Abstract
Background
Advanced glycation/glycoxidation endproducts (AGEs) accumulate in settings of increased oxidative stress – such as diabetes, chronic kidney disease and aging – where they promote vascular stiffness and atherogenesis, but the prospective association between AGEs and cardiovascular events in elders has not been previously examined.
Methods
To test the hypothesis that circulating levels of Nε-carboxymethyl-lysine (CML), a major AGE, increase the risk of incident coronary heart disease and stroke in older adults, we measured serum CML by immunoassay in 2,111 individuals free of prevalent cardiovascular disease participating in a population-based study of U.S. adults ages 65 and older.
Results
During median follow-up of 9.1 years, 625 cardiovascular events occurred. CML was positively associated with incident cardiovascular events after adjustment for age, sex, race, systolic blood pressure, anti-hypertensive treatment, diabetes, smoking status, triglycerides, albumin, and self-reported health status (hazard ratio [HR] per SD [0.99 pmol/l] increase = 1.11, 95% confidence interval [CI]=1.03–1.19). This association was not materially attenuated by additional adjustment for C-reactive protein, estimated glomerular filtration rate (eGFR), and urine albumin/creatinine ratio. Findings were similar for the component endpoints of coronary heart disease and stroke.
Conclusions
In this large older cohort, CML was associated with an increased risk of cardiovascular events independent of a wide array of potential confounders and mediators. Although the moderate association limits CML’s value for risk prediction, these community-based findings provide support for clinical trials to test AGE-lowering therapies for cardiovascular prevention in this population.
Keywords: Advanced glycation endproducts, Carboxymethyl-lysine, Aging, Older Adults, Coronary Heart Disease, Stroke
Introduction
Advanced glycation/glycoxidation endproducts (AGEs) are a diverse class of highly bioactive compounds produced when carbonyl groups on sugars react with amino groups on protein, lipid, or nucleic-acid targets.1 While hyperglycemia can drive the initial reactions on the path to AGE formation – with glycation of hemoglobin being a prime example – subsequent oxidative reactions play a predominant role in the genesis of these molecules.2 Apart from glycoxidation, lipid peroxidation is also involved, leading to the formation of related advanced lipoxidation endproducts (ALEs).2 Accordingly, AGE (and ALE) levels are elevated not only in diabetes, but also in other settings characterized by increased oxidative stress, such as chronic kidney disease (CKD), advancing age, and presence of other cardiovascular risk factors.3 Another major source of AGEs and ALEs is the diet, as such adducts are generated abundantly during cooking of foodstuffs to high temperatures.2
AGEs (and ALEs) have distinctive effects on the vasculature, modifying collagen and other proteins in the arterial wall to increase vascular stiffness, an important risk factor for cardiovascular disease (CVD).4 AGEs also modify lipids and proteins in LDL, fostering LDL trapping in the subendothelial compartment.4 Moreover, AGEs bind to an immunoglobulin superfamily receptor, known as the receptor for AGE (RAGE), which activates nuclear factor kappa B (NFkB), among other pro-oxidant and pro-inflammatory pathways.5 NFkB is a principal orchestrator of the inflammatory response, and RAGE expression in endothelial and vascular smooth muscle cells, as well as leukocytes, promotes atherogenesis.5
Despite experimental evidence implicating AGEs in vascular disease,3, 4 and the prognostic value demonstrated for glycated hemoglobin in longitudinal cohort studies,6 epidemiological data on the relationship between glycoxidative species and clinical CVD outcomes remain sparse. A prospective study of middle-aged adults employed a polyclonal immunoassay to measure circulating AGEs, and found these to be associated with increased risks of cardiovascular and coronary mortality in women, but not men, with7 and without8 diabetes. With the development of an immunoassay for Nε-carboxymethyl-lysine (CML), a dominant AGE and ALE in plasma and tissue proteins,9–11 circulating levels of CML have been associated with all-cause and cardiovascular mortality in moderate-sized population-based cohorts of generally healthy elders12 and disabled older women.13 The same immunoassay was used to measure serum CML, in conjunction with other AGEs, in a modest-sized cohort with CKD, but no association was detected with incident CVD.14 By contrast, different AGEs measured by mass spectrometry/liquid chromatography, including CML, were found to be positively associated with incident CVD in patients with type 1 diabetes.15 The extent to which circulating AGEs influence the risk of new-onset coronary heart disease (CHD) and stroke in older adults, however, has to our knowledge not been previously examined. We undertook to address this question is a prospective cohort study of community-dwelling older men and women.
Methods
Study Population
The Cardiovascular Health Study (CHS) is a population-based investigation of determinants of CVD risk among adults aged 65 and older recruited from Medicare eligibility lists in four U.S. field centers.16 An original cohort of 5,201 subjects was recruited in 1989–90, followed in 1992–93 by a supplementary African-American cohort of 687 individuals. As described previously, participants underwent standardized health evaluations at site clinics at the time of initial enrollment and at follow-up visits.16, 17 The study was conducted in accordance to the Declaration of Helsinki, and all participants provided informed consent.
Of the 5,888 initially recruited subjects, 4,412 out of 4,708 surviving individuals returned for the 1996–97 examination. Among these participants, 2,732 were free of prevalent CVD (CHD, heart failure, atrial fibrillation, stroke, transient ischemic attack, and peripheral arterial disease), as documented by adjudication since the baseline evaluation.17 Exclusion of participants without available serum (n=565) or imputable covariate data (n=56) during a previous multiple imputation procedure18 left 2,111 subjects eligible for the present analyses.
Cardiovascular Events
Surveillance for new-onset cardiovascular events entailed semiannual telephone contacts and/or clinical examinations.19 Medical records were reviewed for potential incident events and all deaths, and events adjudicated by CHS committees according to standardized criteria. Follow-up extending through June 2009 was >97% complete.16, 19, 20 The primary endpoint was a composite of CHD (nonfatal myocardial infarction and fatal coronary events) and nonfatal/fatal stroke, as defined previously.16, 17, 19
Measurement of CML
CML measurement was performed in 2011 with an immunoassay (AGE-CML ELISA, Microcoat, Penzberg, Germany) in serum specimens stored at −70°C since collection in 1996–97. CML is a highly stable chemical species, with mean levels reported to be comparable in plasma samples frozen for 10 years and freshly drawn specimens.21 This immunoassay has similar affinity for protein-bound, peptide-bound, and free CML.22 The minimal detectable level of the assay is 0.02 pmol/l. Intra- and inter-assay analytical coefficients of variation were <5%.
Covariates
Diabetes was defined by fasting glucose ≥7.0 mmol/l or use of glucose-lowering therapy. Measures of body size were determined in standardized fashion.23
Laboratory measurements were obtained on fasting blood samples as detailed previously.24 Homeostasis model assessment of insulin resistance (HOMA2-IR) was calculated using a standard approach,25 as was estimated glomerular filtration rate (eGFR) based on cystatin C.26
Statistical Analysis
Positively skewed variables were logarithmically transformed. Differences in CML concentrations by levels of baseline covariates were assessed with Student’s t test. Cross-sectional associations of CML were assessed with Spearman correlation coefficients and linear regression. The shape of the association between CML and new-onset CHD or stroke was examined with a Cox proportional hazards model adjusted for potential confounders, using a penalized cubic spline. Presence of non-linearity was tested with a partial likelihood ratio test. Testing of the proportional hazards assumption by Schoenfeld’s goodness-of-fit procedures showed no material violation. Missing values for covariates at the 1996–97 examination (n=83) were replaced by values carried over from prior examinations, including those generated by a previous multiple imputation procedure.18
Cox models were initially adjusted for age, sex, and race, and subsequently for additional potential confounders, including measures of body size, smoking habit, diabetes, blood pressure and antihypertensive medications, lipids and lipid-lowering treatment, serum albumin, and self-reported health status. Subsequent models examined the impact of additional factors that could serve as confounders, but might also act to mediate CML’s effects. These consisted of C-reactive protein (CRP), eGFR, and the urine albumin/creatinine ratio (UACR). To test for interactions with key covariates, appropriate cross-product terms were entered using continuous levels where feasible. Sensitivity analyses examined the influence of excluding participants with diabetes, CKD (eGFR <60 mL/min/1.73 m2), macroalbuminuria (UACR>30 mg/mmol), and hemorrhagic stroke.
All analyses were performed with STATA, version 11.0 (College Station, TX), or R version 2.13.0 (http://www.r-project.org). Two-tailed p<0.05 was considered statistically significant.
Results
The baseline characteristics of the study cohort are presented in Table 1, and contrasted with participants excluded for lack of CML or covariate data. Participants in the study sample were younger, more often women and reported better health status, but had similar levels of other cardiovascular risk factors, as compared with excluded participants.
Table 1.
Baseline Characteristics* of Study Cohort and Excluded Participants
| Covariates | Study Cohort (n=2,111) | Excluded Cohort† (n=621) |
|---|---|---|
| Age, years | 78±5 | 80±6 |
| Women | 1,359(64.4) | 450(72.5) |
| Black | 344(16.3) | 113(18.2) |
| BMI, kg/m2 | 27.0±4.6 | 26.3±5.3 |
| Waist-hip ratio | 0.95±0.10 | 0.93±0.10 |
| Systolic blood pressure, mm Hg | 137±20 | 138±20 |
| Diastolic blood pressure, mm Hg | 71±11 | 70±13 |
| Antihypertensive medication | 1,004(47.6) | 241(47.8) |
| Fasting glucose, mmol/l | 5.8±1.6 | 5.8±1.6 |
| Fasting insulin, mU/l | 7.6(5.1–11.6) | 7.1 (4.4–11.2) |
| HOMA2-IR | 0.9(0.6–1.3) | 0.8(0.5–1.3) |
| Diabetes | 324(15.3) | 92(14.8) |
| LDL-C, mmol/l | 3.3±0.9 | 3.4±0.9 |
| HDL-C, mmol/l | 57±16 | 57±16 |
| Triglycerides, mmol/l | 1.3(1.0–1.8) | 1.3(1.0–1.8) |
| Lipid-lowering medication | 167(7.9) | 28(5.6) |
| Albumin, μmol/l | 5.5±0.4 | 5.5±0.4 |
| Current smokers | 161(7.6) | 56(9.0) |
| Alcohol use ≥ 7 drinks/wk | 271(12.8) | 61(9.8) |
| Estrogen replacement (women) | 238(17.5) | 47(12.6) |
| Physical activity, kcal/wk | 803(270–1755) | 510(90–1350) |
| Fair/poor self-reported health status | 384(18.2) | 210(33.8) |
| Unintentional weight loss>4.5 kg | 100 (5.0) | 31(6.8) |
| eGFR (cystatin-based), ml/min/1.73 m2 | 73±19 | 74±21 |
| eGFR <60 ml/min/1.73 m2 | 465(22.0) | 104(16.7) |
| UACR, mg/mmol | 1.0(0.6–2.0) | 1.2(0.7–2.7) |
| Microalbuminuria | 234(11.3) | 26(17.9) |
| Macroalbuminuria | 38(1.8) | 3(2.1) |
| CRP, nmol/l | 21.9(9.5–45.7) | 23.8(10.5–44.8) |
n(%), mean±SD or median(interquartile range)
Involves participants lacking CML (n=565) or imputable covariates (n=56).
Cross-sectional associations of serum CML with baseline covariates are detailed in Table 2. Serum CML showed modest positive correlations with age, systolic blood pressure, HDL-cholesterol, serum albumin and creatinine, and UACR, while exhibiting modest negative correlations with anthropometric parameters, fasting glucose and insulin, LDL-cholesterol and triglycerides, physical activity, and eGFR. Circulating CML levels were higher among participants receiving lipid-lowering medication, those with eGFR<60 mL/min/1.73 m2 and micro- or macroalbuminuria, and those reporting fair/poor health status or previous unintentional weight loss.
Table 2.
Relationship of Serum Carboxymethyl-Lysine with Baseline Covariates
| Covariates | Carboxymethyl-Lysine | |
|---|---|---|
| Correlation Coefficient or Mean* (95% CI) | P | |
| Age | 0.11 | <0.001 |
| Sex | 0.94 | |
| Women (n=1,359) | 2.88(2.84–2.92) | |
| Men (n=752) | 2.88(2.83–2.94) | |
| Race-ethnicity | 0.066 | |
| Black (n=344) | 2.95(2.87–3.04) | |
| Non-Black (n=1,767) | 2.87(2.83–2.90) | |
| BMI | −0.15 | <0.001 |
| Waist-hip ratio | −0.11 | <0.001 |
| Systolic blood pressure | 0.04 | 0.041 |
| Diastolic blood pressure | −0.02 | 0.31 |
| Antihypertensive medication | 0.097 | |
| Yes (n=1,004) | 2.91(2.86–2.96) | |
| No (n=1,107) | 2.85(2.81–2.90) | |
| Fasting glucose | −0.06 | 0.012 |
| Fasting insulin | −0.09 | <0.001 |
| HOMA2-IR† | −0.14 | <0.001 |
| Diabetes | 0.312 | |
| Yes (n=324) | 2.84(2.75–2.93) | |
| No (n=1,787) | 2.89(2.85–2.93) | |
| LDL-C | −0.06 | 0.001 |
| HDL-C | 0.04 | 0.014 |
| Triglycerides | −0.04 | 0.021 |
| Lipid-lowering medication | 0.003 | |
| Yes (n=167) | 3.06(2.93–3.18) | |
| No (n=1,944) | 2.87(2.83–2.90) | |
| Albumin | 0.07 | <0.001 |
| Smoking status | 0.11 | |
| Current (n=161) | 2.79(2.67–2.91) | |
| Never/Ever (n=1,950) | 2.89(2.85–2.92) | |
| Alcohol use | 0.041 | |
| ≥ 7 drinks/wk (n=271) | 2.81(2.63–2.99) | |
| < 7 drinks/wk (n=1,840) | 2.90(2.86–2.95) | |
| Estrogen replacement (women) | 0.37 | |
| Yes (n=238) | 2.84(2.73–2.94) | |
| No (n=1,121) | 2.89(2.85–2.92) | |
| Physical activity (log-kcal/wk) | −0.06 | 0.004 |
| Self-reported health status | 0.002 | |
| Fair/poor (n=384) | 3.00(2.92–3.09) | |
| Excellent/very good/good (n=1,727) | 2.85(2.82–2.89) | |
| Unintentional weight loss>4.5 kg | 0.012 | |
| Yes (n=100) | 3.12(2.93–3.32) | |
| No (n=1,885) | 2.87(2.84–2.91) | |
| eGFR (cystatin-based) | −0.22 | <0.001 |
| CKD | <0.001 | |
| eGFR ≥60 mL/min/1.73 m2 (n=465) | 2.82(2.79–2.85) | |
| eGFR <60 mL/min/1.73 m2 (n=1,646) | 3.11(3.03–3.20) | |
| UACR | 0.08 | <0.001 |
| Albuminuria | 0.008 | |
| None (n=1,796) | 2.87(2.84–2.90) | |
| Microalbuminuria (n=234) | 3.18(2.82–3.59) | |
| Macroalbuminuria (n=38) | 3.14(2.88–3.43) | |
| CRP | <0.01 | 0.98 |
Geometric mean in pmol/l
Log-transformed
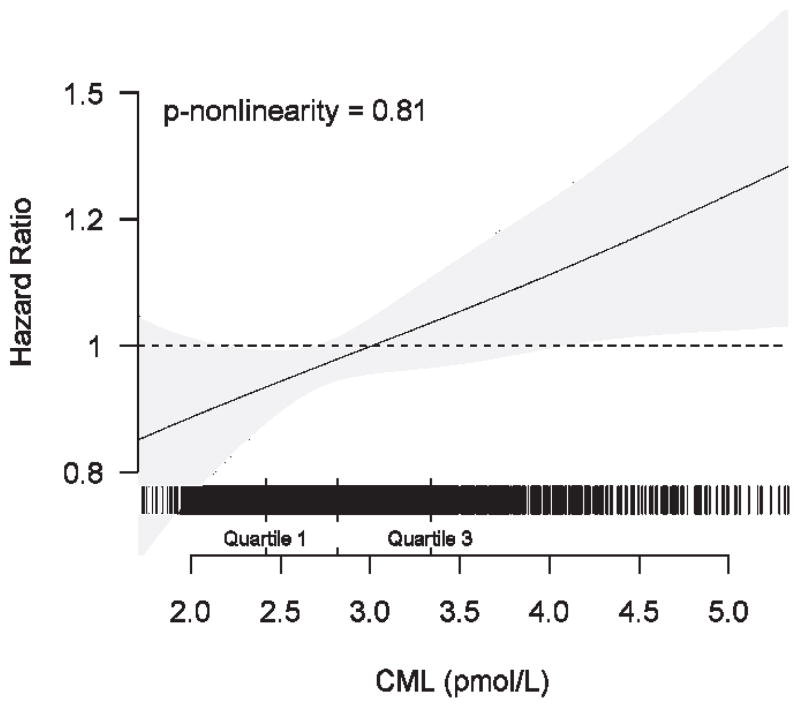
During a median follow-up of 9.1 years, 625 participants experienced at least one incident cardiovascular event (CHD, n=417; stroke, n=274; both, n=66). Adjusted cubic spline analysis was consistent with a linear association between serum CML and incident CHD or stroke (Figure 1). The relative risk estimates for continuous increases in serum CML concentration are given in Table 3. After adjustment for potential confounding (model 2), every SD increase (0.99 pmol/l) in serum CML was associated with a significant 11% increase in the relative risk of the primary composite endpoint. This was not meaningfully altered after additional adjustment for CRP, eGFR and UACR, or when HOMA2-IR was entered as a covariate. As also shown in Table 3, risk estimates were consistent for the individual components, CHD and stroke, both after adjustment for potential confounders and for putative causal intermediates.
Figure 1.
Spline regression graph depicting the adjusted association of continuous CML concentration and cardiovascular events. The horizontal band above the x-axis represents individual participants (vertical lines) with corresponding values for serum CML. The model adjusts for age, sex, race, BMI, systolic blood pressure, antihypertensive medication, diabetes, smoking status, log(triglycerides), serum albumin, and self-reported health status.
Table 3.
Association of Serum Carboxymethyl-Lysine with Incident Cardiovascular Events
| Composite Cardiovascular Endpoint | ||
|---|---|---|
| Incident Events | Incidence Rate* (95% CI) | |
| N | 625 | 35.6(32.9–38.5) |
| HR per SD† (95% CI) | p | |
| Model 1 | 1.09(1.02–1.17) | 0.014 |
| Model 2 | 1.11(1.03–1.19) | 0.004 |
| Model 3 | 1.10(1.02–1.19) | 0.009 |
| Model 4 | 1.10(1.02–1.19) | 0.016 |
| Coronary Heart Disease | ||
| Incident Events | Incidence Rate* (95% CI) | |
| N | 417 | 22.9(20.8–25.2) |
| HR per SD† (95% CI) | p | |
| Model 1 | 1.12(1.03–1.21) | 0.009 |
| Model 2 | 1.13(1.04–1.23) | 0.003 |
| Model 3 | 1.11(1.02–1.21) | 0.019 |
| Model 4 | 1.11(1.01–1.22) | 0.025 |
| Stroke | ||
| Incident Events | Incidence Rate* (95% CI) | |
| N | 274 | 14.1(12.6–15.9) |
| HR per SD† (95% CI) | p | |
| Model 1 | 1.10(0.98–1.22) | 0.093 |
| Model 2 | 1.11(0.99–1.24) | 0.063 |
| Model 3 | 1.13(1.01–1.26) | 0.031 |
| Model 4 | 1.14(1.01–1.28) | 0.027 |
per 1000 person-years.
SD = 0.99 pmol/l
Model 1. Adjusted for age, sex, and race.
Model 2. Adjusted for age, sex, race, BMI, systolic blood pressure, antihypertensive medication, diabetes, smoking status, log(triglycerides), albumin, self-reported health status. Additional adjustment for fasting glucose, other lipid fractions or unintended weight loss did not meaningfully alter the effect estimates.
Model 3. Adjusted for covariates in Model 2 and log(CRP), eGFR and UACR (in subjects with available UACR, n=2,068).
Model 4. Adjusted for covariates in Model 3 and log(HOMA2-IR) (in subjects with available UACR and not receiving diabetes medication [n=1,926]).
There was no evidence of effect-modification of the association of CML with cardiovascular events by age, sex, race, diabetes, BMI, or eGFR (all p >0.65). A significant interaction by UACR was detected (continuous CML × continuous UACR, p<0.001), but this did not persist after removal of extreme upper values of UACR (p=0.57 after exclusion of n=38 participants with macroalbuminuria). Given its tenuous nature, we did not deem this interaction sufficiently compelling to pursue further. Sensitivity analyses showed similar effect estimates after exclusion of participants with macroalbuminuria, eGFR<60 mL/min/1.73 m2 and diabetes, as well as following exclusion or censoring of hemorrhagic stroke.
Discussion
This investigation is to our knowledge the first prospective study to examine the association of serum CML with incident CHD or stroke in a general population-based sample of older adults. It is also the largest study to date to evaluate the relationship between CML and clinical outcomes of any kind. The current analyses document that serum CML is associated with incident cardiovascular events in older men and women independent of an array of potential confounders and/or mediators.
The significant association of serum CML with incident cardiovascular events independent of traditional atherosclerosis risk factors is compatible with the well-documented vascular effects of AGEs in experimental studies.4 As a dominant AGE in human tissues,10, 11 and one whose circulating levels correlate strongly with those of other AGEs,14, 15, 27 CML is deemed an attractive measure of overall AGE burden, vascular and otherwise.
The adverse vascular consequences demonstrated experimentally for AGEs fall broadly into receptor-independent and receptor-dependent effects.1 The first encompass the modification of extracellular matrix proteins, which results in heightened vascular stiffness.4 Receptor-independent effects also involve modification of LDL-cholesterol, impeding its removal from the subendothelial space.4 The second category entails receptor-dependent effects mediated by AGE binding to RAGE, which triggers intracellular pathways that promote formation of reactive oxygen species, deplete nitric oxide, and foster activation of NFkB.1 Together, these pathways promote expression of pro-inflammatory cytokines and cell-adhesion molecules, which play critical roles in atherogenesis.4 The aggregate impact of AGEs on the aorta, as well as the coronary and cerebral circulations, would explain the comparable associations of CML with CHD and stroke here, driven by large-artery atherothrombosis and its sequelae and, especially in the brain, by small-artery disease.4
The current study took care to account for the potential impact of CKD in assessing the association of CML with incident CHD and stroke. Renal disease is an important determinant of AGE formation, marked as it is by the accumulation of reactive carbonyl species and increased oxidative stress.28 Kidney disease also reduces clearance of circulating AGEs,28 both albumin-bound and, especially, small AGE peptides and free adducts.29 Consistent with the relevance of chronic kidney disease to circulating AGE concentrations, CML levels were correlated with eGFR and albuminuria in the present analyses. Adjustment for the latter measures, however, did not substantially attenuate the association of CML with CHD and stroke. While there was a suggestion of interaction by albuminuria, its lack of gradation across increasing UACR levels when a small number of participants with extreme upper values were excluded, makes this finding suspect. Future studies with larger numbers of individuals with severe albuminuria are required to explore the influence, if any, of macroalbuminuria on CML’s relationship with cardiovascular events.
As in previous epidemiological studies that have detected associations between AGEs/CML and mortality,7, 8, 12, 13 the present findings show no obvious differences according to diabetes status. Moreover, consistent with previous reports,7, 30, 31 no positive correlations were observed between serum CML and measures of glycemia. Instead, weak negative correlations with fasting glucose and insulin were apparent. Given that diabetes is a well-established risk factor for AGE formation,1 these findings could relate to the negative correlation of CML with adiposity, whose basis remains uncertain.30 Furthermore, the lack of positive correlations between CML and glycemia could reflect endogenous glycoxidation or lipid peroxidation reactions,31–33 and exogenous dietary intake,27, 34 rather than hyperglycemia-mediated glycation, as principal sources of CML formation.
The results of this study have important clinical implications. The moderate association of CML with incident cardiovascular events indicates that this biomarker would be of limited value for risk prediction. But the independent link uncovered suggests that targeting older adults with elevated CML levels with AGE-lowering interventions could have substantial therapeutic benefits, particularly since elders’ high baseline cardiovascular risk35 yields larger absolute risk reductions. Moreover, the potential of such interventions could extend beyond that suggested by this individual biomarker, because CML is strongly correlated with other major AGEs14, 15, 27 whose levels would be lowered concomitantly.
As relates to AGE/CML lowering strategies, the extent to which dietary AGEs lead to deleterious health consequences in humans has been a matter of controversy,36 but dietary intervention studies have shown AGE-rich meals to be associated with higher circulating levels of AGEs, and measures of oxidative stress, inflammation, and insulin resistance.37, 38 Likewise, pharmacological approaches with AGE cross-link breakers, AGE inhibitors, and dietary AGE binders have shown improvements in vascular function,39, 40 renal function,41 and metabolic or inflammatory derangements42 in small intervention studies. Hence, our findings suggest that studies of dietary AGE restriction or pharmacological interventions targeted to older adults with elevated CML levels could identify effective strategies for CVD reduction in this vulnerable population.
Our study has several limitations. Because CML measurements were available in generally healthier CHS survivors, the results do not necessarily apply to more diseased older populations. An additional limitation is that while CML correlates strongly with other AGEs, it necessarily constitutes a partial marker of global AGE burden. The extent to which other major AGEs, or composite indices thereof, may provide better measures of CVD risk in older adults than CML alone will require further investigation. This question, however, has been previously evaluated in a moderate-sized cohort with type 1 diabetes, where plasma CML, carboxyethyl-lysine, and pentosidine exhibited comparable associations with incident CVD and mortality, and there was no apparent incremental value when the three AGEs were combined.15 Another key limitation is the lack of data on dietary AGE/CML intake in this population, which would have allowed determination of the contribution of exogenous sources to circulating CML levels and their consequences. Unfortunately, a well-validated catalog of the AGE content of specific foods has yet to be developed, owing to the need to employ liquid chromatography/mass spectrometry for such purposes.43 Finally, the study cohort lacked measurement of glycated hemoglobin, which would have provided a comparative measure of longer-term glycemic burden than that available from fasting glucose. Previous studies, however, have found no correlation between serum CML and glycated hemoglobin concentration in cohorts with7, 31 or largely without30 diabetes.
In conclusion, the current findings demonstrate for the first time that serum CML is associated with an increased risk of CHD and stroke in older adults. This prospective association proved independent of a range of traditional atherosclerosis risk factors, including measures of kidney function. The relative modest strength of the overall association indicates that the value of CML measurement does not lie in improvement of risk prediction, but potentially in the identification of individuals who might benefit from AGE-countering therapies. Additional studies are required to define the role of dietary AGE intake to circulating CML levels and associated CVD risk, and to explore the impact of macroalbuminuria on CML’s association with cardiovascular events. Nevertheless, these findings provide impetus for clinical trials to assess the efficacy of dietary or pharmacological interventions to reduce cardiovascular events in elders with elevated CML levels.
Highlights.
Serum levels of the advanced glycoxidation endproduct CML were measured in a large community-based cohort of older adults.
CML was positively correlated with age, blood pressure, lipid treatment, worse health, prior weight loss, and albuminuria.
Negative correlations were seen for CML and adiposity, physical activity, LDL-C, insulin resistance, and eGFR.
CML was associated with higher risk of incident CHD and stroke independent of multiple potential confounders and mediators.
Acknowledgments
This research was supported by R01 HL094555, as well as by contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/PI.htm.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goh SY, Cooper ME. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab. 2008;93:1143–1152. doi: 10.1210/jc.2007-1817. [DOI] [PubMed] [Google Scholar]
- 2.Vlassara H, Striker GE. Age restriction in diabetes mellitus: A paradigm shift. Nature reviews Endocrinology. 2011;7:526–539. doi: 10.1038/nrendo.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol A Biol Sci Med Sci. 2010;65:963–975. doi: 10.1093/gerona/glq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63:582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Basta G. Receptor for advanced glycation endproducts and atherosclerosis: From basic mechanisms to clinical implications. Atherosclerosis. 2008;196:9–21. doi: 10.1016/j.atherosclerosis.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 6.Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilhovd BK, Juutilainen A, Lehto S, Ronnemaa T, Torjesen PA, Hanssen KF, et al. Increased serum levels of advanced glycation endproducts predict total, cardiovascular and coronary mortality in women with type 2 diabetes: A population-based 18 year follow-up study. Diabetologia. 2007;50:1409–1417. doi: 10.1007/s00125-007-0687-z. [DOI] [PubMed] [Google Scholar]
- 8.Kilhovd BK, Juutilainen A, Lehto S, Ronnemaa T, Torjesen PA, Birkeland KI, et al. High serum levels of advanced glycation end products predict increased coronary heart disease mortality in nondiabetic women but not in nondiabetic men: A population-based 18-year follow-up study. Arterioscler Thromb Vasc Biol. 2005;25:815–820. doi: 10.1161/01.ATV.0000158380.44231.fe. [DOI] [PubMed] [Google Scholar]
- 9.Fu MX, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. The advanced glycation end product, nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem. 1996;271:9982–9986. doi: 10.1074/jbc.271.17.9982. [DOI] [PubMed] [Google Scholar]
- 10.Reddy S, Bichler J, Wells-Knecht KJ, Thorpe SR, Baynes JW. N epsilon-(carboxymethyl)lysine is a dominant advanced glycation end product (age) antigen in tissue proteins. Biochemistry. 1995;34:10872–10878. doi: 10.1021/bi00034a021. [DOI] [PubMed] [Google Scholar]
- 11.Dyer DG, Dunn JA, Thorpe SR, Bailie KE, Lyons TJ, McCance DR, et al. Accumulation of maillard reaction products in skin collagen in diabetes and aging. J Clin Invest. 1993;91:2463–2469. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semba RD, Bandinelli S, Sun K, Guralnik JM, Ferrucci L. Plasma carboxymethyl-lysine, an advanced glycation end product, and all-cause and cardiovascular disease mortality in older community-dwelling adults. J Am Geriatr Soc. 2009;57:1874–1880. doi: 10.1111/j.1532-5415.2009.02438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semba RD, Ferrucci L, Sun K, Beck J, Dalal M, Varadhan R, et al. Advanced glycation end products and their circulating receptors predict cardiovascular disease mortality in older community-dwelling women. Aging clinical and experimental research. 2009;21:182–190. doi: 10.1007/bf03325227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busch M, Franke S, Muller A, Wolf M, Gerth J, Ott U, et al. Potential cardiovascular risk factors in chronic kidney disease: Ages, total homocysteine and metabolites, and the c-reactive protein. Kidney international. 2004;66:338–347. doi: 10.1111/j.1523-1755.2004.00736.x. [DOI] [PubMed] [Google Scholar]
- 15.Nin JW, Jorsal A, Ferreira I, Schalkwijk CG, Prins MH, Parving HH, et al. Higher plasma levels of advanced glycation end products are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: A 12-year follow-up study. Diabetes Care. 2011;34:442–447. doi: 10.2337/dc10-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The cardiovascular health study: Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 17.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, et al. Methods of assessing prevalent cardiovascular disease in the cardiovascular health study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 18.Arnold AM, Kronmal RA. Multiple imputation of baseline data in the cardiovascular health study. Am J Epidemiol. 2003;157:74–84. doi: 10.1093/aje/kwf156. [DOI] [PubMed] [Google Scholar]
- 19.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, et al. Surveillance and ascertainment of cardiovascular events. The cardiovascular health study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 20.Longstreth WT, Jr, Bernick C, Fitzpatrick A, Cushman M, Knepper L, Lima J, et al. Frequency and predictors of stroke death in 5,888 participants in the cardiovascular health study. Neurology. 2001;56:368–375. doi: 10.1212/wnl.56.3.368. [DOI] [PubMed] [Google Scholar]
- 21.Beisswenger PJ, Howell SK, Russell GB, Miller ME, Rich SS, Mauer M. Early progression of diabetic nephropathy correlates with methylglyoxal-derived advanced glycation end products. Diabetes Care. 2013;36:3234–3239. doi: 10.2337/dc12-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Frischmann M, Kientsch-Engel R, Steinmann K, Stopper H, Niwa T, et al. Two immunochemical assays to measure advanced glycation end-products in serum from dialysis patients. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2005;43:503–511. doi: 10.1515/CCLM.2005.089. [DOI] [PubMed] [Google Scholar]
- 23.Kizer JR, Biggs ML, Ix JH, Mukamal KJ, Zieman SJ, de Boer IH, et al. Measures of adiposity and future risk of ischemic stroke and coronary heart disease in older men and women. Am J Epidemiol. 2011;173:10–25. doi: 10.1093/aje/kwq311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the cardiovascular health study. Clin Chem. 1995;41:264–270. [PubMed] [Google Scholar]
- 25.Wallace TM, Levy JC, Matthews DR. Use and abuse of homa modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 26.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, et al. Estimating gfr using serum cystatin c alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with ckd. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G, et al. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci. 2007;62:427–433. doi: 10.1093/gerona/62.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vlassara H, Torreggiani M, Post JB, Zheng F, Uribarri J, Striker GE. Role of oxidants/inflammation in declining renal function in chronic kidney disease and normal aging. Kidney international. Supplement. 2009:S3–11. doi: 10.1038/ki.2009.401. [DOI] [PubMed] [Google Scholar]
- 29.Thornalley PJ, Rabbani N. Highlights and hotspots of protein glycation in end-stage renal disease. Seminars in dialysis. 2009;22:400–404. doi: 10.1111/j.1525-139X.2009.00589.x. [DOI] [PubMed] [Google Scholar]
- 30.Semba RD, Arab L, Sun K, Nicklett EJ, Ferrucci L. Fat mass is inversely associated with serum carboxymethyl-lysine, an advanced glycation end product, in adults. J Nutr. 2011;141:1726–1730. doi: 10.3945/jn.111.143172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeuchi M, Makita Z, Yanagisawa K, Kameda Y, Koike T. Detection of noncarboxymethyllysine and carboxymethyllysine advanced glycation end products (age) in serum of diabetic patients. Molecular medicine. 1999;5:393–405. [PMC free article] [PubMed] [Google Scholar]
- 32.Meng J, Sakata N, Takebayashi S, Asano T, Futata T, Nagai R, et al. Glycoxidation in aortic collagen from stz-induced diabetic rats and its relevance to vascular damage. Atherosclerosis. 1998;136:355–365. doi: 10.1016/s0021-9150(97)00238-4. [DOI] [PubMed] [Google Scholar]
- 33.Miyata T, Fu MX, Kurokawa K, van Ypersele de Strihou C, Thorpe SR, Baynes JW. Autoxidation products of both carbohydrates and lipids are increased in uremic plasma: Is there oxidative stress in uremia? Kidney international. 1998;54:1290–1295. doi: 10.1046/j.1523-1755.1998.00093.x. [DOI] [PubMed] [Google Scholar]
- 34.Uribarri J, Peppa M, Cai W, Goldberg T, Lu M, Baliga S, et al. Dietary glycotoxins correlate with circulating advanced glycation end product levels in renal failure patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2003;42:532–538. doi: 10.1016/s0272-6386(03)00779-0. [DOI] [PubMed] [Google Scholar]
- 35.Arnold AM, Psaty BM, Kuller LH, Burke GL, Manolio TA, Fried LP, et al. Incidence of cardiovascular disease in older americans: The cardiovascular health study. J Am Geriatr Soc. 2005;53:211–218. doi: 10.1111/j.1532-5415.2005.53105.x. [DOI] [PubMed] [Google Scholar]
- 36.Pischetsrieder M. Are dietary ages/ales a risk to human health and, if so, what is the mechanism of action? Molecular nutrition & food research. 2007;51:1069–1070. doi: 10.1002/mnfr.200790016. [DOI] [PubMed] [Google Scholar]
- 37.Negrean M, Stirban A, Stratmann B, Gawlowski T, Horstmann T, Gotting C, et al. Effects of low- and high-advanced glycation endproduct meals on macro- and microvascular endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2007;85:1236–1243. doi: 10.1093/ajcn/85.5.1236. [DOI] [PubMed] [Google Scholar]
- 38.Uribarri J, Stirban A, Sander D, Cai W, Negrean M, Buenting CE, et al. Single oral challenge by advanced glycation end products acutely impairs endothelial function in diabetic and nondiabetic subjects. Diabetes Care. 2007;30:2579–2582. doi: 10.2337/dc07-0320. [DOI] [PubMed] [Google Scholar]
- 39.Kass DA, Shapiro EP, Kawaguchi M, Capriotti AR, Scuteri A, deGroof RC, et al. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104:1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- 40.Zieman SJ, Melenovsky V, Clattenburg L, Corretti MC, Capriotti A, Gerstenblith G, et al. Advanced glycation endproduct crosslink breaker (alagebrium) improves endothelial function in patients with isolated systolic hypertension. Journal of hypertension. 2007;25:577–583. doi: 10.1097/HJH.0b013e328013e7dd. [DOI] [PubMed] [Google Scholar]
- 41.Bolton WK, Cattran DC, Williams ME, Adler SG, Appel GB, Cartwright K, et al. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. American journal of nephrology. 2004;24:32–40. doi: 10.1159/000075627. [DOI] [PubMed] [Google Scholar]
- 42.Vlassara H, Uribarri J, Cai W, Goodman S, Pyzik R, Post J, et al. Effects of sevelamer on hba1c, inflammation, and advanced glycation end products in diabetic kidney disease. Clinical journal of the American Society of Nephrology : CJASN. 2012;7:934–942. doi: 10.2215/CJN.12891211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Assar SH, Moloney C, Lima M, Magee R, Ames JM. Determination of nepsilon-(carboxymethyl)lysine in food systems by ultra performance liquid chromatography-mass spectrometry. Amino acids. 2009;36:317–326. doi: 10.1007/s00726-008-0071-4. [DOI] [PubMed] [Google Scholar]