Abstract
Recent evidence suggests a double dissociation of size and spacing limit on letter recognition — it is limited by size in the fovea and critical spacing in the normal periphery. Here, we evaluated whether size or spacing limits letter recognition in people with age-related macular degeneration (AMD) who must use their peripheral vision. We measured the size threshold for recognizing lowercase letters presented alone, or flanked by two letters at various center-to-center nominal letter spacings (multiples of letter size) for 11 observers with AMD. For comparison, similar measurements were obtained at 5 and 10° eccentricity in the nasal and lower visual fields in three older adults with normal vision. Single-letter size thresholds were worse for observers with AMD than at comparable retinal locations in the normal periphery. For flanked letters, size threshold improved with larger nominal spacing up to the critical spacing, beyond which size threshold was unaffected by the flankers. Seven AMD observers had a nominal critical spacing between 1.25× and 1.80×, values close to those in the normal fovea, suggesting that their letter recognition is size-limited; two had a nominal critical spacing of 3–4×, values close to those in the normal periphery, implying that their letter recognition is limited by spacing; and another two had a nominal critical spacing of ~2.3×, implying that their letter recognition is limited by both size and spacing. The wide range of nominal critical spacings observed in our AMD observers may reflect the degree of completeness of their adaptation process to vision loss.
Keywords: letter recognition, crowding, age-related macular degeneration, peripheral vision
INTRODUCTION
To see an object clearly, the object needs to be large enough such that it exceeds the resolution limit of the eye. However, even when an object is large enough to be recognizable on its own, its recognition may still be hampered if it is closely surrounded by other objects. This is the crowding phenomenon (Flom, Heath & Takahashi, 1963; Flom, Weymouth & Kahneman, 1963; Flom, 1991; Levi, 2008; Pelli, Palomares & Majaj, 2004) and is more pronounced in peripheral vision (Jacobs, 1979; Toet & Levi, 1992). Crowding has been suggested as the bottleneck for object recognition (Levi, 2008; Pelli & Tillman, 2008; Pelli, 2008) and the major limiting factor on reading (Pelli et al., 2007; Levi, Song & Pelli, 2007).
The pronounced effect of crowding in the periphery naturally leads to the hypothesis that it is a bottleneck on vision for people who lose their central (foveal) vision and must rely on their peripheral vision. The leading cause of central vision loss is age-related macular degeneration (AMD), which is a leading cause of visual impairment in developed countries, especially for people over the age of 65 (e.g. Congdon et al., 2004; Friedman et al., 2004). The relevance of crowding in limiting vision for people with AMD not only relates to the fact that people with AMD have to rely upon their residual peripheral vision to function, but also because reading is the primary goal of most people with AMD seeking visual rehabilitation (Kleen & Levoy, 1981; Leat & Rumney, 1990; Bullimore & Bailey, 1995) and that crowding has been suggested as the major limiting factor on reading (Pelli et al., 2007; Levi et al., 2007).
If crowding limits reading for people with AMD, then a simple way to minimize crowding and thus improve reading is to increase the separation between adjacent letters in text. A previous attempt in improving reading in AMD through increasing letter spacing in text shows that for all 14 participants in that study, 12 of whom had AMD, the letter spacing that yielded the maximum reading speed, the critical spacing, was essentially the same as the conventional standard spacing used in most printed text (Chung, 2012). This result suggests that there is no need to increase the letter spacing beyond the standard for people with AMD, as long as the print size is large enough for them to achieve their maximum reading speed. In other words, people with AMD do not seem to suffer from as much crowding as would be expected based on the normal periphery. A caveat of this previous study is that the critical spacing was determined for a reading task. Because people can often read a word correctly without having to recognize individual letters correctly (Rawlinson, 1976; Mansfield & Legge, 1999), the critical spacing for reading may differ from that for letter recognition, which represents a more genuine limitation on how object spacing limits our vision (but see Levi et al (2007) and Pelli et al (2007) in which these authors showed that the critical spacing for reading is the same as the critical spacing for letter recognition, a topic that we will discuss in the Discussion). Therefore in this study, we used a letter recognition task to determine the critical spacing. Pelli (2008) proposed that for unimpaired recognition of objects, the minimal separation between each pair of objects must exceed a critical spacing that depends solely on the physical location of the objects in the visual field, but is invariant to the object types or categories. Thus the critical spacing determined in this study would help us understand how object spacing limits object recognition in general, instead of the more specific question of how letter spacing limits letter recognition.
Pelli’s notion suggests that in addition to object size, there is another limitation on object recognition — object spacing. What then, is the primary limiting factor on object recognition — size or spacing, or does that depend on different situations?
By measuring the threshold letter size (the smallest letter size required to reach a given level of recognition accuracy) required for observers to recognize a letter flanked by other letters for a range of letter spacings, Coates, Chin and Chung (2013) and Song, Levi and Pelli (2014) independently showed that the threshold letter size depends on letter spacing, but only when the letter spacing is within the critical spacing. Within this regime, letter recognition is limited by letter spacing but not letter size. When the letter spacing exceeds the critical spacing, threshold letter size is independent on letter spacing, and it is size that limits letter recognition in this regime (see Figures 3 and 4, which will be explained in greater details in Results). These results showed that for any given condition, the primary limiting factor on letter recognition is the greater of the two — size limitation or the critical spacing. At the fovea, these authors found that the critical spacing is very small, consistent with the well-known effect that there is very little crowding at the fovea (Toet & Levi, 1992; Chung, Levi & Legge, 2001; Levi, Klein & Hariharan, 2002). Song et al further showed that when observers were asked to recognize blurry letters at the fovea, the critical spacing increases at the same rate as the threshold letter size. These findings imply that the primary limitation on letter recognition for foveal vision is size (resolution). As long as the size of a letter exceeds the resolution limitation, then observers would be able to recognize the letters well, even in the presence of closely flanked letters. In the normal periphery, the story is different. Although the letter size required for correct recognition of single letters increases with eccentricity (Wertheim, 1980; Jacobs, 1979), the critical spacing increases at a faster rate than that for resolution (Jacobs, 1979; Levi et al, 2007; Coates et al, 2013), with the effect that letters that can be recognized on their own would not be recognizable if other letters are present within a distance that is smaller than the critical spacing at the retinal location of the target letter.
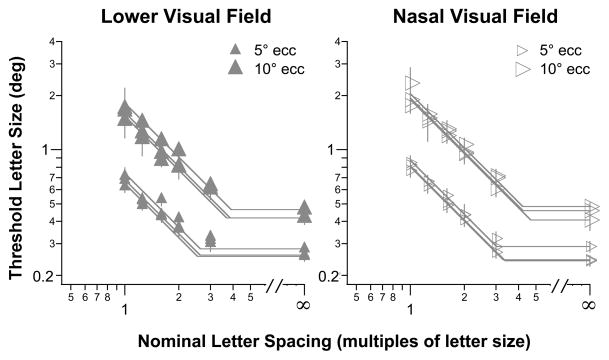
Figure 3.
Threshold letter size (deg) is plotted as a function of nominal letter spacing (multiples of letter size) for the three older adults with normal vision, tested at 5° (smaller symbols) and 10° (larger symbols) in the lower (left panel) and nasal (right panel) visual fields. Both eccentricities in the same visual field are plotted in the same panel, and data from all three observers are plotted (hence, three sets of data for each eccentricity). The straight lines through the data represent the best-fit bilinear function (see text for details) fitted to each set of data (each eccentricity for each observer). The value on the abscissa corresponding to the intersection of the two lines represents the nominal critical spacing. Error bars represent ± 1 SEM of the size-threshold measurement.
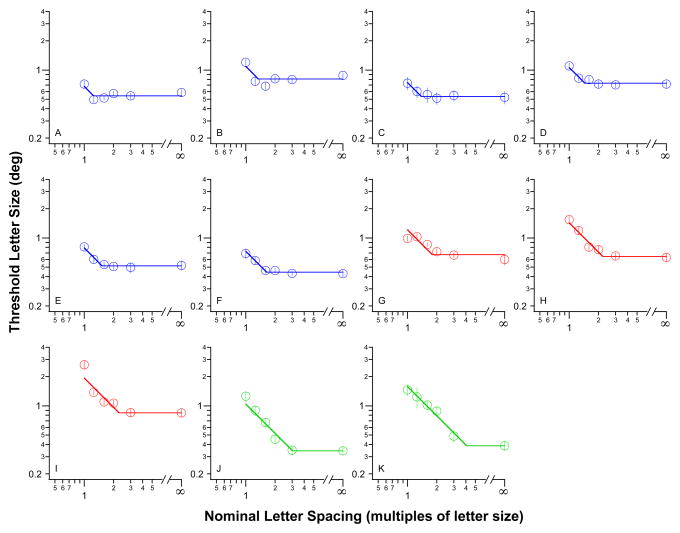
Figure 4.
Threshold letter size (deg) is plotted as a function of nominal letter spacing (multiples of letter size) for the 11 observers with AMD. Each panel shows the data of one observer. The solid lines represent the best-fit bilinear function (see text for details). The value on the abscissa corresponding to the intersection of the two lines represents the nominal critical spacing. Error bars represent ± 1 SEM of the size-threshold measurement.
In this study, we applied a similar methodology and analysis as those of Coates et al (2013) and Song et al (2014) to determine whether size or spacing is the more important limiting factor on letter recognition for people with AMD. On one hand, if size was the primary limiting factor on letter recognition, then as long as the letter size is large enough such that single letters could be recognized, patients with AMD would be able to recognize letters at this size even when they are presented in groups, as in text. The result would also suggest that these patients show the characteristics of the normal fovea, in which size is the primary limiting factor on letter recognition. On the other hand, if spacing was the primary limiting factor on letter recognition, then it means that patients with AMD would benefit more from enlarging the spacing, not the letter size. Considering that the currently available optical and electronic magnifiers enlarge object and the space around an object equally, practically it means that patients would still have troubles recognizing letters that are presented in groups, as in text, even if the letters are made large enough to be recognizable when presented alone. The result would also suggest that these patients exhibit the properties of the normal periphery, in which spacing is the primary factor limiting letter recognition. Clearly, not all patients with AMD would show the same limitation. In the Discussion, we will propose a simple test, based on only two measurements of letter size thresholds, to predict whether letter size or spacing is the more important factor limiting letter recognition for a given patient.
METHODS
Eleven observers with AMD, with age ranging from 66 to 89 years, participated in this study. All had confirmed diagnosis by an ophthamologist or optometrist. The duration for which they had been diagnosed with the disorder ranged from 0.5 to 15 years (see Table 1). Table 1 lists the characteristics, visual acuity as measured using the Bailey-Lovie Acuity Chart and the number of years since the onset of the disorder of these observers. The location of the preferred retinal locus1 for fixation (PRL, a retinal location outside the dysfunctional macular area adopted as the locus for fixation) as measured using a Rodenstock scanning laser ophthalmoscope (SLO) is included for 10 of the 11 observers. Two of these observers showed residual foveal sparing (see footnote b of Table 1). All observers gave written informed consent before the commencement of data collection. This research followed the tenets of the Declaration of Helsinki and was approved by the Committee for Protection of Human Subjects at the University of Houston and the University of California, Berkeley.
Table 1.
Visual characteristics of the 11 observers with AMD. The PRL is only given for the tested eye (PRL was not determined for observer C).
| Observer ID | M/F | Age | Yrs since onset | Acuity (logMAR) | PRL ecc. (deg) | PRL directiona | Letter-size threshold (deg) | Nominal critical spacing (multiples of letter size) | Absolute critical spacing (deg) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| OD | OS | OD | OS | ||||||||
| A | M | 72 | 0.5 | 0.80 | prosthesis | 4.98 | 288° | 0.54 | 1.25 | 0.68 | |
| B | F | 75 | 5 | 0.74 | 0.92 | 5.17 | 94° | 0.81 | 1.35 | 1.09 | |
| C | F | 72 | 4 | 0.62 | 0.64 | - | - | 0.55 | 1.39 | 0.74 | |
| D | M | 84 | 8 | 0.56 | 0.70 | 6.16 | 291° | 0.73 | 1.45 | 1.06 | |
| E | M | 84 | 3 | 0.48 | 0.48 | 2.45 | 298° | 0.52 | 1.53 | 0.79 | |
| F | F | 82 | 9 | 0.50 | 0.52 | 3.49 | 288° | 0.45 | 1.64 | 0.73 | |
| G | F | 73 | 7 | 0.66 | 0.48 | 2.49 | 209° | 0.67 | 1.80 | 1.21 | |
| H | M | 86 | 12 | 0.70 | 0.74 | 6.69 | 259° | 0.64 | 2.23 | 1.43 | |
| I | F | 89 | 15 | 1.04 | 1.06 | 6.50 | 202° | 0.85 | 2.28 | 1.93 | |
| J | F | 74 | 6 | 0.54 | 1.12 | 2.87b | 161° | 0.34 | 3.02 | 1.04 | |
| K | F | 78 | 3 | 0.40 | 0.32 | 4.54b | 143° | 0.39 | 4.07 | 1.58 | |
The direction of the PRL was specified with respect to the fovea (which in all cases, fell within the central scotoma), counter-clockwise from the horizontal 3 o’clock position (0°).
Both observers J and K showed some residual foveal function. Depending on the size of the fixation cross (<1°), the two observers could switch to using their fovea for fixation. They also reported a drastic decrease in vision in dim light, consistent with the shift to a peripheral PRL for visual tasks when the lighting is not sufficient to support foveal vision.
To evaluate whether letter size or spacing is the principal factor limiting letter recognition for people with AMD, we determined the threshold letter size for recognizing the middle letter of sequences of three random lowercase letters (trigrams) for a range of horizontal letter spacings. Threshold letter size was defined as the letter size that yielded a performance accuracy of 52%-correct (50%-correct, after correction for guessing (guessing rate = 1/26)) based on a psychometric function that summarized an observer’s performance (see later). We used lowercase letters instead of the more conventional letter symbols for measuring acuity such as Sloan letters (Song et al, 2014) or Tumbling-E stimuli (Coates et al, 2013) because of our interest in relating the critical spacing for letter recognition with the critical spacing for reading as reported previously (Chung, 2012). Each letter was randomly chosen from the 26 letters of the Times-Roman alphabet, with no repeat allowed within the trigram. We defined letter size according to the x-height in degrees of visual angle, and letter spacing as the center-to-center separation between two adjacent letters, specified as multiples of letter size, or, nominal letter spacing. Nominal letter spacing is a relative measurement and relates to the absolute spacing by multiplying by the letter size. For example, a nominal letter spacing of 1 means that two adjacent letters are very close to one another, regardless of the letter size. A total of six nominal spacings were tested: 1×, 1.25×, 1.6×, 2×, 3× the letter size, along with the single-letter unflanked condition (infinite spacing). Samples of the letter stimuli rendered at different sizes and nominal spacings are given in Figure 1. The order of testing these six nominal spacings was randomized for each participant in the first-half of the testing session. After a short break (~10 min), the six spacings were re-tested in the reverse-order. All participants completed testing in one session (1.5 – 2.5 hrs). Testing was monocular, using the preferred eye (usually but not always the better-seeing eye, see Table 1), with the non-tested eye covered using a standard black cloth eye-patch. The viewing distance ranged from 50 to 100 cm, in order to accommodate the range of letter sizes and spacings to be tested. All participants wore appropriate near corrections for the testing distance.
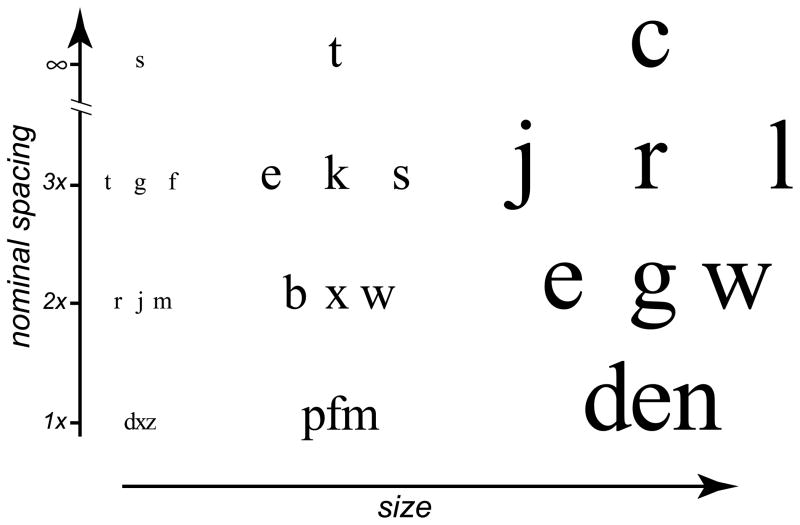
Figure 1.
A schematic figure showing samples of trigram stimuli. We varied letter size (shown along the horizontal dimension in the figure) to assess the letter size threshold for a given nominal letter spacing (shown along the vertical dimension in the figure). Because the nominal letter spacing is specified in multiples of letter size (x-height), the absolute spacing between the letters change as letter size varies. In a given block of trials, only one nominal letter spacing was tested but letters were presented at five letter sizes.
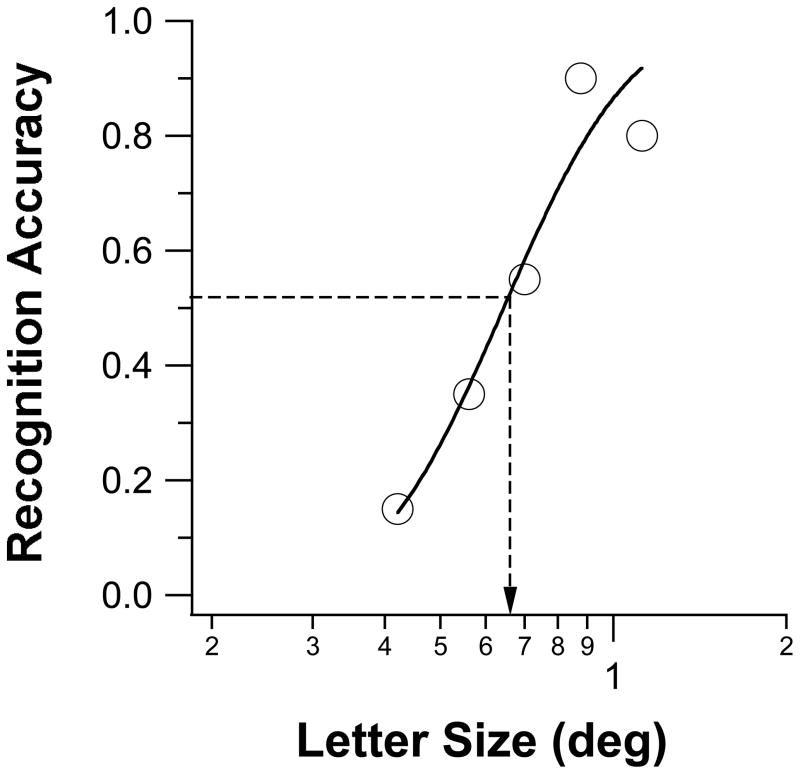
To determine the threshold letter size for a given spacing, we used the Method of Constant Stimuli to present trigrams (or single letters for the unflanked condition) at five letter sizes (20 trigrams per letter size), at each nominal spacing. Each spacing was tested in a separate block of trials. Because the nominal spacing was specified as a multiple of letter size, when the letters became bigger, so did the absolute spacing between letters, with an effect similar to zooming the trigram or moving the participant closer to the display. For a given nominal spacing, the five letter sizes were chosen such that the recognition accuracy spanned a range from close to 0–10% correct to 90–100% correct. Results from the two blocks of the same spacing were combined after the experiment, and a cumulative-Gaussian function was used to fit the data relating recognition accuracy to letter size. Threshold letter size was defined as the letter size that corresponded to 52% accuracy on the fitted function. Figure 2 shows a sample set of data (letter recognition accuracy vs. letter size) with the best-fit cumulative-Gaussian function.
Figure 2.
A sample set of data relating letter recognition accuracy with letter size, for observer F and at a nominal letter spacing of 1×. Each datum was based on 40 trials. The smooth curve drawn through the data points represent the best-fit cumulative- Gaussian function. The threshold letter size is indicated by the arrow associated with the dashed line.
Given that almost all of our participants had central vision loss and had developed a relatively stable and consistent PRL (based on measurements and observations using the Rodenstock SLO), we provided a pair of vertical black lines to guide fixation, akin to the method commonly used in the clinic to guide patients with central scotoma where to look during clinical testing, e.g. with the tangent screen and Amsler’s grid. The two lines were 1 cm thick, subtending an angle of 0.57° at 100 cm or 1.14° at 50 cm, and were separated vertically by a gap of 6 cm, equivalent to 3.43° at 100 cm or 6.84° at 50 cm. This gap size ensured that even with the largest letter size tested, the edge of each black line was still sufficiently separated from the letters to avoid undesirable contour interaction due to the black fixation lines.2 Before the beginning of a trial, observers were asked to direct their gaze to the center of the gap between the two black lines. When the observer was ready, the experimenter initiated a trial and a trigram (or a single letter for the unflanked condition) was presented for 150 ms, with the middle (target) letter presented at the center of the gap between the two black lines (also the center of the display). Observers were first asked to report if they saw three letters (for trigram trials) and if so, they were then asked to verbally report the identity of the target letter. Trials in which observers reported only seeing one or two letters (when three letters were presented) were discarded and repeated. Averaged across observers, this occurred 7.8% of the time (range = 2.7 – 15.6%). Response time was unlimited, although in most cases, observers gave their responses immediately following the offset of the trigram from the display. Following the entry of the response by the experimenter, a brief audio feedback was provided to indicate whether or not the response was correct.
For comparison, the threshold letter size for recognizing the middle letters of trigrams was determined for a range of nominal letter spacings at 5° and 10° eccentricity in the nasal and lower visual fields of three normally sighted older adults (aged 63 – 79; acuity in each eye ≤ 20/20). Because the letters of each trigram were separated horizontally, and because crowding zones in the normal periphery are elongated in shape such that the critical spacing is larger along the radial dimension than the tangential one, testing in the nasal and lower visual fields yielded critical spacings to be compared with those of AMD observers, regardless of whether the PRLs of the AMD observers were radial or tangential or somewhere in-between with respect to the anatomical fovea. Testing was monocular, using only the left eye. The right eye was covered using a standard black cloth eye-patch. Viewing distances were 120 cm and 60 cm for testing at 5° and 10° eccentricity, respectively. Testing procedures were identical to those described above for the testing of observers with AMD, with the only exception that a small fixation target, presented on the left of (for testing the nasal visual field) or directly above (for testing the lower visual field) the center of the middle letter of each trigram, was provided to these normally sighted observers during testing.
Letter Stimuli were generated on a Macintosh G4 computer with software written in MATLAB 5.2.2 (The MathWorks, MA), using the Psychophysics Toolbox extensions (Brainard, 1997; Pelli, 1997), and were displayed on a Sony color monitor (Model # CDP-E540, refresh rate = 75 Hz). Letters were presented as black letters (0.2 cd/m2) on a white background (120 cd/m2). Practice trials were provided to all observers (short blocks of 20 trials at different letter separations) until the observers indicated that they were ready for the actual data collection. Data obtained during practice are not included in this paper.
Curve-fitting
Curve-fitting was carried out using Igor Pro (WaveMetrics Inc., OR), which utilizes a Levenberg-Marquardt iterative algorithm, a form of nonlinear least-squares fitting, to minimize the Chi-square error between the experimental and the model fit. The experimental data were weighted by the inverse of the standard error of each datum during curve-fitting.
RESULTS
We first validate the choice of our stimulus and task by comparing the results obtained in the normal periphery (from older adults with normal vision) with the published data from young adults with normal vision reported in Coates et al (2013) and Song et al (2014). In Figure 3 we plot threshold letter size (deg) as a function of nominal letter spacing (multiples of letter size) obtained at 5° and 10° eccentricity in the lower and nasal visual fields, for each individual control observer. For all observers and retinal locations, threshold letter size decreases with larger nominal letter spacing, up till a point beyond which threshold letter size does not change with larger nominal letter spacing. We adopted the bilinear function (on log-log axes) used by Coates et al (2013) and Song et al (2014) to tease apart the limitations of spacing and size on letter recognition. The premise of the bilinear function is that there is a critical spacing that separates the scenarios in which letter recognition is limited by spacing or size. When the stimulus spacing is smaller than the critical spacing, it is spacing that limits letter recognition. Here, spacing refers to the absolute spacing, which means that it is the separation between adjacent letters in degrees of visual angle that matters, but not letter size. If we express spacing in terms of nominal spacing as we plotted in Figure 3, then we should observe a complete trade-off between letter size and nominal spacing, because absolute spacing is the product of letter size and nominal spacing.
On the other hand, when the stimulus spacing is larger than the critical spacing, then spacing does not pose any limitation on letter recognition, but of course the letter size needs to exceed the resolution limit for the task of letter recognition. The two limitations of spacing and size are represented in Figure 3 by two lines (on log-log axes) with slopes of −1 and 0, respectively, where the intersection of the two lines yields the critical spacing (value on the abscissa) and the estimated optimal threshold letter size (value on the ordinate). For this bilinear fit, we did not constrain the optimal threshold letter size to be the threshold letter size for the unflanked condition.
For our older adults with normal vision, the nominal critical spacing increases from 2.38× the letter size at 5° eccentricity to 3.82× at 10° eccentricity in the lower visual field, and 3.23× at 5° eccentricity to 4.38× at 10° eccentricity in the nasal visual field. These values are very similar to the finding of Coates et al (2013) who reported that the nominal critical spacing ranges from 3–4× the letter size in the normal periphery (3°, 5° and 10° eccentricity). The similar results found in this study and that of Coates et al (2013), despite the differences in the age of the observers and the testing stimuli between the two studies, implies that our stimuli are just as good as the conventional “acuity-chart” stimuli for studying how letter size or spacing limits letter recognition.
We then turn to our observers with AMD. In Figure 4 we plot threshold letter size as a function of nominal letter spacing for each of our 11 observers with AMD.3 Similar to what we observed in the normal periphery, observers with AMD demonstrate a similar relationship between threshold letter size and nominal letter spacing — threshold letter size decreases with larger nominal letter spacing, up till a point beyond which threshold letter size does not change with larger nominal letter spacing. Across observers, the nominal critical spacing ranges from 1.25 to 4.07× the letter size, more than a three-fold change in value. In other words, some observers with AMD could recognize letters in clutter almost just as well as single letters (those whose nominal critical spacing is close to one) while others require letters to be separated by 4× the letter size before they can recognize individual letters presented in groups. What accounts for such a discrepancy in result?
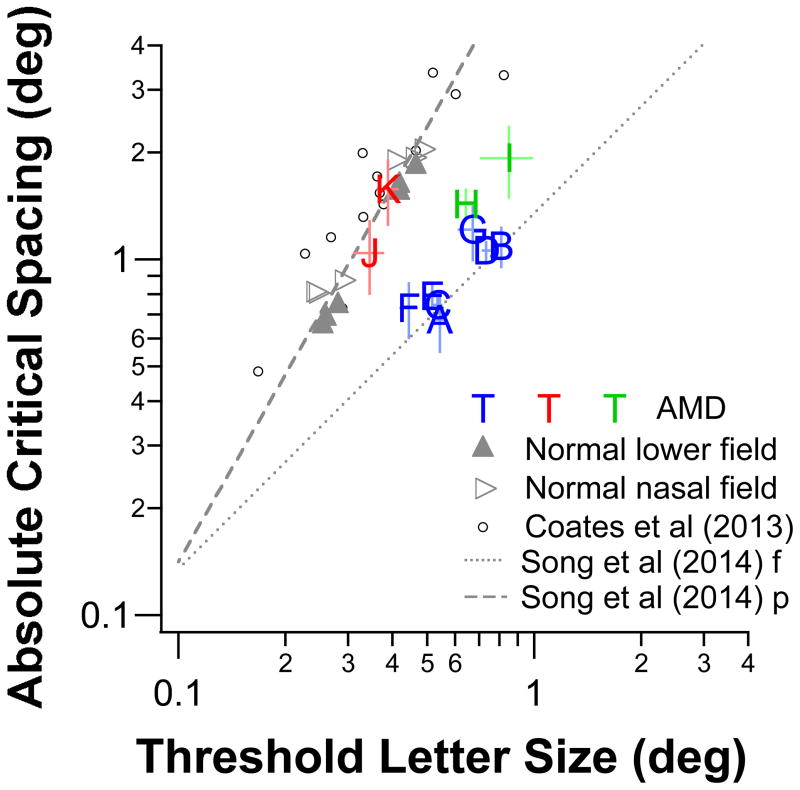
Song et al (2014) reported an even wider range of nominal critical spacing (these authors refer to it as the Spacing:Acuity ratio), from ~1.1 to 9 for people with normal vision. They found that the values are usually small when observers were tested in the fovea, with the values becoming larger in the periphery. A different approach used by Song et al (2014) to illustrate the differences in the nominal critical spacings obtained in the fovea and the periphery is to plot the absolute critical spacing (nominal critical spacing × threshold letter size) as a function of threshold letter size, as shown in Figure 5. The two lines (dotted and dashed) in the figure are essentially the same two lines in Figures 4 and 5 in Song et al (2014), with the dotted line showing the relationship between absolute spacing and acuity in the normal fovea and the dashed line showing the relationship in the normal periphery. In the fovea, the absolute spacing changes with threshold letter size at a similar rate (dotted line), resulting in a slope (on log-log axes) of approximately 1 (the exact value is 0.99±0.02 [SE]: Song et al (2014)). In the periphery, the absolute spacing increases at a faster rate than letter size (dashed line), resulting in a slope (on log-log axes) of greater than 1 (the exact value is 1.75±0.17 [SE]: Song et al (2014)). In other words, the relationship between absolute spacing and acuity indicates whether an observer uses his/her fovea or the periphery. To relate this to our study, we first included in Figure 5 the data of Coates et al (2013) and the data from our older adults to test if the relationship in the normal periphery holds well for other stimuli or ages of observers. These data are plotted as small black circles and gray triangular symbols. Clearly, the data for the normal periphery from the three studies match up reasonably well and follow essentially the same relationship. We then included the data of our 11 observers with AMD, using the individual observer ID as symbols. For seven of these observers (A – G), their symbols fell very close to the dotted “normal fovea” line; while the symbols for observers J and K fell on or very close to the dashed “normal periphery” line. The other two observers (H and I) had their symbols falling between the dotted and the dashed lines. These results imply that despite the presumption that people with AMD use a peripheral retinal location for seeing, some of the our observers (in this case, observers A – G) showed properties of letter recognition resembling those of the normal fovea, instead of the normal periphery. Only two observers showed properties of letter recognition that are consistent with our expectation of the normal periphery. Interestingly, these two observers were the only two who showed evidence of residual foveal function. The significance of these findings will be discussed in the Discussion.
Figure 5.
Absolute critical spacing (deg) is plotted as a function of threshold letter size (deg) for the 11 observers with AMD. Data for observers with AMD are plotted using their observer ID and are color coded according to whether the properties of letter recognition for the observer are consistent with a limitation by size (blue), spacing (red) or both (green). Data obtained from the lower (filled triangles) and nasal (unfilled triangles) visual fields of the three normally sighted observers are also plotted. For comparison, we included the normal peripheral data from Coates et al (2013) obtained from 3 to 10° eccentricity (small unfilled black circles) and the two lines relating the absolute critical spacing with letter size as in Figure 4 in Song et al (2014) (f stands for foveal data and p stands for peripheral data). Despite differences in the experimental paradigm, the three studies (ours, Coates et al’s and Song et al’s) yielded data in the normal periphery that are consistent with one another. Error bars represent ± 1 SEM for both spacing and size estimates.
DISCUSSION
The traditional view on the principal sensory factor limiting object recognition is that the size of the object must exceed the resolution limit of the eye. Recently, emerging evidence suggests that in addition to size, object spacing also plays a significant role in limiting object recognition, especially in the normal periphery and in the presence of certain visual disorders such as amblyopia (Levi et al, 2007; Pelli et al, 2007; Pelli & Tillman, 2008; Song et al, 2014). In their recent paper, Song et al (2014) measured threshold letter size required to recognize a letter in the presence of flanking letters (they used Sloan letters), for different letter spacings, in the normal fovea and periphery and also in a group of observers with amblyopia. By plotting absolute spacing as a function of threshold letter size, the authors showed that their data could be segregated into two relationships (replotted as the dotted and dashed lines in Figure 5 in this paper). One set of data follows the relationship in which spacing increases with letter size at almost the same rate, implying that letter size, not spacing, is the major factor limiting letter recognition. These data include those obtained in the normal fovea and in anisometropic amblyopes. Another set of data follows the relationship in which spacing increases at a faster rate than letter size (also see Jacobs, 1979; Coates et al, 2013), implying that spacing, not size, is the major limiting factor on letter recognition. This includes data obtained in the normal periphery and in strabismic amblyopes. When we plotted our results in the same format (Figure 5), the data of seven of our 11 AMD observers essentially fell along the relationship in which letter size limits letter recognition while the data of two other observers fell along the relationship in which letter spacing limits letter recognition. The data from the other two observers fell between these two limitations, suggesting that their letter recognition might be limited by both letter size and spacing.
People with AMD are believed to suffer from substantial crowding that limits their vision, and require objects to be separated from one another by a large enough distance to minimize the adverse crowding effect (Levi, 2008; Whitney & Levi, 2011; Bailey & Lovie-Kitchin, 2013). The premise is that following the loss of central vision; people with AMD have to use their peripheral vision. If the disorder does not in any means alter the peripheral visual processing in people with AMD, then people with AMD should suffer from substantial crowding, just like the normal periphery, with the result that spacing becomes an important factor limiting letter recognition. Interestingly, seven of the 11 observers who had adopted a peripheral retinal location as their PRL showed that their letter recognition was more limited by letter size, not spacing as would be expected for the periphery. What accounts for such a result?
Figure 5 shows that for the same range of critical spacing (e.g. 0.7 – 2°), the threshold letter size was larger for most observers with AMD than in the normal periphery (gray symbols). Therefore, one explanation for why letter recognition for multiple letters is more limited by letter size instead of spacing for seven of our observers with AMD is that the single-letter size threshold is already elevated, possibly due to the unhealthy retina, thus diminishing the effect of letter spacing. Indeed, we have previously shown that single-letter size threshold measured at the PRL of 17 eyes with macular disorders was always worse than that at the same eccentricity in the normal periphery (Chung, 2013a). Considering that the absolute critical spacing represents a trade-off between letter size and nominal spacing, larger letters mean that they could withstand a smaller nominal letter separation. This may well explain why the critical spacing for seven of our observers with AMD seems to be limited by letter size.
Although the explanation of an increased single-letter size threshold is plausible, what we have learned about the PRL must be taken into account. Recent psychophysical evidence shows that when the disorder has been present for a long time, many people with AMD exhibit characteristics that are more similar to those usually found in the normal fovea, and different from those usually observed in the normal periphery. Such evidence includes the lack of a benefit of reading text with line spacing greater than the standard (Chung et al, 2008; Calabrèse et al, 2010),4 and that the crowding zone measured at the PRL used by people with AMD is isotropic as in the normal fovea (Toet & Levi, 1992); instead of anisotropic in shape (Chung, 2013b). The changes observed in some spatial properties at the PRL of people with AMD have been attributed to the plasticity of the visual system. Indeed, the changes in the two-dimensional shape of the crowding zone at the PRL are difficult to explain without invoking a response to vision loss. Presumably, the constant usage of a peripheral retinal location (PRL) in these observers alter how neurons in the visual cortex projected from the PRL and its vicinity respond to visual stimuli, resulting in changes in certain spatial properties, akin to the underlying mechanism of perceptual learning. However, perceptual learning operates on a much shorter time scale and these changes in response to vision loss take a much longer time. Consequently, we prefer to refer to this process as adaptation. Adaptation to central vision loss is a lengthy process. Patients must learn to use a region in the residual peripheral retina to serve as their reference locus for seeing (the PRL). To date, we know very little about the adaptation process, for example, how long the process takes for completion and what the underlying mechanism is. To relate our findings to the evidence from previous reports, we speculate that observers whose letter recognition is limited by letter size (following the “normal foveal” relationship) are those whose adaptation process is more or less complete. In contrary, observers whose letter recognition is limited by spacing (following the “normal periphery” relationship) are those who have not adapted to their vision status. For those whose data fall between the two relationships, it might simply reflect an ongoing adaptation process, that is, the adaptation is not yet completed (or may never be completed).
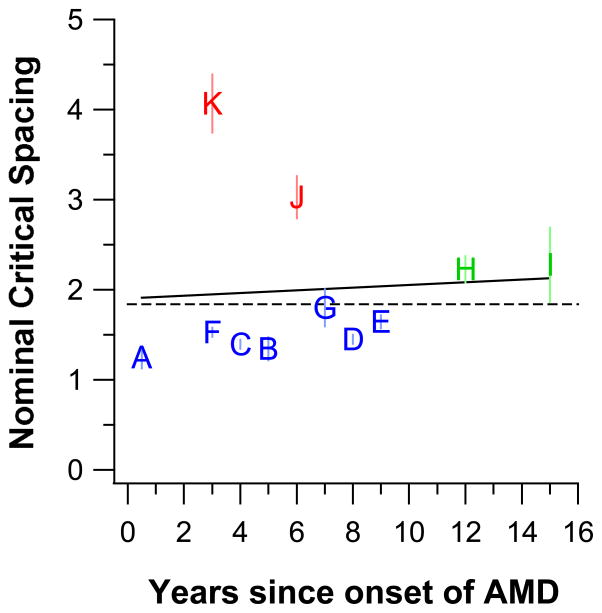
It is reasonable to expect that the degree of completeness of the adaptation process should correlate with the duration since the onset of AMD. In Figure 6, we plot the nominal critical spacing as a function of the number of years since the onset of the disorder. The dashed line represents a nominal critical spacing of 1.84, a criterion used by Song et al (2014) to classify whether letter recognition for an individual is limited more by a size (values < 1.84) or a spacing (values > 1.84) effect (will be explained in greater details in a later section Clinical Implications). There are three noteworthy points from this figure. First, observer A who had the shortest duration since onset (half a year) also had the smallest nominal critical spacing, implying that the adaptation process can be completed in as short as half a year. Second, the duration since onset for observers J and K who showed no sign of adaptation (the properties of letter recognition still followed those of the normal periphery) was within the range of the duration for the seven observers (A – G) who showed a high degree of completeness of adaptation, implying that duration alone cannot predict whether or not the adaptation process is complete. Third, observers H and I who showed partial adaptation also happened to have the longest durations since onset. The latter two points suggest that it does not appear to exist a clear and definitive correlation between the degree of adaptation and the duration since the onset of AMD. However, we acknowledge that there are individual differences in how long it takes for the adaptation process to complete. Thus the duration since onset (especially if the onset is not recent, such as on the scale of weeks or several months) may not be a good indicator of whether or not someone has already completely adapted to the disorder.
Figure 6.
Nominal critical spacing (multiples of letter size) is plotted as a function of the number of years since the onset of AMD. Each observer is represented using his/her ID and color-coded as in Figure 5. The dashed line represents the criterion value of 1.84 adopted by Song et al (2014) to classify whether an observer’s letter recognition is limited more by size (values < 1.84) or spacing (values > 1.84). The solid black line represents the best-fit regression line to the data, with a slope of 0.015±0.068, which is not different from a slope of zero, implying no relationship between the nominal critical spacing and the duration since onset of the disorder. Error bars represent ± 1 SEM.
As we mentioned in the Results, observers J and K were the only two observers who demonstrated residual foveal function, which is not a rare occurrence for patients with AMD. Patients with residual foveal function could continue to enjoy relatively good acuities, however, the field of view for such good vision is usually very small and that their vision drops dramatically under dim light level. Therefore these patients usually would have to rely on two different retinal locations (the fovea and the PRL) for daily functional vision. This might have affected the adaptation process because they are not required to simply use a single retinal location on a constant basis. Indeed, it has been suggested that large-scale cortical reorganization of visual processing only occurs in the complete absence of functional foveal vision (Baker, Dilks, Peli & Kanwisher, 2008).
A caveat with testing observers with residual foveal function is that since we did not formally monitor eye movements, the two observers could have switched to using their fovea during single-letter testing. We cannot rule out this possibility but we do not think it would have a great impact on our results because of the following. We used the Method of Constant Stimuli to present letters of a wide range of sizes to construct a psychometric function, and the larger letters presented would have exceeded the small residual field of view in the fovea. Therefore, these observers probably would have used their peripheral PRL even for single-letter testing. Although they could have switched between the fovea and the peripheral PRL even within a block depending on the letter size, observations of the eye movements of these observers by the experimenter showed that these observers rarely shifted their gaze within a block.
To date, we know very little about the presumed adaptation process. Besides the psychophysical evidence provided above, the only other piece of evidence suggesting changes following vision loss in people with AMD comes from White and Bedell (1990) who studied whether or not there was a shift in the oculomotor reference following the loss of central vision. The authors used a fundus camera to record the retinal location used by observers for fixating a target. Then they asked observers to make a saccade to refixate the target after it has been displaced, and determined whether those saccades were directed toward the PRL, or the anatomical fovea of the observers. Saccades that were directed toward the PRL would indicate an oculomotor adaptation in that there is a shift in the oculomotor reference from the fovea to a non-foveal location. Across observers, they found that only 1 of their 10 observers with AMD demonstrated such a shift in the oculomotor reference, although 6 of their 11 observers with juvenile macular degeneration demonstrated such a shift. Interestingly, the sole observer with AMD who demonstrated a shift in the oculomotor reference was also the one who had the disorder for the longest period of time (17 years). In any case, a shift in the oculomotor reference also implies that the visual system is malleable depending on changes in experience throughout life.
Clinical implications
From a scientific point of view, it is encouraging to know that the visual system, even an aging one, is capable of responding to changes in a person’s environment, including the loss of vision that occurs late in life. However, can we translate this knowledge into clinical practice to benefit patients with AMD? For the individuals whose letter recognition is limited by letter size, practically this means that as long as a letter exceeds the resolution limit to be recognizable on its own, the addition of nearby letters does not affect the recognizability of the letter unless the letter separation is closer than the standard spacing used in common printed text.5 In other words, these individuals should benefit from conventional magnification through the use of, for example, large print or optical magnifiers, and that their response to magnification should be predictable based on the individual’s single-letter size threshold (acuity in the clinical term). In contrast, for the individuals whose letter recognition is limited by letter spacing, what they need is separation between adjacent letters larger than the standard in common printed text. In this case, conventional magnification which provides equal magnification between letter size and spacing may not offer an optimal solution. A better solution is to be able to decouple the magnification of size and spacing such that we can increase the letter spacing beyond the standard spacing. Currently, such a system for magnification is not yet available to patients. To overcome the limitation of spacing, magnification greater than what is required to bring single letters to exceed the resolution limit might be required. This might explain a common clinical observation in that some patients with AMD show a mismatch between their distance and near acuities (for people with normal vision, the distance and near acuities, once converted to the same notation, are very similar). When this happens, patients with AMD almost invariably require magnification higher than what is predicted based on their distance acuity and the size of the print that they would like to see. In clinical terms, they require a larger acuity reserve in order to read comfortably (Whittaker & Lovie-Kitchin, 1993).
Song et al (2014) proposed a quick screening test that includes only two measurements of size thresholds to diagnose if an individual is limited more by a size (resolution) or a spacing (crowding) effect. The two size threshold measurements include one for single-letters and another one for “closely” flanked letters. They suggested any spacing smaller than 1.4× the letter size qualifies as close spacing but they recommended the use of a letter spacing of 1.1× the letter size. The idea is that a template of the bilinear function as used in this study can then be used to fit to the two size thresholds, from which a spacing:acuity ratio (nominal critical spacing) can be derived. They adopted a ratio of 1.84 to separate individuals whose letter recognition is limited by size from those whose performance is limited by spacing. Similarly, we can adopt this method to predict whether patients with AMD have completely adapted to the vision loss and thus show characteristics resembling those of the normal fovea. If so, then they should show a predictable response to magnification. In contrast, patients who have not adapted to the vision loss should show characteristics that resemble the normal periphery. In those cases, they will not respond to magnification as predicted (calculated) and require higher-than-predicted magnification to see their targets clearly. Given our interest in relation to reading, for the AMD patients, we suggest the use of standard letter spacing (averaging 1.13× the x-height for a set of common font, see footnote #3) in common printed text when measuring the closely spaced letter size threshold. Many of the currently available near reading cards for clinical usage such as the Bailey-Lovie Word Reading Card (Bailey & Lovie, 1980) and the MNREAD Acuity Chart (Mansfield, Ahn, Legge & Luebker, 1993) would be suitable for this purpose. Note however, that the measurement of size-threshold for letters presented in groups is not equivalent to measurement of near reading acuity where patients are expected to recognize words, instead of letters.
Applying Song et al’s proposed ratio of 1.84 to our observers would have classified observers A – G (see Table 1) as those whose letter recognition exhibit properties similar to those of the normal fovea. This classification is very consistent with what we reported in the Results earlier, in that when absolute threshold is plotted as a function of letter-size threshold (Figure 5), the data of observers A – G fell very close to the dotted “normal foveal” line. Song et al (2014) showed that the criterion ratio of 1.84, when combined with an appropriate acuity cut-off criterion, was an effective screening tool to identify people who might suffer from an exacerbated crowding effect, including those with strabismic amblyopia (with subnormal acuity) and apperceptive agnosia (with normal acuity). Here, we show that the criterion ratio of 1.84 is equally effective to indicate if an individual with AMD has adapted to his/her vision loss and how his/her response to magnification would be.
Critical spacing for letter recognition vs. critical spacing for reading
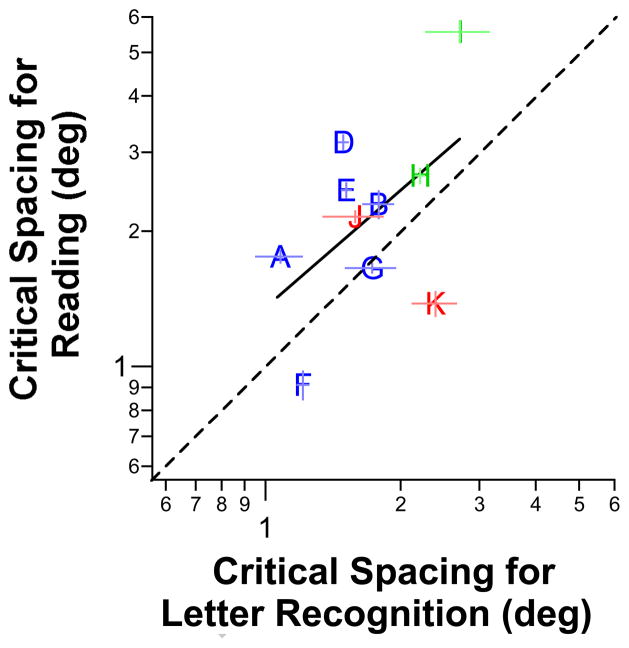
Previously, Pelli et al (2007) and Levi et al (2007) showed that reading is limited by crowding in the normal periphery and in the amblyopic eye, based on the finding that the critical spacing for reading is the same as the critical spacing for letter recognition. If this applied to people with AMD, then we would be able to predict the minimum separation between letters in text required for these people to read at their maximum reading speed, based simply on letter acuities. Although we only measured the critical spacing for letter recognition in this study, we had previously reported the critical spacing for reading for 10 of the 11 observers who participated in this study, thus we could make the comparison, especially since we also used lowercase letters for the task of letter recognition in this study. Figure 6 compares the critical spacing for reading with that for letter recognition. For this comparison, because the critical spacing for reading was obtained for reading speed defined as 80% correct, we reanalyzed our data to derive the letter size thresholds that corresponded to a recognition accuracy of 80% (after correction for guessing) and refit each set of data to obtain the critical spacing for letter recognition (for 80% accuracy). Despite the apparent trend that the critical spacing for reading increases with the critical spacing for letter recognition, because of the substantial individual observer variability, the correlation between the two critical spacings (in log values) is not significant (r = 0.52, tdf=8 = 1.70, p = 0.13). In other words, unlike the relationship reported for the normal periphery and in amblyopes, we cannot simply predict the critical spacing for reading based on that for letter recognition for people with AMD.
Summary
Pelli (2008) suggested that the limitation of spacing applies to object recognition in general, and not solely to letter recognition. Therefore, although our study examined the effects of spacing and size on the task of letter recognition, the results have implications beyond letter recognition. Our finding that many individuals with AMD exhibit a critical spacing smaller than would be predicted based on the normal periphery suggests that these individuals may not suffer from as much crowding in their daily lives as previously postulated. The smaller-than-expected critical spacing is likely to be a consequence of an adaptation process that requires the individual to use a peripheral PRL on a constant basis. In summary, spacing and size both pose a limitation on object recognition for people with AMD, but for those who have adapted to their vision loss, object size seems to be a more important limitation than spacing.
Figure 7.
Absolute critical spacing for reading (deg) is plotted as a function of the absolute critical spacing for letter recognition (deg). Critical spacings for reading were obtained from Chung (2012) while critical spacings for letter recognition were based on an accuracy criterion of 80% and thus are different from the values plotted in Figure 5. Observer C is not included because she did not participate in the study of Chung (2012). The dashed line represents the unity line — the critical spacing for reading is identical to that for letter recognition. Although the black regression line (fitted to log-log data) shows a reasonable trend between the critical spacing for reading and letter recognition, the correlation was not significant (see text for details). Error bars represent ± 1 SEM.
HIGHLIGHTS.
Letter size thresholds obtained in adults with age-related macular degeneration (AMD)
Single letter size thresholds worse in the periphery of AMDs than in normal periphery
Flanked letter size thresholds worsened with spacings smaller than critical spacing
Nominal critical spacing is 1–2× in the normal fovea and 3–4× in the normal periphery
7 of 11 AMDs had a nominal critical spacing comparable with that of the normal fovea
Acknowledgments
This work was supported by research grant R01-EY012810 from the National Eye Institute, NIH. The author thanks Dennis Levi for his helpful comments on an earlier version of the paper and Daniel Coates for the invaluable discussions on the topic.
Footnotes
Following the onset of the central vision loss, patients often adopt a retinal location outside the dysfunctional macular area as the reference locus for seeing. This location is usually referred to as the preferred retinal locus (PRL). There are reports that the PRL may differ for different tasks, here, our defintion of PRL was for a fixation task. However, most of the observers in this study have participated in other studies in our lab in which SLO measurements were obtained. Informal observation showed that in most cases, their PRLs did not change for a fixation, letter recognition and saccadic task.
The size of the gap was equivalent to approximately 4× the x-height of the largest letters tested. In other words, the distance between the edge of each black line and the center of the target letter was approximately 2× the size of the largest letters (even larger nominal separations for smaller letters). This separation exceeded the critical spacing for the threshold letter size (the threshold letter size was always smaller than the largest letter size used in any given block of trials) in all cases and thus should not have caused any undesirable spatial interaction with the letters. For the largest letter sizes, observers’ accuracy for recognizing the middle letters was close to 100%, therefore it was unlikely that the black lines caused much detrimental spatial interaction effect on the recognition of these letters.
In some cases, most notably for observers A–C, their data seem to fit a single line (on log-log axes) with a slope of 0; and for observers J and K, their data seem to fit a single line with a slope of −1. To ensure that our two-line fit best described the data, we compared the goodness-of-fit for the two-line fit, and single-line fit of slope of 0 and −1 respectively. The best fit was defined as the one that yielded the smallest AIC (Akaike Information Criterion) value. For all observers, the AIC value was the smallest, implying that the fit was the best, for the two-line fit.
Also see Blackmore-Wright, Georgeson and Anderson (2013) who reported an improvement in reading speed for the condition in which text was rendered with double spacing between words, and double spacing between lines of text. The effect was greater for low-contrast text. However, the benefit seems to disappear for triple word- or triple line spacing.
We empirically determined the standard letter spacing (ratio of the absolute center-to-center letter spacing to x-height, essentially the same as the definition for nominal letter spacing used in this paper) used in word processing for several commonly used fonts that included fonts with and without serifs, fixed-width and proportional-width: American Typewritter, Arial, Bookman, Courier, Helvetica, Palatino, Times and Times New Roman. Across the eight fonts, the standard letter spacing ranges from 0.98× (Helvetica, a proportional-width font) to 1.32× (Courier, a fixed-width font) the x-height (mean [±SD] = 1.13×[±0.12]).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailey IL, Lovie JE. The design and use of a near near-vision chart. American Journal of Optometry and Physiological Optics. 1980;57:378–387. doi: 10.1097/00006324-198006000-00011. [DOI] [PubMed] [Google Scholar]
- Bailey IL, Lovie-Kitchin JE. Visual acuity testing. From laboratory to the clinic. Vision Research. 2013;90:2–9. doi: 10.1016/j.visres.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Baker CI, Dilks DD, Peli E, Kanwisher N. Reorganization of visual processing in macular degeneration: replication and clues about the role of foveal loss. Vision Research. 2008;48:1910–1919. doi: 10.1016/j.visres.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore-Wright S, Georgeson MA, Anderson SJ. Enhanced text spacing improves reading performance in individuals with macular disease. PLoS One. 2013;8:e80325. doi: 10.1371/journal.pone.0080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Bullimore MA, Bailey IL. Reading and eye movements in age-related maculopathy. Optometry and Vision Science. 1995;72:125–138. doi: 10.1097/00006324-199502000-00011. [DOI] [PubMed] [Google Scholar]
- Calabrèse A, Bernard JB, Hoffart L, Faure G, Barouch F, Conrath J, Castet E. Small effect of interline spacing on maximal reading speed in low-vision patients with central field loss irrespective of scotoma size. Investigative Ophthalmology & Visual Science. 2010;51:1247–1254. doi: 10.1167/iovs.09-3682. [DOI] [PubMed] [Google Scholar]
- Chung STL. Dependence of reading speed on letter spacing in central vision loss. Optometry and Vision Science. 2012;89:1288–1298. doi: 10.1097/OPX.0b013e318264c9dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung STL. The Glenn A. Fry Award Lecture 2012: Plasticity of the visual system following central vision loss. Optometry and Vision Science. 2013a;90:520–529. doi: 10.1097/OPX.0b013e318294c2da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung STL. Cortical reorganization after long-term adaptation to retinal lesions in humans. Journal of Neuroscience. 2013b;33:18080–18086. doi: 10.1523/JNEUROSCI.2764-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung STL, Jarvis SH, Woo SY, Hanson K, Jose RT. Reading speed does not benefit from increased line spacing. Optometry and Vision Science. 2008;85:827– 833. doi: 10.1097/OPX.0b013e31818527ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung STL, Levi DM, Legge GE. Spatial-frequency and contrast properties of crowding. Vision Research. 2001;41:1833–1850. doi: 10.1016/s0042-6989(01)00071-2. [DOI] [PubMed] [Google Scholar]
- Coates DR, Chin JM, Chung STL. Factors affecting crowded acuity: eccentricity and contrast. Optometry and Vision Science. 2013;90:628–638. doi: 10.1097/OPX.0b013e31829908a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon N, O’Colmain B, Klaver CC, Klein R, Muñoz B, Friedman DS, Kempen J, et al. Causes and prevalence of visual impairment among adults in the United States. Archives of Ophthalmology. 2004;122:477–85. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- Flom MC. Contour interaction and the crowding effect. Problems in Optometry. 1991;3:237–257. [Google Scholar]
- Flom MC, Heath GG, Takahashi E. Contour interaction and visual resolution: contralateral effects. Science. 1963;142:979–980. doi: 10.1126/science.142.3594.979. [DOI] [PubMed] [Google Scholar]
- Flom MC, Weymouth FW, Kahneman D. Visual resolution and contour interaction. Journal of the Optical Society of America. 1963;53:1026–1032. doi: 10.1364/josa.53.001026. [DOI] [PubMed] [Google Scholar]
- Friedman DS, O’Colmain BJ, Muñoz B, Tomany SC, McCarty C, de Jong PTVM, Nemesure B, et al. Prevalence of age-related macular degeneration in the United States. Archives of Ophthalmology. 2004;122:564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- Jacobs RJ. Visual resolution and contour interaction in the fovea and periphery. Vision Research. 1979;19:1187–1195. doi: 10.1016/0042-6989(79)90183-4. [DOI] [PubMed] [Google Scholar]
- Kleen SR, Levoy RJ. Low vision care: correlation of patient age, visual goals, and aids prescribed. American Journal of Optometry and Physiological Optics. 1981;58:200–205. [PubMed] [Google Scholar]
- Leat SJ, Rumney NJ. The experience of a university-based low vision clinic. Ophthalmic and Physiological Optics. 1990;10:8–15. [PubMed] [Google Scholar]
- Levi DM, Klein SA, Hariharan S. Suppressive and facilitatory spatial interactions in foveal vision: foveal crowding is simple contrast masking. Journal of Vision. 2002;2(2):140–166. doi: 10.1167/2.2.2. [DOI] [PubMed] [Google Scholar]
- Levi DM, Song S, Pelli DG. Amblyopic reading is crowded. Journal of Vision. 2007;7(2):21, 1–17. doi: 10.1167/7.2.21. [DOI] [PubMed] [Google Scholar]
- Levi DM. Crowding — an essential bottleneck for object recognition: A mini-review. Vision Research. 2008;48:635–654. doi: 10.1016/j.visres.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield JS, Ahn SJ, Legge GE, Luebker A. Ophthalmic and Visual Optics/Noninvasive Assessment of the Visual System Technical Digest. Vol. 3. Optical Society of America; Washington, DC: 1993. A new reading-acuity chart for normal and low vision; pp. 232–235. [Google Scholar]
- Mansfield JS, Legge GE. From letters to words: the role of lexical inference. Investigative Ophthalmology & Visual Science (Suppl) 1999;40:S35. [Google Scholar]
- Pelli DG. The videotoolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Pelli DG. Crowding: a cortical constraint on object recognition. Current Opinion in Neurobiology. 2008;18:445–451. doi: 10.1016/j.conb.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG, Tillman KA, Freeman J, Su M, Berger TD, Majaj NJ. Crowding and eccentricity determine reading rate. Journal of Vision. 2007;7(2):20, 1–36. doi: 10.1167/7.2.20. [DOI] [PubMed] [Google Scholar]
- Pelli DG, Tillman KA. The uncrowded window of object recognition. Nature Neuroscience. 2008;11:1129–1135. doi: 10.1038/nn.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG, Palomares M, Majaj NJ. Crowding is unlike ordinary masking: Distinguishing feature integration from detection. Journal of Vision. 2004;4:1136–1169. doi: 10.1167/4.12.12. [DOI] [PubMed] [Google Scholar]
- Rawlinson GE. Unpublished PhD thesis. University of Nottingham; Nottingham, UK: 1976. The significance of letter position in word recognition. [Google Scholar]
- Song S, Levi DM, Pelli DG. A double dissociation of the acuity and crowding limits to letter identification, and the promise of improved visual screening. Journal of Vision. 2014 doi: 10.1167/14.5.3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toet A, Levi DM. The two-dimensional shape of spatial interaction zones in the parafovea. Vision Research. 1992;32:1349–1357. doi: 10.1016/0042-6989(92)90227-a. [DOI] [PubMed] [Google Scholar]
- Wertheim T. Peripheral visual acuity: Th. Wertheim. American Journal of Optometry and Physiological Optics. 1980;57:915–924. [PubMed] [Google Scholar]
- White JM, Bedell HE. The oculomotor reference in humans with bilateral macular disease. Investigative Ophthalmology & Visual Science. 1990;31:1149–1161. [PubMed] [Google Scholar]
- Whitney D, Levi DM. Visual crowding: a fundamental limit on conscious perception and object recognition. Trends in Cognitive Science. 2011;15:160–168. doi: 10.1016/j.tics.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker SG, Lovie-Kitchin J. Visual requirements for reading. Optometry and Vision Science. 1993;70:54–65. doi: 10.1097/00006324-199301000-00010. [DOI] [PubMed] [Google Scholar]