SUMMARY
Systems biological analysis of immunity to the trivalent inactivated influenza vaccine (TIV) in humans revealed a correlation between early expression of TLR5 and the magnitude of the antibody response. Vaccination of Trl5−/− mice resulted in reduced antibody titers and lower frequencies of plasma cells, demonstrating a role for TLR5 in immunity to TIV. This was due to a failure to sense host microbiota. Thus, antibody responses in germ-free or antibiotic-treated mice were impaired, but restored by oral reconstitution with a flagellated, but not aflagellated, strain of E. coli. TLR5-mediated sensing of flagellin promoted plasma cell differentiation, directly, and by stimulating lymph node macrophages to produce plasma cell growth factors. Finally, TLR5-mediated sensing of the microbiota also impacted antibody responses to the inactivated polio vaccine, but not to adjuvanted vaccines or the live-attenuated yellow fever vaccine. These results reveal an unappreciated role for gut microbiota in promoting immunity to vaccination.
INTRODUCTION
Influenza affects millions of people worldwide, and despite it being one of the most widely targeted viruses through yearly vaccination programs, significant rates of morbidity and mortality persist (CDC, 2013). One of two FDA-approved influenza vaccines in the USA is the TIV. Although the molecular pathways involved in innate sensing of influenza virus and the ensuing adaptive immune response have been studied (Allen et al., 2009; Diebold et al., 2004; Ichinohe et al., 2009), immunological mechanisms by which the inactivated vaccine elicits host immune response remain unclear. TIV is a subunit vaccine composed primarily of HA molecules derived from three different strains of the influenza virus. It is unadjuvanted, yet epidemiological data clearly show a level of effectiveness in populations where vaccination is routinely administered (Kostova et al., 2013). However, neither protection nor effectiveness is complete and a significant proportion of vaccinees, mostly among the young and elderly, remain susceptible to infection. In addition to age, the status of pre-existing immune memory significantly impacts vaccine effectiveness during a given season (Sasaki et al., 2008). Furthermore the molecular mechanisms leading to protective immunity remain poorly studied.
Recently we used a systems biology approach to study the innate and adaptive response induced by vaccination of humans with TIV (Nakaya et al., 2011). An intriguing insight to emerge from this work was that the expression of toll-like receptor 5 (TLR5) within three days after vaccination strongly correlated to the magnitude of the hemagglutination inhibition (HAI) titers 4 weeks after vaccination. TLR5 is a cell-surface receptor specific for flagellin (Hayashi et al., 2001), the monomeric component of bacterial flagellum used for cell motility, and has not been associated with viral infections. Thus, how TLR5 may be involved in the induction of antibody responses to a viral vaccine is unclear.
In this study, we examined whether there was a causal link between TLR5 and TIV-induced humoral immune response. We show that vaccination of Tlr5−/− mice with TIV resulted in strikingly reduced TIV-specific antibody response. Furthermore, we demonstrated that TIV itself did not directly signal through TLR5, but rather that the intestinal microbiota contributed to TLR5-mediated enhancement of immunity to TIV and to the inactivated polio vaccine, another subunit vaccine. These results reveal an unappreciated role for the gut microbiota in modulating vaccine immunity.
RESULTS
TLR5 expression positively correlates with antibody responses to TIV in humans
In a recent clinical study of influenza vaccination in healthy individuals (Nakaya et al., 2011), we identified key gene signatures at early time points following vaccination correlating with the magnitude of the later antibody response. Among these signatures, a striking correlation was observed between the induced level of TLR5 expression on day 3-post vaccination with the magnitude of HAI titers measured at 28 days-post vaccination (Figure S1A) and plasmablast responses on day 7-post vaccination. These correlations were evident across most vaccination seasons, except 2010–2011, and suggest that the induction of TLR5 upon vaccination was not specific for a cohort limited to one particular season.
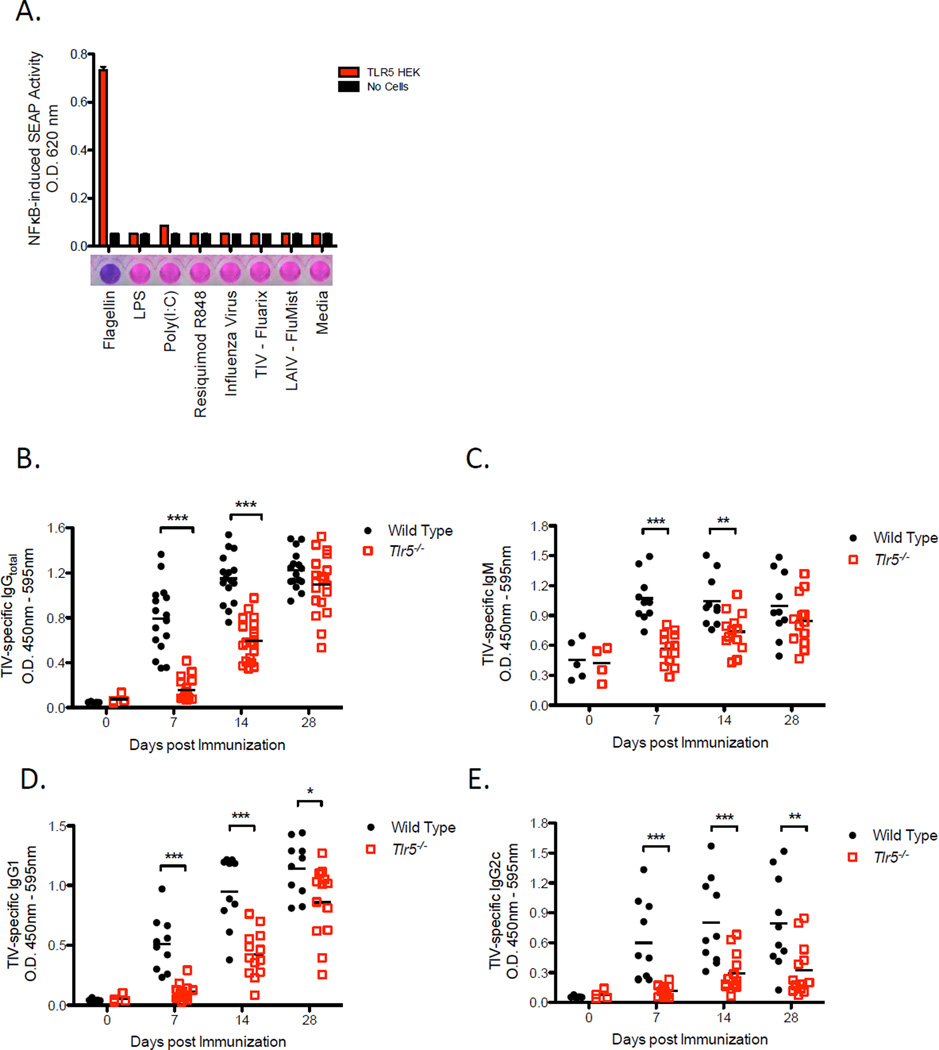
TLR5 is not known to be a sensor of viral stimuli, but rather of bacterial flagellin. We, therefore, determined whether TIV was capable of directly signaling through TLR5 by utilizing the human embryonic kidney cell line, HEK 293, transfected with TLR5 and nuclear factor kappa beta (NF-kB)-inducible reporter gene encoding secreted human alkaline phosphatase (SEAP). Stably transfected cells were cultured with either TIV, Flumist (live-attenuated influenza vaccine), influenza virus (A/Brisbane/59/2007), or a panel of individual TLR agonists that includes flagellin, LPS, PolyI:C and Resiquimod. As expected, flagellin gave a robust activation signal that was evident within 3 hours of incubation with transfected cells. Although cells were incubated further for 20 hours, other ligands including TIV and influenza viruses failed to stimulate TLR5 (Figure 1A). These results suggest that the correlation found between TLR5 expression and the subsequent antibody response cannot be attributed to any type of contaminating source within the vaccine.
Figure 1. TIV induces antibody responses in a TLR5-dependent manner without directly signaling through TLR5.
(A) SEAP concentrations in the supernatant of a TLR5-NFkB reporter cell line co-cultured with the indicated panel of TLR ligands and vaccines. Culture supernatant was assayed for reporter activity at 20 hours of incubation. Data shown are mean O.D. values ± SEM and representative of two independent experiments.
(B–E) TIV-specific IgG total (B), IgM (C), IgG1 (D), IgG2c (E) concentrations in the serum of Tlr5−/− or littermate WT mice. The raw O.D. values shown were obtained using serum (diluted by a factor of 1:200) from three independent experiments assayed concurrently in (B) and two independent experiments in (C–E). Data are represented as the means ± SEM. See also Figure S1.
Influenza-specific antibody responses induced by vaccination is dependent on TLR5 expression
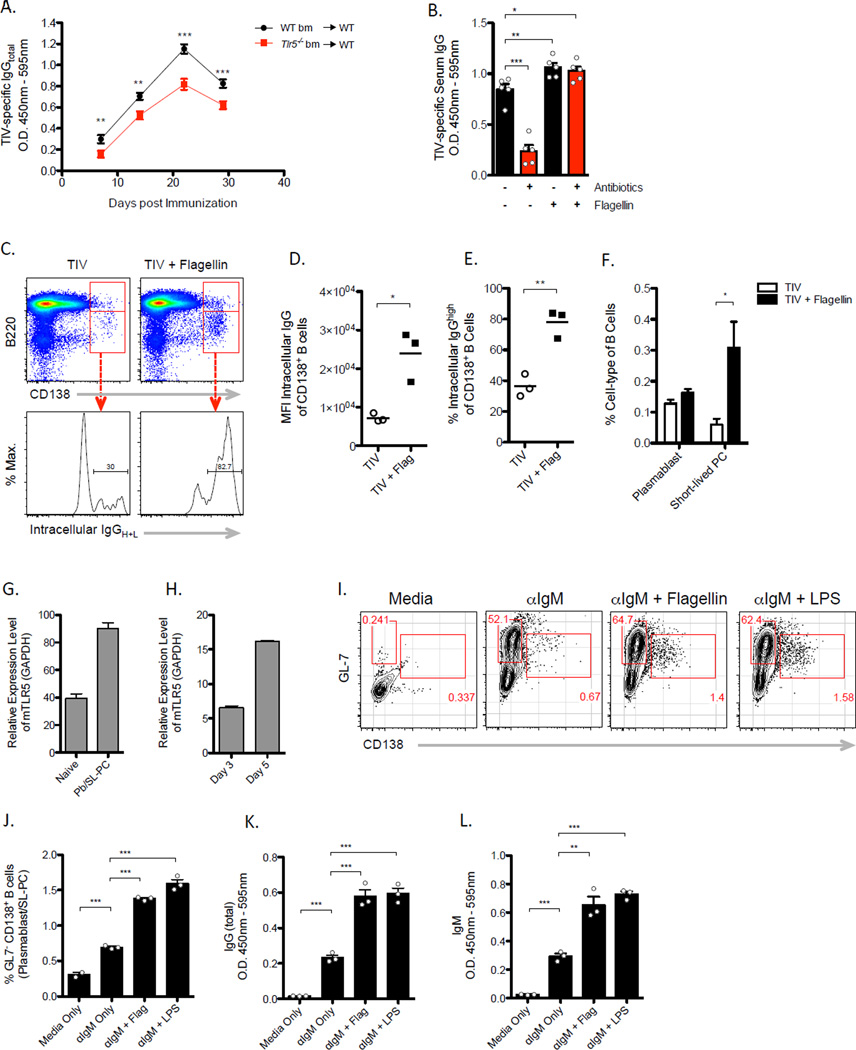
We tested whether TLR5 played a functional role in mediating antibody response to TIV using Tlr5−/− mice. Upon vaccination, TIV-specific immunoglobulin G (IgG) and IgM antibody responses were significantly reduced in Tlr5−/− mice in comparison to responses in littermate wild-type mice (Figures 1B and 1C). The degree of reduction in TIV-specific antibody concentrations was more pronounced during the first 7 days after vaccination than at later time points. Furthermore, TIV-specific IgG1 and IgG2c antibody responses were both significantly impacted by TLR5 deficiency (Figure 1D, 1E, respectively), although the IgG2c antibody response remained considerably impaired throughout the course of the primary immune response. In contrast to vaccine-induced antibody responses, baseline amounts of total IgG in the serum as well as the frequencies of long-lived plasma cells in the bone marrow were comparable between Tlr5−/− mice and wild type littermate mice (Figure S1B and S1C). Together, the data suggest that while Tlr5−/− mice do not exhibit any gross defects or pre-existing immunodeficiencies in the humoral immune system under steady-state conditions, induction of antibody responses following vaccination with TIV is substantially reduced.
Early B cell response to flu vaccination is microbiota-dependent
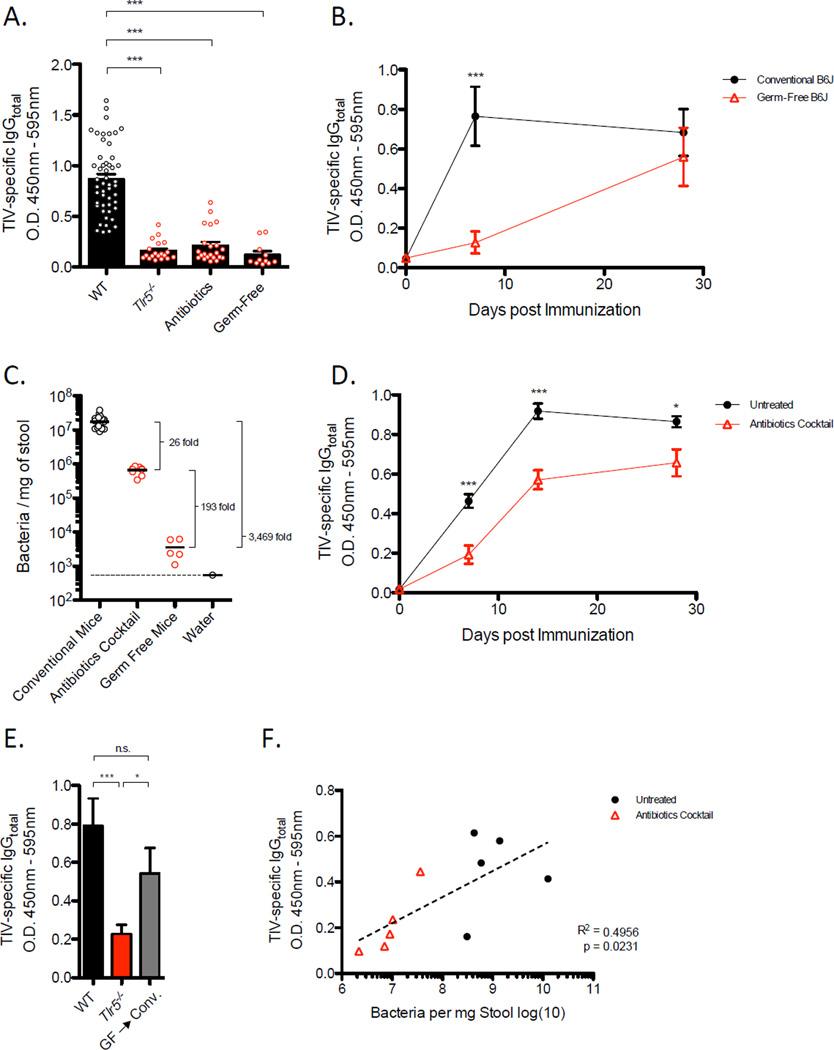
Given that TIV lacked the capacity to directly stimulate TLR5, we hypothesized that an endogenous host derived signal, such as commensal bacteria residing in the gut, was activating the TLR5 pathway. To test this possibility, we compared vaccine-induced responses between germ-free and conventionally housed, specific pathogen free (SPF) B6 mice. Intriguingly, we found that vaccine-specific IgG concentrations were significantly reduced in germ-free mice relative to the response in SPF mice on day 7-post vaccination (Figure 2A). This difference in vaccine-specific antibody levels was less pronounced on day 28 (Figure 2B), which is consistent with the kinetics of the antibody response observed in in Tlr5−/− mice (Figure 1B). These results suggest that microbiota is crucial for the rapid induction of antibody responses following TIV vaccination.
Figure 2. Host Microbiota is Necessary for Early Antibody Response to TIV.
(A) TIV-specific IgG concentrations in serum serum of Trl5−/−, antibiotic-treated, or germ-free, or WT mice on day 7 following vaccination. The raw O.D. values shown were obtained using serum diluted by a factor of 1:200. Data are representative of three independent experiments and shown as means ± SEM.
(B) Kinetics of TIV-specific IgG levels induced in conventional or germ-free mice. The raw O.D. values shown were obtained using serum diluted by a factor of 1:200. Data are representative of three independent experiments and shown as means ± SEM.
(C) Bacteria in stool samples quantified by qPCR.
(D) Kinetics of TIV-specific IgG concentrations induced in antibiotic-treated or untreated mice. The raw O.D. values shown were obtained using serum diluted by a factor of 1:200. Data are representative of five independent experiments and shown as means ± SEM.
(E) TIV-specific IgG concentrations on day 7-post vaccination in Tlr5−/− mice, littermate WT, and conventionalized germ-free mice. Serum samples assayed at1:200 dilution and shown as means ± SEM.
(F) Pair-wise analysis of baseline bacterial with levels of TIV-specific IgG in corresponding mice at day 7-post vaccination. See also Figure S2.
To determine the degree to which microbiota impacts vaccine-induced antibody responses, SPF mice were treated with a cocktail of broad-spectrum antibiotics in the drinking water (Hall et al., 2008; Ichinohe et al., 2011; Rakoff-Nahoum et al., 2004). Following a regimen of 4 weeks of antibiotic treatment, we found approximately 95% of fecal bacteria were eliminated (Figure 2C). Thus, mice were vaccinated with TIV at 4 weeks of treatment and the kinetics of antibodies induced was compared to responses generated in untreated mice. We observed substantial reduction in concentrations of TIV-specific IgG at day 7-post vaccination that steadily increased to amounts comparable in untreated mice by day 28-post vaccination (Figure 2A, 2D). These results demonstrate that antibiotic-mediated depletion of microbiota also impairs vaccine-induced antibody responses to TIV. Similar to Tlr5−/− mice, baseline levels of total serum antibodies were not affected by antibiotic treatment (Figure S1D) or germ-free conditions (Figure S1E), which indicates absence of any gross defects in the immune system. Although the majority of the microbiota was eliminated from the gut following oral antibiotic treatment, we observed that approximately 106 bacteria per milligram of stool still were detectable in antibiotic treated mice (Figure 2C), suggesting that a complete elimination of microbiota is not necessary to impact the capacity to which microbiota influences the immune response to vaccination.
To further establish the role of microbiota following vaccination with TIV, we sought to examine whether allowing commensals to naturally reconstitute a germ-free mouse would rescue antibody responses to TIV. To address this question, germ-free mice were conventionalized via transfer from a germ-free micro-isolator into standard housing conditions. Intriguingly, TIV-specific IgG concentrations in these mice recovered to those comparable in SPF mice (Figure 2E). Furthermore, a pairwise analysis of bacteria numbers obtained from individual stool samples and TIV-specific antibody concentrations from corresponding serum samples revealed a striking positive correlation (Figure 2F) in untreated or antibiotic-treated mice, which is consistent with the interpretation that the increased antibody response observed in naturally reconstituted germ-free mice was due to increased levels of bacteria. Together, these results demonstrate that re-establishment of microbiota promotes antibody responses to TIV.
Microbiota impacts short-lived plasma cell response following TIV vaccination
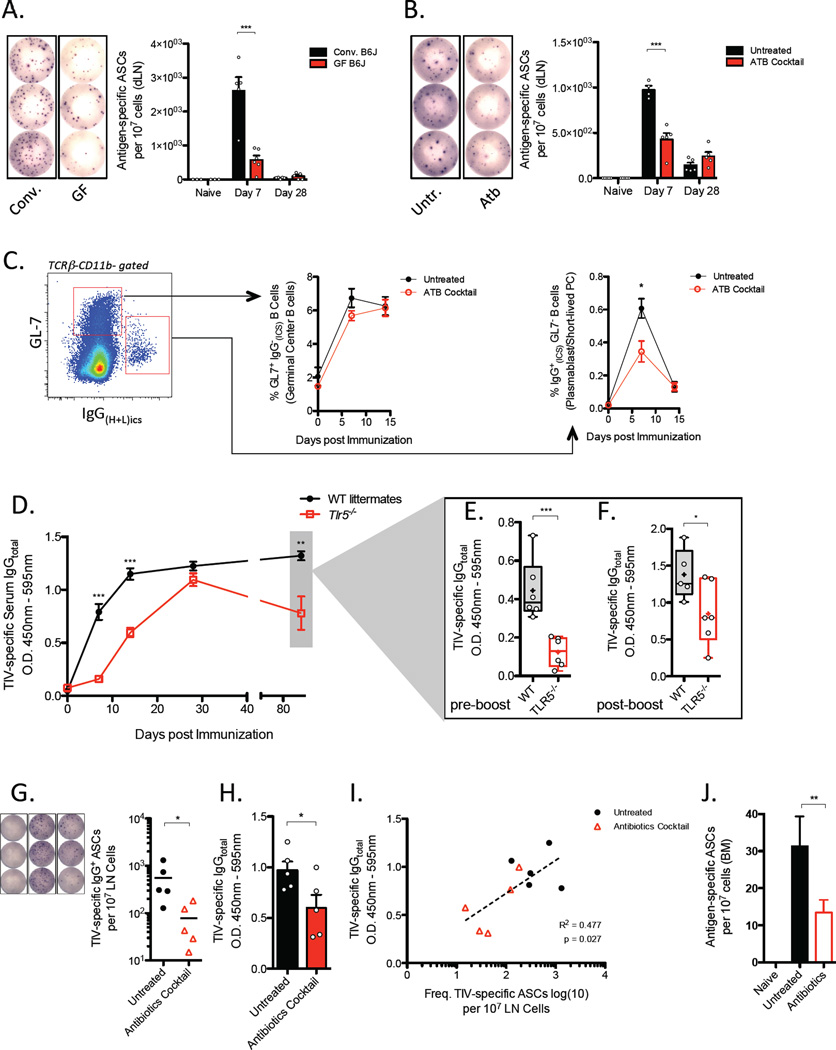
To investigate the cellular mechanisms underlying the impact of microbiota particularly during the early phase of the humoral immune response, we examined B cells in the draining lymph nodes (dLN) through multiple time points after vaccination in either germ-free or antibiotic-treated mice. B cell ELISPOT assays were conducted to measure the frequency of vaccine-specific short-lived plasma cells. Consistent with the amount of TIV-specific antibodies detected in the serum, there was substantial reduction in frequency of TIV-specific antibody secreting cells (ASC) in the dLN of germ-free, antibiotic-treated, and Tlr5−/− mice 7 days-post vaccination (Figure 3A, 3B, S2A respectively). In germ-free mice, the reduction observed in ASC response remarkably reflected the degree to which antigen-specific antibody concentrations in the serum were reduced as compared to SPF mice. A similar trend was also evident in antibiotic-treated mice. Thus, the data suggest that a majority of vaccine-induced IgG antibodies detected during the first 7 days following vaccination are produced by plasmablasts or short-lived plasma cells in the dLN and that the microbiota was critical for the induction of these cell types.
Figure 3. Impact of TLR5 signaling and Microbiota in Primary and Secondary B cell Responses.
(A, B) Frequency of TIV-specific antibody secreting cells (ASC) in the draining LN of germ-free or SPF mice (A) and mice treated with a cocktail of broad-spectrum antibiotics or left untreated
(B). Representative spot formations are shown and total frequencies are expressed as the means ± SEM in the graphs on the right. Data are representative of three independent experiments.
(C) Relative frequencies of germinal center B cells (GL7+, CD138−, intracellular IgG (heavy and light chain) and short-lived plasma cells (GL7−, CD138+, intracellular IgG(heavy and light chain)+) gated from a population of CD11b− TCRb− cells (left and right graphs, respectively).
(D–F) Tlr5−/− and littermate WT mice were vaccinated with TIV and vaccine-specific serum IgG concentrations were measured at the indicated time points following vaccination by serum ELISA. (E) Preboost and (F) day 5-post boost serum concentrations of TIV-specific IgG. Raw O.D. values shown were obtained using serum diluted by a factor of 1:3600 and 1:400, respectively. Data are represented as means ± SEM.
(G) Frequency of TIV-specific ASCs in the LN and (H) levels of TIV-specific IgG at day 5 –post boost injection in antibiotic-treated or untreated mice. Boost dose of TIV was administered 50 days following prime immunization. Representative spot formations are shown on the left and ASC frequencies expressed as means ± SEM in the graph. Raw O.D. values were obtained from serum assayed at a dilution of 1:3200.
(I) Pairwise analysis was conducted using the levels of TIV-specific serum IgG antibodies measured by ELISA corresponding to the frequency of TIV-specific ASCs in the dLN.
(J) Frequency of TIV-specific ASCs in the bone marrow on day 5-post boost injection. See also Figure S3
Furthermore, we characterized the maturation of the B cell response by analyzing B cell subsets in the dLN using flow cytometry. Gating on singlet CD45+ leukocytes, we found that indeed plasmablasts or short-lived plasma cells – as defined by GL7−CD138+ and intracellular IgG(H+L)+ phenotype – were present at lower frequencies on day 7-post vaccination with TIV in antibiotic-treated mice when compared to untreated mice (Figure 3C). Similar observations were made in germ-free mice (data not shown). Intriguingly, in both germ-free or antibiotic-treated mice, these cells did not accumulate significantly at later time points, which suggests that the increasing concentrations of antibodies detected in the serum at day 14 and 28-post vaccination (Figure 2B, D) are not due to a delayed onset of the plasmablast response in these mice. Together, these results support a conclusion that microbiota significantly impacts early induction of ASCs while germinal center B cells responses remain unaffected.
Impact of TLR5 signaling and microbiota on recall response of memory B cells
In humans, TIV is administered seasonally. Thus, the majority of vaccinees examined in the clinical studies described above (Nakaya et al., 2011) are likely to have immunity or immune reactivity against flu antigens from either prior vaccinations or viral exposure. To establish a corollary of the genetic signatures obtained from vaccinated individuals (Figure S1A), we sought to evaluate the role of TLR5 and microbiota on secondary immune responses.
Prior to examining responses to a boost vaccination, however, we found reduced concentrations of TIV-specific antibodies on day 84-post prime vaccination in Tlr5−/− mice (Figure 3D). This was an unexpected observation as comparable amounts of TIV-specific IgG antibodies were detected at 28 days-post vaccination in Tlr5−/− and littermate wild-type mice. These results suggest that the longevity of humoral immune response to a prime vaccination wanes more rapidly in the absence of TLR5 signaling. Curtailed persistence of vaccine-induced antibody concentrations may be due to impaired function or decreased survival of long-lived plasma cells (LL-PC) in the bone marrow. Consistent with this hypothesis, we detected fewer antigen-specific ASCs in the bone marrow of germ-free mice than conventionally housed mice, albeit at very low frequencies (Figure S2B). Furthermore, we detected elevated expression of TLR5 in LL-PCs (Figure S2C–S2E). Together, the data suggest that the longevity of TIV-specific serum antibody titers is governed by the microbiota and, in part, due to defective long-lived plasma cell response following vaccination.
To determine whether there is a similar effect in formation or function of memory B cells, we administered a boost injection into mice using the identical dose and route of the prime vaccination. In contrast to the primary response, TIV-specific IgG antibodies in Tlr5−/− mice were readily detectable at day 7-post boost injection relative to pre-boost concentrations (Figure 3E, F). However, the magnitude of the secondary antibody response was substantially lower in Tlr5−/− mice than in littermate wild-type mice (Figure 3F). These results suggest that TLR5 signaling plays an important role in differentiation or function of memory B cells following vaccination with TIV.
To determine whether antigen-specific ASCs arising from recall response of memory B cells are influenced by the microbiota, antibiotic-treated mice were also given a boost injection as described above. We found fewer TIV-specific IgG+ ASCs and lower serum IgG in antibiotic-treated mice at day 5-post boost injection (Figure 3G, 3H). A pairwise analysis between the corresponding serum ELISA and B cell ELISPOT samples revealed a positive correlation between the frequency of ASC and the magnitude of TIV-specific IgG antibodies in the serum (Figure 3I). Additionally, we found fewer TIV-specific long-lived plasma cells in the bone marrow (Figure 3J). Parenteral vaccination is not expected to affect responses in the bone marrow within 5 days of injection; thus, these results are consistent with our previous observation that fewer long-lived PCs are induced in the absence of microbiota. These results suggest that the magnitude of ASC induction following boost vaccination with TIV is also critically dependent on the microbiota.
Multiple classes of bacteria are necessary to mediate efficient priming of B cell responses to TIV
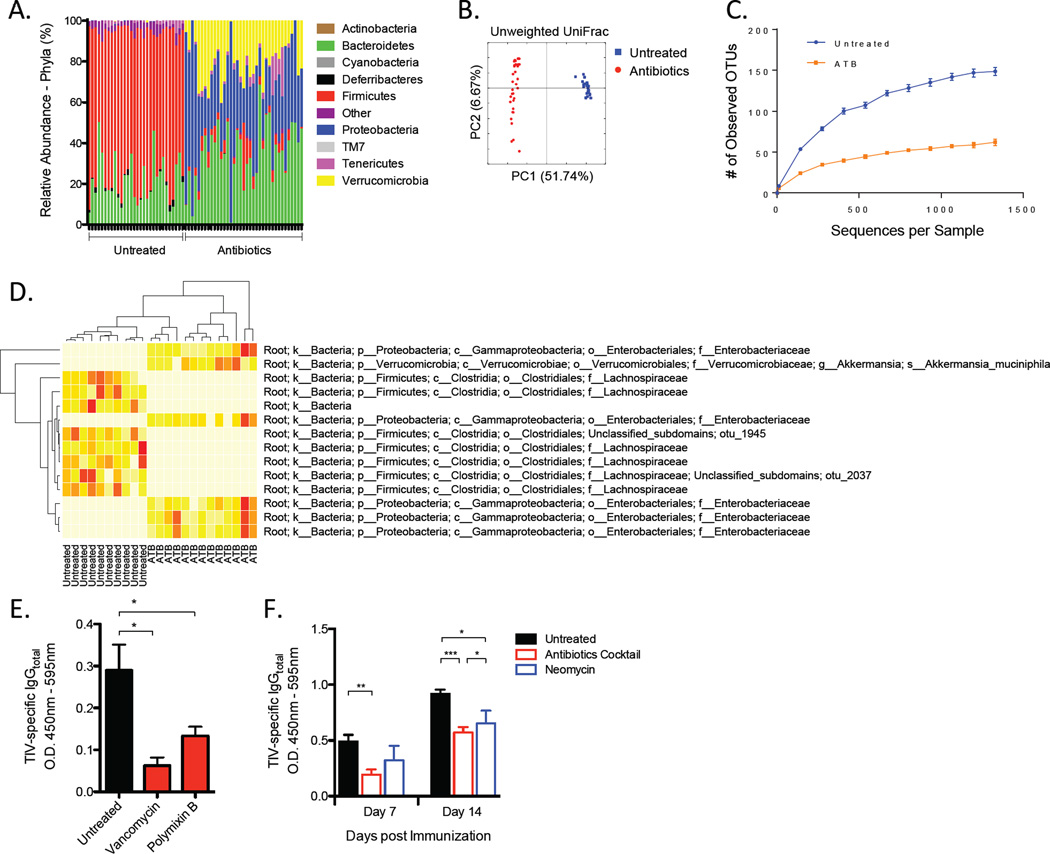
Studies using gnotobiotic techniques have revealed a relationship between a spectrum of different enteric microbial species with the capacity to regulate the local immune system (Atarashi et al., 2013; Atarashi et al., 2011; Ivanov et al., 2008; Mazmanian et al., 2005; Naik et al., 2012). To begin understanding the complexity of microbial species underlying the phenotypes observed in antibiotic-treated mice, we sought to determine which bacteria are responsible for the antibody response to TIV. To do so, we first assessed changes occurring in bacterial communities following antibiotic treatment by characterizing the microbiome and identified key compositional differences between untreated and antibiotic treated mice. Using 454-sequencing, we identified several layers of bacterial taxa groups that differed in relative abundance between untreated and antibiotic-treated groups (Figure 4A-4D). One of the striking differences uncovered was a significant shift in major bacterial phyla. Two of the major phyla present in the mouse microbiota are Bacteroidetes and Firmicutes (Figure 4A), and upon antibiotic treatment, we observed a marked reduction in Firmicutes. Interestingly, although the amount of Bacteroidetes increased following antibiotic treatment, the increase was not proportional to the degree in which Firmicutes were reduced. Instead, emergence of other taxonomic groups was observed, most notably of which was Verrucomicrobia and Proteobacteria.
Figure 4. Multiple Types of Bacteria Are Necessary to Mediate Antibody Response to TIV.
(A–D) Composition of the microbiota in antibiotics-treated or untreated mice. Diversity and specific bacterial components of the host microbiota were assessed using genomic bacterial DNA sequence analyses as described in Experimental Procedures.
(A) Relative abundance of specific bacterial phyla present in the two mouse groups.
(B) Unweighted Unifrac principal coordinate analyses using the QIIME analysis software.
(C) Rarefaction curves for untreated or antibiotics treated (ATB) groups generated based on the sequenced 16S rRNA gene libraries. Data are depicted as the number of unique operational taxonomic units (OTUs).
(D) A “nearest-shrunken centroid” classification analyses identifying bacterial taxa groups highly represented in either of the two clustered groups. Data shown are relative abundance of each OTU identified by this classification method represented in a heat map (white (least abundance) to red (most abundant)).
(E) TIV-specific IgG concentrations on day 7-post vaccination in mice untreated or treated with either vancomycin or polymixin B. Raw O.D. values shown were obtained using serum diluted by a factor of 1:200.
(F) TIV-specific IgG concentrations in mice treated with antibiotics or with neomycin alone. Raw O.D. values shown were obtained using serum diluted by a factor of 1:200 and expressed as means ± SEM. See also Figure S4.
To compare overall microbial community structure, unweighted UniFrac algorithm was applied and visualized using principal coordinate analyses (Figure 4B). Within each of the untreated and antibiotic-treated samples, there was minimal variability in the composition of microbiota. Additionally, compositional differences clustered uniformly according to each of the two types of samples. However, antibiotic treatment resulted in a dramatic decrease in richness and diversity of the microbiota (Figure 4C).
A “nearest-shrunken centroid classification” approach was applied to allow the identification of operational taxonomic units (OTUs) whose abundances significantly differed between each category (untreated vs. antibiotics-treated) with minimal misclassification (Koren et al., 2011; Tibshirani et al., 2002). We found several OTUs that were most abundantly represented in each group (Figure 4D). Specifically, several OTUs belonging to the Lachnospiraceae family of bacteria were well-represented in untreated mice but notably absent following antibiotic treatment. In contrast, emergence of several Enterobacteriaceae and Verrucomicrobiaceae OTUs were detected abundantly in antibiotic-treated mice. Together, the data show that multiple microbial communities are affected by antibiotic treatment and that it remains unknown whether a causal relationship exist between one particular group of bacteria and regulation of immune response to vaccination.
To further dissect the types of bacteria that might be associated with reduced humoral immune response after antibiotic treatment, we administered a panel of different antibiotics and selectively targeted different classes of bacteria. Mice were treated 4–8 weeks with either vancomycin or polymixin B. Vancomycin targets Gram-positive bacteria while polymixin B targets Gram-negative bacteria (Atarashi et al., 2011). Interestingly, treatment with either antibiotic resulted in reduced serum concentrations of TIV-specific IgG (Figure 4E). These results are consistent with multiple classes of bacterial species playing critical roles during vaccine-induced antibody responses.
Additionally, we examined whether gut-resident bacteria or bacteria residing in other mucosal surfaces were necessary in mediating the antibody response to TIV. Neomycin is an antibiotic that is poorly absorbed in the gastrointestinal tract, while ampicillin has been shown to exhibit higher degree of absorption and influence levels of bacteria at distal sites such as the respiratory tract when delivered orally (Ichinohe et al., 2011). Thus, we investigated the effects of targeting strictly gut-resident bacteria using neomycin and compared it to the effects of using our cocktail of antibiotics. Intriguingly, neomycin treatment alone was sufficient to recapitulate the effects observed using the cocktail of antibiotics. Although only a modest reduction in concentration of TIV-specific IgG was observed in neomycin-treated mice on day 7-post vaccination, a clear difference was evident on day 14-post vaccination (Figure 4F). B cell ELISPOT assays in an independent experiment also confirmed that early plasmablast responses in dLN of neomycin-treated mice were comparably diminished to levels observed in mice treated with the cocktail of antibiotics (data not shown). These results suggest that targeting gut-resident bacteria alone is sufficient to impair the early antibody responses to vaccination.
To further demonstrate that the TLR5 signal originated from microbiota in the gut, germ-free mice were inoculated with either flagellated or aflagellated isogenic strains of E. coli (LF82 WT and LF82 FliCmut, respectively) (Figure S3A). Colonization of germ free mice with LF82 WT resulted in higher IgM and IgG antibody responses to TIV than LF82 FliCmut (Figure S3B–S3D). Consistently, oral gavage of LF82 WT in antibiotic-treated mice also resulted in higher antibody responses than when using the aflagellated counterpart (Figure S3E). Together, the data suggest that bacterial flagellin originating from the gut sufficiently mediates antibody response to TIV.
TLR5 expression in hematopoietic population of cells is necessary for TIV-induced antibody responses
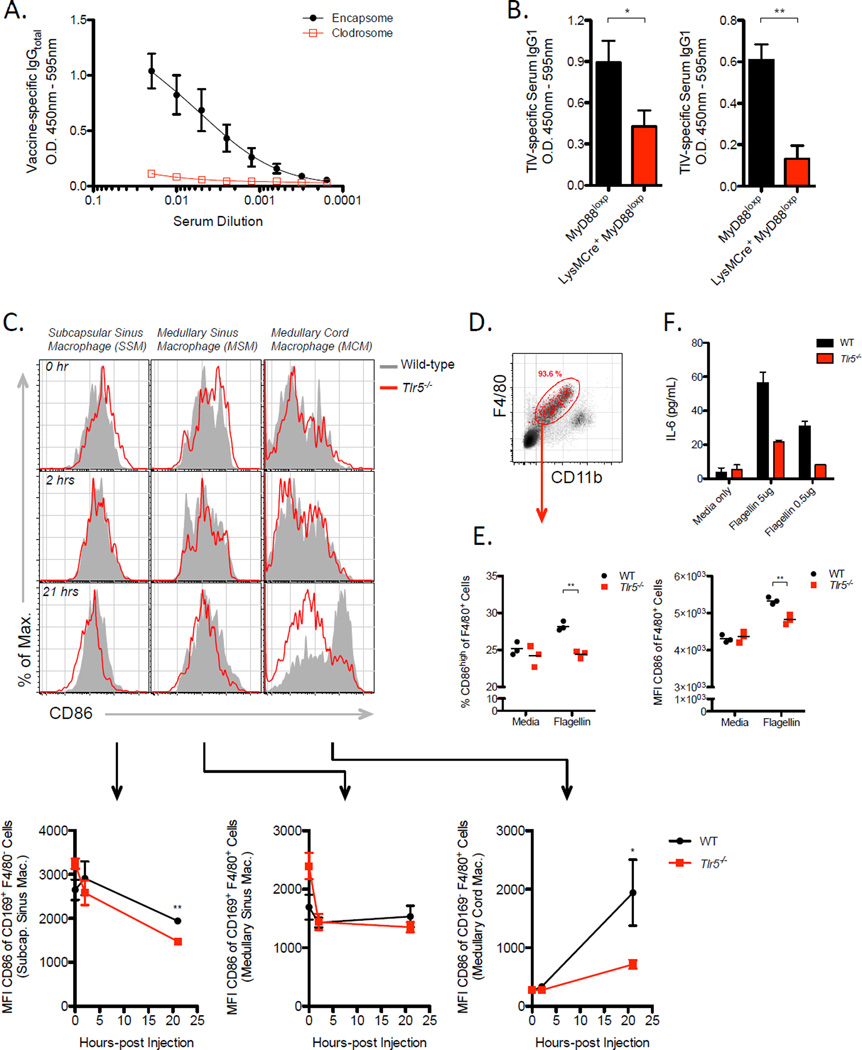
To further investigate the cellular targets of TLR5 signaling during the induction of antibody response to TIV, WT or Tlr5−/− mice were irradiated and reconstituted with either WT or Tlr5−/− donor bone marrow cells. Chimeric mice reconstituted with TLR5-deficient donor cells (WT ➔ KO) mounted significantly lower antibody response to TIV than mice reconstituted with WT donor cells (WT➔WT) (Figure 5A). Consistent with our initial observations in WT and Tlr5−/− mice (Figure 1B), chimeric Tlr5−/− mice reconstituted with Tlr5−/− donor cells (KO➔KO) exhibited significantly lower antibody response to TIV than WT➔WT mice (Figure S4A). In contrast, WT mice reconstituted with Tlr5−/− donor cells (KO➔WT) mounted an antibody response comparable to the KO➔KO mice, which suggest that TLR5 expression in a hematopoietic cell type is critical and its expression in non-hematopoietic cells is insufficient to mediate antibody responses to TIV. Interestingly, the reciprocal chimerism (WT➔KO) resulted in antibody responses similar to the WT➔WT mice, which indicates that TLR5 expression in hematopoietic cell types is sufficient to mediate vaccine-induced antibody response to TIV. Thus, TLR5 expression in non-hematopoietic cells is likely dispensable. Consistent with these results, we found similar levels of TIV-specific antibodies induced in VillinCre Myd88loxp mice, which specifically lack the expression of MyD88 in epithelial cells lining the intestinal tract (Figure S4B). Together, the data suggest a critical role of TLR5 in hematopoietic cells.
Figure 5. Cellular Targets of TLR5 Activation following TIV vaccination.
(A) TIV-specific IgG antibody response in WT or Tlr5−/− bone marrow chimeric mice. Raw O.D. values were obtained using serum diluted at 1:100 and expressed as means ± SEM.
(B) TIV-specific IgG concentrations in antibiotics-treated or untreated mice vaccinated with TIV alone or mixed with flagellin (day 7). Raw O.D. values were obtained using serum diluted at 1:100 and expressed as means ± SEM. Data are representative of three independent experiments. (C–F) WT mice were vaccinated with TIV alone or mixed with flagellin. dLNs were examined on day 7-post vaccination.
(C) Dot plots shown represent a population of CD45+TCRβ− gated cells (top panel) and concentrations of intracellular IgG in CD138+ B cells (bottom panel).
(D) Levels of intracellular IgG in CD138+ B cells are represented as the mean fluorescence intensity values.
(E) Relative frequencies IgG producing CD138+ B cells
(F) Relative frequencies of plasmablasts (CD138+ B220+) and short-lived PCs (CD138+ B220low) as depicted in (C, top panel).
(G) TLR5 expression in naïve B cells and plasmablast/short-lived PC (Pb/SL-PC) cells analyzed by qRT-PCR. Data represent mean TLR5 mRNA (normalized to GAPDH) in arbitrary units ± SEM. Data shown are one of three independently sorted B cell samples assayed in triplicates. (H) TLR5 expression in in vitro stimulated B cells analyzed as in (G). Data shown are representative of two independent experiments.
(I–L) In vitro stimulated B cells in the presence of flagellin or LPS following 6 days in culture.
Data shown are representative of two independent experiments.
(I) Representative dot plots depicting gating and relative frequencies of CD138+ B cells.
(J) Relative frequencies of CD138+ B cells (plasmablast/SL-PC).
(K) Amount of secreted of IgG and IgM (L) in culture supernatant samples assayed by ELISA at a dilution of 1:400. Data are expressed as the means ± SEM. See also Figure S5.
TLR5-mediated signaling pathway does not require dendritic cells
The cellular distribution of TLR5 expression has previously been previously characterized, albeit its functional expression remains unclear (Carvalho et al., 2012; Gewirtz et al., 2001; Gururajan et al., 2007; Letran et al., 2011). The distribution of TLR5 expression can range from cells of the innate immune system such as dendritic cells (DC), macrophage, as well as non-hematopoietic cells such as gut epithelial cells. Given the above findings that TLR5 appears to play a critical role in hematopoietic cells, we hypothesized that it serves important functions in dendritic cells following vaccination. To test this possibility, we utilized mice that selectively lack MyD88 expression in DCs. Targeting MyD88 was ideal in lieu of TLR5 floxed strains, because the alternative receptor for flagellin, NLRC4, was not necessary to mediate TIV-induced antibody responses (Figure S4C). However, vaccination of Cd11cCre Myd88loxp mice resulted in only marginally reduced antibody responses to littermate control mice (Figure S5A). Consistently, activation of DCs in the dLN examined 3 to 18 hours after injection with TIV did and measured by upregulation CD86 and MHC class II molecules was normal (Figure S5B, S5C). Moreover, co-incubation of splenocytes directly with TIV in vitro did not result in CD86 and CD80 upregulation (Figure S5D). Together the data suggest that the TLR5-MyD88 pathway is not critical in DCs during humoral immune responses to TIV. In fact, DT-mediated depletion of DC populations in zDC-DTR bone marrow chimeric mice (Figure S5E) demonstrated that DCs were dispensable during antibody responses to TIV (Figure S5F).
Flagellin enhances the generation of SL-PCs in vivo via direct TLR5 stimulation on activated B cells
To further investigate the direct cellular targets of TLR5 signaling, we examined the effects of exogenously providing the TLR5 signal in vivo. Co-injection of flagellin and TIV in antibiotic-treated mice fully rescued the TIV-specific antibody response within 7 days of vaccination so that is was comparable to that in untreated mice (Figure 5B). These results suggest that the reduced antibody response in antibiotic-treated mice is not due to an inherent defect in the immune system, but rather due to the absence of commensal-derived flagellin. Based on these observations, we further hypothesized that flagellin enhances short-lived plasma cell responses to TIV. Administration of TIV with flagellin resulted in higher frequencies of CD138+ B cells with a greater proportion of these B cells producing isotype-switched IgG antibodies (Figure 5C-5E). Specifically, flagellin enhanced the presence of CD138+ B220low short-lived plasma cells (SL-PC); whereas, CD138+B220+ plasmablasts remained unaffected (Figure 5F). Together, the data suggest that flagellin enhances early antibody responses by promoting the differentiation or survival of activated B cells into SL-PCs.
Therefore, we next explored the possibility that flagellin signaled through TLR5 on B cells, and hypothesized that TLR5 signaling directly enhanced the differentiation of or antibody production in SL-PCs. Although, previous studies by us and other groups have shown that naïve B cells lack TLR5 expression (Dorner et al., 2009; Gururajan et al., 2007), whether TLR5 is expressed in activated B cells following antigen encounter remains unknown. To address this question, purified plasmablasts and SL-PCs (IgM- IgD- CD138+B220+/low) from the dLN of vaccinated mice were analyzed by qRT-PCR. Significant upregulation of TLR5 expression was observed in these cells as compared to naïve B cells (Figure 5G). Consistently, we also found that polyclonal activation of purified splenic B cells in vitro resulted in progressive upregulation TLR5 expression in culture (Figure 5H). TLR5 expression observed in plasma cells is consistent with prior findings in gut B cells and marginal zone B cells (He et al., 2010; He et al., 2007). Intriguingly, stimulated B cells in the presence of flagellin in vitro resulted in enhanced generation of plasma cells and secretion of Ig (Figure 5I-5L). These results are consistent with previous reports on the effects of flagellin in promoting Th2 responses via MyD88-dependent mechanisms (Didierlaurent et al., 2004; Sirard et al., 2009). Together, the data suggest that flagellin directly acts on activated B cells and promotes plasma cell responses.
Macrophages play critical roles in mediating the antibody response to TIV
Although we observed direct effects of flagellin on B cells, vaccination of Cd19Cre Myd88loxp mice resulted in only a modest decrease in magnitude of the antibody response (Figure S5H). This partial phenotype led us to speculate that flagellin may engage multiple cellular pathways and, thus, we explored whether MyD88 signaling in other cell-types were crucial. We have shown data that suggest DCs are not critical in mediating the antibody response to TIV. However, we also have evidence that TIV-specific IgG response is T-cell dependent, because vaccination of Tcrα−/− mice did not yield detectable vaccine-specific IgG (Figure S5G). Therefore, we reasoned that there must be an antigen-presenting cell other than DCs promoting the humoral immune response to TIV. We hypothesized that macrophages served important APC functions towards stimulating B cell responses and targeted these cells for depletion using clodrosome (clodronate-liposomes). Indeed, macrophage-depleted mice failed to mount any detectable vaccine-specific IgG antibody response by day 7 following vaccination (Figure 6A). Furthermore, we found that macrophages expressed more TLR5 relative to naïve B cells (Figure S6A) and, therefore, subsequently tested whether the TLR5-MyD88 signaling pathway played a critical role in macrophages. Vaccination of LysMCre Myd88loxp mice resulted in significantly reduced antibody responses to TIV relative to littermate control mice (Figure 6B). Consistently, we found that macrophages in the dLN were activated by flagellin in vivo. Specifically, cells phenotypically consistent with medullary cord macrophages in the LN (CD 169− F4/80+ of CD11b+CD11c− gated cells) (Gray and Cyster, 2012) were found to express higher amounts of CD86 (Figure 6C) and produce significant quantities of tumor necrosis factor-α (TNFα), interleukin-6 (IL-6), and a proliferation-inducing ligand (APRIL) (Figure S6B–S6E) in a TLR5-dependent manner. Moreover, we found that flagellin directly activated macrophages via TLR5 in vitro (Figure 6D) as marked by upregulation of CD86 (Figure 6E) and enhanced the production of IL-6 (Figure 6F). Together, our results suggest that in addition to the direct effect flagellin on plasma cells, TLR5 signaling on macrophages are critical for antibody production.
Figure 6. Flagellin activates multiple subsets of macrophages in the LN and induces IL-6 production in macrophages via TLR5.
(A) TIV-specific serum IgG levels at day 7-post vaccination in WT mice pre-treated with clodrosome or encapsome. Data shown are representative of two independent experiments and are expressed as the means ± SEM.
(B) TIV-specific serum IgG1 levels in LysMCre+ MyD88loxp or Cre- MyD88loxp mice at day 7-post vaccination. Two independent experiments are shown. Raw O.D. values were obtained using serum diluted by a factor of 1:100.
(C) Levels of CD86 expression on macrophage subsets in the dLN of mice following flagellin injection. The three major macrophage subsets in the LN were examined based on expression profiles of the following surface markers: CD169+F/80− (SSM), CD169+F4/80+ (MSM), CD169-F4/80+ (MCM) within CD11c-Ly6C- gated population of cells. Shown are representative histograms of surface CD86 levels (top) and the kinetics of CD86 MFI values shown as the means ± SEM (bottom).
(D–F) Flow-sorted macrophages were stimulated in vitro with flagellin for 24 hours.
(D) Representative dot plot depicts purity of macrophage isolation expressed in percent of gated cells.
(E) Relative frequencies of CD86+ cells or MFI values of CD86 expression
(F) Levels of IL-6 in culture supernatants expressed as means ± SEM. Data shown are representative of three independent experiments. See also Figure S6.
Antibody responses to adjuvanted or live-attenuated vaccines are TLR5 and microbiota-independent
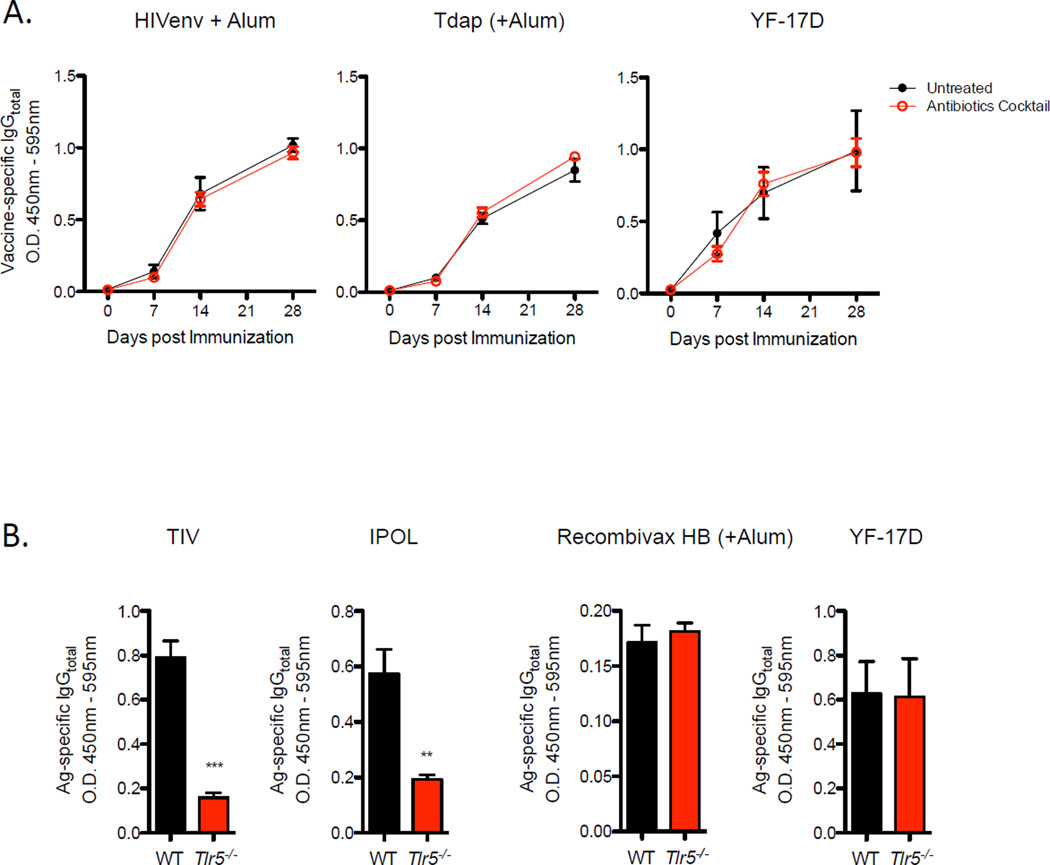
As highlighted above, TIV is an unadjuvanted vaccine. In addition, we demonstrated that co-injection of flagellin with TIV restored antibody responses in antibiotic-treated mice (Figure 5B). Together, it raised the possibility that adjuvanted or live attenuated vaccines may not rely on microbiota-derived signals to promote the antibody response. To address this question, we evaluated immune responses to several types of vaccines. When purified HIVenv protein adsorbed in alum were injected into antibiotic-treated or untreated mice, comparable amounts of antigen-specific IgG responses were observed (Figure 7A). Similarly, antibiotic-treatment did not affect antibody responses to the FDA-approved Tdap vaccine (Tetanus-Diphtheria-Pertussis), which consists of purified toxoids also adsorbed in alum. Additionally, vaccination using the live-attenuated yellow fever vaccine (YF-17D) failed to exhibit differences in magnitudes of antibody responses between untreated and antibiotic-treated mice. Consistently, YF-17D and Recombivax HB (alum-adsorbed recombinant hepatitis B antigens) vaccines induced comparable amounts of antigen-specific IgG responses in Trl5−/− and littermate WT mice (Figure 7B). Together, the data suggest that adjuvanted vaccines are not dependent microbiota-derived TLR5 signals to mediate the antibody response.
Figure 7. Level of Impact Microbiota Invokes on Antibody Responses Is Vaccine-specific.
(A) Magnitude of vaccine-specific IgG responses in antibiotics treated or untreated mice. Raw O.D. values shown were obtained using serum diluted by a factor of 1:100. Data are expressed as the means ± SEM. Data shown are representative of two independent experiments.
(B) Levels of vaccine-specific serum IgG in Tlr5−/− or littermate WT mice. Serum samples diluted by 1:50 were assayed on day 7 (TIV, IPOL) and day 14 (Recombivax HB, YF-17D) post vaccination. Data shown are representative of two independent experiments.
However, whether the dependence on microbiota was specific for TIV-induced antibody responses or a mechanism required by other unadjuvanted protein vaccines remained unknown. To this end, we evaluated immune responses to the polio vaccine, IPOL. The polio vaccine is similar to TIV in regards to being an inactivated, purified viral subunit vaccine. Vaccination of Tlr5−/− with IPOL resulted in significantly reduced IgG antibody response similar to the effects on immunity to TIV (Figure 7B). These results reveal an important role for microbiota in controlling vaccine immunity (Figure S7), particularly on immunity induced by subunit vaccines containing weak or no adjuvants.
DISCUSSION
Emerging evidence diverse roles for the microbiota in influencing host health, the most notable of which involves development and homeostasis of the immune system (Atarashi et al., 2013; Atarashi et al., 2011; Hall et al., 2008; Ivanov et al., 2008; Mazmanian et al., 2005; Rakoff-Nahoum et al., 2004). Recent reports immunity to infections can be impacted by the microbiota (Abt et al., 2012; Ichinohe et al., 2011; Naik et al., 2012). Data from our current study suggest an unappreciated role for the intestinal microbiota in enhancing vaccine immunity. Using both germ-free mice and antibiotics, we demonstrated that the microbiota was critical for the induction of short-lived PC responses as well as long-lived PCs in the bone marrow following vaccination with TIV. Furthermore, the recall of memory B cells was impaired.
In addition, our data suggest that there may be a requirement of multiple types of microbial communities, rather than specific bacterial species. Use of antibiotics, such as vancomycin or polymixin B, to target Gram-positive and Gram-negative bacteria revealed that both types of bacteria play important roles in mediating immune responses against TIV. Therefore, multiple groups or communities of bacteria may be required to support humoral immunity.
A surprising aspect of our studies is the far-reaching effect of intestinal bacteria on immunity to parenteral vaccines. Our studies in mice utilized both i.m. and subcutaneous (s.c.) injections of TIV and IPOL, and we found that vaccine-induced short-lived PC responses were restricted to the locally draining LN (unpublished data). In contrast, a recent study demonstrated that immune responses in skin-draining lymph nodes were influenced by commensal flora of the skin and not by gut-resident microbiota (Naik et al., 2012). However, other studies have shown that depletion of gut-microbiota can enhance host susceptibility to viral infections systemically (Abt et al., 2012) and in the lung (Ichinohe et al., 2011). Hence, capacity of the microbiota to influence immunity may be more pervasive than previously appreciated.
We further demonstrated that TLR5 deficiency or antibiotic treatment did not affect alum-adjuvanted or live-attenuated vaccines, while unadjuvanted vaccines such as TIV and IPOL induced antibody responses through TLR5 and microbiota-dependent mechanisms. However, these results are in contrast to previous reports that show antibiotic treatment diminishes adaptive immune responses to LCMV (Abt et al., 2012) and live influenza viruses (Ichinohe et al., 2011), because live viral pathogens can be expected to signal via multiple innate pathways much like vaccine adjuvants. This apparent discrepancy can be explained, in part, by differences in the route of infection versus immunization utilized in these studies. Furthermore, mechanisms underlying immune responses elicited by vaccine adjuvants may be distinct from pathways affected during an infection by pathogenic agents.
The stimulatory capacity of flagellin to induce both humoral and cellular immune responses has been well characterized (Didierlaurent et al., 2004; McSorley et al., 2002). How flagellin in the gut is influencing B cell response in the periphery remains less clear, but some evidence suggests that direct translocation must occur and that flagellin itself or other downstream signaling pathways following its translocation must be engaged not in the gut associated tissues but rather at the site of B cell priming following vaccination. The first line of evidence supporting this hypothesis is that the expression of TLR5 has been shown on the basolateral surfaces of gut epithelial cells rather than the lumen-facing apical cell surface (Abreu, 2010; Gewirtz et al., 2001). Moreover, signaling via TLR5 in gut-associated tissues may not be necessary or sufficient to mediate the antibody response, because gut-epithelial specific deletion of MyD88 did not affect antibody responses following vaccination. Furthermore, vaccine-specific antibody secreting cells were detected only in dLNs and not in gut-associated tissues such as the mesenteric LNs, peyer’s patch, small and large intestinal lamina propria (unpublished data). These observations suggest that vaccine-specific B cells were not being primed or migrating to sites that might be more proximal to the source of flagellin. Therefore, B cells likely do not come in contact with flagellin that immediately translocated across the intestinal epithelium. Instead, flagellin or other secondary signals likely influence responses in the peripheral LNs
Although we failed to observe a requirement of MyD88 signaling in DCs during antibody responses to TIV, we detected rapid upregulation of TLR5 in activated B cells and enhanced plasma cell responses to flagellin in vivo and in vitro. Previous studies have shown that TLR signaling on B cells is important for mediating antibody responses to certain adjuvanted vaccines (Kasturi et al., 2011; Pasare and Medzhitov, 2005). Consistently, our data support the notion that flagellin acts directly on activated B cells. However, B cell-specific deletion of MyD88 resulted in only modest reduction of TIV-specific antibodies. Thus, we reasoned that there are likely redundant cellular pathways for flagellin and evaluated whether other APCs functioned in TLR5-dependent manner. We showed that macrophages are indeed critical mediators of antibody response to TIV. Although contradictory evidence exists in the literature regarding TLR5 functionality in macrophages, we detected TLR5 expression in macrophage cells in the LN.
A major implication of our findings for global public health is the possibility that the microbiota plays a role in immune responses to vaccines. The status of the host microbiota may be a critical determinant of vaccine efficacy and alteration of mirobiota through antibiotic exposure could negatively impact vaccine efficacy. Furthermore, vaccines are less effective in many parts of developing countries compared to industrialized areas, and this may in part be due to multiple factors affecting the microbiota (Pulendran and Ahmed, 2011). Our results predict that diet, nutrition, metabolic diseases, pre-existing gut-associated pathologies, and other compounding factors affecting the microbiota may in turn affect the capacity of current and future vaccines to establish immunity.
Finally, these results highlight the value of using systems approaches, not only to identify molecular signatures of vaccine efficacy, but also to delineate critical mechanistic insights about host immunity to vaccination (Nakaya et al., 2011; Querec et al., 2009).
EXPERIMENTAL PROCEDURES
Mice and Immunizations
C57BL/6 (Charles River Laboratory, Jackson Laboratory), Tlr5−/− and littermate wild-type mice were bred and housed at Emory University and Georgia State University (Vijay-Kumar et al., 2010a; Vijay-Kumar et al., 2010b), and immunized as indicated in Supplementary information.
Antibiotic Treatment
Mice were treated with a cocktail of broad-spectrum antibiotics (neomycin, 1 g/L; ampicillin, 1 g/L; vancomycin, 0.5 g/L; metronidazole, 1 g/L) (Sigma Aldrich) dissolved in drinking water for four weeks prior to immunizations (Rakoff-Nahoum et al., 2004). Polymixin B Sulfate (USB Corporation) was administered at a concentration of 0.1 g/L.
In vitro Stimulation of B cells
B cells were purified from wild-type spleen using anti-mouse CD19 coated microbeads (Miltenyi). Cells were cultured at 37°C in culture medium containing a cocktail of the following reagents at the indicated final concentrations: rat anti-mouse IgM (2 µg/mL, Southern Biotech), recombinant mouse IL-4 (R&D, 10 ng/mL), recombinant mouse IL-5 (R&D, 10 ng/mL), recombinant mouse CD40L (R&D, 100 ng/mL), and recombinant mouse IFNγ (R&D, 5 ng/mL). Cultured cells were isolated by centrifugation using Histopaque-1077 (Sigma Aldrich).
Macrophage Depletion
Macrophages were depleted using clodronate-liposome (Clodrosome) or control-liposome (Encapsome) reagents purchased from Encapsula NanoSciences. C57BL/6 mice were injected intravenously on days −2 and −1 prior to vaccination.
In vitro Stimulation of Macrophages
Macrophages were purified from collagenase-digested spleens of naïve WT or Tlr5−/− mice using a flow-sorter based on TCRβ-CD19-CD11b-F4/80+ expression profile. Macrophages were stimulated in vitro with flagellin for 20 to 24 hours at 37°C.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Arlette Darfeuille-Michaud for providing LF82 strains; R.L. Toennisson and M. Bower for technical assistance with gnotobiotic studies; B. Cervasi and K.P. Gill for macrophage and B cell sorting. This work was supported by grants from the NIH (HHSN272201400004C, 2U19 AI057266-11, 5R37 DK057665-12, 2R37 AI048638-09) (B.P.). Additional support was provided by grants from the NIH (DK099071 and DK083890) (A.T.G.), Research Fellowship award from the Crohn’s and Colitis Foundation of America (B.C.), NIH (AI085263) and the Burroughs Wellcome Fund (F.Y.), NIH (P40 OD010995, P30 DK34987) and Crohn’s and Colitis Foundation of America (R.B.S., NGRRC), Research Fellowship award from the Influenza Pathogenesis and Immunology Research Center – CEIRS (J.Z.O).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
J.Z.O., B.P. designed experiments and wrote the manuscript. J.Z.O., R.R., B.C., M.S.M., F.Y. conducted experiments. B.C., F.A.C., M.B., K.P.G, provided reagents and technical assistance. P.H. genotyped mice. F.Y., R.B.S., A.T.G., B.P. provided reagents and edited the manuscript.
REFERENCES
- Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nature reviews. Immunology. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, Paley MA, Antenus M, Williams KL, Erikson J, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho FA, Koren O, Goodrich JK, Johansson ME, Nalbantoglu I, Aitken JD, Su Y, Chassaing B, Walters WA, Gonzalez A, et al. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell host & microbe. 2012;12:139–152. doi: 10.1016/j.chom.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices – United States, 2013–14. MMWR. 2013;62(RR07):1–43. [PubMed] [Google Scholar]
- Didierlaurent A, Ferrero I, Otten LA, Dubois B, Reinhardt M, Carlsen H, Blomhoff R, Akira S, Kraehenbuhl JP, Sirard JC. Flagellin promotes myeloid differentiation factor 88-dependent development of Th2-type response. J Immunol. 2004;172:6922–6930. doi: 10.4049/jimmunol.172.11.6922. [DOI] [PubMed] [Google Scholar]
- Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- Dorner M, Brandt S, Tinguely M, Zucol F, Bourquin JP, Zauner L, Berger C, Bernasconi M, Speck RF, Nadal D. Plasma cell toll-like receptor (TLR) expression differs from that of B cells, and plasma cell TLR triggering enhances immunoglobulin production. Immunology. 2009;128:573–579. doi: 10.1111/j.1365-2567.2009.03143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- Gray EE, Cyster JG. Lymph node macrophages. Journal of innate immunity. 2012;4:424–436. doi: 10.1159/000337007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gururajan M, Jacob J, Pulendran B. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PloS one. 2007;2:e863. doi: 10.1371/journal.pone.0000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, Grigg ME, Berzofsky JA, Belkaid Y. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–649. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- He B, Santamaria R, Xu W, Cols M, Chen K, Puga I, Shan M, Xiong H, Bussel JB, Chiu A, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nature immunology. 2010;11:836–845. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, Shan M, Chadburn A, Villanacci V, Plebani A, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. The Journal of experimental medicine. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell host & microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren O, Spor A, Felin J, Fak F, Stombaugh J, Tremaroli V, Behre CJ, Knight R, Fagerberg B, Ley RE, Backhed F. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl 1):4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostova D, Reed C, Finelli L, Cheng PY, Gargiullo PM, Shay DK, Singleton JA, Meltzer MI, Lu PJ, Bresee JS. Influenza Illness and Hospitalizations Averted by Influenza Vaccination in the United States, 2005–2011. PloS one. 2013;8:e66312. doi: 10.1371/journal.pone.0066312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letran SE, Lee SJ, Atif SM, Flores-Langarica A, Uematsu S, Akira S, Cunningham AF, McSorley SJ. TLR5-deficient mice lack basal inflammatory and metabolic defects but exhibit impaired CD4 T cell responses to a flagellated pathogen. J Immunol. 2011;186:5406–5412. doi: 10.4049/jimmunol.1003576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- McSorley SJ, Ehst BD, Yu Y, Gewirtz AT. Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. J Immunol. 2002;169:3914–3919. doi: 10.4049/jimmunol.169.7.3914. [DOI] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, Means AR, Kasturi SP, Khan N, Li GM, et al. Systems biology of vaccination for seasonal influenza in humans. Nature immunology. 2011;12:786–795. doi: 10.1038/ni.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nature immunology. 2011;12:509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nature immunology. 2009;10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Sasaki S, He XS, Holmes TH, Dekker CL, Kemble GW, Arvin AM, Greenberg HB. Influence of prior influenza vaccination on antibody and B-cell responses. PloS one. 2008;3:e2975. doi: 10.1371/journal.pone.0002975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirard JC, Didierlaurent A, Cayet D, Sierro F, Rumbo M. Toll-like receptor 5-and lymphotoxin beta receptor-dependent epithelial Ccl20 expression involves the same NF-kappaB binding site but distinct NF-kappaB pathways and dynamics. Biochimica et biophysica acta. 2009;1789:386–394. doi: 10.1016/j.bbagrm.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Tibshirani R, Hastie T, Narasimhan B, Chu G. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:6567–6572. doi: 10.1073/pnas.082099299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010a;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay-Kumar M, Carvalho FA, Aitken JD, Fifadara NH, Gewirtz AT. TLR5 or NLRC4 is necessary and sufficient for promotion of humoral immunity by flagellin. European journal of immunology. 2010b;40:3528–3534. doi: 10.1002/eji.201040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.