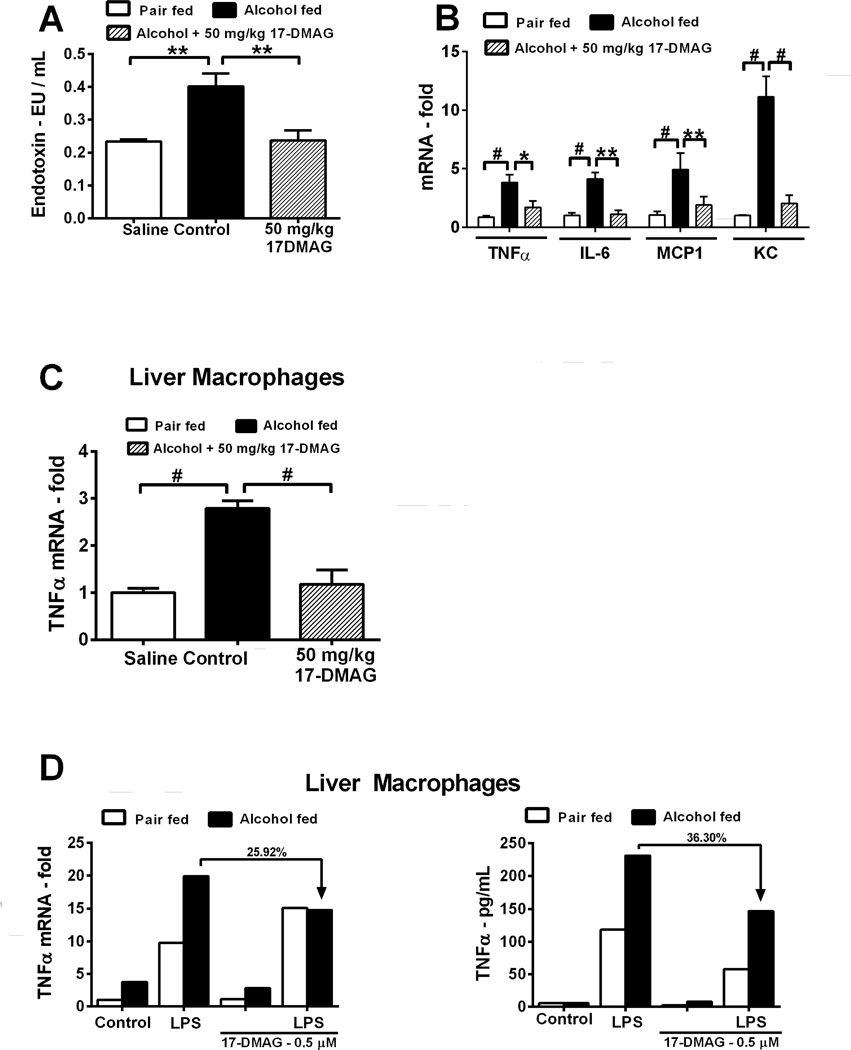
Gut derived circulating endotoxin and liver macrophage cytokine production occurs via CD14 and TLR4 at least in part due to dysregulation of oxidative stress [19]. Inhibition of hsp90 by 17-DMAG lowered serum endotoxin in alcohol fed mice (Fig 3A). Induction of pro-inflammatory cytokines TNFα, MCP1, IL-6 and KC (IL-8) in alcoholic liver was significantly inhibited after 17- DMAG treatment (mRNA: Fig 3B and tissue cytokines: supplementary figure 3C–E).
Figure 3. 17-DMAG treatment inhibits oxidative stress and decreases pro-inflammatory cytokine production.
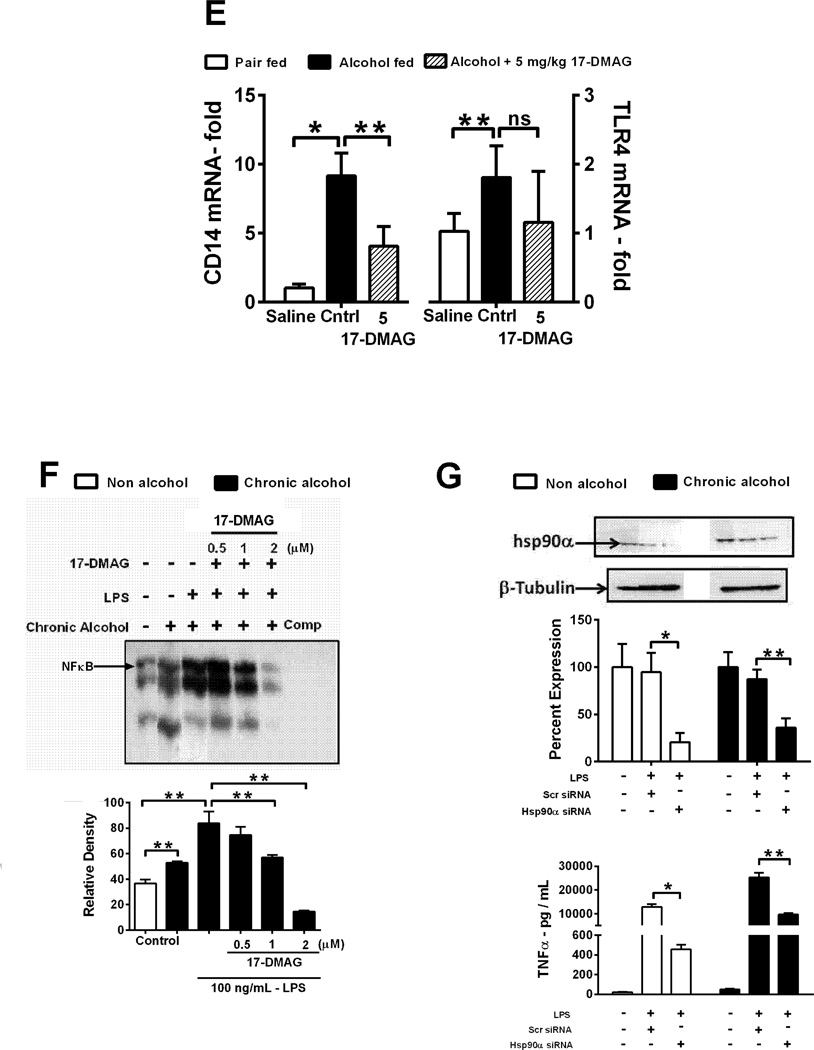
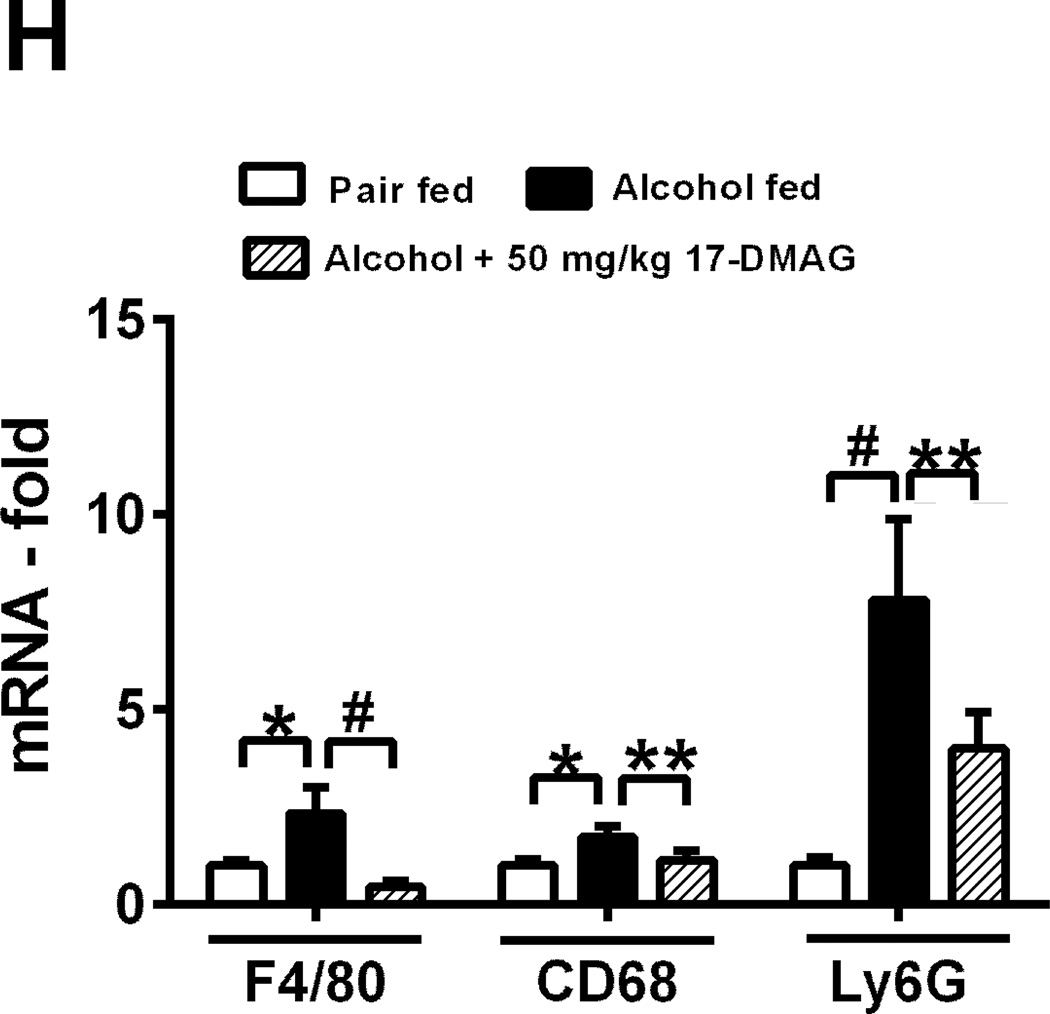
Serum endotoxin (A), liver cytokine mRNA (B) and LM TNFα mRNA (C) analyzed by real time PCR. TNFα mRNA (D:left panel) and protein (D:right panel) was analyzed. LMs were pooled from 8 alcoholic mice stimulated ex-vivo with LPS ± 17-DMAG and percent down-regulation is depicted on each graph. CD14 and TLR4 mRNA (E) analyzed in 17-DMAG treated alcoholic liver. NFκB DNA binding activity (F) analyzed in LPS ± 17-DMAG treated alcoholic RAW macrophages [unlabeled competitor oligonucleotide (Comp)]. RAW macrophages transiently transfected with hsp90α siRNA or scrambled siRNA for 48hrs and knockdown confirmed in cellular lysates (G: upper panel) and supernatants analyzed for TNFα protein (G: lower panel). Hsp90 protein expression normalized to untransfected sample in respective group. Fold change in inflammatory subset cell markers in liver is shown (H). Bars represent mean ± SE, (n=6 mice; n=3 in vitro). **p<0.05, *p<0.0005, #p<0.00005, ns - not significant.
Furthermore, liver macrophages isolated after in vivo treatment of 17-DMAG exhibit downregulation of TNFα mRNA expression (Fig 3C), confirming in vivo suppressive effects of 17-DMAG on pro-inflammatory cytokine production. To analyze whether alcohol-induced sensitization of macrophages to endotoxin/LPS is hsp90 dependent, LMs isolated from chronic alcohol fed mice were treated with LPS (100ng/ml) and/or 17-DMAG (0.5 µM) in vitro. 17-DMAG reduced alcohol mediated increase of LPS-induced TNFα mRNA (Fig 3D) and protein in vitro (Fig 3D). Hsp90 chaperones CD14, which plays a crucial role in LPS sensing [20]. To determine mechanisms associated with inhibition of alcohol mediated macrophage activation by 17-DMAG, we assessed CD14, TLR4 and NFκB. Increased liver CD14 mRNA in alcoholic livers was significantly down-regulated after 17-DMAG treatment (Fig 3E). On the contrary, 17-DMAG did not affect the increase in TLR4 mRNA in alcoholic livers (Fig 3E). Further, 17-DMAG treatment significantly blocked NFκB DNA binding activity in a dose dependent manner in chronic alcohol exposed murine macrophages (Fig 3F). To evaluate specificity of hsp90 inhibition, we transiently transfected hsp90 siRNA in chronic alcohol exposed macrophages followed by LPS treatment. The knockdown of hsp90 by specific siRNA was confirmed at 48 hrs post transfection (Fig 3G). Hsp90 siRNA prevented alcohol mediated increase in LPS-induced TNFα in chronic alcohol exposed macrophages confirming specificity and significance of hsp90 in alcohol mediated macrophage activation (Fig 3G). Chronic alcohol induces inflammatory foci via infiltration and activation of monocyte/macrophages and neutrophils [21]. Figure 3H shows that 17-DMAG inhibits up-regulation of alcohol mediated monocyte/macrophage marker F4/80 and activation marker, CD68 as well as neutrophil marker Ly6G. Thus, hsp90 regulates monocytes/macrophages and neutrophlis in alcoholic liver. Collectively, 17-DMAG treatment decreases macrophage activation and pro-inflammatory cytokine production via CD14 and NFκB.
17-DMAG treatment ameliorates alcohol-induced steatosis by regulation of fatty acid metabolism genes
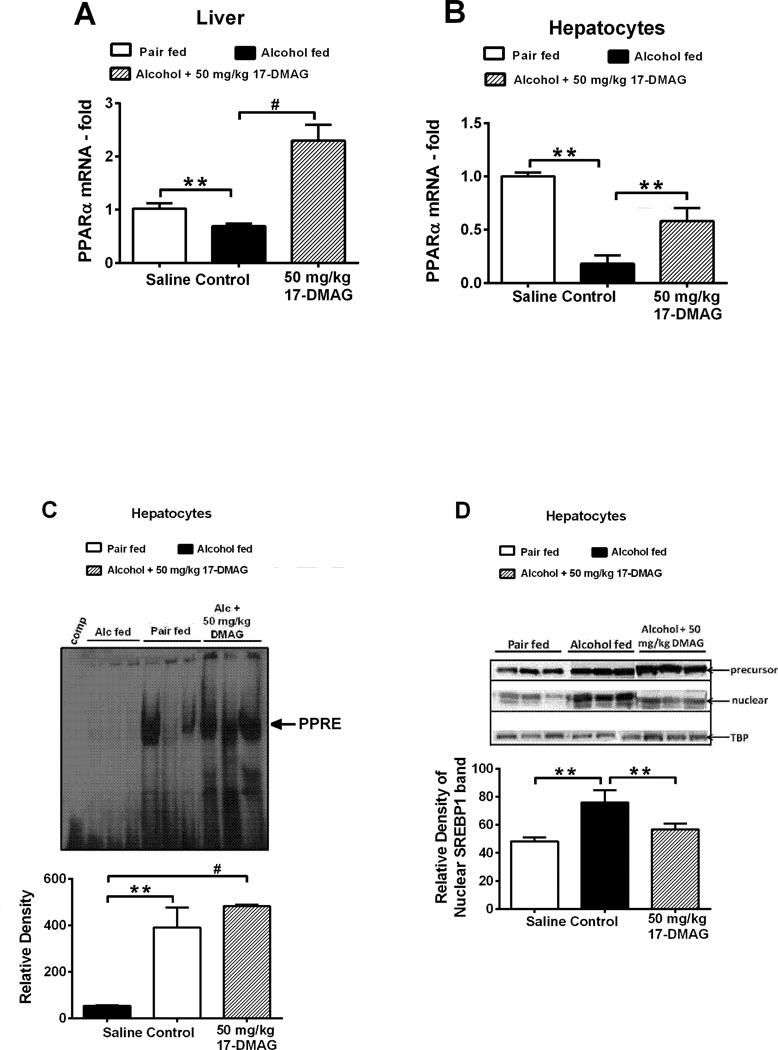
Next, to investigate whether hsp90 plays an important role in alcohol induced hepatic steatosis (Fig 2F–2G), we analyzed genes related to hepatic lipid metabolism. Hsp90 acts as a repressor of PPARα, important transcription factor in fatty acid oxidation [22]. Further, hsp90 is linked to SREBP-1 via mTORC1/Lipin-1 and thus can regulate fatty acid synthesis genes [23]. Figure 4A shows that 17-DMAG treatment prevented down-regulation of PPARα mRNA in alcoholic livers. Similar to whole livers, decrease in PPARα mRNA (Fig 4B) and DNA binding activity (Fig 4C) was detected in isolated hepatocytes after 17-DMAG treatment. In support of these results, chronic alcohol mediated reduction in PPARα target genes such as CPT1a, ACOX, LCAD and MCAD was prevented in hepatocytes (Table 3) and whole livers (Suppl Table 1) after treatment with 17-DMAG. Next, western blot analysis of nuclear SREBP-1 revealed that 17-DMAG reduced low molecular weight mature form of SREBP-1 without significant changes in the high molecular weight precursor (Fig 4D). These results were complimented by down-regulation of chronic alcohol induced lipogenic target genes SREBP1, SCD-1, ACC-1 and FAS upon 17- DMAG treatment (Table 3). These data collectively provide novel evidence for regulation of hepatic lipid metabolism by hsp90 in alcoholic liver.
Figure 4. Hsp90 inhibition alters lipid metabolism genes.
PPARα mRNA in whole liver (A) and isolated hepatocytes (B) after 17-DMAG treatment in chronic-binge alcohol mice. DNA binding activity of PPARα (C) in nuclear extracts of primary hepatocytes detected by EMSA. A representative gel picture is shown with mean relative density ± SE. A 20-fold excess of unlabeled oligonucleotide was included as competitor (Comp). Precursor and mature SREBP1 was detected in nuclear extracts of primary hepatocytes (D). Bars represent mean ± SE, (n=6). **p<0.05, *p<0.0005, #p<0.00005, ns - not significant
Table 3.
Fold changes in the expression level of genes involved in fatty acid oxidation and lipogenesis
| Primary Hepatocytes |
Pair fed | Alcohol fed | Alcohol + 17-DMAG – 50 mg/kg BW |
|
|---|---|---|---|---|
| Oxidation | CPT1a | 1.00 ± 0.11 | 0.75 ± 0.26 | 4.12 ± 1.74c |
| ACOX1 | 1.00 ± 0.04 | 0.32 ± 0.06a | 1.25 ± 0.43c | |
| LCAD | 1.01 ± 0.15 | 0.59 ± 0.34a | 2.18 ± 0.79c | |
| MCAD | 1.00 ± 0.09 | 0.35 ± 0.14a | 1.40 ± 0.58c | |
| Lipogenesis | SREBPF1 | 1.01 ± 0.13 | 2.45 ± 0.78a | 0.99 ± 0.35c |
| ACC1 | 1.01 ± 0.17 | 2.04 ± 0.39a | 0.78 ± 0.53c | |
| FAS | 1.01 ± 0.17 | 1.73 ± 0.36a | 0.92 ± 0.13c | |
| SCD-1 | 1.00 ± 0.08 | 3.07 ± 1.03a | 0.71 ± 0.57c |
Total RNA was isolated from primary hepatocytes and subjected to quantitative RT-PCR for indicated genes. Expression levels were normalized to 18S ribosomal RNA and compared to untreated controls, which were set at 1.0.
p<0.05, pair fed vs alcohol fed
p<0.05 alcohol fed vs alcohol + 50 mg/ kg BW 17-DMAG
Nonparametric ANOVA followed by Kruskal-Wallis test. Values shown are mean ± SE, n=6.
Discussion
The importance of heat shock proteins in liver diseases is emerging. Hsp90 is important in progression of hepatocellular carcinoma (HCC) [10] and hepatitis C virus replication in the liver [12, 13].. The development of hsp90 as an important therapeutic target in chronic diseases and cancer including HCC is evolving [8, 10]. Here, for the first time, we identify the significance of hsp90 as a promising therapeutic target in alcoholic liver disease. Using murine preclinical models of acute and chronic alcoholic liver injury, we show that pharmacological inhibition of hsp90 by 17-DMAG attenuates liver injury by reducing oxidative stress, decreasing macrophage sensitization to LPS leading to diminished pro-inflammatory cytokines and amelioration of alcohol induced steatosis. Our studies reported here, targeting hsp90 in the liver, support feasibility for future clinical development of hsp90 inhibitors in alcoholic liver disease. Here, we present evidence for pathophysiological significance of hsp90 in acute and chronic alcoholic liver injury.
In the present study we show increased expression of hsp90 in human alcoholic cirrhotic livers as well as murine alcoholic liver. Immunohistochemistry revealed diffuse parenchymal and nonparenchymal staining pattern of hsp90 in human and mouse alcoholic livers. Previous studies from our laboratory showed that alcohol induces hsp90 in human monocytes and macrophages [14]. Transcriptional induction of hsp90 in whole livers, isolated hepatocytes and LMs of chronic alcohol fed mice was observed. Western blot analysis confirmed increased protein expression of hsp90 in alcoholic livers, likely regulated by its transcription factor, HSF1, which was upregulated in the nucleus of hepatocytes and LMs. Our results strongly support that alcohol induces hsp90 expression in alcoholic hepatocytes and LMs pointing to its pathological function in liver injury.
The last decade has seen the development of hsp90 inhibitors as an attractive strategy in cancers [24], including HCC [10]. Here, we present novel data identifying the potential application of hsp90 inhibitor as a therapeutic strategy in ALD. Experiments were performed in vivo using 17-DMAG, a water-soluble hsp90 specific inhibitor and geldanamycin derivative, in acute and chronic-binge alcoholic liver injury models. Our data reveals that 17-DMAG treatment prevents and reverses signs of alcoholic liver injury as noted by significant reduction in serum ALT, AST, and liver triglycerides in both pre-clinical alcohol models of liver injury. Bioavailability studies of 17-DMAG by LC-MS/MS confirms its presence in the liver. Based on toxicity data, our studies did not exceed the maximum tolerated dose of 75 mg/kg 17-DMAG, described in supplementary information. Similar to our previous report using a model of endotoxin mediated liver injury [16], 17-DMAG here also led to HSF1 activation and hsp70 induction in alcoholic whole liver, LMs and hepatocytes confirming hsp90 inhibition. Our results provide a basis for future clinical investigation of hsp90 as a therapeutic target in alcoholic liver disease.
The mechanisms associated with alleviation of alcoholic liver injury by 17-DMAG can be multifactorial. Here 17-DMAG treatment during ALD significantly reduced TBARS and HNE, markers of lipid peroxidation, and prevented down-regulation of liver GSH without altering CYP2E1 and ADH1. Earlier studies in HEPG2 cells overexpressing CYP2E1 revealed higher expression of hsp90 which interacts with the membrane associated domain of CYP2E1 [25]. Interestingly, biochemical studies noted that the presence of an ethanol molecule disrupts the interaction between hsp90 and CYP2E1 leading to prevention of proteosomal degradation of CYP2E1 [26]. This may explain the unchanged levels of CYP2E1 in 17-DMAG treated alcoholic livers. Further, 17-DMAG does not utilize GSH for metabolism in the liver likely retaining higher levels of free GSH [27] contributing to decreased alcohol mediated alleviating alcohol mediated oxidative stress in liver. Alcohol mediated oxidative stress at least in part, sensitizes liver macrophages to endotoxin resulting in elevation of pro-inflammatory cytokines and liver injury [19, 28]. Our data shows that 17-DMAG in vivo prevents alcohol mediated elevation of pro-inflammatory cytokine expression likely due to reduced sensitization and activation of LMs, or reduced serum endotoxin. Chronic alcohol mediated increase in circulating endotoxin [29] was inhibited by 17-DMAG. Previous studies have shown hsp90 inhibitors decrease intestinal inflammation and leakage [30] and ameliorate radiation induced small intestinal injury by preventing degenerative changes that can alter gut integrity [31]. Decreased serum endotoxin after 17-DMAG treatment may suggest an important role for hsp90 in alcohol induced gut permeability. The mechanisms associated with decreased liver macrophage activation after 17-DMAG treatment are largely CD14 and NFκB mediated [20]. 17-DMAG reduced CD14 mRNA without significantly affecting alcohol mediated up-regulation of TLR4 mRNA in the liver, similar to our previous observations in an endotoxin liver injury model [16]. Downstream to CD14/TLR4 signaling, alcohol increased direct interaction between IκB kinase (IKK) and hsp90 [14], further contributing to macrophage activation and increased pro-inflammatory cytokine production. Using hsp90 siRNA in RAW 264.7 macrophages, cell line models commonly used to study mechanisms in alcohol and monocyte/macrophage research, confirm specificity and significance of hsp90 in alcohol mediated elevation of pro-inflammatory responses in liver. Finally our results reveal decreased expression of monocyte/macrophage and neutrophil activation markers suggesting that hsp90 inhibition can influence inflammatory foci in alcoholic liver.
The chaperone function of hsp90 in maintaining function of proteins involved in lipid metabolism has been identified. Hsp90 regulates PPARα, important in fatty acid oxidation, by repressing its activity [22]. 17-DMAG prevented decrease in expression and DNA binding activity of PPARα in alcoholic whole liver and isolated hepatocytes, and induction of target genes, CPT-1a, ACOX1, LCAD and MCAD. Previous studies suggested that hsp90 likely regulates SREBP-1, a transcription factor important in fatty acid synthesis, via an mTORC1/lipin-1 axis [23], Hsp90 inhibition has been linked to loss of mTORC1 kinase activity [32]. Such inhibition of hsp90-dependent mTORC1 activity can affect nuclear translocation of SREBP-1 (similar to our results in Fig 4D) via regulation of lipin-1, a crucial mediator of SREBP-1 [23]. Hsp90 inhibition reduced nuclear SREBP-1 and decreased target genes such as SREBPF-1, SCD-1, FAS and ACC-1 in alcoholic livers. These studies point to a direct regulation of hepatic lipid metabolism by hsp90 in alcoholic hepatocytes. Future studies will focused on delineating the precise role of hsp90 in fatty acid metabolism and its contribution to fatty liver disease.
In summary, we demonstrate that hsp90 is induced in human and murine alcoholic liver disease. Hsp90 inhibition in vivo alleviates serum ALT and significantly lowers steatosis in acute and chronic alcoholic liver injury. 17-DMAG treatment decreases serum endotoxin and proinflammatory cytokines likely by down-regulating CD14 expression and NFκB signaling. Finally, inhibition of hsp90 regulates PPARα and SREBP-1 in hepatocytes to influence fatty acid oxidation and synthesis genes in alcoholic liver. Collectively, our findings strongly suggest that hsp90 plays a pivotal role in alcoholic liver disease justifying future testing of clinical efficacy, safety and pharmacokinetics of hsp90 inhibitors.
Supplementary Material
Acknowledgement
The 17-DMAG was generously provided by Bristol-Myers Squibb and the National Cancer Institute, NIH.
Financial Support:
This study was supported by the PHS grant #AA017986 (to PM) from the National Institute of Alcohol Abuse and Alcoholism, National Institutes of Health and Department of Defense grant #W81XWH-11-1-0420 (to PM).
The contents of this article are the sole responsibility of the authors and do not necessarily represent the views of NIAAA.
This work was supported by the University of Massachusetts Center for AIDS Research (P30 AI042845).
Normal and pathologic human liver samples and respective paraffin blocks were obtained through the Liver Tissue Cell Distribution System, [Minneapolis, Minnesota] which was funded by NIH Contract # N01-DK-7-0004/HHSN26700700004C.
List of Abbreviations
- 17-DMAG
17-Dimethylamino-ethylamino-17-demethoxygeldanamycin
- 4-HNE
4-hydroxynonenal
- ACC-1
Acetyl-Coenzyme A carboxylase 1
- ACOX1
Acyl coenzyme A oxidase 1
- ALD
Alcoholic liver disease
- CPT1a
Carnitine palmitoyltransferase 1a
- CYP2E1
Cytochrome P450, family 2, subfamily e, polypeptide 1
- EMSA
Electrophoretic Mobility Shift Assay
- HSF1
Heat Shock Transcription Factor 1
- Hsp70
Heat shock 70kDa protein
- Hsp90
Heat shock 90kDa protein
- LCAD
Long-chain Acyl-Coenzyme A dehydrogenase
- LMs
Liver Macrophages
- MCAD
Medium-chain Acyl-Coenzyme A dehydrogenase
- MCP1/CCL2
monocyte chemoattractant protein 1
- NFκB
Nuclear factor kappa-B
- PPARα
Peroxisome proliferator activated receptor alpha
- TBARS
Thiobarbituric acid reactive substances
- TLR4
Toll like receptor 4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
The authors who contributed to this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
References
- 1.O'Shea RS, Dasarathy S, McCullough AJ. Practice Guideline Committee of the American Association for the Study of Liver Diseases, Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 2.Breitkopf K, Nagy LE, Beier JI, Mueller S, Weng H, Dooley S. Current experimental perspectives on the clinical progression of alcoholic liver disease. Alcohol Clin Exp Res. 2009;33:1647–1655. doi: 10.1111/j.1530-0277.2009.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 4.Frazier TH, Stocker AM, Kershner NA, Marsano LS, McClain CJ. Treatment of alcoholic liver disease. Therap Adv Gastroenterol. 2011;4:63–81. doi: 10.1177/1756283X10378925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naveau S, Chollet-Martin S, Dharancy S, Mathurin P, Jouet P, Piquet MA, et al. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. 2004;39:1390–1397. doi: 10.1002/hep.20206. [DOI] [PubMed] [Google Scholar]
- 6.Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476–3489. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soti C, Nagy E, Giricz Z, Vigh L, Csermely P, Ferdinandy P. Heat shock proteins as emerging therapeutic targets. Br J Pharmacol. 2005;146:769–780. doi: 10.1038/sj.bjp.0706396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacey S, Wilson RH, Walton M, Eatock MM, Hardcastle A, Zetterlund A, et al. A phase I study of the heat shock protein 90 inhibitor alvespimycin (17-DMAG) given intravenously to patients with advanced solid tumors. Clin Cancer Res. 2011;17:1561–1570. doi: 10.1158/1078-0432.CCR-10-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breinig M, Caldas-Lopes E, Goeppert B, Malz M, Rieker R, Bergmann F, et al. Targeting heat shock protein 90 with non-quinone inhibitors: a novel chemotherapeutic approach in human hepatocellular carcinoma. Hepatology. 2009;50:102–112. doi: 10.1002/hep.22912. [DOI] [PubMed] [Google Scholar]
- 11.Myung SJ, Yoon JH, Kim BH, Lee JH, Jung EU, Lee HS. Heat shock protein 90 inhibitor induces apoptosis and attenuates activation of hepatic stellate cells. J Pharmacol Exp Ther. 2009;330:276–282. doi: 10.1124/jpet.109.151860. [DOI] [PubMed] [Google Scholar]
- 12.Bukong TN, Hou W, Kodys K, Szabo G. Ethanol facilitates hepatitis C virus replication via up-regulation of GW182 and heat shock protein 90 in human hepatoma cells. Hepatology. 2013;57:70–80. doi: 10.1002/hep.26010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ujino S, Yamaguchi S, Shimotohno K, Takaku H. Heat-shock protein 90 is essential for stabilization of the hepatitis C virus nonstructural protein NS3. J Biol Chem. 2009;284:6841–6846. doi: 10.1074/jbc.M806452200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandrekar P, Catalano D, Jeliazkova V, Kodys K. Alcohol exposure regulates heat shock transcription factor binding and heat shock proteins 70 and 90 in monocytes and macrophages: implication for TNF-alpha regulation. J Leukoc Biol. 2008;84:1335–1345. doi: 10.1189/jlb.0407256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ambade A, Catalano D, Lim A, Mandrekar P. Inhibition of heat shock protein (molecular weight 90 kDa) attenuates proinflammatory cytokines and prevents lipopolysaccharide-induced liver injury in mice. Hepatology. 2012;55:1585–1595. doi: 10.1002/hep.24802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HR, Kang HS, Kim HD. Geldanamycin induces heat shock protein expression through activation of HSF1 in K562 erythroleukemic cells. IUBMB Life. 1999;48:429–433. doi: 10.1080/713803536. [DOI] [PubMed] [Google Scholar]
- 18.Cohen JI, Chen X, Nagy LE. Redox signaling and the innate immune system in alcoholic liver disease. Antioxid Redox Signal. 2011;15:523–534. doi: 10.1089/ars.2010.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enomoto N, Ikejima K, Yamashina S, Hirose M, Shimizu H, Kitamura T, et al. Kupffer cell sensitization by alcohol involves increased permeability to gut-derived endotoxin. Alcohol Clin Exp Res. 2001;25:51S–54S. doi: 10.1097/00000374-200106001-00012. [DOI] [PubMed] [Google Scholar]
- 20.Vega VL, De Maio A. Geldanamycin treatment ameliorates the response to LPS in murine macrophages by decreasing CD14 surface expression. Mol Biol Cell. 2003;14:764–773. doi: 10.1091/mbc.E02-08-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertola A, Park O, Gao B. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury: A critical role for E-selectin. Hepatology. 2013;58(5):1814–1823. doi: 10.1002/hep.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sumanasekera WK, Tien ES, Davis JW, 2nd, Turpey R, Perdew GH, Vanden Heuvel JP. Heat shock protein-90 (Hsp90) acts as a repressor of peroxisome proliferator-activated receptor-alpha (PPARalpha) and PPARbeta activity. Biochemistry. 2003;42:10726–10735. doi: 10.1021/bi0347353. [DOI] [PubMed] [Google Scholar]
- 23.Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyata Y, Nakamoto H, Neckers L. The therapeutic target Hsp90 and cancer hallmarks. Curr Pharm Des. 2013;19:347–365. doi: 10.2174/138161213804143725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dey A, Kessova IG, Cederbaum AI. Decreased protein and mRNA expression of ER stress proteins GRP78 and GRP94 in HepG2 cells over-expressing CYP2E1. Arch Biochem Biophys. 2006;447:155–166. doi: 10.1016/j.abb.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Kitam VO, Maksymchuk OV, Chashchyn MO. The possible mechanisms of CYP2E1 interactions with HSP90 and the influence of ethanol on them. BMC Struct Biol. 2012;12:33-6807-12-33. doi: 10.1186/1472-6807-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng N, Zou P, Wang S, Sun D. In Vitro Metabolism of 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG) in Human Liver Microsomes. Drug Metab Dispos. 2010 doi: 10.1124/dmd.110.036418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thakur V, McMullen MR, Pritchard MT, Nagy LE. Regulation of macrophage activation in alcoholic liver disease. J Gastroenterol Hepatol. 2007;22(Suppl 1):S53–S56. doi: 10.1111/j.1440-1746.2006.04650.x. [DOI] [PubMed] [Google Scholar]
- 29.Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, et al. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009;50:538–547. doi: 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Y, Huang ZJ, Rahman M, Luo Q, Thorlacius H. Radicicol, an Hsp90 inhibitor, inhibits intestinal inflammation and leakage in abdominal sepsis. J Surg Res. 2013;182:312–318. doi: 10.1016/j.jss.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 31.Lu X, Nurmemet D, Bolduc DL, Elliott TB, Kiang JG. Radioprotective effects of oral 17-dimethylaminoethylamino-17-demethoxygeldanamycin in mice: bone marrow and small intestine. Cell Biosci. 2013;3:36-3701-3-36. doi: 10.1186/2045-3701-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohji G, Hidayat S, Nakashima A, Tokunaga C, Oshiro N, Yoshino K, et al. Suppression of the mTOR-raptor signaling pathway by the inhibitor of heat shock protein 90 geldanamycin. J Biochem. 2006;139:129–135. doi: 10.1093/jb/mvj008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.