Abstract
Background
Fetal alcohol syndrome (FAS) is a leading cause of developmental disability. Active public health surveillance through medical record abstraction has been employed to estimate FAS prevalence rates, typically based on birth cohorts. There is an extended time for FAS characteristics to become apparent in infants and young children, and there are often delays in syndrome recognition and documentation. This methodological paper analyzes the age at case ascertainment in a large surveillance program.
Methods
The Fetal Alcohol Syndrome Surveillance (FASSLink) Project, funded by the Centers for Disease Control and Prevention (CDC), sought to estimate FAS prevalence rates in eight U.S. states. FASSLink used linked abstractions from multiple health care records of suspected cases of FAS. The present paper analyzed data from this effort to determine the child’s age in months at confirming abstraction.
Results
The average age at abstraction for confirmed/probable FAS cases (n=422) was 48.3 (±19.5) months with a range of 0 to 94 months. Age of ascertainment varied by state and decreased with each birth year; the number of cases ascertained also decreased in a steep stepwise gradient over the six birth years in the study.
Discussion
FAS surveillance efforts should screen records of children who are much older than is typical in birth defects surveillance. To best establish rates of FAS using medical records abstraction, surveillance efforts should focus on one-year birth cohorts followed for a fixed number of years or, if using multi-year cohorts, should implement staggered end dates allowing all births to be followed for up to eight years of age.
Keywords: Fetal Alcohol Syndrome, surveillance, record abstraction, age at ascertainment
Introduction
The Fetal Alcohol Syndrome Surveillance (FASSLink) project, funded by the Centers for Disease Control and Prevention (CDC) from 2004 through 2009, sought to determine the prevalence of fetal alcohol syndrome (FAS) among births between 2001 and 2006 in eight states. As prevalence estimates were calculated, there was a clear pattern of apparent decreasing prevalence by year. Those in the 2001 cohort had the highest prevalence (0.52 per 1,000 live births) while those born in 2006 had the lowest (0.123 per 1,000 live births) (Burd et al., 2011). In the absence of any documented secular trend of decreasing alcohol use among pregnant women, it became clear that the decreasing rates were related to study design. The design used abstraction from multiple linked health records for ascertainment of FAS in suspected cases; abstraction was conducted during the years 2004–2009. Case ascertainment required that diagnostic indicators of FAS had emerged, been documented by a physician and subsequently abstracted by members of the project. We hypothesized that in later birth years insufficient time had elapsed to allow the characteristics of FAS to emerge and be identified and documented by medical providers. In this methodological paper, we calculated and analyzed the age of children at the point of FAS ascertainment via record abstraction. The findings have important methodological implications for future FAS surveillance efforts.
Fetal alcohol syndrome (FAS) is a constellation of physical and neurobehavioral deficits resulting from prenatal alcohol exposure. Along with specific facial features, diagnosis depends on deficits in the central nervous system (CNS) that can be structural or functional, including cognitive disability, attention deficit and learning disabilities (Mattson et al., 2010). Secondary characteristics may include mental health disorders, increased risk for alcohol and other drug abuse, and difficulties with school, employment and independent living (Streissguth, 1996; Floyd et al., 2007).
Physically, three distinct criteria are needed to confirm a diagnosis of FAS: a) prenatal and/or postnatal growth retardation; b) central nervous system effects, and c) distinct facial anomalies (i.e. small palpebral fissures, smooth philtrum, thin vermillion) (Hoyme, 2005; CDC, 2009). The presentation of FAS varies tremendously, and may include not only the physical characteristics, but deficits in cognitive and psychosocial functioning that include poor habituation, delays in reaching developmental milestones, learning disabilities and/or developmental disability, and mental health concerns (Wilton and Plane, 2006).
Early and accurate identification and appropriate interventions can lead to a decrease in secondary characteristics and improve the quality of life in individuals with FAS and related conditions collectively known as fetal alcohol spectrum disorders (FASD). Difficulties in recognizing and diagnosing FAS have prevailed since it was first identified, and have led to both over and under identification (Aase, 1994; Cordero et al., 1994; May, 1991). These difficulties are due in part to the subtleties of the physical anomalies, their tendencies to change over time, cultural and ethnic differences, and the varying degrees of severity (Crocker, Vaurio, Riles and Mattson, 2011; Mattson et al., 2010). Many of the characteristics of FAS are not apparent at birth and require physical development to be accurately detected. Furthermore, FAS is a constellation of factors. As such, FAS may not be readily apparent or even diagnosed during annual or routine visits with a child’s health care provider, much less at birth. Identification may require multiple visits to providers from multiple specialties
There are three main research methodologies commonly used to estimate the prevalence of birth defects (including fetal alcohol syndrome) and other health conditions: passive case reporting systems, clinic-based studies, and population-based active case ascertainment and data abstraction (May and Gossage, 2001; Stratton et al., 1996). Passive systems for FAS surveillance have relied on birth defects monitoring programs (BDMPs) that focus primarily on the first year of life or early childhood, or use hospital discharge records (Fox and Druschel, 2003; Hymbaugh et al., 2002). It is generally understood that these systems underestimate the prevalence of FAS (Abel, Martier, Kruger, Ager, and Sokol, 1993; Jones, 1999; Stoler and Holmes, 1999). FAS is a diagnosis that is not easily identified in the first year of life nor do any specific laboratory tests exist that can confirm it by a specific biomarker. In addition, the physical characteristics of FAS rarely lead to hospitalization, thus rendering these reporting avenues less effective (Fox and Druschel, 2003; Hymbaugh et al., 2002).
Clinic-based studies generally take place in prenatal clinics (i.e., Ob/Gyn, family practice, public health) where information is collected from mothers while they are pregnant. In addition to alcohol consumption, other factors that can impact neonatal health including diet, psychological health, tobacco and other drug use, are ascertained using standardized screening instruments and specimen samples (May and Gossage, 2001). While this methodology has many advantages, the inherent disadvantage is that cases presenting in clinic settings are self-selected. However, clinical data can provide one source among others for initial case identification, as was the case in FASSLink.
Active case ascertainment methodologies are considered the ‘gold standard’ in surveillance (Honein and Paulozzi, 1999). These population-based approaches may use active review of records from diverse sources that include medical records, early intervention programs, school records, etc., and may attempt direct physical examination of potential cases following record review (Weiss, Cronk, Mahkorn, Glysch and Zirbel, 2004). The most rigorous approach to active case ascertainment specifically screens entire populations or random samples thereof, such as May et al. (2007), who conducted primary school-based FAS surveillance in South Africa, including complete medical examination of probable cases from first tier screening.
The current study utilized active case ascertainment from existing records in eight US states. Specifically, the FASSLink project used active surveillance with multiple linked record abstractions, with the goal of complete and accurate estimation of prevalence rates within specified geographic areas. Data on all positive (probable or confirmed) cases from the FASSLink project were analyzed for this methodological paper
Methods
The FASSLink project used active surveillance methods for data collection and a software based algorithm for consistent determination of a case as “confirmed” or “probable” for FAS. The FASSLink computer application integrated and processed record abstraction data from multiple sources as entered on each potential case. Details on these methods, adapted from a prior CDC sponsored study, are presented in Hymbaugh et al. (2002) and Cannon et al., (2012).
Each participating state defined case finding procedures to determine which births to prioritize for abstraction. The data access capacity of the states varied; initial identification of potential cases included birth certificate data, diagnostic registries, Medicaid claims data diagnostic codes, review of cases in high risk neo-natal clinics, screening of medical records in high volume obstetric and pediatric units, and review of charts from developmental disability diagnostic clinics. Each participating state developed a strategy to maximize identification of possible cases, within the statutory limits and capacities of their health departments (or designated agents of the state health department). Cases with entry of ICD-9 diagnostic code 760.71 (alcohol affecting fetus or newborn via placenta or breast milk) were considered “pending” cases rather than as confirmed cases; confirmation by standard criteria was required.
Individuals’ status on the three criteria areas for fetal alcohol syndrome diagnosis (facial malformations, central nervous system abnormalities, less than expected growth or size) were entered by FASSLink abstractors. Final status for each child was determined by standard algorithm, collectively combining all abstracted records for each pending case. The criteria were applied cumulatively and did not need to be met simultaneously.
To satisfy the “face” criteria, the algorithm required that the case record included documentation of one of the following:
Facial dysmorphism in at least two separate areas (i.e., short palpebral fissures, thin/narrow upper lip, smooth philtrum); or
Documented notation from a practitioner with an approved medical specialty, stating that the child had facial features consistent with FAS.
To satisfy the CNS criteria, a case record must have included:
Documentation of OFC (occipital frontal circumference) ≤ 10th percentile, or
Diagnosis of attention deficit disorder (ADD) or attention-deficit/hyperactivity disorder (ADHD), or
Diagnosis of developmental delay or intellectual disability.
To satisfy the growth criteria (relative to CDC 2006 national norms), a case record must have documented :
Birth length or weight less than or equal to the 10th centile for age and sex, or
Height or weight less than or equal to the 10th centile for age and sex, or
Weight for height less than or equal to the 10th centile for age and sex.
All cases determined to be “confirmed” (met all three criteria) or “probable” (met the face and either CNS or growth criteria) Fetal Alcohol Syndrome (FAS) based on the FASSLink algorithm (Hymbaugh et al., 2002) were included in this analysis. Additional inclusion/exclusion criteria required that the child be born within the study’s birth cohort period (2001–2006), and that a birth date was available in the final dataset to calculate the age in months of the child at abstraction. An additional requirement for the prevalence study, that biologic parents reside in the specified catchment region, was not relevant to the methodological purpose of the present analysis and was not applied.
All confirmed and probable cases of FAS were retained for analysis. To determine the variable of interest (age at ascertainment abstraction), the date of the earliest abstraction at which the case first satisfied the criteria as confirmed or probable was identified. The child’s age in months at this date was then calculated.
Results
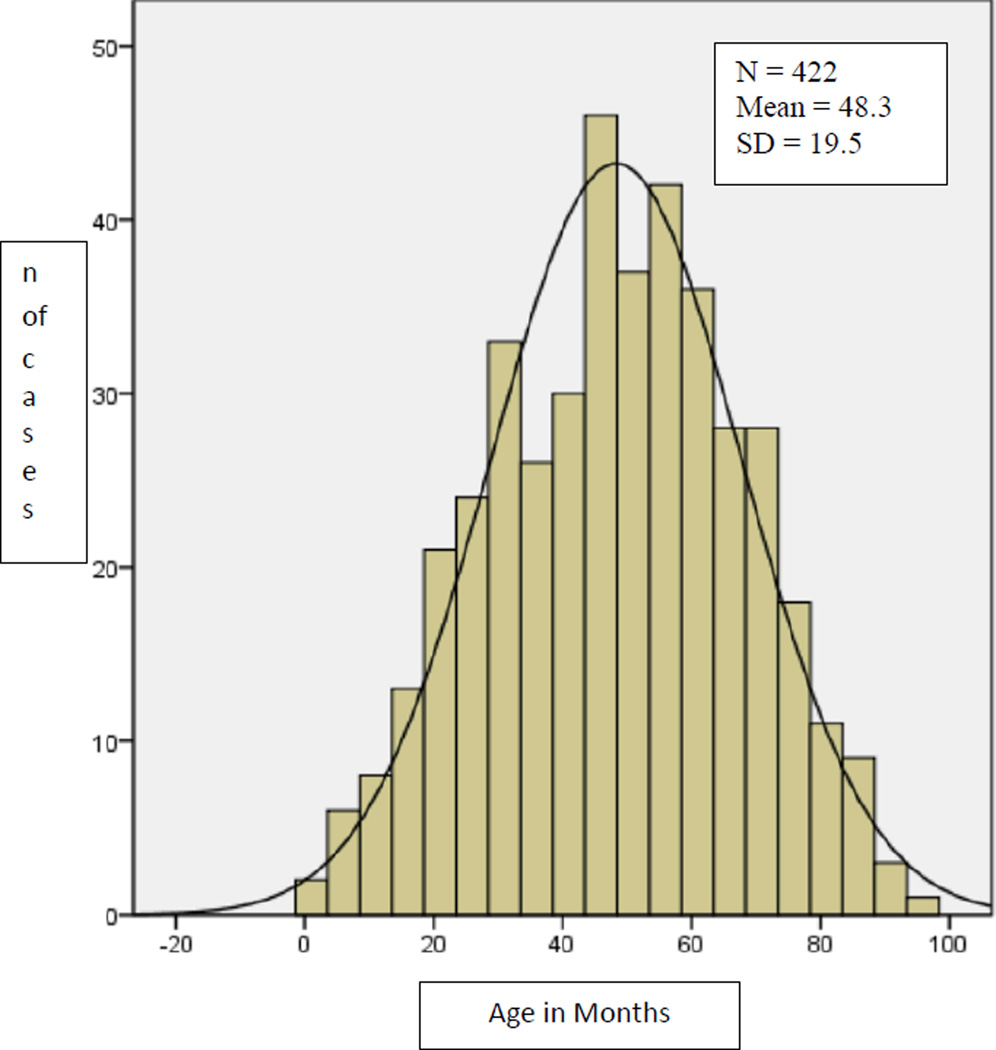
From a birth cohort of 1,322,831 births in years 2001 through 2006 in the participating states’ catchment areas, a total of 6,275 potential cases of FAS were included in the abstraction process. Of these, 422 confirmed (n=277) or probable (n=145) cases of FAS were identified via 969 separate abstractions. The mean age at confirming abstraction was 49.4. months (s.d. =19.1; range 2–94 months) for confirmed cases and 46.1 (s.d.=19.9; range 1–91 months ) for probable cases (Table 1). Since this difference was not significant (t= −1.67, 420 df, p =.096), all probable and confirmed cases were subsequently analyzed together. The final combined mean age at ascertainment was 48.3 (s.d.=19.5) As indicated in Figure 1, the distribution is fairly normal, with a slight right skewing (skewness= −.086) indicating that most children were out of the toddler stage and into the young child stage at the point where they were ascertained by the FASSLink surveillance system.
Table 1.
Age in Months at Abstraction –First Satisfaction of FAS Criteria
| Freq (%) | Age (SD) | |
|---|---|---|
| Probable Cases | 145 ( 34.4) | 49.4 (19.1) |
| Confirmed Cases | 277 ( 65.6) | 46.1 (19.9) |
| All Cases | 422 (100.0) | 48.3 (19.5) |
| Face Criteria Satisfied | 422 (100.0) | 47.5 (19.2) |
| CNS Criteria Satisfied | 376 ( 89.1) | 48.7 (18.7) |
| Growth Criteria Satisfied | 324 ( 76.8) | 44.3 (18.6) |
Figure 1.
Age in Months at Ascertaining Abstraction – Confirmed and Probable Cases of FAS
Table 1 summarizes these data and also provides the age in months at which each one of the three primary criteria were first satisfied. The number of cases is smaller for CNS and growth criteria than for the face criteria since only one of these, plus the facial criteria, was required for designation as a probable case. The growth criteria was satisfied at the earliest mean age (44.3 months), and the CNS criteria at the oldest age (48.7 months).
Among the 422 confirmed and probable cases, 175 (41%) had a formal diagnosis of FAS (ICD-9 code 760.71) in at least one abstracted clinical record. Among all of the 6,275 potential cases identified and abstracted, 488 had documentation in an abstracted record of an FAS diagnosis. Of those cases with a FAS diagnosis noted in a chart, 64 percent (313 cases) did not meet the criteria of the FASSLink algorithm.
We used OLS regression analysis to assess which potentially predictive variables were associated with age at diagnosis. Eight states participated in this FAS surveillance project. Thus there is the possibility of systematic differences due to state-level variability in data sources, case ascertainment processes, or population characteristics. Limited demographic information was available; mother’s race/ethnicity, sex of the child and the year of birth were included in the analysis. There was a large amount of missing data on maternal race/ethnicity, (n=115 or 27% of cases) so “missing race/ethnicity” was included as a category in the analysis. Final FAS status (confirmed versus probable) was also examined. Finally, year of birth was included to assess the extent to which potential elapsed time for abstraction was a factor. Table 2 summarizes these variables, showing both the distribution in the analytic population and the mean age at ascertainment for each characteristic. Note that the distributions in this sample should not be considered a representative sample of children with FAS in the United States due to selection of states and of the sub-populations and catchment areas within states which were targeted for surveillance efforts.
Table 2.
Age (in months) at Confirming Data Abstraction* Confirmed or Probable FAS (n=422)
| Freq (%) | Mean Age (SD) | |
|---|---|---|
| State: | ||
| Colorado | 36 ( 8.5) | 45.1 (21.2) |
| Michigan** | 25 ( 5.9) | 44.0 (16.8) |
| Minnesota | 114 (27.0) | 43.8 (17.7) |
| Missouri | 20 ( 4.7) | 43.2 (17.4) |
| North Dakota | 24 ( 5.7) | 41.4 (19.3) |
| Oregon | 96 (22.7) | 57.2 (19.1) |
| South Dakota | 60 (14.2) | 44.7 (20.6) |
| Wisconsin** | 47 (11.1) | 56.2 (15.8) |
| Race/Ethnicity: | ||
| White/Non-Hispanic | 138 (32.7) | 49.2 (19.6) |
| Black/Non-Hispanic | 54 (12.8) | 46.1 (18.0) |
| Hispanic | 36 ( 8.5) | 49.4 (16.7) |
| American Indian/Non-Hispanic | 55 (13.0) | 44.2 (19.7) |
| Multi Racial/Non-Hispanic | 23 ( 5.4) | 49.0 (18.4) |
| Race Not Stated/Other | 116 (27.5) | 49.4 (20.6) |
| Sex of Child | ||
| Male | 236 (55.9) | 47.9 (18.8) |
| Female | 186 (44.1) | 48.9 (20.4) |
| Year of Birth: | ||
| 2001 | 121 (28.7) | 64.1 (13.7) |
| 2002 | 98 (23.2) | 55.3 (13.4) |
| 2003 | 73 (17.3) | 47.1 (14.3) |
| 2004 | 60 (14.2) | 34.2 (13.3) |
| 2005 | 36 ( 8.5) | 29.4 (10.5) |
| 2006 | 34 ( 8.0) | 19.6 ( 9.5) |
Data abstraction occurred from 2004 through 2009 except in Wisconsin and Oregon which began in 2005.
Surveillance catchment area less than statewide; all others were statewide.
As Table 2 indicates, birth year of the child was associated with decreasing age at ascertainment in a stepwise gradient manner. Of parallel significance is that there was also a steep decrease in the number of cases ascertained with each subsequent annual birth cohort, leading to apparent decreasing prevalence estimates.
Regression results predicting age at confirming abstraction are presented in Table 3. Using South Dakota as the reference category, there were significant differences for several states. The mean age at abstraction in Minnesota was 6.8 months younger than for South Dakota; Oregon and Wisconsin had mean ages at abstraction which were significantly older (13.3 and 7.8 months, respectively). Relative to non-Hispanic white children, children of color did not differ significantly in age at ascertainment. Neither sex of child nor confirmation status (confirmed versus probable) was significantly associated with age at ascertainment. Finally, year of birth (coded from 1 to 6 to correspond to 2001 through 2006) yielded a highly significant regression coefficient (B= − 9.6, p < .001) indicating that on average each subsequent year of birth after 2001 was associated with lower age (of 9.6 months) at ascertainment.
Table 3.
Regression of Age at Confirming Abstraction On Possible Predictors (n=422)*
| B (SE) | t (Sig.) | |
|---|---|---|
| Constant | 63.7 (2.3) | 27.2 (.000) |
| State: | ||
| Colorado | 5.0 ( 2.6) | 1.91 (.06) |
| Michigan | 1.5 (2.9) | 0.52 (.60) |
| Minnesota | −6.8 (2.1) | −3.32 (.001) |
| Missouri | 1.7 ( 3.2) | 0.54 (.59) |
| North Dakota | −0.1 (2.9) | −0.04 (.97) |
| Oregon | 13.3 (2.0) | 6.53 (.001) |
| South Dakota (Reference) | --- | --- |
| Wisconsin | 7.8 (2.4) | 3.18 (.002) |
| Race/Ethnicity: | ||
| White/Non-Hisp (Reference) | --- | --- |
| Black/Non-Hispanic | 0.4 (1.8) | 0.21 (.84) |
| Hispanic | −1.9 ( 2.1) | −0.89 (.37) |
| American Indian/Non-Hispanic | 1.5 (2.1) | 0.75 (.45) |
| Multi Racial/Non-Hispanic | 1.0 (2.6) | 0.39 (.70) |
| Race Not Stated/Other | 0.1 (1.5) | 0.07 (.95) |
| Sex=Male | −0.9 (1.1) | −0.84 (.40) |
| Case confirmed (vs. probable) | −1.4 (1.2) | −1.10 (.27) |
| Year of Birth (coded 1–6) | −9.6 (0.4) | −26.8 (.000) |
R-square=.680; F(15,406 df) = 57.5, p < .001
Discussion
These results indicate that, on average, children were identified through multiple clinical record abstractions as having confirmed or probable FAS shortly after their fourth birthday. Our findings are consistent with literature suggesting that an extended time of physiologic development is required for the visible markers of FAS to become apparent (Burd and Martsolf, 1989). This clearly affected the completeness of case-finding during the latter surveillance years of the FASSLink project. For example, to complete ascertainment at the overall mean age of ascertainment across all years of this study, case ascertainment for the 2006 birth cohort would have had to continue two additional years, into January of 2011 (49 months from December of 2006). Even this two year extension would likely have under-identified cases in this one year birth cohort. If we assume that case ascertainment was relatively complete only in the 2001 birth cohort (which had about 17% of the births but about 30% of all identified cases), the mean age allowed for ascertainment would need to be even greater. In the 2001 birth year cohort, the mean age at ascertainment was 64.1 months.
The oldest age at ascertainment abstraction was 94 months (7 years, 10 months), and conceivably, with longer surveillance periods even more cases would have been found at even older ages. However, allowing for 8 years to complete a surveillance initiative and report results may not be prudent given grant cycles and the need for timely data to inform programs and policies. An alternative might be to develop an algorithm for estimated prevalence, adjusting for the number of months to abstraction with a censored or truncated end point. Targeting an older age cohort, rather than newborns, could also reduce potential under-ascertainment of cases.
In the FASSLink Project the age at ascertainment abstraction for the 70th percentile of cases was 61 months (5 years, 1 month). Prevalence estimates gathered through this age point of abstraction could be adjusted with the assumption that only 70% of likely total case ascertainment had been accomplished. A strategy such as this could allow for the reduction of optimal abstraction time. The methodology for calculating adjusted estimates based on truncated surveillance periods requires further consideration by methodologists, including use of predictive survival analysis, but may be one mechanism to maintain shorter surveillance periods.
Alternatively, a single year birth cohort could be followed for a specified number of years, or examined retrospectively at an older age (as in the CDC’s Autism and Developmental Disabilities Monitoring Network, Rice, 2009), to avoid the problems we have identified in this analysis.
Limitations
The analysis is limited by the lack of definitively complete prevalence data. Data collection for the FASSLink project had a fixed end date (December 31, 2009), applied to the entire cohort of births from years 2001 through 2006. There is a clear drop in numbers ascertained corresponding to decreased age at abstraction as birth year increased. Had abstraction continued, more cases would have been found at a later date, skewing the distribution of age at ascertainment even further to the right and increasing the mean age.
Another limitation, based on the real-world nature of this program, is that not all states began abstraction at the same time or had consistent staffing throughout the project. There was also variation in state capacity, data access, and hence initial targeting of potential cases for abstraction. It is possible that an unknown portion of the positive cases could have been identified earlier, or that the characteristics of the identified case would have been different, given other circumstances.
This analysis did not seek to test the validity of the diagnostic criteria used by practicing physicians and/or of the criteria incorporated into the FASSLink software protocol. However, a serious limitation deserving of future analysis is that 64% of cases abstracted where the FAS diagnosis was present in a chart did not meet the criteria for FAS as defined in this study. Conversely, only 41 percent of the cases determined to be confirmed or probable for FAS via abstraction were found to have an FAS diagnosis in an abstracted chart.
Implications
The implication of this research for surveillance for Fetal Alcohol Syndrome is that a significant amount of time post birth should be allotted for the estimation of true prevalence. The characteristics of FAS are not typically apparent at birth, nor are they consistent between children who are affected. In addition, many medical professionals lack experience in diagnosing FAS, thus limiting the amount of information available in medical records. Multiple visits between primary care and specialty clinics may be needed before documented diagnosis of FAS is given. Cases in the FASSLink project were confirmed as late as approaching the child’s 8th birthday. On average, cases were not identified via abstraction until after the child’s 4th birthday. Traditional birth defects surveillance systems are unlikely to identify such cases. Children born in the 2001 cohort had the highest apparent prevalence (0.52 per 1,000 live births) while those born in 2006 had the lowest (0.12 per 1,000 live births), likely due to the methodological issues analyzed in this paper.
Methodologically, active surveillance efforts may be best suited for single year birth cohorts with a fixed length of time for case ascertainment or ascertainment at a specific age. If multiple year cohorts are used then a rotating end date for each birth year should be used, allowing sufficient and standardized elapsed time for the last year of the cohort to manifest the diagnostic criteria. There is also a need to educate physicians and other health professionals regarding the diagnostic criteria, which may contribute to a reduced age at ascertainment. We trust that future efforts at FAS surveillance will benefit from these results as we seek to refine surveillance methods for this significant health challenge.
Acknowledgements
This study was funded by the Centers for Disease Control and Prevention under the Cooperative Agreement for Fetal Alcohol Syndrome Prevention (PA #03021). The data were collected under the direction of the Fetal Alcohol Syndrome Surveillance Program--FASSLink Team, whose members were: Larry Burd, Ph.D, Department of Pediatrics, University of North Dakota School of Medicine and Health Sciences (ND); Lesa A. Dixon-Gray, MSW, MPH, Women’s Health Program Coordinator, Maternal and Child Health Section, Office of Family Health, Oregon Health Authority (OR); Amy Elliott, Ph.D, Director & Senior Scientist, Center for Health Outcomes and Prevention, Sanford Research (SD); Venkata Garikapaty, PhD, Lead Maternal and Child Health Epidemiologist, Missouri Department of Health and Social Services (MO); Cheryl Lauber, PhD, RN, Nurse Consultant, Lauber Consulting (MI); D. Paul Moberg, Ph.D, Research Professor, Population Health Institute, University of Wisconsin School of Medicine and Public Health (WI); Leslie A. O’Leary, Ph.D, Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities (CDC); Judy Punyko, PhD, State Maternal and Child Health Epidemiologist, Minnesota Department of Health (MN); Margaret F. Ruttenber, MSPH, Research Scientist, Colorado Dept. of Public Health and Environment (CO).
The lead authors (Moberg and Bowser) acknowledge funding support from the Centers for Disease Control and Prevention (CDC) cooperative agreement #U84/CCU524082. The project also benefited from assistance obtained from the Clinical and Translational Science Award (CTSA) program through the National Center for Advancing Translational Sciences (NCATS) grant UL1 TR000427. The content of this article is solely the responsibility of the named authors and does not necessarily represent the official views of the NIH or the Centers for Disease Control and Prevention.
Literature Cited
- Aase JM. Clinical recognition of FAS: Difficulties of detection and diagnosis. Alcohol Health and Research World. 1994;18:5–9. [PMC free article] [PubMed] [Google Scholar]
- Abel EL, Sokol RJ. Fetal alcohol syndrome is now leading cause of mental retardation. Lancet. 1986;328(8517):1222. doi: 10.1016/s0140-6736(86)92234-8. [DOI] [PubMed] [Google Scholar]
- Abel EL, Martier S, Durger M, Ager J, Sokol RJ. Ratings of fetal alcohol syndrome facial features by medical providers and biomedical scientists. Alcoholism: Clinical and Experimental Research. 1993;17:717–721. doi: 10.1111/j.1530-0277.1993.tb00826.x. [DOI] [PubMed] [Google Scholar]
- Burd L, Martsolf JT. Fetal Alcohol Syndrome: Diagnosis and syndromal variability. Physiology and Behavior. 1989;46:39–43. doi: 10.1016/0031-9384(89)90318-1. [DOI] [PubMed] [Google Scholar]
- Burd L, Shin M, Dixon-Gray L, Elliott A, Fershteyn Z, Moberg DP, O’Leary L, Ruttenber M FASSLink Team. Population based surveillance for fetal alcohol syndrome in infants and young children. 2011 Unpublished manuscript. [Google Scholar]
- Cannon MJ, Dominique Y, O’Leary LA, Sniezek JE, Floyd RL. Characteristics and behaviors of mothers who have a child with fetal alcohol syndrome. Neurotoxicology and Teratology. 2012;34:90–95. doi: 10.1016/j.ntt.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Fetal alcohol spectrum disorders competency-based curriculum development guide for medical and allied health education and practice. 2009 http://www.cdc.gov/ncbddd/fasd/curriculum/index.html.
- Cordero JF, Floyd RL, Martin ML, Davis M, Hymbaugh K. Tracking the prevalence of FAS. Alcohol Health and Research World. 1994;18:82–85. [PMC free article] [PubMed] [Google Scholar]
- Crocker N, Vaurio L, Riley EP, Mattson SN. Comparison of verbal learning and memory in children with heavy prenatal alcohol exposure or Attention-Deficit/Hyperactivity Disorder. Alcoholism: Clinical and Experimental Research. 2011;35:1114–1121. doi: 10.1111/j.1530-0277.2011.01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd RL, Sobell M, Velasquez MM, Ingersoll K, Nettleman M, Sobell L, Mullen PD, Ceperich S, Von Sternberg K, Bolton B, Skarpness B, Nagaraja J on behalf of the Project CHOICES Efficacy Study Group. Preventing Alcohol-Exposed Pregnancies: A Randomized Controlled Trial. Am J Prev Med. 2007;32(1):1–10. doi: 10.1016/j.amepre.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox DJ, Druschel CM. Estimating the prevalence of fetal alcohol syndrome (FAS): Effectiveness of a passive birth defects registry system. Birth Defects Research (Part A) 2003;67:604–608. doi: 10.1002/bdra.10108. [DOI] [PubMed] [Google Scholar]
- Honein MA, Paulozzi LJ. Birth defects surveillance assessing the ‘gold standard.’. American Journal of Public Health. 1999;89:1238–1340. doi: 10.2105/ajph.89.8.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme EH, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon NK, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 Institute of Medicine Criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hymbaugh K, Miller LA, Druschel CM, Podvin DW, Meaney FJ, Boyle CA the FASSNet Team. A multiple source methodology for the surveillance of fetal alcohol syndrome: The fetal alcohol syndrome surveillance network (FASSNet) Teratology. 2002;66:S41–S49. doi: 10.1002/tera.90010. [DOI] [PubMed] [Google Scholar]
- Jones KL. Early recognition of prenatal effects: A pediatrician’s responsibility. Journal of Pediatrics. 1999;135:405–406. doi: 10.1016/s0022-3476(99)70157-5. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Ase F, Wutti-Ramo I, Jones KL, May PA, Adnams CM, Konovalova V, Riley EP CIFASD. Toward a neurobehavioral profile of fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research. 2010;34:1640–1650. doi: 10.1111/j.1530-0277.2010.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA. Fetal alcohol effects among North American Indians. Alcohol Health & Research World. 1991;15:239–248. [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome: A summary. Alcohol Research & Health. 2001;25:159–167. [PMC free article] [PubMed] [Google Scholar]
- May, et al. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug and Alcohol Dependence. 2007;88:259–271. doi: 10.1016/j.drugalcdep.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C, et al. Prevalence of autism spectrum disorders—Autism and developmental disabilities monitoring network, United States, 2006. MMWR. 2009;58(SS10):1–20. [PubMed] [Google Scholar]
- Stoler JM, Holmes LB. Under-recognition of prenatal alcohol effects in infants of known alcohol abusing women. Journal of Pediatrics. 1999;135:430–436. doi: 10.1016/s0022-3476(99)70164-2. [DOI] [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia Feds. Fetal Alcohol Syndrome: Diagnosis Epidemiology, Prevention, and Treatment. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Streissguth AP, Giunta CT. Mental health and health needs of infants and preschool children with Fetal Alcohol Syndrome. International Journal of Family Psychiatry. 1988;9:29–47. [Google Scholar]
- Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF. Fetal Alcohol Syndrome in Adolescents and Adults. JAMA. 1991;265(15):1961–1967. [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Kogan J, Bookstein FL. Understanding the occurrence of secondary disabilities in clients with Fetal Alcohol Syndrome (FAS) and Fetal Alcohol Effects (FAE) Centers for Disease Control and Prevention Grant No. 04/CCR008515. 1996 [Google Scholar]
- Weiss M, Cronk CE, Mahkorn S, Glysch R, Zirbel S. The Wisconsin fetal alcohol syndrome screening project. Wisconsin Medical Journal. 2004;103(5):53–60. [PubMed] [Google Scholar]
- Wilton G, Plane MB. The Family Empowerment Network: A service model to address the needs of children and families affected by fetal alcohol spectrum disorders. Pediatric Nursing. 2006;32(4):299–306. [PubMed] [Google Scholar]