Significance
In failing hearts, norepinephrine (NE) net release and endothelin-1 (ET1) levels are increased. ET1 receptor antagonists were successfully used in preclinical heart failure (HF) studies, but clinical studies did not show beneficial effects in HF patients. We found that mice lacking endothelin receptor A (ETA) only in sympathetic neurons but not in cardiomyocytes were protected from the development of HF. Mechanistically, the presynaptic reuptake of NE within the heart was preserved in mice lacking ETA only in sympathetic neurons. These data provide an explanation of why patients in clinical studies do not benefit from ET1 receptor antagonists, because these patients are usually treated with β blockers, which interfere with the mechanism of action identified here.
Keywords: β adrenergic signaling, sympathetic nervous system, norepinephrine reuptake, epigenetic regulation, HDACs
Abstract
In preclinical studies, endothelin receptor A (ETA) antagonists (ETAi) attenuated the progression of heart failure (HF). However, clinical HF trials failed to demonstrate beneficial effects of ETAi. These conflicting data may be explained by the possibility that established HF drugs such as adrenergic receptor blockers interfered with the mechanism of ETAi action in clinical trials. Here we report that mice lacking ETA only in sympathetic neurons (SN-KO) showed less adverse structural remodeling and cardiac dysfunction in response to pathological pressure overload induced by transverse aortic constriction (TAC). In contrast, mice lacking ETA only in cardiomyocytes (CM-KO) were not protected. TAC led to a disturbed sympathetic nerve function as measured by cardiac norepinephrine (NE) tissue levels and [124I]-metaiodobenzylguanidine-PET, which was prevented in SN-KO. In a rat model of HF, ETAi improved cardiac and sympathetic nerve function. In cocultures of cardiomyocytes (CMs) and sympathetic neurons (SNs), endothelin-1 (ET1) led to a massive NE release and exaggerated CM hypertrophy compared with CM monocultures. ETA-deficient CMs gained a hypertrophic response through wild-type SNs, but ETA-deficient SNs failed to mediate exaggerated CM hypertrophy. Furthermore, ET1 mediated its effects indirectly via NE in CM-SN cocultures through adrenergic receptors and histone deacetylases, resulting in activation of the prohypertrophic transcription factor myocyte enhancer factor 2. In conclusion, sympathetic ETA amplifies ET1 effects on CMs through adrenergic signaling pathways. Thus, antiadrenergic therapies may blunt potentially beneficial effects of ETAi. Taken together, this may indicate that patients with β blocker intolerance or disturbed sympathetic nerve function could be evaluated for a potential benefit from ETAi.
Heart failure (HF) is common and, despite therapeutic advancements, is linked to a poor prognosis (1). Various neurohormones are elevated and correlate with the severity of HF (2, 3). Among them, the role of endothelin-1 (ET1) has been extensively studied (4, 5). ET1 was shown to be elevated in blood samples of HF patients, to predict outcome and to play a crucial role for adverse cardiac remodeling (6). In 1996, it was demonstrated that endothelin receptor inhibition is cardioprotective in a preclinical model of HF (7), which was supported by others (8–10). However, despite convincing preclinical data, unspecific ET1 receptor and specific ETA inhibitors (ETAi) showed no beneficial effects in clinical HF trials (11, 12). In contrast to animal studies, HF patients in clinical trials are usually cotreated with other HF drugs such as angiotensin-converting enzyme inhibitors or adrenergic receptor antagonists, raising the possibility that these drugs interfere with the effects of ETAi. Conflicting data came also from genetic studies showing that mice lacking ETA only in cardiomyocytes (CM-KO) were not protected when exposed to cardiac stress (13). Thus, the question has arisen of whether ETA in nonmyocytes affects critical CM signaling pathways via cell–cell communication mechanisms.
ETA is also expressed in neural crest derived tissue such as sympathetic neurons (SNs) that innervate the heart and release norepinephrine (NE) (14, 15), one of the neurotransmitters that regulate cardiac function and growth. The success of HF therapy with adrenergic receptor antagonists that inhibit the target receptor of NE in adult CMs underscores the pivotal role of NE in HF (16). Consequently, the mechanisms by which NE is released from sympathetic neurons impact on signaling cascades in CMs (17–19). We now hypothesized that ETAi mediates their beneficial effects not through the inhibition of ETA on CMs but on SNs and consequently indirectly through an attenuated activation of α-adrenergic receptors (αARs) and β-adrenergic receptors (βARs). In CMs, ETA, and also αARs and βARs, signals to kinases such as protein kinase D (PKD) and calcium calmodulin dependent kinase II (CaMKII). These kinases induce phosphorylation-dependent nucleo-cytoplasmic shuttling of class II histone deacetylases (HDACs) (20, 21), resulting in the activation of the transcription factor myocyte enhancer factor 2 (MEF2), a crucial inductor of pathological cardiac remodeling (22).
In this study, we investigated two KO mouse lines, one lacking ETA only in SNs (SN-KO) and another one lacking ETA only in CMs (CM-KO). We show that SN-ETA but not CM-ETA contributes to adverse cardiac remodeling.
Results
Deficiency of ETA Specifically in SNs but Not in CMs Protects from Pathological Cardiac Remodeling.
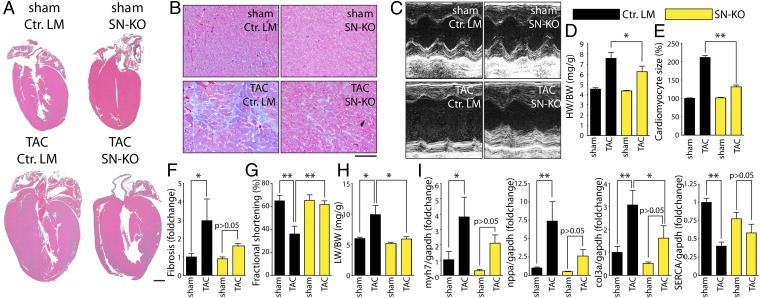
To investigate the role of ETA in specific cell types, we generated conditional mutant mice lacking ETA selectively in SNs (SN-KO) vs. CMs (CM-KO) (Fig. S1A). Quantitative RT-PCR revealed that transgenic expression of Cre under the control of the dopamine-β-hydroxylase promoter (DBH-Cre) (23) led to a substantial loss of ETA in sympathetic ganglia of SN-KO mice, whereas transgenic Cre expression under the control of α-myosin heavy chain promoter (αMHC-Cre) led to a comparable deletion of ETA in the myocardium of CM-KO mice (Fig. S1B). Both lines developed normally without any obvious anatomical abnormalities. We then challenged these lines with pathological pressure overload induced by transverse aortic constriction (TAC) and compared them to control littermates (Ctrl. LM). The degree of TAC was similar in all experimental groups (Fig. S2). CM-KO mice did not show any signs of cardioprotection with regard to cardiac hypertrophy (Fig. S3 A, D, and E), interstitial fibrosis (Figs. S3 B and F and S4), function (Fig. S3 C and G), and pulmonary congestion (Fig. S3H) or gene expression (Fig. S3I). Strikingly, SN-KO mice did not develop massive cardiac hypertrophy (Fig. 1 A, D, and E), interstitial fibrosis (Fig. 1 B and F and Fig. S4), or cardiac dysfunction (Fig. 1 C and G). Pulmonary congestion as an additional sign of severe left ventricular dysfunction was diminished in SN-KO (Fig. 1H). Moreover, dysregulation of genes that are typically associated with adverse cardiac remodeling (including nppa, myh7, Col3a, and Serca) was attenuated in SN-KO after TAC compared with Ctrl. LM (Fig. 1I).
Fig. 1.
Sympathetic ETA is essential for the development of adverse cardiac remodeling. (A) Representative four-chamber view of mice lacking ETA in sympathetic neurons (SN-KO) vs. control littermates with normal ETA expression (Ctrl. LM) 7 wk after sham or TAC surgery. (Scale bar, 1 mm.) (B) Representative trichrome-stained sections. (Scale bar, 100 µm.) (C) Representative transthoracic echocardiography of the left ventricle (M-Mode, midventricular). (D) Heart weight/body weight (HW/BW) ratios (n = 5–7 per group). (E) Quantification of cardiomyocyte size (>80 cells per sample from more than five fields of view; n ≥ 3). (F) Quantification of fibrosis in relation to Ctrl. LM (n ≥ 3 per group). (G) Fractional shortening 3 wk after TAC (n = 5–7 per group). (H) Lung weight/body weight (LW/BW) ratios 7 wk after TAC surgery (n = 5–7 per group). (I) Transcript levels in hearts from Ctrl. LM and SN-KO 7 wk after TAC surgery from genes as indicated in relation to sham-operated Ctrl. LM (n = 5–7 per group). Values are presented as mean ± SEM. *P < 0.05; **P < 0.01.
ETA Impairs Sympathetic Nerve Function in SN-KO After TAC.
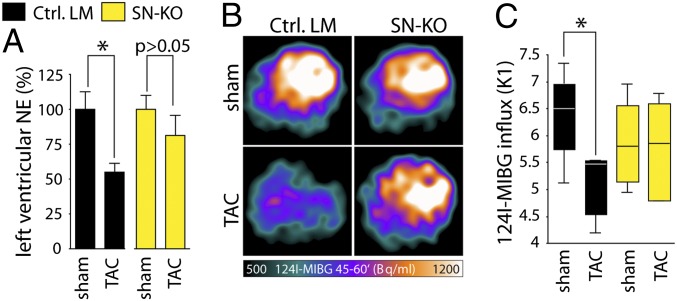
To test whether sympathetic nerve function is preserved in SN-KO after TAC, sympathetic indices including NE tissue levels and metaiodobenzylguanidine (MIBG) uptake were determined. Depleted myocardial NE stores indicated a substantial increase in NE net secretion as an estimate for overactivation of the local cardiac sympathetic nervous system (24, 25). Accordingly, we found left ventricular NE tissue levels to be decreased after TAC in Ctrl. LM but not in SN-KO (Fig. 2A). To address the question of whether depleted myocardial NE stores were mediated—at least in part—by a reduced NE reuptake via the NE transporter (NET), we used the [124I]-MIBG-PET method to assess cardiac NET activity in vivo (17, 18, 26). This method does not directly measure cardiac sympathetic activity but serves as an index for cardiac sympathetic activity. We found that TAC leads to a reduction of MIBG uptake in Ctrl. LM but not SN-KO, strongly suggesting that ET1 impairs sympathetic nerve function in vivo via ETA and NET-mediated NE reuptake (Fig. 2 B and C).
Fig. 2.
Sympathetic ETA impairs sympathetic nerve function. (A) ELISA-based quantification of NE within the left ventricle of SN-KO 7 wk after TAC in relation to sham-operated mice (n = 4–7 per group). (B) Representative PET scan images of [124I]-MIBG uptake in the frame 45–60 min after MIBG injection into sham- and TAC-operated Ctrl. LM vs. SN-KO. (C) Quantitative analysis of the influxes (K1) of [124I]-MIBG via PET scan 7 wk after TAC (controls, n = 4; TAC, n = 6). Values presented as mean ± SEM. *P < 0.05.
ETA Inhibitors Improve Sympathetic Nerve Function in a Rat Model of HF.
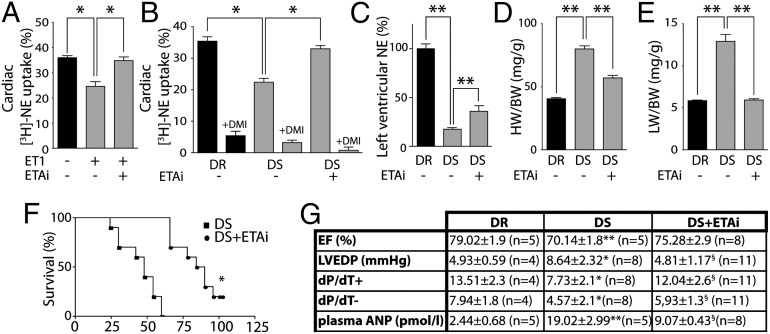
To investigate whether the findings in the mouse models translate to larger rodents, we first perfused isolated hearts of healthy rats with ET1 and found a significantly reduced NE uptake, which could be diminished by pretreatment with darusentan (ETAi) (Fig. 3A). To study whether ETAi normalizes sympathetic nerve function in a rat model of HF, we used salt-sensitive (DS) vs. salt-resistant (DR) Dahl rats, both fed on a high-salt diet (27). DS rats displayed an impaired NE uptake (Fig. 3B) and depleted cardiac NE stores (Fig. 3C). In accordance with the genetic deletion of ETA in TAC mice, ETAi significantly improved NE uptake and NE stores (Fig. 3 B and C), confirming an improved sympathetic nerve function. The improvement of sympathetic nerve function by ETAi was associated with an attenuation of cardiac hypertrophy, a prevention of pulmonary congestion, and a reduced mortality (Fig. 3 D–F). Moreover, we observed further improvements of hemodynamic characteristics and the HF biomarker atrial natriuretic peptide (ANP) (Fig. 3G). Because we focused in the present study on cardiac function, we cannot rule out that the reduced mortality is—at least in part—due to an improved function of the kidney or other organs that are innervated by sympathetic neurons.
Fig. 3.
ETA inhibitors improve sympathetic nerve function in a rat model of HF. (A) [3H]-NE uptake in isolated perfused hearts in the presence/absence of ET1 and ETAi. (B) [3H]-NE uptake with/without ETAi (darusentan) treatment in salt-sensitive Dahl rats (DS) compared with salt-resistant rats (DR). Almost complete inhibition by desipramine (DMI) indicates that the majority of NE is taken up by the NE transporter (NET). (C) Left ventricular NE (tissue level) in DS vs. DR. ETAi treatment attenuates the depletion of left ventricular NE (n = 5–9 per group). (D) ETAi attenuates cardiac hypertrophy (HW/BW) and (E) pulmonary congestion (LW/BW) in DS (n > 10 per group). (F) Kaplan–Meier analysis of DS treated with/without ETAi (n = 10 per group). (G) Left ventricular ejection fraction (EF), left ventricular enddiastolic pressure (LVEDP), contractility index (dP/dT+), relaxation index (and dP/dT−), and plasma levels ANP in DS treated with/without ETAi. Values are presented as mean ± SEM. *P < 0.05; **P < 0.01; and §P < 0.05 vs. DS.
Sympathetic Neurons Regulate Cardiomyocyte Hypertrophy Through Sympathetic ETA.
Next, we aimed at recapitulating the in vivo findings in isolated cell culture systems. Treatment of rat sympathetic neuron-cardiomyocyte cocultures (SN-CM) with 10 nM ET1 led to a fourfold increase of NE net release into the culture medium, which was inhibited by ETAi (Fig. S5A). Although we cannot formally rule out that this effect was mediated by ET1-mediated exocytotic NE release, we propose based on previous experiments (28) that this effect is rather due to inhibition of NE reuptake. The presence of SNs in the SN-CM coculture was confirmed by Western blot analysis using an antibody against the SN-specific marker tyrosine hydroxylase (TH) (Fig. S5B). Remarkably, ET1 led to exaggerated CM hypertrophy in CM-SN cocultures (Fig. S5C). To further support the requirement of the sympathetic ETA for CM hypertrophy, we also performed CM-SN coculture experiments with selective ETA deletions either in CMs or SNs. First, we adenovirally expressed Cre in isolated murine SNs from ETAfl/fl mice (SNKO) and cocultured these cells with WT rat CMs (CMWT). The deletion of SN-ETA abolished exaggerated CM hypertrophy in SNKO-CMWT cocultures (Fig. S5 D and E). Immunocytochemistry with an antibody against ETA illustrates not only a sufficient deletion of ETA through Cre transduction but also that the concentration of ETA on SNs is largely higher than on CMs (Fig. S5D). Second, we adenovirally expressed Cre in isolated murine CMs from ETAfl/fl mice (CMKO) and cocultured these cells with WT rat SNs (SNWT). The deletion of CM-ETA inhibited ET1-induced hypertrophy in monocultures (CMKO) but the addition of SNWT (CMKO-SNWT cocultures) restored the hypertrophic response of CMKO, formally proving that ETA on CMs is dispensable for ET1-induced CM hypertrophy in the presence of SNs (Fig. S5 F and G). Deletion of ETA in CMKO was confirmed by Western blot (Fig. S6).
Sympathetic ETA Regulates CM Hypertrophy Through Adrenergic Receptors.
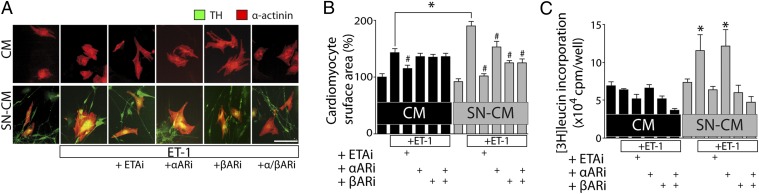
To test whether the SN-dependent increase in CM size depends on adrenergic receptors, we used pharmacological inhibitors for αARs and βARs (αARi and βARi). Strikingly, ET1-induced CM hypertrophy (Fig. 4 A and B) and protein synthesis (Fig. 4C) in CM-SN coculture, but not in CM monoculture, could be attenuated by βARi, whereas β1ARi seems to be sufficient to inhibit exaggerated hypertrophy (Fig. S7). However, αARi was ineffective in this regard. As a control, ETAi sufficiently blocked CM hypertrophy in both culture systems.
Fig. 4.
Sympathetic ETA regulates CM hypertrophy through adrenergic receptors. (A) Immunocytochemistry of neonatal rat ventricular myocytes (NRVMs, CMs) and cocultures of CMs together with neonatal rat sympathetic neurons (SNs) was performed using antibodies recognizing sarcomeric α-actinin (red) and TH (green). Cultured cells were treated for 24 h with 10 nM ET1 in the absence or presence of the inhibitors darusentan (1 μM; ETAi), prazosin (1 μM; αARi), or propranolol (1 μM; βARi). (B) CM cell surface area was quantified. #P < 0.05 vs. ET1-treated; *P < 0.05 ET1-treated SN-CM vs. CM (n = 100–150/per group). (C) [3H]-leucin incorporation in CMs vs. SN-CM with treatment as indicated (ET1: 10 nM 18 h; αAR: 1 μM prazosin; βAR: 1 μM propranolol; 1 h before ET1 treatment). n = 4. *P < 0.05 vs. nontreated SN-CM; #P < 0.05 vs. ET1-treated.
Sympathetic ETA Activates the HDAC–MEF2 Axis in CMs.
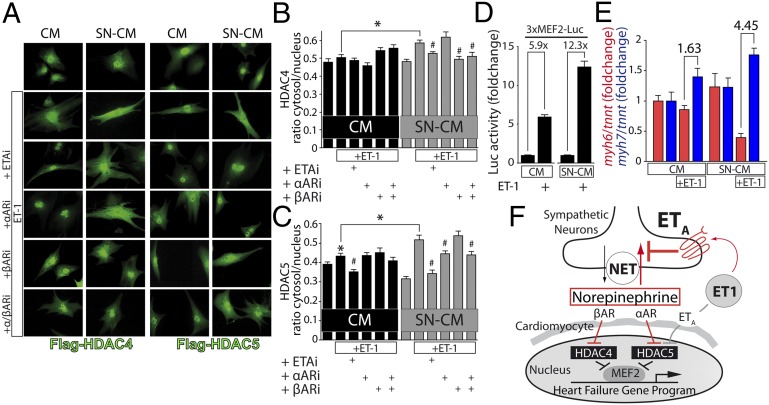
ETA signaling via protein kinases was suggested to result in a phosphorylation-dependent nucleo-cytoplasmic shuttling of class II HDACs, which in turn results in activation of the transcription factor MEF2 and gene programs that lead to cardiomyocyte hypertrophy (20, 22). In SN-CM, ET1 led to a significant increased nuclear export of adenovirally expressed HDAC4 and HDAC5 (Fig. 5 A–C). HDAC4 nuclear export was significantly reduced by βARi, whereas HDAC5 nuclear export was significantly reduced by αARi. Moreover, βAR signaling leads specifically to 14-3-3 binding to HDAC4 but not HDAC5 (Fig. S8). ETAi prevented nuclear export of both HDAC4 and HDAC5. Using MEF2 reporter assays, we found that ET1 induced a higher increase in MEF2 activity in SN-CM coculture compared with CM monoculture (Fig. 5D). A hallmark of transcriptional remodeling is a switch in the expression of myosin heavy chain genes from myh6 to myh7 (29). ET1 caused a moderate myh6/myh7 switch in CM monoculture but, remarkably, a dramatic switch in SN-CM coculture (Fig. 5E).
Fig. 5.
Sympathetic ETA activates the HDAC-MEF2 axis in CMs. (A) CMs were transduced with adenoviruses harboring Flag-HDAC4 or -HDAC5, starved for 4 h, and then treated for 12 h with 10 nM ET1 in the absence or presence of the inhibitors darusentan (1 μM; ETAi), prazosin (1 μM; αARi), or propranolol (1 μM; βARi). Immunocytochemistry was performed using antibodies recognizing Flag. (B and C) Quantitative analysis of cytosolic vs. nuclear localized HDACs. *P < 0.05 ET1-treated vs. nontreated group and SN-CM vs. CM. #P < 0.05 inhibitor treated vs. ET1-treated group (n ≥ 40 cells per group). (D) MEF2 activity was measured by MEF2 luciferase reporter assay (n = 4). *P < 0.05. (E) Transcripts of myh6 and myh7 after 24-h treatment with10 nM ET1 (n = 6). *P < 0.05. (B–E) Values are presented as mean ± SEM. (F) Working model: ET1 stimulates ETA on CMs but to a larger extent on SNs. ET1 leads via SN-ETA to a substantial increase of NE net release by inhibiting NET-dependent NE reuptake, which in turn leads to an activation of αARs and βARs and nuclear export of HDAC5 and HDAC4 through distinct signaling pathways (pathway highlighted in red). Thus, the involvement of adrenergic receptors amplifies ET1-driven activation of MEF2.
Discussion
In this study, we demonstrate that ET1 mediates adverse cardiac remodeling not through CM-ETA but through SN-ETA.
Sympathetic ETA Is Required for Abnormal Sympathetic Nerve Function and Pathological Cardiac Remodeling.
We have shown previously in isolated perfused hearts that ET1 impairs NET-dependent NE reuptake but not exocytotic NE release (28). However, isolated heart perfusion in combination with ETAi did not allow the determination of the underlying critical cell types or the investigation of disease-relevant remodeling processes. Now we demonstrate that SN-ETA determines the cardiac vulnerability to pathological pressure overload. Based on our previous data (28) and on the MIBG uptake measurements, we conclude that inhibition of SN-ETA leads to an attenuation of adrenergic neurotransmission and to cardioprotection. In contrast to SN-KOs, CM-KOs were not protected from pathological cardiac stress. The latter finding is consistent with the prior finding that CM-KO mice did not show an improvement of cardiac function after angiotensin or isoproterenol infusion (13) and highlights the critical role of ETA in nonmyocytes. We cannot exclude that ETB in CMs plays a critical role during pathological cardiac remodeling because the role of the ETB has not been investigated in conditional KO models. However, some evidence is provided from mice lacking ETB globally. These mice develop intestinal aganglionosis and megacolon with early death, which could be rescued by reexpression of the ETB under the control of the dopamine-β-hydroxylase promoter, indicating that the SN-ETB may also be important for sympathetic nerve function (15, 30). Previous pharmacological data indicate that sympathetic ETB does not influence NE reuptake but inhibits exocytotic NE release in the heart (28). Through the use of CM-SN cocultures, we provide additional evidence that sympathetic ETA is required for the transmission of ET1-dependent adrenergic and hypertrophic signals to CMs. These results explain how ETAi improves local NE homeostasis as also shown in DS rats on a high-salt diet. However, why did selective ETAi not result in reduced plasma NE levels in HF patients (11, 31)? This discrepancy might be explained by the idea that plasma NE levels are not ideal surrogates for the local cardiac adrenergic drive but rather serve as a marker for a generalized sympathetic activation. In fact, it was shown that the cardiac adrenergic drive independently alters from the generalized sympathetic tone (32).
Role of Nonmyocytes in the Regulation of ET1-Dependent Signal Transduction in CMs.
Until now, it was assumed that ET1 mediates its hypertrophic effects through ETA on CMs (33), which mainly is based on the observation that ET1 exerts hypertrophy of cultured CMs. However, CM primary cultures are heterogenous and contain—dependent on the type of isolation and duration of cultivation—variable amounts of nonmyocytes such as fibroblasts, endothelial cells, smooth muscle cells, and intrinsic neurons (34). This heterogeneity is potentially one of the reasons for variable results from isolation to isolation as experienced by many investigators. The results of this study demonstrate that SN-ETA is required for pathological remodeling of the heart and that CM-ETA is dispensable in the presence of SNs. In this regard, previous studies on ET1-induced CM hypertrophy usually used relatively high concentrations of ET1 (10–100 nM). Accordingly, we observed robust CM hypertrophy in CM monocultures only with 100 nM ET1. In cocultures, by contrast, 10 nM ET1 was sufficient to induce robust CM hypertrophy, suggesting that the ETA on SNs is more sensitive than on CMs. The reason might lie in the relative expression pattern. Fig. S5D implies that the ETA is expressed to a larger extent in SNs as compared to CMs. Likewise, we reported previously that ET1 concentrations around 100 pM affect NE reuptake in isolated perfused hearts (28). Thus, the possibility exists that physiological ET1 levels that were reported to lie in the picomolar range predominantly act via SN-ETA rather than CM-ETA. These findings are not surprising from a developmental point of view: SNs are derived from neural crest cells and loss-of-function studies have unmasked that the deletion of ETA in neural crest results in severe cardiac phenotypes (35), whereas the CM deletion of ETA did not result in an obvious phenotype in classical pathological models (13).
Divergent and Convergent Signaling of ET1.
ETA and αAR signal via PKD toward HDAC5 to derepress MEF2 target genes that drive adverse cardiac remodeling (20, 36). We now show that ET1 signals via SN-ETA indirectly to CM-αAR and then converges with CM-ETA on HDAC5 signaling. However, our results also revealed that SN-ETA transduces its signals via βARs to HDAC4, indicating the involvement of other kinases, because PKD is not a downstream kinase of βARs (37, 38). These data are consistent with our previous report that CaMKII selectively signals to HDAC4 (21, 39). In contrast to PKD, CaMKII is well known to be activated by βARs (40, 41). How βAR signaling acts on HDAC5 is under debate (42–44). In our hands, βAR activation leads to more 14-3-3 binding (as a measure of cytosolic accumulation of HDACs) of HDAC4 but to less 14-3-3 binding to HDAC5, suggesting that βARs lead to (i) cytosolic accumulation of HDAC4 possibly by CaMKII and (ii) nuclear accumulation of HDAC5. The latter might be explained by a previously reported inhibitory effect of protein kinase A (a major downstream mediator of βAR signaling) on PKD (44, 45). Based on our data, we propose a model (Fig. 5F), by which CM-ETA signals specifically through PKD to HDAC5, whereas SN-ETA signals indirectly via NE through αARs to HDAC5 but through βARs to HDAC4. Why does ET1 signaling diverge to adrenergic receptors that in turn converge on distinct class II HDAC family members? Both, HDAC4 and HDAC5 have been demonstrated to inhibit MEF2 (46). Thus, our findings provide a mechanistic basis by which SN-ETA amplifies de-repression of MEF2 by removing more than one HDAC family member from the nucleus.
Potential Clinical Implications.
An improved NE reuptake by ETAi leads to restoration of NE stores, which was reported to be associated with an increased exercise tolerance in patients (24). Thus, we hypothesize that exercise tolerance may be an additional clinical readout for future ETAi studies. Based on the findings of the present study, we hypothesized that individual patients not tolerating β blockers might benefit from ETAi. Likewise, studies in which only up to 56% of the patients received β blockers showed a significant benefit from ETAi or unspecific endothelin receptor inhibitors (Table S1). In studies in which more patients received antiadrenergic therapies, no significant benefit was observed, suggesting that β blockers indeed blunt the potential therapeutic effects of ETAi. We also aimed at retrieving supporting information from the largest ETAi trial on HF, namely the endothelin-a receptor antagonist trial in heart failure trial (11). However, the size of the subgroups (in particular patients without antiadrenergic therapy) did not allow a statistically reliable analysis. Thus, an ETAi trial with this small HF patient cohort might be an interesting approach to test the hypothesis that patients without antiadrenergic therapy might benefit from ETAi. Moreover, imaging techniques such as [123I]-MIBG uptake (17, 18, 26) that allow an assessment of the sympathetic nerve function in patients may serve as a tool for the selection of patients that benefit in particular from ETAi.
In conclusion, we unmasked, by the use of cell type-specific in vivo and ex vivo gene deletion tools, a critical role for sympathetic ETA in the development and progression of HF. ET1 amplifies its effect on CMs indirectly by the stimulation of adrenergic neurotransmission and the activation of αARs and βARs and subsequently distinct HDAC- and MEF2-dependent signaling pathways. Other than hypothesized before, ETA on CMs does not seem to critically contribute to the development of HF.
Materials and Methods
Experimental Animals.
All experiments were performed in accordance with relevant guidelines and regulations. This investigation followed the Principle of Laboratory Animal Care (NIH Publication No. 86-23, revised 1985) and was approved by the authorities of the Regierungspräsidium Karlsruhe. For detailed information, see SI Materials and Methods.
[124I]MIBG-PET.
The precursor resin for the production of ultratrace [124I]MIBG was a kind gift from James F. Kronauge (Molecular Insight Pharmaceuticals, Cambridge, MA). To prevent eventual saturation of the norepinephrine transporter by MIBG in the animal model, MIBG of high specific activity was produced. For this purpose, the solid labeling approach was applied (47). For detailed information, see SI Materials and Methods.
Isolated Heart Perfusion.
Hearts were perfused according to the Langendorff method as previously published (27). For detailed information, see SI Materials and Methods.
Determination of Neurohormones.
Measurement of neurohormones followed the protocol as previously described (27). For detailed information, see SI Materials and Methods.
Transthoracic Echocardiography.
Echocardiographic evaluation was performed using a VisualSonicsVevo 2100 echocardiograph. For detailed information, see SI Materials and Methods.
Isolation of Primary Cells.
SNs were prepared and cultured from newborn Sprague–Dawley rats or ETAfl/fl mice as described previously (48). Briefly, freshly isolated superior cervical ganglia from 1- to 3-d-old pups were dissected, incubated for 45 min in 1.5 mg/mL collagenase, titrated, and then preplated on cultural plastic to permit the attachment of nonneuronal cells. After 1 h, SNs were plated with CM medium (SI Materials and Methods) and cocultured for 3–5 d with an additional 10 ng/mL NGF (rat recombinant). After 24 h in coculture, 1 µM cytosine arabinofuranoside was added to stop cell division of potential contaminating fibroblasts. The isolation of CMs is described in detail in SI Materials and Methods.
Plasmids and Reagents.
For detailed information, see SI Materials and Methods.
Indirect Immunofluorescence, Western Blot Analysis and Coimmunoprecipitation, Histology, RNA Analysis, Reporter Assays, [3H]-Leucine Incorporation, and Adenovirus Production.
For detailed information, see SI Materials and Methods.
Statistics.
The results are expressed as the mean ± SEM. Statistical analysis was performed using SPSS 12.0 software (SPSS). Differences between groups were tested by one-way ANOVA with post hoc comparisons by Bonferroni’s multiple comparison test or unpaired Student t test where appropriate. Kaplan–Meier survival analysis was performed using the log-rank test. In the immunofluorescence experiments, the nucleocytoplasmatic distribution of HDAC4 and -5 was analyzed in >40 cells per experiment, whereas a ratio was built between fluorescence in nucleus vs. cytosol using ImageJ software. In all tests, P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Michaela Oestringer, Claudia Heft, and Ulrike Oehl for expert technical assistance and David Stanmore for editing the manuscript. Darusentan was a gift from Klaus Muenter. The precursor resin for the production of ultratrace MIBG was a gift from James F. Kronauge. Adenovirus for Cre recombinase was a gift from Oliver Müller. Floxed ETA mice were forwarded from Rohini Kuner. J.B. was supported by Deutsche Forschungsgemeinschaft Grants BA 2258/2-1 and SFB 1118, European Commission Grants FP7-Health-2010 and MEDIA-261409, the Deutsches Zentrum für Herz-Kreislauf-Forschung [DZHK (German Centre for Cardiovascular Research)], and the German Ministry of Education and Research. L.H.L. was supported by the “Karin-Nolte-Stiftung” and is recipient of the Heidelberg Research Center for Molecular Medicine Career Development fellowship. J.S.R. was supported by the Otto Hess Scholarship of the German Cardiac Society. M.Y. was supported by the Japan Society for the Promotion of Science through the Funding Program for World-Leading Innovative R&D on Science and Technology, initiated by the Council for Science and Technology Policy.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1409026111/-/DCSupplemental.
References
- 1.Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Executive summary: Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation. 2012;125(1):188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.Benedict CR, et al. SOLVD Investigators Relation of neurohumoral activation to clinical variables and degree of ventricular dysfunction: A report from the Registry of Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1994;23(6):1410–1420. doi: 10.1016/0735-1097(94)90385-9. [DOI] [PubMed] [Google Scholar]
- 3.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415(6868):206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 4.Yanagisawa M, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 5.Barton M, Yanagisawa M. Endothelin: 20 years from discovery to therapy. Can J Physiol Pharmacol. 2008;86(8):485–498. doi: 10.1139/Y08-059. [DOI] [PubMed] [Google Scholar]
- 6.McMurray JJ, Ray SG, Abdullah I, Dargie HJ, Morton JJ. Plasma endothelin in chronic heart failure. Circulation. 1992;85(4):1374–1379. doi: 10.1161/01.cir.85.4.1374. [DOI] [PubMed] [Google Scholar]
- 7.Sakai S, et al. Inhibition of myocardial endothelin pathway improves long-term survival in heart failure. Nature. 1996;384(6607):353–355. doi: 10.1038/384353a0. [DOI] [PubMed] [Google Scholar]
- 8.Chen XC, et al. Effects of endothelin receptor A antagonist FR139317 on rats with congestive heart failure. Acta Pharmacol Sin. 2001;22(10):896–900. [PubMed] [Google Scholar]
- 9.Mulder P, et al. Long-term survival and hemodynamics after endothelin-a receptor antagonism and angiotensin-converting enzyme inhibition in rats with chronic heart failure: Monotherapy versus combination therapy. Circulation. 2002;106(9):1159–1164. doi: 10.1161/01.cir.0000027138.07524.38. [DOI] [PubMed] [Google Scholar]
- 10.Yamauchi-Kohno R, et al. Role of endothelin in deterioration of heart failure due to cardiomyopathy in hamsters: Increase in endothelin-1 production in the heart and beneficial effect of endothelin-A receptor antagonist on survival and cardiac function. Circulation. 1999;99(16):2171–2176. doi: 10.1161/01.cir.99.16.2171. [DOI] [PubMed] [Google Scholar]
- 11.Anand I, et al. EARTH investigators Long-term effects of darusentan on left-ventricular remodelling and clinical outcomes in the EndothelinA Receptor Antagonist Trial in Heart Failure (EARTH): Randomised, double-blind, placebo-controlled trial. Lancet. 2004;364(9431):347–354. doi: 10.1016/S0140-6736(04)16723-8. [DOI] [PubMed] [Google Scholar]
- 12.Coletta A, Thackray S, Nikitin N, Cleland JG. Clinical trials update: Highlights of the scientific sessions of The American College of Cardiology 2002: LIFE, DANAMI 2, MADIT-2, MIRACLE-ICD, OVERTURE, OCTAVE, ENABLE 1 & 2, CHRISTMAS, AFFIRM, RACE, WIZARD, AZACS, REMATCH, BNP trial and HARDBALL. Eur J Heart Fail. 2002;4(3):381–388. doi: 10.1016/s1388-9842(02)00040-5. [DOI] [PubMed] [Google Scholar]
- 13.Kedzierski RM, et al. Cardiomyocyte-specific endothelin A receptor knockout mice have normal cardiac function and an unaltered hypertrophic response to angiotensin II and isoproterenol. Mol Cell Biol. 2003;23(22):8226–8232. doi: 10.1128/MCB.23.22.8226-8232.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isaka M, Kudo A, Imamura M, Kawakami H, Yasuda K. Endothelin receptors, localized in sympathetic nerve terminals of the heart, modulate norepinephrine release and reperfusion arrhythmias. Basic Res Cardiol. 2007;102(2):154–162. doi: 10.1007/s00395-006-0623-2. [DOI] [PubMed] [Google Scholar]
- 15.Lehmann LH, Stanmore DA, Backs J. The role of endothelin-1 in the sympathetic nervous system in the heart [published online ahead of print March 13, 2014] Life Sci. 2014 doi: 10.1016/j.lfs.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Anonymous Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353(9169):2001–2007. [PubMed] [Google Scholar]
- 17.Nakata T, et al. Cardiac death prediction and impaired cardiac sympathetic innervation assessed by MIBG in patients with failing and nonfailing hearts. J Nucl Cardiol. 1998;5(6):579–590. doi: 10.1016/s1071-3581(98)90112-x. [DOI] [PubMed] [Google Scholar]
- 18.Ogita H, et al. Prognostic significance of cardiac (123)I metaiodobenzylguanidine imaging for mortality and morbidity in patients with chronic heart failure: A prospective study. Heart. 2001;86(6):656–660. doi: 10.1136/heart.86.6.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreusser MM, et al. Injection of nerve growth factor into stellate ganglia improves norepinephrine reuptake into failing hearts. Hypertension. 2006;47(2):209–215. doi: 10.1161/01.HYP.0000200157.25792.26. [DOI] [PubMed] [Google Scholar]
- 20.Vega RB, et al. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol. 2004;24(19):8374–8385. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest. 2006;116(7):1853–1864. doi: 10.1172/JCI27438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim Y, et al. The MEF2D transcription factor mediates stress-dependent cardiac remodeling in mice. J Clin Invest. 2008;118(1):124–132. doi: 10.1172/JCI33255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parlato R, Otto C, Begus Y, Stotz S, Schütz G. Specific ablation of the transcription factor CREB in sympathetic neurons surprisingly protects against developmentally regulated apoptosis. Development. 2007;134(9):1663–1670. doi: 10.1242/dev.02838. [DOI] [PubMed] [Google Scholar]
- 24.Rundqvist B, Eisenhofer G, Elam M, Friberg P. Attenuated cardiac sympathetic responsiveness during dynamic exercise in patients with heart failure. Circulation. 1997;95(4):940–945. doi: 10.1161/01.cir.95.4.940. [DOI] [PubMed] [Google Scholar]
- 25.Backs J, et al. The neuronal norepinephrine transporter in experimental heart failure: Evidence for a posttranscriptional downregulation. J Mol Cell Cardiol. 2001;33(3):461–472. doi: 10.1006/jmcc.2000.1319. [DOI] [PubMed] [Google Scholar]
- 26.Carrió I, Cowie MR, Yamazaki J, Udelson J, Camici PG. Cardiac sympathetic imaging with mIBG in heart failure. JACC Cardiovasc Imaging. 2010;3(1):92–100. doi: 10.1016/j.jcmg.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Buss SJ, et al. Spironolactone preserves cardiac norepinephrine reuptake in salt-sensitive Dahl rats. Endocrinology. 2006;147(5):2526–2534. doi: 10.1210/en.2005-1167. [DOI] [PubMed] [Google Scholar]
- 28.Backs J, Bresch E, Lutz M, Kristen AV, Haass M. Endothelin-1 inhibits the neuronal norepinephrine transporter in hearts of male rats. Cardiovasc Res. 2005;67(2):283–290. doi: 10.1016/j.cardiores.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Nadal-Ginard B, Mahdavi V. Molecular basis of cardiac performance. Plasticity of the myocardium generated through protein isoform switches. J Clin Invest. 1989;84(6):1693–1700. doi: 10.1172/JCI114351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosoda K, et al. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994;79(7):1267–1276. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 31.Lüscher TF, et al. Heart Failure ET(A) Receptor Blockade Trial Hemodynamic and neurohumoral effects of selective endothelin A (ET(A)) receptor blockade in chronic heart failure: The Heart Failure ET(A) Receptor Blockade Trial (HEAT) Circulation. 2002;106(21):2666–2672. doi: 10.1161/01.cir.0000038497.80095.e1. [DOI] [PubMed] [Google Scholar]
- 32.Rundqvist B, Elam M, Bergmann-Sverrisdottir Y, Eisenhofer G, Friberg P. Increased cardiac adrenergic drive precedes generalized sympathetic activation in human heart failure. Circulation. 1997;95(1):169–175. doi: 10.1161/01.cir.95.1.169. [DOI] [PubMed] [Google Scholar]
- 33.Vega RB, Bassel-Duby R, Olson EN. Control of cardiac growth and function by calcineurin signaling. J Biol Chem. 2003;278(39):36981–36984. doi: 10.1074/jbc.R300023200. [DOI] [PubMed] [Google Scholar]
- 34.Armour JA. Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp Physiol. 2008;93(2):165–176. doi: 10.1113/expphysiol.2007.041178. [DOI] [PubMed] [Google Scholar]
- 35.Clouthier DE, et al. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development. 1998;125(5):813–824. doi: 10.1242/dev.125.5.813. [DOI] [PubMed] [Google Scholar]
- 36.Fielitz J, et al. Requirement of protein kinase D1 for pathological cardiac remodeling. Proc Natl Acad Sci USA. 2008;105(8):3059–3063. doi: 10.1073/pnas.0712265105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avkiran M, Rowland AJ, Cuello F, Haworth RS. Protein kinase d in the cardiovascular system: Emerging roles in health and disease. Circ Res. 2008;102(2):157–163. doi: 10.1161/CIRCRESAHA.107.168211. [DOI] [PubMed] [Google Scholar]
- 38.Cuello F, et al. Protein kinase D selectively targets cardiac troponin I and regulates myofilament Ca2+ sensitivity in ventricular myocytes. Circ Res. 2007;100(6):864–873. doi: 10.1161/01.RES.0000260809.15393.fa. [DOI] [PubMed] [Google Scholar]
- 39.Backs J, et al. The delta isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci USA. 2009;106(7):2342–2347. doi: 10.1073/pnas.0813013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bers DM. CaMKII inhibition in heart failure makes jump to human. Circ Res. 2010;107(9):1044–1046. doi: 10.1161/CIRCRESAHA.110.231902. [DOI] [PubMed] [Google Scholar]
- 41.Grimm M, Brown JH. Beta-adrenergic receptor signaling in the heart: Role of CaMKII. J Mol Cell Cardiol. 2010;48(2):322–330. doi: 10.1016/j.yjmcc.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang CW, et al. Acute β-adrenergic activation triggers nuclear import of histone deacetylase 5 and delays G(q)-induced transcriptional activation. J Biol Chem. 2013;288(1):192–204. doi: 10.1074/jbc.M112.382358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haworth RS, Stathopoulou K, Candasamy AJ, Avkiran M. Neurohormonal regulation of cardiac histone deacetylase 5 nuclear localization by phosphorylation-dependent and phosphorylation-independent mechanisms. Circ Res. 2012;110(12):1585–1595. doi: 10.1161/CIRCRESAHA.111.263665. [DOI] [PubMed] [Google Scholar]
- 44.Sucharov CC, Dockstader K, Nunley K, McKinsey TA, Bristow M. β-Adrenergic receptor stimulation and activation of protein kinase A protect against α1-adrenergic-mediated phosphorylation of protein kinase D and histone deacetylase 5. J Card Fail. 2011;17(7):592–600. doi: 10.1016/j.cardfail.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haworth RS, Roberts NA, Cuello F, Avkiran M. Regulation of protein kinase D activity in adult myocardium: Novel counter-regulatory roles for protein kinase Cepsilon and protein kinase A. J Mol Cell Cardiol. 2007;43(6):686–695. doi: 10.1016/j.yjmcc.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 46.Lu J, McKinsey TA, Nicol RL, Olson EN. Signal-dependent activation of the MEF2 transcription factor by dissociation from histone deacetylases. Proc Natl Acad Sci USA. 2000;97(8):4070–4075. doi: 10.1073/pnas.080064097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hunter DH, Zhu X. Polymer-supported radiopharmaceuticals: [131I]MIBG and [123I]MIBG. J Labelled Comp Radiopharm. 1999;42(7):653–661. [Google Scholar]
- 48.Lockhart ST, Turrigiano GG, Birren SJ. Nerve growth factor modulates synaptic transmission between sympathetic neurons and cardiac myocytes. J Neurosci. 1997;17(24):9573–9582. doi: 10.1523/JNEUROSCI.17-24-09573.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.