Abstract
Social evaluative threat (SET) is a potent stressor in humans linked to autonomic and endocrine responses, and multiple health problems. Neuroimaging has recently begun to elucidate the brain correlates of SET, but as yet little is known about the mediating cortical-brainstem pathways in humans. This paper replicates and extends findings in a companion paper (Wager et al., submitted) using an independent cohort of participants and different image acquisition parameters. Here, we focused specifically on relationships between the medial prefrontal cortex (MPFC), midbrain periaqueductal gray (PAG), and heart rate (HR). We applied multi-level path analysis to localize brain mediators of SET effects on HR and self-reported anxiety. HR responses were mediated by opposing signals in two distinct sub-regions of the MPFC—increases in rostral dorsal anterior cingulate cortex (rdACC) and deactivation in ventromedial prefrontal cortex (vmPFC). In addition, HR responses were mediated by PAG. Additional path analyses provided support for two cortical subcortical pathways: one linking vmPFC, PAG, and HR, and another linking rdACC, thalamus, and HR. PAG responses were linked with HR changes both before and during SET, whereas cortical regions showed stronger connectivity with HR during threat. Self-reported anxiety showed a partially overlapping, but weaker, pattern of mediators, including the vmPFC, dorsomedial PFC (dmPFC), and lateral frontal cortex, as well as substantial individual differences that were largely unexplained. Taken together, these data suggest pathways for the translation of social threats into both physiological and experiential responses, and provide targets for future research on the generation and regulation of emotion.
Introduction
The study of brain-body relationships has been an enduring theme in psychology and neuroscience. It encompasses research on brain regulation of the autonomic, endocrine, and immune systems, and has broad implications for understanding both human emotion (Ekman, Levenson, & Friesen, 1983) and psychological influences on health (Glaser & Kiecolt-Glaser, 2005; Lane et al., in press). Understanding brain-body communication is also important for improving prevention and treatment of a number of disorders, including some with an obvious neuro-psychological component, such as depression (Kirsch et al., 2008; Sneed et al., 2008), anxiety (Etkin & Wager, 2007), and pain (Loggia, Schweinhardt, Villemure, & Bushnell, 2008; Wager, Scott, & Zubieta, 2007), and others in which the focus has traditionally been exclusively physiological, including cardiac health (Rozanski et al., 1988), Parkinson’s disease (Benedetti et al., 2004; de la Fuente-Fernandez et al., 2001), asthma (Kemeny et al., 2007), wound healing (Godbout & Glaser, 2006), and resistance to infection (Cohen et al., 2002). Much work has been devoted to understanding psychological effects on health, but very little of it has yet involved detailed analysis of brain circuits and mechanisms in humans.
Threats to the self access brain-body communication systems in a particularly powerful and health-relevant way. Projections from brainstem nuclei that are relatively conserved across species, including the periaqueductal gray (PAG), multiple hypothalamic nuclei, and other brainstem nuclei together coordinate patterns of behavioral, autonomic, and endocrine responses to threats. Descending projections modulate activity in the body, and ascending projections modulate cognition, perception, and behavior.
In addition to being centrally involved in regulating other brain systems, these systems are themselves remarkably sensitive to conceptual thought and activity in “higher” cortical regions. Simply being asked to remember a series of numbers, for example, produces changes in heart rate, pupil diameter, and other peripheral effects in less than one second. Autonomic responses to threat depend not only on the perception of threat in the environment, but also on an organism’s appraisal of resources and options for “coping” with the threat (Tomaka, Blascovich, Kibler, & Ernst, 1997). The modulation of threat responses by perceived resources is reflected in the activation of different cortical-PAG circuits (Bandler, Keay, Floyd, & Price, 2000). There appears to be a close correspondence between the systems involved in generating emotional experience1 and regulating peripheral physiology. The first set of systems evaluates stimuli in the environment and within the body, relates them to the “self” (i.e., evaluate prospects for the organism’s future survival, security, and well being), and generates feelings. The second set of systems is involved in generating autonomic and endocrine responses. The medial prefrontal cortex (MPFC), insula, amygdala, hypothalamus, and PAG have emerged as key players in both (Kober, Barrett, Joseph, Bliss-Moreau et al., 2008; T. Wager et al., 2008), and some contemporary theories relate the origins of emotion and social behavior to the systems involved in regulating the body (Porges, 2003).
From this vantage point, it may appear unsurprising that in modern contemporary society, physiological threat responses (and their health consequences) are often driven by emotional appraisals of abstract information (Lazarus, 1991). Being laughed at or held in contempt by one’s peers, feeling excluded, isolated, judged, or frustrated, and other sorts of abstract appraisals can strongly shape brain-body communication. The notion of “stress” and, more recently, allostatic load—the need to adapt metabolic and brain processes to a changing environment— capture the idea that such appraisals of threat to both the social and physical self can have adverse health consequences. “Threat” in this sense may be distinct from physical threat in its brain etiology and consequences, and it may be qualitatively different from fear conditioning (Etkin & Wager, in press; T. Wager et al., 2008). Moreover, though the generation of social threat in humans may share common features with systems involved in generating multiple types of threat responses in animals, its genesis in the human brain is likely to be qualitatively distinct from that in other animals.
Social threat and other conceptually driven threats are thus an important topic for study using human neuroimaging. Whereas a number of human neuroimaging studies have focused on physical threats (like shock), only a handful have studied the neural bases of SET or related threats to intellectual competence (Critchley, 2003; Dedovic et al., 2005; Eisenberger, Taylor, Gable, Hilmert, & Lieberman, 2007; P. J. Gianaros, F. M. Van Der Veen, & J. R. Jennings, 2004; Kern et al., 2008; Wang et al., 2005).
In a companion paper (Wager et al., submitted), we found evidence that two separate regions of the medial prefrontal cortex (mPFC) had separable, opposite effects on the generation of social threat responses. We used fMRI combined with a novel multi-level path analysis approach to identify brain mediators of the effects of a public speech preparation task (social evaluative threat, SET) on heart rate (HR). A region in the anterior/dorsal midcingulate cortex, around the pregenual anterior cingulate (pgACC), responded with activity increases to the SET challenge, mediated SET-evoked HR changes, and was more strongly activated in individuals with high HR reactivity to the challenge. Conversely, activity decreases in a right ventromedial/medial orbital (vmPFC/mOFC) region were observed with SET. The magnitude of deactivation across time mediated HR responses during the task, and this SET-brain-heart pathway was more strongly deactivated in those with high HR reactivity.
A pattern of increases and decreases in dorsal and ventral MPFC sub-regions—increases in dorsal anterior cingulate cortex (dACC) or pgACC and decreases in vmPFC/mOFC—has been a consistent, though perhaps underappreciated, finding in SET tasks (Critchley et al., 2003; Eisenberger et al., 2007; P. Gianaros, F. M. Van Der Veen, & J. R. Jennings, 2004; Gianaros, Derbyshire et al., 2005). Perceived stress during SET has been associated with increased cortisol and HR reactivity, which is correlated with increased dACC activity (Eisenberger et al., 2007) and decreased vmPFC and mOFC activity (Eisenberger et al., 2007; Pruessner et al., 2008). Similarly, dACC increases during performance stress correlate with measures of sympathetic activation, whereas mOFC decreases correlate with decreases in measures of parasympathetic activation (P. Gianaros et al., 2004), both of which are independent contributors to HR increases during speech preparation (Berntson et al., 1994). The dorsal/ventral increase/decrease pattern we observed is paralleled by similar findings in human and animal fear conditioning: conditioned cues have replicably elicited dACC increases and vmPFC/mOFC decreases (Milad et al., 2007; Phelps, Delgado, Nearing, & LeDoux, 2004; Schiller, Levy, Niv, LeDoux, & Phelps, 2008). A similar pattern has been found in meta-analyses of valenced emotional states, with more dorsal regions associated with negative emotional experience and medial orbital regions associated with positive experience (T. Wager et al., 2008). Together, these finding suggest that these two dissociable sub-regions of mPFC have differential roles in affective appraisal, with consequences for threat-induced cardiac reactivity.
A second consistent finding, however, differentiates SET from fear conditioning. SET manipulations have typically produced decreased activity subcortical regions, including the amygdala and hippocampus (Dedovic et al., 2005; Pruessner et al., 2008). The findings in our companion paper are consistent with this pattern (Wager et al., submitted). A possible explanation is that fMRI responses in human amygala are driven by salient sensory cues that predict affective value under conditions of uncertainty (Amaral, 2003; Herry et al., 2007; Kim et al., 2004; Paton, Belova, Morrison, & Salzman, 2006; Phan, Wager, Taylor, & Liberzon, 2004; Whalen et al., 1998), and thus plays a key role in conditioned “fear” responses, but not unconditioned threat states more generally (Davis, 1998; Walker & Davis, 1997; Wallace & Rosen, 2001). One notable exception to pattern of amygdala decreases in SET is a paper by Gianaros et al. (Gianaros et al., 2008) that found correlations between blood pressure reactivity to a performance stress task and amygdala activation. Interestingly, in this study, interference-task (“Stroop”) trials were presented in a temporally unpredictable fashion, and it is possible that these stimuli act in a manner similar to predictive cues in fear conditioning and related paradigms.
The goal of this paper was to replicate the findings in the companion study using an independent sample (collected on a different scanner and using a different fMRI pulse sequence) and extend them in several important ways. First, none of the studies cited above make strong contact with a large animal literature that implicates lower subcortical and brainstem regions in the generation of threat responses (Bandler et al., 2000; Barbas, Saha, Rempel-Clower, & Ghashghaei, 2003; Devinsky, Morrell, & Vogt, 1995; Saper, 2002), These studies have focused on two systems critical for coordinating central and peripheral responses: the hypothalamus and PAG. The PAG in particular is considered critical for shaping autonomic activity—including cardiac responses—largely through nuclei in the medulla, including the dorsal vagal motor nucleus, parabrachial complex, and rostral ventral medulla. SET influences on functional connections between the cortex, PAG, and autonomic output have not been examined with human imaging, and this was a goal of the present study.
Second, extant data have not examined whether the autonomic and experiential correlates of SET are mediated by similar or distinct systems. Public-speaking SET challenges evoke fear/anxiety, anger, and disgust (as assessed by analysis of facial expressions), and fear/anxiety expressions in particular are correlated with physiological responses (Lerner, Dahl, Hariri, & Taylor, 2007). As noted above, changes in autonomic responses have been associated with the dorsal cingulate and MPFC as well as putative visceromotor regions in rdACC/pgACC and vmPFC. Activity in MPFC, rdACC, and dorsal and orbital lateral prefrontal cortex have also been associated with evoked emotional experience (Kober, Barrett, Joseph, Bliss-Moreau et al., 2008; T. Wager et al., 2008), and ‘anxiety-like’ anticipatory processes in particular (Porro, 2003; Waugh, Wager, Fredrickson, Noll, & Taylor, 2008)2. It is not yet clear, however, whether different systems mediate HR and reported anxiety responses to SET, and we tested these relationships. Third, the location of brain activity in the ventral MPFC may be subject to distortions and signal loss depending on the pulse sequence used; here, we used a different pulse sequence from that in the companion paper.
The multilevel path modeling approach
The current study was designed to address these two issues by identifying cortical-subcortical and cortical-brainstem pathways that mediate the effects of SET on changes in HR and self-reported experience. We used multi-level mediation effect parametric mapping (M-MEPM)— an extension of standard path analysis techniques (Baron & Kenny, 1986; Hyman, 1955; MacCorquodale & Meehl, 1948) suitable for analyzing path models and mediation effects within-persons—to characterize the dynamic relationships between SET, brain, and physiological and experiential measures of response across time. This approach is described in more detail in the companion paper (Wager et al., submitted), but we briefly recap some of the main points here.
A traditional activation-based hypothesis might predict only that SET should activate some regions and deactivate others. A mediation hypothesis would also make this prediction, but only as part of a larger set of interconnected predictions about pathways. if a SET-brain region-HR pathway exists with a specific brain region as a mediator, three significant statistical relationships should be observed: 1) SET should activate the brain region (Path a in Figure 1B); 2) Activity in the brain region should predict HR controlling for SET (Path b); and 3) The SET-HR relationship should be significantly reduced when controlling for activity in the brain region, which we refer to as the mediation test or the a*b effect.
Figure 1.
Social evaluative threat (SET)-related outcomes and mediation model. A) Group-averaged (N = 18) heart-rate changes (red) and self-reported anxiety changes (blue) across time. Anxiety ratings were interpolated from ratings made every 20 sec. The colored bars at the bottom show the phases of the experiment, with non-threat phases in gray, threat phases in pink, and instruction phases in yellow. Shaded regions indicate standard errors of the mean. B) Mediation path diagram showing the mediation effect search strategy. The initial variable (left) was experimentally induced SET across time. The outcome variable (right) was fluctuations in heart rate across time. The mediation effect parametric mapping analysis strategy involved searching for brain voxels in which fMRI time courses mediated the SET-heart rate relationship. A voxel was considered a significant mediator if it showed a significant a path across subjects, indicating brain responses to SET; a significant b path, indicating brain-heart rate correlation, controlling for SET; and a significant mediation effect, defined as the product of path coefficients a and b. Path c reflects cardiovascular responses to the SET challenge. All path analyses controlled for activity related to vascular responses in large vessels, visual and motor activity, and head movement (see Figure 6).
To test these effects, the M-MEPM analysis extends traditional concepts in path modeling in two ways. First, path models were originally formulated for a single level of analysis—i.e., relationships across time within a single participant. However, we were interested in making inferences about variations in SET, brain, and HR across time (within-person) and their generalizability to a population, which requires a multi-level analysis. This allows us to take advantage of the many repeated observations across time in fMRI, and investigate the dynamic relationships between SET, brain, and HR. Effects (Path a, Path b, a*b) are estimated on within-person changes across time, and their statistical significance is tested across persons, treating participant as a random effect (Kenny, Korchmaros, and Bolger, 2003). Second, path models and related structural equation models traditionally test relationships among variables (e.g., brain regions) specified a priori. However, while the general location of mediating pathways can be specified, exactly which voxels are mediators of SET effects are unknown. Most human neuroimaging studies analyze voxel-by-voxel maps of brain activity, because even if one has a relatively precise anatomical hypothesis (e.g., the PAG) it is often difficult to specify exactly which voxels in that area should show the effect. The M-MEPM framework (and software) facilitates performing mediation analysis voxel-by-voxel in a region of interest (ROI) or across the brain, allowing researchers to locate multiple brain regions that satisfy the statistical criteria for mediation.
We used the M-MEPM framework to test several hypotheses about mediators of SET effects on HR. First, we expected a bi-valent response to SET in two separate sub-regions of the medial prefrontal cortex/anterior cingulate. We expected activation in pgACC/rdACC to mediate increases in HR, and we expected deactivation in vmPFC/mOFC to mediate increases in HR. Second, we expected activation in the PAG to mediate increases in HR. Third, we expected PAG to mediate the relationship between cortical activity in one or both sub-regions and HR.
Methods
Participants
Participants were 18 healthy, right-handed, native English speakers (mean age 21 years, 9 males) recruited at Columbia University. Individuals with a prior history of neurological or psychiatric illness or current or prior psychoactive medication use were excluded. Participants were asked to abstain from tobacco and caffeine use for 24 hours before scanning. All participants gave written informed consent, and the study was approved by the Columbia University Institutional Review Board.
Procedure and fMRI task design
Before participants entered the scanner, they were informed that during scanning they would be given two 2-minute periods to mentally prepare two different speeches. They were given the following instructions prior to scanning: a) Speeches should be 7 minutes long, and will be presented to two different audiences after scanning is complete. One speech will be given before a panel of professors and experts in the law and business, and the second will be scored by a computer analysis program, Latent Semantic computer Analysis (LSA), which they were told could grade college-level essays. Pictures and biographies of panelists were shown. B) You will be given the speech topics during fMRI scanning. C) For control purposes, there was a small chance you will not have to give any speech. (No participants actually gave speeches.)
A schematic of the design is shown in Figure 1A. After an initial anatomical scan, baseline physiological and brain data were acquired for 120 seconds. Then, for 15 seconds the first speech topic was presented on the screen and participants then had 2 minutes to mentally prepare it. After 2 minutes, participants viewed on-screen instructions with the topic of the second speech. Again, they were given two minutes to prepare it mentally. After this, every participant was told that they were randomly selected to not give a speech, and asked to relax for the remaining 2 minutes. This period was used to measure recovery. The two speech topics were, “the effects of interest rates on stock prices,” and “the relationship between tariffs and free trade.” These topics were selected based on a pilot study evaluating a separate group of participants’ anticipated anxiety ratings to a number of possible topics (data not shown). Assignment of both topics and audiences to the first or second speech preparation period were counterbalanced across participants.
Every 20 sec during baseline, speech preparation, and recovery, participants were visually cued to provide a current subjective anxiety rating on a 5-point Likert scale that ranged from “no anxiety” to “extremely anxious.” Participants made ratings on a continuous visual analogue scale using an MR-compatible trackball (Resonance Technologies, Inc.) with the right hand. These ratings were interpolated to the TR (2 sec) using linear interpolation for time series analysis. All in-scanner stimuli were presented by a digital projection onto a screen placed in the scanner room. Stimulus presentation was controlled by E-prime software (Psychology Software Tools Inc.).
Data acquisition and analysis
Heart rate was collected continuously with a sampling rate of 100 Hz during fMRI acquisition using photoplethysmography on the left index finger. Successive R-wave peaks were identified using a custom algorithm identifying deviations from a moving average baseline (TDW) implemented in Matlab (Matworks Inc, Natick, MA, USA). The automatic beat detection algorithm was manually reviewed and corrected by a coder blind to condition. HR time series were reconstructed from the R-R intervals and interpolated to the TR. Skin conductance data were collected (leads were placed on the volar surfaces of the first and third fingers of the left hand), but not analyzed in this report.
MR images were collected on a 1.5 GE Signa Twin Speed Excite HD scanner (GE Medical Systems). Structural images were acquired using high-resolution T1 spoiled gradient recall images (SPGR) for anatomical localization and warping to a standard space. Functional images were acquired with a T2*-sensitive EPI BOLD pulse-sequence (TR = 2000 ms, TE = 40 ms, flip angle = 60°), sensitive to blood-oxygen-level-dependent (BOLD) magnetic susceptibility. For each participant we obtained 266 brain volumes during the scanning run (24 ascending odd/even interleaved slices, 3.4375 × 3.475 × 4.5 mm).
Functional images were subjected to standard preprocessing. First, slice timing acquisition correction and realignment of the functional images to correct for head movement were performed using FSL (FMRIB centre, University of Oxford). Remaining preprocessing steps were performed using the Statistical Parametric Mapping analysis package (SPM2, Wellcome Department of Cognitive Neurology, London, UK). The realigned images from each participant were coregistered to the anatomical space of the structural image. Structural images were normalized to the Montreal Neurological institute (MNI) template space (avg152t1.img). Finally, the normalized functional images were smoothed with an 8-mm (FWHM) Gaussian smoothing kernel to facilitate inter-subject registration in group analysis.
Following this pre-processing, we used linear regression to remove several sources of nuisance variance from each voxel of each participant’s time series data. One set of regressors modeled activity during rating probes and instruction periods, convolved with the canonical SPM hemodynamic response function, and their first and second derivatives. In addition, we identified voxels in the major arteries, which were apparent on the SPGR images, as shown in Figure 2. The first three principal components from the vascular time series (generally from the internal carotid, middle cerebral, and anterior cerebral arteries) were also included as nuisance regressors, along with global signal values and a linear drift regressor. Finally, we included the 6 standard head-movement related estimates from realignment (x, y, and z translation, roll, pitch, and yaw), as well as their derivatives, squares, and squared derivatives (24 movement-related regressors total). This procedure helped to minimize the chances that results would be related to artifactual sources of variance or vascular blood flow effects.
Figure 2.
Covariates removed from each participant’s brain, HR, and anxiety time series data prior to multi-level path analysis. The top left panels show a structural T1-weighted image for one representative participant. Major arteries identified using a custom segmentation procedure are shown in red in the panels below, and on a left medial surface representation at right. The bottom panel shows an example of covariates for one participant, with time on the x-axis. These included the first three principal component scores from the participants’ vascular voxels and their derivatives, visual stimulation during instructions periods and rating periods and their derivatives, global signal and linear drift, and covariates related to head movement. ACA, anterior cerebral artery; ICA, internal carotid artery; MCA, middle cerebral artery.
Statistical analysis: Multi-level path analysis
Statistical analysis was performed using the M-MEPM toolbox. Mathematical details of the multi-level model can be found in the companion study (Wager et al., submitted). Briefly, the mediation analysis can be conceptualized as a series of analyses testing different components of the mediation hypothesis in each voxel within brain regions of interest. The multi-level path model evaluated at each voxel tests the following effects in the context of a single, multi-equation model: (1) Brain responses to the SET challenge, as evidenced by increases in the [Speech Prep – Baseline] contrast within-participants (Path a in Figure 1B). (2) Correlations between brain and HR changes across time, within-participants, controlling for SET (Path b in Figure 1B). (3) A mediation test for whether the brain voxel explains a significant amount of the SET-HR covariance. This is accomplished by testing the product of path coefficients a*b using a bootstrap test. (4) After identifying mediating regions, we tested whether activity in each pathway was moderated by SET—that is, whether the brain-HR relationship was significantly stronger during threat than baseline. Whereas a mediation effect can identify functional pathways, a moderation effect can test whether brain-heart effects hold across all task conditions or are specific to the SET period.
Though the model used was identical to that in the companion paper (Wager et al., submitted), in this paper, a bootstrap test was used to test the significance of a, b, and a*b effects, which provides a more sensitive test of mediation than the approximate test based on normality assumptions (Shrout & Bolger, 2002). 4000 bootstrap samples were used at each voxel. We also note that the combination of the bootstrap test and the integration of individual differences variables is not yet implemented in the MEPM toolbox, so HR was not entered as an individual differences predictor at the second level. Future versions will allow both bootstrapping and individual differences covariates.
For each whole-brain M-MEPM analysis, we controlled the false positive rate using False Discovery Rate control at q < .05 (Genovese, Lazar, & Nichols, 2002), across images related to all effects in the path model (a, b, and a*b effects). The interpretation is thus that the expected false positive rate is 5% of ‘significant’ voxels across the whole analysis. We focused on regions that showed FDR-corrected results in each of the three effects.
Moderation analysis: variations in brain-HR connectivity across task states
This analysis is conceptually similar to testing whether the brain-HR connectivity was stronger during Speech Preparation than during Baseline and Recovery periods. Though simpler analyses on individual brain-HR correlations during different task phases yielded the same results in all ROIs (data not shown), the multi-level model is statistically preferable because it includes weights based on within-subjects variance, and we describe it below.
A [Speech Preparation – Baseline]×brain activity interaction regressor was created in the following way. First, brain activity was averaged over voxels in the region of interest separately for each participant, yielding a set of 18 region-average time series. Second, a matrix of nuisance covariates (Q) was removed from the brain activity, in order to prevent differences across task periods to drive the interaction results. Q included indicator (dummy-coded) vectors coding for each of the task periods relative to the initial baseline (including Speech Preparation, Relief instructions, and Recovery periods). Additional movement-related and vascular covariates were removed prior to analysis as well. Third, the brain time series for each participant was multiplied with the contrast-coded [Speech Preparation – Baseline] time series for each participant to create series of 18 interaction regressors for each participant. Fourth, and finally, a 60 s Tukey window was applied to each interaction regressor, to ensure that transitions between task periods did not drive the interaction results. Once these interactions were created, they were entered into a multi-level mixed effects GLM. The HR time series for the 18 participants (expressed as change from the individual’s baseline HR) was the outcome variable to be predicted.
Limitations on causal inference
In the present study, SET was experimentally manipulated in an off-on-off design, unconfounding it with processes like fatigue that change monotonically over time. SET-induced brain activity can thus be reasonably interpreted as caused by SET. Brain relationships with HR could plausibly be either causes of HR change, effects of HR as perceived by the brain, or both; however, the locations of active regions in “visceromotor” cortex and known brainstem modulators of HR favor brain-to-heart causality. For these reasons, although the path models we use are directional in form, we do not interpret the directionality of brain-heart connections.
Results
Physiological and subjective effects of SET
Compared with pre- and post-stress baselines, speech preparation induced reliable increases in HR (6.22 beats per minute, BPM, t = 7.68, p < .0001) and subjective anxiety (1.39 units on a 5-point scale, t = 3.83, p = 0.002), as shown in Figure 1A. HR increases were positively correlated with Spielberger Trait Anxiety scores (STAI, r = 0.64, p < .05), but anxiety increases were not (r = 0.25, n.s.). The first speech preparation period induced larger increases in HR than the second period (3.56 BPM, t = 3.34, p = .005), though HR increases were significant for the second preparation period alone (3.80 BPM, t = 4.88, p = .0003). Anxiety ratings did not vary between the two speeches (−.046 points, n.s.). After the second preparation period and instructions indicating that no speech would be delivered, participants' HR returned to baseline. There was no difference between initial-baseline and post-stress baseline values (−1.31 BPM, n.s.). Anxiety ratings showed some evidence for a drop during the post-stress baseline below their initial values (−0.70 points, t = −2.00, p = .066). Within participants, HR changes were positively associated with anxiety changes over time (b = 2.61 BPM/unit anxiety, t = 3.11, p = .007), indicating correlated time courses likely induced by the speech preparation. Individual differences in HR reactivity and anxiety reactivity to the task were uncorrelated (r = 0.07, n.s.).
Physiological analyses reported above controlled for gender, STAI, target audience (Panel vs. Computer scoring), and speech topic (“interest rates” vs. “tariffs”; see Methods), leaving 13 error degrees of freedom for all analyses. None of these variables3 had significant effects on HR, anxiety reports, or activity in key brain regions (all p’s > .1); thus, we do not report on them in detail here.
fMRI results
Our analysis proceeded in the following stages:
(1) First, we searched for brain mediators of the relationship between SET and HR within participants (across time), as diagrammed in Figure 1B, using the M-MEPM toolbox. (2) Second, we further characterized key brain regions from this analysis by testing whether brain-HR correlations varied across task states (Baseline, Speech Prep, and Recovery). That is, we tested whether task state moderated the brain-HR relationship. (3) We performed the MEPM analysis on the PAG, a key brainstem region of interest, to localize PAG voxels that mediated the SETHR relationship. (4) We performed a series of path analyses to test for hypothesized relationships between SET, key frontal and brainstem regions, and HR. These analyses differed from the MEPM analysis in that they considered multiple brain regions in the same path models, and could thus test for independent effects of multiple regions. This set of analyses also included a post hoc search for additional brainstem mediators of cortical activity not mediated by the PAG. (5) We performed an MEPM analysis to search for brain mediators of the SET-reported anxiety relationship within participants. In the following, we present results from each of these analyses.
1. Mediators of SET effects on HR
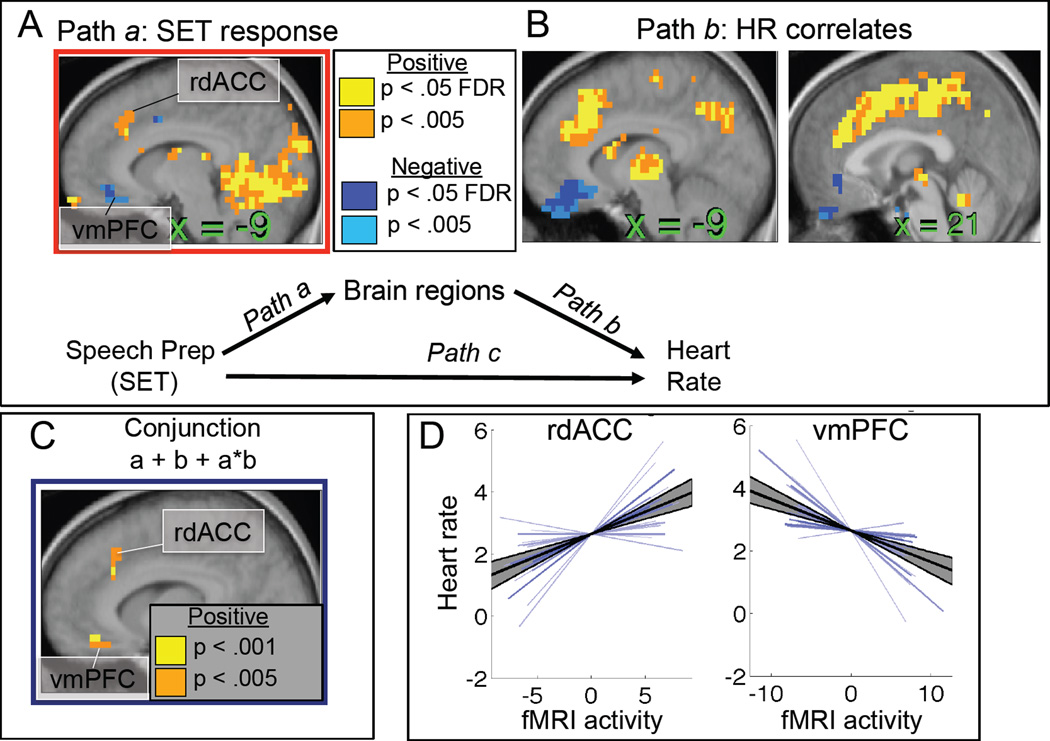
Our first analysis searched for mediators of the SET-HR relationship, including regions identifying SET effects on fMRI activity (Path a), fMRI correlates of HR across time (Path b), and the a*b mediation effect. We performed a whole-brain search at q < 0.05 (False Discovery Rate [FDR] corrected, corresponding to P < 0.0012) with 3 contiguous voxels in each effect. Regions that showed evidence for all three effects are of primary interest, as they show the strongest evidence for mediation. In particular, we sought to test whether the pattern found in our previous study (Wager et al., submitted) of more dorsal MPFC (pgACC and rdACC) increases and mOFC/vmPFC decreases would mediate the Speech Preparation-HR relationship.
The brain regions that responded to the [Speech Preparation – Baseline] comparison (Path a) are shown in the left panel of Figure 3, and a complete listing with statistics is reported in Supplementary Table S1. As in the companion study, a number of regions responded to the SET challenge, including increases in rdACC and decreases in vmPFC/mOFC, but also including activity in the lateral PFC, insula, medial temporal lobes, occipital cortex, medial cerebellum, and other regions likely to be related to various task demands (such as visual stimulation during instruction presentation, etc.).
Figure 3.
Results of the mediation effect parametric mapping search. A) Path a results. Saggital slice showing regions whose activity increased (yellow/orange) or decreased (blue) in response to the social evaluative threat (SET) challenge. Significant regions of 3 or more contiguous voxels at q < .05 False Discovery Rate corrected, and voxels contiguous with these regions at p < .005, are shown. rdACC: rostral dorsal anterior cingulate cortex; vmPFC, ventromedial prefrontal cortex. B) Path b results. Sagittal slices showing significant positive (yellow/orange) or negative (blue) correlates of heart rate changes over time, controlling for the time course of the SET manipulation. C) Significant mediators, showing the conjunction of significance in the a path, the b path, and the mediation test on the a*b product. D) Examples of individual subjects’ partial regression slopes (blue lines) for the brain-heart relationship (b path) for each significant mediating region. The span of each line along the x-axis reflects the 95% confidence interval of fMRI activity for that participant. The thickness and darkness of the line reflects the weight assigned to that participant based on the within-subject variability, with darker and thicker lines indicating lower within-subject error and more weight in the group analysis. The black line shows the group average, and the shaded gray area shows the 95% confidence interval for the regression slopes. Variability in the intercept values across participants has been removed for display purposes.
Brain correlates of HR controlling for SET are shown in the right panels of Figure 2 and listed in Supplementary Table S2. These included the rdACC and a large area of the dorsal anterior and posterior cingulate cortices, as well as the PAG, mediodorsal thalamus, caudate, lateral frontal cortex, and medial cerebellum. Negative associations with HR were found in the vmPFC/mOFC.
Rather than discussing these regions in detail, we focus on those with consistent relationships with both SET and HR, as well as evidence for a significant mediation effect. Only two areas of the brain showed evidence for the conjunction of both SET (Path a) and HR (Path b) effects and the a*b mediation effect. As shown in Figure 3C, areas with at least 3 voxels at P < .001 in each of the three tests included the rdACC and vmPFC/mOFC. Individual participant slope plots are shown in Figure 3D for these two regions, and statistical details are presented in Table 1. Both areas showed positive mediation effects, which is consistent with the directions of the a and b Paths reported above. In the rdACC, SET caused increased brain activity, which was associated with increased HR. In the vmPFC, SET caused decreases in brain activity, and greater decreases were associated with increased HR. Thus, because both Path a and b links were negative in the vmPFC, the a*b product that reflects the mediation test was positive.
Table 1.
| 1st-level: within-subjects | Path a (SET) | Path b (HR) | Mediation (a*b) | Sig. Voxels | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Name | x | y | z | Vol. (mm3) | max Z | P | max Z | P | max Z | P | P<.001 | P<.005 |
| Medial frontal cortex | |||||||||||||
| VMPFC/mOFC | −10 | 38 | −14 | 1010 | −3.39 | 0.0007 | −3.44 | 0.0006 | 3.58 | 0.0003 | 4 | 19 | |
| rdACC/MCC | −7 | 24 | 40 | 1170 | 3.03 | 0.0024 | 3.53 | 0.0004 | 3.58 | 0.0003 | 6 | 22 | |
| Periaqueductal gray (PAG) region of interest | P < .05 | P < .10 | |||||||||||
| PAG | −3 | −28 | −14 | 319 | 1.54 | 0.12 | 2.70 | 0.0069 | 2.45 | 0.01 | 2 | 6 | |
Note. Brain mediators of social evaluative threat (SET) effects on heart rate (HR), including regions showing significant effects in all three tests of interest (Paths a, b, and a*b). Statistics are reported only for voxels with the peak effect size for the a*b effect. Sig. voxels: the number of significant voxels at each threshold (two tailed) in the mediation (a*b) effect. Abbreviations: mOFC, medial orbitofrontal cortex; MCC, mid-cingulate cortex; PAG, periaqueductal gray; rdACC, rostral dorsal anterior cingulate; VMPFC, ventromedial prefrontal cortex.
Figure 4A shows the time courses of activity in the rdACC (red) and vmPFC (blue). The rdACC shows evidence for positive responses during both speech preparation periods, with a break during the instruction period for the second speech preparation. The vmPFC shows prominent decreases that are driven primarily by the first speech preparation period. HR was markedly higher during the first speech period and habituated to some degree, though reported anxiety did not (Figure 1).
Figure 4.
Time course of mediating regions and moderation of brain-heart connectivity by task phase. A) Group average time course of fMRI activity in the rostral dorsal cingulate (rdACC, red) and ventromedial prefrontal cortex (vmPFC, blue). Each region is shown on a saggital slice to the right of the plot. Task periods are shown at the bottom of the plot, as in Figure 1. The rdACC time course shows significant elevation during both speeches. The vmPFC time course shows evidence for increases before the beginning of speech preparation and a large negative deflection during the first speech preparation period. Shaded areas show standard errors of the mean, and thicker orange and green lines indicate periods in which the group average differed significantly from zero. All covariates were removed prior to averaging (see Figure 6). To facilitate visualization of low-frequency changes related to task phases, the time series were smoothed with a 60 sec Gaussian full-width half-max moving average. B) Moderation of brain-heart connectivity by the task phase. The plots show the average brain-heart correlation for each task phase, after conversion to Fisher’s Z values to normalize the distribution across participants. Correlations between the rdACC and heart rate (left panel) varied as a function of task phase, with the strongest correlations during speech preparation. Correlations between the vmPFC and heart rate (right panel) did not differ significantly across task periods, but appeared to be strongest before and during preparation. Error bars show standard errors of the mean.
2. Moderation analysis: changes in brain-HR correlations across time
Figure 4B shows the results of moderation analyses that tested the strength of brain-HR correlations across different phases of the task (pre-preparation baseline, preparation, and post-preparation recovery). Statistical details are presented in Table 2. Our hypotheses were directional, so one-tailed tests were performed. We expected stronger positive connectivity in rdACC, and stronger negative connectivity in vmPFC, during speech preparation.
Table 2.
Moderation analyses: Brain-HR correlation as a function of task period
| Average time series correlation | Mixed-effects model [Speech Prep -Baseline] |
[Baseline - Recovery] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Baseline | Speech Prep |
Recovery | difference | STE | t | P | difference | STE | t | P |
| vmPFC/mOFC | −0.1 | −0.12 | 0 | −0.05 | 0.03 | −1.54 | 0.072 | −0.09 | 0.09 | −1.5 | 0.080 |
| rdACC | 0.05 | 0.16 | 0.11 | 0.08 | 0.03 | 2.93 | 0.005 | −0.06 | 0.07 | −0.8 | 0.220 |
| PAG | 0.06 | 0.06 | 0.07 | 0.02 | 0.02 | 1.36 | 0.096 | −0.01 | −0 | −0.2 | 0.421 |
Note. Brain-heart rate (HR) association as a function of task period. Variations in association strength constitute a moderation by task state. STE, standard error. Other abbreviations are as in Table 1.
Correlations between the rdACC and heart rate (Figure 3B, left panel) varied as a function of task phase, with the strongest correlations during speech preparation. This result indicates that the rdACC-HR pathway was significantly engaged by SET. By contrast, correlations between the vmPFC and heart rate (right panel) showed a non-significant trend across task periods, but appeared to be strongest before and during preparation. Overall, the results suggest that the balance of (negative) vmPFC contributions and (positive) rdACC contributions to HR changes across time, with vmPFC effects more pronounced earlier and rdACC effects dominating later.
3. PAG region of interest analysis and inter-region connectivity
To test for predicted mediation in the PAG, we conducted an ROI analysis specifically on a pre-defined area surrounding the PAG at a reduced statistical threshold (P < .05 in each of the three critical tests). Figure 4A shows the extent and location of the PAG ROI (left panels). We found evidence for significant relationships in PAG voxels in all 3 effects. As shown in the right panels of Figure 4A, PAG increased to SET (Path a), predicted HR controlling for SET (Path b), and showed evidence for mediation (a*b). Statistics for the region showing the overlap of all three effects are presented in Table 1. Notably, the PAG-HR connectivity did not vary substantially across task periods (Table 2, Figure 4).
4. Path models of cortical-brainstem-HR connectivity
We focused our brain hypotheses on three kinds of pathways concerning cortical-subcortical connectivity hypothesized a priori4:
Whether vmPFC and rdACC were independent mediators of SET effects on HR
Whether PAG is a mediator of the relationship between rdACC and/or vmPFC and HR
Whether cortical regions mediated SET effects on PAG
These tests are commonly described as tests of “effective connectivity” because they test directional relationships while using regression to control for hypothesized potential common causes (i.e., common inputs from other variables). However, as both brain and heart data were observed rather than experimentally manipulated, we do not provide a strong interpretation of causality or directness (i.e., no 3rd-variable common cause) for brain-heart relationships.
To test hypothesis 1, we constructed a path model with SET as the initial variable, both vmPFC and rdACC as mediators, and HR as the outcome. The results showed that each was an independent mediator. vmPFC showed negative path coefficients, indicating that vmPFC decreases mediated increases in HR (Za = −3.72, Zb = −3.52, Zab =3.70, all P < .001), whereas rdACC paths were positive (Za = −3.48, Zb = −3.62, Zab =3.42, all P < .001). Thus, although rdACC and vmPFC were significantly negatively coupled (b = −.05, t(17) = −2.28, p < .05), they independently mediated some of the SET-HR covariance.
To test hypothesis 2, we constructed a path model with each of rdACC and vmPFC as initial variables, PAG as a mediator, and HR as an outcome, controlling for SET. PAG mediated the vmPFC-HR connection (Za = −3.59, Zb = 2.48, Zab =−2.38, all P < .02). However, rdACC did not, primarily because it was not associated with PAG (Za = 0.96, n.s., Zb = 2.58, P < .01, Zab =0.32, n.s.).
To test hypothesis 3, we constructed a path model with SET as the initial variable, each of rdACC and vmPFC as mediators, and PAG as an outcome. vmPFC showed strong connectivity with both SET and PAG, and a trend towards significant mediation (Za = −3.41, P<.001, Zb = −3.54, P < .001, Zab =1.79, P = .07). It was also a complete mediator of SET effects on PAG (direct SET-PAG Zc’ = 0.37, n.s.). rdACC, by contrast, did not mediate SET effects on PAG. Though it showed a strong response to SET, as in other models, it was not connected with PAG (Za = 3.49, P<.001, Zb = 0.84, n.s., Zab =1.04, n.s.).
Thus, a consistent picture from these three models is that the vmPFC is most strongly connected to PAG and has an inhibitory effect, whereas rdACC is not strongly connected with PAG. These relationships are summarized graphically in Figure 6.
Figure 6.
Summary of cortical-brainstem and cortical-heart rate connections. Green lines show positive links, and blue lines show negative links. The causal directionality of effects, except for SET effects, cannot be determined from the data alone (especially as the vast majority brain pathways include bidirectional projections) and so are not marked with arrows. Experimentally induced social evaluative threat (SET) induced increases in rostral dorsal cingulate cortex (rdACC) and periaqueductal gray (PAG), both shown in yellow, and decreases in ventromedial prefrontal cortex (vmPFC). All three areas were significant partial mediators of the SET effect on heart rate (HR). The rdACC influence on HR was partly mediated by a negative coupling with the vmPFC, but a strong direct positive influence on HR remained that was partially mediated by the thalamus, but not the PAG. The vmPFC was negatively coupled with the PAG, which was a strong but partial mediator of the vmPFC-HR relationship. These results are broadly consistent with recent meta-analytic results that show preference for positive valence in the vmPFC and negative valence in the dorsomedial PFC (dmPFC; inset panel, left), and coactivation across studies between the rdACC and dmPFC cortical regions and and the PAG and medial thalamus (inset panel, right). Overall, the results suggest that multiple medial frontal regions differentially contribute to the generation of stress-induced heart-rate increases.
This result begs the question of which brainstem regions might mediate rdACC relationships with HR, as brainstem nuclei are known to be proximal mediators from animal studies. We performed an additional post hoc MEPM analysis within a mask including the brainstem and thalamus. In this analysis, rdACC was the initial variable, HR was the outcome, and we searched for brain mediators of the rdACC-HR relationship. The FDR-corrected threshold across the search space and contrasts was P < .0075. Only two regions showed significant effects in all three path coefficients (Path a, rdACC-brain; Path b, brain-HR; and the a*b mediation test). These regions were in the left and right vental thalamus. For the left thalamus, MNI coordinates were [x, y, z] = [−14, −17, 4], 3031 mm3 (57 contiguous voxels). For the right thalamus, [x, y, z] = [17, −17, 4], 638 mm3 (12 voxels). These regions are shown in Figure 6. The thalamic regions were strongly coupled with both rdACC and PAG controlling for other key regions (t(17) = 6.34, and t(17) = 11.16, respectively, both P < .001). It did not show significant effective connectivity with vmPFC, however (t(17) = −1.66, n.s.).
5. Brain mediators of subjective anxiety
As Figure 1 shows, the temporal profiles of HR and anxiety responses were correlated, but they also differed substantially. Therefore, to test whether the same or different brain areas would be associated with anxiety reports, we conducted an additional M-MEPM analysis to examine the brain areas that mediated subjective anxiety reports across time.
The results from this analysis were not as strong as for the HR analysis: no regions were significant with FDR correction. No results were significant in all three effects (Path a, SETbrain, Path b, brain-anxiety, a*b) at P < .001 or even at P < .005, indicating less strong and straightforward relationships linking SET, brain activity, and reported anxiety (as compared with HR).
To provide an exploratory analysis of mediators of anxiety, we focused on the a*b effect alone, which can, in a multi-level context, reveal evidence for functional pathways that vary across individual as well as those that are consistent in the group. The results are listed in Table 3, and shown in Figure 7. A number of regions showed evidence for significant a*b effects at P < .001, including effects in dorsolateral prefrontal cortex and right IFG just anterior to Broca’s area, vmPFC/mOFC, temporal poles, caudate head, and the dmPFC superior to rdACC and anterior to pre-SMA. Notably, they overlapped only partially with predictors of HR, most notably in the vmPFC.
Table 3.
Mediators (a*b) of subjective anxiety profiles
| 1st-level: within-subjects | Path a (SET) | Path b(HR) | Mediation (a*b) | r with anxiety reactivity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Name | x | y | z | Vol. (mm3) | max Z | P | max Z | P | max Z | P | Cov(a,b) | Path a | Path b | a*b |
| Medial frontal | |||||||||||||||
| DMPFC | 14 | 48 | 45 | 3829 | 0.36 | 0.72 | −1.68 | 0.09 | 3.58 | 0.0003 | 0.66 | −0.22 | −0.05 | 0.16 | |
| R DMPFC | 17 | 48 | 22 | 1276 | 0.17 | 0.86 | −0.71 | 0.47 | 3.57 | 0.0004 | 0.46 | −0.04 | 0.29 | 0.08 | |
| L pgACC/VMPFC | −3 | 41 | −14 | 6966 | 0.31 | 0.76 | −0.02 | 0.98 | 3.58 | 0.0003 | 0.54 | 0.13 | −0.36 | 0.45 | |
| R aVMPFC | 14 | 55 | −14 | 266 | −0.76 | 0.44 | −0.40 | 0.69 | 3.56 | 0.0004 | 0.64 | −0.16 | −0.52* | 0.16 | |
| Lateral frontal | |||||||||||||||
| L DLPFC | −52 | 14 | 32 | 425 | 0.51 | 0.61 | 0.07 | 0.94 | 3.55 | 0.0004 | 0.64 | 0.12 | 0.01 | 0.37 | |
| L DLPFC | −34 | 38 | 40 | 3988 | 0.07 | 0.94 | −0.42 | 0.67 | 3.58 | 0.0003 | 0.48 | −0.07 | 0.39 | 0.18 | |
| L IFG | −62 | 24 | 9 | 425 | 2.21 | 0.03 | 1.13 | 0.26 | 3.58 | 0.0003 | 0.50 | 0.30 | 0.76* | 0.21 | |
| Medial temporal | |||||||||||||||
| R TP | 41 | 7 | −40 | 4041 | −1.25 | 0.21 | −2.49 | 0.01 | 3.58 | 0.0003 | 0.61 | −0.14 | −0.29 | 0.21 | |
| L TP | −38 | 14 | −45 | 1595 | −1.33 | 0.18 | −0.60 | 0.55 | 3.56 | 0.0004 | 0.34 | −0.18 | −0.47* | 0.49* | |
| R uncus | 17 | 3 | −40 | 213 | −0.90 | 0.37 | −1.37 | 0.17 | 3.58 | 0.0003 | 0.46 | −0.13 | 0.10 | 0.06 | |
| R PHCP | 17 | −14 | −32 | 904 | −1.02 | 0.31 | −2.59 | 0.0096 | 3.57 | 0.0004 | 0.28 | 0.27 | −0.26 | 0.09 | |
| Lateral temporal | |||||||||||||||
| R aSTS | 55 | 10 | −27 | 532 | −0.17 | 0.87 | −0.57 | 0.57 | 3.56 | 0.0004 | 0.73 | −0.10 | −0.16 | 0.36 | |
| Parietal | |||||||||||||||
| L IPL | −55 | −48 | 58 | 266 | −1.09 | 0.28 | 0.15 | 0.88 | 3.50 | 0.0005 | 0.57 | 0.09 | −0.21 | −0.53* | |
| Occipital | |||||||||||||||
| L OCC | −17 | −82 | 0 | 1542 | 3.49 | 0.0005 | 2.23 | 0.03 | 3.58 | 0.0003 | 0.54 | −0.21 | −0.09 | −0.23 | |
Note. Mediators of subjective anxiety changes across time, as reflected in significant a*b effects. The Cov(a,b) column lists the estimated covariance between a and b effects. Large values suggest the presence of coherent pathways that may vary in sign across individuals. The rightmost columns list Pearson's r values for the correlation between path coefficients and 'anxiety reactivity,' or overall increases in anxiety for [Speech Preparation - Baseline].
p < .05.
DLPFC, dorsolateral prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; IFG, inferior frontal gyrus; PHCP, parahippocampal cortex; OCC, occipital cortex. Other abbreviations are as in Table 1.
Figure 7.
Mediators of reported anxiety. These regions showed evidence for an a*b mediation effect, but most did not show evidence for average Path a and Path b effects (See Table 3). This implies that functional pathways linking social threat, brain activity, and anxiety reports in these regions were variable in sign and/or strength across individuals. Top right: A positive correlation between anxiety reactivity (x-axis) and right inferior frontal gyrus activity (y-axis) suggests that those who report that the task is more anxiogenic show links between social threat, inferior frontal activity, and anxiety. Bottom right: A negative correlation between between anxiety reactivity and anterior ventromedial prefrontal activity suggests that those who report that the task is more anxiogenic show links between social threat, vmPFC deactivation, and anxiety. Yellow: P < .001; Orange: P < .005; Pink: P < .01.
Though these results are candidate mediators of reported anxiety responses to social threat, the interpretation of these mediation effects requires some further explanation. The groupaverage a and b paths were significant for very few of these regions (see Table 3), indicating a lack of consistent relationships with SET and anxiety across participants. A significant a*b effect without significant a and b effects is only possible in a multi-level mediation context (Kenny, Korchmaros, & Bolger, 2003), and it indicates that there may be coherent relationships that vary in direction across individuals. For example, consider that some individuals may show brain increases to the SET challenge, and others may show decreases. If activity predicts increased anxiety (positive Path b) for the participants who show increases (positive Path a), and stronger decreases predict anxiety (negative Path b) for the participants who show decreases (negative Path a), then the net result will be consistent mediation, but in different directions (positive vs. negative) for different individuals. This consistency is reflected in the covariance of a and b paths, which were significantly positive for most regions showing significant mediation effects (r values ranged from .27 to .73; see Table 3). Under what circumstances might this occur? One is if the brain regions have a variable role in shaping anxiety reports—i.e., if they are involved in an appraisal process that increases anxiety for some participants but decreases it for others. We return to this issue in the discussion.
To help interpret the functional role of brain activity in these regions in shaping anxiety, we examined correlations between path coefficients and the strength of overall reported anxiety reactions during speech preparation (as compared with baseline and recovery periods). Few regions showed correlations with anxiety reactivity, but those that did provide some clues. In the IFG, for example, high reported anxiety was associated with positive IFG-anxiety correlations across time. Low reported anxiety was associated with negative IFG-anxiety correlations. The vmPFC showed the opposite pattern, suggesting that as in the HR prediction analyses, decreases in vmPFC are coupled with high anxiety in “reactors.” In high reactors, vmPFC decreases were associated with anxiogenic responses.
Discussion
Negative evaluation by other individuals is a potent laboratory and real-life stressor, likely because it threatens self-esteem—or, more precisely, one’s perceived prospects for future access to social and material resources. It is particularly relevant for health in modern industrialized countries, in which acute physical threat is relatively rare, but many individuals experience stresses related to social well being and status on a regular basis. Work in non-human animals has elucidated several kinds of deleterious physiological effects of threat and stress, including SET in particular (Blascovich, Mendes, Tomaka, Salomon, & Seery, 2003; Cohen et al., 2000; Kemeny, 2003; Mcewen, 2007; Thayer & Sternberg, 2006), and important work has been devoted to the brain systems that generate and mediate threat (Critchley, 2003; Eisenberger et al., 2007; Gianaros, Jennings, Sheu, Derbyshire, & Matthews, 2007; Kern et al., 2008).
Human neuroimaging research on brain-peripheral relationships can provide important information on the brain systems that generate (and perceive) physiological responses to social threat. It can also help to integrate human and non-human research that has focused largely on different levels of the neuraxis. Animal research has identified specific roles for brainstem nuclei such as the PAG, parabrachial complex, solitary nucleus, and dorsal vagal motor nucleus in threat (Bandler & Shipley, 1994; Behbehani, 1995; Janig & Habler, 2000; Keay & Bandler, 2001; Saper, 2002). By contrast, human research has focused largely on the cortex, basal ganglia, and amygdala. We focused on the PAG because it is both heavily implicated in central control of lower brainstem autonomic effectors under threat (Bandler et al., 2000; Price, 2005; Verberne & Owens, 1998), and its activity is likely to be more detectable in fMRI than lower brainstem nuclei. PAG appears to be reliably activated in human imaging studies of pain and threat (Bingel, Lorenz, Schoell, Weiller, & Buchel, 2006; Derbyshire et al., 2002; Fairhurst, Wiech, Dunckley, & Tracey, 2007; Mobbs et al., 2007; Mohr et al., 2008; Valet et al., 2004; Wager, Rilling et al., 2004; Wager et al., 2007), as well as other studies elicting negative emotional experiences (T. Wager et al., 2008). In a recent meta-analysis, we found that studies that activated dorsal MPFC PAG were more likely to activate PAG during the same conditions (Kober, Barrett, Joseph, Bliss-Moreau et al., 2008). Our main goals were to replicate and extend the results of our companion paper on SET effects, focusing in particular on cortical-PAG pathways. Our main findings can be summarized briefly as follows.
A dual-process model of HR control: Mediation by reciprocal MPFC subregions
As in the companion study (Wager et al., submitted), we found activity increases in rdACC and decreases in vmPFC/mOFC during a SET speech preparation challenge, which independently mediated effects on HR during speech preparation. In both studies, activity in the more dorsal mid-rostral/pregenual cingulate region (rdACC or pgACC) and vmPFC/mOFC were negatively correlated, but each was also an independent predictor of HR increases during SET. The findings suggest that different regions of the MPFC have qualitatively different (and perhaps opposite) roles in generating and regulating autonomic responses to SET.
In the present study, we found that PAG was also a mediator of the relationship between SET and HR increases. Subsequent confirmatory path models suggested that the MPFC-HR connection in the more ventral vmPFC/mOFC region in particular was mediated by the PAG. The rdACC-HR connection was not mediated by PAG, but was mediated by thalamic activity that was itself connected with PAG. These findings confirm the role of the PAG in human socially generated threat, and provide some preliminary steps towards building a model of cortical-brainstem-autonomic pathways in humans. Based on these findings, it appears that such a model is likely to involve at least two different and opposed systems in the MPFC, a third contribution from the basal ganglia (putamen), and separable mediators at the brainstem/diencephalic level. These results help to pave the way for examining both more fine-grained patterns of individual differences in response to threat (Blascovich et al., 2003; Tugade & Fredrickson, 2004) and psychosocial interventions and manipulations that affect threat responses (al'Absi, Bongard, & Lovallo, 2000; Eisenberger et al., 2007; Fredrickson, Mancuso, Branigan, & Tugade, 2000).
The more dorsal of the two MPFC regions we have identified across studies is isomorphic with either the anterior portion of the anterior mid-cingulate cortex (what we have termed rdACC) or the pgACC as identified by Vogt and colleagues (Palomero-Gallagher, Vogt, Schleicher, Mayberg, & Zilles, 2008; Vogt, 2005). The findings in the companion paper (Wager et al., submitted) were somewhat more ventral, centered on the pgACC, but this may be due more to differences in the image acquisition techniques than differences in the localization of brain activity. Anatomical localization in the ventral part of the MPFC is non-trivial; it is well-known that magnetic field inhomogeneity caused by air sinuses causes MR signal loss and spatial distortion in the vmPFC in particular (Du, Dalwani, Wylie, Claus, & Tregellas, 2007; Glover & Law, 2001). For this reason, we considered it important to replicate the SET effects we report here in two separate samples, using two different kinds of pulse sequences. In the companion paper, we used spiral in-out imaging (Glover & Law, 2001), which shows less spatial distortion but more spatial blurring than the EPI sequence used in the present paper. It is possible that the results in the present study, and other previous studies that have used predominantly EPI, appear more dorsal than the actual location of neural activity due to EPI distortion, and thus pgACC is a better estimate of the positive cortical generator of HR. However, high-resolution imaging studies with low image distortion, and converging evidence from other imaging modalities, are needed to be more certain.
The complexities of localization nothwithstanding, these results can help to localize the principal correlates of cardiovascular responses within the MPFC. Precise localization is required if relationships with animal models are to be established and if the regions are to be developed as prospective measures or diagnostic criteria. We note that correlations between dorsal MPFC and cardiovascular reactivity (HR or blood pressure) in several recent studies (Critchley, Tang, Glaser, Butterworth, & Dolan, 2005; P. Gianaros et al., 2004; Gianaros et al., 2008) have been localized primarily to the pre-SMA (Brodmann’s Area 6), an area broadly associated with behavioral ‘energization’ (Stuss & Alexander, 2007) that accompanies diverse forms of working memory and attentional demand (van Snellenberg & Wager, in press; Wager, Reading, & Jonides, 2004; Wager & Smith, 2003). Pre-SMA cardiac correlations are not specific to evaluative threat, as pre-SMA correlates with cardiac responses to exercise (Critchley, Corfield, Chandler, Mathias, & Dolan, 2000; Critchley et al., 2005; Wong, Masse, Kimmerly, Menon, & Shoemaker, 2007). Thus, it is the more ventral aspect of the cingulate—the pre-genual cingulate (pgACC, Areas 25/32) and ventro-medial prefrontal cortex/medial orbitofrontal cortex (vmPFC/mOFC, Areas 13, 14, and 11m), in which the mediators in the present study are located, that may be most critical in SET. Unlike pre-SMA, these areas are homologues to the “visceromotor” cortex in monkeys (Devinsky et al., 1995; Öngür, Ferry, & Price, 2003).
It is also the more ventral MPFC regions that are most closely linked in animal models to the PAG, though dorsomedial connections exist as well (Bandler et al., 2000). Specifically, Dorsal MPFC anterior to pre-SMA (Areas 9 and 24b) project to the lateral PAG and dorsal hypothalamus; vmPFC (Areas 25, 32, and medial 10) projects to the dorsolateral PAG and medial hypothalamus; and medial and lateral OFC project to the ventrolateral PAG and lateral hypothalamus. The pre-SMA (Area 6) does not project to PAG. It is worth noting here that meta-analyses of human neuroimaging studies have shown co-activation between the PAG and the pgACC (Area 24b/32) and dorsal MPFC (Area 9), but in a region clearly anterior to the pre- SMA (Kober, Barrett, Joseph, Bliss-Moreau et al., 2008). Thus, in sum, pre-SMA activity is more likely to be associated with behavioral energization of the skeletomotor system, whereas more rostral and ventral MPFC sub-regions are likely drivers of psychosocially mediated visceromotor responses.
Subcortical mediators: Striatum and PAG but not amygdala
In this study, we did not attempt a systematic characterization of all subcortical regions of interest that may partially mediate physiological responses to social threat. We focused specifically on the PAG due to its role in coordinating autonomic and behavioral responses. However, two points on subcortical mediators are worth noting. First, de-activation in the right putamen was the only subcortical mediator of HR increases in both studies that passed our stringent correction for multiple comparisons. This activity was located in the mid-dorsal region in the companion study, but in the ventral striatum proper in this study.
Secondly, notably absent in both studies was the amygdala, which was de-activated (not activated) during speech preparation in both studies. These decreases were not strongly enough so to meet the threshold for whole-brain corrected significance in either study, but are apparent in region of interest analyses in both studies. The amygdala has been consistently linked with responses to and learning of predictive cues in fear conditioning (LeDoux, 2000; Phelps et al., 2004), increased activation in anxiety disorders (Etkin & Wager, 2007; Nitschke et al., 2009), and negative emotional experience reported to photographs (Ochsner & Gross, 2008; Phan, 2004; Wager, Hughes, Davidson, Lindquist, & Ochsner, 2008). However, amygdala responses in human imaging studies may be linked more tightly to short-term predictive cues than to the core experience of threat or anxiety per se (Amaral, 2003; Anderson et al., 2003; Anderson & Phelps, 2002; Ewbank, Barnard, Croucher, Ramponi, & Calder, 2009; Phan et al., 2003; T. D. Wager et al., 2008; Whalen et al., 1998). For example, meta-analyses have found that the stimuli that most reliably elicit amygdala activation in studies of human emotion is viewing faces of other individuals showing fearful expressions (T. Wager et al., 2008). In nonhuman animals, the amydala does not appear to be essential for context-driven “anxiety-like” states or responses to unconditioned fear cues such as predator odors (Davis & Lee, 1998; Wallace & Rosen, 2001). Our results support a distinction between SET, which engages MPFC-brainstem responses, and fear conditioning and related paradigms, which clearly involve amygdala-PAG pathways.
Overlapping, but distinct, mediators of anxiety reports
This pattern of results diverged substantially from the brain mediators of reported anxiety. Anxiety reports measured across time were positively correlated with HR responses across time (both increased substantially during speech preparation and returned to or below baseline levels afterwards). It is notable that we observed amygala de-activation during speech preparation, while reported anxiety increased. Therefore, the processes that are abnormal in patients with anxiety disorders (evidenced in part by increased responsivness in amygdala and insula (Etkin & Wager, 2007) may be quite different from the anxiety reported in normal individuals during SET.
In the SET challenge, brain mediators of anxiety included the vmPFC, in approximately the same location as the region mediating HR responses. However, other mediators of anxiety were divergent from the mediators of HR. One mediator of anxiety was the most dorsal part of the dmPFC. This region has been linked to mentalizing about others and their knowledge and intentions (Mitchell, Macrae, & Banaji, 2004; Ochsner & Gross, 2005; Rilling, Dagenais, Goldsmith, Glenn, & Pagnoni, 2008), anxiety-generating cognitive appraisals (Kalisch, Wiech, Critchley, & Dolan, 2006), and negative emotional experience more generally in meta-analyses (T. Wager et al., 2008). Other mediators of anxiety included the left IFG, dorsolateral PFC, and temporal poles. Lateral PFC activity has been linked to both the generation and regulation of negative emotional appraisals (Bishop, Duncan, Brett, & Lawrence, 2004; Kober, Barrett, Joseph, Blissmoreau et al., 2008), which might suggest a variable role in mediating anxiety responses depending on the contents (anxiogenic or anxiolytic) of task or goal representations maintained in the PFC.
Indeed, in our study, the mediation effect in these regions showed evidence for variability across individuals. Only those who reported strong increases in anxiety (anxiety ‘reactors’), for example, showed the pathway evident in the mediation of HR: a pathway linking SET, vmPFC decreases, and increased anxiety. In addition, only anxiety ‘reactors’ showed evidence for a positive SET-IFG-anxiety pathway. While it is difficult to interpret these effects post hoc with certainty, there is a precedent for believing that anxiety reports do not mean the same thing for all participants. “Repressors” experience anxiety but do not report it (Weinberger, Schwartz, & Davidson, 1979), and in several studies implicit measures—but not self-reported emotion—have been found to predict physiological responses to SET challenges (Egloff, Wilhelm, Neubauer, Mauss, & Gross, 2002; Lerner et al., 2007). One explanation for the variable mediation results in vmPFC, for example, is that some individuals are “repressors” for whom vmPFC deactivation does not predict anxiety because they do not accurately report the anxiety they feel. This explanation is consistent with the negative anxiety reactivity-Path b correlation shown in Figure 7.
It is also possible that the vmPFC plays a role in interoception—the perception of physiological changes in the body—and the generation of subjective anxiety based on these signals. However, the anterior insula is the region most closely associated with interoception in the literature to date (Craig, 2003), and we did not find anxiety mediation in the insula. A promising future approach might be to assess anxiety using implicit measures.
Strengthening of cortical, but not PAG, connectivity with HR during SET
An additional extension in this paper was to test whether brain-HR correlations in the vmPFC, rdACC, and PAG were stronger during (moderated by) speech preparation itself than during pre-stress baseline or post-stress recovery. We found evidence that speech preparation strengthened connectivity in both cortical areas, but that PAG was coupled with HR during all task states. The implication is that PAG signal is more closely coupled with HR irrespective of cognitive processes, whereas the coupling between cortical regions and HR is driven by conceptual processing during SET and/or anticipatory anxiety (in the case of the vmPFC).
Whereas mediation implies that SET produces changes in brain activity, which in turn drives HR, moderation is a test of the SET x brain interaction on HR. A form of such a moderation test is implemented in popular SPM software as a “psychophysiological interaction” analysis, and is commonly interpreted as evidence for a task-specific functional pathway. However, there are several possible interpretations. The most straightforward one is that the regions are mediators, and that with low SET, restricted range keeps them from correlating with HR as strongly as they otherwise would—thus, the relationship is stronger during threat. Nonlinearity in the SET-brain and brain-HR relationships could also create an interaction. Alternatively, a second brain region activated by SET could be a common cause of both brain and HR increases. Finally, the functional role of the region could be changing during SET, by virtue of its participation in another functional network, or overlapping signals related to other brain processes either added or removed during SET. While these alternatives are impossible to disentangle without converging evidence, we note that stronger brain-HR connectivity during SET is consistent with these regions’ role as mediators.
Desiderata on the single-epoch and path modeling approaches
The paucity of neuroimaging studies of SET is perhaps due to the fact that studying SET involves several unique challenges, which require innovative approaches to the design and analysis of neuroimaging studies. We describe these, and our approach, briefly below.
A key, relatively novel aspect of our task design in this and the companion paper (Wager et al., submitted) was that we used a single-epoch approach to eliciting a threat state over a brief (2 min) period. This is an atypical fMRI task design, as the vast majority of blood oxygen level dependent (BOLD) fMRI studies have studied emotional responses by using briefly presented affective stimuli, or by contrasting emotional manipulation or performance stress with a control condition in alternating blocks (typically every 20–30 sec). The use of a single-epoch design was important because it is likely that social threat responses cannot be reliably turned on and off multiple times during scanning: Participants habituate to even a single repetition of a SET challenge (Berntson et al., 1994; Cohen et al., 2000; Kelsey et al., 1999). Experimental designs with prolonged challenges (i.e., low temporal frequency) more closely mirror emotion induction procedures in non-imaging settings (Fredrickson & Levenson, 1998; Fredrickson et al., 2000) and are more likely to induce strong changes in emotional states. Indeed, the notion that emotional states evolve more gradually over time has motivated the use of novel fMRI techniques (Wang et al., 2005) and positron emission tomography (Kern et al., 2008; Phan, Wager, Taylor, & Liberzon, 2002; Pruessner et al., 2008) in the study of stress and emotion.
An fMRI study by Preussner et al. (Pruessner et al., 2008) that compared alternating blocks of math performance under stressful and non-stressful control conditions (the “Montreal Neuroimaging Stress Test”) illustrates this difficulty. The stress vs. control comparison revealed increased fMRI signal in areas associated with math performance and controlled response selection—premotor cortex, caudal dorsal cingulate, and occipital association areas—but none of the areas associated with emotional experience in humans (T. Wager et al., 2008) or stress generation and modulation in animals. The use of single-epoch, low-frequency designs is not completely novel (Breiter et al., 1997; Eisenberger, Lieberman, & Williams, 2003), but demonstrating that standard BOLD fMRI can capture responses to a single-epoch SET challenge suggests that this may be a promising way to characterize brain systems that perceive and respond to social threat.
Another key choice in this paper was the choice to avoid the standard method of making inferences about psychological mechanisms based solely on subtraction methods and logic. The subtraction method has been used to compare stressful performance with non-stressful task performance (Gianaros, May, Siegle, & Jennings, 2005; Kern et al., 2008; Pruessner et al., 2008). However, it is difficult to conceive of control conditions that isolate the essential affective experience component of SET responses that is likely to lead to physiological changes. For example, Preussner et al. compared stressful mental arithmetic, which includes time deadlines, negative performance feedback, and expectations about normative performance, with a control condition without these elements. It is difficult to determine which brain correlates of SET are related to aspects of the emotional state that generate subjective feelings and shape autonomic activity, and which reflect changes in how arithmetic operations are performed.
A complete parsing of brain activity induced by “stressor” tasks into cognitive, emotional, motoric, and autonomic afferent and efferent components is still likely to be a long way off. For example, our results do not inform on whether MPFC activity is linked to physiology because it is the seat of subjective emotional experience or some physiological mechanism that shapes homeostatic and metabolic processes largely outside of conscious awareness. Rather than attempting to isolate subjective experiences of emotion, we attempted to localize close correlates of integrated autonomic output. This output is of interest in its own right because of its consequences for the body, but it may also aid in the study of emotional experience, if only by identifying a potential alternative explanation for “emotional” as well as “cognitive” activations in the VMPFC.
Conclusions
In sum, this paper and its companion report several findings that may assist the integration of human and non-human approaches to studying brain-body communication and its effects on health and health-related physiological processes. First, they demonstrate that a single-epoch social threat challenge can be meaningfully studied using BOLD fMRI. Second, they demonstrate that the multi-level path modeling approach can be used to both constrain inferences on how brain responses to SET are interpreted and to establish relationships between experimental manipulations, brain activity, and peripheral physiology. Third, they establish a bivalent pattern of cortical and subcortical changes that mediate HR increases during SET, including activity increases in the pgACC/rdACC and PAG, and de-activation in the vmPFC/mOFC and putamen. The papers also establish the localization of vmPFC-PAG-HR and rdACC-thalamus-HR that are likely locations for cortical-brainstem pathways that translate mental appraisals into adaptive physiological responses. These responses are likely to result in allostatic load on the body (Mcewen, 2007), and the heart in particular (Jiang et al., 1996; Rozanski et al., 1988).
Supplementary Material
Figure 5.
Mediation of the social evaluative threat (SET) effect on heart rate (HR) by the periaqueductal gray (PAG). A) the PAG region of interest (ROI) is shown in green. A) Left: Voxels showing significant responses to SET (a path) at a threshold of p < .025 (yellow) and p < .05 (orange; one-tailed) within the PAG ROI. Center: Voxels showing a significant relationship with HR, controlling for SET (b path). Right: Voxels showing a significant mediation effect (a*b product). B) The time course of activity in the PAG, which shows the largest peaks during the first 30 sec and the last 30 sec of the first speech. Details of the plot are as in Figure 3A. C) The average PAG brain activity – HR correlation during each task phase. Details are as in Figure 3B. The PAG shows a consistent positive relationship with HR across task phases.
Acknowledgments
We would like to thank Niall Bolger for helpful discussion on path analysis, and the authors of SPM software for making it freely available. This paper was made possible with the support of grant funding from NSF 0631637 (T.W.), NIH Grant MH076136 (T.W.), NIH Grant MH082308 (T.W.), and NIH Grant MH076137 (K.O.).
Footnotes
Author contributions: Design: T.D.W. and K.O. Data collection: B.H. and M.D. Analysis: T.D.W., M.D., and M.L. Writing: T.D.W., V.A.V.A., and K.O. Matlab code implementing mediation analyses is freely available at http://www.columbia.edu/cu/psychology/tor/.
Some working definitions of terms are as follows: By “emotion,” we mean integrated processes that involve the generation of valenced (good/bad) feelings over a period of seconds to hours. Emotions are constructed from basic affective elements interpreted in the context of relationships between the self and environment. “Threat” might be considered an affective or perhaps a primitive emotional response, which may be elaborated into different emotions (“fear,” “anxiety,” or even “anger”) depending on the contextual appraisal involved. “Stress” is a loose construct that refers to cumulative emotional or physiological demands over time.
Two caveats are in order. First, it seems premature to differentiate specific emotions based on the gross anatomical location of activation (Wager et al., 2008a). Second, the neural correlates of anxiety have not been definitively localized in part because different kinds of ‘anxiety’ may differ substantially in their neuroanatomical bases. Among clinical anxiety disorders, for example, the evidence to date suggests that only patients with specific phobias reliably show greater dACC activity than controls in emotional probe tasks (social anxiety patients do not) (Etkin & Wager, 2007).
The one exception was that the group assigned to prepare the first speech for computer analysis scoring showed larger HR responses during preparation of speeches for both Computer and Panel audiences, but this effect is not likely to bear on the within-participant analyses we report here. HR and anxiety responses were not significantly different for speeches delivered to Computer and Panel audiences, controlling for task order.
We believe that structural models assessing ‘direct’ connectivity among each of these key regions would depend heavily on the assumptions of the linear model. Because there would be many variables in such a model, and inferences about direct connectivity depends on assuming an absence of nonlinear effects and higher-order interactions, the results of such a model are less likely to be stable across studies. Thus, we restricted ourselves to a small set of a priori tests.
References
- al'Absi M, Bongard S, Lovallo WR. Adrenocorticotropin responses to interpersonal stress: effects of overt anger expression style and defensiveness. Int J Psychophysiol. 2000;37(3):257–265. doi: 10.1016/s0167-8760(00)00108-2. [DOI] [PubMed] [Google Scholar]
- Amaral DG. The amygdala, social behavior, and danger detection. Ann N Y Acad Sci. 2003;1000:337–347. doi: 10.1196/annals.1280.015. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, et al. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 2003;6(2):196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Is the human amygdala critical for the subjective experience of emotion? Evidence of intact dispositional affect in patients with amygdala lesions. J Cogn Neurosci. 2002;14(5):709–720. doi: 10.1162/08989290260138618. [DOI] [PubMed] [Google Scholar]
- Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull. 2000;53(1):95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17(9):379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC neuroscience. 2003;4:25. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46(6):575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Colloca L, Torre E, Lanotte M, Melcarne A, Pesare M, et al. Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nat Neurosci. 2004;7(6):587–588. doi: 10.1038/nn1250. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Binkley PF, Uchino BN, Quigley KS, Fieldstone A. Autonomic cardiac control. III. Psychological stress and cardiac response in autonomic space as revealed by pharmacological blockades. Psychophysiology. 1994;31(6):599–608. doi: 10.1111/j.1469-8986.1994.tb02352.x. [DOI] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120(1–2):8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7(2):184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Blascovich J, Mendes WB, Tomaka J, Salomon K, Seery M. The robust nature of the biopsychosocial model challenge and threat: a reply to Wright and Kirby. Pers Soc Psychol Rev. 2003;7(3):234–243. doi: 10.1207/S15327957PSPR0703_03. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19(3):591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Cohen S, Hamrick N, Rodriguez MS, Feldman PJ, Rabin BS, Manuck SB. The stability of and intercorrelations among cardiovascular, immune, endocrine, and psychological reactivity. Ann Behav Med. 2000;22(3):171–179. doi: 10.1007/BF02895111. [DOI] [PubMed] [Google Scholar]
- Cohen S, Hamrick N, Rodriguez MS, Feldman PJ, Rabin BS, Manuck SB. Reactivity and vulnerability to stress-associated risk for upper respiratory illness. Psychosom Med. 2002;64(2):302–310. doi: 10.1097/00006842-200203000-00014. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13(4):500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Critchley H. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126(10):2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol (Lond) 2000;523(Pt 1):259–270. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126(Pt 10):2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Tang J, Glaser D, Butterworth B, Dolan RJ. Anterior cingulate activity during error and autonomic response. Neuroimage. 2005;27(4):885–895. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biological Psychiatry. 1998;44(12):1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Davis M, Lee YL. Fear and anxiety: Possible roles of the amygdala and bed nucleus of the stria terminalis. Cognition & Emotion.Special Issue: Neuropsychological perspectives on affective and anxiety disorders. 1998;12(3):277–305. [Google Scholar]
- de la Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: mechanism of the placebo effect in Parkinson's disease. Science. 2001;293(5532):1164–1166. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, Pruessner JC. The Montreal Imaging Stress Task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J Psychiatry Neurosci. 2005;30(5):319–325. [PMC free article] [PubMed] [Google Scholar]
- Derbyshire SW, Jones AK, Creed F, Starz T, Meltzer CC, Townsend DW, et al. Cerebral responses to noxious thermal stimulation in chronic low back pain patients and normal controls. Neuroimage. 2002;16(1):158–168. doi: 10.1006/nimg.2002.1066. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Du YP, Dalwani M, Wylie K, Claus E, Tregellas JR. Reducing susceptibility artifacts in fMRI using volume-selective z-shim compensation. Magn Reson Med. 2007;57(2):396–404. doi: 10.1002/mrm.21150. [DOI] [PubMed] [Google Scholar]
- Egloff B, Wilhelm FH, Neubauer DH, Mauss IB, Gross JJ. Implicit anxiety measure predicts cardiovascular reactivity to an evaluated speaking task. Emotion. 2002;2(1):3–11. doi: 10.1037/1528-3542.2.1.3. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302(5643):290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35(4):1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Levenson RW, Friesen WV. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221(4616):1208–1210. doi: 10.1126/science.6612338. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. Am J Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Simpson HB, Neria Y, Lewis-Fernandez R, Schneier F, editors. Brain systems underlying anxiety disorders: A view from neuroimaging. Understanding Anxiety. (in press). [Google Scholar]
- Ewbank MP, Barnard PJ, Croucher CJ, Ramponi C, Calder AJ. The amygdala response to images with impact. Soc Cogn Affect Neurosci. 2009 doi: 10.1093/scan/nsn048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhurst M, Wiech K, Dunckley P, Tracey I. Anticipatory brainstem activity predicts neural processing of pain in humans. Pain. 2007;128(1–2):101–110. doi: 10.1016/j.pain.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Levenson RW. Positive emotions speed recovery from the cardiovascular sequelae of negative emotions. Cognition and Emotion. 1998;12(2):191–220. doi: 10.1080/026999398379718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Mancuso RA, Branigan C, Tugade MM. The Undoing Effect of Positive Emotions. Motivation and Emotion. 2000;24(4):237–258. doi: 10.1023/a:1010796329158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gianaros P, Van Der Veen FM, Jennings JR. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology. 2004;41(4):521–530. doi: 10.1111/1469-8986.2004.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Derbyshire SW, May JC, Siegle GJ, Gamalo MA, Jennings JR. Anterior cingulate activity correlates with blood pressure during stress. Psychophysiology. 2005;42(6):627–635. doi: 10.1111/j.1469-8986.2005.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Derbyshire SW, Matthews KA. Heightened functional neural activation to psychological stress covaries with exaggerated blood pressure reactivity. Hypertension. 2007;49(1):134–140. doi: 10.1161/01.HYP.0000250984.14992.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, May JC, Siegle GJ, Jennings JR. Is There a Functional Neural Correlate of Individual Differences in Cardiovascular Reactivity? Psychosom Med. 2005;67(1):31–39. doi: 10.1097/01.psy.0000151487.05506.dc. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J Neurosci. 2008;28(4):990–999. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Van Der Veen FM, Jennings JR. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during workingmemory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology. 2004;41(4):521–530. doi: 10.1111/1469-8986.2004.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5(3):243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46(3):515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Glaser R. Stress-induced immune dysregulation: implications for wound healing, infectious disease and cancer. J Neuroimmune Pharmacol. 2006;1(4):421–427. doi: 10.1007/s11481-006-9036-0. [DOI] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, et al. Processing of temporal unpredictability in human and animal amygdala. Journal of Neuroscience. 2007;27(22):5958. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman HH. Survey Design and Analysis: Principles, Cases, and Procedures. Free Press; 1955. [Google Scholar]
- Janig W, Habler HJ. Specificity in the organization of the autonomic nervous system: a basis for precise neural regulation of homeostatic and protective body functions. Prog Brain Res. 2000;122:351–367. doi: 10.1016/s0079-6123(08)62150-0. [DOI] [PubMed] [Google Scholar]
- Jiang W, Babyak M, Krantz DS, Waugh RA, Coleman RE, Hanson MM, et al. Mental stress--induced myocardial ischemia and cardiac events. Jama. 1996;275(21):1651–1656. doi: 10.1001/jama.275.21.1651. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Critchley H, Dolan R. Levels of appraisal: A medial prefrontal role in high-level appraisal of emotional material. Neuroimage. 2006;30(4):1458–1466. doi: 10.1016/j.neuroimage.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neuroscience and biobehavioral reviews. 2001;25(7–8):669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- Kelsey RM, Blascovich J, Tomaka J, Leitten CL, Schneider TR, Wiens S. Cardiovascular reactivity and adaptation to recurrent psychological stress: effects of prior task exposure. Psychophysiology. 1999;36(6):818–831. [PubMed] [Google Scholar]
- Kemeny ME. The psychobiology of stress. Current Directions in Psychological Science. 2003 [Google Scholar]
- Kemeny ME, Rosenwasser LJ, Panettieri RA, Rose RM, Berg-Smith SM, Kline JN. Placebo response in asthma: a robust and objective phenomenon. J Allergy Clin Immunol. 2007;119(6):1375–1381. doi: 10.1016/j.jaci.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Kenny DA, Korchmaros JD, Bolger N. Lower level mediation in multilevel models. Psychol Methods. 2003;8(2):115–128. doi: 10.1037/1082-989x.8.2.115. [DOI] [PubMed] [Google Scholar]
- Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33(4):517–529. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, et al. Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci. 2004;16(10):1730–1745. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5(2):e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Functional grouping and cortical-subcortical interactions in emotion: A meta-analysis of neuroimaging studies. 42 Cong. Rec. 2008:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: A meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R, Waldstein SR, Critchley HD, Derbyshire S, Drossman D, Wager TD, et al. The rebirth of neuroscience in psychosomatic medicine, part II: Clinical applications and implications for research. Psychsomatic Medicine. doi: 10.1097/PSY.0b013e318198a11f. (in press) [DOI] [PubMed] [Google Scholar]
- Lazarus RS. Cognition and motivation in emotion. Am Psychol. 1991;46(4):352–367. doi: 10.1037//0003-066x.46.4.352. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lerner JS, Dahl RE, Hariri AR, Taylor SE. Facial expressions of emotion reveal neuroendocrine and cardiovascular stress responses. Biological Psychiatry. 2007;61(2):253–260. doi: 10.1016/j.biopsych.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Loggia ML, Schweinhardt P, Villemure C, Bushnell MC. Effects of psychological state on pain perception in the dental environment. J Can Dent Assoc. 2008;74(7):651–656. [PubMed] [Google Scholar]
- MacCorquodale K, Meehl PE. On a distinction between hypothetical constructs and intervening variables. Psychological Review. 1948;55(2):307–321. doi: 10.1037/h0056029. [DOI] [PubMed] [Google Scholar]
- Mcewen B. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiological Reviews. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Milad M, Wright C, Orr S, Pitman R, Quirk G, Rauch S. Recall of Fear Extinction in Humans Activates the Ventromedial Prefrontal Cortex and Hippocampus in Concert. Biological Psychiatry. 2007;62(5):446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Encoding-specific effects of social cognition on the neural correlates of subsequent memory. J Neurosci. 2004;24(21):4912–4917. doi: 10.1523/JNEUROSCI.0481-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, et al. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317(5841):1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr C, Leyendecker S, Mangels I, Machner B, Sander T, Helmchen C. Central representation of cold-evoked pain relief in capsaicin induced pain: an eventrelated fMRI study. Pain. 2008;139(2):416–430. doi: 10.1016/j.pain.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, et al. Anticipatory Activation in the Amygdala and Anterior Cingulate in Generalized Anxiety Disorder and Prediction of Treatment Response. Am J Psychiatry. 2009 doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K, Gross J. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Currents Directions in Psychological Science. 2008;17(1):153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öngür D, Ferry A, Price J. Architectonic subdivision of the human orbital and medial prefrontal cortex. J. Comp. Neurol. 2003;460(3):425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K. Receptor architecture of human cingulate cortex: Evaluation of the four-region neurobiological model. Hum Brain Mapp. 2008 doi: 10.1002/hbm.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439(7078):865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21(2):768–780. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Decker LR, Noll DC, Nichols TE, et al. Activation of the medial prefrontal cortex and extended amygdala by individual ratings of emotional arousal: a fMRI study. Biol Psychiatry. 2003;53(3):211–215. doi: 10.1016/s0006-3223(02)01485-3. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroimaging studies of human emotions. CNS Spectr. 2004;9(4):258–266. doi: 10.1017/s1092852900009196. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Porges SW. The Polyvagal Theory: phylogenetic contributions to social behavior. Physiol Behav. 2003;79(3):503–513. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- Porro C. Functional activity mapping of the mesial hemispheric wall during anticipation of pain. NeuroImage. 2003;19(4):1738–1747. doi: 10.1016/s1053-8119(03)00184-8. [DOI] [PubMed] [Google Scholar]
- Price JL. Free will versus survival: brain systems that underlie intrinsic constraints on behavior. J Comp Neurol. 2005;493(1):132–139. doi: 10.1002/cne.20750. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, et al. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry. 2008;63(2):234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Dagenais JE, Goldsmith DR, Glenn AL, Pagnoni G. Social cognitive neural networks during in-group and out-group interactions. Neuroimage. 2008;41(4):1447–1461. doi: 10.1016/j.neuroimage.2008.03.044. [DOI] [PubMed] [Google Scholar]
- Rozanski A, Bairey CN, Krantz DS, Friedman J, Resser KJ, Morell M, et al. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med. 1988;318(16):1005–1012. doi: 10.1056/NEJM198804213181601. [DOI] [PubMed] [Google Scholar]
- Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From fear to safety and back: reversal of fear in the human brain. J Neurosci. 2008;28(45):11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7(4):422–445. [PubMed] [Google Scholar]
- Sneed JR, Rutherford BR, Rindskopf D, Lane DT, Sackeim HA, Roose SP. Design makes a difference: a meta-analysis of antidepressant response rates in placebo-controlled versus comparator trials in late-life depression. Am J Geriatr Psychiatry. 2008;16(1):65–73. doi: 10.1097/JGP.0b013e3181256b1d. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philos Trans R Soc Lond B Biol Sci. 2007;362(1481):901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Sternberg E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci. 2006;1088:361–372. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- Tomaka J, Blascovich J, Kibler J, Ernst JM. Cognitive and physiological antecedents of threat and challenge appraisal. J Pers Soc Psychol. 1997;73(1):63–72. doi: 10.1037//0022-3514.73.1.63. [DOI] [PubMed] [Google Scholar]
- Tugade MM, Fredrickson BL. Resilient individuals use positive emotions to bounce back from negative emotional experiences. J Pers Soc Psychol. 2004;86(2):320–333. doi: 10.1037/0022-3514.86.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, et al. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain--an fMRI analysis. Pain. 2004;109(3):399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- van Snellenberg JX, Wager TD. Cognitive and Motivational Functions of the Human Prefrontal Cortex. In: Goldberg E, Bougakov D, editors. Luria's Legacy in the 21st Century. Oxford: Oxford University Press; (in press) [Google Scholar]
- Verberne AJ, Owens NC. Cortical modulation of the cardiovascular system. Progress in Neurobiology. 1998;54(2):149–168. doi: 10.1016/s0301-0082(97)00056-7. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6(7):533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T, Barrett L, Bliss-Moreau E, Lindquist K, Duncan S, Kober H, et al. The handbook of emotion. Guilford Press; 2008. The neuroimaging of emotion. [Google Scholar]
- Wager TD, Hughes B, Davidson M, Lindquist ML, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Reading S, Jonides J. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22(4):1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proceedings of the National Academy of Sciences. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a metaanalysis. Cogn Affect Behav Neurosci. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wager TD, Waugh C, Lindquist M, Noll DC, Fredrickson BL, Taylor SE. Brain mediators of cardiovascular responses to social threat, Part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage. doi: 10.1016/j.neuroimage.2009.05.043. (submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. Journal of neuroscience the official journal of the Society for Neuroscience, The. 1997;17(23):9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace KJ, Rosen JB. Neurotoxic lesions of the lateral nucleus of the amygdala decrease conditioned fear but not unconditioned fear of a predator odor: comparison with electrolytic lesions. J Neurosci. 2001;21(10):3619–3627. doi: 10.1523/JNEUROSCI.21-10-03619.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, et al. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci USA. 2005;102(49):17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh CE, Wager TD, Fredrickson BL, Noll DC, Taylor SF. The neural correlates of trait resilience when anticipating and recovering from threat. Social Cognitive and Affective Neuroscience. 2008;3(4):322–332. doi: 10.1093/scan/nsn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DA, Schwartz GE, Davidson RJ. Low-anxious, high-anxious, and repressive coping styles: psychometric patterns and behavioral and physiological responses to stress. J Abnorm Psychol. 1979;88(4):369–380. doi: 10.1037//0021-843x.88.4.369. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18(1):411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S, Masse N, Kimmerly D, Menon R, Shoemaker J. Ventral medial prefrontal cortex and cardiovagal control in conscious humans. Neuroimage. 2007;35(2):698–708. doi: 10.1016/j.neuroimage.2006.12.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.