SUMMARY
Trans -autophosphorylation is among the most prevalent means of protein kinase activation, yet its molecular basis is poorly defined. In Toll-like receptor and interleukin-1 receptor signaling pathways, the kinase IRAK4 is recruited to the membrane proximal adapter MyD88 through death domain (DD) interactions, forming the oligomeric Myddosome and mediating NF-κB activation. Here we show that unphosphorylated IRAK4 dimerizes in solution with a Kd of 2.5 μM and that Myddosome assembly greatly enhances IRAK4 kinase domain (KD) autophosphorylation at sub-Kd concentrations. The crystal structure of the unphosphorylated IRAK4KD dimer captures a conformation that appears to represent the actual trans-autophosphorylation reaction, with the activation loop phosphosite of one IRAK4 monomer precisely positioned for phosphotransfer by its partner. We show dimerization is crucial for IRAK4 autophosphorylation in vitro and ligand-dependent signaling in cells. These studies identify a mechanism for oligomerization-driven allosteric autoactivation of IRAK4 that may be general to other kinases activated by autophosphorylation.
INTRODUCTION
Toll-like receptors (TLRs) and Interleukin-1 (IL-1) receptors (IL-1Rs) form a large family of transmembrane receptors that function in inflammatory and innate immune signaling (Kawai and Akira, 2010; Netea et al., 2012). TLR family members are expressed in a variety of immune cells, each recognizing a distinct pathogen-associated molecular pattern such as dsRNA, lipopolysaccharides, flagellin, and unmethylated CpG DNA using extracellular leucine-rich repeat (LRR) domains. IL-1R family members recognize cognate agonistic cytokines such as IL-1β and IL-18 using extracellular immunoglobulin-like domains. Structural information on TLR-ligand complexes reveals that LRR domains form similar M-shaped dimers that bring their C-termini in close proximity to each other for signal initiation (Jin and Lee, 2008). Crystal structures of a ternary complex comprised of IL-1R, the co-receptor IL-1RAcP, and IL-1β also demonstrate adjacent receptor and co-receptor C-termini (Thomas et al., 2012; Wang et al., 2010).
Members of the TLR and IL-1R families share a common cytoplasmic Toll/IL-1 receptor (TIR) domain that is essential for the initiation of intracellular signaling (Ferrao et al., 2012; Netea et al., 2012). Ligand binding promotes receptor oligomerization, resulting in juxtaposition of receptor TIR domains and recruitment of TIR containing adapter proteins. MyD88 is the most critical of these adaptors, being essential for IL-1R signaling and all TLR family members except TLR3. MyD88 recruits IL-1R associated kinase (IRAK) family members, directly interacting with IRAK4, the most proximal IRAK, followed by the downstream kinases IRAK1 and IRAK2. Activation of IRAKs results in formation of the TRAF6-TAK1-IKK signalosome for induction of the NF-κB pathway through polyubiquitination and kinase activation (Ferrao et al., 2012). The importance of MyD88 and IRAK4 in human diseases is exemplified by the prevalence of gain of function MyD88 mutations in diffuse large B-cell lymphoma and Waldenström’s macroglobulinemia (Ngo et al., 2011; Treon et al., 2012), as well as loss of function MyD88 and IRAK4 mutations in children with recurrent pyogenic bacterial infections (Picard et al., 2003; von Bernuth et al., 2008).
MyD88 and IRAKs all contain a death domain (DD) (Figure 1A), a small α-helical domain that is highly prevalent in immune signaling complexes. Recruitment of IRAKs to MyD88 at the membrane is mediated by formation of an oligomeric DD scaffold, the Myddosome (Motshwene et al., 2009). Our previous crystal structure of this scaffold revealed 6 MyD88 DDs, 4 IRAK4 DDs, and 4 IRAK2 DDs in a helically symmetrical sequential arrangement, providing insight into the elegant ordered assembly mechanism (Lin et al., 2010). Consistent with previous results, IRAK4 is the most upstream kinase in the pathway and is capable of autophosphorylation (Cheng et al., 2007; Li et al., 2002). In addition, activated IRAK4 phosphorylates the activation loop of the downstream kinases IRAK1 and IRAK2, and MyD88 has been shown to promote this phosphorylation (Li et al., 2002).
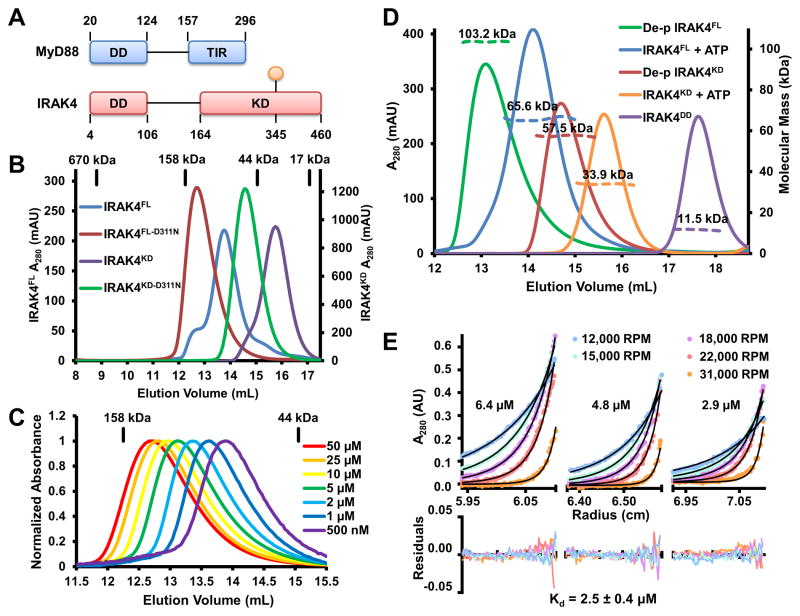
Figure 1. Unphosphorylated IRAK4 is Dimeric.
(A) Domain organization of IRAK4 and MyD88. Approximate domain boundaries are labeled with residue numbers and the prototypical phosphorylation site at T345 is shown in orange.
(B) Size-exclusion chromatograms of full-length and kinase domain of IRAK4 (IRAK4FL and IRAK4KD), and their catalytically inactive forms (IRAK4FL-D311N and IRAK4KD-D311N). Elution volumes of protein standards are indicated above.
(C) Normalized size-exclusion chromatograms of IRAK4FL-D311N at different concentrations.
(D) Size-exclusion chromatograms (solid lines) and molecular mass as measured by multi-angle light scattering (MALS) (dotted lines) of dephosphorylated (de-p) IRAK4FL and IRAK4KD, IRAK4FL and IRAK4KD after ATP/Mg2+ incubation, and the IRAK4 death domain (IRAK4DD).
(E) Sedimentation equilibrium analytical ultracentrifugation of IRAK4FL-D311N at 6.4 μM, 4.8 μM, and 2.9 μM. Samples were run at five different speeds shown in revolutions per minute (RPM). Solid black lines correspond to the fitting of a monomer-dimer self-association model, which produced a dimerization Kd of 2.5 ± 0.4 μM. Residuals are plotted below.
See also Figure S1.
Many immune signaling complexes are now known to be higher-order assemblies that function as platforms for the activation of enzymes such as kinases and caspases (Wu, 2013). Oligomerization-driven kinase activation may be mediated by autophosphorylation between two identical kinase molecules, likely in trans, although activation in cis has been implicated in other systems (Hu et al., 2013; Pellicena and Kuriyan, 2006). In the case of IRAK4, a previous report implicated a cis-autophosphorylation mechanism as wild-type (WT) IRAK4 was unable to phosphorylate a kinase dead IRAK4 in their experiments (Cheng et al., 2007).
Here, we used a combination of biochemistry, structural biology and cell biology to reveal the molecular mechanism of IRAK4 autoactivation in the Myddosome. We showed that surprisingly, unphosphorylated but not phosphorylated IRAK4 kinase domain (KD) is dimeric in solution with a modest dimerization constant. Previous structural studies on kinase autophosphorylation have been hampered by the transient nature of the process, and relied on analysis of crystal lattice interactions. The crystal structure of the IRAK4 dimer revealed in this study instead allowed visualization of the native interactions in solution, and permitted direct assessment of the role of IRAK4 dimerization in autophosphorylation and signaling. We demonstrated that IRAK4 undergoes trans-, rather than cis-autophosphorylation, and that the asymmetric unphosphorylated IRAK4KD dimer structure caught in an enzyme-substrate embrace represents a bona fide trans-autophosphorylation conformation that is promoted by the Myddosome. Our studies uncovered the proximity-enhanced allosteric changes required for IRAK4 activation, which may represent a common mechanism of kinase trans-autophosphorylation.
RESULTS
Unphosphorylated, not Phosphorylated IRAK4 Forms Dimers in Solution
In order to capture the conformation of IRAK4 in the Myddosome during the initial autophosphorylation event, we reasoned that IRAK4 should be unphosphorylated. We therefore mutated the conserved Asp311 in the catalytic loop of full-length IRAK4 (IRAK4FL) to an Asn (IRAK4FL-D311N). This residue interacts with the attacking hydroxyl side chain of the substrate and is required for phosphotransfer (Nolen et al., 2004). We expressed His-tagged IRAK4FL-D311N and co-expressed a His-IRAK4FL-D311N/MyD88DD complex in insect cells (Figure S1A) with the goal of crystallization. Surprisingly, the size-exclusion chromatography (SEC) elution position of IRAK4FL-D311N suggested that it was dimeric in solution (Figure 1B). When analyzed at different concentrations with SEC, IRAK4FL-D311N eluted as single peaks rather than two distinct monomer and dimer peaks (Figure 1C). These peaks shifted from a dimeric position towards a monomeric one as the concentration was lowered, suggestive of a dynamic monomer/dimer equilibrium.
We initially speculated that this dimerization was mediated by the DD of IRAK4, as many DDs are capable of homo-oligomerization (Ferrao and Wu, 2012). To test this hypothesis, we expressed and purified the IRAK4 KD containing the D311N mutant (IRAK4KD-D311N). Unexpectedly, IRAK4KD-D311N also appeared dimeric by SEC analysis (Figure 1B), suggesting that IRAK4 dimerization is mediated by the KD. In agreement, a construct containing only the DD of IRAK4 has an experimental molecular mass of 11.5 kDa (3.0% error) as measured by multi-angle light scattering (MALS), consistent with the 11.9 kDa calculated monomeric molecular mass (Figure 1D).
Because previously crystallized phosphorylated IRAK4 KD (p-IRAK4KD) was monomeric in solution (Kuglstatter et al., 2007), we suspected that IRAK4 dimerization is affected by the phosphorylation state of the kinase. Therefore, we expressed and purified WT IRAK4FL and IRAK4KD from insect cells. IRAK4FL was judged to be active by phosphorylation of an IRAK1 activation loop peptide (Figure S1B). The IRAK4 activation loop contains three phosphorylation sites, T342, T345 and S346 (Cheng et al., 2007), with T345 as the prototypical phosphoresidue responsible for phosphorylation-dependent activation loop stabilization and kinase activation (Kuglstatter et al., 2007). Western blot analysis with an antibody generated to react with an IRAK4 pT345-containing phosphopeptide showed that IRAK4FL was natively phosphorylated, and that this phosphorylation could be removed by treatment with λ-phosphatase (Figure S1C). Upon incubation of IRAK4FL with ATP/Mg2+, T345 phosphorylation increased (Figure S1D). In comparison, IRAK4FL-D311N had inappreciable levels of native T345 phosphorylation, and no additional phosphorylation was detected upon ATP/Mg2+ incubation (Figure S1D). Liquid chromatography-tandem mass spectrometry (LC-MS/MS) revealed that IRAK4FL is phosphorylated at T345, T342 and S346, with the most significant phosphorylation occurring at the prototypical T345 residue (Figure S1E). Importantly, both natively phosphorylated IRAK4FL and IRAK4KD appeared primarily monomeric when subjected to SEC analysis (Figure 1B).
To further determine if IRAK4 phosphorylation states play a role in the monomer to dimer transition, we first dephosphorylated IRAK4FL with λ-phosphatase. Dephosphorylated IRAK4FL was analyzed by mass spectrometry, and no residual phosphorylation was detected on any of the activation loop residues (Figure S1E). Dephosphorylated IRAK4FL is dimeric in solution, with an experimental molecular mass of 103.2 kDa (0.8% error) as measured by MALS (Figure 1D), approximating the calculated dimer molecular mass of 103.8 kDa. Incubation with ATP/Mg2+ results in autophosphorylation of IRAK4FL and a shift towards a monomeric molecular mass, 65.6 kDa (1.0% error) by MALS (Figure 1D). Similarly, dephosphorylated IRAK4KD is also dimeric with a measured molecular mass of 57.5 kDa (0.5% error) (Figure 1D), in comparison with the calculated monomer molecular weight of 34.8 kDa. Following treatment with ATP/Mg2+, autophosphorylated IRAK4KD becomes monomeric, with an experimental molecular mass of 33.9 kDa (0.8% error) (Figure 1D).
In order to investigate the thermodynamic parameters of IRAK4 dimerization, we performed sedimentation equilibrium analytical ultracentrifugation (SE-AUC) on IRAK4FL-D311N at concentrations of 6.4 μM, 4.8 μM, and 2.9 μM (Figure 1E). Centrifugation at five speeds followed by global fitting of a monomer-dimer self-association model resulted in a measured dimerization constant (Kd) of 2.5 ± 0.4 μM, a modest affinity in protein-protein interactions.
IRAK4 Trans-autophosphorylation is Enhanced by MyD88
The dimerization constant and the equilibrium behavior of IRAK4 during SEC suggest a dynamic dimerization that may not occur in cells in the resting state. Because IRAK4 gets recruited to the Myddosome through DD/DD interactions with MyD88, we next sought to understand the effect of MyD88 on IRAK4 autophosphorylation, and therefore signaling-dependent IRAK4 activation. An in vitro assay of IRAK4 autophosphorylation would require soluble recombinant MyD88. Although MyD88DD can form a well-defined complex when co-expressed with IRAK4FL-D311N (Figure S1A), MyD88DD alone has the propensity to form insoluble aggregates. Even the best behaved construct of MyD88 (20–154), containing the DD and additional linker residues, still exhibits poor solubility and forms high molecular weight aggregates when analyzed by SEC (Figure 2A).
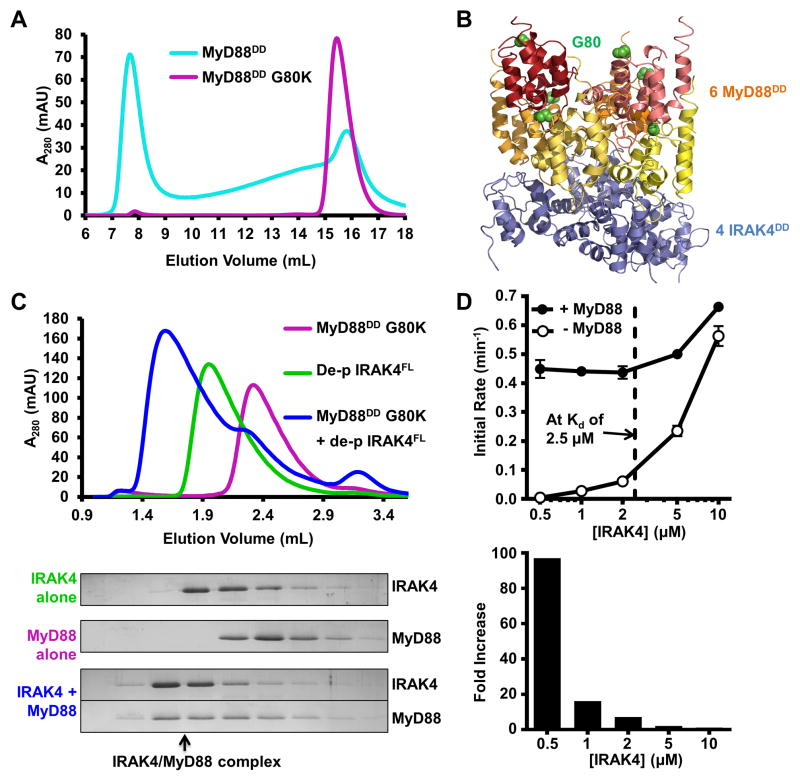
Figure 2. MyD88 Enhances IRAK4 Trans-autophosphorylation.
(A) Size-exclusion chromatograms of MyD88DD (cyan) and MyD88DD with a solubilizing mutation (G80K, purple).
(B) Structure of the Myddosome DD complex containing MyD88DD (warm colors) and IRAK4DD (blue). Positions of MyD88 residue G80 are shown as green spheres.
(C) Size-exclusion chromatograms of MyD88DD G80K (purple), dephosphorylated IRAK4FL (green), and the IRAK4/MyD88 complex (blue) formed in vitro (top). SDS-PAGE of the fractions is shown with Coomassie blue staining (below). Black arrow indicates co-migration of IRAK4 and MyD88 in a high molecular weight complex.
(D) Autophosphorylation rates of dephosphorylated IRAK4FL at different concentrations, with (black circle) and without (open circle) pre-incubation with MyD88DD G80K. Dotted black line indicates the Kd of IRAK4 dimerization. Data represent mean ± SEM.
See also Figure S2.
In the crystal structure of the ternary Myddosome DD complex (Lin et al., 2010), a layer of MyD88 interacts with a layer of IRAK4 using one surface, while simultaneously utilizing a distinct interface to mediate MyD88 homotypic interactions (Figure 2B). Therefore, we postulated that the ability of MyD88 to aggregate was dependent on the interaction between these two MyD88 layers. To disrupt MyD88 self-association, we selected residues at the MyD88/MyD88 interface while leaving the MyD88/IRAK4 interface intact (Figure 2B). The MyD88 G80K mutation successfully disrupted aggregation as assayed by SEC (Figure 2A). Importantly, MyD88DD-G80K retained the ability to assemble into a high molecular weight complex with dephosphorylated IRAK4FL (Figure 2C).
In order to understand the relationship between IRAK4 dimerization and activation, we performed autophosphorylation assays using [γ-32P]ATP with different concentrations of dephosphorylated recombinant IRAK4FL (Figure S2A). At high concentrations, IRAK4FL efficiently underwent rapid autophosphorylation (Figure 2D). The rate of autophosphorylation was highly dependent on concentration. As the concentration of IRAK4FL was lowered below that of the dimerization Kd the rate of autophosphorylation decreased significantly. (Figure 2D). At 500 nM, the rate of autophosphorylation is approximately 120-fold slower than at 10 μM. The correspondence between the concentration dependence of autophosphorylation and dimerization suggests that dimerization is a prerequisite for autophosphorylation.
Concentration-dependent autophosphorylation is indicative of trans-, rather than cis-autophosphorylation as suggested previously from the inability of WT IRAK4 to phosphorylate a kinase inactive mutant IRAK4FL-K213A/K214A (Cheng et al., 2007). However, this experiment was performed at concentrations below 400 nM for WT and mutant IRAK4, which would exhibit a negligible rate shown in the concentration series of IRAK4 autophosphorylation (Figure 2D). In further support of trans-autophosphorylation, WT IRAK4FL robustly phosphorylated the kinase inactive mutant IRAK4KD-D311N (Figure S2B, and see below).
Addition of MyD88DD-G80K to the reaction resulted in dramatic increases of autophosphorylation rates (Figure 2D). At high concentrations, the effect of MyD88DD-G80K was subtle, resulting in a 1.2-fold increase at 10 μM. The effect of rate acceleration of MyD88DD-G80K was much more pronounced at low IRAK4FL concentrations. At 500 nM IRAK4FL, addition of MyD88DD-G80K resulted in a near 100-fold increase in the autophosphorylation rate. The enhancement of IRAK4FL autophosphorylation by MyD88 is dependent on the IRAK4 DD, as MyD88 did not interact with (Figure S2C) or promote the autophosphorylation of IRAK4KD (Figure S2D). These data support the model in which kinase dimerization is required for efficient trans-autophosphorylation. Dimerization can be promoted either by increasing the kinase concentration above the dimerization Kd, or by the incorporation of IRAK4 into the oligomeric Myddosome, effectively increasing the local concentration and promoting proximity induced autophosphorylation. The linker between IRAK4 DD and KD contains approximately 57 residues; assuming an extended linker conformation, the lower limit of the local concentration of the kinase domain in the Myddosome would be ~150 μM.
Asymmetric “Enzyme-Substrate” Embrace in the IRAK4 Dimer Crystal Structure
We initially attempted crystallization of both IRAK4FL-D311N alone and the IRAK4FL-D311N/MyD88DD complex (Figure S1A), with the former giving promising initial hits. However, SDS-PAGE analysis of the IRAK4FL-D311N crystals revealed that they contained a fragment of IRAK4 that is consistent in molecular weight to the kinase domain (Figure S3A), suggesting that the protein had been proteolyzed in the linker between the DD and the KD during crystallization (Figure 1A). Given that Myddosome-induced dimerization and associated allosteric changes may be recapitulated in IRAK4KD-D311N at the high protein concentration used in vitro, we pursued crystal optimization and structure determination of the IRAK4KD-D311N dimer. Crystals of IRAK4KD-D311N in complex with a pan-kinase inhibitor, the natural microbial alkaloid staurosporine, were obtained in space group P6122, a crystal form distinct from those of WT p-IRAK4KD reported previously (Kuglstatter et al., 2007; Wang et al., 2006). The addition of staurosporine to IRAK4KD-D311N does not induce dimerization, and in fact may slightly inhibit dimer formation at low IRAK4KD-D311N concentrations (Figure S3D). The structure was solved with molecular replacement and refined to a resolution of 2.65 Å (Table 1). The asymmetric unit contains two kinase monomers (A and B), each with staurosporine bound in the active site (Figure 3A).
Table 1.
Crystallographic Statistics
| IRAK4KD-D311N Native | IRAK4KD-D311N Sulfur Anomalous | |
|---|---|---|
| Constructs | Residues R164 - S460 | Residues R164 - S460 |
| Structure determination | Molecular Replacement | Molecular Replacement |
| Data collection | ||
| Beamline | NSLS X29 | NSLS X4A |
| Space group | P6122 | P6122 |
| Cell dimensions | ||
| a, b, c (Å) | 87.6, 87.6, 421.6 | 87.5, 87.5, 424.0 |
| Wavelength (Å) | 1.075 | 2.0736 |
| Resolution (Å) | 41.81 – 2.65 (2.75 – 2.65) | 38.88 – 2.80 (2.90 – 2.80) |
| CC1/2 | 1 (0.985) | 0.999 (0.473) |
| Rmerge (%) | 12.1 (207) | 18.3 (412) |
| Mean I/σI | 37.2 (4.8) | 45.8 (2.0) |
| Completeness (%) | 100.0 (99.9) | 97.3 (79.8) |
| Total Reflections | 2,422,721 (244,494) | 5,227,241 (94,043) |
| Unique Reflections | 29,127 (2,830) | 24,250 (1,929) |
| Redundancy | 83.2 (86.4) | 212.6 (62.4) |
| Refinement | ||
| Resolution (Å) | 41.81 – 2.65 (2.75 – 2.65) | 38.88 – 2.80 (2.90 – 2.80) |
| Rwork / Rfree (%) | 20.26 / 25.06 | 18.02 / 23.97 |
| Number of atoms | 4,446 | 4,349 |
| Proteins | 4,264 | 4,264 |
| Ligands | 85 | 85 |
| H2O | 97 | 0 |
| Average B-Factor (Å2) | 62.60 | 107.10 |
| Proteins | 63.20 | 107.20 |
| Ligands | 54.10 | 100.60 |
| RMSDs | ||
| Bond lengths (Å) / Angles (°) | 0.008 / 1.11 | 0.008 / 1.15 |
| Ramachandran Plot | ||
| Favored / Outliers (%) | 95 / 0 | 91 / 0.38 |
Numbers in parentheses are for the highest resolution shell.
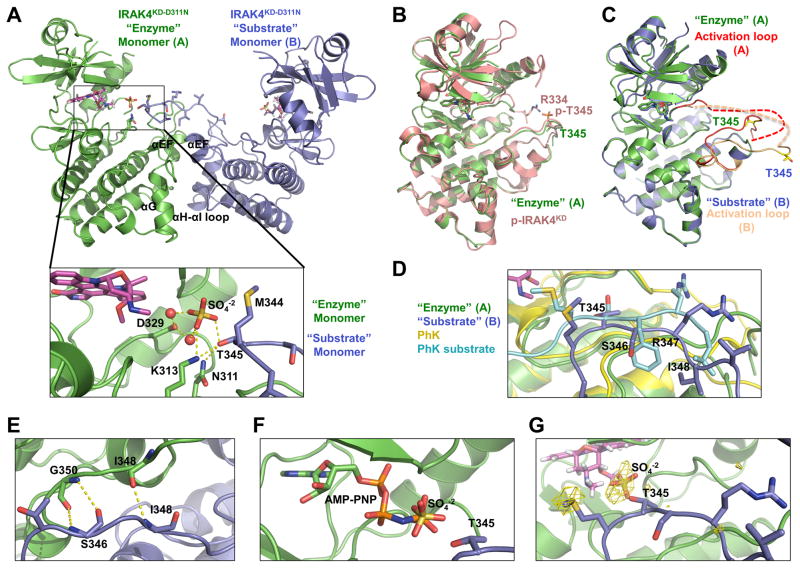
Figure 3. Asymmetric Enzyme-Substrate Embrace in the IRAK4 Dimer Structure.
(A) Ribbon diagram of IRAK4KD-D311N dimer structure, shown in green for the “enzyme” monomer A and blue for the “substrate” monomer B. The pan-kinase inhibitor staurosporine (magenta), sulfate ion (red and yellow), “enzyme” monomer catalytic residues, and “substrate” monomer activation loop side chains are shown as sticks. Detailed interactions at the active site of the “enzyme” monomer are shown (inset). Hydrogen bonds are shown as dotted yellow lines. T345 is the prototypical phosphorylation residue.
(B) Superposition of IRAK4KD-D311N “enzyme” monomer (green) with phosphorylated IRAK4KD structure (p-IRAK4KD, PDB: 2NRY) (pink).
(C) Superposition of IRAK4KD-D311N “enzyme” monomer A (green) with “substrate” monomer B (blue). Monomer A activation loop (red) and monomer B activation loop (peach) adopt distinct conformations. Side chains of T345 are represented as yellow sticks to highlight differences in conformation.
(D) Superposition of IRAK4KD-D311N “enzyme” monomer with phosphorylase kinase (PhK) (yellow) bound to a peptide substrate (teal) (PDB: 2PHK). Side chains of “substrate” monomer and PhK peptide are shown as sticks. Labels correspond to IRAK4KD-D311N “substrate” monomer residues.
(E) Anti-parallel hydrogen bonding interactions between IRAK4KD-D311N “enzyme” monomer (green) and “substrate” monomer (blue).
(F) Modeled ATP analogue AMP-PNP in the active site of IRAK4KD-D311N “enzyme” monomer based on superposition with p-IRAK4KD/AMP-PNP complex structure (PDB: 2OID). AMP-PNP, sulfate ion and T345 of the “substrate” monomer are shown as sticks.
(G) Sulfur anomalous difference Fourier of IRAK4KD-D311N structure contoured at 2.5σ (yellow).
Remarkably, the two IRAK4 monomers form an asymmetric dimer with a conformation resembling an “enzyme-substrate” embrace, in which monomer A acts as the enzyme and monomer B acts as the substrate (Figure 3A). The “enzyme” monomer contains an ordered DFG motif and an activation loop conformation similar to the p-IRAK4KD structure in complex with staurosporine (PDB code 2nry) (Figure 3B, S3B). Therefore, the enzyme monomer adopts a conformation typical of phosphorylated active IRAK4 without the prerequisite phosphorylation. Among the three phosphorylation sites of IRAK4, T345 is the prototypical phosphoresidue required for the active kinase conformation (Kuglstatter et al., 2007; Wang et al., 2006). Residue R334, which stabilizes the active conformation through its interaction with the phosphate of p-T345 in the p-IRAK4KD structure (Kuglstatter et al., 2007; Wang et al., 2006), is disordered in the enzyme monomer of the IRAK4KD-D311N structure (Figure 3B, S3B). The other two sites at T342 and S346 are nonprototypical sites of phosphorylation, and do not make crucial intramolecular interactions for maintaining the active conformation in the crystal structures of p-IRAK4KD (Kuglstatter et al., 2007; Wang et al., 2006).
Although the overall conformation of the “substrate” monomer in the asymmetric dimer is similar to the enzyme monomer with a RMSD of 0.77 Å for 248 superimposed Cα positions, part of the activation segment including the DFG motif and the activation loop showed striking differences (Figure 3C, S3C). In the substrate monomer, the activation loop is extended outward towards the active site of its partner, the enzyme monomer. Superposition of the enzyme monomer with the structure of phosphorylase kinase (PhK) bound to a peptide substrate shows a similarity in position and conformation between the PhK peptide substrate and the activation loop of the IRAK4 substrate monomer (Figure 3D), supporting the enzyme-substrate interpretation of the asymmetric IRAK4 dimer. Residues 348–350 of the enzyme monomer activation loop form an anti-parallel β-sheet with residues 346–348 of the substrate monomer activation loop (Figure 3E), consistent with the archetypal interactions involved in substrate recognition by protein kinases (Knighton et al., 1991).
Strikingly, T345 of the substrate monomer is positioned at the active site of the enzyme monomer, immediately adjacent to a bound sulfate ion in the crystal structure (Figure 3A). When AMP-PNP is modeled into the active site by superposition of the enzyme monomer with p-IRAK4 bound to the nucleotide (RMSD 0.30 Å) (PDB code 2OID) (Kuglstatter et al., 2007), the γ-phosphate is located in approximately the same position as this sulfate (Figure 3F). Additionally, T345 of the substrate kinase makes hydrogen bonds with the catalytic base residue, here mutated to N311, as well as K313 and the sulfate ion (Figure 3A). K313 interacts with the sulfate ion in the same manner that it uses to stabilize the negative charge of the γphosphate of ATP. The mode of interaction of T345 with the catalytic base residue and a positively charged residue is similar to substrate recognition by the tyrosine kinases insulin receptor kinase (Hubbard, 1997) and insulin-like growth factor 1 receptor kinase (IGF1-RK) (Favelyukis et al., 2001). In the absence of Mg2+, the Mg2+ coordinating residue D329 instead forms water-mediated hydrogen bonds with the sulfate ion. Therefore, T345 is bound in a position and environment poised for the phosphotransfer reaction.
Because the residue preceding T345 is a Met (M344), we used sulfur anomalous diffraction to verify the correct placement of the substrate monomer activation loop. Highly redundant diffraction data were collected at 6 keV, where the calculated anomalous scattering factor f″ is equal to 0.95 (Table 1). The structure was solved by molecular replacement and peaks in the sulfur anomalous difference Fourier density were readily apparent. Importantly, the anomalous difference map showed the location of the sulfur atom in the side chain of M344, confirming that the assignment of the activation loop is correct (Figure 3G).
Symmetric, Exo-site IRAK4 Dimerization at the C-terminal Lobe
The IRAK4 dimer buries ~1,160 Å2 surface area from each of the monomers as calculated using the online program PDBePISA (Krissinel and Henrick, 2007). In addition to the asymmetric activation loop interactions, the C-terminal lobes of the two kinases interact with each other at exo-sites across a non-crystallographic two-fold symmetry axis (Figure 3A, 4A). The non-crystallographic two-fold symmetry is almost perfect, with a 175.0° rotation between the two monomers. The C-terminal lobe contacts contain interactions between residues on helix αEF (the αEF exo-site), and interactions between αG of one monomer and the αH-αI loop of the other monomer (the αG exo-site) (Figure 4A). At the αEF exo-site, residues L360 and R361 contribute large buried surface area through both hydrophobic and polar interactions (Figure 4B). The interaction at the αG exo-site is highly charged, consisting of the acidic αG helix surface of one monomer and basic surface of the αH-αI loop on the opposing monomer (Figure 4C, S4A). As previously reported, helix αG exists in an unusual conformation having swung out 10 Å away from αEF (Kuglstatter et al., 2007; Wang et al., 2006). This position of αG is unique to the IRAK4 kinase and is critical for the observed dimerization interface.
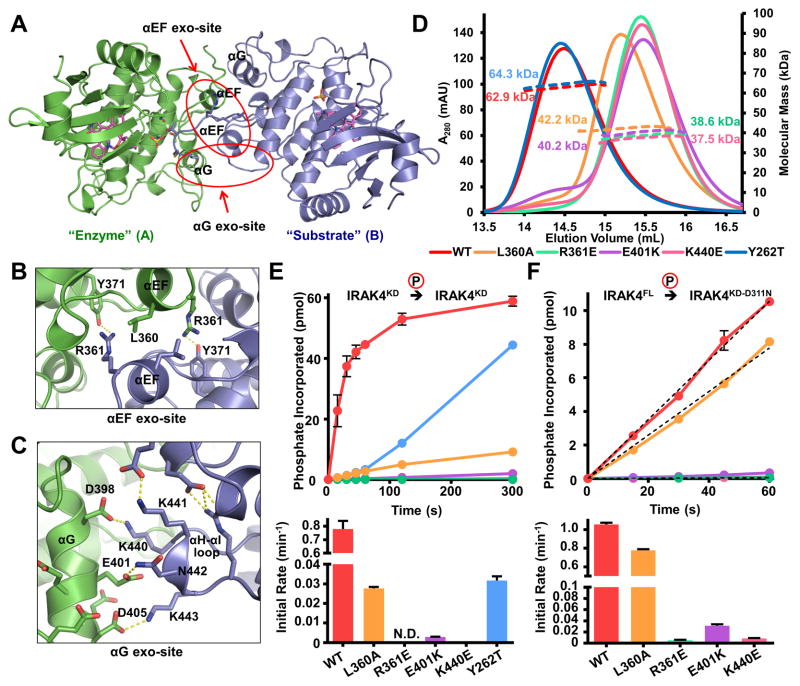
Figure 4. Symmetrical Exo-site Interactions in IRAK4 Dimerization and Autophosphorylation.
(A) Ribbon diagram of the IRAK4KD-D311N dimer viewed down the approximate dimer axis, colored as in Figure 3A. Positions of the αG and αEF helices are indicated.
(B) Detailed interactions at the αEF exo-site. Residues L360, R361, and Y371 of both monomers are labeled.
(C) Detailed interactions at the αG exo-site. Side chains from negatively charged surface of αG and positively charged αH-αI loop are represented as sticks.
(D) Size-exclusion chromatograms (solid lines) and molecular masses as measured by MALS (dotted lines) of αEF exo-site (L360A, R361E), αG exo-site (E401K, K440E) and gatekeeper (Y262T) mutations on a IRAK4KD-D311N background (WT).
(E) Autophosphorylation of 10 μM IRAK4KD (WT), IRAK4KD with αEF exo-site, αG exo-site and gatekeeper mutations as measured by autoradiography (top) and the initial rates of autophosphorylation (bottom). Data represent mean ± SEM.
(F) Trans-phosphorylation of IRAK4KD-D311N with or without αEF exo-site or αG exo-site mutations (10 μM) by IRAK4FL (1 μM) (top) and the initial rates of trans-phosphorylation (bottom). Data represent mean ± SEM.
See also Figure S4.
Analysis of residues at the dimerization interface indicates conservation among IRAK4 proteins from different species (Figure S4B), suggesting that IRAK4 dimerization is a general feature of its activation mechanism. There is no apparent sequence similarity of the IRAK4 activation loop with a physiological IRAK4 substrate, the activation loop of IRAK1 (Hekmat-Nejad et al., 2010). This observation suggests that the IRAK4 activation loop is not an optimal IRAK4 substrate, and that the exo-site interactions are needed to facilitate IRAK4 trans-autophosphorylation.
Mutations that Disrupt IRAK4 Dimerization Inhibit Autophosphorylation in Vitro
To test the importance of the structurally observed IRAK4 dimer, we used mutagenesis to disrupt IRAK4 dimerization and studied the effects of mutations on IRAK4 autophosphorylation. Residues L360 and R361 of the αEF helix, E401 of the αG helix, and K440 of the αH-αI loop were targeted for mutagenesis based on their buried surface area and predicted contribution to the interface. These mutations were introduced into the dimeric IRAK4KD-D311N. All selected mutations disrupted dimerization when analyzed by MALS (Figure 4D). The more conservative mutation, L360A, was less disruptive to dimerization than the charge reversal mutations, and resulted in a less monomeric phenotype. These data confirmed the observed dimerization interface in the crystal.
Mutations that disrupt IRAK4 dimerization were then tested for their effect on IRAK4 autophosphorylation. The interface mutations were introduced into WT IRAK4KD. Each kinase mutant was purified and dephosphorylated with λ-phosphatase. Autophosphorylation of these mutants was then assayed using [γ-32P]ATP (Figure 4E). WT IRAK4KD showed robust autophosphorylation. IRAK4KD-L360A was partially defective, while the charge reversal mutations R361E, E401K, and K440E resulted in either extremely low or undetectable levels of autophosphorylation. The differential severity between the L360A and charge reversal autophosphorylation phenotypes corresponds with the partial and complete dimerization disruption observed in these mutants. In addition, we introduced these mutations into the inactive IRAK4KD-D311N construct. They were then subjected to trans-phosphorylation by WT IRAK4FL (Figure 4F). Robust trans-phosphorylation was detected when phosphorylating catalytically inactive IRAK4KD-D311N. However the dimerization interface mutants showed attenuated phosphorylation by WT IRAK4, suggesting that disruption of dimerization impaired the ability of IRAK4 to act as a substrate. Again, the severity of the trans-autophosphorylation phenotypes correlated with the degree of dimerization disruption. The mutational effects on autophosphorylation (Figure 4E) are more severe than on trans-phosphorylation (Figure 4F), likely because in autophosphorylation, the dimerization interfaces on both partners in the dimer are compromised. Taken together, these data demonstrate that dimerization is necessary for IRAK4 autophosphorylation.
Disruption of IRAK4 Dimerization Results in Defective Signaling in Cells
To test whether disruption of dimerization impairs the ability of IRAK4 to perform its role in signaling, we utilized an IRAK4-deficient human fibroblast cell line derived from a patient with recurrent pyogenic infections (Picard et al., 2003). These IRAK4-deficient human fibroblasts were reconstituted with either Flag-tagged WT human IRAK4FL, the kinase inactive mutant IRAK4FL-K213M, IRAK4FL-L360A, or IRAK4FL-R361E. K213 is a critical ATP-binding residue at the N-terminal lobe, which is properly oriented for catalysis by an ion pair with the αC-helix conserved in all protein kinases (Nolen et al., 2004). These cells were then stimulated with IL-1β and the signaling properties were observed (Figure 5).
Figure 5. Disruption of IRAK4 Dimerization Results in Defective Signaling in Cells.
(A) Human IRAK4-deficient fibroblasts infected with retroviruses containing empty vector construct (Vector), Flag-tagged IRAK4FL wild-type (WT) and mutants (K213M, L360A, R361E, and Y262T) were treated with IL-1β (1 ng/ml) for the indicated times, followed by Western blot analyses using antibodies against p-IKKα/β, p-JNK, p-IκBα, IκBα, Flag and actin.
(B, C) RT-PCR analyses for the fold induction of TNFα (B) and IL-8 (C) expression in the same reconstituted human fibroblasts following treatment with IL-1β for the indicated times. The experiment was repeated three times. Data represent mean ± SEM.
See also Figure S5.
As reported previously, in comparison with IRAK4WT, reconstitution with IRAK4FL-K213M compromised IKK phosphorylation, IκBα degradation, and JNK phosphorylation (Figure 5A) due to decreased TAK1-dependent IKK and MAP kinase activation (Fraczek et al., 2008; Yao et al., 2007). However, lack of IRAK4 kinase activity did not significantly affect IκBα phosphorylation due to an alternative MEKK3-dependent pathway that phosphorylates but does not degrade IκBα (Fraczek et al., 2008; Yao et al., 2007). Additionally, decreases in the levels of TNFα and IL-8 mRNA were observed in the IRAK4FL-K213M reconstituted cell line (Figure 5B, 5C). Remarkably, reconstitution with the dimerization-deficient mutants IRAK4FL-L360A and IRAK4FL-R361E resulted in phenotypes similar to those of kinase inactive IRAK4FL-K213M with respect to IKK and JNK phosphorylation, IκBα degradation (Figure 5A), as well as TNFα (Figure 5B) and IL-8 mRNA expression levels (Figure 5C). Introduction of mutations into the IRAK4 kinase domain did not affect the interaction with MyD88 (Figure S5). These data demonstrate that IRAK4 dimerization is absolutely required for IRAK4 kinase activation and the associated biological functions.
Small and Wide Angle X-ray Scattering (SAXS/WAXS) of the Myddosome
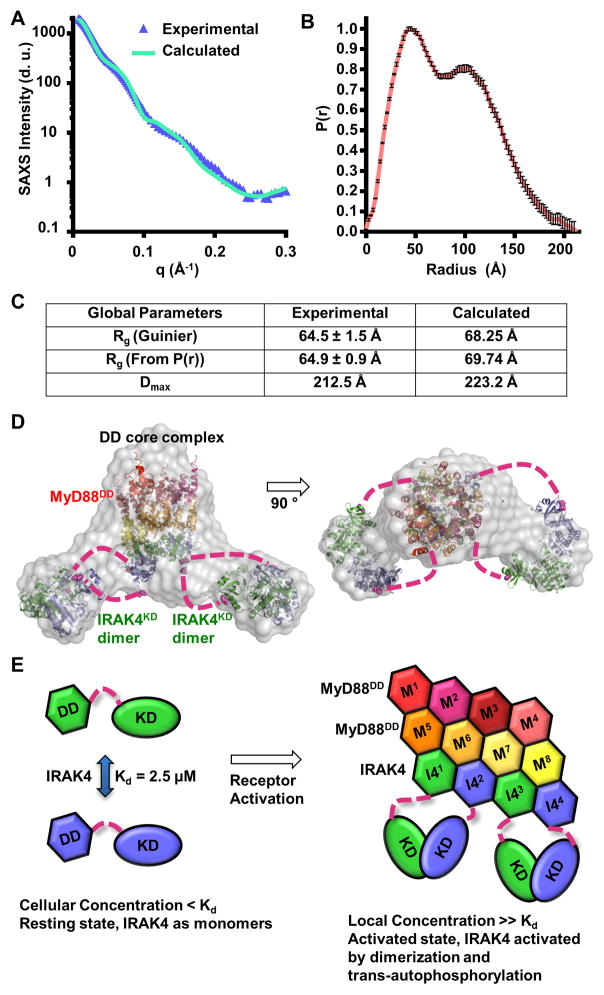
We next sought to obtain structural information on the Myddosome complex containing MyD88DD and IRAK4FL in order to understand the positioning of the kinase domains relative to the DD core. MyD88DD was co-expressed with IRAK4FL-D311N in insect cells. The complex was purified to homogeneity (Figure S1A) and subjected to SAXS/WAXS. Guinier analysis at low scattering angles indicated that the sample was free of aggregation (Figure 6A) and provided an estimated radius of gyration (Rg) of 64.5 ± 1.5 Å. The pair-distance distribution function (P(r)) obtained via indirect Fourier transformation possessed a bimodal shape (Figure 6B). The best P(r) function was obtained with a maximum linear dimension (Dmax) of 212.5 Å. The Rg of 64.9 ± 0.9 Å obtained through indirect transform closely approximated the estimated value obtained with Guinier analysis (Figure 6C).
Figure 6. Small and Wide Angle X-ray Scattering (SAXS/WAXS) of the Myddosome.
(A) Experimental scattering profile of the IRAK4FL-D311N/MyD88DD complex as a function of the scattering vector q (q = 4πsin(θ/2)/λ), where θ is the scattering angle and λ is the X-ray wavelength) after solvent background subtraction (blue), superimposed with the scattering profile calculated from the IRAK4FL-D311N/MyD88DD model in (D) (green). d.u.: detector unit
(B) Pair-distance distribution function (P(r)) of the IRAK4FL-D311N/MyD88DD complex. Data represent calculated P(r) values ± SD.
(C) Experimental and calculated radii of gyration (Rg) and maximum linear dimension (Dmax). Data represent calculated values ± SD.
(D) The chevron shaped molecular envelope (grey) from ab initio reconstruction fitted with structures of the binary Myddosome DD complex (PDB: 3MOP, 8 MyD88DD in warm colors and 4 IRAK4DD in green and blue) and 2 IRAK4KD dimers (green and blue). The IRAK4 DDs and KDs presumed to be of the same polypeptides are shown in the same green or blue colors.
(E) Schematic model of IRAK4 dimerization and activation in the Myddosome in a signal-dependent manner.
The P(r) function obtained through indirect Fourier transform was then subjected to ab initio molecular envelope calculation. Twenty independent models were generated from different random seed starting points. Of the twenty initial models, one fell outside two standard deviations and was omitted from the ensemble. The nineteen remaining models were averaged and filtered to an appropriate volume. The resulting envelope contained a central core flanked by two lobes on either side (Figure 6D). We reasoned that the envelope represented the central DD core of the Myddosome, flanked by two IRAK4 kinase dimers. Weaker densities were observed between the central core and the flanked lobes, suggesting the location of the linker between IRAK4 DD and KD. High resolution crystal structures were modeled into this molecular envelope. In the docked model, the N-terminal lobes of the KD dimers face towards the core DD complex. The distances between C-terminal tails of IRAK4 DDs and N-terminal residues of KDs are between 48 Å to 77 Å, which can be easily accommodated by the linker of ~57 residues between the two domains. The docked model shows good agreement with experimental data (Figure 6A, 6C). Formation of IRAK4 KD dimers in the Myddosome supports proximity-driven dimerization and IRAK4 activation (Figure 6E).
Molecular Dynamics (MD) Simulations
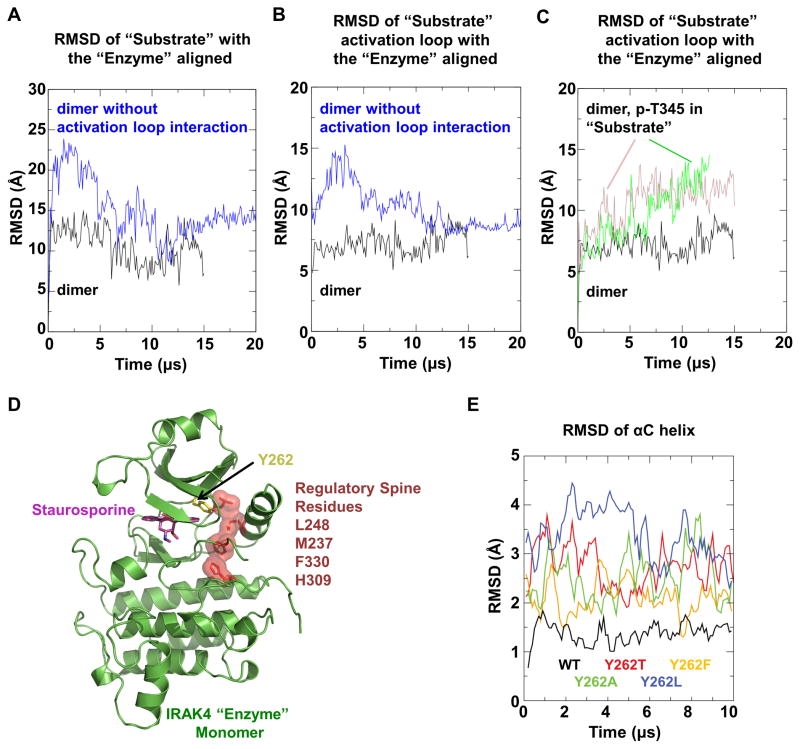
Our mutagenesis data showed that the exo-site interactions are crucial for IRAK4 dimer formation. However, direct mutagenesis of activation loop residues may lead to unforeseen effects on enzyme kinetics in addition to effects on the observed enzyme-substrate embrace. We thus turned to long-timescale MD simulations, which can reveal conformational changes on microsecond to millisecond time scales (Shaw et al., 2009). We first simulated the asymmetric IRAK4 dimer and verified its stability over the course of the MD simulation (Figure 7A-B). We then generated a hypothetical symmetrical dimer by replacing the substrate monomer with a superimposed enzyme monomer, effectively removing the asymmetric activation loop interaction while preserving symmetric exo-site interactions. MD simulations of this symmetrical dimer resulted in rapid deviations in structure, which were much more substantial than those observed in the asymmetric dimer simulations, suggesting that the activation loop interaction is important for maintaining the observed dimer configuration.
Figure 7. Molecular Dynamics (MD) Simulations.
(A, B) MD simulation of the asymmetric IRAK4KD-D311N dimer structure (black) and the hypothetical symmetrical dimer structure without activation loop interactions (blue). RMSD values of Cα atoms between the initial and simulated “substrate” monomer (A) or its activation loop (residue 329–354) (B) are shown when the “enzyme” monomer is aligned.
(C) MD simulation of the IRAK4KD-D311N dimer structure hypothetically phosphorylated at T345 in the “substrate” monomer, with (pink) and without (green) bound ADP. RMSD values of Cα atoms between the initial and simulated “substrate” monomer activation loop are shown when the “enzyme” monomer is aligned.
(D) Ribbon diagram of “enzyme” monomer in the IRAK4KD-D311N dimer structure, showing the regulatory spine residues (dark red) and the gatekeeper residue Y262 (yellow).
(E) MD simulation of the “enzyme” monomer with the hypothetical mutations in the gatekeeper tyrosine residue in comparison with WT (black). RMSD values of Cα atoms in the αC helix (residue 222–239) are shown when the initial and simulated structures are aligned with all Cα atoms.
See also Figure S6.
Our biochemical data established that activation loop phosphorylation dissociates IRAK4 dimers (Figure 1D). To deduce if T345, the canonical phosphorylation site in the activation loop of IRAK4, may be responsible for this dissociation, we simulated the stability of the enzyme-substrate embraced dimer upon T345 phosphorylation. Consistent with our biochemical data, addition of the phosphate group to T345 induced large deviations of the substrate kinase activation loop (Figure 7C), suggesting dissociation would occur at longer timescales. These data provide evidence that phosphorylation at T345 is a mechanism of IRAK4 dimer dissociation.
DISCUSSION
Kinase activities are highly regulated and, in a large subset of kinases, this regulation is implemented by phosphorylation within the activation loop (Taylor and Kornev, 2011). In these kinases, phosphorylated residues within the activation loop mediate specific intramolecular interactions that stabilize the active kinase conformation. For many kinases, this phosphorylation event is realized by trans-autophosphorylation between two identical kinase molecules, although examples of cis-autophosphorylation are also known (Hu et al., 2013).
We hypothesize that autophosphorylating kinases sample the active conformation more frequently than their non-autophosphorylating counterparts, even in the absence of activation loop phosphorylation. Protein kinases contain a highly conserved hydrophobic regulatory spine that anchors important elements for catalysis (Meharena et al., 2013; Taylor and Kornev, 2011). IRAK4 contains a unique gatekeeper residue Y262 (Kuglstatter et al., 2007; Wang et al., 2006), which packs against the regulatory spine (Figure 7D) and may stabilize the active conformation. In MD simulations, mutation of the unique Y262 to Ala or common gatekeeper residues such as Thr, Phe, or Leu, led to destabilization of the active conformation in the absence of phosphorylation (Figure 7E). Additionally, IRAK4KD-Y262T is defective for in vitro autophosphorylation (Figure 4E) without disrupting dimerization (Figure 4D), and reconstitution of IRAK4-deficient human fibroblasts with IRAK4FL-Y262T does not restore WT signaling (Figure 5). Taken together, these data suggest that this unique tyrosine gatekeeper is critical for IRAK4’s autophosphorylation activity.
Molecular mechanisms of trans-autophosphorylation have been pursued extensively. Structurally, analysis of crystal packing has revealed that several kinases known to autophosphorylate form activation loop swapped, symmetrical crystallographic dimers. These include the Ser/Thr kinase TBK1, important for interferon activation in innate immunity, the DNA damage checkpoint Ser/Thr kinase CHK2, and the tyrosine kinase IGF1-RK (Cai et al., 2009; Ma et al., 2012; Wu et al., 2008). Therefore, it has been proposed that reciprocal exchange of activation segments might be a common mechanism of trans-autophosphorylation (Oliver et al., 2007), which may explain the lack of sequence similarity between activation loops and substrates. However, because neither of the active sites is fully formed in these symmetrical dimers, it raises the question of how catalysis of trans-autophosphorylation is achieved in these conformations.
Formation of unphosphorylated IRAK4KD dimers in solution gave us the unique opportunity to unambiguously correlate the dimer interface observed in the crystal with dimerization in solution, trans-autophosphorylation in vitro with and without MyD88, and signaling in cells. Our studies led to an elegant, yet simple mechanism of trans-autophosphorylation in which one kinase assumes the active enzyme conformation while the partner kinase inserts its activation loop as a substrate. Curiously, the exo-site interactions in the IRAK4 dimer represent a commonly used allosteric site (Goldsmith et al., 2007), and likely further promote adoption of an active conformation of the enzyme kinase. For example, a similar exo-site containing the αG helix is used in recognition of the substrate eIF2α by protein kinase RNA-activated (PKR) (Seo et al., 2008) (Figure S6A). The modest dimerization constant renders IRAK4 in an inactive state at physiological concentrations and safeguards against spontaneous ligand independent autoactivation. Recruitment of IRAK4 to MyD88 to form the Myddosome dramatically increases the local concentration of IRAK4, promoting dimerization and allosteric activation (Figure 6E). Addressing allosteric changes responsible for enzyme activation in oligomerization-driven biological systems remains an important and exciting challenge for structural biologists.
The mode of trans-autophosphorylation elucidated here has been proposed previously for p21-activated kinases (PAKs) (Pirruccello et al., 2006). The active conformation of IRAK4 in the absence of phosphorylation is reminiscent of PAK1, which showed an active conformation without the cognate Thr phosphorylation (Lei et al., 2005). An asymmetric enzyme-substrate dimer was also observed in the crystal lattice of the unphosphorylated PAK1 structure (Wang et al., 2011) (Figure S6B), which suggests a common mechanism of trans-autophosphorylation. Unlike the IRAK4 dimer, the exo-site interactions in the PAK1 dimer are not symmetrical. In both cases, the extensive exo-site interactions may facilitate the positioning of the activation loops as substrates, explaining the lack of sequence similarity between activation loops and substrates. Given the highly dynamic nature of protein kinases, it is likely that a variety of different structural features are utilized to achieve autophosphorylation.
EXPERIMENTAL PROCEDURES
Cloning and Protein Purification
Human IRAK4, FL, KD, and all mutants were expressed in insect cells, while MyD88DD MyD88DD-G80K and IRAK4DD were expressed in E. coli. Proteins were purified with HisPur™ Cobalt Resin (Thermo) followed by anion exchange and size exclusion chromatography (Superdex 200 10/300 GL, GE Healthcare).
Multi-Angle Light Scattering
Samples were applied over a Superdex 200 10/300 GL column coupled to a three-angle light scattering detector (mini-DAWN TRISTAR) and a refractive index detector (Optilab DSP) (Wyatt Technology).
Sedimentation Equilibrium Analytical Ultracentrifugation
IRAK4FL-D311N was subjected to ultracentrifugation until equilibrium was reached in a Beckman Coulter Optima XL-A ultracentrifuge with an An-60 Ti rotor using six-channel centerpieces and quartz glass.
Crystallization, Data Collection and Structure Determination
IRAK4KD-D311N was co-crystallized with staurosporine in 1.6–1.9 M ammonium sulfate and 100 mM Hepes-NaOH at pH 7. Crystals were cryoprotected and flash frozen in liquid nitrogen. Data collection was performed at the National Synchrotron Light Source (NSLS).
Kinase Assays
Dephosphorylated IRAK4 was subjected to auto- and trans-phosphorylation experiments with or without precincubation with MyD88DD-G80K using [γ-32P]ATP. Phosphoproteins were separated from free nucleotides by SDS-PAGE and visualized by autoradiography.
Plasmids and Retroviruses
IRAK4 WT and mutants were cloned into pMXs-IRES-Puro and transfected into Phoenix cells for viral packaging. Human IRAK4-deficient fibroblasts were infected by the packaged retrovirus and stable viral integration was selected by puromycin.
Immunoblotting and Co-immunoprecipitation
Cell lysates were separated by 10% SDS-PAGE, transferred to Immobilon-P membranes (Millipore), and subjected to immunoblotting. For co-immunoprecipitations, cell lysates were incubated with 20 μl of protein A-Sepharose beads with anti-Flag antibody before immunoblotting.
Quantitative Real-Time PCR
Total RNA was isolated using TRIzol reagent (Invitrogen) and subjected to reverse transcription using SuperScript-reverse transcriptase (Invitrogen). Quantitative PCR was performed on an AB 7300 RealTime PCR System.
Small- and Wide-Angle X-Ray Scattering
Data were collected in triplicate on the IRAK4FL-D311N/MyD88DD complex at concentrations of 2, 1, and 0.5 mg/mL. Data were merged and scaled, followed by evaluation of the particle distance distribution function P(r) and ab initio modeling.
Molecular Dynamics Simulations
Equilibrium molecular dynamics simulations were performed on the special-purpose molecular dynamics machine Anton (Shaw et al., 2009).
Supplementary Material
Figure S1. Characterization of IRAK4 Activity and Phosphorylation State, Related to Figure 1
(A) Anion-exchange chromatography of co-expressed IRAK4FL-D311N/MyD88DD complex. A280 absorption profile (blue) shows major elution peak at a conductivity of approximately 35 mS/cm (red). SDS-PAGE of anion-exchange fractions followed by Coomassie blue staining indicates that the peak contains both IRAK4FL-D311N and MyD88DD.
(B) Phosphorylation of 160 μM Smt3 tagged IRAK1 activation loop peptide (362–380) by 300 nM IRAK4FL. Samples at various time points were separated by SDS-PAGE, visualized by autoradiography (top) and quantified (bottom).
(C) Dephosphorylation of IRAK4FL. Natively phosphorylated IRAK4FL was incubated with λ-phosphatase at 30 °C. Samples at various time points were analyzed by SDS-PAGE followed by either Coomassie blue staining (top) or Western blot using a phospho-specific antibody for IRAK4 pT345 (bottom).
(D) Phosphorylation of insect cell purified IRAK4FL and IRAK4FL-D311N examined before and after incubation with 5 mM ATP at 25 °C. Samples were separated by SDS-PAGE and visualized by anti-IRAK4 pT345 Western blot (top) and Coomassie blue staining (bottom).
(E) Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS) of λ-phosphatase treated IRAK4FL, with and without incubation with ATP/Mg2+. Shown are the significant phosphorylation events on the IRAK4 activation loop residues, T342, T345, and S346, along with the expectation value of each ion.
Figure S2. MyD88 Enhances Trans-autophosphorylation of IRAK4, Related to Figure 2
(A) Representative autoradiography images of IRAK4FL autophosphorylation with and without MyD88DD-G80K. IRAK4FL samples at indicated concentrations were incubated with [γ-32P]ATP and Mg2+ at 25 °C. IRAK4FL was pre-incubated with or without MyD88DD-G80K. Timepoints were subjected to SDS-PAGE and visualized by autoradiography.
(B) Trans-phosphorylation of IRAK4KD-D311N (10 μM) by IRAK4FL (1 μM). The same data is shown as part of Figure 4F. Data represent mean ± SEM.
(C) Pull-down assay of 100 μM 6xHis tagged MyD88DD-G80K with 25 μM dephosphorylated IRAK4DD, IRAK4KD, or IRAK4FL. Red asterisks indicate successful pull-down of IRAK4 construct. I: input, P: pulldown.
(D) Autophosphorylation of 2 μM dephosphorylated IRAK4FL or IRAK4KD with and without 50 μM MyD88DD-G80K preincubation. Data represent mean ± SEM.
Figure S3. Activation Loop of Each IRAK4 Monomer Adopts a Distinct Conformation, Related to Figure 3
(A) Coomassie stained SDS-PAGE of IRAK4FL-D311N crystals and the crystallization drop. Several crystals were harvested and washed with reservoir solution before addition of SDS-PAGE loading buffer (Harvested Crystals). This was compared to the protein composition of the complete drop (Whole Drop), indicating that the crystals are enriched for the IRAK4 degradation product that is consistent in molecular weight with the IRAK4 kinase domain.
(B) Superposition of IRAK4KD-D311N “enzyme” monomer A with p-IRAK4KD as in Figure 3B. The canonical phosphorylated residue, pT345 of p-IRAK4KD and T345 of IRAK4KD-D311N are shown as sticks. R334 of p-IRAK4KD forms a salt bridge with the phosphate group of pT345, but is disordered in the structure of IRAK4KD-D311N.
(C) Superposition of IRAK4KD-D311N “enzyme” monomer A, “substrate” monomer B, and p-IRAK4KD. The activation loop conformation of IRAK4KD-D311N “substrate” monomer B is distinct from that of IRAK4KD-D311N “enzyme” monomer A and p-IRAK4KD. Activation loop residue side chains are shown as sticks and labeled for the IRAK4FL-D311N structure.
(D) Normalized size-exclusion chromatograms of IRAK4KD-D311N at indicated concentrations incubated with either DMSO or 500 μM staurosporine.
Figure S4. Residues at IRAK4 Dimer Interface are Highly Conserved, Related to Figure 4
(A) Surface electrostatics of αG and αH-I exo-sites at the IRAK4KD-D311N dimer interface. The interfaces are composed of highly negatively charged αG helix and highly positively charged αH-αI loop. In the dimer, each surface is juxtaposed with the complimentary surface of its partner.
(B) Alignment of IRAK4KD orthologs from various species. Surfaces involved in IRAK4 dimerization, including the activation loop, αEF helix, αG helix, and αH-αI loop are highlighted in cyan, green, red and blue, respectively. Pink asterisks indicate sites of mutagenesis.
Figure S5. IRAK4 Kinase Domain Mutations do not Disrupt interaction with MyD88, Related to Figure 5
IRAK4-deficient human fibroblasts were infected with retroviruses containing empty vector construct (Vector), Flag-tagged IRAK4FL wild-type (WT) and mutants (K213M, L360A, R361E, and Y262T). Cells were treated with IL-1β (1 ng/mL) for the indicated times, followed by immunoprecipitation (IP) with Flag antibody and Western blot for MyD88. Western blot of whole cell lysate (WCL) was included to control for MyD88 and Flag-IRAK4 expression levels.
Figure S6. Comparison of the IRAK4 Asymmetric Enzyme-Substrate Dimer with PKR and PAK1, Related to Figure 7
(A) The KD of PKR (yellow) is superimposed with the IRAK4KD-D311N “enzyme” monomer. The PKR substrate eIF2α also interacts with the PKR αG helix. Similarly, the IRAK4 “substrate” monomer interacts with the IRAK4 “enzyme” monomer αG helix.
(B) The “enzyme” monomers of PAK1 (yellow) and IRAK4KD-D311N (green) are superimposed. The “substrate” monomers of PAK1 (pink) and IRAK4KD-D311N (blue) are in distinct locations, illustrating the large differences between the dimerization interfaces.
HIGHLIGHTS.
Unphosphorylated IRAK4 kinase domain dimerizes in solution and in the Myddosome.
MyD88 enhances IRAK4 autophosphorylation at concentrations below dimerization Kd.
Crystal structure of IRAK4 kinase domain dimer reveals an enzyme-substrate complex.
Dimer interface mutations compromise autophosphorylation and signal transduction.
Acknowledgments
We thank Dr. Wayne Hendrickson for suggesting the use of X4A beamline of NSLS for sulfur anomalous diffraction, Dr. Qun Liu for collecting highly redundant sulfur anomalous data on IRAK4 crystals, Dr. Venkatesh Mysore for assistance in performing the MD simulation, Dr. Stewart Shuman and Dr. Heather Ordonez for their technical support and advice involving radiolabeled kinase assays, Dr. Stephen Harrison and Dr. Yoana Dimitrova for access to the SE-AUC equipment and assistance with the experiment, and the National Institutes of Health for funding support (AI050872 to HW).
Footnotes
AUTHOR CONTRIBUTIONS
R.F. performed protein purification, SEC, MALS, AUC, in vitro kinase assays, crystallization, structure determination, and SAXS, including Figures 1, 2, 3, 4, 6, S1, S2, S3, S4, and S6. H.Z. and X.L. performed and oversaw respectively cellular signaling analyses, western blots, RT-PCR, and Co-IP, including Figures 5 and S5. Y.S. performed MD simulations, including Figure 7. Q.L. expressed protein in insect cells. H.W. supervised the project. R.F., H.W., H.Z., Y.S., and X.L. wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cai Z, Chehab NH, Pavletich NP. Structure and activation mechanism of the CHK2 DNA damage checkpoint kinase. Mol Cell. 2009;35:818–829. doi: 10.1016/j.molcel.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Cheng H, Addona T, Keshishian H, Dahlstrand E, Lu C, Dorsch M, Li Z, Wang A, Ocain TD, Li P, et al. Regulation of IRAK-4 kinase activity via autophosphorylation within its activation loop. Biochem Biophys Res Commun. 2007;352:609–616. doi: 10.1016/j.bbrc.2006.11.068. [DOI] [PubMed] [Google Scholar]
- Favelyukis S, Till JH, Hubbard SR, Miller WT. Structure and autoregulation of the insulin-like growth factor 1 receptor kinase. Nat Struct Biol. 2001;8:1058–1063. doi: 10.1038/nsb721. [DOI] [PubMed] [Google Scholar]
- Ferrao R, Li J, Bergamin E, Wu H. Structural insights into the assembly of large oligomeric signalosomes in the toll-like receptor-interleukin-1 receptor superfamily. Sci Signal. 2012;5:re3. doi: 10.1126/scisignal.2003124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrao R, Wu H. Helical assembly in the death domain (DD) superfamily. Curr Opin Struct Biol. 2012;22:241–247. doi: 10.1016/j.sbi.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraczek J, Kim TW, Xiao H, Yao J, Wen Q, Li Y, Casanova JL, Pryjma J, Li X. The kinase activity of IL-1 receptor-associated kinase 4 is required for interleukin-1 receptor/toll-like receptor-induced TAK1-dependent NFkappaB activation. J Biol Chem. 2008;283:31697–31705. doi: 10.1074/jbc.M804779200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith EJ, Akella R, Min X, Zhou T, Humphreys JM. Substrate and docking interactions in serine/threonine protein kinases. Chem Rev. 2007;107:5065–5081. doi: 10.1021/cr068221w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekmat-Nejad M, Cai T, Swinney DC. Steady-state kinetic characterization of kinase activity and requirements for Mg2+ of interleukin-1 receptor-associated kinase-4. Biochemistry. 2010;49:1495–1506. doi: 10.1021/bi901609m. [DOI] [PubMed] [Google Scholar]
- Hu J, Stites EC, Yu H, Germino EA, Meharena HS, Stork PJ, Kornev AP, Taylor SS, Shaw AS. Allosteric activation of functionally asymmetric RAF kinase dimers. Cell. 2013;154:1036–1046. doi: 10.1016/j.cell.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard SR. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. EMBO J. 1997;16:5572–5581. doi: 10.1093/emboj/16.18.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin MS, Lee JO. Structures of TLR-ligand complexes. Curr Opin Immunol. 2008;20:414–419. doi: 10.1016/j.coi.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Knighton DR, Zheng JH, Ten Eyck LF, Xuong NH, Taylor SS, Sowadski JM. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991;253:414–420. doi: 10.1126/science.1862343. [DOI] [PubMed] [Google Scholar]
- Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Kuglstatter A, Villasenor AG, Shaw D, Lee SW, Tsing S, Niu L, Song KW, Barnett JW, Browner MF. Cutting Edge: IL-1 Receptor-Associated Kinase 4 Structures Reveal Novel Features and Multiple Conformations. J Immunol. 2007;178:2641–2645. doi: 10.4049/jimmunol.178.5.2641. [DOI] [PubMed] [Google Scholar]
- Lei M, Robinson MA, Harrison SC. The active conformation of the PAK1 kinase domain. Structure. 2005;13:769–778. doi: 10.1016/j.str.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Li S, Strelow A, Fontana EJ, Wesche H. IRAK-4: a novel member of the IRAK family with the properties of an IRAK-kinase. Proc Natl Acad Sci U S A. 2002;99:5567–5572. doi: 10.1073/pnas.082100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Lo YC, Wu H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature. 2010;465:885–890. doi: 10.1038/nature09121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Helgason E, Phung QT, Quan CL, Iyer RS, Lee MW, Bowman KK, Starovasnik MA, Dueber EC. Molecular basis of Tank-binding kinase 1 activation by transautophosphorylation. Proc Natl Acad Sci U S A. 2012;109:9378–9383. doi: 10.1073/pnas.1121552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meharena HS, Chang P, Keshwani MM, Oruganty K, Nene AK, Kannan N, Taylor SS, Kornev AP. Deciphering the structural basis of eukaryotic protein kinase regulation. PLoS biology. 2013;11:e1001680. doi: 10.1371/journal.pbio.1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motshwene PG, Moncrieffe MC, Grossmann JG, Kao C, Ayaluru M, Sandercock AM, Robinson CV, Latz E, Gay NJ. An oligomeric signaling platform formed by the Toll-like receptor signal transducers MyD88 and IRAK-4. J Biol Chem. 2009;284:25404–25411. doi: 10.1074/jbc.M109.022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Wijmenga C, O’Neill LA. Genetic variation in Toll-like receptors and disease susceptibility. Nat Immunol. 2012;13:535–542. doi: 10.1038/ni.2284. [DOI] [PubMed] [Google Scholar]
- Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH, Kohlhammer H, Xu W, Yang Y, Zhao H, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen B, Taylor S, Ghosh G. Regulation of protein kinases; controlling activity through activation segment conformation. Mol Cell. 2004;15:661–675. doi: 10.1016/j.molcel.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Oliver AW, Knapp S, Pearl LH. Activation segment exchange: a common mechanism of kinase autophosphorylation? Trends Biochem Sci. 2007;32:351–356. doi: 10.1016/j.tibs.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Pellicena P, Kuriyan J. Protein-protein interactions in the allosteric regulation of protein kinases. Curr Opin Struct Biol. 2006;16:702–709. doi: 10.1016/j.sbi.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Picard C, Puel A, Bonnet M, Ku CL, Bustamante J, Yang K, Soudais C, Dupuis S, Feinberg J, Fieschi C, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299:2076–2079. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- Pirruccello M, Sondermann H, Pelton JG, Pellicena P, Hoelz A, Chernoff J, Wemmer DE, Kuriyan J. A dimeric kinase assembly underlying autophosphorylation in the p21 activated kinases. J Mol Biol. 2006;361:312–326. doi: 10.1016/j.jmb.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Seo EJ, Liu F, Kawagishi-Kobayashi M, Ung TL, Cao C, Dar AC, Sicheri F, Dever TE. Protein kinase PKR mutants resistant to the poxvirus pseudosubstrate K3L protein. Proc Natl Acad Sci U S A. 2008;105:16894–16899. doi: 10.1073/pnas.0805524105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw DE, Dror RO, Salmon JK, Grossman JP, Mackenzie KM, Bank JA, Young C, Deneroff MM, Batson B, Bowers KJ, et al. Millisecond-scale molecular dynamics simulations on Anton. Proceedings of the Conference on High Performance Computing Networking, Storage and Analysis; Portland, Oregon: ACM; 2009. pp. 1–11. [Google Scholar]
- Taylor SS, Kornev AP. Protein kinases: evolution of dynamic regulatory proteins. Trends Biochem Sci. 2011;36:65–77. doi: 10.1016/j.tibs.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Bazan JF, Garcia KC. Structure of the activating IL-1 receptor signaling complex. Nat Struct Mol Biol. 2012;19:455–457. doi: 10.1038/nsmb.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treon SP, Xu L, Yang G, Zhou Y, Liu X, Cao Y, Sheehy P, Manning RJ, Patterson CJ, Tripsas C, et al. MYD88 L265P somatic mutation in Waldenstrom’s macroglobulinemia. N Engl J Med. 2012;367:826–833. doi: 10.1056/NEJMoa1200710. [DOI] [PubMed] [Google Scholar]
- von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, Chrabieh M, Mustapha IB, Ghandil P, Camcioglu Y, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Zhang S, Li L, Liu X, Mei K, Wang X. Structural insights into the assembly and activation of IL-1beta with its receptors. Nat Immunol. 2010;11:905–911. doi: 10.1038/ni.1925. [DOI] [PubMed] [Google Scholar]
- Wang J, Wu JW, Wang ZX. Structural insights into the autoactivation mechanism of p21-activated protein kinase. Structure. 2011;19:1752–1761. doi: 10.1016/j.str.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Wang Z, Liu J, Sudom A, Ayres M, Li S, Wesche H, Powers JP, Walker NP. Crystal structures of IRAK-4 kinase in complex with inhibitors: a serine/threonine kinase with tyrosine as a gatekeeper. Structure. 2006;14:1835–1844. doi: 10.1016/j.str.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Wu H. Higher-order assemblies in a new paradigm of signal transduction. Cell. 2013;153:287–292. doi: 10.1016/j.cell.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Li W, Craddock BP, Foreman KW, Mulvihill MJ, Ji QS, Miller WT, Hubbard SR. Small-molecule inhibition and activation-loop trans-phosphorylation of the IGF1 receptor. EMBO J. 2008;27:1985–1994. doi: 10.1038/emboj.2008.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Kim TW, Qin J, Jiang Z, Qian Y, Xiao H, Lu Y, Qian W, Gulen MF, Sizemore N, et al. Interleukin-1 (IL-1)-induced TAK1-dependent Versus MEKK3-dependent NFkappaB activation pathways bifurcate at IL-1 receptor-associated kinase modification. J Biol Chem. 2007;282:6075–6089. doi: 10.1074/jbc.M609039200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Characterization of IRAK4 Activity and Phosphorylation State, Related to Figure 1
(A) Anion-exchange chromatography of co-expressed IRAK4FL-D311N/MyD88DD complex. A280 absorption profile (blue) shows major elution peak at a conductivity of approximately 35 mS/cm (red). SDS-PAGE of anion-exchange fractions followed by Coomassie blue staining indicates that the peak contains both IRAK4FL-D311N and MyD88DD.
(B) Phosphorylation of 160 μM Smt3 tagged IRAK1 activation loop peptide (362–380) by 300 nM IRAK4FL. Samples at various time points were separated by SDS-PAGE, visualized by autoradiography (top) and quantified (bottom).
(C) Dephosphorylation of IRAK4FL. Natively phosphorylated IRAK4FL was incubated with λ-phosphatase at 30 °C. Samples at various time points were analyzed by SDS-PAGE followed by either Coomassie blue staining (top) or Western blot using a phospho-specific antibody for IRAK4 pT345 (bottom).
(D) Phosphorylation of insect cell purified IRAK4FL and IRAK4FL-D311N examined before and after incubation with 5 mM ATP at 25 °C. Samples were separated by SDS-PAGE and visualized by anti-IRAK4 pT345 Western blot (top) and Coomassie blue staining (bottom).
(E) Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS) of λ-phosphatase treated IRAK4FL, with and without incubation with ATP/Mg2+. Shown are the significant phosphorylation events on the IRAK4 activation loop residues, T342, T345, and S346, along with the expectation value of each ion.
Figure S2. MyD88 Enhances Trans-autophosphorylation of IRAK4, Related to Figure 2
(A) Representative autoradiography images of IRAK4FL autophosphorylation with and without MyD88DD-G80K. IRAK4FL samples at indicated concentrations were incubated with [γ-32P]ATP and Mg2+ at 25 °C. IRAK4FL was pre-incubated with or without MyD88DD-G80K. Timepoints were subjected to SDS-PAGE and visualized by autoradiography.
(B) Trans-phosphorylation of IRAK4KD-D311N (10 μM) by IRAK4FL (1 μM). The same data is shown as part of Figure 4F. Data represent mean ± SEM.
(C) Pull-down assay of 100 μM 6xHis tagged MyD88DD-G80K with 25 μM dephosphorylated IRAK4DD, IRAK4KD, or IRAK4FL. Red asterisks indicate successful pull-down of IRAK4 construct. I: input, P: pulldown.
(D) Autophosphorylation of 2 μM dephosphorylated IRAK4FL or IRAK4KD with and without 50 μM MyD88DD-G80K preincubation. Data represent mean ± SEM.
Figure S3. Activation Loop of Each IRAK4 Monomer Adopts a Distinct Conformation, Related to Figure 3
(A) Coomassie stained SDS-PAGE of IRAK4FL-D311N crystals and the crystallization drop. Several crystals were harvested and washed with reservoir solution before addition of SDS-PAGE loading buffer (Harvested Crystals). This was compared to the protein composition of the complete drop (Whole Drop), indicating that the crystals are enriched for the IRAK4 degradation product that is consistent in molecular weight with the IRAK4 kinase domain.
(B) Superposition of IRAK4KD-D311N “enzyme” monomer A with p-IRAK4KD as in Figure 3B. The canonical phosphorylated residue, pT345 of p-IRAK4KD and T345 of IRAK4KD-D311N are shown as sticks. R334 of p-IRAK4KD forms a salt bridge with the phosphate group of pT345, but is disordered in the structure of IRAK4KD-D311N.
(C) Superposition of IRAK4KD-D311N “enzyme” monomer A, “substrate” monomer B, and p-IRAK4KD. The activation loop conformation of IRAK4KD-D311N “substrate” monomer B is distinct from that of IRAK4KD-D311N “enzyme” monomer A and p-IRAK4KD. Activation loop residue side chains are shown as sticks and labeled for the IRAK4FL-D311N structure.
(D) Normalized size-exclusion chromatograms of IRAK4KD-D311N at indicated concentrations incubated with either DMSO or 500 μM staurosporine.
Figure S4. Residues at IRAK4 Dimer Interface are Highly Conserved, Related to Figure 4
(A) Surface electrostatics of αG and αH-I exo-sites at the IRAK4KD-D311N dimer interface. The interfaces are composed of highly negatively charged αG helix and highly positively charged αH-αI loop. In the dimer, each surface is juxtaposed with the complimentary surface of its partner.
(B) Alignment of IRAK4KD orthologs from various species. Surfaces involved in IRAK4 dimerization, including the activation loop, αEF helix, αG helix, and αH-αI loop are highlighted in cyan, green, red and blue, respectively. Pink asterisks indicate sites of mutagenesis.
Figure S5. IRAK4 Kinase Domain Mutations do not Disrupt interaction with MyD88, Related to Figure 5
IRAK4-deficient human fibroblasts were infected with retroviruses containing empty vector construct (Vector), Flag-tagged IRAK4FL wild-type (WT) and mutants (K213M, L360A, R361E, and Y262T). Cells were treated with IL-1β (1 ng/mL) for the indicated times, followed by immunoprecipitation (IP) with Flag antibody and Western blot for MyD88. Western blot of whole cell lysate (WCL) was included to control for MyD88 and Flag-IRAK4 expression levels.
Figure S6. Comparison of the IRAK4 Asymmetric Enzyme-Substrate Dimer with PKR and PAK1, Related to Figure 7
(A) The KD of PKR (yellow) is superimposed with the IRAK4KD-D311N “enzyme” monomer. The PKR substrate eIF2α also interacts with the PKR αG helix. Similarly, the IRAK4 “substrate” monomer interacts with the IRAK4 “enzyme” monomer αG helix.
(B) The “enzyme” monomers of PAK1 (yellow) and IRAK4KD-D311N (green) are superimposed. The “substrate” monomers of PAK1 (pink) and IRAK4KD-D311N (blue) are in distinct locations, illustrating the large differences between the dimerization interfaces.