Abstract
Aims
In the HORIZONS trial, in-hospital treatment with bivalirudin reduced bleeding and mortality in primary percutaneous coronary intervention (PCI) compared with heparin and routine glycoprotein IIb/IIIa inhibitors (GPI). It is unknown whether this advantage of bivalirudin is observed in comparison with heparins only with GPI used as bailout.
Methods and results
In the EUROMAX study, 2198 patients with ST-segment elevation myocardial infarction (STEMI) were randomized during transport for primary PCI to bivalirudin or to heparins with optional GPI. Primary and principal outcome was the composites of death or non-CABG-related major bleeding at 30 days. This pre-specified analysis compared patients receiving bivalirudin (n = 1089) with those receiving heparins with routine upstream GPI (n = 649) and those receiving heparins only with GPI use restricted to bailout (n = 460). The primary outcome death and major bleeding occurred in 5.1% with bivalirudin, 7.6% with heparin plus routine GPI (HR 0.67 and 95% CI 0.46–0.97, P = 0.034), and 9.8% with heparins plus bailout GPI (HR 0.52 and 95% CI 0.35–0.75, P = 0.006). Following adjustment by logistic regression, bivalirudin was still associated with significantly lower rates of the primary outcome (odds ratio 0.53, 95% CI 0.33–0.87) and major bleeding (odds ratio 0.44, 95% CI 0.24–0.82) compared with heparins alone with bailout GPI. Rates of stent thrombosis were higher with bivalirudin (1.6 vs. 0.6 vs. 0.4%, P = 0.09 and 0.09).
Conclusion
Bivalirudin, started during transport for primary PCI, reduces major bleeding compared with both patients treated with heparin only plus bailout GPI and patients treated with heparin and routine GPI, but increased stent thrombosis.
Keywords: Bivalirudin, Glycoprotein IIb/IIIa inhibitors, ST-segment elevation myocardial infarction, Primary percutaneous coronary intervention, Heparins
See page 2448 for the editorial comment on this article (doi:10.1093/eurheartj/ehu223)
Introduction
According to the current guidelines of the European Society of Cardiology (ESC) for patients with acute myocardial infarction with persistent ST-segment elevation, intravenous anticoagulation is recommended in all patients undergoing primary percutaneous coronary intervention (PCI).1 Unfractionated heparin (UFH) has traditionally been used in the catheterization laboratory as the anticoagulant of choice during primary PCI to prevent thrombosis and ischaemic events. However, UFH has several pharmacological limitations including a high intra- and inter-individual variability.2 Bivalirudin is a thrombin-specific anticoagulant, which has the ability to inactivate both free and fibrin-bound thrombin.3 In several randomized clinical trials, bivalirudin has demonstrated efficacy and safety in patients undergoing PCI.4–6 In the HORIZONS trial, bivalirudin reduced the primary combined endpoint including death, myocardial infarction and bleeding complications compared to heparin plus routine glycoprotein IIb/IIIa inhibitors (GPI) in patients undergoing primary PCI.6 Moreover, cardiovascular mortality was reduced after 30 days and at 3-year follow-up.7 However, the use of GPI is not always routine in clinical practice anymore and might have contributed to the increase in bleeding complications seen in the control arm. In the EUROMAX trial, the use of GPI was not mandated, but was left to the standard practice of participating centres.8,9 This provided the opportunity to compare bivalirudin to heparins without planned use of GPI.
Methods
Study population
The EUROMAX study design has been previously described in detail.8 In brief, the study enrolled patients 18 years of age or older, presenting within 12 h after the onset of symptoms with a presumed diagnosis of ST-segment elevation myocardial infarction (STEMI). All patients had to be scheduled for angiography with the intention of performing primary PCI within 2 h after the first medical contact. Patients were identified, initial consent was obtained, randomization was performed, and study drug administration was initiated in the ambulance or in a non-PCI hospital. Patients were transported urgently to the primary PCI hospital, where treatment was continued and outcomes data collected. All patients provided written, informed consent and the study was approved by local ethics committees and health authorities.
Study treatments
Patients were randomized to treatment with either bivalirudin or heparins (control group). Bivalirudin was to be administered as a bolus of 0.75 mg/kg, followed by an infusion of 1.75 mg/kg/h. The protocol specified that the infusion should be continued for at least 4 h after PCI at a dose of be 0.25 mg/kg/h; however, continuation of the full dose (1.75 mg/kg/h) used during PCI was also permitted.
Both the decision for the use and the selection of GPI agent were left up to the investigator's discretion and preference. The use of GPI was classified as routine when treatment commenced before or during angiography but not after the start of PCI. Bailout use of GPI was permitted after the commencement of PCI in both bivalirudin- and heparin-treated patients, but was limited according to the protocol only for the presence of giant thrombus or no-reflow during or after the index procedure.
Patients who were assigned to the heparins group (control group) were to receive either UFH or low-molecular-weight heparin (LMWH). Unfractionated heparin was to be administered at a dose of 100 IU/kg without a GPI or 60 IU/kg with a GPI; LMWH was to be given as a bolus of 0.5 mg/kg.
All patients received aspirin and an approved P2Y12 inhibitor as early as possible after the first medical contact. Decisions regarding access site, performance of thrombus aspiration, and stent type were left to physician preference.
Study outcomes
The primary outcome was the composite of death from any cause or protocol major bleeding not related to coronary-artery bypass grafting (CABG) at 30 days. The principal secondary 30-day outcome was a composite of death from any cause, myocardial re-infarction, or non-CABG major bleeding. Pre-specified secondary endpoints included major adverse cardiovascular events [MACE; death, reinfarction, ischaemia-driven revascularization (IDR), or stroke], net adverse clinical events (NACE; composite of major adverse cardiovascular events and non-CABG major bleeding), each of the components of the primary and principal secondary outcomes, IDR, and stent thrombosis (as defined by the Academic Research Consortium).10
Protocol-defined major bleeding was characterized as bleeding unrelated to CABG surgery that included intracranial, retroperitoneal, or intraocular bleeding; access-site haemorrhage requiring radiological or surgical intervention; a reduction in the haemoglobin level of more than 4 g/dL (2.5 mmol/L) without an overt source of bleeding; a reduction in the haemoglobin level of >3 g/dL (1.8 mmol/L) with an overt source of bleeding; reintervention for bleeding; or use of any blood-product transfusion. Note that access-site haematomas which did not require surgical or radiological intervention were not counted as major bleeds regardless of their size.
All cases of deaths, bleeding events, reinfarction, IDR, stent thrombosis, and stroke were adjudicated by a blinded, independent clinical-events committee.
In the present pre-specified analysis, we compared outcomes among patients with routine bivalirudin treatment to patients in the control arm according to GPI use (heparin plus routine GPI vs. heparin without routine GPI). In patients randomized to the control arm, the use of a GPI was classified as either ‘routine’ (treatment of patients before or during angiography but not once PCI has commenced) or ‘bailout’ (treatment of patients during or after PCI).
Statistical analysis
Analyses were performed in the intention-to-treat (ITT) population, which was defined as all patients who underwent randomization and provided written informed consent. The chi-square test was used for comparisons of event rates or, in the case of sparse data, Fisher's exact test was used. Log-rank test was used to compute the significance of time-to-event data. Continuous variables are reported as medians and interquartile ranges. A P-value of <0.05 was considered significant. Categorical variables are reported as frequencies and percentages. To account for differences in baseline characteristics in this post-randomization subgroup analysis, a logistic regression analysis was performed. Variables in the logistic regression model included: age over 65 years, female sex, anaemia, hypertension, hyperlipidaemia, diabetes, smoking, creatinine clearance ≤60 mL/min, choice of P2Y12 inhibitor, prior myocardial infarction, prior CABG, prior PCI, Killip class ≥II, access-site (femoral vs. radial), pre-PCI TIMI Flow 0/1, single-vessel disease, placement of a drug-eluting stent, and treatment with bivalirudin vs. heparins with routine GPI vs. heparins with bailout GPI only. Analyses were performed with the use of SAS software, version 9.2.
Results
Patients
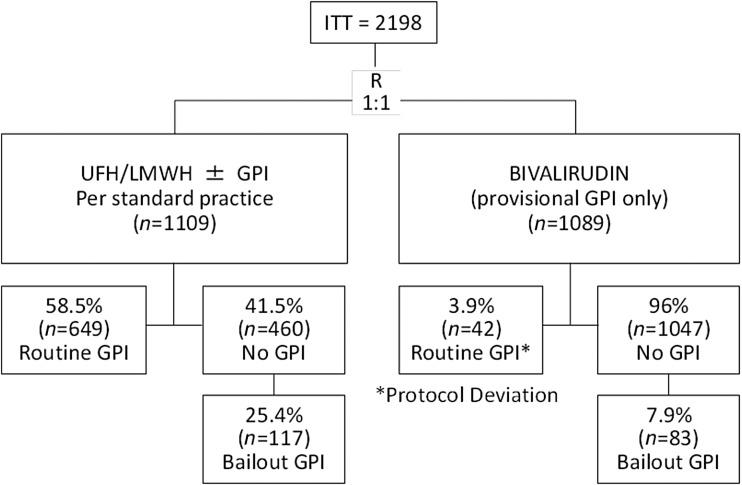
A total of 2198 patients (1089 in the bivalirudin group and 1109 in the control group) provided formal informed consent and comprised the ITT population. A breakdown of GPI use by treatment arm is presented in Figure 1. In the control arm, 649 (58.5%) patients received routine GPI and 460 (41.5%) did not. In the routine GPI group, GPI treatment was initiated in the ambulance in 68% and during coronary angiography in 32% of patients. Of the 460 patients who did not receive routine use of a GPI, 117 (25.4%) patients received it as bailout during PCI and most frequently due to the presence of giant thrombus. In the bivalirudin group, 1047 (96%) patients did not receive a routine GPI, while 42 (3.9%) patients did, despite the fact that this was a deviation from the protocol. Of the 1047 patients who did not receive routine GPI, 83 (7.9%) received it as bailout also more commonly due to the presence of giant thrombus.
Figure 1.
GPI use by randomized treatment group. GPI, glycoprotein IIb/IIIa inhibitor; ITT, intent-to-treat; LMWH, low-molecular-weight heparin; UFH, unfractionated heparin.
The baseline characteristics of patients in the three groups were generally well matched between the groups, although there was a higher rate of hypertension and lower rates of Killip class II–IV and creatinine clearance ≤60 mL/min in the heparins with routine GPI use treatment group compared with the heparins with bailout GPI use group (Table 1).
Table 1.
Baseline characteristics
| Characteristics | Bivalirudin (N = 1089) n/N (%) |
Heparins + routine GPI (N = 649) n/N (%) |
Heparins + bailout GPI (N = 460) n/N (%) |
|---|---|---|---|
| Bailout GPIa | 83/1046 (7.9) | N/A | 117/460 (25.4) |
| Age, median (IQR), years | 61 (52, 71) | 61 (52, 71) | 62 (53, 73) |
| Age >65 years | 394 (36.2) | 245 (37.8) | 189 (41.1) |
| Female | 275 (25.3) | 144 (22.2) | 104 (22.6) |
| Diabetes | 127 (11.7) | 89/648 (13.7) | 80 (17.4) |
| Current smoker (within past 30 days) | 453 (41.6) | 271/648 (41.8) | 201 (43.7) |
| Hypertension† | 459 (42.2) | 261/648 (40.3) | 243 (52.8) |
| Previous myocardial infarction | 80 (7.4) | 65/648 (10.0) | 48 (10.4) |
| Previous PTCA/PCI | 97 (8.9) | 57/648 (8.8) | 51 (11.1) |
| Previous CABG | 18 (1.7) | 14/648 (2.2) | 15 (3.3) |
| Hyperlipidaemiab | 398 (36.6) | 222/648 (34.3) | 195 (42.4) |
| Killip class II–IV† | 77/996 (7.7) | 27/568 (4.8) | 42/432 (9.7) |
| Creatinine clearance ≤60 mL/min† | 147/1001 (14.7) | 81/617 (13.1) | 84/381 (22.0) |
| Creatinine clearance >60 mL/min | 854/1001 (85.3) | 536/617 (86.9) | 297/381 (78.0) |
| Anaemia | 129/987 (13.1) | 86/606 (14.2) | 62/383 (16.2) |
CABG, coronary artery bypass graft surgery; GPI, glycoprotein IIb/IIIa inhibitor; IQR, interquartile range; N/A, not applicable; PCI, percutaneous coronary intervention; PTCA, percutaneous transluminal coronary angioplasty.
aData are provided for patients who were eligible for bailout use of a GPI (i.e. those who did not receive the drug routinely).
bKnown hyperlipidaemia or on lipid-lowering drugs.
†P < 0.05.
Procedures and treatments
Study medications and procedural details are presented in Table 2. Enoxaparin was used in 51/649 (8%) and 43/460 (9%) of patients treated with routine or with bailout GPI, respectively. A similar percentage of patients in each group received a loading dose of a P2Y12 inhibitor, with clopidogrel as the most frequently given agent. A significantly higher percentage of patients in the heparins plus bailout GPI group received prasugrel compared with the heparins plus routine GPI group. Conversely, ticagrelor was more commonly given to patients in the heparins plus routine GPI compared with heparins with bailout GPI group.
Table 2.
Treatment and procedural characteristics
| Variable | Bivalirudin (N=1089) n/N (%) |
Heparins + routine GPI (N = 649) n/N (%) |
Heparins + bailout GPI (N = 460) n/N (%) |
|---|---|---|---|
| P2Y12 loading dose | 1048/1066 (98.3) | 629/643 (97.8) | 429/440 (97.5) |
| Clopidogrel | 524/1048 (50.0) | 329/627 (52.5) | 216/429 (50.3) |
| Prasugrel* | 323/1048 (30.8) | 119/627 (19.0) | 187/429 (43.6) |
| Ticagrelor* | 201/1048 (19.2) | 179/627 (28.5) | 26/429 (6.1) |
| P2Y12 maintenance dose | 958/1065 (90.0) | 585/643 (91.0) | 384/439 (87.5) |
| Clopidogrel | 377/955(39.5) | 220/583 (37.7) | 187/381 (49.1) |
| Prasugrel* | 321/955 (33.6) | 135/583 (23.2) | 163/381 (42.8) |
| Ticagrelor* | 257/955(26.9) | 228/583 (39.1) | 31/381 (8.1) |
| Catheter access site | |||
| Femoral* | 558/1069 (52.2) | 374/631 (59.3) | 208/453 (45.9) |
| Radial | 510/1069 (47.7) | 257/631 (40.7) | 245/453 (54.1) |
| Single-vessel disease* | 591/1069 (55.3) | 348/631 (55.2) | 208/453 (45.9) |
| Pre-PCI TIMI flow | |||
| 0 to 1* | 593/931 (63.7) | 319/563 (56.7) | 244/369 (66.1) |
| 2 | 143/931 (15.4) | 112/563 (19.9) | 46/369 (12.5) |
| 3 | 195/931 (20.9) | 132/563 (23.4) | 79/369 (21.4) |
| Post-PCI TIMI flow | |||
| 0 to 1 | 18/930 (1.9) | 7/563 (1.2) | 9/369 (2.4) |
| 2 | 29/930 (3.1) | 22/563 (3.9) | 9/369 (2.4) |
| 3 | 883/930 (94.9) | 534/563 (94.8) | 351/369 (95.1) |
| Drug-eluting stent* | 538/943 (57.1) | 364/573 (63.5) | 165/373 (44.2) |
| Thrombectomy | 304/943 (32.2) | 192/573 (33.5) | 106/373 (28.4) |
| Balloon angioplasty only | 48/943 (5.1) | 29/573 (5.1) | 13/373 (3.5) |
GPI, glycoprotein IIb/IIIa inhibitor; PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction.
*P < 0.05.
Femoral artery access, drug-eluting stent use, and the presence of single-vessel disease were all more common in the heparins plus routine GPI group, while pre-PCI TIMI flow of 0 or 1 was more frequent among the heparins with bailout GPI patients.
Outcomes
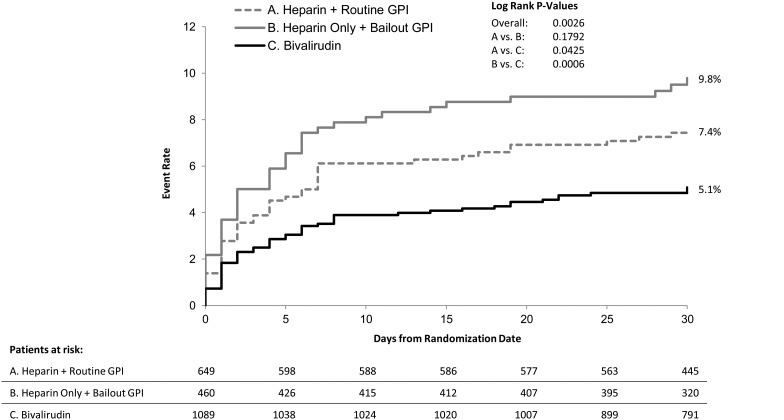
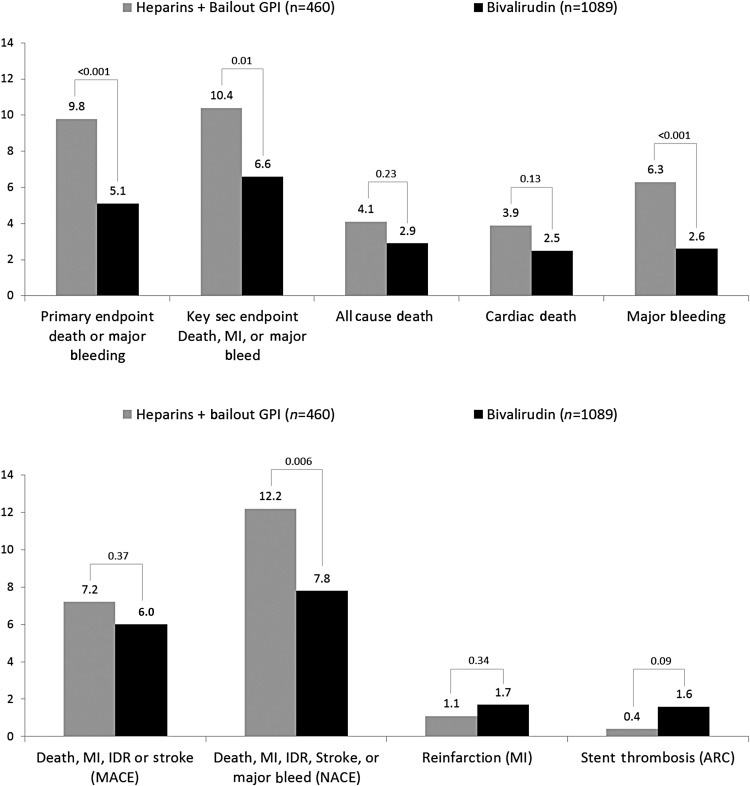
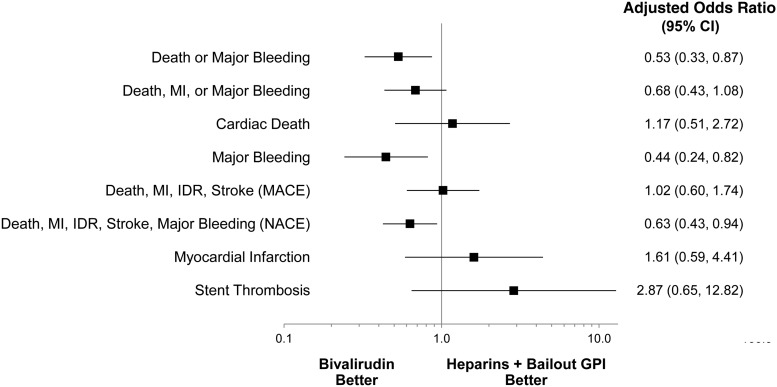
Comparisons of unadjusted event rates between the three treatment groups are shown in Table 3. Among patients treated with heparins, there were no significant differences observed in the rates of either the primary or key secondary outcomes according to the use of GPI. The composite of ischaemic events (MACE), however, was significantly lower in patients treated with heparins plus routine GPI compared with patients treated with heparin and bailout GPI (4.3 vs. 7.2%, P = 0.04). In the comparison between bivalirudin and either of the heparins arms the results were consistent with the overall results of the main trial. Specifically, bivalirudin resulted in significantly lower rates of the primary outcome and protocol major bleeding (Table 3, Figure 2), as well as a higher rate of stent thrombosis. After adjustment, the benefit of bivalirudin over heparins with bailout GPI for the primary outcome, major bleeding, and NACE was maintained (Figures 3 and 4).
Table 3.
Primary and secondary outcomes by treatment group
| Parameter | Bivalirudin (N = 1089) n (%) |
Heparins + routine GPI (N = 649) n (%) |
Relative risk (95% CI)a |
P-valuea | Heparins + bailout GPI (N = 460) n (%) |
Relative risk (95% CI)b |
P-valueb | Relative risk (95% CI)c |
P-valuec |
|---|---|---|---|---|---|---|---|---|---|
| Primary endpoint: death or major bleeding | 55 (5.1) | 49 (7.6) | 0.67 (0.46, 0.97) | 0.03 | 45 (9.8) | 0.52 (0.35, 0.75) | 0.0006 | 1.30 (0.88, 1.91) | 0.19 |
| Key secondary endpoint: death, MI, or major bleeding | 72 (6.6) | 54 (8.3) | 0.79 (0.57, 1.12) | 0.18 | 48 (10.4) | 0.63 (0.45, 0.90) | 0.01 | 1.25 (0.87, 1.82) | 0.23 |
| Death | 32 (2.9) | 15 (2.3) | 1.27 (0.69, 2.33) | 0.44 | 19 (4.1) | 0.71 (0.41, 1.24) | 0.23 | 1.79 (0.92, 3.48) | 0.09 |
| Cardiac cause | 27 (2.5) | 15 (2.3) | 1.07 (0.58, 2.00) | 0.83 | 18 (3.9) | 0.63 (0.35, 1.14) | 0.13 | 1.69 (0.86, 3.32) | 0.13 |
| Protocol major bleeding | 28 (2.6) | 38 (5.9) | 0.44 (0.27, 0.71) | 0.0007 | 29 (6.3) | 0.41 (0.25, 0.68) | 0.0005 | 1.08 (0.67, 1.72) | 0.76 |
| Death, MI, IDR, or stroke (MACE) | 65 (6.0) | 28 (4.3) | 1.38 (0.90, 2.13) | 0.14 | 33 (7.2) | 0.83 (0.56, 1.25) | 0.37 | 1.66 (1.02, 2.71) | 0.04 |
| Death, MI, IDR, stroke, or major bleeding (NACE) | 85 (7.8) | 62 (9.6) | 0.82 (0.60, 1.12) | 0.21 | 56 (12.2) | 0.64 (0.47, 0.88) | 0.006 | 1.27 (0.91, 1.79) | 0.16 |
| Myocardial infarction (MI) | 19 (1.7) | 5 (0.8) | 2.26 (0.85, 6.04) | 0.10 | 5 (1.1) | 0.61 (0.60, 4.27) | 0.34 | 1.41 (0.41, 4.85) | 0.58 |
| Stent thrombosis | 17 (1.6) | 4 (0.6) | 2.53 (0.86, 7.49) | 0.09 | 2 (0.4) | 3.59 (0.83, 15.48) | 0.09 | 0.71 (0.13, 3.84) | 0.69 |
CABG, coronary artery bypass graft; CI, confidence interval; GPI, glycoprotein IIb/IIIa inhibitor; IDR, ischaemia-driven revascularization; MACE, major adverse cardiovascular events; N/A, not applicable; NACE, net adverse clinical events.
aRelative risk (95% CI) and P-value comparing bivalirudin with heparins + routine GPI.
bRelative risk (95% CI) and P-value comparing bivalirudin with heparins + bailout GPI.
cRelative risk (95% CI) and P-value comparing heparins + bailout GPI with heparins + routine GPI.
Figure 2.
Kaplan–Meier curves of the primary composite endpoint. GPI, glycoprotein IIb/IIIa inhibitor.
Figure 3.
Unadjusted outcomes of bivalirudin vs. heparins + bailout GPI. ARC, Academic Research Consortium; GPI, glycoprotein IIb/IIIa inhibitor; IDR, ischaemia-driven revascularization; MACE, major adverse cardiac events; MI, myocardial infarction; NACE, net adverse clinical events.
Figure 4.
Adjusted ORs of bivalirudin vs. heparin + bailout GPI. GPI, glycoprotein IIb/IIIa inhibitor; IDR, ischaemia-driven revascularization; MACE, major adverse cardiac events; MI, myocardial infarction; NACE, net adverse clinical events.
A total of 94 patients in the control group received enoxaparin, 62 (66%) of the latter were treated with routine GPI. The rates of the primary endpoint were not different between the patients treated with enoxaparin and UFH (9.6 vs. 8.2%, odds ratio 1.16, 95% CI 0.60–2.24, P-value) (see Supplementary material online, Table S1).
Discussion
The main finding of this pre-specified subgroup analysis of the EUROMAX trial is that a strategy of upstream bivalirudin with bailout use of GPI in <10% of patients reduces the composite endpoint of death or major bleeding compared with a strategy of heparins plus bailout use of GPI and a strategy of heparins plus a routine upstream use of GPI in patients with STEMI scheduled for primary PCI. Although this analysis was pre-specified, it still represents a post-randomization subgroup comparison within an open-label trial and should therefore be considered as hypothesis generating rather than definitive.
In the HORIZONS-AMI trial, bivalirudin administered in the hospital reduced the combined clinical endpoint of death, myocardial infraction, and major bleeding compared with UFH and routine use of GPI.6 However, contemporary practice has since evolved to include earlier treatment with initiation in the pre-hospital phase, increasing use of prasugrel and ticagrelor and a rise in the adoption of the radial approach. One point of criticism on HORIZONS-AMI has been the routine use of GPI, which is not recommended in the current ESC-STEMI guidelines1 and many operators have adopted a bailout only strategy for GPI. It has been argued that the routine use of GPI may have contributed to the higher bleeding rate, without any benefit on ischaemic outcomes. However, several randomized trials have consistently shown the benefit of GPI when added to heparin in this setting.11–13 In the ON TIME-2 trial, upstream tirofiban given in the ambulance was beneficial compared with selective use during primary PCI.14
The EUROMAX trial tested the hypothesis that bivalirudin, started upstream before primary PCI, is beneficial in the context of the use of the new antiplatelet drugs prasugrel and ticagrelor and a high use of radial access.9 The results of this pre-specified subgroup analysis are consistent with the overall trial results and provide evidence that the benefits of bivalirudin over heparins are consistent regardless of the use of GPI. Interestingly, the strategy of upstream heparins alone with bailout GPI was associated even with higher ischaemic and bleeding complications compared with the routine use of heparin and upstream GPI, replicating the results of ON-TIME 2.14 In a previous randomized trial comparing bivalirudin vs. heparin during PCI in patients with acute coronary syndromes, bivalirudin reduced ischaemic complications (6.2 vs. 7.9% , P = 0.039) and bleeding complications (3.5 vs. 9.3%, P < 0.001) compared with heparin.15 A recent meta-analysis found a consistent reduction of bleeding complications of bivalirudin vs. heparin regardless of the bleeding risk of the patients.16 The advantage of bivalirudin was observed regardless of the planned (OR = 0.58, 95% CI 0.47–0.72) or provisional use (OR = 0.40, 95% CI 0.32–0.51).
Importantly, patients treated with bivalirudin were at higher risk for acute stent thrombosis, an observation consistent with the results of HORIZONS-AMI. The excess risk for acute stent thrombosis was limited to the first 4 h after the index procedure and was probably the result of the combination of the short half-life and rapid clearance of bivalirudin and the delayed bioavailability of the oral P2Y12 inhibitors, including the newer agents prasugrel and ticagrelor.16 Possible treatments that could mitigate this risk could include co-administration of UFH, prolongation of the bivalirudin infusion at the PCI dose for the first few hours after the procedure, or the use of an immediate acting P2Y12 inhibitor such as cangrelor; however, they will need to be tested in prospective trials.
Limitations
The data presented derive from a pre-specified but post-randomization analysis. The decision to use an upstream therapy with heparin only or heparin plus routine GPI was completely left to the discretion of the investigators and therefore, the equilibrium of randomization in baseline characteristics is potentially lost. Therefore, these results should be considered as hypothesis generating rather than definitive. Since enoxaparin was given in only 94 (8.4%) patients the results apply only to the use of UFH, which has been shown to be inferior to enoxaparin in the ATOLL trial.17 EUROMAX was an open-label trial due to the logistic difficulties related to implementation of complex antithrombotic regimens in the pre-hospital setting while rushing patients to primary PCI. However, all events were reviewed by a central adjudication committee blinded to treatment allocation.
Conclusion
In this pre-specified subgroup analysis from EUROMAX, pre-hospital bivalirudin reduced the composite outcome of death or major bleeding compared with both heparins with routine GPI and heparins with only bailout GPI , an effect largely driven by marked reductions in major bleeding.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by The Medicines Company, Parsippany, NJ, USA. Funding to pay the Open Access publication charges for this article was provided by The Medicines Company, New York, USA.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the excellent editing work of Jayne Prats, employee of The Medicines Company, by the preparation of the manuscript.
Conflict of interest: J.D., E.N.D, and D.B. are employees of the Medicines Company. U.Z., A. H., L.N., P. C., K.H., P. G., C.H. and P.G. S, have received speakers honoraria from the Medicines Company.
References
- 1.Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van 't Hof A, Widimsky P, Zahger D. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 2.Hirsh J, Warkentin TE, Shaughnessy SG, Anand SS, Halperin JL, Raschke R, Granger C, Ohman EM, Dalen JE. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing considerations, monitoring, efficacy, and safety. Chest. 2001;119(1 suppl):64S–94S. doi: 10.1378/chest.119.1_suppl.64s. [DOI] [PubMed] [Google Scholar]
- 3.Bates S, Weitz JI. Direct thrombin inhibitors for treatment of arterial thrombosis: potential differences between bivalirudin and hirudin. Am J Cardiol. 1998;82:12P–18P. doi: 10.1016/s0002-9149(98)00660-2. [DOI] [PubMed] [Google Scholar]
- 4.Lincoff AM, Bittl JA, Harrington RA, Feit F, Kleiman NS, Jackman JD, Sarembock IJ, Cohen DJ, Spriggs D, Ebrahimi R, Keren G, Carr J, Cohen EA, Betriu A, Desmet W, Kereiakes DJ, Rutsch W, Wilcox RG, de Feyter PJ, Vahanian A, Topol EJ REPLACE-2 Investigators. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA. 2003;289:853–863. doi: 10.1001/jama.289.7.853. [DOI] [PubMed] [Google Scholar]
- 5.Stone GW, McLaurin BT, Cox DA, Bertrand ME, Lincoff AM, Moses JW, White HD, Pocock SJ, Ware JH, Feit F, Colombo A, Aylward PE, Cequier AR, Darius H, Desmet W, Ebrahimi R, Hamon M, Rasmussen LH, Rupprecht HJ, Hoekstra J, Mehran R, Ohman EM ACUITY Investigators. Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006;355:2203–2216. doi: 10.1056/NEJMoa062437. [DOI] [PubMed] [Google Scholar]
- 6.Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Dangas G, Wong SC, Fahy M, Parise H, Mehran R HORIZONS-AMI Trial Investigators. Heparin plus a glycoprotein IIb/IIIa inhibitor versus bivalirudin monotherapy and paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction (HORIZONS-AMI): final 3-year results from a multicentre, randomised controlled trial. Lancet. 2011;377:2193–2204. doi: 10.1016/S0140-6736(11)60764-2. [DOI] [PubMed] [Google Scholar]
- 7.Steg PG, van ‘t Hof A, Clemmensen P, Lapostolle F, Dudek D, Hamon M, Cavallini C, Gordini G, Huber K, Coste P, Thicoipe M, Nibbe L, Steinmetz J, Ten Berg J, Eggink GJ, Zeymer U, Campo dell’ Orto M, Kanic V, Deliargyris EN, Day J, Schuette D, Hamm CW, Goldstein P. Design and methods of European Ambulance Acute Coronary Syndrome Angiography Trial (EUROMAX): an international randomized open-label ambulance trial of bivalirudin versus standard-of-care anticoagulation in patients with acute ST-segment-elevation myocardial infarction transferred for primary percutaneous coronary intervention. Am Heart J. 2013;166:960–967. doi: 10.1016/j.ahj.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 8.Steg PG, van ‘t Hof A, Hamm CW, Clemmensen P, Lapostolle F, Coste P, Ten Berg J, Van Grunsven P, Eggink GJ, Nibbe L, Zeymer U, Campo dell’ Orto M, Nef H, Steinmetz J, Soulat L, Huber K, Deliargyris EN, Bernstein D, Schuette D, Prats J, Clayton T, Pocock S, Hamon M, Goldstein P EUROMAX Investigators. Bivalirudin started during emergency transport for primary PCI. N Engl J Med. 2013;369:2207–2217. doi: 10.1056/NEJMoa1311096. [DOI] [PubMed] [Google Scholar]
- 9.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 10.Bovill EG, Terrin ML, Stump DC, Berke AD, Frederick M, Collen D, Feit F, Gore JM, Hillis LD, Lambrew CT, et al. Hemorrhagic events during therapy with recombinant tissue-type plasminogen activator, heparin, and aspirin for acute myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI), Phase II Trial. Ann Intern Med. 1991;115:256–265. doi: 10.7326/0003-4819-115-4-256. [DOI] [PubMed] [Google Scholar]
- 11.The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329:673–682. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- 12.Montalescot G, Barragan P, Wittenberg O, Ecollan P, Elhadad S, Villain P, Boulenc JM, Morice MC, Maillard L, Pansiéri M, Choussat R, Pinton P ADMIRAL Investigators. Abciximab before Direct Angioplasty and Stenting in Myocardial Infarction Regarding Acute and Long-Term Follow-up. Platelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarction. N Engl J Med. 2001;344:1895–1903. doi: 10.1056/NEJM200106213442503. [DOI] [PubMed] [Google Scholar]
- 13.van 't Hof AWJ, ten Berg JM, Heestermans AA, et al. Ongoing Tirofiban In Myocardial infarction Evaluation (On-TIME) 2 study group. Prehospital initiation of tirofiban in patients with ST-elevation myocardial infarction undergoing primary angioplasty (On-TIME 2): a multicentre, double-blind, randomised controlled trial. Lancet. 2008;372:537–546. doi: 10.1016/S0140-6736(08)61235-0. [DOI] [PubMed] [Google Scholar]
- 14.Bittl JA, Chaitman BR, Feit F, Kimball W, Topol EJ. Bivalirudin versus heparin during coronary angioplasty for unstable or postinfarction angina: final report reanalysis of the bivalirudin angioplasty study. Am Heart J. 2001;142:952–959. doi: 10.1067/mhj.2001.119374. [DOI] [PubMed] [Google Scholar]
- 15.Tarantini G, Brener SJ, Barioli A, Gratta A, Parodi G, Rossini R, Navarese EP, Niccoli G, Frigo AC, Musumeci G, Iliceto S, Stone GW. Impact of baseline hemorrhagic risk on the benefit of bivalirudin versus unfractionated heparin in patients treated with coronary angioplasty: a meta-regression analysis of randomized trials. Am Heart J. 2014;167:401–412. doi: 10.1016/j.ahj.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Parodi G, Valenti R, Bellandi B, Migliorini A, Marcucci R, Comito V, Carrabba N, Santini A, Gensini GF, Abbate R, Antoniucci D. Comparison of prasugrel and ticagrelor loading doses in ST-segment elevation myocardial infarction patients RAPID (Rapid Activity of Platelet Inhibitor Drugs) primary PCI study. J Am Coll Cardiol. 2013;61:1601–1606. doi: 10.1016/j.jacc.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Montalescot G, Zeymer U, Silvain J, et al. Intravenous enoxaparin or unfractionated heparin in primary percutaneous coronary intervention for ST-elevation myocardial infarction: the international randomised open-label ATOLL trial. Lancet. 2011;378:693–703. doi: 10.1016/S0140-6736(11)60876-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.