Abstract
Female mice exhibit a better survival rate than males after infection, but if infection follows an ozone-induced oxidative stress, male survival exceeds that of females. Our goal was to study bronchoalveolar lavage factors that contribute to these sex differences in outcome. We studied parameters at 4, 24, and 48 hours after ozone exposure and infection, including markers of inflammation, oxidative stress, and tissue damage, and surfactant phospholipids and surfactant protein A (SP-A). A multianalyte immunoassay at the 4 hr time point measured 59 different cytokines, chemokines, and other proteins. We found that: 1) Although some parameters studied revealed sex differences, no sex differences were observed in LDH, total protein, MIP-2, and SP-A. Males showed more intragroup significant differences in SP-A between filtered air- and ozone-exposed mice compared to females. 2) Oxidized dimeric SP-A was higher in FA-exposed female mice. 3) Surfactant phospholipids were typically higher in males. 4) The multianalyte data revealed differences in the exuberance of responses under different conditions - males in response to infection and females in response to oxidative stress. These more exuberant, and presumably less well-controlled responses associate with the poorer survival. We postulate that the collective effects of these sex differences in response patterns of lung immune cells may contribute to the clinical outcomes previously observed.
Keywords: Oxidative stress, surfactant, SP-A, pneumonia, innate immunity
1. Introduction
As the lung performs its role in respiration, it is vulnerable to infection and damage from inhaled pathogens, allergens, and toxic gases or particles. In most air-breathing organisms an efficient and well-regulated innate host defense system eliminates these threats and preserves the delicate structure of the lung. However, an overly exuberant response to microbial or environmental threats, such as an excessive respiratory burst producing reactive oxidant species, could potentially damage tissue and interfere with respiration.
Innate host defense in the alveoli is provided primarily by alveolar macrophages. Their function is highly regulated by the alveolar microenvironment, including other lung cells, such as type 1 and type 2 alveolar epithelial cells. The alveolar microenvironment includes pulmonary surfactant and other regulatory molecules and secretions from all of the above cell types, as well as other proteins that enter the alveolus from the circulation or the lung interstitium. The influence of surfactant on the macrophage is complex, and involves the surfactant proteins, especially surfactant protein A (SP-A), as well as the lipid constituents of surfactant. Numerous papers have demonstrated a regulatory role for SP-A on the function of macrophages and their production of chemokines and cytokines [1-7]. Certain surfactant phospholipids also have a profound influence on the function of the macrophage, and often oppose the action of SP-A [2,8-13]. The importance of the contribution of SP-A becomes evident with the increased susceptibility of SP-A knockout mice to a number of different infections [14-18]. There have also been a number of studies demonstrating that SP-A can be oxidized and its function compromised by exposure to air pollutants such as ozone or to other materials containing reactive oxidant species [19-25]. More recently it has become clear that SPA also plays a role in regulating the expression of a number of macrophage gene products, and these in turn may regulate reactive oxidant species in the alveolar space or participate in maintaining protease/antiprotease balance in the lung [3,4].
In a series of publications we have described sex differences [18,21,22,26,27] in outcome after infecting mice with Klebsiella pneumoniae, with or without a prior oxidative stress in the form of an acute ozone exposure. The above studies include differences in survival and phagogcytosis of pathogens by alveolar macrophages [18,21,25] as well as differences in histopathology and dissemination of the resulting infection [22,27]. Moreover, we have shown that the sex differences persist and are accentuated in mice lacking SP-A or after the oxidative modification of SP-A [18,21]. The sex differences in survival have been shown to be dependent upon gonadal hormones [26]. A further insight into the molecular basis of the sex differences has been gained via the study of the proteome of alveolar macrophages from mice lacking SP-A and after a “rescue” of these mice with exogenous SP-A [3,4].
In the present study we continue to study the basis for the sex differences by studying a number of parameters in the BAL after filtered air (FA) or ozone exposure during the initial phases of infection. Endpoints studied included total and differential BAL cell counts, lactate dehydrogenase (LDH), total protein, total oxidized protein, phospholipid, SP-A, oxidized SP-A, and MIP-2. In an effort to gain a more global insight into the BAL factors that may contribute to the observed differences we performed immunoassays on 59 different analytes in BAL, including a variety of inflammatory mediators, proteins involved in the acute phase response and coagulation, and proteins involved in cell growth and differentiation.
2. Material and Methods
2.1 Animals
Male and female C57BL/6 mice (from Jackson Laboratory (Bar Harbor, ME)) were used at 8-12 weeks of age. The Penn State University Institutional Animal Care and Use Committee approved all procedures involving animals. Animals were exposed to FA or ozone, infected with K. pneumonia, and sacrificed at the indicated time for further study as described.
2.2 Exposure of mice to ozone
Mice were exposed to ozone (2 ppm for 3 h) or to FA (control) at the same time in separate chambers. Each chamber consisted of a 3.7 liter closed glass vessel into which glass containers with wire mesh tops were placed. The temperature was maintained at 25°C, humidity was set to 50 %, and the flow rate was 15 L/min through each (FA and ozone) chamber. Air flow and ozone content were continually monitored. All FA and ozone exposures were conducted in parallel. For various experiments 3-6 mice were treated with either ozone or FA.
The ozone dose/duration (2 ppm for 3 h) has been used by other laboratories [28-30] and was chosen in our preliminary work as being optimal [31] for further investigations. The rational for this dose is based on a study by Hatch et al [32] in which they reported that experimental animals required higher ozone concentrations than humans to deliver comparable amounts of ozone to the distal lung. This determination was based on numbers of neutrophils and macrophages, as well as the protein content of BAL.
2.3 Preparation of bacteria
Klebsiella pneumoniae bacteria (ATCC 43816) were purchased from the American Tissue Culture Collection (Rockville, MD), then grown and prepared as described previously [21]. Bacteria were grown for 18 hr in tryptic soy broth (TSB) media at 37°C until they reached stationary phase. The suspension of bacteria was diluted until the OD660 was equal to 0.4. We used a 200 μl aliquot of this dilution to inoculate 50 ml of fresh TSB for sub-cultivation for 3 h, resulting in a culture that was in the mid-log phase of growth. We then placed the sub-culture on ice to stop growth. Using cold PBS, the culture was serially diluted to obtain ~ 9 × 103 CFU/ml, and mice were infected by injecting 50 μl of this bacterial suspension (containing ~ 450 CFU) intratracheally. CFU per ml values were calculated from the OD660 of the bacterial suspension, and an aliquot was also spread on tryptic soy agar (TSA) plates to confirm CFU estimates.
2.4 Infection of mice with
K. pneumoniae
Infection was performed as described previously [21]. Briefly, the animals were anesthetized, the trachea was surgically exposed, and ~ 450 CFU/mouse were inoculated intratracheally in 50 μl of PBS. If any mice died within the first 12 hr post-infection, we considered the death to be due to the surgical procedure rather than resulting from the infection and we excluded those mice from the study. In cases where mice were moribund with no chance of recovery, the mice were euthanized to prevent unnecessary suffering according to Penn State University Institutional Animal Care and Use Committee recommendations and were included with the natural deaths. After exposure to FA or ozone and subsequent infection (or instillation with vehicle), mice were subjected to bronchoalveolar lavage and various parameters were analyzed, as described below.
2.5 BAL analyses
The lungs of the mice were subjected to bronchoalveolar lavage (BAL) (3 times with 0.5 ml of 0.9% NaCl) at the 4, 24, and 48 hr post-infection time points, as described [31]. Three independent experiments were performed for each time point; each experiment involved 5 mice exposed to ozone and 5 mice exposed to FA, or a total of 83 male mice [42 FA-exposed and 41 ozone-exposed], and 74 female mice [39 FA-exposed and 35 ozone-exposed]. The BAL fluids were centrifuged (150 × g, 5 min, 4°C) and the cell pellets resuspended in 0.9% NaCl. Cell-free supernatants were frozen at – 80°C until subsequent analyses were performed as described below.
2.5.1 Cell and biochemical analyses of BAL fluid
Total cell counts were performed immediately after BAL using a hemocytometer. For the differential cell counts, slides were prepared using a cytocentrifuge and stained with a Hema-3 Stain Kit (Fisher Scientific, Pittsburgh, PA), and analyzed by light microscopy [21]. Total protein concentration was determined using the Micro BCA Protein Assay (Pierce Biotechnology, Rockford, IL). For determination of total phospholipids, 100 μl of BAL supernatant were lyophilized and then assayed using the Phospholipids B Assay (WAKO Chemicals Inc, Richmond, VA). Lactate dehydrogenase (LDH) was measured on 50 μL of each BAL sample using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI) following the manufacturer's instructions.
2.5.2 MIP-2 cytokine concentration measurement
MIP-2 concentration in the cell free BAL was measured using the Quantikine M mouse MIP-2 enzyme-linked immunosorbent assay (ELISA) kit (R&D systems, McKinley Place, NE) according to the manufacturer's protocol. For the analysis, a 500 μl aliquot of cell-free BAL was lyophilized and reconstituted to 100 μl with the assay diluent buffer included in the kit. Plates were read at 450 nm using the SPECTRA Fluor Plus ELISA plate reader (Tecan US, Research Triangle Park, NC).
2.5.3 Detection of total oxidized proteins
Oxidized proteins were detected using the OxyBlot Oxidized Protein Detection Kit (Intergen, Purchase, NY) as described [33]. This kit detects carbonyl groups that have been introduced into proteins through oxidation. In brief, 25 μl of BAL sample were denatured by adding an equal volume of 12% SDS. Samples were then derivatized with 2.5 μl of 10X 2, 4-dinitriphenylhydrazine (DNPH) solution and incubated for 10 min at room temperature. Derivatization was stopped with the addition of 25 μl of neutralization solution. Samples were then analyzed by dot blot. Aliquots containing the DNPH-derivatized proteins were brought up to a volume of 500 μl with 0.01 M phosphate buffered saline (pH 7.2) and 200 μl of each sample were blotted onto nitrocellulose by vacuum using a 96-well dot-blot apparatus (Bio-Rad). Immunodetection of oxidized proteins was performed according to the manufacturer's instructions, although the rabbit anti-DNP and goat anti-rabbit IgG (HRP-conjugated) antibodies supplied were used at half the recommended concentrations. Enhanced chemiluminescence (ECL) was used to detect antibody binding and blots were exposed to XAR film (Eastman Kodak Co., Rochester, NY). The film was developed and carbonylated protein levels were quantified by laser densitometry.
2.5.4 Detection of oxidized and total SP-A
Detection of oxidized and total SP-A was performed as described [33]. In brief, 200 μl aliquots of BAL samples were concentrated by lyophilization. Protein samples were subjected to electrophoresis on 12.5% SDS polyacrylamide gels. Two separate gels were run and the separated proteins transferred either to a nitrocellulose membrane for SP-A detection or to a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA) for oxidized SP-A detection. Immunodetection of total SP-A was done, as described previously [33]. Briefly, after blocking, the blots were incubated with polyclonal rabbit anti-SP-A IgG (1:10,000) for 1 hr at room temperature and washed. The blots were then incubated with secondary antibody (goat anti-rabbit IgG HRP conjugate; 1:25,000) (Bio-Rad) for 1 hr at room temperature and washed again. Antibody binding was detected by enhanced chemiluminescence (ECL). Blots were incubated with ECL solutions (PerkinElmer Life Sciences, Boston, MA) and exposed to Kodak X-Omat XAR films (Eastman Kodak Co., Rochester, NY). The films were developed and SP-A levels were quantified by laser densitometry. Bands representing the SP-A monomers and SP-A dimers were quantified separately. Total SP-A was the sum of the two determinations.
Oxidized SP-A was detected as described previously [33]. Briefly, blots were dehydrated for 1 min with 100% methanol, washed for 5 min in 0.02 M Tris (pH 7.5) with 20% methanol, 5 min with 2 N hydrochloric acid (HCl), and then treated with 100 μg DNPH/ml in 2 N HCl for 5 min. The membranes were again washed 3 times with 2 N HCl, 7 times with 100% methanol, and one time with 0.02 M TBS (5 min each wash), and blocked overnight. Immunodetection of oxidized proteins was done, as described above in section C for the detection of total oxidized proteins.
2.6 Multi-analyte immunoassay (RodentMAP v.2.0) analysis
The RodentMAP version 2.0 (antigens) analysis of mouse BALs was performed by Rules-Based Medicine (RBM) (Austin, TX, USA). For the assay, BALs from untreated, unexposed mice were used as an additional control. Non-infected mice were intratracheally inoculated after FA or ozone exposure with 50 μl of PBS rather than with 50 μl of bacterial suspension. For RodentMAP analysis the 4 hr time point was used with a total of 30 mice, or 15 male and 15 female mice [6 FA-exposed (3 mice infected with K. pneumoniae and 3 mice non-infected), 6 ozone-exposed (3 mice infected and 3 mice non-infected), and 3 untreated (not exposed and non-infected) for each sex]. Fifty-nine analytes were quantified by the RodentMAP assay.
2.7 Statistics
All data were analyzed with a simple t-test. Results were considered statistically significant when p < 0.05.
3. Results
Two major groups of experiments were performed. In the first one a number of parameters were measured at 4, 24, and 48 hours after exposure to either ozone or filtered air (FA) and infection with K. pneumoniae. All mice in this group of experiments were infected via an intratracheal instillation of bacteria. In the second group a multi-analyte immunoassay was performed at the 4 hr time point to measure the levels of 59 analytes in BAL, in order to assess early events. This type of analysis included mice exposed to FA or ozone that were either infected or lacked infection plus untreated mice (not exposed, not infected).
3.1 Measurements
3.1.1 BAL cellular content
3.1.1.1 Total cells:
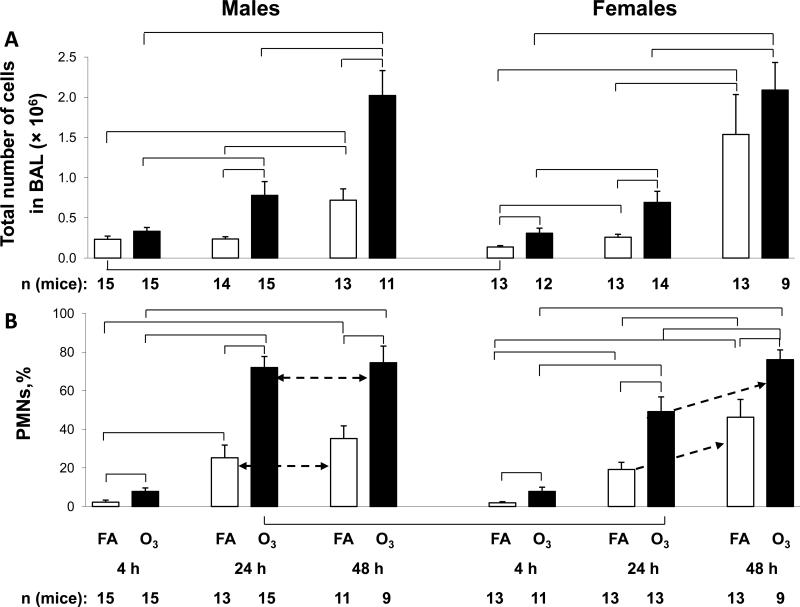
The total number of cells in BAL was determined (Figure 1A). With the exception of the 4 hr time point in the total number of cells in FA-exposed and infected mice, there were no other differences between male and female mice. The effect of infection on total cell number was seen by comparing the cell numbers in FA-exposed mice. FA-exposed males showed no significant increases between 4 and 24 hours, but a significant increase was observed in cell numbers at the 48 hr time point compared to the 4 hr and 24 hr time points. FA-exposed females, on the other hand, showed a significant increase between 4 and 24 hours, followed by an additional significant increase between 24 and 48 hours. However, ozone exposure had a pronounced effect on cell numbers. Cell counts were significantly higher in ozone-exposed mice than their FA-exposed counterparts in all cases except at the 4 hr time point in male mice, and the 48 hr time point in females.
Figure 1.
BAL cell counts. Panel A. Total BAL cells from male and female mice were counted by hemocytometer and values from male and female are shown on separate graphs. The y-axis shows the total number of cells × 106. Brackets indicate groups that differ significantly from one another (p<0.05). Panel B. After performing a differential cell count on cytospin preparations, the % polymorphonuclear leukocytes (PMN) was determined and is graphed on the y-axis. Values from FA-exposed mice are shown in white bars and ozone-exposed mice in black bars. The differences between 24 and 48 h time points are shown with the dotted arrow. Exposure condition, time elapsed, and number of mice per point are shown below the x-axis.
These data indicate that infection (with prior FA exposure) does not have a major impact on cell number until 48 hr after its initiation, whereas infection after ozone exposure has an effect as early as 4 hr (in females) after termination of exposure and the initial infection.
3.1.1.2 Percentage of PMNs
Differential cell counts were performed to determine the basis for the increased cell numbers, with particular emphasis on the percentage of PMNs (%PMN) (Figure 1B). In the FA-exposed mice there was a progressive increase in the %PMN as the time after infection progressed. Comparisons between the time points showed significant increases between nearly all time points in both sexes. There were no sex differences in the FA-exposed mice.
There was definitely a greater ozone effect on the %PMN in ozone-exposed mice compared to FA-exposed mice at all time points and in both sexes. The %PMN in the ozone-exposed mice also underwent a progressive, and in most cases a significant increase, similar to that seen in the FA-exposed mice. The %PMN reached ~75-80% at the 48 hr time point in both sexes. At 24 hr, the ozone-exposed male mice were already approaching that level (75-80%) so there was no significant change between 24 and 48 hr. However, the %PMN in males was significantly higher than in females at the 24 hr time point.
The data show that although infection after FA exposure has an impact on %PMN, this is significantly increased when infection is preceded by an ozone exposure. Our previous work [22] where bacterial load was assessed at 24 and 48 hr after the instillation of bacteria and after FA or ozone exposure, indicates that the increased %PMN after ozone exposure may be a consequence of increased bacterial CFU (or decreased clearance).
3.1.2 BAL LDH levels
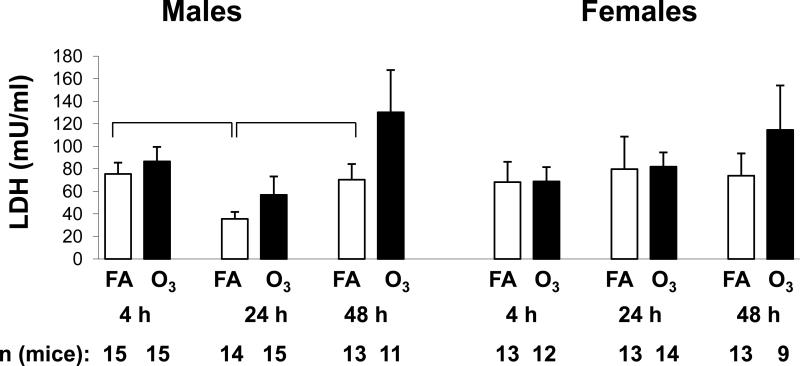
We measured LDH in the BAL fluid (Figure 2) as an index of tissue damage. LDH levels were very similar in most of the FA-exposed samples. In males, the 24 hr FA sample was significantly lower than the FA-exposed samples from 4 and 48 hr males and showed a trend toward being lower than all of the other samples, including the female samples. There were no significant differences between FA- and ozone-exposed samples from males and females at each time point studied. There was a trend toward higher LDH levels in both male and female mice 48 hr after ozone exposure although it did not reach significance.
Figure 2.
LDH levels in BAL. LDH levels were determined in BAL samples from male and female mice. Values (mUnits/ml) are graphed on the y-axis. X-axis, number of mice, and significance are as described in Figure 1.
3.1.3 Total BAL protein
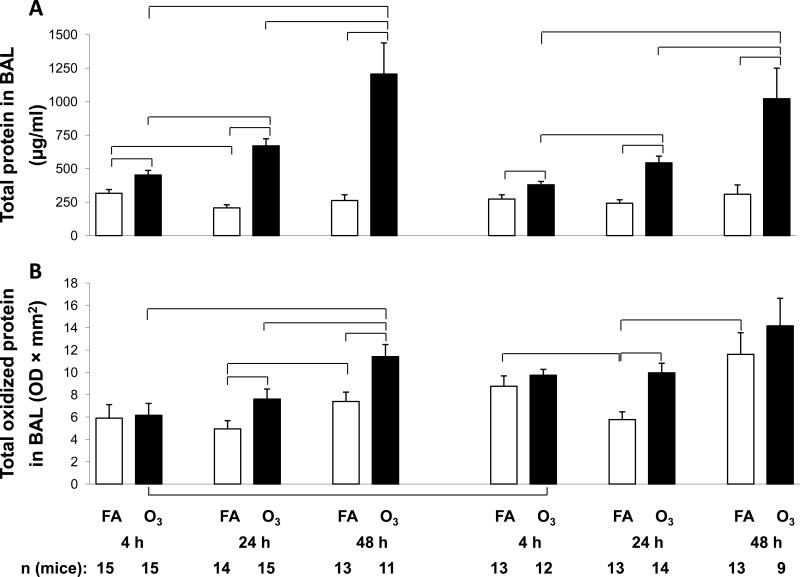
The protein content of the BAL fluid of FA-exposed mice (Figure 3A) was similar at nearly all time points, although levels in males at 4 hr were significantly higher than at 24 hr. As with cell counts, ozone exposure had a very profound effect and levels of total BAL protein were significantly higher than FA-exposed levels at all time points and in both sexes. In both sexes there was a progressive increase in protein content of the ozone-exposed mice with a significant increase between time points as time elapsed. There were no sex differences in either FA- or ozone-exposed mice.
Figure 3.
Total and oxidized BAL protein. Panel A. BAL samples from male and female mice were subject to protein determinations and values (μg/ml) are graphed on the y-axis. Panel B. Total oxidized protein was determined by treating an aliquot of BAL protein with the OxyBlot Oxidized Protein Detection Kit and the densitometric values (OD × mm2) are graphed on the y-axis. X-axis and significance for both panels are as described in Figure 1.
These results show that infection with prior FA exposure has a very limited effect on total BAL protein, but ozone exposure followed by infection results in significant changes, as we have previously demonstrated in uninfected mice exposed to ozone [31].
3.1.4 Total oxidized BAL protein
We also measured levels of proteins in the BAL that had undergone oxidation (carbonylation) as a consequence of infection and exposure to ozone (Figure 3B). In the FA-exposed mice there was a slight reduction in total oxidized protein from the 4 hr to the 24 hr time point that reached significance in the females, but not in the males. There were significant increases in both sexes between the 24 and 48 hr time points. This late increase may be due to the progression of infection.
In mice that were ozone-exposed prior to infection there was a progressive increase in oxidized protein from 4 to 24 to 48 hr. In all cases the levels of oxidized protein exceeded those seen in the comparable FA-exposed mice, and these changes were significant at 24 hr in both sexes and at 48 hr in males only. No sex differences were observed except that ozone-exposed values at 4 hr were significantly higher in females than in males.
3.1.5 Surfactant constituents
3.1.5.1 SP-A
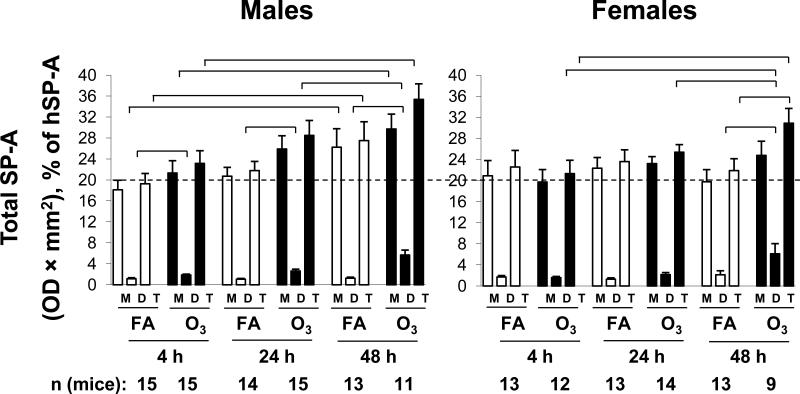
SP-A in the BAL samples was quantified by densitometry (Figure 4) of immunostained gels. For each sample the monomeric and dimeric forms were quantified separately and the total SP-A was calculated by adding the two. Although dimeric SP-A was a small fraction of the total SP-A (~5-15%) it underwent the largest relative changes.
Figure 4.
SP-A determinations. Aliquots of BAL samples from male and female mice were subjected to electrophoresis and immunostained with an antiserum to SP-A, followed by a horseradish-peroxidase-conjugated secondary antibody. Detection of immune complexes was done by enhanced chemiluminescence. Bands were quantified by laser densitometry and are shown for SP-A monomer (M), SP-A dimer (D), and total (T) SP-A (monomer + dimer). X-axis, number of mice, and significance are as described in Figure 1. The data from different blots were normalized by comparison with a positive control lane containing a sample of human SP-A that was run on all gels. The dotted reference line at 20% is shown to facilitate comparison between points.
In male FA-exposed mice, there was a trend toward increasing monomeric and total SP-A values as the time increased after infection, although a significant increase was observed only between 4 and 48 hours. This trend was not seen in female mice and there were no significant differences between FA-exposed female mice at any of the time points. A trend similar to that seen in monomeric SP-A was not seen in dimeric SP-A from either sex.
In ozone-exposed male mice all SP-A values were higher than the corresponding FA-exposed values. In male mice all dimeric SP-A values were significantly higher in ozone-exposed mice than in FA-exposed mice. In females the difference in dimeric SPA between FA- and ozone-exposed mice was only significant at 48 hours and this resulted in a significant difference in total SP-A values between of FA- and ozone-exposed female mice. This was the only case where a significant difference in total SPA values occurred between FA- and ozone-exposed mice. The 48 hour dimeric SP-A value in ozone-exposed females was also significantly higher when compared to ozone-exposed females at 4 and 24 hours. Ozone-exposed males also had significantly higher SP-A dimer levels at the 48 hour time point than ozone-exposed values at 24 hours. There were no significant differences between males and females.
3.1.5.2 Oxidized SP-A
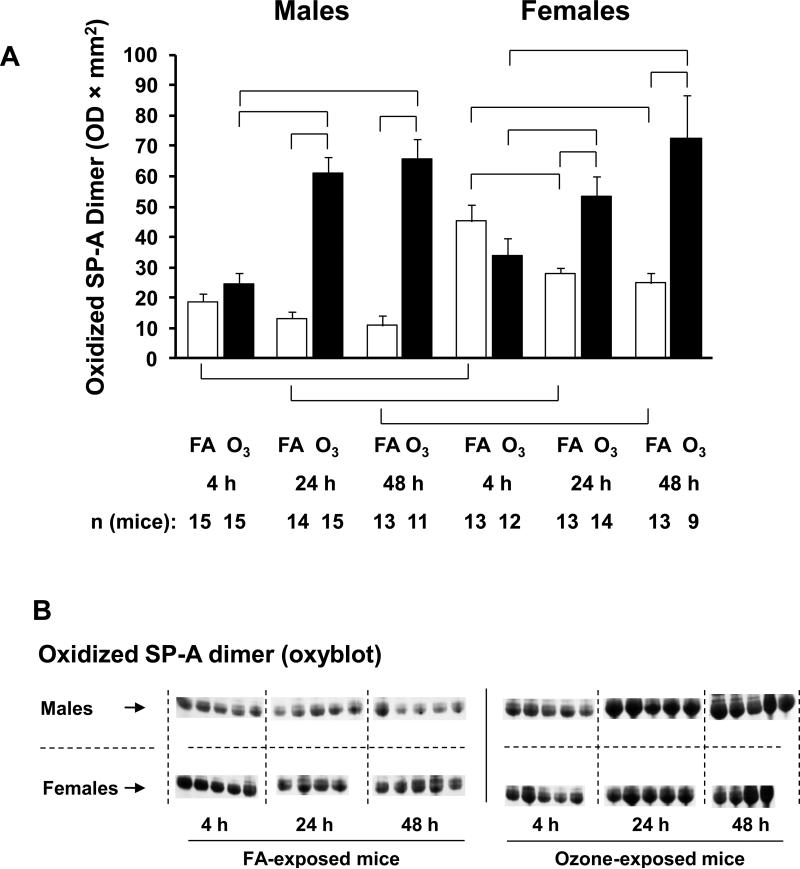
We next measured oxidized (carbonylated) SP-A (Figure 5A) because in previous studies we have demonstrated that SP-A is susceptible to oxidation and that oxidation compromises its function. Oxidation by carbonylation was found primarily in dimeric SP-A. A representative gel in which SP-A dimer bands have been immunostained with the OxyBlot Protein Detection Kit is depicted in Figure 5B. After FA exposure in males there were no changes in oxidized SP-A at any time point. However, in female mice the oxidized SP-A value was significantly higher at 4 hours after FA exposure and infection than at 24 and 48 hours. At all three time points oxidized SP-A was significantly higher in females than in males (Figure 5A).
Figure 5.
Oxidized SP-A measurements in BAL samples from males and females. Panel A. Oxidized SP-A was determined by treating a Western blot (identical to that used in Figure 4) with the OxyBlot Protein Detection Kit and having its bands quantified by laser densitometry. Oxidation is only seen in the dimeric form of SP-A, and the dimeric SP-A values (OD × mm2) are graphed on the y-axis. X-axis, number of mice, and significance are as described in Figure 1. Panel B. A representative Western blot stained with the OxyBlot Kit is shown.
After ozone exposure in both sexes oxidized SP-A was significantly higher than with FA exposure at the 24 and 48 hour time points. In addition, at the 24 and 48 hour time points, the oxidized SP-A values in both sexes were significantly higher than the 4 hour time point. There were no sex differences in oxidized SP-A values after ozone exposure.
3.1.5.3 Phospholipids
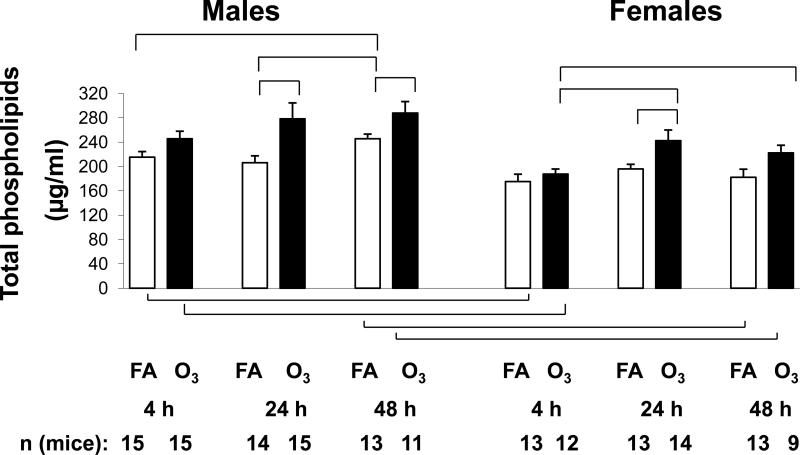
We also measured BAL surfactant phospholipid levels (Figure 6). The 48 hr time point in FA-exposed males was the highest value measured, and it was significantly different from both the 4 hr and 24 hr FA points. In females there were no significant differences between the FA-exposed time points. Both the 4 and 48 hr time points in FA-exposed males were significantly higher than the corresponding female levels.
Figure 6.
Total phospholipids. The phospholipid content of BAL was determined in males and females using the Phospholipids B assay. Values (μg/ml) are graphed on the y-axis. X-axis, number of mice, and significance are as described in Figure 1.
In all cases levels of BAL phospholipids in ozone-exposed mice were higher than in the corresponding FA-exposed mice, but they only reached significance in the 24 hr (both sexes) and the 48 hr (male) time points. Levels from ozone-exposed males were significantly greater than those from females at both 4 and 48 hr.
3.1.6 BAL MIP-2 levels
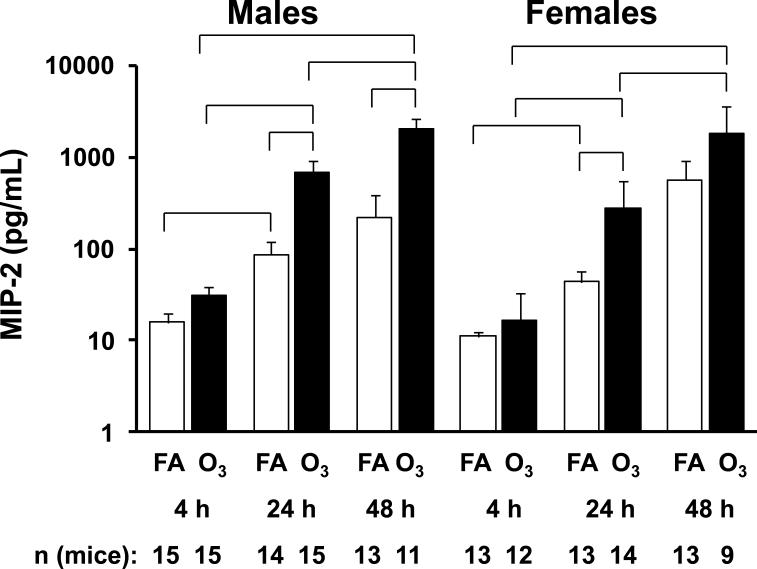
We studied MIP-2 levels in the BAL fluid (Figure 7) to determine whether this neutrophil chemoattractant might be responsible for the changes that we observed. There were progressive increases in MIP-2 over time in the FA-exposed mice of both sexes. Significant increases were observed with FA in both sexes between 4 and 24 hr.
Figure 7.
MIP-2 levels. MIP-2 levels in BAL fluid were determined by ELISA. Values (pg/ml) for males and females are graphed using a logarithmic scale on the y-axis. X-axis, number of mice, and significance are as described in Figure 1.
The same picture was seen when ozone-exposed samples from different time points were compared, with significant increases being observed between 4 and 24 hr, 24 and 48 hr, and 4 and 48 hr time points in both sexes. Ozone exposure, compared to FA-exposure, increased the levels of MIP-2 and significant differences were observed for the 24 hr (males and females) and the 48 hour time points (males). Thus, infection increases MIP-2 over time in both sexes, and these changes are increased further in the ozone-exposed and infected mice.
3.1.7 Multi-analyte immunoassay (RodentMAP) measurements
In order to obtain a more comprehensive view of molecules that may be contributing to the changes described above and help explain the mechanism(s) for the sex differences observed previously in this animal model, we used a multi-analyte immunoassay to measure 59 different analytes (see Supplementary Table), including MIP-2, which was analyzed by ELISA above. All samples were obtained at a single 4 hr time point after FA or ozone exposure and instillation of either bacteria or vehicle. The assay and time point were chosen so that we could assess early events that followed exposure and infection. In addition to studying FA- or ozone-exposed infected mice, we also examined the impact of bacteria by comparing mice instilled with bacteria and mice that were instilled with vehicle but no bacteria.
To determine whether sex differences exist at baseline (i.e. without any manipulation), we carried out the multi-analyte immunoassay with untreated male and female mice (Table, baseline). This comparison revealed only 4 significant differences. These were in GM-CSF, IL-18, VEGF, and von Willebrand factor. In all cases male values were higher than female values.
3.2 Comparisons
3.2.1. Filtered Air
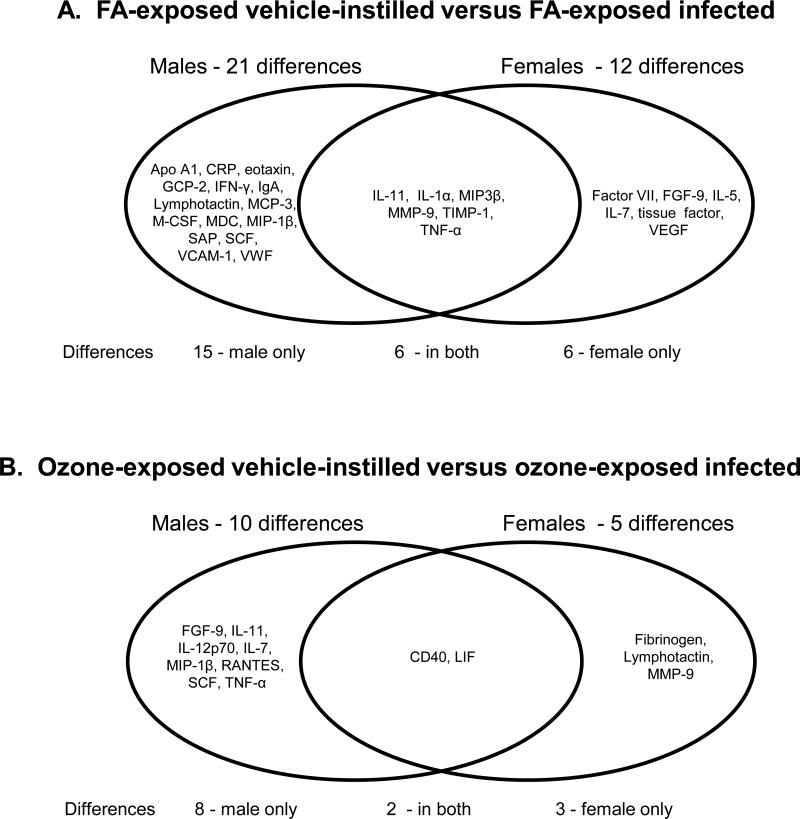
FA-exposed and vehicle-instilled versus FA-exposed and infected - We instilled FA-exposed mice with vehicle after the termination of FA exposure and compared them to FA-exposed mice that received an instillation of bacteria. With this comparison, significant differences were assumed to be a consequence of infection. We found 21 significant differences (Figure 8A) when FA-exposed infected male mice were compared to FA-exposed vehicle-instilled male mice. In a similar comparison in female mice there were only 12 significant differences. There were 6 common responses including: IL-11, IL-1α, MIP-3β, MMP-9, TIMP-1, TNF-α, which all increased in the infected mice.
Figure 8.
Venn diagram of differences due to infection. Panel A. A Venn diagram shows the significant differences between FA-exposed, vehicle-instilled samples and FA-exposed infected mice. Differences in males are shown on the left and differences in females on the right. The overlapping area shows the changes seen in both sexes. Panel B. A similar diagram is shown depicting the differences between ozone-exposed, vehicle-instilled mice and ozone-exposed infected mice. Differences in males are shown on the left and differences in females on the right. The overlapping area shows the changes seen in both sexes.
Of the 27 proteins that were significantly increased in one or both sexes (Figure 8A, Supplementary Table), 19 underwent larger increases in males compared to females, 5 increased more in females than in males (factor VII, FGF-9, IL-5, IL-7, tissue factor), and three proteins underwent equal changes in both sexes (GCP-2, IL-1α, MIP-1β). Of the 27 proteins, most were chemokines and cytokines that probably play a role in host defense processes. There were also increases in a couple of acute phase proteins (C reactive protein and serum amyloid P) and several proteins involved in coagulation (Factor VII, tissue factor, and von Willebrand factor).
3.2.1.1 Sex differences
A direct comparison of samples from male and female mice after FA and in the absence of pathogen (i.e vehicle-instilled) revealed 3 significant changes (M-CSF, tissue factor, TNF-α) (Table, FA-vehicle) and all were at higher levels in males. Moreover, when we examined the entire set, of the 59 analytes, 42 were higher (albeit not significantly) in males. When samples from FA-exposed males that had been infected were compared with FA-exposed and infected females (Table, FA-infected) there were 12 significant changes (ApoA, IFN-γ, IgA, IL-10, MCP-3, M-CSF, MDC, MIP-3β, MMP-9, RANTES, tissue factor, TNF-α). All but IL-10 were at higher levels in males than in females. However, it was interesting to note that among the infected vs vehicle-instilled comparisons (Figure 8A), there were more significant differences in males than in females (FA-vehicle-instilled vs. FA-infected), 21 in males vs. 12 in females).
3.2.2 Ozone
Ozone-exposed vehicle-instilled vs. ozone-exposed infected mice - A similar analysis as described above for the FA-exposed mice was performed for the ozone-exposed mice. We compared ozone-exposed mice that were instilled with vehicle without bacteria with ozone-exposed mice that had been infected (Figure 8B). In males there were 10 significant differences (CD40, FGF-9, IL-11, IL-12p70, IL-7, LIF, MIP-1β, RANTES, SCF, TNF-α) and in females there were 5 (CD40, fibrinogen, LIF, lymphotactin, MMP-9). In most cases infection caused an increase in the levels of the analyte, and the levels of the analytes and the magnitude of the response for the most part was greater in males than in females. These data (Figure 8) indicate that in the presence of infection males exhibit a larger number of changes in BAL and this is independent of whether there was a prior ozone exposure.
3.2.2.1 Sex differences
When samples from ozone-exposed males receiving vehicle were compared directly to their female counterparts there were 3 significant differences (KC/GRO-α, MIP-1α, SAP) (Table, ozone-vehicle). The comparison of the ozone-exposed infected male and female mice revealed 12 significant changes (EGF, fibrinogen, haptoglobin, IL-17, Interferon γ inducible protein (IP)-10, KC/GRO-α, MCP-5, M-CSF, MIP-2, RANTES, SCF, TNF-α) (Table, ozone-infected).
3.2.3. FA and ozone
3.2.3.1 FA-exposed, vehicle-instilled vs ozone-exposed, vehicle-instilled
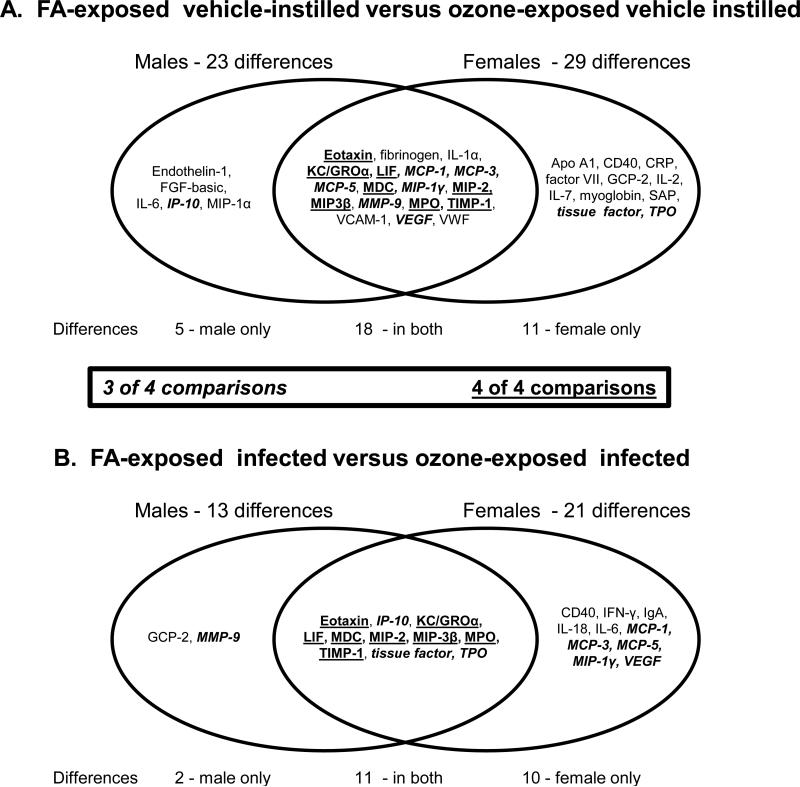
We next compared the responses of both sexes between FA- and ozone-exposed mice (Figure 9). When we compared FA-exposed to ozone-exposed mice instilled with vehicle (uninfected) there were 23 differences in males and 29 in females (Figure 9A). Of these, 18 exhibited significant changes in both sexes. In addition, five significant changes in males (that did not occur in females) included: endothelin-1, FGF-basic, IL-6, IP-10, MIP-1α. In all cases the trend was the same in females. Eleven significant changes in females (that did not occur in males) included: Apo A1, CD40, CRP, factor VII, GCP-2, IL-2, IL-7, myoglobin, SAP (serum amyloid P), tissue factor, TPO (thrombopoietin) and nearly all of these followed the same trend in males.
Figure 9.
Venn diagram of differences due to ozone exposure. Panel A. A Venn diagram shows the significant differences between FA- and ozone-exposed samples instilled with vehicle (uninfected). Differences in males are shown on the left and differences in females on the right. The overlapping area shows the changes seen in both sexes. Panel B. A similar diagram is shown depicting the differences between FA- and ozone-exposed that have been infected with pathogen. Differences in males are shown on the left and differences in females on the right. The overlapping area shows the changes seen in both sexes. Analytes that were significantly different in 3 of 4 comparisons (i.e. FA-exposed vs ozone-exposed – vehicle-instilled: 1) in males; 2) in females; FA-exposed vs ozone-exposed – infected – 3) in males; 4) in females) are italicized. Differences that were significant in all 4 comparisons are underlined.
3.2.3.2 FA-exposed, infected vs. ozone-exposed, infected
Similarly, comparing infected FA-exposed infected to ozone-exposed females to their male counterparts there were more significant changes in females (Figure 9B) than males (21 changes in female and 13 in males). In all cases the levels of the proteins increased. Eleven of these changes were significant in both sexes. Common changes included eotaxin, KC/GRO-α, IP-10, LIF, MDC, MIP-2, MIP-3β, MPO, TIMP-1, tissue factor, TPO. The 8 underlined proteins in both Fig. 9A and 9B were significantly different from FA to ozone in both males and females with and without bacteria and their expression differed significantly in all 4 comparisons (Male FA vs ozone – vehicle-instilled; male FA vs ozone – infected; female FA vs ozone –vehicle-instilled; female FA vs ozone – infected). Nine of the total changes were significant (IP-10, MCP-1, MCP-3, MCP-5, MIP-1γ, MMP-9, tissue factor, TPO, VEGF) in 3 out of 4 the comparisons (italicized items in Figures 9A and 9B). Two significant changes in infected males that did not occur in females included: GCP-2 and MMP-9. Ten significant changes in infected females that did not occur in infected males included: CD40, IFN-γ, IgA, IL-18, IL-6, MCP-1, MCP-3, MCP-5, MIP-1γ, VEGF. These data (Figure 9) indicate that in response to ozone-induced oxidative stress females exhibit a larger number of changes and that this type of response appears to be independent of the presence or absence of infection.
4. Discussion
We have shown that male and female mice differ in their abilities to combat infection, and that oxidative stress, in the form of acute ozone exposure, affects this response [18,21,22,26,27]. Our goal in the current study was to study BAL factors that may contribute to these differences. For this we used our well-characterized model of FA or ozone exposure and bacterial infection. In addition, BAL samples obtained from an additional group of mice, including mice that received vehicle but no bacteria, were subjected to a multi-analyte immunoassay analysis for a variety of proteins.
As anticipated, both infection and ozone exposure resulted in increases in widely used markers of lung inflammation and tissue damage. Total BAL cells, BAL neutrophils, and total protein showed increases, often presenting as a progressive increase after infection, with greater increases observed in the ozone-exposed mice. This was also the case in terms of levels of LDH, a widely used index of tissue damage, as well as MIP-2, a potent neutrophil chemoattractant. However, with the exception of sporadic sex differences, none of the above parameters showed consistent sex differences, as males and females exhibited similar levels and similar changes. We concluded that under the studied experimental conditions the BAL cellular composition and markers of tissue damage and PMN recruitment may not explain the previously observed sex differences in survival [18,21].
Because a more pronounced response was observed in infected mice that were ozone-exposed, measurements of oxidized BAL protein were carried out. These showed a trend toward higher values in both FA- and ozone-exposed females compared to males as time after exposure increased. However, this reached significance only at 4 hours after ozone exposure with females exhibiting higher levels. The oxidative changes observed indicate that females may exhibit a more robust macrophage anti-microbial activity with regards to oxidative burst.
Because AM activity has been shown to be regulated by surfactant constituents, most notably SP-A (which enhances host defense function) and the surfactant lipids [2,34], the levels of SP-A and surfactant lipids were investigated. Although there were no sex differences in SP-A levels at any of the time points, males and females seemed to exhibit different response patterns with males showing more significant intragroup differences. Females, on the other hand, did not show the same trend with the exception of the 48 hour ozone-exposed mice. Whether this indicates that males may be more sensitive to infection remains to be determined, although a poorer survival rate in FA-exposed infected males has been observed [21]. Moreover, survival under these conditions has been shown to be further decreased in mice that lacked SP-A [18]. In contrast, the levels of oxidized SP-A, and specifically the levels of the dimeric form of SP-A, were significantly higher in FA-exposed, infected female mice compared to their male counterparts. Whether this is a consequence of a robust macrophage antimicrobial activity (as suggested above for the oxidation of total BAL protein) resulting in SP-A functional deficits [20,23,25,35] or whether the oxidized dimeric form mediates important signaling pathways is not known. However, SP-A and some other proteins (as proposed by us and others) may serve as sacrificial antioxidants [33,36-39]. These proteins are highly susceptible to oxidation and may thereby lower the overall oxidant burden by reacting with reactive oxidants. Thus, the higher levels of oxidized SP-A in FA-exposed infected females may represent a protective response, and may contribute to the higher survival seen previously under the same conditions in females [21]. However, this was not the case when an additional oxidative stress was added (i.e. ozone exposure). In this case no significant differences were observed between male and female in the oxidized dimeric form of SP-A. This may explain, in part, the loss of the female advantage in survival in response to infection and ozone exposure [21].
Modulation of the SP-A host defense activity by surfactant phospholipids has been demonstrated in a number of studies [9,10,40-42]. In the present study at both 4 and 48 hour time points, in both FA- and ozone-exposed mice, the males had significantly higher levels of BAL phospholipids than females. It is possible that the higher levels in males inhibit the ability of the macrophages to clear bacteria, resulting in a more severe infection. For example, the higher levels of lipids may inhibit the respiratory burst [12,13] induced in response to infection and thereby reduce the levels of reactive oxidant species in males necessary to kill and clear bacteria. Together, these data indicate that surfactant components, including SP-A and lipids, may have a role to play in innate immunity and in infection, in particular, where sex differences in the response to infection are observed. Moreover, it appears that infection enhances the differences between FA- and ozone, because no differences were observed in a previous study between uninfected FA- and ozone-exposed mice [31].
A multi-analyte immunoassay was employed in an attempt to gain a more global insight into the early events of the previously observed sex differences in clinical outcome after exposure to ozone and infection in mice [18,21]. The analytes included cytokines and chemokines, proteins involved in the acute phase response and the coagulation cascade, and a number of other proteins associated with cell growth and differentiation and lung function. In the baseline determination (untreated male and female mice) we found that only 4 of the 59 analytes exhibited sex differences. Of these GM-CSF and IL-18 were at higher levels in males and these may be relevant to host defense function. GM-CSF enhances macrophage activity [43] and IL-18 plays a central role in initiating and regulating the inflammatory cascade [44]. Although higher levels of IL-18 have been associated with decreased survival in male mice in a model of endotoxin-induced systemic inflammation [44], no sex differences in IL-18 levels were seen in our experimental model of infection in the presence or absence of ozone exposure. This indicates that when other or multiple insults such as oxidative stress and infection are present, other pro-inflammatory factors may play a more important role.
Similarly, comparisons of FA-exposed male and female mice instilled with vehicle revealed only 3 significant differences (M-CSF, TNF-α, tissue factor) and all were at higher levels in males. Moreover, when we examined the entire set, of the 59 analytes, 42 were higher (albeit not significantly) in males. When we infected FA-exposed male mice, 21 of the analytes undergoing changes in male FA-exposed uninfected mice underwent additional significant increases compared to their uninfected levels. In nearly all cases the levels were higher in males compared to females and 11 of these were significantly higher. Cytokines and chemokines were likely increased by the infection to activate defense processes. MMP-9 was probably increased to aid in PMN migration to the site of infection [45] and its action was balanced by an increase in TIMP-1, its inhibitor. Acute phase (C-reactive protein and serum amyloid P) and coagulation proteins (Factor VII, tissue factor, von Willebrand factor) are probably increased in an attempt to limit tissue damage that may result from the newly initiated infection [46,47]. It is unclear why, if all of these important regulatory molecules are at higher levels in the male, the male exhibits poorer survival in our infection model [18,21]. We speculate that this more vigorous, and presumably less well-controlled inflammatory response is less effective in fighting infection. It is also possible that the male disadvantage may result from differential expression of some other (yet unknown) factor related to host defense.
Although FA-exposed and infected males have been shown to have a lower survival rate than their female counterparts, exposure to ozone has been shown to have a greater negative effect on females than it does on males [18,21]. In the present study, in the uninfected female mice, 29 (vs 23 in males) of the 59 analytes were significantly different when FA and ozone exposed mice were compared. These demonstrate that although oxidative stress has a profound effect on the expression of immunoregulatory molecules in both sexes, females appear to have a more exuberant response to oxidative stress (Figure 9A). When a second hit (i.e. infection) was added, again a larger number of significant changes were observed in females when FA- and ozone-exposed mice were compared (21 in females, 13 in males). Because the number of changes was somewhat lower when a second hit (i.e. infection) was added (Figure 9B), we postulate that infection may directly or indirectly alter the expression of many of these (and other) molecules. This, in turn, may result in a reduced number of changes in ozone-exposed infected mice compared to uninfected mice.
However, in both cases (with or without infection) females in the presence of ozone-induced oxidative stress showed a more exuberant response compared to males as assessed by the larger number of analytes (Figure 9) showing altered levels. In contrast, males showed a more exuberant response to infection regardless of the presence or absence of oxidative stress (Figure 8). Thus, the multi-analyte analysis indicates that, based on the number of analytes with changed levels, males and females under different conditions mount responses with various degrees of exuberance. We speculate that the more vigorous response occurs in the sex less capable of effectively handling the insult (infection in males; oxidative stress in females) and that the more vigorous response is less likely well-controlled and thus, in turn, may negatively affect survival. In fact, in the present study the more exuberant response in infected males (Figure 8A) and the more exuberant response in ozone-exposed infected females (Figure 9B) correlates with poorer survival [18,21,22]. Moreover, males after FA exposure and infection, in addition to a poorer survival [21], have been shown to have more extrapulmonary lesions in liver and spleen compared to the FA-exposed infected females [27]. The latter may compromise the ability of the spleen to mount an appropriate immune response in males in response to infection. Similarly, ozone-exposed infected females have been identified with a more pronounced decrease in pulmonary bacterial clearance, a higher increase in lung weight [22], and excessive lung inflammation as assessed by lung histopathology [27]. Of interest, females have been shown to be susceptible to other oxidative stresses including cigarette smoke [48]. Although the detailed mechanisms that could explain the previously observed differences in survival are not fully understood, the sex hormones have been shown to play a role [26].
5. Conclusion
The present data indicate that exuberant and perhaps less well-controlled responses of males and females in response to infection (males) and/or oxidative stress (females) may have negative consequences and may contribute to sex differences observed in survival in our previous studies [18,21,22,27]. Furthermore, the surfactant components, SP-A and phospholipids, may be contributing factors to the differential outcomes observed between males and females.
Supplementary Material
Highlights.
Based on the number of BAL molecules with changed levels, males appear to exhibit a more exuberant in response to infection.
Based on the number of BAL molecules with changed levels, females exhibit a more vigorous response following ozone exposure.
Surfactant lipid levels differ between males and females in response to infection and/or ozone-induced oxidative stress.
Oxidized dimeric SP-A levels differ between male and females in response to infection and/or ozone-induced oxidative stress.
BAL expression levels differ between sexes under different conditions i.e. infection and/or ozone-induced oxidative stress.
Acknowledgements
This work was supported in part by NIH HL 34788 and R01 ES009882 from the National Heart, Lung and Blood Institute and the National Institute of Environmental Health Sciences and Xiaozhuang Gan was supported by the China Scholarship Council, Beijing, China.
Abbreviations
- SP-A
surfactant protein A
- BAL
bronchoalveolar lavage
- PMN
polymorphonuclear leukocytes
- LDH
lactate dehydrogenase
- MIP-2
macrophage inflammatory protein-2
- FA
filtered air
- CFU
colony forming units
- PBS
phosphate buffered saline
- DNP
2, 4-dinitriphenylhydrazine
- ECL
enhanced chemiluminescence
- SDS
sodium dodecyl sulphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Current addresses: Rizwanul Haque, PhD, Dept. of Biotechnology, School of Earth, Biological and Environmental Science, Central University of Bihar, Patna, 800014, India. Xiaozhuang Gan, MD, Capital Institute of Pediatrics, Beijing, China. Guirong Wang, PhD, Department of Surgery, SUNY Upstate Medical University, Syracuse, NY 13210.
Reference List
- 1.Floros J, Phelps DS. Pulmonary surfactant protein A; structure, expression, and its role in innate host defense. In: Nakos G, Lekka M, editors. Update of intensive care medicine. Vol. 6. University of Ioannina; Ioannina, Greece: 2002. pp. 87–102. [Google Scholar]
- 2.Phelps DS. Surfactant regulation of host defense function in the lung: A question of balance. Pediatr Pathol Mol Med. 2001;20:269–292. [PubMed] [Google Scholar]
- 3.Phelps DS, Umstead TM, Quintero OA, Yengo CM, Floros J. In vivo rescue of alveolar macrophages from SP-A knockout mice with exogenous SP-A nearly restores a wild type intracellular proteome; actin involvement. Proteome Sci. 2011;9:67. doi: 10.1186/1477-5956-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phelps DS, Umstead TM, Floros J. Sex differences in the response of the alveolar macrophage proteome to treatment with exogenous surfactant protein-A. Proteome Sci. 2012;10:44. doi: 10.1186/1477-5956-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crouch E, Wright JR. Surfactant proteins a and d and pulmonary host defense. Annu Rev Physiol. 2001;63:521–554. doi: 10.1146/annurev.physiol.63.1.521. [DOI] [PubMed] [Google Scholar]
- 6.Wright JR. Pulmonary surfactant: a front line of lung host defense. J Clin Invest. 2003;111:1453–1455. doi: 10.1172/JCI18650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 8.Huang W, Wang G, Phelps DS, Al Mondhiry H, Floros J. Combined SP-A-bleomycin effect on cytokines by THP-1 cells: impact of surfactant lipids on this effect. Am J Physiol Lung Cell Mol Physiol. 2002;283:L94–L102. doi: 10.1152/ajplung.00434.2001. [DOI] [PubMed] [Google Scholar]
- 9.Koptides M, Umstead TM, Floros J, Phelps DS. Surfactant protein A activates NF-kappa B in the THP-1 monocytic cell line. Am J Physiol. 1997;273:L382–L388. doi: 10.1152/ajplung.1997.273.2.L382. [DOI] [PubMed] [Google Scholar]
- 10.Kremlev SG, Phelps DS. Effect of SP-A and surfactant lipids on expression of cell surface markers in the THP-1 monocytic cell line. Am J Physiol. 1997;272:L1070–L1077. doi: 10.1152/ajplung.1997.272.6.L1070. [DOI] [PubMed] [Google Scholar]
- 11.Kremlev SG, Phelps DS. Surfactant protein A stimulation of inflammatory cytokine and immunoglobulin production. Am J Physiol. 1994;267:L712–L719. doi: 10.1152/ajplung.1994.267.6.L712. [DOI] [PubMed] [Google Scholar]
- 12.Kerecman J, Mustafa SB, Vasquez MM, Dixon PS, Castro R. Immunosuppressive properties of surfactant in alveolar macrophage NR8383. Inflamm Res. 2008;57:118–125. doi: 10.1007/s00011-007-7212-1. [DOI] [PubMed] [Google Scholar]
- 13.Tonks A, Parton J, Tonks AJ, Morris RH, Finall A, Jones KP, et al. Surfactant phospholipid DPPC downregulates monocyte respiratory burst via modulation of PKC. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1070–L1080. doi: 10.1152/ajplung.00386.2004. [DOI] [PubMed] [Google Scholar]
- 14.LeVine AM, Kurak KE, Bruno MD, Stark JM, Whitsett JA, Korfhagen TR. Surfactant protein-A-deficient mice are susceptible to Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol. 1998;19:700–708. doi: 10.1165/ajrcmb.19.4.3254. [DOI] [PubMed] [Google Scholar]
- 15.LeVine AM, Hartshorn K, Elliott J, Whitsett J, Korfhagen T. Absence of SP-A modulates innate and adaptive defense responses to pulmonary influenza infection. Am J Physiol Lung Cell Mol Physiol. 2002;282:L563–L572. doi: 10.1152/ajplung.00280.2001. [DOI] [PubMed] [Google Scholar]
- 16.LeVine AM, Bruno MD, Huelsman KM, Ross GF, Whitsett JA, Korfhagen Tr. Surfactant protein A-deficient mice are susceptible to group B streptococcal infection. J Immunol. 1997;158:4336–4340. [PubMed] [Google Scholar]
- 17.LeVine AM, Gwozdz J, Stark J, Bruno M, Whitsett J, Korfhagen T. Surfactant protein-A enhances respiratory syncytial virus clearance in vivo. J Clin Invest. 1999;103:1015–1021. doi: 10.1172/JCI5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mikerov AN, Haque R, Gan X, Guo X, Phelps DS, Floros J. Ablation of SP-A has a negative impact on the susceptibility of mice to Klebsiella pneumoniae infection after ozone exposure: sex differences. Respir Res. 2008;9:77. doi: 10.1186/1465-9921-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang W, Wang G, Phelps DS, Al Mondhiry H, Floros J. Human SP-A genetic variants and bleomycin-induced cytokine production by THP-1 cells: effect of ozone-induced SP-A oxidation. Am J Physiol Lung Cell Mol Physiol. 2004;286:L546–L553. doi: 10.1152/ajplung.00267.2003. [DOI] [PubMed] [Google Scholar]
- 20.Janic B, Umstead TM, Phelps DS, Floros J. Modulatory effects of ozone on THP-1 cells in response to SP-A stimulation. Am J Physiol Lung Cell Mol Physiol. 2005;288:L317–L325. doi: 10.1152/ajplung.00125.2004. [DOI] [PubMed] [Google Scholar]
- 21.Mikerov AN, Gan X, Umstead TM, Miller L, Chinchilli VM, Phelps DS, et al. Sex differences in the impact of ozone on survival and alveolar macrophage function of mice after Klebsiella pneumoniae infection. Respir Res. 2008;9 doi: 10.1186/1465-9921-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mikerov AN, Hu S, Durrani F, Gan X, Wang G, Umstead TM, et al. Impact of sex and ozone exposure on the course of pneumonia in wild type and SP-A (−/−) mice. Microb Pathog. 2012;52:239–249. doi: 10.1016/j.micpath.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang G, Umstead TM, Phelps DS, Al Mondhiry H, Floros J. The effect of ozone exposure on the ability of human surfactant protein A variants to stimulate cytokine production. Environ Health Perspect. 2002;110:79–84. doi: 10.1289/ehp.0211079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis IC, Zhu S, Sampson JB, Crow JP, Matalon S. Inhibition of human surfactant protein A function by oxidation intermediates of nitrite. Free Radic Biol Med. 2002;33:1703–1713. doi: 10.1016/s0891-5849(02)01170-x. [DOI] [PubMed] [Google Scholar]
- 25.Mikerov AN, Umstead TM, Gan X, Huang W, Guo X, Wang G, et al. Impact of ozone exposure on the phagocytic activity of human surfactant protein A (SP-A) and SP-A variants. Am J Physiol Lung Cell Mol Physiol. 2008;294:L121–L130. doi: 10.1152/ajplung.00288.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durrani F, Phelps DS, Weisz J, Silveyra P, Hu S, Mikerov AN, et al. Gonadal hormones and oxidative stress interaction differentially affects survival of male and female mice after lung Klebsiella Pneumoniae infection. Exp Lung Res. 2011;38:165–172. doi: 10.3109/01902148.2011.654045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mikerov AN, Cooper TK, Wang G, Hu S, Umstead TM, Phelps DS, et al. Histopathologic evaluation of lung and extrapulmonary tissues show sex differences in Klebsiella pneumoniae - infected mice under different exposure conditions. Int J Physiol Pathophysiol Pharmacol. 2011;3:176–190. [PMC free article] [PubMed] [Google Scholar]
- 28.Hollingsworth JW, Maruoka S, Li Z, Potts EN, Brass DM, Garantziotis S, et al. Ambient ozone primes pulmonary innate immunity in mice. J Immunol. 2007;179:4367–4375. doi: 10.4049/jimmunol.179.7.4367. [DOI] [PubMed] [Google Scholar]
- 29.Kierstein S, Krytska K, Sharma S, Amrani Y, Salmon M, Panettieri RA, Jr., et al. Ozone inhalation induces exacerbation of eosinophilic airway inflammation and hyperresponsiveness in allergen-sensitized mice. Allergy. 2008;63:438–446. doi: 10.1111/j.1398-9995.2007.01587.x. [DOI] [PubMed] [Google Scholar]
- 30.Hulo S, Tiesset H, Lancel S, Edme JL, Viollet B, Sobaszek A, et al. AMP-activated protein kinase deficiency reduces ozone-induced lung injury and oxidative stress in mice. Respir Res. 2011;12:64. doi: 10.1186/1465-9921-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haque R, Umstead TM, Ponnuru P, Guo X, Hawgood S, Phelps DS, et al. Role of surfactant protein-A (SP-A) in lung injury in response to acute ozone exposure of SP-A deficient mice. Toxicol Appl Pharmacol. 2007;220:72–82. doi: 10.1016/j.taap.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatch GE, Slade R, Harris LP, McDonnell WF, Devlin RB, Koren HS, et al. Ozone dose and effect in humans and rats. A comparison using oxygen-18 labeling and bronchoalveolar lavage. Am J Respir Crit Care Med. 1994;150:676–683. doi: 10.1164/ajrccm.150.3.8087337. [DOI] [PubMed] [Google Scholar]
- 33.Haque R, Umstead TM, Ahn MH, Phelps DS, Floros J. Effect of low doses of lipopolysaccharide prior to ozone exposure on bronchoalveolar lavage: Differences between wild type and surfactant protein A-deficient mice. Pneumon. 2009;22:143–155. [PMC free article] [PubMed] [Google Scholar]
- 34.Floros J, Wang G, Mikerov AN. Genetic complexity of the human innate host defense molecules, surfactant protein A1 (SP-A1) and SP-A2--impact on function. Crit Rev Eukaryot Gene Expr. 2009;19:125–137. doi: 10.1615/critreveukargeneexpr.v19.i2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang G, Bates-Kenney SR, Tao JQ, Phelps DS, Floros J. Differences in biochemical properties and in biological function between human SP-A1 and SP-A2 variants, and the impact of ozone-induced oxidation. Biochemistry. 2004;43:4227–4239. doi: 10.1021/bi036023i. [DOI] [PubMed] [Google Scholar]
- 36.Kim IG, Park SY, Oh TJ. Dithiothreitol induces the sacrificial antioxidant property of human serum albumin in a metal-catalyzed oxidation and gamma-irradiation system. Arch Biochem Biophys. 2001;388:1–6. doi: 10.1006/abbi.2000.2255. [DOI] [PubMed] [Google Scholar]
- 37.Burcham PC. Potentialities and Pitfalls Accompanying Chemico-Pharmacological Strategies against Endogenous Electrophiles and Carbonyl Stress. Chem Res Toxicol. 2008 doi: 10.1021/tx700399q. [DOI] [PubMed] [Google Scholar]
- 38.Sayed AA, Cook SK, Williams DL. Redox balance mechanisms in Schistosoma mansoni rely on peroxiredoxins and albumin and implicate peroxiredoxins as novel drug targets. J Biol Chem. 2006;281:17001–17010. doi: 10.1074/jbc.M512601200. [DOI] [PubMed] [Google Scholar]
- 39.Umstead TM, Freeman WM, Chinchilli VM, Phelps DS. Age-related changes in the expression and oxidation of bronchoalveolar lavage proteins in the rat. Am J Physiol Lung Cell Mol Physiol. 2009;296:L14–L29. doi: 10.1152/ajplung.90366.2008. [DOI] [PubMed] [Google Scholar]
- 40.Kremlev SG, Umstead TM, Phelps DS. Effects of surfactant protein A and surfactant lipids on lymphocyte proliferation in vitro. Am J Physiol. 1994;267:L357–L364. doi: 10.1152/ajplung.1994.267.4.L357. [DOI] [PubMed] [Google Scholar]
- 41.Kremlev SG, Umstead TM, Phelps DS. Surfactant protein A regulates cytokine production in the monocytic cell line THP-1. Am J Physiol. 1997;272:L996–1004. doi: 10.1152/ajplung.1997.272.5.L996. [DOI] [PubMed] [Google Scholar]
- 42.Song M, Phelps DS. Comparison of SP-A and LPS effects on the THP-1 monocytic cell line. Am J Physiol Lung Cell Mol Physiol. 2000;279:L110–L117. doi: 10.1152/ajplung.2000.279.1.L110. [DOI] [PubMed] [Google Scholar]
- 43.Trapnell BC, Whitsett JA. Gm-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol. 2002;64:775–802. doi: 10.1146/annurev.physiol.64.090601.113847. [DOI] [PubMed] [Google Scholar]
- 44.Aoyama M, Kotani J, Usami M. Gender difference in granulocyte dynamics and apoptosis and the role of IL-18 during endotoxin-induced systemic inflammation. Shock. 2009;32:401–409. doi: 10.1097/SHK.0b013e31819c358a. [DOI] [PubMed] [Google Scholar]
- 45.Bradley LM, Douglass MF, Chatterjee D, Akira S, Baaten BJ. Matrix metalloprotease 9 mediates neutrophil migration into the airways in response to influenza virus-induced toll-like receptor signaling. PLoS Pathog. 2012;8:e1002641. doi: 10.1371/journal.ppat.1002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mercer PF, Chambers RC. Coagulation and coagulation signalling in fibrosis. Biochim Biophys Acta. 2013;1832:1018–1027. doi: 10.1016/j.bbadis.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 47.Abrams ST, Zhang N, Dart C, Wang SS, Thachil J, Guan Y, et al. Human CRP defends against the toxicity of circulating histones. J Immunol. 2013;191:2495–2502. doi: 10.4049/jimmunol.1203181. [DOI] [PubMed] [Google Scholar]
- 48.Ben Zaken CS, Pare PD, Man SF, Sin DD. The growing burden of chronic obstructive pulmonary disease and lung cancer in women: examining sex differences in cigarette smoke metabolism. Am J Respir Crit Care Med. 2007;176:113–120. doi: 10.1164/rccm.200611-1655PP. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.