Abstract
Vision is important for locomotion in complex environments. How it is used to guide stepping is not well understood. We used an eye search coil technique combined with an active marker-based head recording system to characterize the gaze patterns of cats walking over terrains of different complexity: (1) on a flat surface in the dark when no visual information was available, (2) on the flat surface in light when visual information was available but not required, (3) along the highly structured but regular and familiar surface of a horizontal ladder, a task for which visual guidance of stepping was required, and (4) along a pathway cluttered with many small stones, an irregularly structured surface that was new each day. Three cats walked in a 2.5 m corridor, and 958 passages were analyzed. Gaze activity during the time when the gaze was directed at the walking surface was subdivided into four behaviors based on speed of gaze movement along the surface: gaze shift (fast movement), gaze fixation (no movement), constant gaze (movement at the body’s speed), and slow gaze (the remainder). We found that gaze shifts and fixations dominated the cats’ gaze behavior during all locomotor tasks, jointly occupying 62–84% of the time when the gaze was directed at the surface. As visual complexity of the surface and demand on visual guidance of stepping increased, cats spent more time looking at the surface, looked closer to them, and switched between gaze behaviors more often. During both visually guided locomotor tasks, gaze behaviors predominantly followed a repeated cycle of forward gaze shift followed by fixation. We call this behavior “gaze stepping”. Each gaze shift took gaze to a site approximately 75–80 cm in front of the cat, which the cat reached in 0.7–1.2 s and 1.1–1.6 strides. Constant gaze occupied only 5–21% of the time cats spent looking at the walking surface.
Keywords: locomotion, eye movement, head movement, constant gaze, travel fixation, motor control
INTRODUCTION
Vision is important for successful locomotion, especially when navigating through complex natural environments. Many studies were conducted with a goal to determine how visual information is collected during locomotion, and three major types of gaze behaviors were described: fixations on objects, gaze shifts, and constant gaze. Fixations are believed to be periods when visual information is gathered about objects (e.g., Land and Hayhoe, 2001). Constant gaze occurs when during locomotion a subject looks a fixed distance ahead (Fowler and Sherk, 2003); this behavior is also called “travel fixation” in humans (Patla and Vickers, 1997, 2003). During constant gaze, images of objects travel across the retina in a constant pattern, and many studies suggest that such “optic flow” provides useful information about both the objects in the environment and the subject’s own movement (Gibson, 1958; Lee, 1980). Gaze shifts, also known as gaze saccades, are rapid gaze movements between fixations and constant gaze episodes, and visual sampling has been shown to be significantly suppressed during gaze shifts (Bridgeman et al., 1975; rev. in Wurtz, 2008). While both gaze fixations and constant gaze are thought to be behaviors, during which visual information is collected, reports on how much gaze fixations and constant gaze are used during locomotion differ.
In both humans and animals, a substantial amount of data suggest that constant gaze and optic flow information play major, if not the dominant, roles in guiding locomotion (e.g., Sun et al., 1992; Sherk and Fowler, 2000; Warren et al., 2001; Srinivasan and Zhang, 2004; Mulavara et al., 2005). This was reported to be the case not only for determining the direction or speed of locomotion, but also for gathering information about irregularities on the walking surface for accurate foot placement. For example, Sherk and Fowler (2001) showed that strobe lighting that disrupts optic flow also interferes with cats’ ability to step accurately on a cluttered pathway. The same authors, inferring cats’ gaze movement from movement of the head, reported that cats rarely fixate on any point during walking along that cluttered pathway and spend 48–71% of time in constant gaze (Fowler and Sherk, 2003). Similarly, when studying how people use vision during stepping on irregularly placed targets, Patla and Vickers (2003) found that subjects use ~60% of their time on constant gaze, while fixating gaze on stepping targets only 14–16% of the time. It was also shown that obstacles suddenly appearing on the walking path can be successfully overstepped during locomotion even if gaze had never fixated on them (Marigold et al., 2007). These observations lead many researchers to believe that fixation of gaze on the walking pathway or on objects on this pathway is not required for accurate stepping on complex surfaces, and that both humans and animals gather most of the visual information necessary to guide stepping on such surfaces from optic flow. However, the results of other studies suggest that the ability of individuals to rely on optic flow for accurate stepping depends on the complexity of the walking surface. For example, when people were instructed to avoid stepping on cracks in the pavement, they fixated gaze on the walkway approximately two times per step (Land, 2006); and when walking on complex multi-surface pathways, which included slippery, tilted, and rocky patches, they spent less than 1% of the time in constant gaze, and made approximately three fixations of 100 ms or longer for every meter of traversed pathway (Marigold and Patla, 2007). Similarly, when people needed to step accurately on a series of irregularly placed stepping stones, they made a saccade to each stone in the sequence and fixated gaze on it before making a step (Hollands et al., 1995; Hollands and Marple-Horvat, 1996, 2001). Based on these data, it is tempting to conclude that during relatively simple tasks, constant gaze is the strategy of choice, while in comparatively difficult tasks, the constant gaze strategy is abandoned and gaze fixations and shifts prevail.
However, in addition to the above studies that focused on gaze patterns in rich visual environments, several studies have shown that both humans and animals move their gaze substantially even during the simplest locomotor task possible from a visuomotor coordination perspective, walking on a flat surface in darkness. For example, Collewijn (1977a, b) found that the gaze behavior of cats and rabbits moving around in a darkened box consisted almost entirely of shifts and fixations. Experimenting with rhesus and cynomolgus monkeys running on a circular platform and not facing any requirements for accurate foot placement, Solomon and Cohen (1992b) found that animals moved their gaze continuously, even in the darkness.
The considerable diversity in experimental tasks, data recording, and analysis techniques in previous studies and disagreement of their results is sufficient for one to still wonder what gaze behaviors take place during normal unobstructed locomotion when there is no specific visual task and how these behaviors are modified with the introduction of objects and stepping targets. Accurate recording of gaze in unrestrained subjects has consistently proven to be a challenge, but advancements in wearable scleral search coil-based eye-tracking technology has made high frequency and precision recordings of eye movements in freely walking subjects possible (Ogorodnikov et al., 2006). A miniature head-mounted magnetic field generator and eye coil signal decoder for recording the rotation of the eye in the orbit, coupled with a three-dimensional head movement-tracking technology have allowed us to record both eye and head movement during overground locomotion in the unrestrained cat at high temporal resolution (200 Hz) and to calculate gaze direction and gaze/ground intercept locations with high accuracy. Using this new technology we have re-examined the gaze strategy during walking. We hypothesized that cats will exhibit distinct gaze behaviors in environments where the visual complexity of the walking surface and the accuracy requirements for foot placement differ. We have studied cats because they are the closest animals to humans whose unconstrained locomotion behavior can be fully researched in the laboratory, and because they are classic subjects for studies in both visual and motor systems.
In this report, we first describe the gaze behaviors of cats walking on a flat surface in complete darkness, where visual information was neither needed nor available to guide walking. We then describe the gaze behaviors employed during locomotion over the same surface in light, where visual information was available but not necessary to guide stepping. Next, we present data on the cat gaze behavior during walking along the highly but regularly structured and familiar surface of a horizontal ladder, a task for which we have previously shown that step-by-step visual guidance is required. Finally, we demonstrate how cats use their gaze when traversing a walkway cluttered with many small stones placed in a new haphazard pattern every trial, a complex but natural everyday task for land-living creatures. Our main finding is that, in all these environments, cats predominantly use gaze shifts and fixations, not constant gaze, to aid their walking, and that, as the visual complexity of the environment and the demand on visual guidance of stepping increases, cats spend more time looking at the surface, look closer to themselves, and shift between gaze behaviors more frequently.
A brief account of this study was published in abstract form (Rivers et al., 2009).
EXPERIMENTAL PROCEDURES
Recordings were obtained from three adult cats (two females: cat 1 (3.7 kg) and cat 3 (3.0 kg) and a male, cat 2 (4.0 kg)). All experiments were conducted in accordance with NIH guidelines and with the approval of the Barrow Neurological Institute Animal Care and Use Committee.
Locomotion tasks
Positive reinforcement via food was used to adapt cats to the experimental situation and engage them in locomotor behavior (Skinner, 1938; Pryor, 1975). Cats walked in a 2.5 m long by 0.6 m wide chamber (Fig. 1A–C). A longitudinal wall divided the chamber into two corridors that cats passed sequentially and repeatedly in the counter-clockwise direction. One long outer side of the chamber was constructed of clear acrylic plastic to permit recording of cat movements (see below), while other sides were opaque. The floor in the entire chamber was covered with rubberized black material. The passage of the cat through the beginning and the end of each corridor was monitored using photo-sensors paired with infrared light-emitting diodes (LEDs). LEDs had emission wavelengths of 850–900 nm, which is outside of the visible spectral range of the cat (Guenther and Zrenner, 1993).
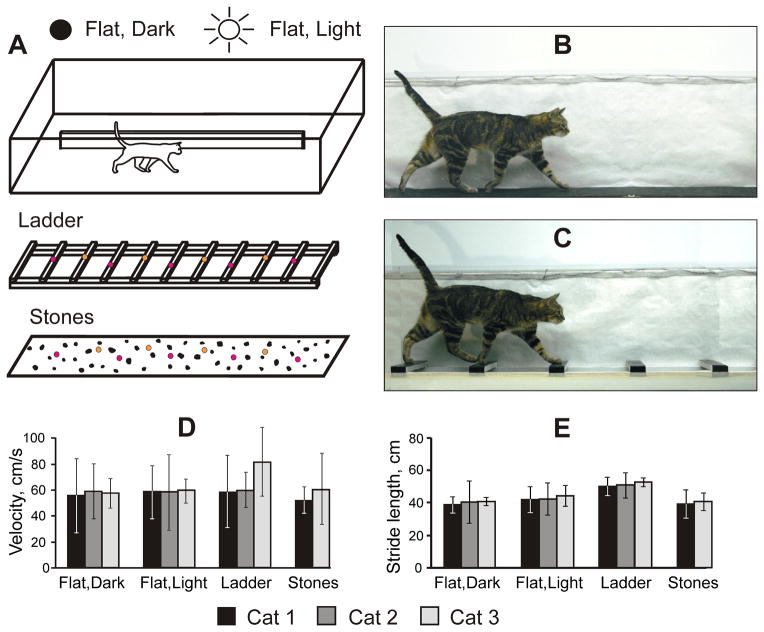
Figure 1.
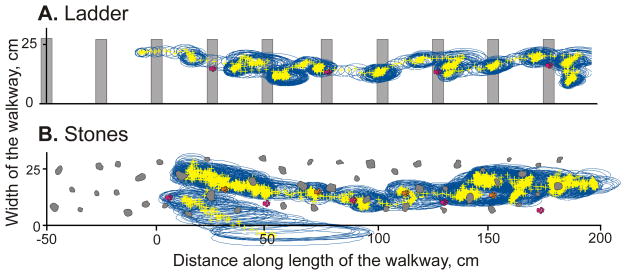
Locomotion tasks. A: A box with corridors 2.5 m long and 0.3 m wide served as an experimental chamber. Four locomotor tasks with different demands on visual guidance of stepping were studied: (1) walking on a flat surface in the dark: simple surface and no visual input; (2) walking on a flat surface in the light: simple surface and visual information is available but is not required for locomotion; (3) walking on crosspieces of a horizontal ladder: highly structured surface, but regular and familiar; and (4) walking through a pathway with many small stones (gray shapes, n=51): highly structured surface, which is irregular and new every day. Colored circles on the crosspieces of the ladders and between stones schematically show placements of cat right (pink) and left (orange) forelimb paws, not to scale. B: Photograph of cat 3 walking in the chamber on the flat surface in the light. C: Photograph of the same cat walking on crosspieces of horizontal ladder. B, C: Photographs were taken after end of experiments and removal of implants. D: Walking velocity of different cats during the four locomotor tasks. E: Stride length of cats during the four locomotor tasks. D, E: Error bars are SDs.
Cats were presented with four locomotor tasks of different visual complexity and demand on visual guidance of stepping (Fig. 1A–C):
Walking on a flat surface in complete darkness. Recordings during this task provided background information on gaze behaviors during locomotion that were not related to visual sampling of the environment. For this task, all sources of visible light inside the room were either extinguished or covered. There were no windows in the room, the computer screen for monitoring recording was placed outside the room, and the room’s doors were light-proofed. The only source of light in the room was a set of computer-controlled standard fluorescent bulbs in a ceiling fixture 2 m above the chamber’s floor that, when turned on, provided approximately 500 lux of illuminance. On randomly selected trials, these lights were turned off, triggered by the cat passing by the LED at the beginning of the clear-sided corridor. Within 17 ms, the level of illumination in the room fell to less than 0.01 lux as measured by T-10 illuminance meter (Konica Minolta, Ramsey, NJ), and judged by human observers – to less than 10−3 lux (because for 15 min dark-adapted humans the room appeared darker than a moonless night with a clear sky, which has illumination of around 10−3 lux; Vatsia et al., 1972). Lights came back on when the cat reached the LED at the end of the corridor, typically in less than 4 s. A photocell was used to record the state of illumination. Although, when fully dark-adapted, cats require only 9.92*10−8 ± 0.92*10−8 mL (approximately 3*10−7 lux) to see, almost six times less light than humans (Gunter, 1951), it takes cats at least 5 minutes to display the first signs of dark adaptation in behavioral testing and at least 30 minutes to fully dark adapt (Lamotte and Brown, 1970; Lankheet et al., 1996). Dark adaptation increases cat light sensitivity by a factor of ~10000, so the light adapted cat is only able to see if there is more than 10−3 lux. Thus, the very short period of darkness used in our experiments did not allow for much dark adaptation; and for the walking cat, the room could be considered to be completely dark. We have previously shown that, in a similar experimental chamber, cats readily walk on a flat surface in the dark (Armer et al., 2013).
Walking on the flat surface in the light. Recordings during this task provided information on gaze behaviors in an environment where visual information was available but was not necessary for successful locomotion. On trials randomly alternated with those presented in the dark, the lights stayed on and cats walked on the same surface illuminated by the overhead bulbs. Since cats could successfully walk on the flat surface in the dark, it is clear that visual information was not required for this task, however, when the lights were on, there were many potential areas of visual interest for the cats - from the walkway’s surface to items in the laboratory.
Walking along a horizontal ladder. Recordings during this task gave information on gaze behaviors during walking along a complex surface where visual information was required for successful stepping (Beloozerova and Sirota, 2003). The ladder’s surface, however, was regularly structured and, because of extensive prior training, was very familiar to the cats. For this task, during selected blocks of trials conducted under the same illumination as the flat surface “light” trials, a horizontal ladder was placed in the transparent-sided corridor, and cats walked on tops of the crosspieces. The crosspieces were flat and 5 cm wide. This width slightly exceeded the cat’s mean foot support area of ~3 cm in diameter. Crosspieces were spaced 25 cm apart. Positions of the crosspieces were recorded using infrared LEDs placed on their outer edges. The tops of the crosspieces were 7 cm above the floor of the chamber and were covered with the same rubberized black material as the floor. A detailed description of biomechanics in cats during walking on the flat surface and the horizontal ladder in our experimental setup can be found in a previous report (Beloozerova et al., 2010). In short, during walking along the ladder, as compared to flat surface, cats assume a more bent-forward posture by lowering the center of mass, rotating the neck and head down, and increasing flexion of the distal joints. They step on the crosspieces with much less spatial variability. Out of 229 tested mechanical parameters, however, the overwhelming majority do not differ between flat surface and ladder locomotion.
Walking along a stones-cluttered pathway. Recordings during this task gave information on gaze behaviors during walking on another highly complex surface where visual information was needed for successful locomotion. Unlike the ladder, however, the stones-cluttered pathway was irregularly structured and new every day. For this task, during selected blocks of trials that were conducted under the same illumination as the “light” flat surface and ladder trials, 51 small stones (1–2 cm high and 1.5–4 cm in diameter) were placed pseudo-randomly on the surface of the walkway in the transparent-sided corridor. A placement method similar to that of Sherk and Fowler (2001) was used. Four different cardboard templates, each 60 cm long, were placed in the walkway, and stones were placed on the walkway’s floor through holes in the templates. Four templates could be placed in four different locations and with four different orientations yielding a total of 6,144 possible stones layouts. The layout of the stones was different on every block of trials although the density of stones remained constant. The position of the stones was recorded via both digital photography and the Vizualeyez system (VZ-4000, Phoenix Technologies Inc., Canada). Stones occupied only ~7% of the walkway surface but considerably restricted foot placement. Calculations show that, if the cat did not modify their steps to avoid stepping on the stones, it would step on a stone, on average, once in every step (23% chance of intersection per limb, per step), or, on average, in 97% of all passages through the corridor. In fact, however, they performed considerably better than that – kicking a stone only once every three or four passages. Only cats 1 and 3 were tested during this task (Table 1).
Table 1.
| Task
| |||||
|---|---|---|---|---|---|
| Behavior | Cat # | Flat in dark | Flat in light | Ladder | Stones |
| Passages | 1 | 57 | 71 | 99 | 64 |
| 2 | 60 | 56 | 86 | 0 | |
| 3 | 106 | 96 | 112 | 151 | |
|
| |||||
| Total | 223 | 223 | 297 | 215 | |
|
| |||||
| Gaze shifts Away | 1 | 198 | 75 | 1035 | 766 |
| 2 | 328 | 490 | 730 | 0 | |
| 3 | 59 | 254 | 556 | 504 | |
|
| |||||
| Total | 585 | 819 | 2321 | 1270 | |
|
| |||||
| Gaze shifts Toward | 1 | 208 | 80 | 427 | 543 |
| 2 | 438 | 439 | 341 | 0 | |
| 3 | 117 | 188 | 298 | 384 | |
|
| |||||
| Total | 763 | 707 | 1066 | 927 | |
|
| |||||
| Fixations | 1 | 203 | 98 | 1804 | 951 |
| 2 | 339 | 185 | 1159 | 0 | |
| 3 | 161 | 150 | 834 | 1064 | |
|
| |||||
| Total | 703 | 433 | 3797 | 2015 | |
|
| |||||
| Constant gaze | 1 | 135 | 13 | 606 | 115 |
| 2 | 134 | 56 | 516 | 0 | |
| 3 | 57 | 71 | 272 | 464 | |
|
| |||||
| Total | 326 | 140 | 1394 | 579 | |
|
| |||||
| Slow gaze Away | 1 | 61 | 6 | 218 | 74 |
| 2 | 65 | 17 | 234 | 0 | |
| 3 | 33 | 18 | 95 | 207 | |
|
| |||||
| Total | 159 | 41 | 547 | 281 | |
|
| |||||
| Slow gaze Toward | 1 | 108 | 56 | 682 | 160 |
| 2 | 181 | 89 | 402 | 0 | |
| 3 | 34 | 48 | 158 | 272 | |
|
| |||||
| Total | 323 | 193 | 1242 | 432 | |
With each cat, experiments were conducted every other day for approximately three weeks. Blocks of trials (flat-dark/flat-light, ladder, or stones) consisted of 30–100 passages through the clear-sided corridor and were randomized across days. Two or three locomotor tasks were presented on each day. The passage through the return corridor was always accomplished in the light, and no obstructions were presented in that corridor. The food reward was always given in the same corner of the return corridor. Cats were trained on the same schedule for at least one month before data collection was initiated. They were accustomed to wearing a cotton jacket, LEDs and electro-mechanical sensors on their forelimb paws for recording the swing and stance phases of the stride (see below), and a light backpack with preamplifiers.
Surgical procedures
After cats were trained, surgery was performed under Isoflurane anesthesia using aseptic procedures. The left eye of the cat was implanted with a scleral search coil (Robinson, 1963). The conjunctiva was cut around the iris, and a 21 mm in diameter three-turn coil made of Teflon-coated stainless steel wire (Cooner Wire, AS-634) was positioned symmetrically around it and sewn to the sclera at three or four points. The leads of the coil were led subcutaneously along the lateral aspect of the head and connected to a connector on the head base. The resistance of the coil with leads was 15–20 Ohms.
To form the head base, the skin and fascia were retracted from the dorsal surface of the skull. At ten points around the circumference of the head, stainless steel screws were screwed into the skull and connected together with a wire; the screw heads and the wire were then inserted into a plastic cast to form the circular base (Beloozerova and Sirota, 1993; Prilutsky et al., 2005). The plane of the top of the base was made parallel to the stereotaxical horizontal plane. Later, awake cats were rigidly held by the base during calibration of the eye coil signal and head position in space before testing gaze behavior during locomotion. The base was also used to attach a miniature, portable magnetic field generator and eye coil signal decoder (Ogorodnikov et al., 2006) to record the rotation of the eye in the orbit.
Coordinate frames
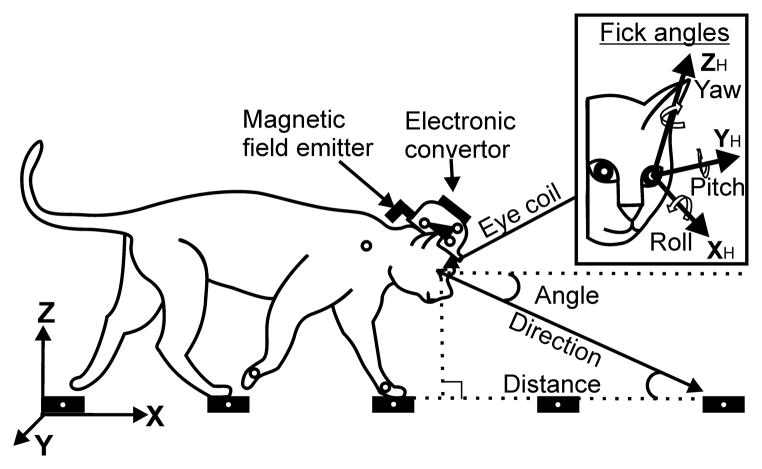
In a freely moving subject, gaze direction results from a combination of the eye rotation in the orbit and the head rotation in space (Fig. 2). Therefore, two coordinate frames were defined: a walkway-related “head-in-space” or “global” coordinate frame (X, Y, Z) and a head-related “eye-in-head” coordinate frame (XH, YH, ZH; Fig. 2 insert). The head-in-space coordinate frame originated on the left front of the transparent side of the chamber’s corridor on the level of the walkway’s surface. The X-axis was parallel to the transparent side of the chamber and floor (along the length of the chamber), the Y-axis was perpendicular to X-axis and run along the width of the chamber (positive to the right), and the Z-axis was orthogonal to the XY-plane and was directed upward (height). The eye-in-head coordinate frame originated in the center of the left eyeball, which was assumed to coincide with the center of the left orbit at all times. It was defined in accordance with the adopted Fick scheme in stereotaxical planes as follows: the XH (roll or torsion) axis was parallel to the rostro-caudal line, the YH (pitch) axis was orthogonal to the rostro-caudal line and parallel to the inter-aural line (positive rotation was upward), and the ZH (yaw) axis was orthogonal to those two and was directed upward (positive rotation was to the cat’s left). As eye roll does not affect the direction of gaze but would have required an implantation of an additional eye coil to be measured, it was neither measured nor included in the calculations.
Figure 2.
Coordinate frames. The chamber-related global (X, Y, Z; “head-in-space”) coordinate system is shown in the left bottom corner, and the head-related “Fick” coordinate system (“eye-in-head”; XH, YH, ZH; Fick, 1954) is shown in the insert. Positions of the magnetic field emitter and electronic converter on the cat’s head are indicated. The emitter antenna is placed behind the head and oriented orthogonally to the plane of the eye search coil. Approximate positions of LEDs on the head, forelimb right shoulder, right and left wrists, and cross-pieces of the ladder are shown by small circles. Gaze angle, direction, and distance to intersect with the walking surface are schematically shown for the XZ plane.
Eye movement recording
Movement of the left eye in the orbit was recorded using a modified scleral coil technique (Ogorodnikov et al., 2006). A high-frequency magnetic field generator (10 and 11 MHz) was positioned on the head base. The generator’s emitting antenna was placed behind the head of the cat and oriented orthogonally to the eye coil (Fig. 2). The signal from the coil was decoded and pre-amplified using an electronic module positioned on the head base. The voltage output was sampled at frequency of 200 Hz and recorded using a data acquisition system Power1401/Spike2 (Cambridge Electronic Design, Cambridge, UK). Pitch and yaw eye movement components according to the adopted Fick scheme (Fick, 1854; Haslwanter, 1995) were recorded only (Fig. 2, insert).
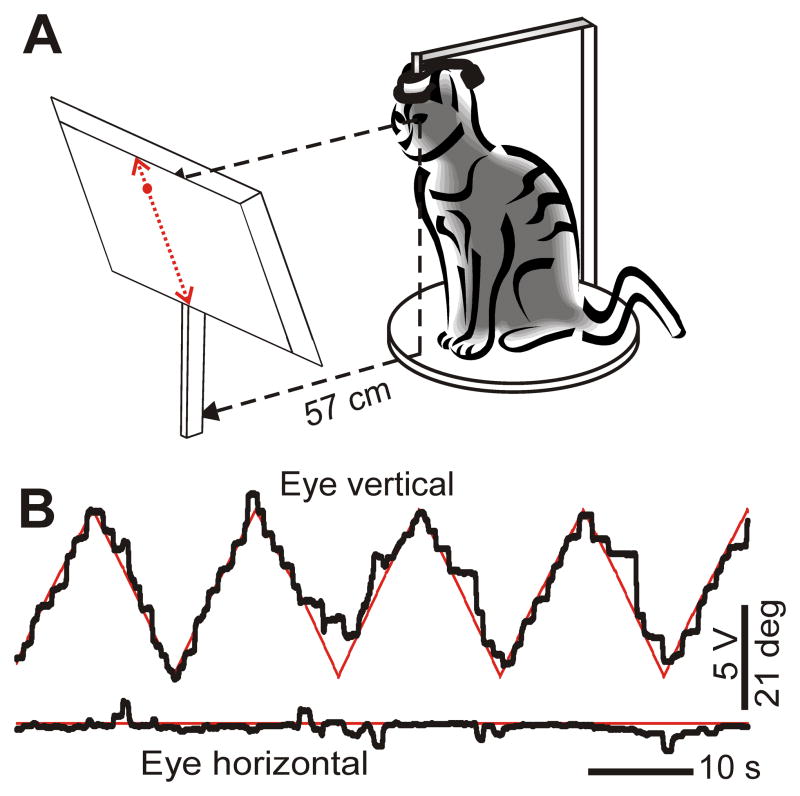
For calibration of the eye movement in relation to the head, the cat was seated in a comfortable position with its head fixed by the head base to an external frame so that the natural position of the head in the sitting cat was approximated (rotated 17 deg nose down). A 20″ computer screen was placed 57 cm in front of the cat’s eyes with the screen perpendicular to the plane of the head base (Fig. 3A). At this distance, 1 degree of angular eye movement results in 1 cm of gaze displacement on the screen. Position of the eyeball when the cat was looking at the center of the screen was taken as the zero degree coordinate along both the YH and Z H axes (Fig. 2, insert). From this central position, the screen allowed for testing the eye rotation in approximately ±20 degrees range. All eye movements during locomotion were within these limits (e.g., Fig. 4A, B). A calibration technique adopted from Kang and Malpeli (2003) was used. First, a rough estimate of the correspondence between the eye coil voltage output and position of gaze on the screen was made by presenting a toy to the cat at different locations along the screen and recording the signal from the coil when the cat appeared to look at the toy. Next, a 2.5 mm diameter round target was presented on the screen. It moved horizontally or vertically along a standard grid at a speed of 3–7 degrees/s. Using positive reinforcement via food, the cat was trained to follow the moving target with its gaze. For holding the gaze for 1s within ±2.5 degrees of the target, which would project its image onto the region of cat’s retina that is specialized for high visual resolution, the area centralis (e.g. Rapaport and Stone, 1984), the cat was rewarded with pasted chicken delivered to its mouth by a computer-controlled pneumatic pump.
Figure 3.
Calibration of eye movement. A: The cat was seated in a comfortable position with its head fixed by the head base to an external frame so that the cat’s eyes were 57 cm away from a 20″ computer screen. A 2.5 cm in diameter round target was presented on the screen and moved with a speed of 3–7 deg/s either horizontally or vertically along a standard grid. Using positive reinforcement (food), cats were trained to follow the target with their gaze. B: A sample record of eye movement of the cat when the cat was visually tracking the target in the vertical (pitch) plane. When calibration coefficients (gain and offset, see text) were taken into account, the eye movement (black W-shaped trace) closely followed, albeit in small steps, the moving visual target (red W-shaped trace). At the same time, in the horizontal (yaw) plane the eye (black lower trace) stayed quite precisely together with the target (red lower trace).
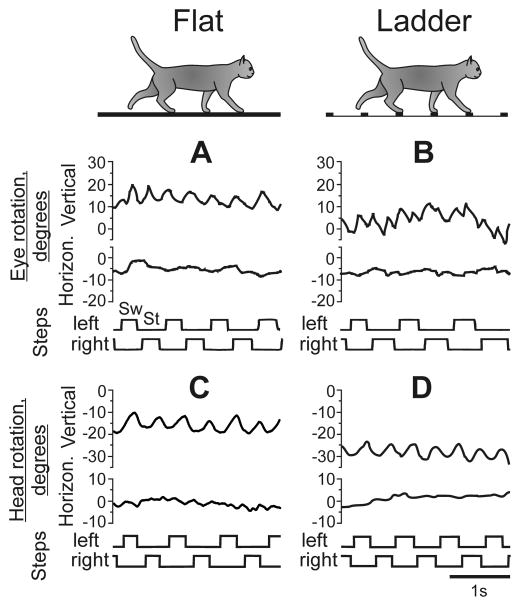
Figure 4.
Eye and head movements during locomotion. A, B: Vertical (pitch) and horizontal (yaw) components of eye rotation in the orbit are shown along with the right and left forelimb swing (deflection up, Sw) and stance (deflection down, St) phases of the stride as a cat walks along the flat surface in the light (A) and on the horizontal ladder (B). During flat surface locomotion the cat makes many vertical eye movements while horizontal position of the eye remains rather stable. During ladder locomotion vertical eye movements are even more numerous and less regular, while horizontal movements are still very small. C, D: Vertical and horizontal components of head rotation in the walking chamber-related global coordinate system (X, Y, Z in Fig. 2) are shown as the cat walks along the flat surface in the light (C) and on the horizontal ladder (D). During both flat and ladder locomotion, the rotation of the head in the vertical plane is significantly larger than in the horizontal one.
The gain and offset calibration adjustments of the eye coil signal were performed offline by matching the horizontal and vertical positions of the cat’s eye to the target’s position on the screen when the cat was following the target. A typical example of a calibrated recording of eye movement is shown in Figure 3B. Within the studied range of the eye movement, there was a linear correspondence between voltage output from the eye coil recording system and degrees of eye rotation in the orbit. Since the emitting antenna was placed behind the head in an orientation that was very close to perpendicular to the eye search coil, there were no “cross-talk” between vertical and horizontal channels in eye coil recordings (Fig. 3B). Eye rotation calibration was performed immediately before or after each locomotion test. We found that the voltage-to-degree recalculation coefficients (gain values) were stable over the course of weeks. The accuracy of eye rotation recording was about 1.0 degree which is less than calibration error. The sensitivity of the eye tracker was 0.1 degrees.
To validate our calibration of the eye movement, during a terminal experiment performed under deep anesthesia, we mechanically rotated the cat’s left eye while recording voltage output from the scleral search coil. The voltage-to-degree recalculation coefficients obtained during this experiment closely matched those determined using the behavioral method.
Head movement recording
During locomotion, the position and angular movement of the head in the chamber (in “global” X, Y, Z coordinates, Fig. 2) was recorded using the computerized, active-marker three-dimensional real-time motion capture and analysis system Visualeyez (VZ-4000, Phoenix Technologies Inc., Canada). Three wide-angle, six-chip infrared LEDs with wavelengths of 755–785 nm, which are not visible to cats when lit (Guenther and Zrenner, 1993), were permanently placed on the head implant in a non-collinear fashion 3–8 cm apart (Fig. 2). Distances between LEDs and the center of the left eye orbit were obtained from X-ray images. The recording cameras of the Visualeyez apparatus were positioned approximately 2.5 m from the transparent side of the walking chamber, and calibration was performed according to manufacturer specifications. Before each locomotion test, the cat was seated next to the chamber in a comfortable position and its head was fixed to an external frame with zero degrees in yaw, zero degrees in roll, and −17 degrees in pitch (nose down) angles in the global (X, Y, Z; Fig. 2) reference frame. To calculate both the position of the center of the left eye orbit and the rotation angles of the head (roll, pitch, and yaw) in global coordinates, we used functions provided by the VZ Analyzer software package. To calculate the position of the orbit in the global coordinate system, the three LEDs on the head were used to create an ‘object’ (black triangle on the head in Fig. 2). The ‘object’ function finds coordinates of the center of mass of a triangle formed by non-collinear LEDs, and these coordinates can be then transposed to the known position of the center of the orbit. To calculate the head rotation values, a ‘rigid body’ was created based on the LEDs. According to manufacturer’s specifications, the VZ system error for recording position of an LED was 0.5 mm.
Recording during locomotion
After calibration was completed, the cat was released into the walking chamber, where it continuously walked through corridors, stopping only briefly after each round in one of the corners for a food reward. Signals from the eye coil decoder on the head base (yaw and pitch) were led to a connector in cat’s backpack and then through a cable to an analog-to-digital converter board, and recorded using Power1401/Spike2 system at a frequency of 200 Hz. Figure 4A, B shows a representative record of the eye movement of the cat walking on the flat surface (A) and along the ladder (B) together with the swing and stance phases of the right and left forelimb, which were monitored by measuring the electrical resistance between electromechanical sensors on the foot and the electro-conductive rubberized floor cover (Beloozerova and Sirota, 1993; Beloozerova et al., 2010). It can be seen that during both locomotor tasks, the cat stepping pattern was very regular, and horizontal (yaw) eye movements were small and varied little between tasks. In contrast, vertical (pitch) eye movements were quite substantial and differed noticeably between tasks: while on the flat surface, eye pitch pattern was smooth and quasi-sinusoidal; on the ladder, it had many sharp angles and was less regular. Head movements were quite regular during both locomotor tasks but were also significantly larger in the vertical than horizontal plane (Fig. 4C, D). It is because of the much greater magnitude of vertical eye and head movements as compared to horizontal ones, and the clear dependence of the vertical eye movement on locomotor task, that we have focused this study on gaze behaviors in the vertical plane.
In addition to electromechanical sensors on the feet for recording swing and stance phases of the step cycle, an LED similar to those on the head base was placed on each forelimb wrist and the right shoulder to record foot and shoulder position in three-dimensional space (Fig. 2). Signals from all LEDs were sampled at a frequency of 200 Hz and saved to a hard disk. Synchronization between the VZ-4000 and Power1401/Spike2 systems was achieved through a linked electrical channel.
We refer to the full movement cycle of one limb (e.g., beginning of swing to beginning of next swing of the same limb) as a “step cycle” or “stride” (and use them interchangeably); and to one half of such cycle as a “step.”
Calculation of gaze direction and gaze/surface intercept point
In walking subjects, gaze direction results from a combination of the eye rotation in the head and the head rotation in space (Fig. 2). Figure 5 shows representative eye pitch, head pitch, and combined eye-and-head (that is, gaze) pitch angular movements during different locomotor tasks. On the flat surface in the dark, eye pitch movement was often sinusoidal and in opposite phase with the head pitch movement, so the cat gaze angle was sometimes stable (Fig. 5A). On the same surface in the light, the gaze pitch angle often changed rapidly, and an episode of a rapid gaze shift is encircled in Figure 5B. On the ladder and stones-cluttered pathway, the gaze pitch movement had a more complex pattern than on the flat surface (Fig. 5C, D).
Figure 5.
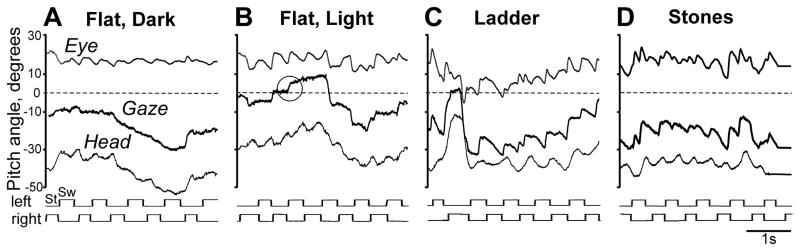
Representative vertical (pitch) gaze patterns during different locomotor tasks. The gaze pitch angle is the sum of the eye-in-head and head-in-space pitch angles. A gaze pitch angle of zero degrees indicates that the cat is looking horizontally. The stride phases for both forelimbs are shown below the graphs. In B, a circle is highlighting a period when gaze pitch rapidly changes.
To determine the gaze intersection with the walkway’s surface, we used a rotation matrix algorithm (see Appendix for details and an example calculation). First, we calculated the direction vector of the eye in the head (line of sight) using the eye rotation values in the head coordinate system. Next, we rotated this vector by the head rotation values within the global coordinate system to obtain the spatial gaze direction vector. Then, using head coordinates in space, we determined the intercept point of the gaze direction vector with the walking surface. Calculations were carried out using a custom MatLab program (Mathworks, Lowell, MA). Figure 6 shows an example of the gaze intersect position along the walkway’s surface during 1s of walking. Since cats have a retinal focus area (area centralis) of approximately 5 degrees (Rapaport and Stone, 1984), a ±2.5 degrees window around the gaze vector and the surface area, with which that “ray of clear sight” intersected, was calculated.
Figure 6.
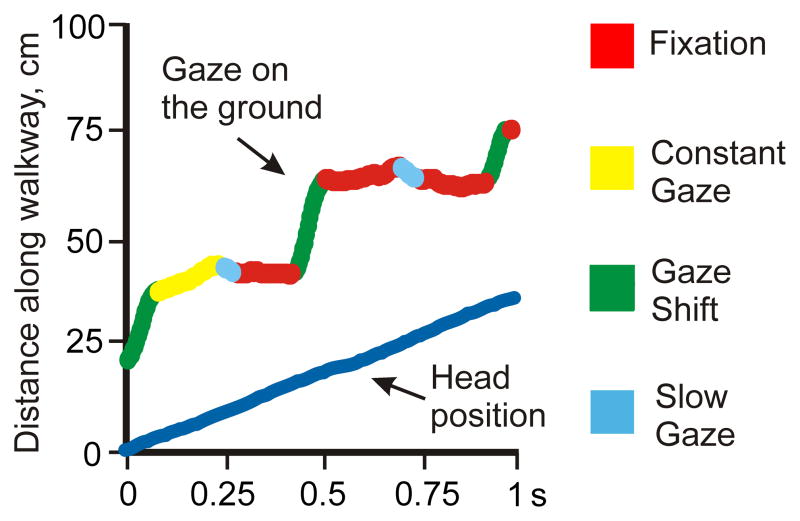
The four gaze behaviors. An example of sequential positions of the gaze along the walkway is shown, and the four gaze behaviors indicated with different colors. Fixation (red) occurs when the gaze is not moving along the ground. Constant gaze (yellow) occurs when the gaze and the cat (“eye position” blue line) are moving at approximately same speed. Gaze shift (green) occurs when the gaze is moving along the walkway much faster or much slower than the cat is. Slow gaze (light blue) encompasses the remainder of the gaze behavior.
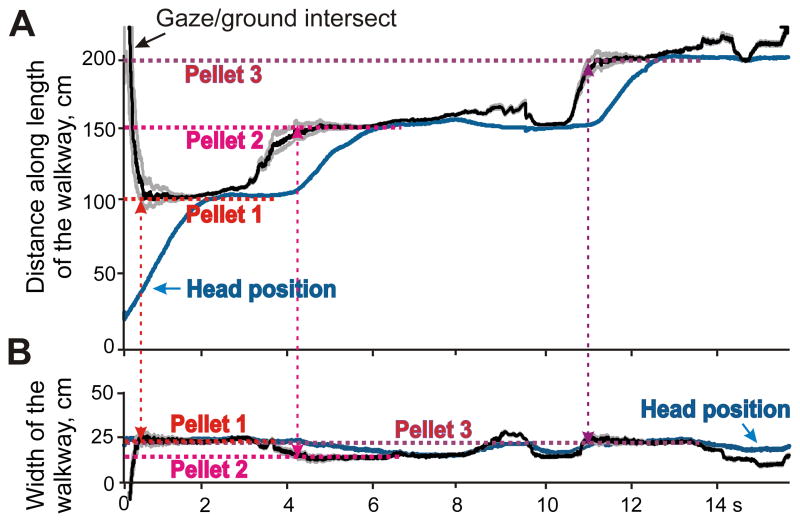
We noted that cats often fixate gaze on their food while approaching it, and took advantage of this to confirm our calculations. We placed 3×3×5 mm food pellets in known positions along the walkway and had cats collect them while we were recording their gaze (Fig. 7, Video 1). As cats approached a food pellet, they almost continuously looked at it, and a correct gaze calculation resulted in the gaze/surface intersect point (black line) coinciding with the pellet position (red dashed lines).
Figure 7.
Validation of calculation of the gaze/surface intersect location using a behavioral “food pellet” test. Black lines show sequential intersections of the gaze with the surface of the walkway along the X (A) and Y (B) axes when the cat walks on the flat surface where three food pellets were placed in different locations. Grey lines around the black one outline the area that projected to the area centralis of the retina. Positions of the food pellets are shown by dashed red, pink, and purple lines. An overlap of gaze and pellet lines along both axes indicates gaze fixation on the pellet, and vertical dashed arrows align beginnings of gaze fixations on each of the three pellets. The blue line shows the position of the cat’s eye. See also supplemental Video 1; it shows the movement of cat’s gaze along the surface and the movement of the head (the center of the left eye) in the corridor during a trial, in which a single food pellet was placed on the floor of the walkway.
In this paper we will only describe gaze behaviors as they were observed in the pitch plane, and only for the periods of time when gaze intersected the walking surface.
Classification of gaze behaviors
For each passage down the test corridor, the entire time when the gaze intersected the walking surface was analyzed. To classify gaze behaviors, we first used a custom MatLab program, a modified version of an algorithm for detection of microsaccades (Engbert and Mergenthaler, 2006), to determine all inflection points on the line of sequential gaze/surface intersect positions plotted against time, between which the line was linear for at least three data points (15 ms). An inflection point was recognized when the change in gaze velocity (acceleration) along the surface exceeded ±5 cm/s2 for more than 30 ms. For every period between two neighboring inflection points, the velocity of the gaze moving along the surface (X, Y plane) was divided by the velocity of the cat walking during that time. Based on the gaze/cat velocity proportion, we recognized the following four gaze behaviors; they are color-coded in Figure 6:
Fixation: the gaze was not moving along the surface, and the gaze/cat velocity proportion was 0±0.5.
Constant gaze: the gaze on the surface and the cat were moving at approximately same speed, and the gaze/cat velocity proportion was between 0.5 and 1.5.
Gaze shift: the gaze was moving along the surface much faster or much slower than the cat, with the gaze/cat velocity proportion greater than ±2.
Slow gaze: the gaze was moving along the surface and relative to the cat but not fast/slow enough to be termed “gaze shift” under the definition above and the gaze/cat velocity proportion was between 1.5 and 2, or between −0.5 and −2.
If neighboring gaze behaviors were classified similarly, they were combined. Then for each gaze behavior episode we determined: (i) the distance of the gaze/surface intersection point from the cat at the beginning of the episode, expressed in centimeters, milliseconds, and number of strides that cat needed to reach it, (ii) the duration, and when applicable, (iii) the amplitude, the distance the gaze traveled along the walkway’s surface. We also calculated the percentage of time the cat spent exhibiting each type of gaze behavior during each locomotor task. We then characterized and compared gaze behaviors across different locomotor tasks.
Statistics
To compare parameters of strides and each of the gaze behaviors between locomotor tasks a generalized linear model (GLM) was used. When the treatment effects were significantly different, post-hoc least square difference contrasts were conducted (SPSS 20). Unless noted otherwise, for all mean values, the standard deviation (SD) is given. When data were categorical, Z tests for proportions were performed.
RESULTS
The database used for this study is summarized in Table 1. Between 56 and 151 passages by each cat through the test corridor during each locomotor task were analyzed (cat 2 was not recorded on the stones-cluttered walkway). These passages yielded 59–1035 gaze shifts, 161–1804 fixations, 13–606 constant gaze episodes, and 6–682 slow gaze episodes per cat per task.
Walking on a flat surface in the dark
The cats’ first locomotor task was to walk along a flat surface in complete darkness. During this task vision could neither be used nor was needed for a successful locomotion (Armer et al., 2013). Cats were accustomed to brief periods of darkness while walking around the chamber, and, when lights were turned off, they always continued moving without interruption. Cats walked with similar velocities of 55–57 cm/s and mean stride lengths of 39–41 cm (Fig. 1D, E).
During walking in the dark, cats directed their gaze at the walkway’s surface 34–48% of the time (Fig. 8A–C, left bars). Their gaze would either fixate on a spot (fixation), travel at the same velocity as the cat (constant gaze), or move rapidly (gaze shift) or slowly (slow gaze) along the walkway and in relation to the cat. A representative record of the gaze of one cat (cat 1) is shown in Figure 9A. At time zero, when the light was turned off, the cat was looking ahead toward the end of the walkway. After about 1.3 s, however, the cat turned its gaze down toward the invisible surface of the walkway about 125 cm ahead. The line of gaze intersection with the surface shows periods of gaze shifts, fixations, constant gaze episodes, and slow gaze episodes in different colors as defined in Figure 6. More than 30% of the time the cat spent shifting its gaze along the invisible surface (green). It split the rest approximately equally between gaze fixations (red), constant gaze (yellow), and slow gaze (blue). This example shows that, even during locomotion in complete darkness with no use of vision, cats very often shifted their gaze from one location on the surface to another and quite often fixated gaze on some invisible points, while producing only infrequent and rather short-duration constant gaze episodes. Overall, characteristics of the four gaze behaviors were as follows.
Figure 8.
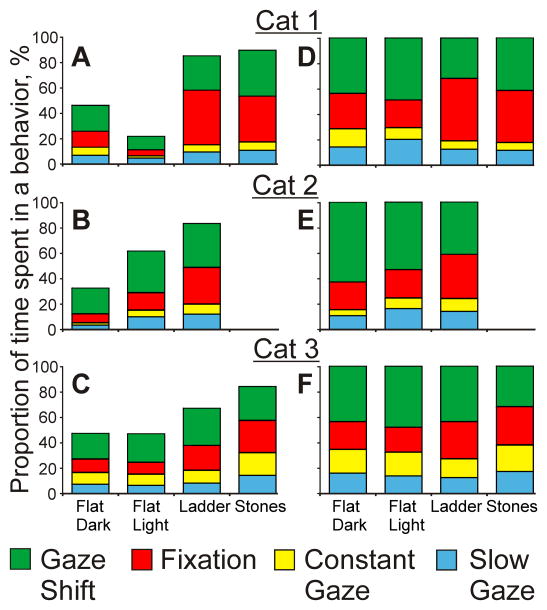
Proportion of time a gaze behavior of a cat took within cat’s total walking time (A–C) and within the time when cat’s gaze intersected the walking surface (D–F) during each locomotor task. In A–C, the total height of the multicolor bar shows the percentage of time the cats’ gaze intercepted the walking surface. Gaze behaviors are color-coded as indicated at the bottom of the figure. Cat 2 was not recorded during the stony pathway task.
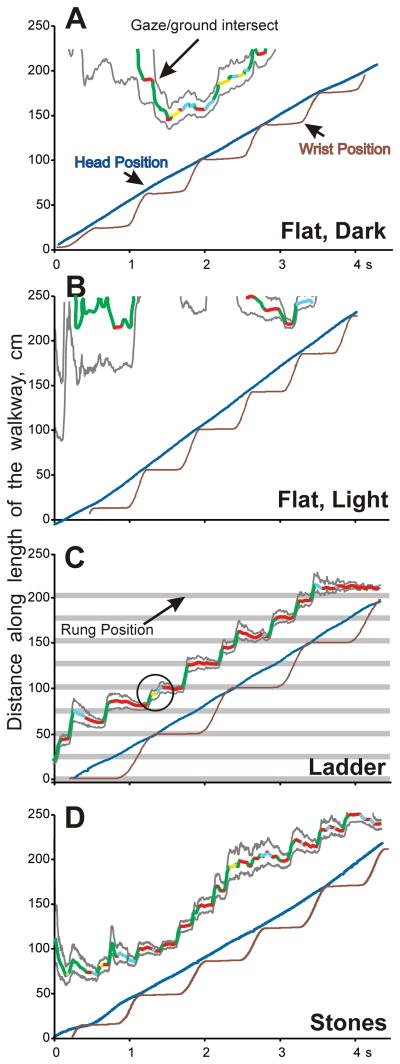
Figure 9.
Representative examples of gaze patterns along the length of the walkway (X-axis) during the four locomotor tasks. All records were obtained from cat 1. Sequential positions of the gaze along the walkway (gaze/surface intersect) are shown by the multicolored line, where different colors indicate different gaze behaviors (green shows gaze shifts, red highlights periods of gaze fixation, yellow shows constant gaze episodes, and light blue indicates periods of slow gaze; see Methods and Fig. 6 for definition and classification of gaze behaviors). Gray lines around the multicolor line outline the area that projected to the retina’s area centralis. The dark blue line shows position of the eye, and brown line indicates position of the wrist of the right forelimb. For the ladder task, the shaded areas with pink edges indicate positions of crosspieces. Gaze and wrist sequential positions form horizontal lines when the gaze and wrist are not moving forward: that means fixation for the gaze and stance phase of the stride cycle for the forelimb. In C, a period of constant gaze is encircled.
Gaze Shifts
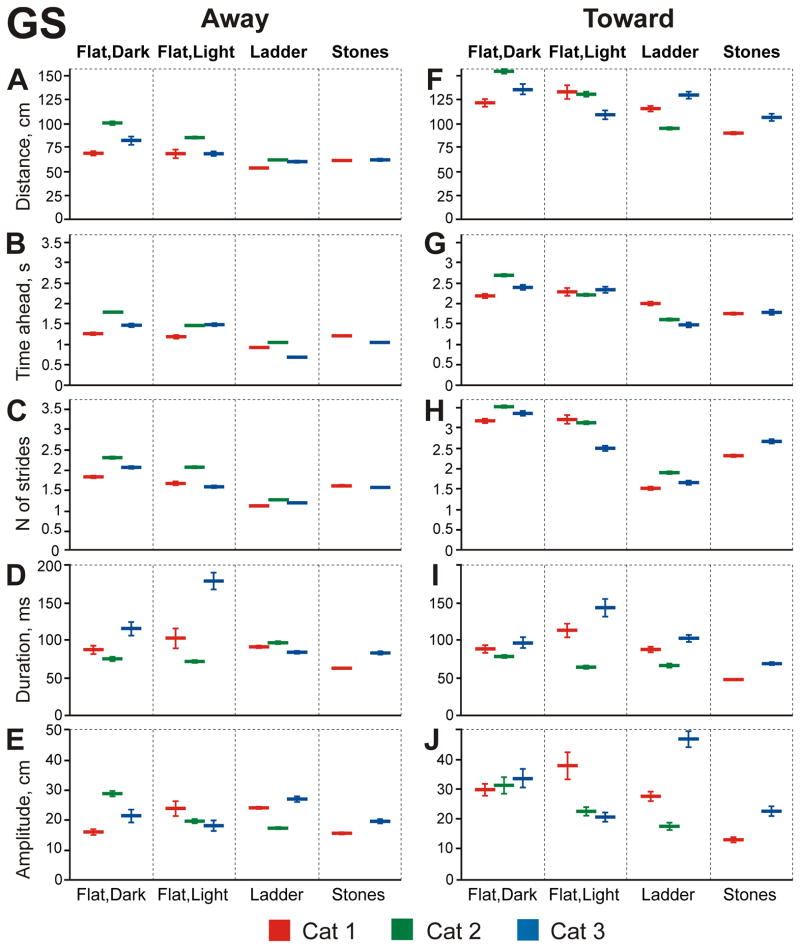
These rapid changes of gaze position on the walking surface were the dominant gaze behaviors observed in cats walking in the dark (Z test, p<0.0001). In all cats they comprised ~20% of the total walking time (Fig. 8A–C) and 43–62% of the time when cats’ gaze was directed at the surface (Fig. 8D–F, left bars). Gaze shifts could either take the gaze/surface intersect point further away from the cat (“away” shifts) or bring it closer (“toward” shifts).
Away gaze shifts accounted for 38–49% of all shifts. They typically started 71–101 cm away from the cat (Fig. 11A), and it took cats 1.3–1.8 s and 1.8–2.3 strides to arrive to the away gaze shift start point (Fig. 11B, C). Away shifts lasted 76–116 ms, and their amplitudes were between 16 and 29 cm (Fig. 11D, E), so they ended 104±43 cm away from the cat. Toward gaze shifts occurred slightly more often than away ones, representing 57–62% of all gaze shifts (Z test, p<0.001). They started at much greater distances than away ones, 122–155 cm away from the cat (GLM, p=0.026; Fig. 11F). Consequently, it took cats longer, 2.2–2.7s and 3.2–3.5 strides, to reach the toward gaze shift starting point (Fig. 11G, H). End points of these shifts were also further away from the cat than those of away shifts (109±43 cm; GLM, p =0.03).
Figure 11.
Characteristics of gaze shifts (GS) during the four locomotor tasks. A–E: Gaze shifts moving gaze/surface intersect point further away from the cat. F–J: Gaze shifts moving gaze/surface intersect point toward the cat. Data obtained from individual cats are color-coded as indicated at the bottom of the figure; data are shown as mean ± SEM. A, F: Distance ahead of the cat, at which the gaze shifts were initiated. B, G: Time needed for the cat to reach the gaze shift initiation point. C, H: Number of steps required from the cat to reach the gaze shift initiation point; calculated by dividing the mean needed time by the mean step duration. D, I: Duration of the gaze shift. E, J: Amplitude of a gaze shift showing how far along the surface the gaze moved during the shift.
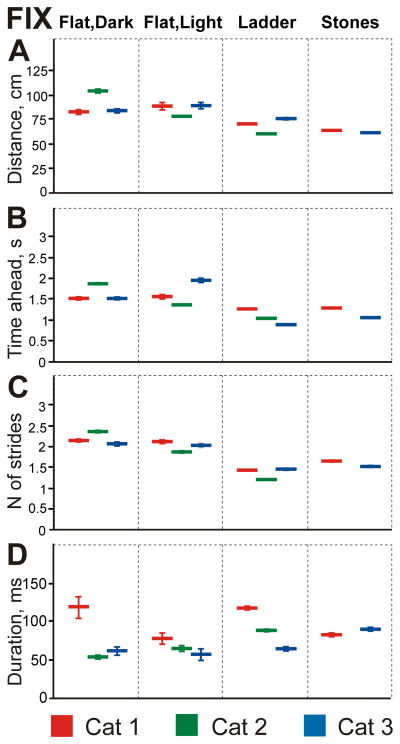
Fixations occupied 7–13% of the total walking time (Fig. 8A–C) or 22–28% of the time when cats were looking at the walkway’s surface (Fig. 8D–F). They were made at a rate of 1.5–5.6 per passage. Cats fixated gaze on points initially located 83–105 cm away (Fig. 12A), which took 1.5–1.8 s and 2–2.4 strides to reach (Fig. 12B, C). Fixation durations were similar in cats 2 and 3 (54 and 61 ms, respectively) but were longer in cat 1 (118 ms; Fig. 12D).
Figure 12.
Characteristics of gaze fixations (FIX) during the four locomotor tasks. Designations are as in Fig. 11.
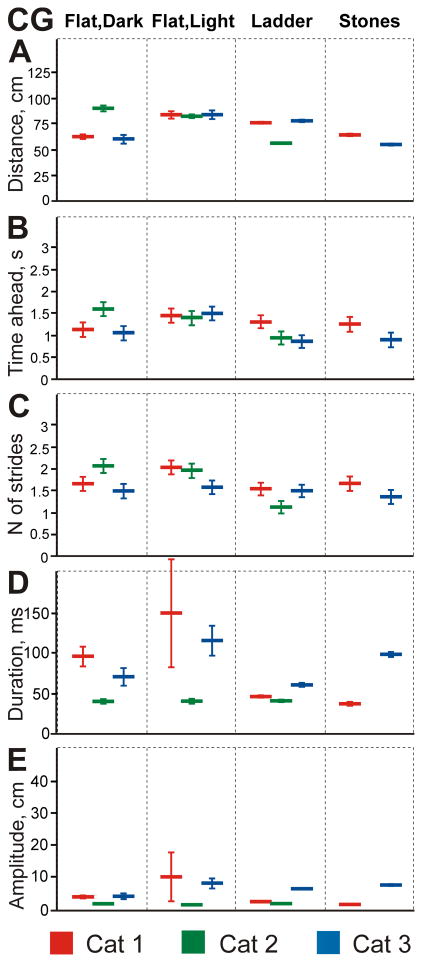
Constant gaze was only seen infrequently, comprising 2–9% of the total walking time (Fig. 8A–C) or 5–19% of the time when cats looked at the surface (Fig. 8D–F). In cats 1 and 3, periods of constant gaze occurred when they looked at the walkway at a similar distance ahead (60 and 62 cm, respectively), while cat 2 looked further away (90 cm; Fig. 13A). It took cats 1.1–1.6 s and 1.5–2.0 strides to reach the constant gaze start point (Fig. 13B, C). Constant gaze episodes were very short in all cats (Fig. 13D), so cats only traveled 2.1–4.2 cm along the walkway during an average episode (Fig. 13E).
Figure 13.
Characteristics of constant gaze (CG) during the four locomotor tasks. Designations are as in Fig. 11.
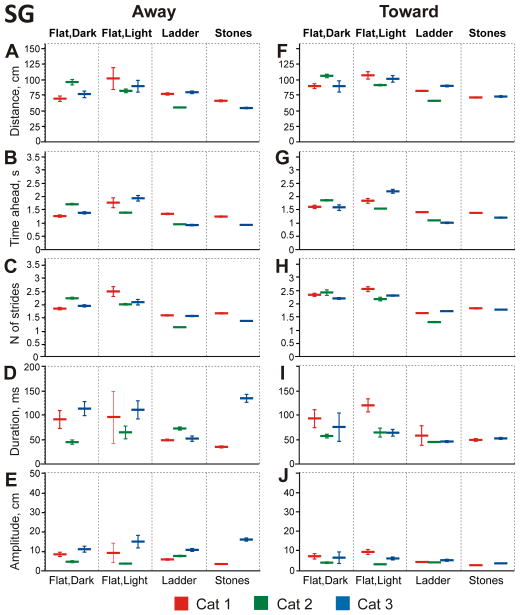
Slow gaze occupied 4–8% of total walking time (Fig. 8A–C) or 11–16% of time when cats’ gaze was directed at the surface (Fig. 8D–F). Cats more often moved gaze “slowly” toward them than away (in 60–74% of cases; Z test, p<0.0001). At the beginning of toward slow gaze episode cats typically looked 89–106 cm ahead; and it took them 1.6–1.9 s and 2.2–2.4 strides to reach this point (Fig. 14F–H). Toward slow gaze episodes lasted 58–93 ms, and their amplitudes were 4.1–7.2 cm (Fig. 14I, J).
Figure 14.
Characteristics of slow gaze (SG) episodes during the four locomotor tasks. A–E: Slow gaze episodes, during which the gaze/surface intersect point moved further away from the cat. F–J: Slow gaze episodes during which the gaze/surface intersect point moved toward to the cat. Designations are as in Fig. 11.
Away slow gaze events started slightly closer to the cat (69–96 cm; GLM, p<0.001; Fig. 14A, B, C). In cats 1 and 3 they lasted 92 and 113 ms, respectively, but were shorter in cat 2 (46 ms; Fig. 14D). Accordingly, the amplitude of away slow gaze events was larger in these cats (8.3 and 11.0 cm, respectively) than in cat 2 (4.6 cm; Fig. 14E).
Walking on the flat surface in the light
During walking on the flat surface in the light visual information was available to cats but was not required for successful locomotion. Cats walked at identical speeds (58–59 cm/s), which were similar to their walking paces in the dark (Fig. 1D). Stride lengths were also rather similar among cats, 42 – 44 cm, but in cats 1 and 3 they were larger than during walking in the darkness by 3.3 cm (Fig. 1E). A comparative description of forelimb kinematics in cats during walking on the flat surface in the light and darkness in our experimental setup can be found in our recent report (Armer et al., 2013). In short, movements of the paw were identical in all parameters tested. Position of the scapula was 2.3±1.3 mm (mean ± SEM) higher throughout most of the step cycle during walking in the light compared to the darkness, while vertical velocities of the scapula were similar.
Cats differed in the amount of time which they spent looking at the walkway surface: while cat 1 only looked at it 22% of the time, significantly less than during walking in the dark (Z test, p<0.0001), two other cats looked considerably more (62% and 47%, respectively; Z test, p<0.0001), with cat 2 looking at the surface more in the light than in the dark (Fig. 8A–C). A representative record of the gaze of one cat (cat 1) during walking on the flat surface in the light is shown in Figure 9B. The cat only looked at the surface of the walkway at the beginning of the passage and at the end, spending only about half of its walking time on this. The only gaze behaviors that it expressed were gaze shifts interspaced with fixations, and a short slow gaze episode at the end.
Overall, during walking on the flat surface in the light, when visual information was available but not required for locomotion, cats’ gaze behavior was in many respects similar to that seen in the darkness. Namely, during the occasions that cats looked at the walkway surface, they often shifted their gaze and occasionally fixated on some points, while constant gaze episodes were similarly infrequent and short. Unlike in the dark, however, cats made slightly more away gaze shifts than towards ones, and all gaze shifts were made between points nearer to the cat. Detailed characteristics of the four gaze behaviors were as follows.
Gaze shifts
As during walking in the dark, gaze shifts were the most frequent gaze behaviors observed when cats walked on the flat surface in the light (Z test, p<0.0001). In the light, however, cats varied more in the amount of time they spent making gaze shifts, with this time ranging between 11 and 33% of total walking time (Fig. 8A–C). Whereas cats made slightly more toward than away gaze shifts during walking in the dark, they made approximately equal numbers of gaze shifts in the two directions during walking in the light (47–52%). Two of three cats started away gaze shifts closer to them than in the dark, at 69–86 cm away (GLM, p<0.0001; Fig. 11A). Across all subjects, it took cats 1.2–1.5 s and 1.6–2.1 strides to reach the away gaze shift start point (Fig. 11B, C). Durations of away gaze shifts varied greatly between cats in the range of 72–180 ms, while amplitudes were much more similar ranging from 18 to 24 cm (Fig. 11D, E).
The same two cats that were starting away gaze shifts closer to them also started toward gaze shifts closer, while the other cat began them slightly further away than in the dark (Fig. 11F). For all cats, the starting point of toward gaze shifts was much further away than the typical starting point of an away gaze shift (GLM, p<0.001; compare to Fig. 11A), and it took cats 2.2–2.4 s and 2.5–3.5 strides to reach it (Fig. 11G, H). Toward and away gaze shifts ended at similar distances from the cat: 98±38 and 95±41 cm away, respectively (GLM, p=0.12). Overall, toward gaze shifts on the flat surface in the light had the longest durations among all tasks, ranging from 65 to 144 ms (GLM, p<0.0001 Fig. 11I); their amplitudes, however, varied between 21 and 38 cm, and in two cats were smaller than in the dark (Fig. 11J).
Fixation
During walking on the flat surface in the light cats fixated on the surface 5–14% of the total walking time (Fig. 8A–C) or 20–22% of the time spent looking toward the ground (Fig. 8D–F). This was similar to their behavior during walking in the dark (Z test, p=0.2). Also similar to walking in the dark, they were made at a rate of 1.4–3.3 per passage. Cats fixated on points located 79–90 cm away, which for two of them was similar to fixation distances in the dark, while one cat fixated on closer points (Fig. 12A). Among cats, it took 1.3–1.9 s and 1.9–2.1 strides to reach the fixation point (Fig. 12B, C). Fixations lasted 55–78 ms (Fig. 12D).
Constant gaze was seen as rarely in cats walking on the flat surface in the light as it was in the dark. It occupied only 2–9% of walking time or 8–19% of the time when cats looked at the surface (Fig. 8). Cat 1 only had 15 constant gaze episodes during 71 passages. During these episodes, cats looked 69–83 cm ahead (Fig. 13A), slightly further away than in the dark (GLM, p=0.007), and it took cats 1.4–1.5 s and 1.6–2.0 strides to reach the constant gaze start point (Fig. 13B, C). Duration of constant gaze episodes varied among cats, ranging greatly from 42 to 240 ms (Fig. 13D). Distances that cats traveled during constant gaze episodes, as in the dark, were quite small: 2–10 cm (Fig. 13E).
Slow gaze
In the light, cats continued to spend little time in slow gaze, using only 4–10% of their total walking time or 14–21% of the time when they looked at the surface (Fig. 8). Slow gaze events started at distances 81–106 cm away (Fig. 14A, F) and, unlike in the dark, most of the time (63–91%) were directed away from the cat (Z test, p<0.0001). It took cats 1.3–2.2 s and 1.9–2.5 strides to reach the slow gaze start point (Fig. 14B, C, G, H). Slow gaze episodes lasted 63–120 ms, and their amplitudes were 3–15 cm (Fig. 14D, E, I, J).
Walking along the horizontal ladder
The horizontal ladder presented cats with a highly uneven but regularly structured surface, which, despite being very familiar to the cats, required that they see it for successful locomotion (Beloozerova and Sirota, 2003). On the ladder, cats 1 and 2 walked at a similar pace of 58–59 cm/s, while cat 3 walked faster (81 cm/s; Fig. 1D). Stride lengths were determined by spacing of the ladder’s crosspieces and were 50–53 cm (Fig. 1E). A detailed description of biomechanics in cats during walking on the flat surface and the horizontal ladder in our experimental setup can be found in our previous report (Beloozerova et al., 2010).
Unlike during walking on the flat surface in either darkness or light, cats spent the vast majority of their time looking at the walking surface (the plane along the top of the ladder crosspieces), significantly more than in those two conditions (67–85%; Z test, p<0.0001; Fig. 8A–C). A representative record of the gaze of one cat (cat 1) during walking along the ladder is shown in Figure 9C. During this passage, the cat was looking at the ladder’s surface the entire time. As it walked, it fixated its gaze on or near a crosspiece 1–1.5 strides or 2–3 steps away and then shifted it to the next crosspiece. There was only one very short period of constant gaze. A birds-eye view of this passage is shown in Figure 10A, and supplemental Video 2 shows three-dimensional positions of the gaze, left eye, and right forelimb wrist in real time and at a slower rate. Overall, cats’ gaze behavior during walking on the ladder predominantly followed a cycle: forward gaze shift followed by a fixation, than another forward gaze shift and another fixation.
Figure 10.
Representative examples of gaze intersect with walking surface during ladder (A) and stones (B) locomotor tasks, a “bird’s-eye view”. The record in A is a “bird’s-eye view” representation of a trial shown in Figure 9C, and record in B is a “bird’s-eye view” representation of a trial shown in Figure 9D. The position and dimensions of crosspieces of the ladder and stones are to scale and are marked in gray. Crosses indicate position of the calculated center of gaze every 5 ms, and blue circles show the surface area that projected onto the retina’s area centralis. The paw placement locations are shown for the right (pink; in A and B) and left (orange; in B only) forelimbs. See also supplemental Video 2, which shows movement of cat’s gaze along the surface of the ladder, movement of the head (the center of the left eye) in the corridor, and movement of the right paw from one crosspiece of the ladder to another one during the trial that is shown here in A and also in Figure 9C.
In summary, when walking along the horizontal ladder, which required vision for accurate stepping, cats looked at the plane of the ladder surface more than they looked on the flat surface. Cats spent from 1/4 to 1/3 of their walking time making gaze shifts along this plane, and unlike on the flat surface, made many more away than toward shifts. All gaze shifts on the ladder had shorter durations, were made closer to the cat, and their start points were reached faster than on the flat surface. There were also many more gaze fixations than on the flat surface, which were significantly longer and were made on points closer to the cat, and these points were reached faster than on the flat surface. Constant gaze episodes occupied less than 10% of walking time and were of shorter durations and amplitudes than on the flat surface. Detailed characteristics of the four gaze behaviors were as follows.
Gaze shifts
During walking along the ladder, cats spent more time shifting their gaze than they did when walking on the flat surface in either the dark or light: 27–34% of the total walking time and 32–44% of the time when cats’ gaze intersected the top-of-crosspieces plane (Z test, p<0.0001; Fig. 8). In sharp contrast with their behavior during walking on the flat surface in either darkness or light, cats made noticeably more away than towards gaze shifts during walking along the ladder (Z test, p<0.0001): among cats, proportion of away shifts ranged between 63% and 72%.
Cats started away gaze shifts nearer to them than on the flat surface in either the dark or light, at 55–63 cm ahead (GLM, p<0.0001; Fig 11. A), and it took cats 0.7–1.1 s and 1.1–1.2 strides to reach this point (Fig 11. B, C). This was the shortest time and the smallest number of strides among all tasks (t test, p<0.0001). Away gaze shifts lasted 72–92 ms, which for two of the cats was much shorter than on the flat surface in the light (GLM, p<0.0001, Fig. 11D). Away gaze shifts amplitudes were 18–27 cm, with cat 1 data averaging at 25 cm, which was the exact distance between crosspieces of the ladder (Fig. 11E).
At the beginning of toward gaze shifts, two of the cats looked closer to them than on the flat surface, while one cat looked further away, about same distance as in the dark (Fig. 11F). Across all cats, toward gaze shift start distances ranged between 95 and 130 cm, and cats reached these points in 1.4–2.0 s and 1.2–2.5 strides (Fig. 11G, H). This was again the shortest time and the smallest number of strides between all tasks (t test, p<0.0001). In two cats, toward gaze shifts lasted shorter on the ladder than on the flat surface in the light (GLM, p<0.001; Fig 11. I). Amplitudes differed substantially among cats, with two of them making smaller shifts on the ladder than on the flat surface and one making larger shifts (Fig. 11J). Toward gaze shifts terminated at the same distance in front of the cat as did the away gaze shifts, at 79±35 and 79±32 cm away, respectively.
Fixation
Cats fixated gaze much more on the plane of the ladder than on the flat surface, spending in fixation 19–43% of their total walking time and 29–49% of the time when they looked at ladder surface plane (Z tests, p<0.0001; Fig. 8). The frequency of fixations was 3–5 times higher than during walking on the flat surface either in the darkness or light and ranged between 7.4 and 18.2 per passage. Cats fixated on points at distances 61–77 cm away (Fig. 12A). For all cats, this was a closer distance than during walking on the flat surface either in the dark or light (GLM, p<0.0001). Interestingly, although cat 3 walked faster than cats 1 and 2 (Fig. 1D), all cats were reaching the point of gaze fixation on the ladder in a similar time and number of strides (in 0.9–1.3 s and 1.2–1.5 strides; Fig. 12B, C). For all cats this was the shortest time and the smallest number of strides to reach the point of fixation as compared to walking on the flat surface either in the dark or light (t test, p<0.0001). In all cats, gaze fixations lasted longer on the ladder than on the flat surface in the light, ranging from 64 to 118 ms (GLM, p<0.0001; Fig. 12D).
Constant gaze
During walking on the ladder, cats spent only 6–10% of the total walking time and 7–15% of the time when their gaze intersected the plane of the ladder in constant gaze (Fig. 8). Mean start distances of constant gaze ranged from 56 to 78 cm ahead of the cat, and it took cats 0.9–1.3 s and 1.1–1.5 strides to reach that start point (Fig. 13A–C). For all cats, this was a closer distance ahead (GLM, p<0.01), shorter time, and smaller number of strides compared to walking on the flat surface in the light (t test, p<0.0001). In two of the cats, constant gaze episodes were shorter than on the flat surface either in the dark or light, ranging between 42 and 62 ms (GLM, p<0.0001; Fig. 13D). Also, in two cats constant gaze amplitudes were smaller than on the flat surface in the light (2–7 cm; GLM, p<0.0001; Fig. 13E).
Slow gaze
During ladder locomotion, cats spent only 8–12% of their total walking time and 13–15% of the time they were looking at the plane of the ladder in slow gaze (Fig. 8). As on the flat surface in the light, on the ladder all cats had noticeably more away (ranging between 65–77% among cats) than toward slow gaze episodes (Z test, p<0.0001). Across all slow gaze events, start distances for all cats were closer to the cats on the ladder than on the flat surface in the light (GLM, p<0.0001; Fig. 14A, F). It took cats 0.9–1.4 s and 1.1–1.7 strides to reach the slow gaze start point, again a shorter time and a smaller number of strides than during walking on the flat surface in the light (t test, p<0.0001; Fig. 14B, C, G, H). Slow gaze events lasted 46–73 ms and, in all but one case, were shorter than on the flat surface in either dark or light conditions (Fig. 14D, I). Slow gaze amplitudes were all below 10 cm (Fig. 14E, J).
Walking along the stones-cluttered pathway
Unlike the ladder, which presented cats with a highly uneven but regularly structured and familiar surface, the stones-cluttered pathway challenged cats with irregularly placed obstacles whose layouts were new every day. Only cats 1 and 3 were tested during this task (Table 1). Cat 3 walked faster than cat 1 (60 vs. 51 cm/s; GLM, p<0.001), but their stride lengths were similar (t test, p=0.069) and slightly shorter than during walking on the flat surface in the light (t test, p=0.023; Fig. 1D, E).
Similarly to their behavior on the ladder, during walking along the stony pathway cats spent most of their time looking at the surface; again, much more than during walking on the flat floor either in the dark or light (84–90%; Z test, p<0.0001; Fig. 8A, C). In fact, cat 3 spent more time looking at the surface during this task than on the ladder (Z test, p<0.0001). A representative record of the gaze of one cat (cat 1) is shown in Figure 9D. During this passage, the cat was looking at the surface the entire time. As it walked, it fixated the gaze on points 50–100 cm away before shifting it to the next point. There were only two short constant gaze episodes. As with walking along the ladder, gaze shifts and fixations dominated the gaze behavior. A birds-eye view of this passage is shown in Figure 10B.
Overall, when walking along the stones-cluttered pathway, which was irregularly structured and perpetually novel, cats looked at the surface more often than during walking on the flat surface, and one of the cats looked even more than on the regular surface of the ladder. Cats spent as much time making gaze shifts along the stony pathway as along the ladder, but their gaze shifts were often shorter in duration, covered smaller distances, and were made closer to cats than on the ladder. Cats made as many gaze fixations as on the ladder, but these fixations, likewise, began at closer points. Constant gaze episodes tended to occupy more time and were of larger amplitude than during any other task but still were rather infrequent and short. Detailed characteristics of the four gaze behaviors were as follows.
Gaze Shifts
During walking along the stony pathway, cat 1 spent more time in gaze shifts than during walking on the flat surface either in the dark or light (36% total, 41% intercepting; Z test, p<0.0001; Fig. 8A), while cat 3 used approximately same proportion of time (27% total, 32% intercepting; Fig. 8C). Cats tended to make slightly more away than toward gaze shifts (ranging between 57–58% among cats; Z test, p<0.0001). Cats initiated them at distances comparable to those seen during the ladder task (GLM; p=0.1; Fig. 11A). It took cats 1.1–1.2 s and 1.5–1.6 strides to reach the away gaze shift start point, similar to when walking on the flat floor in the light, but slightly longer than on the ladder (GLM, p<0.0001; Fig. 11B, C). Away gaze shift durations in one cat were the shortest of all tasks, while in the other cat they were as short as on the ladder, and thus much shorter than when walking on the flat surface (62–84 ms; GLM, p<0.0001; Fig. 11D). Away gaze shift amplitudes were the smallest of all tasks (<20 cm, GLM; p<0.0001; Fig. 11E).
At the beginning of toward gaze shifts, both cats looked closer to their current position than during any other task: 91–107 cm away (GLM, p<0.0001; Fig. 11F), and it took cats 1.8 s and 2.3–2.7 strides to reach the toward gaze shift start point (Fig. 11G, H). Toward gaze shifts on the stony pathway were the shortest among all tasks, ranging from 49 to 69 ms (GLM, p<0.0001; Fig. 11I). Their amplitudes were also small and, for cat 1, they were the smallest observed (13 cm; GLM, p<0.0001; Fig. 11J). Both toward and away gaze shifts terminated at the same distance from the cats: at 75±25 and 76±28 cm away, respectively (GLM, p=0.24).
Fixations
During walking along the stony pathway, cats fixated on the surface 25–36% of the time, or 30–41% of time when looking towards the surface (Fig. 8), which was similar to their behavior during walking along the ladder. Fixations were made at a rate of 7–14 per passage, as often as during walking on the ladder. Cats fixated on points 63–65 cm away, which, for both cats, was closer than during any other task (Fig. 12A). Cats reached the fixation point in 1.0–1.3 s and 1.5–1.7 strides, taking just slightly longer than on the ladder (GLM, p=0.0005; Fig. 12B, C). In cat 1, fixations were of shorter duration than on the ladder (83 ms), while in cat 3 they were longer (90 ms; GLM, p<0.027; Fig. 12D).
Constant gaze
Both cats tended to spend slightly more time in constant gaze during walking along the stony pathway than during any other task. They differed, however, with cat 3 spending 17% of its total walking time and 21% of the time when its gaze intersected the surface in constant gaze, while cat 1 spent only 7% and 8%, respectively (Fig. 8). Cats typically started constant gaze 55–64 cm in front of them (Fig. 13A), which required 0.91–1.3 s and 1.3–1.6 strides to reach this point (Fig. 13B, C). Constant gaze episodes of cat 3 were longer than on the ladder (99 ms), while in cat 1 they were the shortest of all tasks (38 ms; GLM, p<0.0001; Fig. 13D). Constant gaze amplitudes were small in both cats, ranging between 2 and 8 cm (Fig. 13E).
Slow gaze
Cats spent 11–15% of total walking time and 12–17% of the time when their gaze intersected the surface in slow gaze (Fig. 8). There were more toward than away slow gaze episodes (54–73% vs. 27–46%, respectively, Z test, p<0.0001). Away slow gaze events started 54–63 cm in front of the cat (Fig. 14A), and it took cats 0.9–1.2 s and 1.3–1.6 strides to reach this point (Fig. 14B, C). Cats differed in duration and amplitude of away slow gaze episodes (Fig. 14D, E). Toward slow gaze events were started 71–73 cm away (Fig. 14F), and these points were reached in 1.2–1.4 s and 1.8 strides (Fig. 14G, H). Slow gaze episodes lasted 50–53 ms, and their amplitudes were 3–4 cm (Fig. 14I, J).
Across-task overview
Although cats differed in some aspects of their gaze behaviors, they shared most features. In the section below, we compare gaze behaviors across locomotor tasks using their pooled data.
Cats spent much more time looking at the surface during walking along the ladder (67–85%) and stony pathway (84–90%) than on the flat surface either in the dark (23–47%) or light (21–62%; Z test, p=0.0001; Fig. 8A–C, Fig. 15A). Together, gaze shifts and fixations dominated gaze behavior during all tasks (62–84%) while constant gaze episodes occupied a substantially smaller portion of time (Z test, p=0.0001; Fig. 8D–F).
Figure 15.
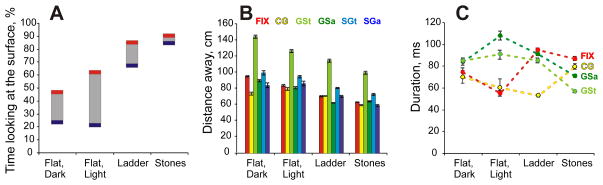
Changes in the gaze behavior with increase in visual complexity of walking environment and demand on visual guidance of stepping. A: The minimal (blue) and maximal (red) time spent looking at the walking surface across all cats. Grey boxes highlight spread between minimal and maximal values. B: Distance in front of the cat where different gaze behaviors began. C: Duration of gaze behaviors during different locomotor tasks. In B, C: mean ± SEM values across all cats are shown for different gaze behaviors. GSa - away gaze shifts (purple), GSt - toward gaze sifts (blue), FIX – gaze fixations (red), CG – constant gaze (yellow), SGa – slow gaze directed away from the cat (green), SGt – slow gaze directed toward the cat (light green). In C, a dotted line of a corresponding color highlights a trend for the parameter.
Gaze Shifts
Cats started away gaze shifts the furthest distance away when they walked on the flat surface in the dark (89±40 cm; GLM, p<0.001). That distance decreased progressively as the visual complexity of walking environment and demand on visual guidance of stepping increased, first on the flat surface in the light (80±36 cm), and then on the ladder and stones-cluttered pathway (61±27 and 63±25 cm, respectively; GLM, p<0.001; Fig. 15B). Cats went from looking 2.2 strides ahead on the flat surface in the dark, to 1.9 strides ahead in the light, and 1.2–1.6 strides on the ladder and stony pathway. During walking along the ladder, cats made away gaze shifts of the largest amplitude and second longest duration (23±18 and 91±56 ms; GLM, p<0.001), while on the stony pathway, the duration of away gaze shifts were the shortest (71±50 ms; GLM, p<0.041).
Similarly to away gaze shifts, across tasks, cats started toward gaze shifts from the furthest distance away while on the flat surface in the dark (143±58 cm; GLM, p<0.001), moving closer as the visual complexity of the environment increased in the light (126±58 cm), then on the ladder (114±57 cm), and finally on the stony pathway (98±58 cm; GLM, p<0.001; Fig. 15B). During all tasks, cats needed to make more than 2 strides to reach the toward gaze shift start. Toward gaze shifts lasted a similar amount of time during walking on the flat surface in the dark (85±63 ms), light (91±105 ms), and on the ladder (85±75 ms), but were much shorter for the stony pathway (57±43 ms; GLM, p<0.001; Fig. 15C). The stones task was also associated with the smallest amplitude of toward gaze shifts (17±27 cm; GLM, p<0.001).
Fixation
Cats fixated on the farthest points during walking on the flat surface in the dark (94±25 cm away), they fixated slightly closer while on the flat surface in the light (83±29 cm), still closer when on the ladder (70±24 cm), and closest on the stones-cluttered pathway (63±20 cm; GLM, p<0.001; Fig. 15B). Fixations were short during walking on the flat surface in the dark (74±119 ms) and even shorter on the flat surface in the light (55±61 ms), but were much longer during walking on the ladder and the stony pathway (95±91 and 87±80 ms, respectively; GLM, p=0.001; Fig. 15C). Cats reached the fixation point by 1.6 strides on the stony pathway and by 1.4 strides on the ladder, while taking 1.9 strides on the flat surface in the light, and 2.4 strides in the dark. So, as the visual complexity of the walking environment and demand on visual guidance of stepping was increasing, cats fixated for longer periods and on closer points, and were reaching these points faster.
Constant Gaze was relatively rarely observed during any task encompassing only 4–21% of time the gaze intersected the walkway. During constant gaze episodes, cats looked the greatest distance away during walking on the flat surface in the light (79±32 cm), tended to look some closer - in the dark (73±36 cm) and on the ladder (70±26 cm), and looked much closer - on the stony pathway (58±22 cm; GLM, p<0.05; Fig. 15B). These distances were covered in 1.8 strides on the flat surface in both the light and dark, and in 1.4 and 1.5 strides on the ladder and stony pathway, respectively. Durations of constant gaze episodes ranged from 53 to 79 ms and were significantly longer during walking along the stony pathway than during walking along the ladder or the flat surface in the light (GLM, p=0.004; Fig. 15C). Their amplitudes were small, ranging from 3.4 to 5.7 cm, and were similar across all tasks except the stony pathway, where they were slightly larger (5.7±6.7 cm; GLM, p<0.001).
DISCUSSION
The main finding of this study is that gaze shifts and fixations, not constant gaze, dominate the gaze behavior of walking cats. Our main errors resulted from projection of the two-dimensional gaze movement along the walking surface onto the line where this surface intersected with the sagittal plane (X, Z in Fig. 2). Although when walking, cats move both the head and eyes in the vertical direction (pitch, results in forward-backward movement of the gaze along the walking surface) much more than in the lateral direction (yaw, Fig. 4; for head movement, also see Fowler and Sherk, 2003), such projection certainly caused misclassification of slower oblique gaze shifts into the “slow gaze” group and the probably rare strictly lateral gaze shifts and slow gaze events into gaze fixations. This projection of gaze, however, did not affect our judgment and classification of constant gaze episodes. In addition, the range, within which we considered the gaze to be “constant” (gaze/cat velocity ratio between 0.5–1.5), was, in our opinion, quite generous. Moreover, while other authors have in the past restricted duration of a constant gaze episode to some minimum, for example, to 2–3 frames of a 30 Hz video recording that corresponds to 66–99 ms (Patla and Vickers, 1997, 2003; Fowler and Sherk, 2003), we used a much looser temporal restriction when defining constant gaze events, where even gaze shifts as short as 15 ms were counted (three data points of our 200 Hz recording). In spite of these allowances, we saw no walking task in which the proportion of constant gaze exceeded either gaze fixation or gaze shifts, although it was close in cat 3 when it walked along the stony pathway. Thus, even with the number, duration, and amplitude of gaze shifts underestimated (mostly in favor of slow gaze events), while all rather broadly defined constant gaze episodes, including very short ones, counted, we still found that gaze shifts and fixations, and not constant gaze, dominate the gaze behavior of walking cats.
Our finding that gaze shifts and fixations dominate gaze behavior during locomotion is in considerable contrast with a number of previous reports in both humans (Patla and Vickers, 1997, 2003) and cats (Sherk and Fowler, 2003), which, while noting that frequent gaze shifts and occasional gaze fixations were of course observed, concluded that constant gaze comprised a major portion of gaze behavior during locomotion, even on terrains where accurate stepping was required. We believe that this difference in findings is most likely due to the fact that we recorded gaze with higher temporal resolution and, thus, could document gaze behaviors that were smaller in amplitude and shorter lasting. Fowler and Sherk (2003) studied cats walking along a walkway cluttered with many small objects using video recording of cats’ head only. To calculate the gaze, these authors considered eyes to be stationary within the head when the head was not moving, an assumption based on a prior report by Guitton and colleagues (1984) that cats rarely deviate their eyes more than 2 degrees from the center without also moving the head. The study by Guitton and colleagues (1984), however, explored only horizontal eye and head movements and only in the sitting cat. Even if their findings could be fully applied to the vertical gaze of the walking cat, one should consider that if the eye is positioned 20 cm above the ground (a typical height of the eye in the walking cat) and the gaze is directed 60 cm down the walkway (a closer end limit to the distance at which cats typically look during walking both along the ladder and the stony walkways; Figs. 11–14, also Fowler and Sherk, 2003) a mere 3 degree rotation of the eye results in a 12.6 cm displacement of the gaze on the surface, which is approximately 1/3 of an average limb transfer distance during a stride. This is a significant distance from the behavioral point of view. Fowler and Sherk (2003) acknowledged that they were uncertain about the eyes and the total gaze movement during periods when the head was moving, and thus have excluded these periods from their analysis. They argued that since cats blinked often during head shifts, gaze behaviors occurring during these head movements did not add significantly to the total useful gaze anyway. Also, the smallest gaze saccades, which these authors were able to record, were 3.5 degrees, while the largest ones, those more than ~15 degrees in amplitude, they have classified separately as “glances”. Therefore, because of all methodical approaches used, the time that cats spent making gaze shifts during locomotion was almost certainly underestimated in the Fowler and Sherk (2003) study. However, even with such underestimation these authors still found that cats spend ~30% of locomotion time shifting their gaze along the walking surface. In respect to gaze fixations, Fowler and Sherk (2003) acknowledged that, because measuring small head rotations with their technique was imprecise, they could have misclassified brief gaze fixations as constant gaze episodes. In contrast to this study in cats, which was based on recording and evaluation of movement of the head only, the study of gaze patterns during locomotion in humans performed by Patla and Vickers (2003) employed a very precise recording of eyes movement in the orbit by a device from Applied Science Laboratories (Bedford, MA, USA), but detected head movements by visual evaluation of the blurriness of video images obtained from a head mounted camera - not a very accurate method. We have accurately recorded movements of both the eyes and the head. Our error at recording the head three-dimensional position was 0.5 mm, the error at recording eye position in the orbit was 1.0 degree. The accuracy of recording is critical because, in the walking subject, substantial gaze shifts can occur in result of small angular changes in the eye and/or head position. Our findings agree more with those of other authors who have observed significant gaze shifting and many gaze fixations in walking subjects (Collewijn, 1977a, b; Grossman et al., 1989; Solomon and Cohen, 1992a, b; Crane and Demer, 1997; Turano et al., 2002). None of these authors, however, researched gaze behavior in conditions when subjects need to step accurately on the surface.
Another possible explanation for our failure to find as much use of the constant gaze behavior as Fowler and Sherk (2003) is that neither our ladder with crosspieces spaced far apart nor our sparsely scattered stones presented a surface complex enough to engage the constant gaze mechanism. Indeed, the cluttered pathway task that Fowler and Sherk (2003) used had ~25% of walking surface covered with objects, while in our experiments the stones took only ~7% of the surface. Moreover, we ourselves observed more constant gaze episodes when cats walked along the stony pathway than along the ladder, a task that presented more objects (Fig. 15C). It is possible that when there are too many objects to look at individually during the time available, as likely was the case in studies by Dr. Sherk’s group (Sherk and Fowler, 2001; Fowler and Sherk 2003), the visual sampling leans toward the use of optic flow information. This view would explain why people used a “saccade and fixation” gaze strategy rather than relying on optic flow information when stepping on just a few stepping stones (Hollands et al., 1995; Hollands and Marple-Horvat, 1996, 2001), avoiding some cracks in the pavement (Land, 2006), or selecting where to step on a multi-surface pathway consisting of only 15 different plates (Marigold and Patla, 2007). Our unpublished observation that cats use constant gaze more when they walk faster along both our horizontal ladder and stony pathway is also consistent with this view.
One of the prominent findings of this study is that, while walking along the flat surface in the complete darkness, cats spent 43–62% of the time, which their gaze intercepted the plane of the invisible walkway, shifting their gaze, and in addition they spent 22–28% of the time in gaze fixations (Fig. 8). These gaze shifts and fixations were not related to visual sampling of the walkway because in the dark there was nothing for the cats to see. Rather, they were generated in response to vestibular, somatosensory, and/or internal signals. In their studies of gaze behavior during locomotion in primates, Solomon and Cohen (1992a, b) found that the velocity storage mechanism, a brainstem-cerebellar mechanism, which retains velocity of the head and surroundings and then drives the eyes after the head and surroundings have stopped moving (Cohen et al., 1977; Raphan et al., 1979), plays the major role in determining gaze behavior during locomotion. Indeed, they found that when monkeys run in a circle, their gaze moved in a series of saccadic shifts in the direction of progression, with each shift followed by a fixation, even in complete darkness. They further found that when the platform, upon which the monkeys run, was rotated in the opposite direction, resulting in monkeys making less progress in space while running at the same velocity in respect to the platform, their gaze still moved in a similar “nystagmic” pattern. Another innate mechanism for gaze stabilization during locomotion in the absence of vision was described in the tadpole. It was shown that, in the tadpole isolated spinal-brainstem preparation, locomotion-related rhythmicity can be readily recorded from oculomotor nerves (Stehouwer, 1987; Combes et al., 2008; Lambert et al., 2012). Because there are no sensory inputs in such a preparation from the vestibular apparatus, body or eyes, the spinal locomotor pattern generator (CPG), in addition to sending signals to the body, must be also sending signals to the eyes. During actual locomotion, these signals move the eyes in the direction opposite to that of the head. In the cat, an animal that has a prominent neck, it is very likely that signals from the spinal locomotor CPG can not only drive the eyes, but also significantly determine the movement of the head. Indeed, in all species tested, the movement of the head during locomotion was found to be synchronized with the movement of limbs (e.g., Pozzo et al., 1990; Xiang et al., 2008, Dunbar et al., 2008). We believe that it is very possible that the gaze behaviors, which we have observed in the cat during locomotion on the flat surface in the darkness, were at least partially driven by the spinal locomotor CPG. Directing gaze at the walking surface for a significant portion of time even in the darkness is likely to be beneficial because such behavior keeps subjects ready to immediately see the surface as soon as lighting conditions improve.
During walking on the flat surface in the light, in addition to the velocity storage and spinal locomotor CPG efference copy signals, visual information could influence cats’ gaze behavior. Similarly to their behavior during walking in the darkness, cats still looked at the surface, at most only slightly more than half of the time (22–62%, Fig. 8 and 15A). The main difference from their behavior in the dark was that they looked at closer points on the walkway (Fig. 15B). This suggests that when visual information was available, cats were occasionally visually sampling the walking surface in front of them, and that the distance of 83±29 cm was more beneficial for their visual system as compared to the 94±25 cm distance observed in the darkness (Fig. 15B), the distance that was probably “hardwired” within innate mechanisms. In addition, during walking in the light, cats made significantly more away gaze shifts than toward ones; so they were also overriding the innate mechanisms by making more away shifts than would occur otherwise, likely in order to maintain the optimal distance for visual inspection of the surface as the body progressed forward. Solomon and Cohen (1992b) also found that during walking in the light monkeys made substantially more gaze shifts than in the darkness.
During walking on the horizontal ladder, cats had to see in order to step accurately on the crosspieces (Beloozerova and Sirota, 2003). Thus, the gaze behavior during ladder locomotion, in addition to following the innate mechanisms, was also visual sampling-related. In our previous study (Beloozerova et al., 2010) we have researched the kinematics of the cat limbs and body movements during walking on the flat surface and along a horizontal ladder similar to that used in this study. We found that on the ladder, cats assumed a more bent-forward posture by lowering the center of mass of both the trunk and the head/neck segments by 1–2 cm, by rotating both the head and the neck nose-down by 5–10 degrees, by increasing flexion of the distal joints, and, in some cases, by rotating the trunk’s rostral side down by several degrees. Consequently, during ladder locomotion the long axis of the head, if continued forward, intersected the ground much closer to the cat than on the flat surface. This finding is supported by our current study, in which we have directly calculated gaze/surface intersect position during different locomotor tasks and found that on the ladder it was 70±24 cm away from the cat, about 10 cm closer than during walking on the flat surface (83±29 cm; Fig. 15B). The finding that during locomotion cats adjust the posture and movement of their entire body to aid vision corresponds well with similar observations in people (Imai et al., 2001; Mulavara and Bloomberg, 2002–2003; Mulavara et al., 2005; Land, 2004, 2009). In our 2010 report, we suggested that cats adjust posture during locomotion in order to bring eyes closer to the ground so that retinal image of stepping targets is larger, as a better view of the targets is likely to make guidance of paw placements easier during an accuracy demanding task.
It appears, however, that this need to look at closer points during a more difficult locomotor task is not only related to a requirement for better vision, but also, and possibly mainly, is linked with an optimal visuo-motor information processing time that is needed for generation of adjustments of limb movements according to information obtained by vision. For example, our cat #3 walked faster than other cats (Fig. 1D), but she also looked further away, and in result reached the viewed sites only a little bit faster than her slower walking peers, at just under one second (Figs. 11–14). The “around one second” time to reaching for manipulation of a viewed object appears to be a “golden rule” for timing of visual sampling for adjustment/generation of a wide variety of movements. It was reported that, when walking in a cluttered environment, cats look 1.3 s ahead (Fowler and Sherk, 2003; Wilkinson and Sherk, 2005) and people look 0.8–1.2 s ahead (Grasso et al., 1998; Patla and Vickers, 2003; Land, 2006; Marigold and Patla, 2007). Also, people take 0.75 s to start steering a car after seeing a target (Land and Lee, 1994), articulating a sound after seeing a letter (rev. in Rayner, 1998), and 0.8 s to strike a key after observing a musical note (Furneaux and Land, 1999). For both walking cats and humans, “around one second” typically corresponds to about 1–1.5 strides or 2–3 steps, and Wilkinson and Sherk (2005) found that this is exactly how long cats remember viewed targets during locomotion. Our finding that during walking on surfaces where accurate stepping is required cats look ahead of them, not at their feet, confirms a similar observation by Dr. Sherk (Fowler and Sherk, 2003; Wilkinson and Sherk, 2005) and further supports the notion that during locomotion vision is used in a “feed-forward” rather than “feed-back” mode, meaning that visual information is utilized for planning of future steps rather than for an online correction and guidance of the current one (Hollands et al., 1995; Hollands and Marple-Horvat, 1996, 2001).
In addition to looking nearer at the surface during walking along the ladder than the flat surface, on the ladder cats made many more away gaze shifts. The ratio of away gaze shifts to toward ones was much higher during this task than during walking on the flat surface. This was likely due to the fact that when the gaze intersects the surface closer to the cat so the cat reaches the closer point faster, there is a need to move gaze forward more frequently. A high number of away gaze shifts during locomotion along the ladder (5–10 per passage accomplished in 4 strides, Fig. 1A, Table 1) also suggests that during locomotion in environments with a high demand on visual guidance of stepping, eye movements are associated closely, and may be programmed jointly, with limb movements, as was previously suggested for humans (Hollands et al., 1995; Hollands and Marple-Horvat, 1996, 2001). Our preliminary data on the correlation between timing of eye and limb movements during locomotion along the horizontal ladder and stones-cluttered pathway showed that they are tightly linked in cats as well (Rivers et al., 2010). A relatively low number of toward gaze shifts indicated that cat’s gaze fixation on the ladder was behaviorally rather effective, so only few “catch-up” gaze shifts directed toward the cat were needed as the cat progressed ahead.
Despite the need to move gaze forward more frequently along the ladder, cats fixated their gaze on the surface of the ladder much more often and for longer periods of time than on the flat surface (Fig. 12). This suggests that they were collecting visual information for guidance of stepping during these periods of time. The finding by Wilkinson and Sherk (2005) that cats can successfully make 1–1.5 strides or 2–3 steps on a cluttered pathway without hitting an object after lights are turned off indicates that during locomotion cats normally use central, not peripheral, vision to obtain information about the walking surface. Among our cats, the typical fixation time on the ladder was in the range of 64–118 ms, which thus reveals the time that cats needed to obtain adequate visual information for an accurate limb transfer. This time was much longer than during walking on the flat surface (55±61 ms, Fig 15C). Patla and colleagues (1996) also found that in conditions more demanding for accurate stepping, people look at the walking pathway more frequently and for a longer time. The entire pattern of cats gaze behavior during walking on the ladder (Figs. 9C, 10A, and Video 2), which can be identified as “gaze stepping”, suggests that cats were gathering visual information about the ladder on a step-by-step basis.
Unlike the ladder that presented cats with a highly uneven but regularly structured surface, the stones-cluttered pathway offered cats irregularly placed obstacles, the layout of which was new every training or testing day. This substantially complicated stepping on the surface using memory. Thus, the gaze behavior during walking along the stony pathway, while still possibly reflecting some innate mechanisms, was significantly determined by the necessity to select suitable foot placements in between many small objects. Cats tended to look at the haphazardly organized stones-cluttered pathway more than on the regular surface of the horizontal ladder (Fig. 15A), and they also tended to look slightly closer to themselves (Fig. 15B). This was likely because on the stony pathway there were more step-relevant objects on the surface and they were located closer to cats. But although the stones-cluttered pathway presented a more complex terrain as compared to the ladder because of its irregularity and unfamiliarity, and cats’ strides were shorter on the stony pathway (Fig. 1E), it took cats only slightly longer to reach the gaze fixation points on this walkway as compared to the ladder (Fig. 12B, C). Fowler and Sherk (2003) also found that their cats were reaching the viewed points while walking along a pathway cluttered with even more small objects than ours in a similar time and number of steps. This supports the “one second” rule for the time required for the visual information-to-action transformation that we have noted above.
Cats spent as much time making gaze shifts along the stones-cluttered surface as they did on the ladder, but their gaze shifts were of shorter duration and covered smaller distances. This was likely due to the fact that, on the stones-cluttered pathway, objects important for accurate stepping were located closer to each other than on the ladder. The frequencies of gaze fixation and their durations were similar between the stones-cluttered pathway and the ladder. The finding that gaze behavior on the stony pathway and the horizontal ladder were quite similar except for the features that could be easily explained by a different location of objects on the two pathways suggests that the ladder was for the cats as complex a task for accurate stepping as the stony-cluttered pathway, and that cats did not step on the ladder using their memory either, even though the ladder had regularly spaced crosspieces and was very familiar to them, but used vision to plan every step.
Constant gaze episodes tended to occupy more time and be longer during walking along the stones-cluttered pathway than during any other task but still were rather infrequent and short. This, as we’ve suggested above, could be because cats did not have enough time to look at every single object and were beginning to utilize optic flow.
Stabilization of the world’s image on the retina during locomotion is difficult. But how much stabilization is actually needed? One can substantially reduce the demand for gaze stabilization by resorting to a strategy of using only the shortest fixations that are absolutely needed for an image processing (64–118 ms for our cats), and then letting the gaze shift according to the whole-body locomotion synergy, just adjusting it slightly to fall on areas of interests as needed. This would be similar to how accurate limb positioning during locomotion over a complex terrain appears to be accomplished: by a slight adjustment of the limbs’ movements to accurately step on the correct sites while generally following the standard pattern of locomotion (Beloozerova et al., 2010). Rieiro and colleagues (2012) have recently found that stimuli are most salient, at least for people, when they are presented only very briefly, for 50–100 ms at a time, and their perceived contrast decreases if they are visible for longer periods. It is well known that visibly flickering stimuli appear enhanced (Bartley, 1939). So, short times of fixations might be even beneficial. It was shown that visual guidance of foot positioning during locomotion on a horizontal ladder depends mainly upon static visual cues sampled with a low frequency (Assaiante et al., 1989), and that free active gaze behavior that includes saccades and fixations is more beneficial for performance even in such “optic flow” dominated environment as simulated driving (Wilkie and Wann, 2003; Wilkie et al., 2008, 2011).
Conclusion
A novel technique for simultaneous recording of eye and head movement in unrestrained cats with high accuracy and at high frequencies has allowed us to obtain a detailed description of cat vertical gaze behaviors during locomotion in environments with different visual complexities and demands on visual guidance of stepping. We found that during all locomotor tasks tested, including during walking in the darkness, gaze shifts and fixations, not constant gaze, dominate cats’ gaze behaviors, and that during walking on complex surfaces, cats look at the surface for nearly the entire time, fixating for ~100 ms on sites approximately one second away, and moving gaze forward in a step-like manner as they progress forward.
Supplementary Material
Highlights.
Vision is critical for walking on complex surfaces, but its use is poorly known.
Cats walked on flat surface in the light and dark, along a ladder and a stony path.
An eye search coil method combined with active marker-based head tracking was used.
Gaze shifts and fixations dominated cats’ gaze behavior during all walking tasks.
Constant gaze occupied only 5–21% of the time cats spent looking at the surface.
Acknowledgments
The authors are deeply indebted to Drs. Steven Macknik, Susana Martinez-Conde, Jorge Orero-Millan, and Mr. Hector Rieiro for assistance with eye-movement calibration and programming for data analyses; to Mr. Peter Wettenstein for exceptional engineering support, to Dr. Mark Pruel for X-ray imaging, and to Dr. Jessica Li for help with statistical analysis. We also thank Dr. Vladimir Marlinski for many helpful discussions of the data and critical review of the manuscript, and Dr. Sergei Yakushin for discussion and review of the manuscript’s later version. Supported by grant R01 NS-058659 to Dr. Irina Beloozerova.
ABBREVIATIONS
- CG
constant gaze
- CPG
central pattern generator
- FIX
gaze fixation
- GLM
generalized linear model
- GSa
gaze shift away from the cat
- GSt
gaze shift toward the cat
- LED
light emitting diode
- SGa
slow gaze away from the cat
- SGt
slow gaze toward the cat
- St
stance
- Sw
swing
Appendix. Calculation of the gaze/surface intersect
Head position and orientation in space is recorded in the walking chamber-related coordinate system (X, Y, Z; Fig. 2) using the motion capture and analysis system Visualeyez.
Horizontal (yaw) and vertical (pitch) eye angles in Fick coordinates within the head are recorded in the head-centered coordinate system (XH, YH, ZH; Fig. 2, insert) using the magnetic scleral search coil eye tracking system, and rotation (direction) of the eye is calculated as a function of these two angles.
Horizontal (yaw), vertical (pitch), and lateral (roll) head angles are calculated/measured. A rotation matrix algorithm is applied to the eye rotation in the head to combine it with the head rotation and obtain the final gaze direction vector in the walking chamber-related coordinate system.
Intersect of the gaze direction vector with the walking surface is calculated.
Gaze intersect calculations
-
θ - Recorded horizontal eye angle
φ - Recorded vertical eye angle
-
Convert from spherical to Cartesian coordinates (ρ, θ, φ) → (x, y, z), assume ρ = 1,
(This conversion uses the complementary angles to those usually shown in the Cartesian to spherical coordinate conversion).
Create a line of sight vector, V = (Vx, Vy, Vz) from the center of the eye through the center of the pupil. Standard tip minus tail subtraction are used to generate the vector, and the vector is normalized to unit length. -
The following three rotation matrices were applied to transform line of sight within the head to final gaze direction, V′.
The three rotation matrices, Rx(τ), Ry(δ), and Rz(μ) rotate the line of sight vector about the labeled axis (x, y, and z by angles τ, γ, and μ respectively):Final gaze direction is calculated by the product of the line of sight vector with the rotation matrices.The sequence of rotations is important to preserve correct system orientation. Specifically, the order of rotations is: roll is first to preserve the axis where the head rolls towards the shoulder with eyes forward in the orbit, second is pitch to preserve the vertical rotation axis of the head, and finally is yaw to preserve the side rotation or ‘no’ motion.
The resulting vector V′ is the combination of the angular displacement of the eye and the head. This is the final line of sight, and can be used to model the gaze direction in a freely moving subject. For example, to calculate where the cat gaze intercepts the ground, the line of sight V′ can be translated into a convenient global reference frame where the position of the eye and the ground is known, and subsequently, the magnitude of the line of sight vector can be scaled appropriately.
In the calculation below the position of the eye in walking chamber-related coordinates is known, the final gaze vector is known, and the ground is Zground = 0. The scaling factor, k, is determined through algebra.
Example Calculation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armer MC, Nilaweera WU, Rivers TJ, Dasgupta NM, Beloozerova IN. Effect of light on the activity of motor cortex neurons during locomotion. Behav Brain Res. 2013;250:238–250. doi: 10.1016/j.bbr.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaiante C, Marchand AR, Amblard B. Discrete visual samples may control locomotor equilibrium and foot positioning in man. J Mot Behav. 1989;21(1):72–91. doi: 10.1080/00222895.1989.10735466. [DOI] [PubMed] [Google Scholar]
- Bartley SH. Some effects of intermittent photic stimulation. J Exp Psychol. 1939;25(5):462–480. [Google Scholar]
- Beloozerova IN, Sirota MG. The role of the motor cortex in the control of accuracy of locomotor movements in the cat. J Physiol. 1993;461:1–25. doi: 10.1113/jphysiol.1993.sp019498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloozerova IN, Sirota MG. Integration of motor and visual information in the parietal area 5 during locomotion. J Neurophysiol. 2003;90(2):961–971. doi: 10.1152/jn.01147.2002. [DOI] [PubMed] [Google Scholar]
- Beloozerova IN, Farrell BJ, Sirota MG, Prilutsky BI. Differences in movement mechanics, electromyographic, and motor cortex activity between accurate and non-accurate stepping. J Neurophysiol. 2010;103:2285–2300. doi: 10.1152/jn.00360.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgeman B, Hendry D, Stark L. Failure to detect displacement of the visual world during saccadic eye movements. Vision Res. 1975;15(6):719–722. doi: 10.1016/0042-6989(75)90290-4. [DOI] [PubMed] [Google Scholar]
- Cohen B, Matsuo V, Raphan T. Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic after-nystagmus. J Physiol. 1977;270(2):321–344. doi: 10.1113/jphysiol.1977.sp011955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collewijn H. Eye- and head movements in freely moving rabbits. J Physiol. 1977a;266(2):471–498. doi: 10.1113/jphysiol.1977.sp011778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collewijn H. Gaze in freely moving subjects. In: Baker R, Berthoz A, editors. Control of gaze by brain stem neurons. Developments in neuroscience. Vol. 1. 1977b. pp. 13–22. [Google Scholar]
- Combes D, Le Ray D, Lambert FM, Simmers J, Straka H. An intrinsic feed-forward mechanism for vertebrate gaze stabilization. Curr Biol. 2008;18(6):R241–243. doi: 10.1016/j.cub.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Crane BT, Demer JL. Human gaze stabilization during natural activities: translation, rotation, magnification, and target distance effects. J Neurophysiol. 1997;78(4):2129–2144. doi: 10.1152/jn.1997.78.4.2129. [DOI] [PubMed] [Google Scholar]
- Dunbar DC, Macpherson JM, Simmons RW, Zarcades A. Stabilization and mobility of the head, neck and trunk in horses during overground locomotion: comparisons with humans and other primates. J Exp Biol. 2008;211(Pt 24):3889–3907. doi: 10.1242/jeb.020578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engbert R, Mergenthaler K. Microsaccades are triggered by low retinal image slip. PNAS. 2006;103:7192–7197. doi: 10.1073/pnas.0509557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick AE. Die bewegungen des menschlichen augapfels. Zeitschrift fur rationale Medizin. 1854;4:109–128. [Google Scholar]
- Fowler GA, Sherk HA. Gaze during visually-guided locomotion in cats. Behav Brain Res. 2003;139(1–2):83–96. doi: 10.1016/s0166-4328(02)00096-7. [DOI] [PubMed] [Google Scholar]
- Furneaux S, Land MF. The effects of skill on the eye-hand span during musical sight-reading. Proc Biol Sci. 1999;266(1436):2435–2440. doi: 10.1098/rspb.1999.0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JJ. Visually controlled locomotion and visual orientation in animals. Br J Psychol. 1958;49(3):182–194. doi: 10.1111/j.2044-8295.1958.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Grasso R, Prévost P, Ivanenko YP, Berthoz A. Eye-head coordination for the steering of locomotion in humans: an anticipatory synergy. Neurosci Lett. 1998;253(2):115–118. doi: 10.1016/s0304-3940(98)00625-9. [DOI] [PubMed] [Google Scholar]
- Grossman GE, Leigh RJ, Bruce EN, Huebner WP, Lanska DJ. Performance of the human vestibuloocular reflex during locomotion. J Neurophysiol. 1989;62(1):264–272. doi: 10.1152/jn.1989.62.1.264. [DOI] [PubMed] [Google Scholar]
- Guitton D, Douglas RM, Volle M. Eye-head coordination in cats. J Neurophysiol. 1984;52(6):1030–1050. doi: 10.1152/jn.1984.52.6.1030. [DOI] [PubMed] [Google Scholar]
- Gunter R. The absolute threshold for vision in the cat. J Physiol. 1951;114:8–15. doi: 10.1113/jphysiol.1951.sp004599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther E, Zrenner E. The spectral sensitivity of dark- and light-adapted cat retinal ganglion cells. J Neurosci. 1993;13(4):1543–1550. doi: 10.1523/JNEUROSCI.13-04-01543.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslwanter T. Mathematics of three-dimensional eye rotations. Vision Res. 1995;35(12):1727–1739. doi: 10.1016/0042-6989(94)00257-m. [DOI] [PubMed] [Google Scholar]
- Hollands MA, Marple-Horvat DE. Visually guided stepping under conditions of step cycle-related denial of visual information. Exp Brain Res. 1996;109(2):343–356. doi: 10.1007/BF00231792. [DOI] [PubMed] [Google Scholar]
- Hollands MA, Marple-Horvat DE. Coordination of eye and leg movements during visually guided stepping. J Mot Behav. 2001;33(2):205–216. doi: 10.1080/00222890109603151. [DOI] [PubMed] [Google Scholar]
- Hollands MA, Marple-Horvat DE, Henkes S, Rowan AK. Human eye movements during visually guided stepping. J Mot Behav. 1995;27(2):155–163. doi: 10.1080/00222895.1995.9941707. [DOI] [PubMed] [Google Scholar]
- Imai T, Moore ST, Raphan T, Cohen B. Interaction of the body, head, and eyes during walking and turning. Exp Brain Res. 2001;136(1):1–18. doi: 10.1007/s002210000533. [DOI] [PubMed] [Google Scholar]
- Kang I, Malpeli JG. Behavioral calibration of eye movement recording systems using moving targets. J Neurosci Methods. 2003;124(2):213–218. doi: 10.1016/s0165-0270(03)00019-0. [DOI] [PubMed] [Google Scholar]
- Lambert FM, Combes D, Simmers J, Straka H. Gaze stabilization by efference copy signaling without sensory feedback during vertebrate locomotion. Curr Biol. 2012;22(18):1649–1658. doi: 10.1016/j.cub.2012.07.019. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Brown JL. Dark adaptation and spectral sensitivity in the cat. Vision Res. 1970;10(8):703–716. doi: 10.1016/0042-6989(70)90017-9. [DOI] [PubMed] [Google Scholar]
- Land MF. The coordination of rotations of the eyes, head and trunk in saccadic turns produced in natural situations. Exp Brain Res. 2004;159(2):151–160. doi: 10.1007/s00221-004-1951-9. [DOI] [PubMed] [Google Scholar]
- Land MF. Eye movements and the control of actions in everyday life. Prog Retin Eye Res. 2006;25(3):296–324. doi: 10.1016/j.preteyeres.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Land MF. Vision, eye movements, and natural behavior. Vis Neurosci. 2009;26(1):51–62. doi: 10.1017/S0952523808080899. [DOI] [PubMed] [Google Scholar]
- Land MF, Lee DN. Where we look when we steer. Nature. 1994;369:742–744. doi: 10.1038/369742a0. [DOI] [PubMed] [Google Scholar]
- Land MF, Hayhoe M. In what ways do eye movements contribute to everyday activities? Vision Res. 2001;41:3559–3565. doi: 10.1016/s0042-6989(01)00102-x. [DOI] [PubMed] [Google Scholar]
- Lankheet MJ, Rowe MH, van Wezel RJ, van de Grind WA. Horizontal cell sensitivity in the cat retina during prolonged dark adaptation. Vision Neurosi. 1996;13(5):885–896. doi: 10.1017/s0952523800009135. [DOI] [PubMed] [Google Scholar]
- Lee DN. The optic flow field: the foundation of vision. Philos Trans R Soc Lond B Biol Sci. 1980;290(1038):169–179. doi: 10.1098/rstb.1980.0089. [DOI] [PubMed] [Google Scholar]
- Marigold DS, Patla AE. Gaze fixation patterns for negotiating complex ground terrain. Neuroscience. 2007;144(1):302–313. doi: 10.1016/j.neuroscience.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Marigold DS, Weerdesteyn V, Patla AE, Duysens J. Keep looking ahead? Re-direction of visual fixation does not always occur during an unpredictable obstacle avoidance task. Exp Brain Res. 2007;176(1):32–42. doi: 10.1007/s00221-006-0598-0. [DOI] [PubMed] [Google Scholar]
- Moore ST, Hirasaki E, Cohen B, Raphan T. Effect of viewing distance on the generation of vertical eye movements during locomotion. Exp Brain Res. 1999;129(3):347–361. doi: 10.1007/s002210050903. [DOI] [PubMed] [Google Scholar]
- Mulavara AP, Bloomberg JJ. Identifying head-trunk and lower limb contributions to gaze stabilization during locomotion. J Vestib Res. 2002–2003;12(5–6):255–269. [PubMed] [Google Scholar]
- Mulavara AP, Houser J, Miller C, Bloomberg JJ. Full-body gaze control mechanisms elicited during locomotion: effects of VOR adaptation. J Vestib Res. 2005;15(5–6):279–289. [PubMed] [Google Scholar]
- Mulavara AP, Richards JT, Ruttley T, Marshburn A, Nomura Y, Bloomberg JJ. Exposure to a rotating virtual environment during treadmill locomotion causes adaptation in heading direction. Exp Brain Res. 2005;166(2):210–219. doi: 10.1007/s00221-005-2356-0. [DOI] [PubMed] [Google Scholar]
- Ogorodnikov D, Tarasenko S, Yakushin S, Cohen B, Raphan T. Head fixed field coil system for measuring eye movements in freely moving monkeys. Conf Proc IEEE Eng Med Bio Soc. 2006;1:5567–5570. doi: 10.1109/IEMBS.2006.260407. [DOI] [PubMed] [Google Scholar]
- Patla AE, Vickers JN. Where and when do we look as we approach and step over an obstacle in the travel path? Neuroreport. 1997;8(17):3661–3665. doi: 10.1097/00001756-199712010-00002. [DOI] [PubMed] [Google Scholar]
- Patla AE, Vickers JN. How far ahead do we look when required to step on specific locations in the travel path during locomotion? Exp Brain Res. 2003;148(1):133–138. doi: 10.1007/s00221-002-1246-y. [DOI] [PubMed] [Google Scholar]
- Patla AE, Adkin A, Martin C, Holden R, Prentice S. Characteristics of voluntary visual sampling of the environment for safe locomotion over different terrains. Exp Brain Res. 1996;112(3):513–522. doi: 10.1007/BF00227957. [DOI] [PubMed] [Google Scholar]
- Pozzo T, Berthoz A, Lefort L. Head stabilization during various locomotor tasks in humans. I. Normal subjects. Exp Brain Res. 1990;82(1):97–106. doi: 10.1007/BF00230842. [DOI] [PubMed] [Google Scholar]
- Prilutsky BI, Sirota MG, Gregor RJ, Beloozerova IN. Quantification of motor cortex activity and full-body biomechanics during unconstrained locomotion. J Neurophysiol. 2005;94(4):2959–2969. doi: 10.1152/jn.00704.2004. [DOI] [PubMed] [Google Scholar]
- Pryor K. Lads before the wind. New York: Harper and Row; 1975. [Google Scholar]
- Rapaport DH, Stone J. The area centralis of the retina in the cat and other mammals: focal point for function and development of the visual system. Neuroscience. 1984;11(2):289–301. doi: 10.1016/0306-4522(84)90024-1. [DOI] [PubMed] [Google Scholar]
- Raphan T, Matsuo V, Cohen B. Velocity storage in the vestibulo-ocular reflex arc (VOR) Exp Brain Res. 1979;35(2):229–248. doi: 10.1007/BF00236613. [DOI] [PubMed] [Google Scholar]
- Rayner K. Eye movements in reading and information processing: 20 years of research. Psychol Bull. 1998;124(3):372–422. doi: 10.1037/0033-2909.124.3.372. [DOI] [PubMed] [Google Scholar]
- Rieiro H, Martinez-Conde S, Danielson AP, Pardo-Vazquez JL, Srivastava N, Macknik SL. Optimizing the temporal dynamics of light to human perception. Proc Natl Acad Sci USA. 2012;109(48):19828–19833. doi: 10.1073/pnas.1213170109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers TJ, Sirota MG, Guttentag AI, Ogorodnikov DA, Beloozerova IN. Program No. 454.14. 2009 Neuroscience Meeting Planner. Chicago, IL: SFN; 2009. Gaze behaviors of freely walking cats. Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers TJ, Shaw NA, Sirota MG, Beloozerova IN. Program No. 278.3. 2010 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2010. The relationship between vertical gaze shifts and stride cycle in freely walking cats. Online. [Google Scholar]
- Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Sherk H, Fowler GA. Optic flow and the visual guidance of locomotion in the cat. Int Rev Neurobiol. 2000;44:141–170. doi: 10.1016/s0074-7742(08)60741-2. [DOI] [PubMed] [Google Scholar]
- Sherk H, Fowler GA. Visual analysis and image motion in locomoting cats. Eur J Neurosci. 2001;13(6):1239–1248. doi: 10.1046/j.0953-816x.2001.01493.x. [DOI] [PubMed] [Google Scholar]
- Skinner BF. The Behavior of Organisms: an experimental analysis. New York: Appleton-Century-Crofts, Inc; 1938. [Google Scholar]
- Solomon D, Cohen B. Stabilization of gaze during circular locomotion in light. I. Compensatory head and eye nystagmus in the running monkey. J Neurophysiol. 1992a;67(5):1146–1157. doi: 10.1152/jn.1992.67.5.1146. [DOI] [PubMed] [Google Scholar]
- Solomon D, Cohen B. Stabilization of gaze during circular locomotion in darkness. II. Contribution of velocity storage to compensatory eye and head nystagmus in the running monkey. J Neurophysiol. 1992b;67(5):1158–1170. doi: 10.1152/jn.1992.67.5.1158. [DOI] [PubMed] [Google Scholar]
- Srinivasan MV, Zhang S. Visual motor computations in insects. Annu Rev Neurosci. 2004;27:679–696. doi: 10.1146/annurev.neuro.27.070203.144343. [DOI] [PubMed] [Google Scholar]
- Stehouwer DJ. Compensatory eye movements produced during fictive swimming of a deafferented, reduced preparation in vitro. Brain Res. 1987;410(2):264–268. doi: 10.1016/0006-8993(87)90323-4. [DOI] [PubMed] [Google Scholar]
- Sun HJ, Carey DP, Goodale MA. A mammalian model of optic-flow utilization in the control of locomotion. Exp Brain Res. 1992;91(1):171–175. doi: 10.1007/BF00230026. [DOI] [PubMed] [Google Scholar]
- Turano KA, Geruschat DR, Baker FH. Fixation behavior while walking: persons with central visual field loss. Vision Res. 2002;42(23):2635–2644. doi: 10.1016/s0042-6989(02)00299-7. [DOI] [PubMed] [Google Scholar]
- Vatsia ML, Stich UK, Dunlap D. Night-Sky Radiant Sterance from 450 to 2000 Nanometers. Defense Technical Information Center; 1972. p. 42. [Google Scholar]
- Warren WH, Jr, Kay BA, Zosh WD, Duchon AP, Sahuc S. Optic flow is used to control human walking. Nat Neurosci. 2001;4(2):213–216. doi: 10.1038/84054. [DOI] [PubMed] [Google Scholar]
- Wilkie RM, Wann JP. Eye-movements aid the control of locomotion. J Vis. 2003;3(11):677–684. doi: 10.1167/3.11.3. [DOI] [PubMed] [Google Scholar]
- Wilkinson EJ, Sherk HA. The use of visual information for planning accurate steps in a cluttered environment. Behav Brain Res. 2005;164(2):270–274. doi: 10.1016/j.bbr.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Wurtz RH. Neuronal mechanisms of visual stability. Vision Res. 2008;48(20):2070–2089. doi: 10.1016/j.visres.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Yakushin SB, Kunin M, Raphan T, Cohen B. Head stabilization by vestibulocollic reflexes during quadrupedal locomotion in monkey. J Neurophysiol. 2008;100(2):763–780. doi: 10.1152/jn.90256.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.