Abstract
The introduction of reduced intensity conditioning regimens (RIC) made it possible to offer allogeneic hematopoietic cell transplantation (alloHCT) to older patients with myelodysplastic syndromes (MDS). However, the relative risks and benefits of alloHCT compared to novel non-transplant therapies continue to be the source of considerable uncertainty. We will perform a prospective biologic assignment trial to compare RIC alloHCT to non-transplant therapies based on donor availability. Primary outcome is 3-year overall survival. Secondary outcomes include leukemia-free survival, quality of life, and cost-effectiveness. Four hundred patients will be enrolled over roughly 3 years. Planned subgroup analyses will evaluate key biologic questions, such as the impact of age & response to hypomethylating agents on treatment effects. Findings from this study potentially may set a new standard of care for older MDS patients who are considered candidates for alloHCT.
Keywords: Biologic assignment, MDS, transplantation
Introduction
Myelodysplastic syndromes (MDS) represent a heterogeneous group of acquired malignant bone marrow disorders characterized by high rates of apoptosis leading to ineffective hematopoiesis.1 An acquired bone marrow failure picture ensues and leads to varying degrees of peripheral blood cytopenias and potentially fatal complications, including infection and bleeding.1, 2 MDS is most often diagnosed in elderly individuals with a median age of 76 years at diagnosis,3, 4. Overall, about 30% of individuals with MDS progress to acute myeloid leukemia (AML), although the probability of progression is largely determined by disease risk at presentation4, 5 (e.g. the 2-year risk of progression to AML is 80% for those with high-risk disease but only 10% among those with low risk disease).6
The most widely used prognostic classification system for MDS is the International Prognostic Scoring System (IPSS), which takes into account the number of bone marrow blasts, cytogenetic abnormalities, and cytopenias.5 The IPSS classifies patients into low-risk, intermediate-1, intermediate-2, and high-risk stages. The median survival ranges from 5.7 years for those with low-risk disease to being only measured in months for those with high-risk disease.5 Very recently, a newer IPSS was published (IPSS-R),7 but to date most clinical experience and conduct of investigative clinical trials for MDS have used the original IPSS.
A wide range of therapeutic approaches exist for patients with MDS, which are typically selected based on the patient's estimated risk of death.5-9 And treatment guidelines have been developed by independent groups.10, 11 Introduction of hypomethylating agents (HMA) prolongs progression-free survival,12 overall survival, 13, 14 and delays transformation to AML.12-14 However, to date, allogeneic hematopoietic cell transplantation (alloHCT) remains the only curative therapeutic modality available. Despite its curative potential, because of the risk of non-relapse mortality with alloHCT in a population of mostly older individuals, many patients with MDS are still not referred for transplant evaluation.15 A recent query of transplantation activity reported to the Center for International Blood & Marrow Transplant Research (CIBMTR) showed that out of a total of 3,101 alloHCTs performed in the US between 2000 and 2010 for MDS, only 232 (7.5%) were among persons aged 65 years and older (unpublished data; W.S., personal communication). Recent studies, however, have shown that among patients who were considered to be candidates for alloHCT and were referred to the transplant programs, age was not an important predictor of post-transplant outcomes.16-18 With the introduction of reduced intensity conditioning regimens (RIC) alloHCT, which have been shown to be associated with promising results in MDS,19-21 as well as expanded coverage for the alloHCT by Medicare under the Coverage-with-Evidence-Development (CED) mechanism,22 more patients are now undergoing this curative therapy.23
To better define the value of alloHCT comparative analyses are needed. Few such analyses have been performed. In a retrospective cohort analysis, alloHCT (n=103) recipients were 70% less likely to die (p=.007) compared to patients that only received hypomethylating agents (HMA).24 However, this particular study did not control for lead time bias,24 and therefore, the results should be interpreted with great caution.25 In a recent retrospective analysis, the investigators employed a multistate statistical model to define the optimal timing of alloHCT for MDS patients aged 60-70 (n=514).26 This analysis demonstrated that among those with low risk MDS (IPSS low-risk/intermediate-1), non-transplant therapies provided a higher life expectancy, while among those with high risk MDS (IPSS intermediate-2/high-risk) proceeding immediately to alloHCT was associated with higher life expectancy than non-transplant approaches.26 A small prospective study compared alloHCT to non-transplant approaches using a “donor vs. no donor” comparison.27 One hundred and sixty three patients with intermediate/high risk MDS were enrolled. The distribution of donor status was as follows: 34 had no donors; 115 had a human leukocyte antigen (HLA) matched donor (identical sibling or well matched (HLA 10/10) unrelated donor [WMUD]); 14 had partially matched (HLA 9/10) unrelated donor (PMUD). The primary outcome (overall survival at 48 months) was significantly different among the three groups (p=0.01). The corresponding survival probabilities were 17% (95% confidence intervals [CI] 6-43), 35% (95% CI 26-49), and 8% (95% CI 1-55). Whether the difference between the no donor arm and the HLA matched donor group was significant is unclear, however. Given the wide confidence intervals around these estimates, these results need confirmation in larger studies.
Given the lack of definitive prospective data evaluating the relative risks/benefits of alloHCT compared to non-transplant approaches among older MDS patients, and as a response to the Centers for Medicare & Medicaid Services (CMS) CED for National Coverage Determination of Stem Cell Transplantation, The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) has launched BMT CTN 1102 prospective study to address this knowledge gap.28 In this paper, we discuss the design of BMT CTN 1102 and steps taken to address potential sources of bias.
Study Overview
The fundamental question being addressed is whether patients aged 50-75 years with high-risk MDS referred to transplantation centers, and for whom a suitable donor is available, have a 3-year survival advantage with RIC alloHCT compared to non-transplant based therapies (offered to those without a suitable donor but transplant eligible). Other key outcomes include leukemia-free survival, quality of life, and cost-effectiveness.
Figure 1 depicts the overall study schema. Subjects are enrolled at the referral visit to the transplant center. Subjects for whom a search for an unrelated donor was initiated prior to their referral to the transplant center will not be eligible for participation in this study. However, subjects for whom sibling tissue typing was initiated prior to their referral will be allowed to participate. All subjects are initially assigned to the no donor arm at the time of enrollment. Subjects with a suitable donor will be reassigned to the donor arm when a donor is identified. A suitable donor is defined as either an HLA-matched related donor, or an 8/8 (HLA-A, -B, -C, and -DRB1) matched unrelated donor (by confirmatory typing). If no suitable donor is identified during a 90-day interval (from enrollment), subjects will be permanently assigned to the no donor arm. The 90-day interval was chosen based on prior experience from the National Marrow Donor Program that if a donor is not found in this time frame, the subsequent likelihood of finding a donor is very small.
Figure 1. Study Schema.
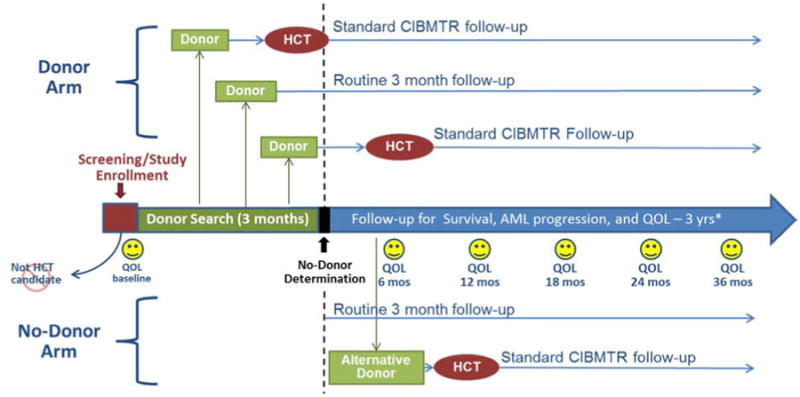
Key Issues in Study Design
Randomization vs. Biologic Assignment
Randomized controlled trials (RCT) represent the gold standard design when one wishes to compare two therapies. However, among patients who are referred for transplant evaluation, and for whom an HLA matched donor has been identified, it is generally viewed as not feasible to conduct a true RCT (i.e. randomize patients with HLA matched donors to alloHCT vs. non transplant).29, 30 An alternative approach is to conduct a biological assignment trial, which means that patients with a suitable HLA-matched donor are assigned to the alloHCT arm, while patients who do not have such a donor are assigned to the non-transplantation arm. Biologic assignment trials have been used successfully to evaluate the role of alloHCT across multiple hematologic malignancies.31-36. With this design, accrual is significantly enhanced, and therefore this is a more feasible design compared to a RCT.37 Given prior beliefs and misconceptions regarding the value of alloHCT among those with a donor, biologic assignment design provides a reasonable platform upon which the study can be performed.37 BMT CTN 1102 is designed as a prospective, comparative biologic assignment study of RIC alloHCT from related and unrelated donors vs. hypomethylating therapy/best supportive care among patients with intermediate-2/high-risk de novo MDS.
Although selection bias can arise in biologic assignment, it is the most feasible design for this trial. The study was carefully designed and adjusted analysis was planned to minimize potential bias as discussed below.
Enrolling at Referral to Transplant Program vs. Other Time points (e.g. at Diagnosis or at Complete Remission)
Since most newly diagnosed MDS patients are treated by community oncologists, it is difficult to recruit patients at diagnosis before they have received therapy.
Unlike acute leukemias where a complete remission is the objective of induction chemotherapy (and therefore would be an ideal time to enroll patients onto a study that evaluates consolidative strategies), induction chemotherapy is rarely used at the time of initial MDS diagnosis and a complete remission occurs in a minority of MDS patients on HMAs, rendering enrollment at CR neither a clinically meaningful nor a feasible time point.12, 14 BMT CTN 1102 is addressing the fundamental question of whether RIC alloHCT offers a survival advantage compared to non-transplant therapies, among older MDS patients who are felt to be transplantation candidates. Therefore, enrolling patients at the time of referral would seem to be a reasonable approach.
Enrollment Bias and Prognostic Factor Imbalances
Given the lack of randomization, biologic assignment trials are inherently vulnerable to enrollment bias and prognostic factor imbalances. A detailed discussion of enrollment bias in the setting of biologic assignment trials has been published elsewhere.37 In order to reduce bias and assemble the proper control group for the “donor-arm,” it is critical to enroll patients without knowledge of whether a donor is available or not. Therefore, patients whose tissue typing for unrelated donors was initiated prior to referral will not be eligible. Patients whose tissue typing for a sibling donor was initiated prior to referral will still be eligible. The latter should not risk enrollment bias, because for those patients in whom the tissue typing rules out available sibling donors, they will still be considered for a HLA-matched unrelated donor transplant. Additionally, a multivariate analysis is planned that will adjust for any serious imbalances in baseline characteristics.
Potential Bias Introduced During Donor Search
Excessive early deaths (early indicates no suitable donor is identified and the 90-day window is not yet reached) could potentially bias the study in favor of the RIC alloHCT arm, since subjects who die before a donor is identified will be analyzed in the no-donor arm. Conversely, subjects with an already identified sibling donor (which means immediate assignment to the donor arm) who are referred to the transplant center who experience early death (early indicates death occurring less than 90 days from enrollment and before RIC alloHCT is actually performed) are analyzed in the donor group, which could bias the study in favor of the non-transplant arm. The protocol team will monitor the rates of early death carefully and will report them to the Data Safety Monitoring Board. The protocol team expects that these early deaths rates will be very small given that these patients were felt to be eligible to undergo alloHCT within the preceding 2-3 months.
Eligibility Criteria
Patients with de novo MDS are eligible irrespective of how long they had MDS.38 Patients must have (or previously had) an intermediate-2 or high-risk IPSS stage.5 Our intent is to include patients in whom a RIC alloHCT is preferred based on physician's assessment. Patients younger than 50 years of age typically undergo high intensity conditioning alloHCT.23 Therefore, subjects aged 50-75 years will be eligible to participate.
For subjects to be assigned to the donor arm, a suitable donor must be identified, which as previously stated is defined as either HLA matched related donor or 8/8 HLA well matched unrelated donor. Two recent analyses informed the decision to restrict donor types to only these two. We recently reported outcomes of 701 MDS patients (median age 53 years (range 22-78)) after HLA-identical sibling versus 8/8 (HLA-A, -B, -C, and DRB1) matched unrelated donor vs. 7/8 HLA-partially matched unrelated donor alloHCT.39 In multivariate analysis, HLA-identical sibling HCT recipients had similar survival compared to 8/8 matched unrelated donor HCT recipients, and both HLA-identical sibling and 8/8 matched unrelated donor groups had superior survival compared to 7/8 HLA-partially matched unrelated HCT recipients (Table 1).39
Table 1. Multivariate Analysis of a Cohort of Adult MDS Patients who Underwent HLA-Identical Sibling (MRD) HCT or 8/8 or 7/8 Matched Unrelated Donor (MUD) HCT from 2002-2006.
| Transplant related mortality RR (95 % CI) | P value | Relapse RR (95 % CI) | P value | Treatment Failure (Death or Relapse) RR (95 % CI) | P value | Mortality RR (95% CI) | P value | |
|---|---|---|---|---|---|---|---|---|
| 8/8 MUD vs. MRD | 1.43 (1.06-1.95) | 0.02 | 0.85 (0.60-1.18) | 0.33 | 1.13 (0.91-1.42) | 0.26 | 1.24 (0.98-1.56) | 0.06 |
| 7/8 MUD vs. MRD | 1.80 (1.23-2.63) | 0.002 | 1.02 (0.66-1.60) | 0.91 | 1.47 (1.10-1.96) | 0.008 | 1.62 (1.21-2.17) | 0.001 |
| 7/8 MUD vs. 8/8 MUD | 1.25 (0.91-1.72) | 0.16 | 1.21 (0.81-1.81) | 0.35 | 1.29 (1.00-1.66) | 0.04 | 1.30 (1.01-1.68) | 0.03 |
Adapted from Saber et al.39
Additionally, using data from the CIBMTR, we recently conducted an exploratory analysis to determine the impact of different donor sources on post alloHCT outcomes in patients with MDS (unpublished data). We selected patients who were at least 21 years of age that received an alloHCT for MDS between 2000 and 2010 in the United States. Donor sources were: HLA-identical sibling (n=1458), well matched unrelated donors (n=1091), partially matched unrelated donors (n=273), cord blood (n=153), and haploidentical donors (n=95). Median age at HCT was 54 (range 21-81) and age distribution was similar across the 5 groups. In univariate analysis, survival was significantly lower for partially matched unrelated donors, umbilical cord graft and haploidentical donor alloHCT compared to HLA-identical sibling and well matched unrelated donor alloHCT (p<0.001). Survival was not significantly different (p=0.56) among the three alternative donor groups (Table 1s).
Together, these analyses suggest that transplantation from alternative donors is associated with significantly poorer outcomes than transplantation from HLA-identical sibling and well matched unrelated donor. Therefore, only HLA-identical siblings or 8/8 HLA well matched unrelated donors are considered suitable donor types in this study. Physicians and patients participating in this study should have no intention to pursue alternative donor HCT if a suitable donor is not available. In addition, if a suitable donor is available, the intention should be to proceed to RIC alloHCT as soon as possible.
The outcomes of therapy-related MDS with non-transplant therapies are quite dismal. A recent analysis by Bally et al. suggests a 2-year survival of 14% with DNA hypomethylating agents.40 Given the grim prognosis with HMA, these patients may benefit from an alloHCT from an alternative donor even if a suitable donor is not available, and therefore patients with therapy-related MDS are excluded from this study.
RIC AlloHCT Regimens
The actual choice of regimen is left to the discretion of the treating physician. However, regimens must be declared by the center as a preferred regimen in order to assure that alloHCT is performed according to the institutional standard. The rationale for not specifying a particular RIC regimen is further supported by recent analysis of data reported to the CIBMTR (unpublished). In univariate analysis of outcomes of MDS patients aged 50-75 that underwent RIC alloHCT between 2005 and 2011, and controlling for donor type, we found no significant difference in survival between the different reduced intensity conditioning regimens (Table 2s).
Non-Transplant Regimens
The protocol does not mandate particular non-transplant therapy. However, we expect that the vast majority of patients will be treated with HMA. We acknowledge that non-transplant treatments used for patients in the control arm may vary. However, there are no non-transplant therapies that produce cures in patients with high-risk MDS, and most non-transplant patients will receive the few FDA-approved and available therapies when appropriate. The poor outcomes associated with these therapies are demonstrated in Table 3s. Data on the type of non-transplant therapies will be captured (i.e. whether HMA were received or not, number of cycles, duration of therapy).
Feasibility
Based on historical CIBMTR data and assuming an accrual rate of 40%, we expect annual enrollment of 84 patients to the RIC alloHCT arm. The length of time required to accrue the targeted sample size for this study depends on the proportion of enrolled patients with a suitable donor. Table 4s provides estimated annual accruals for various proportions of donor availability. Based on these assumptions, it is estimated that 2.5-3.5 years of accrual will be necessary to enroll the targeted sample size.
Data Collection for Subjects Assigned to the No Donor Arm
Subjects assigned to the no donor arm will continue to be followed by their primary hematologists. The HCT centers which enrolled and registered the patients will be responsible for periodic contact (every 3 months for Year 1 and 2, every 6 months in Year 3: +/- 1 month) with the primary hematologists. Documentation of transformation to AML will be requested, as well as treatment history. Vital status (death or alive), and date of the last follow up or death will be recorded.
Data Collection for Subjects Assigned to the Donor Arm
The schedule of follow up for subjects assigned to the donor arm but had not yet undergone alloHCT will follow the same schedule outlined above for the no-donor arm. Once transplanted, the follow up will be through submission of CIBMTR pre- and post-transplant comprehensive Report Forms. In the event that patients with donors DO NOT undergo transplantation, the follow up will remain the responsibility of the transplant center, with the same schedule outlined above for the no-donor arm.
Key Issues in Study Analysis
Intention-To-Treat vs. As Treated Analysis
To minimize bias that may occur post assignment, intention to treat (ITT) analysis is planned as the primary analysis. Table 2 gives examples that illustrate how the ITT principle will be maintained when events occur during the 90-day interval and beyond. Additional sensitivity analyses excluding patients who died or dropped out before 90 days from enrollment as well as a secondary analysis using as-treated principle will also be conducted.
Table 2. Examples of Treatment Arm Assignment and Subsequent Analysis Based on Suitable Donor Identification.
| Scenario | Arm |
|---|---|
| A transplant donor is identified 30 days from consent. The subject undergoes transplantation 70 days from consent. | RIC alloHCT |
| A transplant donor is identified 30 days from consent. The subject receives 4 cycles of hypomethylating therapy and undergoes transplantation 200 days from consent. | RIC alloHCT |
| A transplant donor is identified 30 days from consent. The subject receives 4 cycles of hypomethylating therapy and expires related to an infection prior to transplantation. | RIC alloHCT |
| The patient has an identified sibling donor on the date of consent. The patient eventually declines transplantation. | RIC alloHCT |
| A donor search is begun after enrollment, and the subject begins hypomethylating therapy. The subject expires 80 days after consent without a donor being identified. | Non-Transplant Arm |
| A donor search is begun after enrollment, and the subject begins hypomethylating therapy. No donor is identified after a 90 day search. The subject continues on hypomethylating therapy. | Non-Transplant Arm |
| A donor search is begun after enrollment, and the subject begins hypomethylating therapy. No donor is identified after a 90 day search. The subject progresses and undergoes alternative donor transplantation 150 days from consent. | Non-Transplant Arm |
Statistical Power
Secondary analyses of published data from CIBMTR for high-risk MDS patients older than age 50 suggest three-year OS estimates between 35-40%.39 Based on data from a compassionate use program of DNA hypomethylating agents, the three-year OS probabilities for the non-transplant arm are estimated to range between 20% and 25%.41 Based on these data, we expect to observe an absolute difference of 15% in three-year OS probabilities in favor for patients assigned to the RIC alloHCT. Table 5s gives the estimated sample size and power (at least 80% power) for various combinations of baseline survival probability and donor availability. It is expected that 60% - 70% of patients will have a donor. The required sample size increases with higher percentage of donor availability.
Planned Subgroup Analyses
The value of HMA therapy pre-HCT- and more specifically, the “optimal” timing to refer a patient on HMA therapy for transplantation evaluation- remains unknown. Retrospective analyses have examined the impact of pre-HCT HMA therapy on post-HCT outcomes. The largest study included 163 individuals who underwent HCT after azacitidine, after leukemia-type induction chemotherapy. There were no differences in post-HCT outcomes.42 A smaller study from Seattle demonstrated a slight advantage to pre-HCT therapy with azacitidine over induction chemotherapy, potentially because of reduced toxicity.43 Both of these studies, however, lack the size of the original patient population initially considered for transplantation—the actual denominator—without which it is impossible to determine the role of one pre-HCT approach vs another. Other retrospective analyses compared pre-HCT HMA therapy with no treatment, and showed no benefit to HMA therapy. These studies were affected by similar selection biases as mentioned above.44, 45 Predefined subgroup analyses to determine the impact of pre-HCT HMA therapy (including response to HMA therapy) on the study outcomes will be performed to address this important question.
Additional predefined subgroup analyses will examine the impact of the following factors on treatment effect: patient age (<65 years vs. ≥65 years); disease duration; IPSS and IPSS-R.
Discussion/Conclusions
Understanding the relative risks/benefits of alloHCT can move the field significantly forward. We believe that the current study design affords the best practical approach to achieving this goal in a relatively unbiased fashion. Despite potential limitations discussed above, the strength of the current design is that it is likely to result in successful accrual by: 1) having eligibility criteria that will capture a large segment of the patients referred for transplantation; 2) allowing flexibility in both transplant and non-transplant therapies administered; 3) minimizing the data collection burden; and, 4) providing the optimal comparator groups given the constraints discussed above. It also allows for quality-of-life and cost-effectiveness studies, which are being planned in a “real-world” population of patients. Recently, a Data Safety Monitoring Board recommended that NHLBI prematurely terminate BMT CTN 0901 (Cinicaltrials.gov identifier: NCT0133991046) due to preliminary data suggesting that high intensity regimens were associated with superior outcomes compared to RIC regimens permitted in the study among patients with AML and MDS who were eligible to get either regimen intensities. Since the data were not sufficiently mature to perform subgroup analysis based on disease type, it is not known currently whether these preliminary findings are consistent among AML vs. MDS patients. These analyses will be performed in the future. We acknowledge that these preliminary results might lead some physicians to prefer higher intensity regimens in some patients otherwise eligible for BMT CTN 1102. However, we believe the proportion will be relatively low, since the eligibility criteria for BMT CTN 1102 from the start were only patients felt to be candidates for RIC alloHCT and NOT high intensity regimens (because of comorbidities or age). Patients who are candidates for higher intensity regimens are NOT currently eligible for enrollment onto BMT CTN 1102.
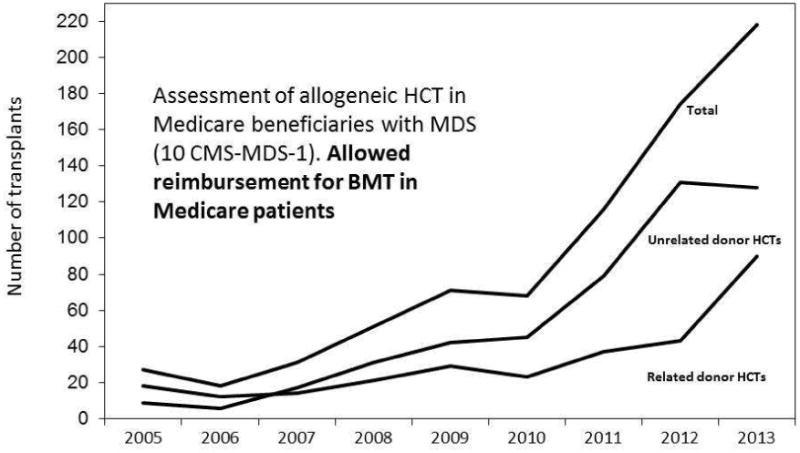
The results of BMT CTN 1102 have the potential to change practice. If this study demonstrates a significant survival advantage with alloHCT, we expect that the number of patients transplanted would increase significantly, setting the stage for more refined studies. In fact, emerging data from an ongoing single arm prospective study conducted at the CIBMTR to evaluate safety of alloHCT for older MDS patients,18 also made possible by the expanded coverage for the alloHCT by Medicare under the CED mechanism,22 clearly show that under this coverage mechanism the number of HCTs have risen dramatically (Figure 2). These data strongly support that barriers to access to alloHCT care is affecting the decision to refer patients for transplant evaluation. BMT CTN 1102 has recently gained approval by CMS, and therefore, costs of alloHCT for Medicare beneficiaries enrolled onto this study will be covered. If the study demonstrates survival advantage with alloHCT, this will have significant implications with respect to coverage of costs of alloHCT for MDS by CMS for all Medicare beneficiaries in the future. Alternatively, if the study fails to show an advantage to allogeneic HCT, it will make us dramatically rethink how we approach the problem of MDS in this patient population. Finally, if the study fails to accrue adequate numbers of patients for completion a timely manner, there is the definite risk that Medicare will choose to no longer continue coverage and we will never know the answer.
Figure 2. US Allogeneic Transplants for MDS in patients older than 65, 2005 - 2013.
Supplementary Material
Table 1s. Survival Probabilities by Donor Type
Table 2s. Univariate Analysis of Transplantation Outcomes for MDS Patients by Conditioning Regimen and Donor Type
Table 3s. Outcomes of Non-Transplant Therapy in MDS
Table 4s: Estimated Annual Accrual
Table 5s: Power to Detect 15% Increase in OS Probability in the Transplant Arm for Various Survival Probabilities and Proportions of Donor Availability
Acknowledgments
The design and conduct of BMT CTN 1102 is supported by a Grant/Cooperative Agreement U10 HL069294 from the National Heart, Lung, and Blood Institute (NHLBI) and the National Cancer Institute (NCI) of the National Institutes of Health (NIH).
Data collection on alloHCT recipients will be through the CIBMTR, which is supported by Public Health Service Grant/Cooperative Agreement U24 CA076518 from the NCI, the NHLBI and the National Institute of Allergy and Infectious Diseases (NIAID); a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-12-1-0142 and N00014-13-1-0039 from the Office of Naval Research; and grants from Allos Therapeutics, Inc.; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Celgene Corporation; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;Gentium SpA; Genzyme Corporation; GlaxoSmithKline; HistoGenetics, Inc.; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Remedy Informatics; Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; TerumoBCT; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Raza A, Galili N. The genetic basis of phenotypic heterogeneity in myelodysplastic syndromes. Nat Rev Cancer. 2012 Dec;12(12):849–859. doi: 10.1038/nrc3321. [DOI] [PubMed] [Google Scholar]
- 2.Scott BL, Deeg HJ. Myelodysplastic syndromes. Annu Rev Med. 2010;61:345–358. doi: 10.1146/annurev.med.051308.132852. [DOI] [PubMed] [Google Scholar]
- 3.Ma X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes: incidence and survival in the United States. Cancer. 2007 Apr 15;109(8):1536–1542. doi: 10.1002/cncr.22570. [DOI] [PubMed] [Google Scholar]
- 4.Rollison DE, Howlader N, Smith MT, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001-2004, using data from the NAACCR and SEER programs. Blood. 2008 Jul 1;112(1):45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997 Mar 15;89(6):2079–2088. [PubMed] [Google Scholar]
- 6.Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007 Aug 10;25(23):3503–3510. doi: 10.1200/JCO.2006.08.5696. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012 Sep 20;120(12):2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Manero G. Myelodysplastic syndromes: 2012 update on diagnosis, risk-stratification, and management. Am J Hematol. 2012 Jul;87(7):692–701. doi: 10.1002/ajh.23264. [DOI] [PubMed] [Google Scholar]
- 9.Kantarjian H, O'Brien S, Ravandi F, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer. 2008 Sep 15;113(6):1351–1361. doi: 10.1002/cncr.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg PL, Attar E, Bennett JM, et al. Myelodysplastic syndromes. J Natl Compr Canc Netw. 2013 Jul 1;11(7):838–874. doi: 10.6004/jnccn.2013.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malcovati L, Hellstrom-Lindberg E, Bowen D, et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood. 2013 Oct 24;122(17):2943–2964. doi: 10.1182/blood-2013-03-492884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lubbert M, Suciu S, Baila L, et al. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol. 2011 May 20;29(15):1987–1996. doi: 10.1200/JCO.2010.30.9245. [DOI] [PubMed] [Google Scholar]
- 13.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009 Mar;10(3):223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002 May 15;20(10):2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 15.Sekeres MA, Schoonen WM, Kantarjian H, et al. Characteristics of US patients with myelodysplastic syndromes: results of six cross-sectional physician surveys. J Natl Cancer Inst. 2008 Nov 5;100(21):1542–1551. doi: 10.1093/jnci/djn349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim Z, Brand R, Martino R, et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol. 2010 Jan 20;28(3):405–411. doi: 10.1200/JCO.2009.21.8073. [DOI] [PubMed] [Google Scholar]
- 17.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010 Apr 10;28(11):1878–1887. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atallah E, Pedersen TL, Warlick ED, et al. The Outcome of Hematopoietic Cell Transplantation (HCT) for Myelodysplastic Syndrome (MDS) in Adults >=65 Years of Age: First Report of the Coverage with Evidence Development (CED) in Medicare Beneficiaries. ASH Annual Meeting Abstracts. 2012 Nov 16;120(21):1983. 2012. [Google Scholar]
- 19.Laport GG, Sandmaier BM, Storer BE, et al. Reduced-intensity conditioning followed by allogeneic hematopoietic cell transplantation for adult patients with myelodysplastic syndrome and myeloproliferative disorders. Biol Blood Marrow Transplant. 2008 Feb;14(2):246–255. doi: 10.1016/j.bbmt.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura R, Rodriguez R, Palmer J, et al. Reduced-intensity conditioning for allogeneic hematopoietic stem cell transplantation with fludarabine and melphalan is associated with durable disease control in myelodysplastic syndrome. Bone Marrow Transplant. 2007 Nov;40(9):843–850. doi: 10.1038/sj.bmt.1705801. [DOI] [PubMed] [Google Scholar]
- 21.Oran B, Giralt S, Saliba R, et al. Allogeneic hematopoietic stem cell transplantation for the treatment of high-risk acute myelogenous leukemia and myelodysplastic syndrome using reduced-intensity conditioning with fludarabine and melphalan. Biol Blood Marrow Transplant. 2007 Apr;13(4):454–462. doi: 10.1016/j.bbmt.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allogeneic hematopoietic stem cell transplantation for the treatment of myelodysplastic syndromes. [Accessed August 8, 2013]; http://www.cms.gov/Medicare/Coverage/Coverage-with-Evidence-Development/Allogeneic-Hematopoietic-Stem-Cell-Transplantation-for-the-treatment-of-Myelodysplastic-Syndromes-.html.
- 23.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides. [Accessed June 16, 2011];2010 http://www.cibmtr.org.
- 24.Platzbecker U, Schetelig J, Finke J, et al. Allogeneic hematopoietic cell transplantation in patients age 60-70 years with de novo high-risk myelodysplastic syndrome or secondary acute myelogenous leukemia: comparison with patients lacking donors who received azacitidine. Biol Blood Marrow Transplant. 2012 Sep;18(9):1415–1421. doi: 10.1016/j.bbmt.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brand R, Putter H, van Biezen A, et al. Comparison of allogeneic stem cell transplantation and non-transplant approaches in elderly patients with advanced myelodysplastic syndrome: optimal statistical approaches and a critical appraisal of clinical results using non-randomized data. PLoS One. 2013;8(10):e74368. doi: 10.1371/journal.pone.0074368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koreth J, Pidala J, Perez WS, et al. Role of reduced-intensity conditioning allogeneic hematopoietic stem-cell transplantation in older patients with de novo myelodysplastic syndromes: an international collaborative decision analysis. J Clin Oncol. 2013 Jul 20;31(21):2662–2670. doi: 10.1200/JCO.2012.46.8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robin M, Porcher R, Ades L, et al. Outcome Of Patients With IPSS Intermediate (int) Or High Risk Myelodysplastic Syndrome (MDS) According To Donor Availability: A Multicenter Prospective Non Interventional Study For The SFGM-TC and GFM. Paper presented at: 55th ASH Annual Meeting and Exposition; December 6-10, 2013; New Orleans. [Google Scholar]
- 28.ClinicalTrials.gov. Allo vs. Hypomethylating/Best Supportive Care in MDS (Blood and Marrow Transplant Clinical Trials Network #1102) [Accessed February 14 2014]; http://clinicaltrials.gov/ct2/show/NCT02016781?term=02016781&rank=1.
- 29.Wheatley K, Gray R. Commentary: Mendelian randomization--an update on its use to evaluate allogeneic stem cell transplantation in leukaemia. Int J Epidemiol. 2004 Feb;33(1):15–17. doi: 10.1093/ije/dyg313. [DOI] [PubMed] [Google Scholar]
- 30.Burnett AK, Hills RK, Milligan DW, et al. Attempts to optimize induction and consolidation treatment in acute myeloid leukemia: results of the MRC AML12 trial. J Clin Oncol. 2010 Feb 1;28(4):586–595. doi: 10.1200/JCO.2009.22.9088. [DOI] [PubMed] [Google Scholar]
- 31.Krishnan A, Pasquini MC, Logan B, et al. Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011 Dec;12(13):1195–1203. doi: 10.1016/S1470-2045(11)70243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cassileth PA, Harrington DP, Appelbaum FR, et al. Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission. N Engl J Med. 1998 Dec 3;339(23):1649–1656. doi: 10.1056/NEJM199812033392301. [DOI] [PubMed] [Google Scholar]
- 33.Reiffers J, Stoppa AM, Attal M, et al. Allogeneic vs autologous stem cell transplantation vs chemotherapy in patients with acute myeloid leukemia in first remission: the BGMT 87 study. Leukemia. 1996 Dec;10(12):1874–1882. [PubMed] [Google Scholar]
- 34.Woods WG, Neudorf S, Gold S, et al. A comparison of allogeneic bone marrow transplantation, autologous bone marrow transplantation, and aggressive chemotherapy in children with acute myeloid leukemia in remission. Blood. 2001 Jan 1;97(1):56–62. doi: 10.1182/blood.v97.1.56. [DOI] [PubMed] [Google Scholar]
- 35.Mohty M, de Lavallade H, Ladaique P, et al. The role of reduced intensity conditioning allogeneic stem cell transplantation in patients with acute myeloid leukemia: a donor vs no donor comparison. Leukemia. 2005 Jun;19(6):916–920. doi: 10.1038/sj.leu.2403770. [DOI] [PubMed] [Google Scholar]
- 36.Oosterveld M, Suciu S, Verhoef G, et al. The presence of an HLA-identical sibling donor has no impact on outcome of patients with high-risk MDS or secondary AML (sAML) treated with intensive chemotherapy followed by transplantation: results of a prospective study of the EORTC, EBMT, SAKK and GIMEMA Leukemia Groups (EORTC study 06921) Leukemia. 2003 May;17(5):859–868. doi: 10.1038/sj.leu.2402897. [DOI] [PubMed] [Google Scholar]
- 37.Logan B, Leifer E, Bredeson C, et al. Use of biological assignment in hematopoietic stem cell transplantation clinical trials. Clin Trials. 2008;5(6):607–616. doi: 10.1177/1740774508098326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009 Jul 30;114(5):937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 39.Saber W, Cutler CS, Nakamura R, et al. Impact of donor source on hematopoietic cell transplantation outcomes for patients with myelodysplastic syndromes (MDS) Blood. 2013 Jul 11; doi: 10.1182/blood-2013-04-496778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bally C, Thepot S, Quesnel B, et al. Azacitidine in the treatment of therapy related myelodysplastic syndrome and acute myeloid leukemia (tMDS/AML): a report on 54 patients by the Groupe Francophone Des Myelodysplasies (GFM) Leuk Res. 2013 Jun;37(6):637–640. doi: 10.1016/j.leukres.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 41.Itzykson R, Thepot S, Quesnel B, et al. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood. 2011 Jan 13;117(2):403–411. doi: 10.1182/blood-2010-06-289280. [DOI] [PubMed] [Google Scholar]
- 42.Damaj G, Duhamel A, Robin M, et al. Impact of azacitidine before allogeneic stem-cell transplantation for myelodysplastic syndromes: a study by the Societe Francaise de Greffe de Moelle et de Therapie-Cellulaire and the Groupe-Francophone des Myelodysplasies. J Clin Oncol. 2012 Dec 20;30(36):4533–4540. doi: 10.1200/JCO.2012.44.3499. [DOI] [PubMed] [Google Scholar]
- 43.Gerds AT, Gooley TA, Estey EH, Appelbaum FR, Deeg HJ, Scott BL. Pretransplantation therapy with azacitidine vs induction chemotherapy and posttransplantation outcome in patients with MDS. Biol Blood Marrow Transplant. 2012 Aug;18(8):1211–1218. doi: 10.1016/j.bbmt.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lubbert M, Bertz H, Ruter B, et al. Non-intensive treatment with low-dose 5-aza-2′-deoxycytidine (DAC) prior to allogeneic blood SCT of older MDS/AML patients. Bone Marrow Transplant. 2009 Nov;44(9):585–588. doi: 10.1038/bmt.2009.64. [DOI] [PubMed] [Google Scholar]
- 45.Field T, Perkins J, Huang Y, et al. 5-Azacitidine for myelodysplasia before allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2010 Feb;45(2):255–260. doi: 10.1038/bmt.2009.134. [DOI] [PubMed] [Google Scholar]
- 46.ClinicalTrials.gov. Reduced Intensity Conditioning Versus Myeloablative Conditioning for Acute Myeloid Leukemia or Myelodysplastic Syndrome (MAvRIC) Trial. [Accessed May 28, 2014]; http://clinicaltrials.gov/show/NCT01339910.
- 47.Prebet T, Gore SD, Esterni B, et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. J Clin Oncol. 2011 Aug 20;29(24):3322–3327. doi: 10.1200/JCO.2011.35.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Itzykson R, Thepot S, Quesnel B, et al. Long-term outcome of higher-risk MDS patients treated with azacitidine: an update of the GFM compassionate program cohort. Blood. 2012 Jun 21;119(25):6172–6173. doi: 10.1182/blood-2012-04-422204. [DOI] [PubMed] [Google Scholar]
- 49.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006 Apr 15;106(8):1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 50.Cutler CS, Lee SJ, Greenberg P, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004 Jul 15;104(2):579–585. doi: 10.1182/blood-2004-01-0338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1s. Survival Probabilities by Donor Type
Table 2s. Univariate Analysis of Transplantation Outcomes for MDS Patients by Conditioning Regimen and Donor Type
Table 3s. Outcomes of Non-Transplant Therapy in MDS
Table 4s: Estimated Annual Accrual
Table 5s: Power to Detect 15% Increase in OS Probability in the Transplant Arm for Various Survival Probabilities and Proportions of Donor Availability


