Abstract
Immunoglobulin class-switch recombination (CSR) requires activation-induced cytidine deaminase (AID). Deamination of DNA by AID in transcribed switch (S) regions leads to double-stranded breaks in DNA that serve as obligatory CSR intermediates. Here we demonstrate that the catalytic and regulatory subunits of protein kinase A (PKA) were specifically recruited to S regions to promote the localized phosphorylation of AID, which led to binding of replication protein A and subsequent propagation of the CSR cascade. Accordingly, inactivation of PKA resulted in considerable disruption of CSR because of decreased AID phosphorylation and recruitment of replication protein A to S regions. We propose that PKA nucleates the formation of active AID complexes specifically on S regions to generate the high density of DNA lesions required for CSR.
Antibodies are composed of immunoglobulin heavy chains and light chains, with an amino-terminal variable (V) region involved in antigen binding and a carboxy-terminal constant (C) region required for the effector function. During early B cell development in fetal liver and adult bone marrow, the genes encoding the heavy-chain and light-chain V regions are assembled from component V, diversity (D) and joining (J) gene segments by the process of V(D)J recombination1. The productive assembly of heavy chains and light chains generates a mature B cell that expresses immunoglobulin M (IgM) on its surface. The mature B cells subsequently migrate to the secondary lymphoid organs such as the spleen and lymph nodes, where they encounter antigens and undergo two additional rounds of genetic alteration in the forms of class-switch recombination (CSR) and somatic hypermutation (SHM)2–5.
CSR results in a change in the heavy-chain constant region (CH) from Cµ to other downstream CH regions (Cγ3, Cγ1, Cγ2b, Cγ2a, Cε and Cα, all encoded in the mouse Igh locus) so that secondary isotypes (IgG, IgE and IgA) with different effector functions are generated3,4. CSR occurs in repetitive DNA elements 1–12 kilobases in length, called ‘switch’ (S) regions, that precede each CH gene. The CH genes are organized as independent transcription units3,4. Transcription is initiated from a cytokine-inducible intronic (I) promoter upstream of an I-exon, proceeds through the S regions and terminates downstream of the CH exons. The primary transcript is spliced to remove the intronic S region, thereby joining the I and CH exons to generate a polyadenylated, noncoding germline transcript. Mutations of promoters or enhancers that impair germline transcription through particular CH genes impair CSR to that particular isotype, which shows that transcription serves an important mechanistic function in CSR3,4.
In addition to S regions and transcription, CSR absolutely requires participation of the activated B cell–specific protein AID6,7. AID is a single-stranded DNA (ssDNA)-specific DNA cytidine deaminase8–12, and the ability of AID to deaminate its target sequences is intimately linked to transcription3. Transcription through S regions, which are rich in guanosine and cytidine with a characteristic guanosine-rich nontemplate strand, generates RNA-DNA hybrid structures, such as R loops, that displace the nontemplate strand as ssDNA13–15. In vitro studies have shown that AID can efficiently deaminate cytidine to uridine in R loops10. The deaminated S regions are then processed by proteins of the base excision–repair pathway (such as uracil DNA glycosylase) and mismatch-repair pathway (such as Msh2) to ultimately generate DNA double-stranded breaks (DSBs)16–19. General DNA-repair pathways synapse and join DSBs between two broken S regions to complete CSR3.
Mature B cells also undergo SHM, a process by which point mutations are introduced at a very high rate into V-region exons of the heavy-chain and light-chain genes, leading to the selection of antibodies with higher affinity2,5. The mechanism by which SHM is initiated is related to CSR in that it requires AID and transcription, in this case through the V-gene segments. However, unlike S regions, V genes are not rich in guanosine and cytidine and therefore do not readily provide ssDNA substrates for AID. Biochemical studies have shown that AID interacts with replication protein A (RPA), and the AID-RPA complex can bind to and stabilize ssDNA in transcribed V genes to mediate deamination20. Thus, although AID is essential for both CSR and SHM6,7, the mechanism by which AID accesses the target sequences is different for the two reactions3.
Although immunoglobulin sequences are the main physiological targets of AID, several transcribed non-immunoglobulin genes are also mutated by AID21–23. Non-immunoglobulin gene targets of AID are of particular relevance to B cell lymphomagenesis, as most mature B cell lymphomas have mutations and translocations of oncogenes, such as MYC and BCL6, and AID has been directly linked to the initiation of such oncogenic events24–27. AID activity and expression is thus carefully regulated at many levels, including B cell–specific expression28 and destabilization of its mRNA mediated by microRNA29,30, as well as proteolysis31 and phosphorylation20,32–35. However, none of those regulatory processes can explain how AID-induced DSBs are restricted mainly to S regions in a B cell actively undergoing CSR when AID and its cofactor RPA are abundant. Here we propose that the specificity of AID-induced DNA lesions is achieved in part by the recruitment of its activating kinase, protein kinase A (PKA), exclusively to S regions during CSR.
RESULTS
Phosphorylation of AID at Ser38 by PKA regulates CSR
AID in activated B cells is phosphorylated at the serine residue at position 38 (Ser38)20,32,34,36. Ser38 is in a consensus cAMP-dependent PKA-phosphorylation site. Recombinant AID can be phosphorylated in vitro at Ser38 by PKA, and the phosphorylated AID (pSer38-AID) acquires the ability to interact with RPA and mediate deamination of SHM targets32.We substituted the serine at the Ser38 phosphorylation site with alanine, followed by retroviral expression of the resulting mutant protein AID(S38A) in AID-deficient mouse splenic B cells. In agreement with early published observations32,34,35 and in contrast to subsequent reports37, AID(S38A) was substantially impaired in its ability to restore CSR to AID-deficient splenic B cells (Fig. 1a).
Figure 1.
Phosphorylation of AID by PKA modulates CSR. (a) Flow cytometry of GFP and surface IgG1 on Aicda−/− splenic B cells stimulated with LPS plus IL-4 and left uninfected or infected with retroviral vector expressing GFP alone (pMIG) or AID(WT) or AID(S38A) and a GFP indicator, assessed 96 h after infection. Numbers in quadrants indicate percent cells in each; numbers in parentheses indicate IgG1+ cells in the GFP+ gate. Data are representative of at least five independent experiments. (b) GFP+ IgG1+ cells in the total GFP+ population of wild-type splenic B cells stimulated with LPS plus IL-4 and infected with retrovirus vector control (pMIG) or retrovirus expressing PKA-Cα. *, P < 0.01 (two-tailed Student’s t-test). Data are representative of four independent experiments (mean and s.d.).
Additional evidence that PKA activates CSR was provided by in vitro overexpression studies. After retroviral overexpression of an epitope-tagged PKA catalytic subunit (PKA-Cα; A001914) in splenic B cells stimulated with lipopolysaccharide (LPS) and interleukin 4 (IL-4), immunoblot analysis showed that expression of the exogenous PKA-Cα was approximately twofold more than that of endogenous PKA-Cα (Supplementary Fig. 1 online). Wild-type B cells that overexpressed PKA-Cα had a percentage of IgG1+ cells approximately 40–50% higher than that of cells expressing the vector control (Fig. 1b and Supplementary Fig. 2 online). The greater CSR frequency was not due to enhanced B cell proliferation (Supplementary Fig. 3a,b online) or germline transcription (Supplementary Fig. 4 online). Experiments with B cells that expressed a catalytically inactive PKA-Cα mutant (K73M)38 were inconclusive, as we were unable to detect the mutant protein in the retrovirally infected B cells (Supplementary Fig. 1). Overexpression of Pim1 (Supplementary Fig. 1), a kinase that does not phosphorylate AID, did not increase CSR (Supplementary Fig. 5 online). Thus, many lines of evidence suggest that phosphorylation of AID at Ser38 by PKA is important in CSR in vivo.
AID phosphorylation is not needed for S-region binding
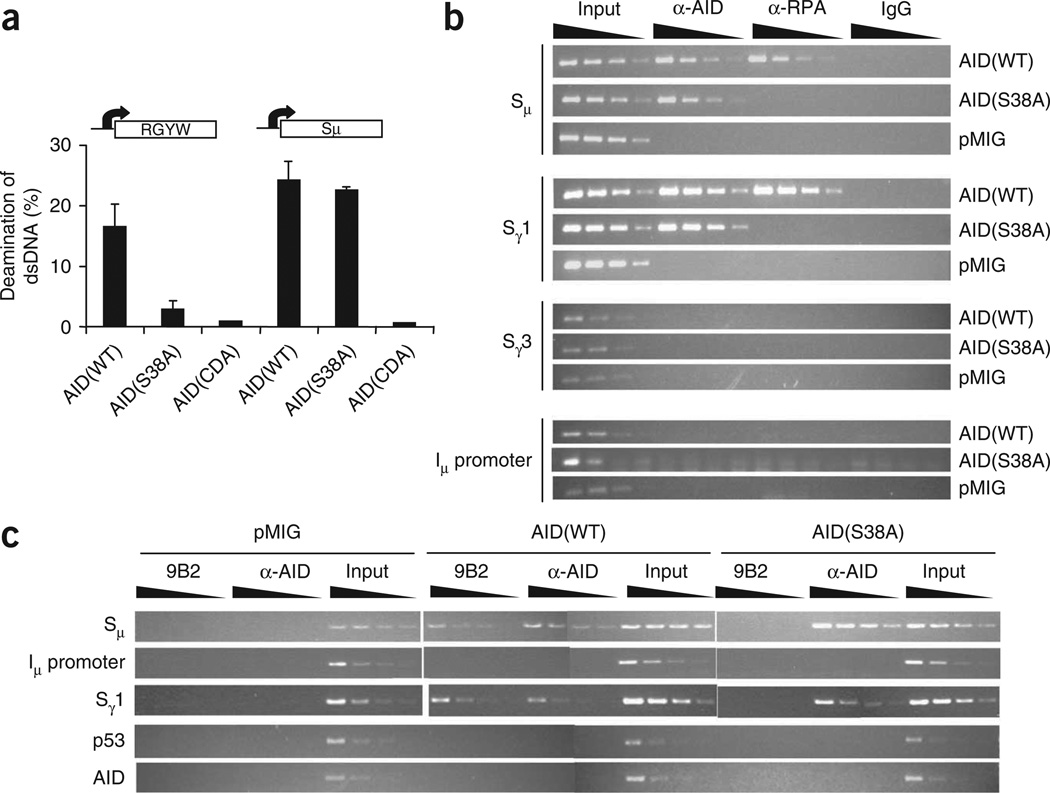
The substantial CSR defect in cells expressing AID(S38A) would not have been predicted by prevailing CSR models, which posit that ssDNA in the context of R loops represents a suitable AID target and thus would not require RPA-mediated stabilization20. Indeed, whereas AID(S38A) purified from B cells was considerably compromised in its ability to deaminate an SHM target in vitro, the mutant protein was as competent as wild-type AID (AID(WT)) in mediating deamination of a transcribed S region (Fig. 2a). These results reiterate the idea that phosphorylation of AID is dispensable for deamination of R loop–forming substrates. To determine if the CSR defect in AID(S38A) cells was due to less binding of AID(S38A) to S regions, we did chromatin immunoprecipitation (ChIP) experiments. We cultured AID-deficient splenic B cells ex vivo and infected them with retrovirus expressing AID(WT) or AID(S38A) or with the control retroviral vector (pMIG), then immunoprecipitated cross-linked DNA-protein complexes with polyclonal AID-specific antibodies. We analyzed the immunoprecipitated DNA for the presence of Sµ and Sγ1, regions that are active for CSR in conditions of stimulation. We found that AID(S38A) and AID(WT) associated with Sµ and Sγ1 to a similar extent (Fig. 2b). There was no detectable binding of AID to either Sγ3, an S region that is not a recombination target in cells cultured with LPS plus IL-4, or the Igh Iµ promoter. These results demonstrate that phosphorylation of AID at Ser38 is not essential for its association with S regions. However, in B cells expressing AID(S38A), RPA could not be recruited to S regions (Fig. 2b), consistent with the idea that the interaction between AID and RPA is phosphorylation dependent20,39. Overall, these experiments indicate that the defect in CSR in AID(S38A)-expressing cells was not due to a failure of AID to access S regions but instead was due to an inability to recruit RPA.
Figure 2.
Phosphorylation of AID is not required for interaction with S-region DNA. (a) Deaminase activity in Aicda−/− splenic B cells infected with retrovirus expressing AID(WT), AID(S38A) or an AID cytidine deaminase mutant containing H56R-E58Q substitutions (AID(CDA))10; nuclear extracts were assayed for transcription-coupled DNA deaminase activity on a T7 RNA polymerase–transcribed SHM substrate composed of multiple RGYW ‘hotspot’ sequences (left) or an Sµ fragment (right), as described20, in the presence of recombinant RPA. Data are representative of three independent experiments. (b,c) ChIP analysis of AID-deficient splenic B cells infected with pMIG or retrovirus expressing AID(WT) or AID(S38A); DNA immunoprecipitated 48 h after infection with anti-AID (α-AID), anti-RPA (α-RPA), IgG (control) or anti-pSer38-AID (9B2), or input DNA, was serially diluted 1:3 (wedges above lanes) and various genomic DNA sequences (left margin) were amplified with specific primers. Results are representative of three independent experiments.
The ability of AID(S38A) to efficiently associate with S regions prompted us to investigate if pSer38-AID was present at S regions. For this, we raised a monoclonal antibody (9B2) to pSer38-AID. In immunoblots of protein extracts derived from cells expressing both AID and PKA-Cα, 9B2 reacted specifically with a polypeptide that migrated with the same mobility as AID (Supplementary Figs. 6 and 7 online). There was no reactivity with AID(S38A) (Supplementary Figs. 6 and 7) or with an unphosphorylated peptide encompassing the Ser38 residue (Supplementary Fig. 8 online), which indicated that the antibody was specific to pSer38-AID. In ChIP experiments, 9B2 readily detected pSer38-AID at Sµ and Sγ1, but not at the control regions p53 and AID, in AID-deficient B cells infected with retrovirus expressing AID(WT) (Fig. 2c). There was no amplification of S regions in immunoprecipitated DNA from AID-deficient B cells transduced with AID(S38A) or the control vector pMIG (Fig. 2c). These results demonstrate that pSer38-AID is present on S regions undergoing CSR.
PKA subunits are recruited to S regions during CSR
The results presented above suggested that PKA activates CSR by phosphorylating, and thus regulating, AID activity. As PKA is a generally expressed kinase with many characterized substrates40 and AID can deaminate ssDNA indiscriminately in vitro10–12, we reasoned that specificity for AID activity might be restricted in vivo by localized phosphorylation of AID bound to S regions in B cells undergoing CSR. Notably, PKA, which is typically anchored in subcellular compartments to limit its kinase activity, is recruited to actively transcribed genes41. In unactivated cells, the PKA holoenzyme consists of two regulatory and two catalytic subunits; the phosphotransferase activity of the catalytic subunits is inhibited by the regulatory subunits. Activation and release of the catalytic subunit is mediated by cAMP, which binds allosterically to the regulatory subunits in the holoenzyme42. Mouse splenic B cells expressed mRNA encoding the regulatory subunits PKA-RIα (A000049) and PKA-RIIα (A001918), as well as the catalytic subunits PKA-Cα and PKA-Cβ (A001915); however, they did not express mRNA encoding the regulatory subunits PKA-RIβ and PKA-RIIβ (A000050; Supplementary Fig. 9 online). In CSR-stimulated splenic B cells, PKA subunits were present in the cytoplasm, nucleus and chromatin fractions (Supplementary Fig. 10 online).
To determine whether PKA subunits are recruited to S regions during CSR, we did ChIP experiments with antibodies to PKA subunits, using wild-type B cells stimulated with LPS plus IL-4. We found the catalytic subunit PKA-Cα at Sµ and Sγ1 but not at Sγ3 (Fig. 3a). Notably, the regulatory subunit PKA-RIα also bound the active S regions (Fig. 3a), which suggested that the PKA holoenzyme was associated with the Igh locus. ChIP experiments with antibodies to the subunits PKA-Cβ and PKA-RIIα were not reproducible and the association of these subunits with S regions could not be determined (data not shown). There was no detectable binding of the PKA subunits to other control genomic sequences, such those derived from Aicda (which encodes AID) or Trp53 (which encodes p53; Fig. 3a), or to several other genes that are upregulated or downregulated in cells undergoing CSR (Supplementary Fig. 11 online).
Figure 3.
Recruitment of PKA subunits to S regions during CSR. (a) ChIP analysis of wild-type splenic B cells stimulated for 48 h with LPS plus IL-4; input or immunoprecipitated DNA (antibodies, above lanes) was serially diluted 1:3 (wedges) and various genomic DNA sequences (left margin) were amplified with specific primers. (b) ChIP analysis, as described in a, of wild-type splenic B cells or T cells purified and stimulated for 48 h in culture with anti-CD40 or with anti-CD3 plus anti-CD28, respectively, or of unstimulated B cells (top). Histone H3, an integral component of chromatin, serves as a positive control. (c) ChIP analysis, as described in a, of AID-deficient splenic B cells stimulated for 48 h with LPS plus IL-4. Results are representative of three independent experiments.
The association of PKA subunits with S regions was specific to cells activated for CSR and could be detected as early as 13 h after stimulation (Supplementary Fig. 12 online). We did not detect PKA at S regions in unstimulated splenic B cells or in B cells cultured ex vivo with antibody to CD40 (anti-CD40), which induces proliferation but not CSR (Fig. 3b). Additionally, there were no PKA subunits at S regions in proliferating splenic T cells (Fig. 3b). As AID has been found to interact with the PKA holoenzyme32,33 (Supplementary Fig. 6), we tested the possibility that the association of PKA with S regions is mediated by AID. ChIP experiments with activated AID-deficient B cells showed that the PKA subunits were still bound to S regions (Fig. 3c), which indicated that the binding was AID independent. Similar to results obtained with unstimulated wild-type B cells, we did not detect PKA at S regions in unstimulated splenic B cells derived from AID-deficient mice (Supplementary Fig. 13 online). The experiments reported above demonstrate that the subunits PKA-Cα and PKA-RIα are specifically recruited to S regions in B cells activated for CSR and that the formation of the PKA–S region complex is AID independent.
PKA, AID and RPA form a complex on S regions
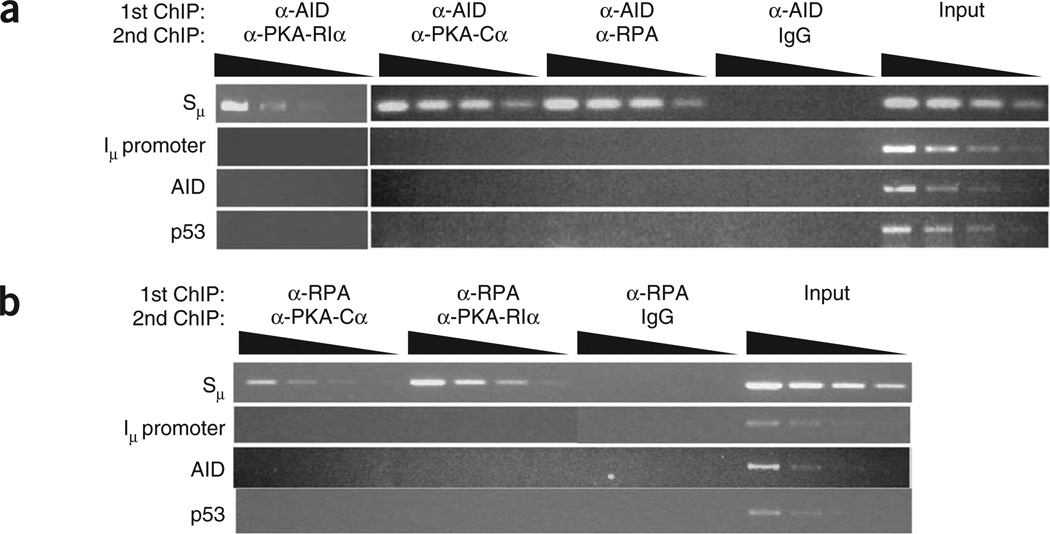
To determine if the proteins (PKA, RPA and AID) shown to be associated with S regions are in the same macromolecular complex, we did two-step ChIP experiments. In these assays, we purified DNA-protein complexes with one set of antibodies (anti-AID or anti-RPA) and subjected them to a second round of ChIP with a different set of antibodies (anti-PKA-RIα, anti-PKA-Cα or anti-RPA). We used nonspecific IgG in the second round of ChIP as a control for the ‘carryover’ of antibody from the first ChIP. In experiments in which the first ChIP used anti-AID, followed by secondary ChIP with anti-PKA-RIα or anti-PKA-Cα, Sµ was readily detected in the DNA-protein complex (Fig. 4a), which indicated that AID was bound to Sµ that also contained both PKA subunits. Likewise, two-step ChIP experiments with anti-RPA as the primary ChIP antibody showed that RPA, PKA-Cα and PKA-RIα were in a complex with Sµ (Fig. 4b). As binding of RPA to S regions depends on pSer38-AID, and phosphorylation of AID by PKA-Cα requires the release of PKA-RIα from the PKA holoenzyme, phosphorylation of AID must occur without physical displacement of the PKA-RIα subunit from the chromatin. Whether PKA, AID and RPA are directly bound to each other on the DNA remains unclear; however, our data suggest that a complex of AID, RPA and PKA is assembled at the S regions to locally phosphorylate AID and initiate the early stages of CSR.
Figure 4.
PKA, AID and RPA form a macromolecular complex on S regions. Two-step ChIP analysis of wild-type splenic B cells stimulated for 48 h with LPS plus IL-4; after a first round of ChIP with anti-AID (a) or anti-RPA (b) and a second round of ChIP with various antibodies (above lanes), input or immunoprecipitated DNA was diluted 1:3 (wedges) and various genomic DNA sequences (left margin) were amplified with specific primers. Results are representative of three independent experiments.
Genetic inactivation of PKA impairs CSR
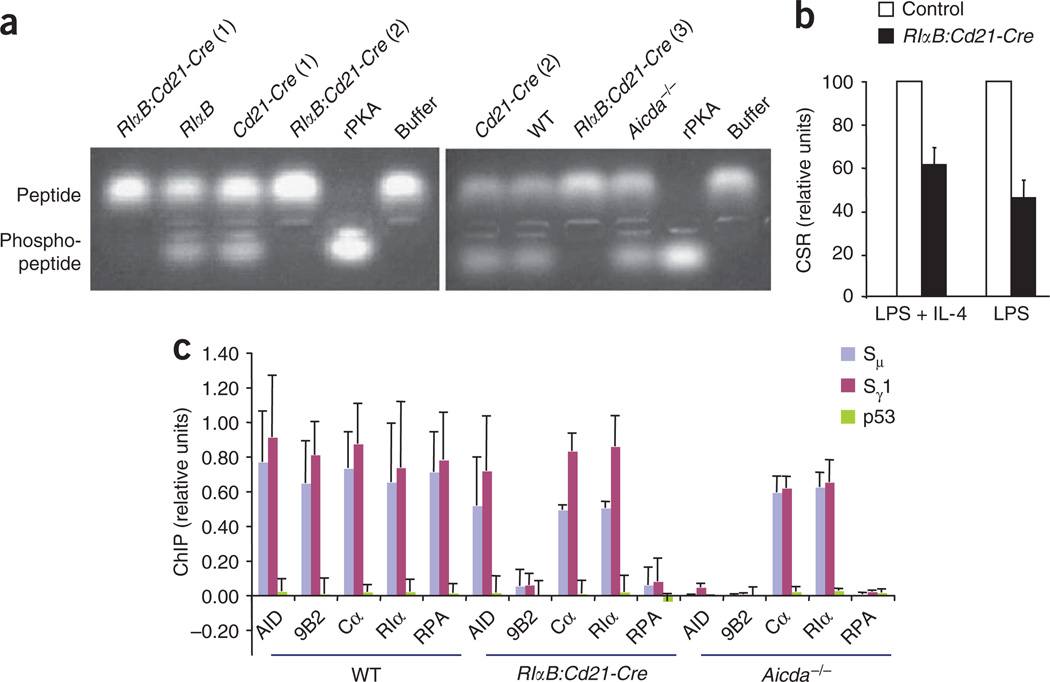
To directly demonstrate that PKA activity regulates CSR, we used the ‘RIαB’ mutant mouse, which contains a G324A substitution in the cAMP-binding site of PKA-RIα that diminishes the affinity of PKA-RIα for cAMP by two orders of magnitude43. Thus, the mutant PKA-RIα protein remains bound to the catalytic subunit at relatively high concentrations of cellular cAMP, which effectively decreases cellular PKA activity. We bred RIαB mice to Cd21-Cre-transgenic mice (which express Cre recombinase driven by the Cd21 promoter) to delete the neomycin-resistance cassette in the mutated RIαB allele (RIαB:Cd21-Cre; Supplementary Fig. 14 online) specifically in mature B cells44. B cells from RIαB:Cd21-Cre mice had much less overall PKA activity (Fig. 5a). Next, we activated splenic B cells from RIαB:Cd21-Cre mice ex vivo with LPS or with LPS plus IL-4 to promote CSR to IgG3 or IgG1, respectively. B cells from RIαB:Cd21-Cre mice had 35–50% less CSR than did control cells from their wild-type littermates (Fig. 5b). The diminished CSR was not accompanied by less AID or PKA protein or germline transcription or less cell proliferation (Supplementary Figs. 15–17 online). Thus, genetic suppression of PKA activity in B cells impairs CSR.
Figure 5.
Conditional inactivation of PKA activity impairs CSR. (a) PKA activity in whole-cell extracts of splenic B cells (identified above lanes) stimulated for 48 h with LPS plus IL-4, analyzed with a Kemptide assay. rPKA, recombinant PKA catalytic subunit (positive control); Buffer, reaction without any added protein; numbers in parentheses identify different samples. Results are representative of at least three independent experiments. (b) Class switching to IgG1 or IgG3, respectively, in splenic B cells from RIαB:Cd21-Cre and control littermate mice (wild-type, RIαB and Cd21-Cre) stimulated for 4 d with LPS plus IL-4 or with LPS alone. CSR for RIαB:Cd21-Cre mice is presented as a fraction of CSR for control mice, set as 100. Data are from at least four independent experiments (mean and s.d.). (c) ChIP analysis of splenic B cells from Aicda−/−, RIαB:Cd21-Cre and wild-type (WT) littermate control mice; immunoprecipitated DNA (antibodies, horizontal axis) was amplified by real-time PCR with primers specific to genomic DNA sequences (key). Data represent three independent experiments (mean and s.d.).
Finally, we did ChIP experiments to determine how the lower PKA activity affected the recruitment of various CSR factors to S regions. For this set of experiments, we analyzed input and immunoprecipitated DNA samples by quantitative real-time PCR. The lower PKA activity had no effect on the amount of PKA subunits associated with S regions (Fig. 5c). In addition, the amount of AID bound to S regions was similar in wild-type and RIαB:Cd21-Cre cells, consistent with the data reported above demonstrating that phosphorylation of AID at Ser38 did not regulate its recruitment to the Igh locus (Fig. 2b). Notably, however, there was much less association of phosphorylated AID and RPA with S regions (Fig. 5c), which provides unequivocal genetic evidence that impaired PKA activity leads to less phosphorylated AID at S regions, with concomitantly less recruitment of RPA and CSR. The hypomorphic nature of the RIαB mutation allowed measurable phosphorylation of AID and recruitment of RPA to S regions (Fig. 5c and Supplementary Fig. 18 online) and could account for the finding that the CSR defect in RIαB:CdD21-Cre cells was less severe than that in B cells expressing AID(S38A).
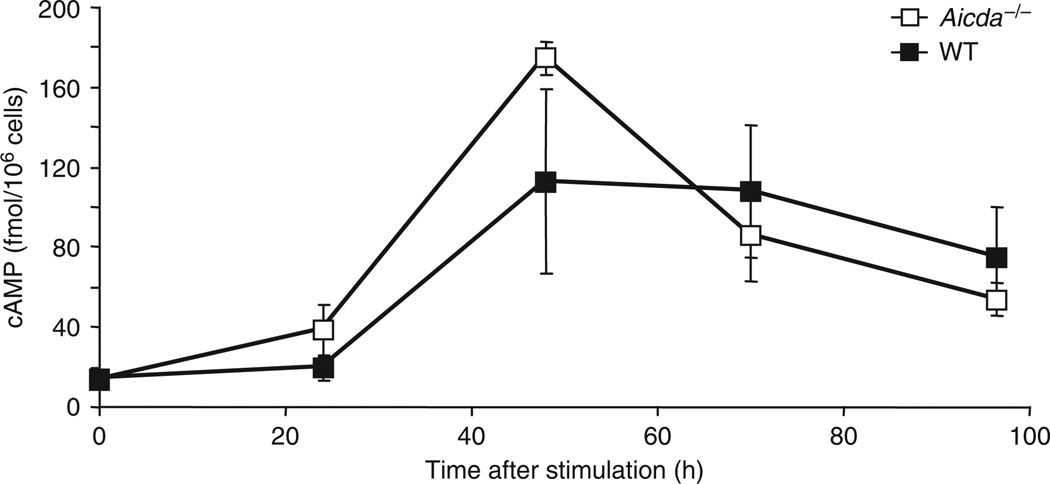
The results reported above led us to propose a model in which a CSR stimulus assembles AID and PKA at activated S regions (Supplementary Fig. 19 online). The PKA-RIα subunit associated with the PKA holoenzyme maintains AID in an unphosphorylated state until a burst of cAMP activates PKA-Cα, which leads to phosphorylation of AID, recruitment of RPA and activation of CSR (Supplementary Fig. 19). In agreement with our model, both wild-type and AID-deficient splenic B cells stimulated to undergo CSR had a three- to fourfold increase in cAMP between 24 and 48 h, followed by a gradual decrease between 48 and 96 h (Fig. 6). Overall, our findings that AID can be recruited to S regions without phosphorylation at Ser38, the underlying prediction that PKA phosphorylates S region–bound AID, and the observed increase in cAMP at a time that coincides with CSR provide a satisfactory model whereby synchronous phosphorylation of multiple AID molecules generates the high density of DSBs required for efficient CSR45.
Figure 6.
Induction of cAMP in activated splenic B cells. Enzyme immunoassay of cAMP in wild-type and Aicda–/– splenic B cells stimulated for various times (horizontal axis) with LPS. Data are representative of three experiments (mean and s.d. of three independent cultures).
DISCUSSION
AID can deaminate S regions in vitro and bind to S regions in B cells independently of its phosphorylation status. Yet efficient CSR requires phosphorylation of AID at Ser38 (refs. 32–35,46), which suggests a phosphorylation-dependent post-deamination function for AID. The only known biochemical function for phosphorylation of AID at Ser38 is to promote interaction with RPA, as compensatory substitutions in AID(S38A) that allow constitutive binding to RPA can restore CSR activity39. Thus, AID ‘activity’ during CSR includes both its enzymatic activity (DNA deamination) as well as its ability to recruit RPA to S regions.
The precise function of RPA in CSR is not clear, but it may act ‘downstream’ of deamination, as in the recruitment of uracil DNA glycosylase or mismatch-repair proteins, which convert deaminated cytidines to DSBs4. Additionally, RPA might recruit proteins such as 53BP1 and H2AX to DSBs to promote synapsis between distal broken S regions before their ligation during CSR. The known requirements for uracil DNA glycosylase, mismatch-repair proteins, 53BP1 and H2AX in CSR and the reported interactions of those proteins with RPA support the idea that RPA is involved ‘downstream’ of DNA deamination3. Failure to recruit RPA to S regions could thus impair conversion of the deaminated residues into DSBs or the interaction between distal DSBs. Either defect or both defects could be manifested as a considerable defect in CSR. The residual CSR in cells expressing AID(S38A) probably reflects the ability of unphosphorylated AID to bind to and deaminate S regions, a few of which could be converted into nicks and DSBs, and a productive recombination reaction with a ‘downstream’ S region by an RPA-independent mechanism. Thus, it is possible that low frequencies of CSR could occur in the absence of assembled PKA complexes. That idea is consistent with the observation that the artificial generation of DSBs in S regions can allow CSR, albeit less CSR, in the absence of AID47. Thus, the function of RPA in CSR is possibly distinct from that in SHM, in which the AID-RPA interaction is needed to permit access of AID to transcribed V genes20.
The PKA-dependent activation of AID has several potential implications for the prevention of inadvertent DNA lesions. First, the sequestration of AID in an inactive PKA holoenzyme complex32,33 could effectively limit the total cellular concentration of active AID. Second, the specific recruitment of both AID and PKA to S regions ensures activity only in the context of physiological targets. Thus, whereas AID can deaminate and mutate several transcribed genes in germinal center B cells at a very low rate23, its full DSB-generating activity, as required for CSR, is ‘unmasked’ only when it is recruited together with PKA, which thereby effectively restricts AID-dependent mutagenesis at non-immunoglobulin genes. Finally, the AID-independent recruitment of PKA to activated S regions and the ability of AID to interact with PKA lead us to hypothesize that PKA itself could be the factor that targets AID to S regions during CSR.
The data presented here provide evidence of a mammalian serine-threonine kinase that is recruited to S regions to activate CSR. The mechanism by which the PKA subunits are ‘ferried’ and tethered to the S regions is unclear; however, PKA (and possibly AID) may associate with a specific transcription factor or an unknown ‘targeting factor’ that recruits it (or them) to the S regions during CSR. In this context, binding sites for the cAMP-response element–binding protein, a well characterized PKA substrate, have been found in the germline promoters of Sα and Sγ1, and cAMP-response element– binding protein has been shown to regulate germline transcription ‘downstream’ of transforming growth factor-β stimulation48. Alternatively, the recruitment of PKA to S regions may be mediated by A kinase–anchoring proteins. Hypothetical B cell A kinase–anchoring proteins would coordinate the enzymes and substrates required for CSR to permit efficient activation of AID by juxtaposing AID with its substrate (DNA), its activator (PKA) and its cofactor (RPA). Regardless of the mechanism of the recruitment of PKA to S regions, the association of PKA with S regions is consistent with the hypothesis that localized activation of PKA through cAMP microdomains directs PKA activity toward specific substrates. The phosphorylation and activation of AID by PKA directly on the target DNA thus sets a paradigm for the way a generally expressed kinase achieves specificity for its substrate by concerted recruitment of both to a specific subcellular compartment.
METHODS
Mouse models, B cell purification and retroviral infection
Aicda−/− mice were a gift from T. Honjo. Wild-type BALB/c and Cd21-Cre mice were from The Jackson Laboratory. RIαB mice were generated as described43. All animals were maintained according to guidelines for animal welfare of the Memorial-Sloan Kettering Research Animal Resource Center.
Mouse splenic B cells were purified by negative selection with anti-CD43 magnetic beads (Miltenyi Biotec) and were stimulated with LPS (10 µg/ml), with LPS (10 µg/ml) plus IL-4 (12.5 µg/ml) or with anti-CD40 (1 µg/ml; HM40-3; eBioscience) plus IL-4. Cells were labeled with the cytosolic dye CFSE (carboxyfluorescein diacetate succinimidyl diester) as described49. Mouse splenic T cells were purified by negative selection with the Pan T Cell Isolation kit (Miltenyi Biotec) and were stimulated with plate-bound anti-CD3 and anti-CD28 (a gift from J. Allison). Retroviral infection was done as described50.
ChIP
ChIP was done as described51. PCR primers are in Supplementary Table 1 online). Two-step ChIP was done as described52.
A LightCycler DNA Master SYBR Green I kit (Roche) was used for real-time PCR of immunoprecipitated DNA (primers, Supplementary Table 1). Real-time PCR products were analyzed for incorporation of SYBR Green and crossing points were obtained with the Lightcycler software. Melting-curve analysis and agarose gels confirmed the presence of a single PCR product of the predicted size. ‘Relative units’ for ChIP were calculated by normalization of the crossing point of ChIP with each specific antibody to the crossing point of the input DNA; the inverse of the input DNA–normalized crossing point was then calculated. The ‘IgG-preclear’ value (inverse of the input DNA–normalized crossing point) was subtracted from value obtained for ChIP with each specific antibody (inverse of the input DNA–normalized crossing point) to obtain the ‘relative units’ for ChIP.
Plasmids
The retroviral vector pMIG has been described50. Additional plasmid descriptions are in the Supplementary Methods online. The cDNA encoding PKA-Cα, PKA-Cβ, PKA-RIα and PKA-RIIα was obtained by RT-PCR of splenic B cell RNA and was subsequently cloned into pCDNA3.1/HisC (Invitrogen) to obtain Xpress epitope–tagged PKA subunits.
Xpress-tagged PKA-Cα and PKA-Cβ were cloned into the multiple cloning sites of pMIG; untagged PKA-RIα and PKA-RIIα were cloned into pMIG. The cDNA encoding mouse AID (AID(WT) or AID(S38A)) with both Flag and hemagglutinin epitope tags on the amino terminus was generated by PCR amplification and was subsequently cloned into the multiple cloning site of pMIG. The plasmid pMIG-hPim1 was obtained from P. Rothman.
Reagents and antibodies
Antibodies for ChIP were as follows: anti-PKA-Cα (sc-903; Santa Cruz Biotechnology), anti-PKA-RIα (sc-28893; Santa Cruz Biotechnology), anti-AID (as described10), anti-RPA (NA19L; Oncogene), anti-H3 (ab12079; Abcam) and anti–rabbit IgG (I5006; Sigma). Antibodies for flow cytometry were as follows: allophycocyanin–anti-IgG1 (X56), phycoerythrin-indotricarbocyanine–anti-B220 (RA3-6B2) and fluorescein isothiocyanate– anti-IgG3 (R40–82; all from BD Pharmingen). Antibodies for immunoblot analysis were as follows: anti-Xpress (R910-25; Invitrogen), anti-PKA-Cα (610981; BD Transduction), anti-PKA-RIα (610610; BD Transduction), anti-PKA-RIIα (612242; BD Transduction), anti-hemagglutinin (3F10; Roche), anti-α-tubulin (T9026; Sigma), anti-β-tubulin (sc-5274; Santa Cruz Biotechnology), anti-Flag (M2; Sigma), anti-GFP (33–2600; Zymed) and anti-Pim-1 (sc-28777; Santa Cruz Biotechnology). Additional antibody descriptions are in the Supplementary Methods.
LPS was from Sigma (L7261), IL-4 was from R&D Systems (404-ML-050), anti-CD40 was from eBiosciences (HM40-3), and anti-CD3 and anti-CD28 were a gift from J. Allison. Additional antibody descriptions are in the Supplementary Methods.
Armenian hamster monoclonal antibody to pSer38-AID (9B2) was generated by the Monoclonal Antibody Core Facility (Memorial Sloan-Kettering Cancer Center). A peptide phosphorylated (*) at Ser38 (CYVVKRRDS*ATS), synthesized at the Keck Biotechnology Resource Center at Yale University, was conjugated to keyhole limpet hemocyanin before immunization of hamsters.
Cell extract fractionation
Cells were separated into cytoplasmic and nuclear fractions as described53. Chromatin fractions were obtained by resuspension of the nuclear lysis pellet in five volumes of HCl lysis buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol and 1.5 mM phenylmethyl sulfonyl fluoride). Hydrochloric acid was added to a final concentration of 0.2 M and the samples were kept at 4 °C for 30 min before being spun at 20,000g in a refrigerated micro-centrifuge. The pellet was discarded and the supernatant (chromatin extract) was neutralized with 0.5 volumes of 1 M Tris, pH 8.0.
Assay of cAMP
The amount of cAMP was determined with the cAMP Biotrak Enzymeimmunoassay system (Amersham). B cells (1 × 106) in a volume of 1 ml in a 24-well plate were stimulated for various times with LPS plus IL-4. For calculation of cAMP amounts, the absorbance at 450 nm at each time point was compared with a cAMP standard curve.
PKA activity assay
PKA activity was measured with the PepTag Non-Radioactive Protein Kinase assay (Promega). In this ‘Kemptide’ assay, phosphorylation of a PKA peptide substrate causes it to move toward the positive electrode during electrophoresis through an agarose gel. Whole-cell extracts were prepared by lysis of B cells stimulated (48 h) for CSR in a Nonidet-P40 lysis buffer (0.5% (vol/vol) Nonidet-P40, 50 mM Tris, pH 7.5, 100 mM NaCl, 0.1 mM EDTA and 10% (vol/vol) glycerol); 10 µg was used in each assay. Recombinant PKA (NEB) was used as a positive control.
Accession codes
UCSD-Nature Signaling Gateway (http://www.signaling-gateway.org): A001914, A000049, A001918, A001915 and A000050.
Supplementary Material
ACKNOWLEDGMENTS
We thank T. Honjo (Kyoto University) for AID-deficient mice; J. Allison (Memorial Sloan-Kettering Cancer Center) for anti-CD3 and anti-CD28; P. Rothman (University of Iowa) for the plasmid pMIG-hPim1; and L. Denzin and D. Sant’Angelo, as well as members of their laboratories and members of the Chaudhuri laboratory, for discussions and technical assistance. Supported by the US National Institutes of Health (T32CA09149 to B.V.), the Damon Runyon Cancer Research Foundation (J.C.), the Bressler Scholars Foundation (J.C.), the Frederick Adler Chair for Junior Faculty (J.C.) and the Sloan-Kettering Institute (J.C.).
Footnotes
Note: Supplementary information is available on the Nature Immunology website.
References
- 1.Jung D, Giallourakis C, Mostoslavsky R, Alt FW. Mechanism and control of V(D)J recombination at the immunoglobulin heavy chain locus. Annu. Rev. Immunol. 2006;24:541–570. doi: 10.1146/annurev.immunol.23.021704.115830. [DOI] [PubMed] [Google Scholar]
- 2.Papavasiliou FN, Schatz DG. Somatic hypermutation of immunoglobulin genes: merging mechanisms for genetic diversity. Cell. 2002;109:S35–S44. doi: 10.1016/s0092-8674(02)00706-7. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhuri J, et al. Evolution of the immunoglobulin heavy chain class switch recombination mechanism. Adv. Immunol. 2007;94:157–214. doi: 10.1016/S0065-2776(06)94006-1. [DOI] [PubMed] [Google Scholar]
- 4.Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu. Rev. Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peled JU, et al. The biochemistry of somatic hypermutation. Annu. Rev. Immunol. 2008;26:481–511. doi: 10.1146/annurev.immunol.26.021607.090236. [DOI] [PubMed] [Google Scholar]
- 6.Muramatsu M, et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 7.Revy P, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 8.Petersen-Mahrt SK, Harris RS, Neuberger MS. AID mutates E. coli suggesting a DNA deamination mechanism for antibody diversification. Nature. 2002;418:99–103. doi: 10.1038/nature00862. [DOI] [PubMed] [Google Scholar]
- 9.Ramiro AR, Stavropoulos P, Jankovic M, Nussenzweig MC. Transcription enhances AID-mediated cytidine deamination by exposing single-stranded DNA on the nontemplate strand. Nat. Immunol. 2003;4:452–456. doi: 10.1038/ni920. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhuri J, et al. Transcription-targeted DNA deamination by the AID antibody diversification enzyme. Nature. 2003;422:726–730. doi: 10.1038/nature01574. [DOI] [PubMed] [Google Scholar]
- 11.Dickerson SK, Market E, Besmer E, Papavasiliou FN. AID mediates hypermutation by deaminating single stranded DNA. J. Exp. Med. 2003;197:1291–1296. doi: 10.1084/jem.20030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bransteitter R, Pham P, Scharff MD, Goodman MF. Activation-induced cytidine deaminase deaminates deoxycytidine on single-stranded DNA but requires the action of RNase. Proc. Natl. Acad. Sci. USA. 2003;100:4102–4107. doi: 10.1073/pnas.0730835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian M, Alt FW. Transcription-induced cleavage of immunoglobulin switch regions by nucleotide excision repair nucleases in vitro. J. Biol. Chem. 2000;275:24163–24172. doi: 10.1074/jbc.M003343200. [DOI] [PubMed] [Google Scholar]
- 14.Yu K, Chedin F, Hsieh CL, Wilson TE, Lieber MR. R-loops at immunoglobulin class switch regions in the chromosomes of stimulated B cells. Nat. Immunol. 2003;4:442–451. doi: 10.1038/ni919. [DOI] [PubMed] [Google Scholar]
- 15.Shinkura R, et al. The influence of transcriptional orientation on endogenous switch region function. Nat. Immunol. 2003;4:435–441. doi: 10.1038/ni918. [DOI] [PubMed] [Google Scholar]
- 16.Schrader CE, Edelmann W, Kucherlapati R, Stavnezer J. Reduced isotype switching in splenic B cells from mice deficient in mismatch repair enzymes. J. Exp. Med. 1999;190:323–330. doi: 10.1084/jem.190.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rada C, et al. Immunoglobulin isotype switching is inhibited and somatic hypermutation perturbed in UNG-deficient mice. Curr. Biol. 2002;12:1748–1755. doi: 10.1016/s0960-9822(02)01215-0. [DOI] [PubMed] [Google Scholar]
- 18.Rada C, Di Noia JM, Neuberger MS. Mismatch recognition and uracil excision provide complementary paths to both Ig switching and the A/T-focused phase of somatic mutation. Mol. Cell. 2004;16:163–171. doi: 10.1016/j.molcel.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Bardwell PD, et al. Altered somatic hypermutation and reduced class-switch recombination in exonuclease 1-mutant mice. Nat. Immunol. 2004;5:224–229. doi: 10.1038/ni1031. [DOI] [PubMed] [Google Scholar]
- 20.Chaudhuri J, Khuong C, Alt FW. Replication protein A interacts with AID to promote deamination of somatic hypermutation targets. Nature. 2004;430:992–998. doi: 10.1038/nature02821. [DOI] [PubMed] [Google Scholar]
- 21.Shen HM, Peters A, Baron B, Zhu X, Storb U. Mutation of BCL-6 gene in normal B cells by the process of somatic hypermutation of Ig genes. Science. 1998;280:1750–1752. doi: 10.1126/science.280.5370.1750. [DOI] [PubMed] [Google Scholar]
- 22.Pasqualucci L, et al. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature. 2001;412:341–346. doi: 10.1038/35085588. [DOI] [PubMed] [Google Scholar]
- 23.Liu M, et al. Two levels of protection for the B cell genome during somatic hypermutation. Nature. 2008;451:841–845. doi: 10.1038/nature06547. [DOI] [PubMed] [Google Scholar]
- 24.Ramiro AR, et al. AID is required for c-myc/IgH chromosome translocations in vivo. Cell. 2004;118:431–438. doi: 10.1016/j.cell.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Unniraman S, Zhou S, Schatz DG. Identification of an AID-independent pathway for chromosomal translocations between the Igh switch region and Myc. Nat. Immunol. 2004;5:1117–1123. doi: 10.1038/ni1127. [DOI] [PubMed] [Google Scholar]
- 26.Ramiro AR, et al. Role of genomic instability and p53 in AID-induced c-myc-Igh translocations. Nature. 2006;440:105–109. doi: 10.1038/nature04495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasqualucci L, et al. AID is required for germinal center-derived lymphomagenesis. Nat. Genet. 2008;40:108–112. doi: 10.1038/ng.2007.35. [DOI] [PubMed] [Google Scholar]
- 28.Muramatsu M, et al. Specific expression of activation-induced cytidine deaminase (AID), a novel member of the RNA-editing deaminase family in germinal center B cells. J. Biol. Chem. 1999;274:18470–18476. doi: 10.1074/jbc.274.26.18470. [DOI] [PubMed] [Google Scholar]
- 29.Dorsett Y, et al. MicroRNA-155 suppresses activation-induced cytidine deaminase-mediated Myc-Igh translocation. Immunity. 2008;28:630–638. doi: 10.1016/j.immuni.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teng G, et al. MicroRNA-155 is a negative regulator of activation-induced cytidine deaminase. Immunity. 2008;28:621–629. doi: 10.1016/j.immuni.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aoufouchi S, et al. Proteasomal degradation restricts the nuclear lifespan of AID. J. Exp. Med. 2008;205:1357–1368. doi: 10.1084/jem.20070950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basu U, et al. The AID antibody diversification enzyme is regulated by protein kinase A phosphorylation. Nature. 2005;438:508–511. doi: 10.1038/nature04255. [DOI] [PubMed] [Google Scholar]
- 33.Pasqualucci L, Kitaura Y, Gu H, Dalla-Favera R. PKA-mediated phosphorylation regulates the function of activation-induced deaminase (AID) in B cells. Proc. Natl. Acad. Sci. USA. 2006;103:395–400. doi: 10.1073/pnas.0509969103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McBride KM, et al. Regulation of hypermutation by activation-induced cytidine deaminase phosphorylation. Proc. Natl. Acad. Sci. USA. 2006;103:8798–8803. doi: 10.1073/pnas.0603272103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McBride KM, et al. Regulation of class switch recombination and somatic mutation by AID phosphorylation. J. Exp. Med. 2008;205:2585–2594. doi: 10.1084/jem.20081319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chatterji M, Unniraman S, McBride KM, Schatz DG. Role of activation-induced deaminase protein kinase A phosphorylation sites in Ig gene conversion and somatic hypermutation. J. Immunol. 2007;179:5274–5280. doi: 10.4049/jimmunol.179.8.5274. [DOI] [PubMed] [Google Scholar]
- 37.Shinkura R, Okazaki IM, Muto T, Begum NA, Honjo T. Regulation of AID function in vivo. Adv. Exp. Med. Biol. 2007;596:71–81. doi: 10.1007/0-387-46530-8_7. [DOI] [PubMed] [Google Scholar]
- 38.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. The transcriptional activity of NF-kB is regulated by the IkB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell. 1997;89:413–424. doi: 10.1016/s0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 39.Basu U, Wang Y, Alt FW. Evolution of phosphorylation-dependent regulation of activation-induced cytidine deaminase. Mol. Cell. 2008;32:285–291. doi: 10.1016/j.molcel.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shabb JB. Physiological substrates of cAMP-dependent protein kinase. Chem. Rev. 2001;101:2381–2411. doi: 10.1021/cr000236l. [DOI] [PubMed] [Google Scholar]
- 41.Pokholok DK, Zeitlinger J, Hannett NM, Reynolds DB, Young RA. Activated signal transduction kinases frequently occupy target genes. Science. 2006;313:533–536. doi: 10.1126/science.1127677. [DOI] [PubMed] [Google Scholar]
- 42.Kim C, Cheng CY, Saldanha SA, Taylor SS. PKA-I holoenzyme structure reveals a mechanism for cAMP-dependent activation. Cell. 2007;130:1032–1043. doi: 10.1016/j.cell.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 43.Howe DG, et al. Inhibition of protein kinase A in murine enteric neurons causes lethal intestinal pseudo-obstruction. J. Neurobiol. 2006;66:256–272. doi: 10.1002/neu.20217. [DOI] [PubMed] [Google Scholar]
- 44.Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Iga/b heterodimer. Cell. 2004;117:787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Odegard VH, Schatz DG. Targeting of somatic hypermutation. Nat. Rev. Immunol. 2006;6:573–583. doi: 10.1038/nri1896. [DOI] [PubMed] [Google Scholar]
- 46.Cheng H-L. Integrity of AID serine-38 phosphorylation site is critical for class switch recombination and somatic hypermutation in mice. Proc. Natl. Acad. Sci. USA. doi: 10.1073/pnas.0812304106. (in the press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zarrin AA, et al. Antibody class switching mediated by yeast endonuclease generated DNA breaks. Science. 2007;315:377–381. doi: 10.1126/science.1136386. [DOI] [PubMed] [Google Scholar]
- 48.Lin YC, Shockett P, Stavnezer J. Regulation of transcription of the germline immunoglobulin alpha constant region gene. Curr. Top. Microbiol. Immunol. 1992;182:157–165. doi: 10.1007/978-3-642-77633-5_19. [DOI] [PubMed] [Google Scholar]
- 49.Reina-San-Martin B, et al. H2AX is required for recombination between immunoglobulin switch regions but not for intra-switch region recombination or somatic hypermutation. J. Exp. Med. 2003;197:1767–1778. doi: 10.1084/jem.20030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Parijs L, et al. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity. 1999;11:281–288. doi: 10.1016/s1074-7613(00)80103-x. [DOI] [PubMed] [Google Scholar]
- 51.Nambu Y, et al. Transcription-coupled events associating with immunoglobulin switch region chromatin. Science. 2003;302:2137–2140. doi: 10.1126/science.1092481. [DOI] [PubMed] [Google Scholar]
- 52.Ju BG, et al. A topoisomerase IIb-mediated dsDNA break required for regulated transcription. Science. 2006;312:1798–1802. doi: 10.1126/science.1127196. [DOI] [PubMed] [Google Scholar]
- 53.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.