Significance
Antiinfectives, drugs that inhibit virulence strategies of microbial pathogens without affecting bacterial growth, may prevent hospital-acquired infections caused by antibiotic-resistant Staphylococcus aureus. We used virtual screening and synthetic optimization to identify 3,6-disubstituted triazolothiadiazole compounds as inhibitors of sortase, an enzyme that incorporates surface proteins into the staphylococcal envelope. Other Gram-positive bacteria also use sortase for protein assembly in the envelope and disease pathogenesis, suggesting that sortase inhibitors could protect high-risk patients against infection with many nosocomial pathogens.
Keywords: nosocomial infection, LPXTG motif, antivirulence, computational screening
Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) is the most frequent cause of hospital-acquired infection, which manifests as surgical site infections, bacteremia, and sepsis. Due to drug-resistance, prophylaxis of MRSA infection with antibiotics frequently fails or incites nosocomial diseases such as Clostridium difficile infection. Sortase A is a transpeptidase that anchors surface proteins in the envelope of S. aureus, and sortase mutants are unable to cause bacteremia or sepsis in mice. Here we used virtual screening and optimization of inhibitor structure to identify 3-(4-pyridinyl)-6-(2-sodiumsulfonatephenyl)[1,2,4]triazolo[3,4-b][1,3,4]thiadiazole and related compounds, which block sortase activity in vitro and in vivo. Sortase inhibitors do not affect in vitro staphylococcal growth yet protect mice against lethal S. aureus bacteremia. Thus, sortase inhibitors may be useful as antiinfective therapy to prevent hospital-acquired S. aureus infection in high-risk patients without the side effects of antibiotics.
The gram-positive bacterium Staphylococcus aureus colonizes the human skin and nares yet also causes invasive diseases such as skin and soft tissue infections, osteomyelitis, pneumonia, bacteremia, sepsis, and endocarditis (1). Methicillin-resistant S. aureus (MRSA) acquired resistance against many different drugs, including β-lactam, cephalosporin, fluoroquinolone, aminoglycoside, tetracycline, macrolide, trimethoprim-sulfamethoxazole, and vancomycin antibiotics (2). In the United States, MRSA isolates are responsible for >50% of S. aureus infections in hospitals and long-term care facilities (3). Individuals at high risk of MRSA infection include very-low-birth-weight neonates, elderly, and patients with indwelling catheters, endotracheal intubation, medical implantation of foreign bodies (prosthetic joints, implants and heart valves), trauma, surgical procedures, diabetes, dialysis, and immunosuppressive or cancer therapy (4). Antibiotic prophylaxis is designed to mitigate the risk of S. aureus infection, especially in surgical patients; however, this frequently fails due to drug resistance (5). Importantly, antibiotic therapy suppresses human microbiota and promotes Clostridium difficile infection, which is also associated with increased morbidity and mortality (6, 7). Several trials for vaccines and immune therapeutics were designed to prevent MRSA infection in hospital settings; these efforts have thus far failed to meet their study end points (4).
Surface proteins of S. aureus are secreted as precursors with C-terminal sorting signals that are cleaved by sortase A (SrtA) between the threonine (T) and the glycine (G) residues of their LPXTG motif (8, 9). The active site cysteine residue of sortase forms an acyl enzyme intermediate that is relieved by the nucleophilic attack of the amino group (pentaglycine crossbridge) in peptidoglycan synthesis precursors (10). Surface proteins attached to peptidoglycan precursors are subsequently incorporated into the cell wall envelope and displayed on the staphylococcal surface (9). Genome sequencing revealed that all S. aureus isolates encode 17–21 surface proteins with LPXTG sorting signals, which fulfill diverse functions during the infectious process (11). SrtA mutants cannot assemble surface proteins into their envelope and are unable to form abscess lesions in organ tissues or cause lethal bacteremia when inoculated into the bloodstream of mice (12, 13). In contrast, mutations that abrogate the expression of secreted virulence factors may cause attenuation but do not abrogate the ability of S. aureus to cause infectious diseases (12).
We reasoned that small molecule inhibitors blocking SrtA may be useful as antiinfectives to prevent S. aureus infection without affecting the growth of other bacteria. If so, such compounds could be used to reduce the incidence of MRSA infections without the side effects of antibiotics.
Results
Identifying Sortase Inhibitors.
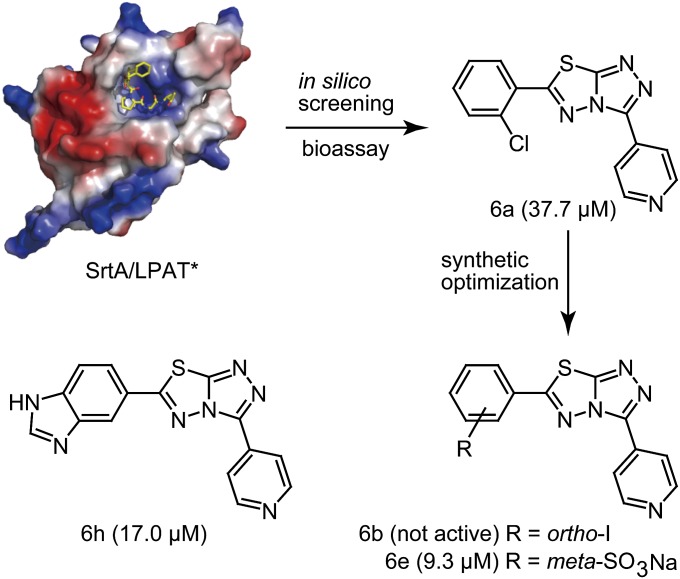
We used the structural coordinates from the SrtA substrate complex [SrtA/LPAT*; Protein Data Bank (PDB) ID code 2KID] to model the enzyme active site as a target for computational screening (14). The scaffold of topsentin A, a natural product that inhibits sortase A in vitro (15), was used as a model ligand. Scaffold hopping and molecular docking were combined for the virtual screening of the drug-like Specs database (www.specs.net), which contains about 300,000 compounds, for compounds that bind the active site (Fig. 1). After virtual screening, 105 compounds were selected for experimental validation using purified recombinant sortase (SrtAΔN24) (10). The Km of sortase-catalyzed hydrolysis of an internally quenched fluorescent peptide substrate (abz-LPATG-dnp) was 17.5 μM, and percent inhibition of sortase activity was measured at 100 μM compound concentration (Fig. S1 A and B). Compounds with ≥50% inhibition were analyzed with an orthogonal HPLC assay to quantify SrtAΔN24 cleavage of abz-LPATG-dnp substrate, and IC50 values were calculated. The hit compound 6a exhibited an IC50 of 37.7 μM for S. aureus sortase (Fig. 1 and Table S1). To improve the inhibitory activity, we performed synthetic optimization of the chemical structure of compound 6a (Scheme S1) (16). This synthesis afforded compound 6e [3-(4-pyridinyl)-6-(2-sodiumsulfonatephenyl)[1,2,4]triazolo[3,4-b][1,3,4]thiadiazole] with an IC50 of 9.3 μM (Fig. 2A), which represents a fourfold improvement over the screening hit, compound 6a (Fig. 1 and Table S1).
Fig. 1.
Screening and optimization of sortase inhibitors. Structure-based in silico screening of small molecule library for compounds that bind the active site of S. aureus SrtA identifies hit compound 6a (IC50 value in parentheses). Synthetic optimization of the 3,6-disubstituted triazolothiadiazole scaffold generated 14 different compounds including 6b, 6h, and 6e.
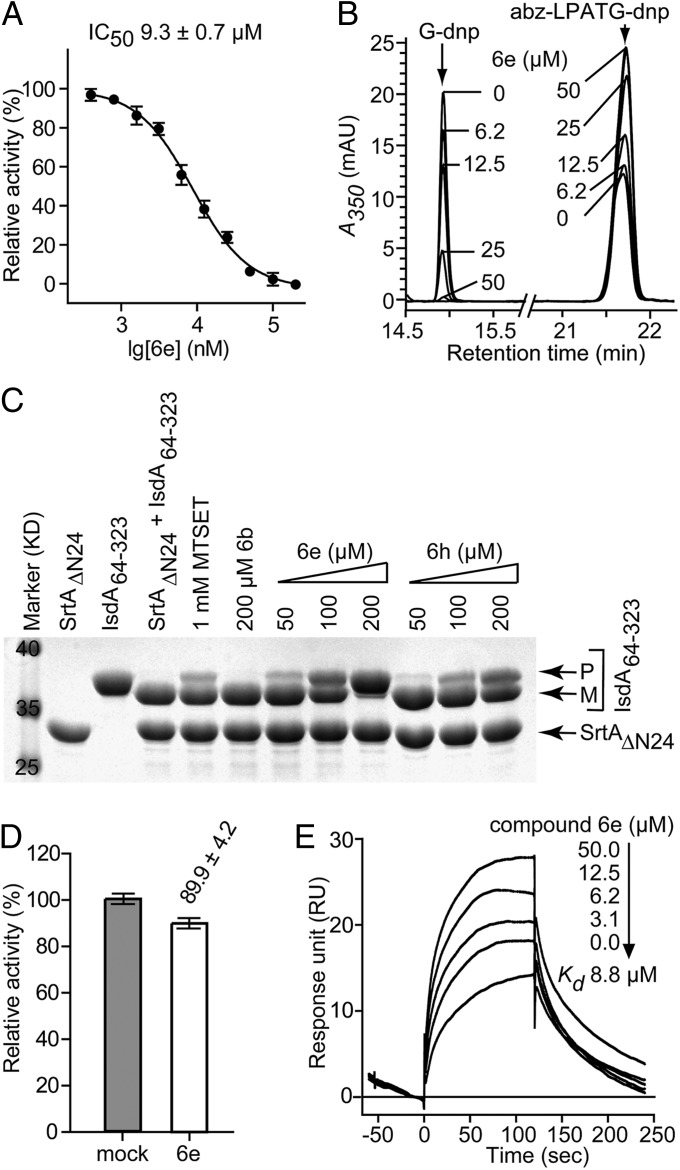
Fig. 2.
Inhibition of sortase function in vitro. (A) Purified recombinant S. aureus sortase (SrtAΔN24) was incubated with fluorogenic substrate abz-LPATG-dnp and relative activity, i.e., substrate cleavage, measured in the presence of variable concentrations of compound 6e. Each reaction condition was assayed in triplicate, and average values and SEMs were determined. (B) SrtAΔN24 catalyzed transpeptidation with abz-LPATG-dnp and Gly3 generates abz-LPAT-Gly3 and G-dnp was perturbed with increasing concentrations of compound 6e and relative inhibitory rates calculated. Representative HPLC trace shows the substrate and the dnp-containing product. (C) SDS/PAGE analysis of transpeptidation reactions; 10 μg SrtAΔN24, 10 μg IsdA64–323, and 3 mM Gly3 were incubated for 2 h at 37 °C with variable concentrations of compound 6b, 6h, or 6e. Migratory positions of IsdA64–323 substrate precursor (P) and mature transpeptidation product (M) are indicated. (D) SrtAΔN24 was incubated with buffer alone or with compound 6e at 10 × IC50 concentration and diluted, and sortase activity was measured as abz-LPATG-dnp cleavage. Control (mock) sample was assigned 100% activity. Eighty-nine percent (±4.2) activity were recovered from SrtAΔN24 treated with 6e inhibitor. (E) Compound 6e binding to SrtAΔN24 was analyzed with surface plasmon resonance, and the dissociation constant (Kd = 8.8 µM) was calculated.
Inhibition of Sortase-Catalyzed Transpeptidation.
Sortase-mediated anchoring of surface proteins involves a transpeptidation reaction (17) but is not associated with the release of cleaved surface proteins into the extracellular medium (18). We therefore asked whether the inhibitors identified above also block sortase-catalyzed transpeptidation. SrtAΔN24 cleavage of the abz-LPATG-dnp peptide and amide bond formation with the NH2-Gly3 nucleophile generates products abz-LPAT-Gly3 and G-dnp, which can be quantified by HPLC and MS (Fig. S2) (19). Compound 6e is active in a dose-dependent manner with 10.8–93.6% inhibition at 6.25–50 μM, respectively (Fig. 2B). The calculated IC50 (17.7 μM) is in agreement with the IC50 (9.3 μM) derived from the fluorescence-based assay (Fig. S1C). Surface proteins IsdA and SasX are expressed by Chinese MRSA isolates (20). Incubation of affinity-purified IsdA64–323 (P) or SasX30–178 precursor (P) with purified SrtAΔN24 and NH2-Gly3 nucleophile resulted in sorting signal cleavage to yield the transpeptidation product (M), which could be blocked with the noncompetitive inhibitor N,N,N-trimethyl-2-(methylsulfonylthio)ethanaminium chloride (MTSET) (Fig. 2C and Fig. S3) (21). Sortase cleavage of sorting signals was blocked in a dose-dependent manner by compounds 6h and 6e, but not by compound 6b (Fig. 2C and Fig. S3).
Reversible Inhibition of Sortase with 3,6-Disubstituted Triazolothiadiazole.
Because of its high inhibitory activity and aqueous solubility, compound 6e was analyzed for its mechanism of sortase inhibition. Following dilution of SrtAΔN24/inhibitor at 10-fold IC50, 89.9 ± 4.2% of sortase activity was recovered compared with mock-treated sortase (Fig. 2D). This result suggested that compound 6e functions as a reversible inhibitor that does not covalently modify the active site cysteine of sortase (22). Inhibitor binding to sortase was tested with surface plasmon resonance (SPR) experiments, which revealed the direct binding of compound 6e to SrtAΔN24 with a Kd of 8.8 μM (Fig. 2E). Circular dichroism (CD) spectroscopy was used to monitor changes in sortase structure on binding inhibitor. As expected, SrtAΔN24 exhibited a negative band near 200 nm, indicative of its mixed β-sheet secondary structure (23, 24) (Fig. S4). Addition of 200 μM compound 6e to purified SrtAΔN24 (100-fold excess of inhibitor vs. enzyme) caused minor changes in secondary structure content (Fig. S4), indicating that the inhibitor does not promote protein aggregation. Together these data suggest that compound 6e binds reversibly to the active site of sortase and likely interferes with the enzyme’s ability to recognize and cleave its substrates.
Inhibition of Sortase Activity in Staphylococci.
During bacterial growth, sortase catalyzes the assembly of surface proteins into the cell wall at the septal and polar compartments of S. aureus (25). Sortase-mediated cleavage of surface protein sorting signals can be measured with pulse-chase experiments, which permit an analysis of precursor processing using [35S]Met/Cys radiolabeling of staphylococcal proteins and immunoprecipitation (26). Immediately following ribosomal synthesis, the P1 precursor species of protein A (SpA) are secreted and their N-terminal signal peptides are removed to generate P2 intermediates. Sortase cleaves the P2 precursor at its LPXTG motif and anchors mature SpA (M) in the cell wall envelope (Fig. 3A) (26). As expected, treatment of staphylococci with 2 mM MTSET abolishes sortase-mediated cleavage of P2 precursors (21). Treatment of staphylococci with compound 6e blocked P2 precursor cleavage by sortase A (Fig. 3B). By varying the concentration of the inhibitor, we calculated the IC50 68.7 µM of compound 6e for the in vivo inhibition of SrtA.
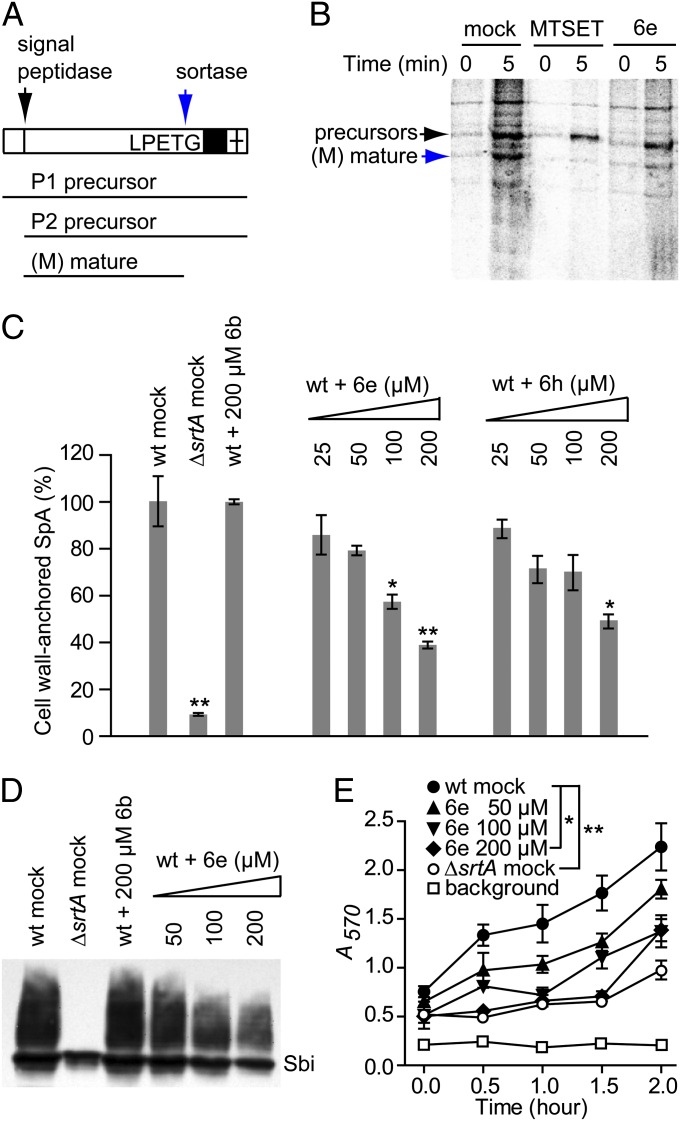
Fig. 3.
In vivo inhibition of staphylococcal sortase. (A) Diagram illustrating SpA precursors P1 and P2 and the sortase-catalyzed mature anchored product (M). (B) Pulse-chase experiment with [35S]Met/Cys (0 and 5 min) reveals the migratory positions of the P1/2 precursors and mature (M) species of S. aureus on SDS/PAGE. Treatment of S. aureus with the noncompetitive inhibitor MTSET and with compound 6e causes accumulation of SpA precursors, revealing the inhibition of SrtA activity. (C) The abundance of Ig binding to SpA in the bacterial cell wall envelope was quantified with FITC-labeled human IgG and washed S. aureus cells from cultures grown in the absence of inhibitor (control) or in the presence of variable concentrations of compounds 6b, 6h, and 6e; values for the srtA deletion mutant are included as a control. Statistical significance (*P < 0.05, **P < 0.01) was determined using the unpaired, two-tailed Student t test (n = 3, brackets identify the mean and the SEMs). (D) The abundance of SpA in the bacterial cell wall envelope was quantified by SDS/PAGE immunoblotting with SpA-reactive antibodies using S. aureus cultures grown in the absence of inhibitor (S. aureus Newman) or in the presence of variable concentrations of compounds 6b and 6e; the srtA deletion mutant was included as control. The migratory position of cross-reactive Sbi is identified. (E) Binding of S. aureus Newman cells grown for indicated amounts of time (hours) in the presence of variable concentrations of compound 6e or mock control to fibrinogen-coated microtiter plates was quantified with crystal violet staining and absorbance measurements (A570). As control, the srtA deletion mutant cannot anchor fibrinogen binding surface proteins (ClfA and ClfB) in the bacterial cell wall. Microtiter plate staining without staphylococci was used to determine background signal. Statistical significance (*P < 0.05, **P < 0.01) was determined using the unpaired, two-tailed Student t test (n = 3, brackets identify the mean and the SEMs).
In S. aureus Newman, SrtA anchors 19 different surface proteins in the bacterial envelope (27), including SpA, a molecule that binds the Fcγ and Fab domains of host immunoglobulins (28), as well as clumping factor A and B, which bind to the γ- and α-chains of host fibrinogen, respectively (29, 30). Treatment of S. aureus cultures with the sortase inhibitor 6e reduced the incorporation of SpA into the bacterial envelope (Fig. 3 C and D). Similarly, treatment with compound 6e reduced staphylococcal association with fibrinogen (Fig. 3E), a key mechanism for the pathogenesis of bloodstream infections (13). We noticed a slight reduction in the abundance of Sbi (staphylococcal binder of Ig), a secreted protein that is not cleaved by SrtA, both in the ΔsrtA mutant and in staphylococci that had been treated with compound 6e (Fig. 3D); the molecular basis for this phenotype is not known.
Antiinfective Therapy with 3,6-Disubstituted Triazolothiadiazole.
Deletion of the SrtA gene does not affect the in vitro growth of S. aureus Newman (9), a human clinical isolate (31), or of the laboratory strain S. aureus RN4220 (32). Unlike MTSET, which reacts with all available thiolate moieties and rapidly kills staphylococci (21), the addition of compound 6e concentrations inhibitory for SrtA (up to 200 μM) to staphylococcal cultures did not affect the growth of S. aureus Newman (Fig. 4A). Using the microtiter broth dilution method, we measured the minimal inhibitory concentration of compound 6e to be >15 mg/mL (>40 mM). These results indicate that compound 6e selectively inhibits sortase activity and does not function as an antibiotic for S. aureus.
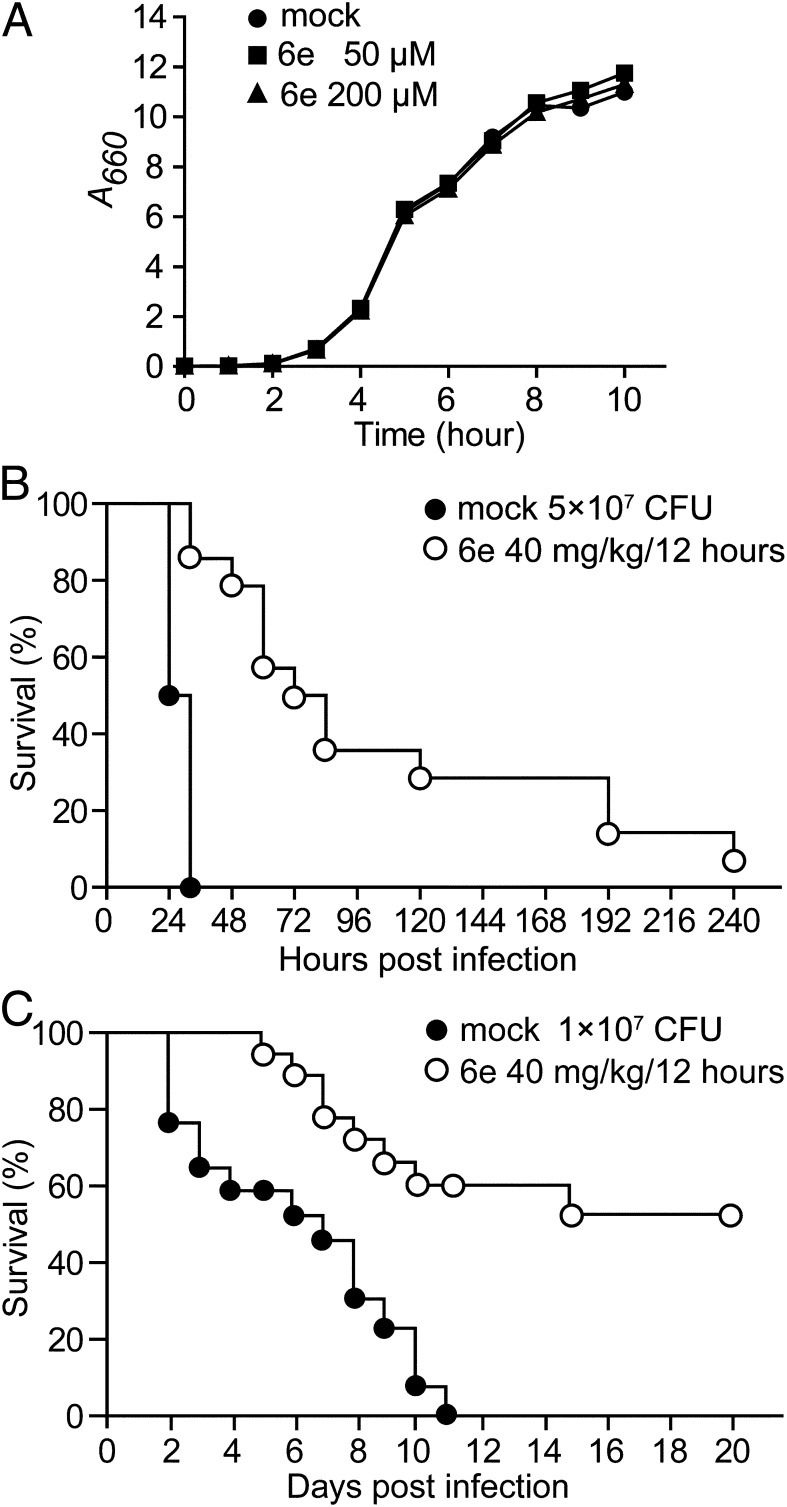
Fig. 4.
Antiinfective therapy with 3,6-disubstituted triazolothiadiazole. (A) The addition of sortase inhibitors does not affect the growth of S. aureus. (B) BALB/c mice (n = 14) received mock or compound 6e (40 mg/kg body weight) treatment via i.p. injection at 12-h intervals for 5 d. Four hours after the first injection, animals were challenged by i.v. injection with 5 × 107 CFU S. aureus Newman, and survival was recorded. (C) BALB/c mice (n = 15) were treated as in B and challenged with 1 × 107 CFU S. aureus Newman. Statistical significance was examined with the log-rank test (mock vs. compound 6e, P < 0.001).
Cohorts of BALB/c mice (n = 14) received i.p. injections with either drug vehicle (mock) or with compound 6e (40 mg/kg body weight) in 12-h intervals for 120 h (5 d). Four hours following the initiation of treatment, mice were infected via i.v. inoculation of 5 × 107 colony-forming units (CFUs) S. aureus Newman, a challenge dose that is lethal for healthy animals (33). Mock-treated mice died of staphylococcal bacteremia within 32 h, whereas animals that had received treatment with compound 6e displayed increased time to death and 10% survival (mock vs. compound 6e, P < 0.001; Fig. 4B). This experiment was repeated (n = 15) with a lower challenge dose (1 × 107 CFUs) of S. aureus Newman. As before, all of the mock-treated animals succumbed to staphylococcal bacteremia, whereas more than half (8/15) of the compound 6e-treated mice survived staphylococcal bacteremia (mock vs. compound 6e, P < 0.001; Fig. 4C). These data suggest that sortase inhibitors may be useful as antiinfective therapy to prevent S. aureus bloodstream infections in hospital settings.
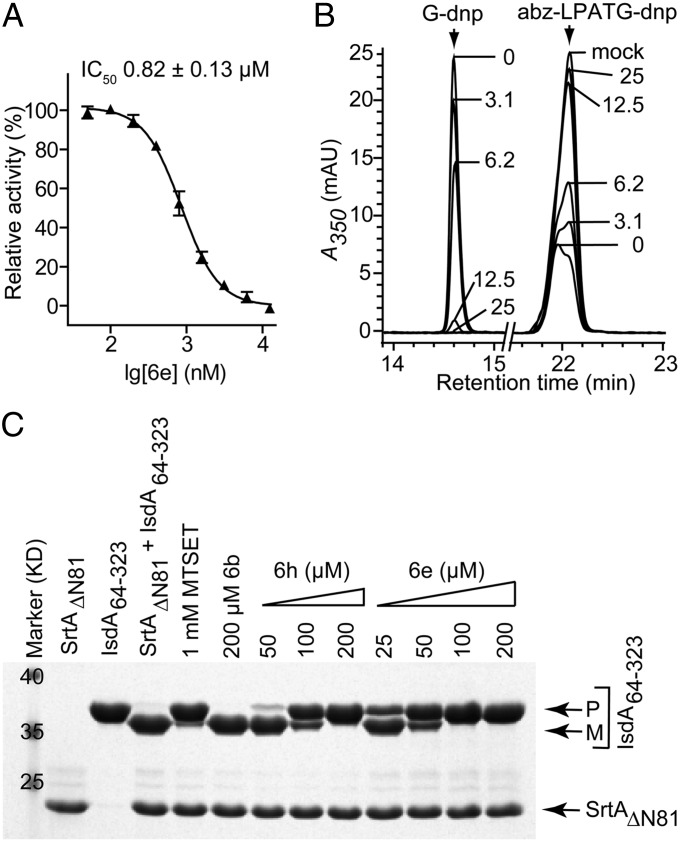
Inhibition of Sortase from Different Gram-Positive Bacteria.
Most Gram-positive bacteria assemble surface proteins in their cell wall envelope via a sortase-catalyzed mechanism (34). The LPXTG motif is conserved in a wide variety of surface proteins (35); however, the chemical structure of the peptidoglycan crossbridge varies between different bacterial species (36). Although structurally similar (37–40), the amino acid identity between sortases from different Gram-positive bacteria is limited to a few key residues at or near the active site (34). We therefore asked whether compound 6e can inhibit sortase from three different microbes: Bacillus anthracis, Streptococcus pneumoniae, and Streptococcus pyogenes. Compounds 6e and 6h both inhibited purified, recombinant sortase from S. pyogenes. Using the fluorescence-based assay, we calculated the compound 6e IC50 of 0.82 μM, revealing a 10-fold higher inhibitory activity for S. pyogenes sortase than for the staphylococcal enzyme (Fig. 5A); this result was confirmed with the orthogonal HPLC assay (Fig. 5B). The structural analogs 6d, 6h, 6j, 6l, and 6n also displayed inhibitory activities for sortases from S. pyogenes, S. pneumoniae, and B. anthracis (Table S2). S. pyogenes sortase-catalyzed cleavage of the IsdA surface protein from S. aureus was inhibited in a dose-dependent manner by compounds 6e and 6h (Fig. 5C). These data suggest that 3,6-disubstituted triazolothiadiazole inhibit sortase enzymes from different Gram-positive bacteria.
Fig. 5.
Inhibition of SrtA from Streptococcus pyogenes. (A) Purified recombinant S. pyogenes sortase (SrtAΔN81) was incubated with fluorogenic substrate abz-LPATG-dnp, and relative activity was measured in the presence of variable concentrations of compound 6e. Each reaction condition was assayed in triplicate, and average values and SEMs were determined. (B) S. pyogenes SrtAΔN81 catalyzed transpeptidation with abz-LPATG-dnp and Gly3 generates abz-LPAT-Gly3 and G-dnp, which was perturbed with increasing concentrations of compound 6e. The relative inhibitory rate was calculated (IC50 = 7.8 μM). (C) SDS/PAGE analysis of transpeptidation reactions with 10 μg S. pyogenes SrtAΔN81, 10 μg S. aureus IsdA64–323, and 3 mM Gly3 were incubated for 2 h at 37 °C with variable concentrations of compound 6b, 6h, or 6e. Migratory positions of IsdA64–323 substrate precursor (P) and mature transpeptidation product (M) are indicated.
Discussion
Hospital-acquired infections with multidrug-resistant bacteria represent a global public health threat, and MRSA is currently the most frequent cause of morbidity and mortality (41). The emergence of multidrug-resistant MRSA isolates acquiring glycopeptide resistance during vancomycin therapy documents the urgent need for controlling the use of antibiotics (42). Recent research efforts have been directed at developing antiinfective therapies against S. aureus, focusing on small molecules that interfere with virulence gene regulation (43). S. aureus agr, a four-gene operon, promotes the constitutive synthesis and secretion of the AgrB-AgrD–derived autoinducing pheromone (AIP) (44), which activates the sensory kinase-response regulator AgrC-AgrA at threshold concentrations to promote staphylococcal expression and secretion of exotoxins (45). AIP-mediated activation of AgrC can be inhibited with peptide analogs and small molecules, which diminishes S. aureus colonization and invasion of skin and soft tissues (43, 46). AIP is inactivated by neutrophil Nox2 NADPH oxidase modification and apolipoprotein B binding (47, 48), which interferes with agr-mediated quorum sensing when S. aureus enters the bloodstream (49, 50). In agreement with this model, AgrC inhibitors do not affect staphylococcal load, abscess formation, or disease outcome when mice are challenged by i.v. inoculation with S. aureus (51).
In contrast to agr-controlled virulence gene expression, sortase-mediated assembly of surface proteins in the bacterial envelope is essential for the pathogenesis of abscess formation and lethal bacteremia following i.v. inoculation of the pathogen (12, 13). Earlier work used in vitro (inhibition of fluorogenic substrate cleavage) and virtual screening of compound libraries to identify sortase inhibitors (15, 52–56). Although these studies identified both competitive and noncompetitive inhibitors (57, 58), isolated compounds have not yet been shown to inhibit in vivo sortase activity in staphylococci, i.e., the cleavage of sorting signals or the assembly of surface proteins into the bacterial cell wall (54, 59–62). Many of the isolated compounds diminish or block staphylococcal growth, indicating that they cannot function as selective inhibitors of S. aureus sortase (53, 61, 63, 64). Thus, the efficacy of sortase inhibitors as antiinfective therapeutics was heretofore not demonstrated (62, 65).
We used virtual screening for compounds that bind the active site of sortase and experimental validation to identify 3,6-disubstituted triazolothiadiazole compounds as a new class of sortase inhibitors. SARs were studied to improve the efficacy of sortase inhibitors, which characterized 3-(4-pyridinyl)-6-(2-sodiumsulfonatephenyl)[1,2,4]triazolo[3,4-b][1,3,4]thiadiazole (compound 6e) as the most potent inhibitor. Of note, compound 6e blocked sortase-catalyzed cleavage and transpeptidation reactions with LPXTG substrate peptides and inhibited the incorporation of surface proteins into the staphylococcal envelope. Although not optimized for its pharmacokinetic and pharmacodynamics attributes, compound 6e displayed efficacy as an antiinfective in preventing the lethal outcome of S. aureus bacteremia in mice. Earlier work synthesized a wide variety of 3,6-disubstituted triazolothiadiazole derivatives and examined these compounds for their antibacterial, antifungal, and analgesic attributes (16). Although certain members of this class of compounds can inhibit bacterial or fungal growth, antibiotic or analgesic effects are not universal attributes of 3,6-disubstituted triazolothiadiazole compounds (16), which may be explored further for clinical development of antiinfectives (16).
Sortases and cell wall-anchored surface proteins contribute to the virulence strategies of many different bacterial pathogens (34). Sortases and surface proteins with LPXTG sorting signals are found in other nosocomial pathogens, for example, Enterococcus faecalis, Enterococcus faecium, and Clostridium difficile (66–68). Earlier work revealed the contribution of sortase toward enterococcal virulence and the pathogenesis of urinary tract infections or endocarditis (67, 69, 70). Thus, antiinfective therapy with sortase inhibitors may be useful to broadly prevent nosocomial infections with antibiotic-resistant Gram-positive bacteria.
Materials and Methods
In Vivo Inhibition of Staphylococcal Sortase A.
S. aureus cultures were pulse-labeled with [35S]Met/Cys for 1 min, and all further incorporation of radioactive amino acids into proteins was quenched by the addition of excess unlabeled Met/Cys (chase). At timed intervals, 0 and 5 min after the addition of the chase, culture aliquots were precipitated with trichloroacetic acid, washed in acetone, and dried, and the cell wall peptidoglycan was digested with lysostaphin. MTSET (100 mM in water) was added at a final concentration of 2 mM 10 s after labeling with [35S]Met/Cys had commenced. Compound 6e (100 mM in water) was added at a final concentration of 200 µM 10 min before pulse-labeling with [35S]Met/Cys. SpA was immunoprecipitated with SpA-specific antibodies, and radiolabeled polypeptides were analyzed by 10% SDS/PAGE and PhosphoImager.
Animal Model of S. aureus Infection.
Lethal challenge experiments were performed at the Shanghai Public Health Clinical Center following animal care and use protocols that were reviewed, approved, and supervised by the Committee for Animal Experiments at Fudan University. Overnight cultures of S. aureus Newman were diluted 1:1,000 into 30 mL fresh tryptic soy broth (TSB) and grown with rotation at 37 °C for 3 h. Bacteria were centrifuged at 3,000 × g, washed, and suspended in PBS to A600 0.8 or 1.6. Cohorts of BALB/c mice (6-wk-old females; Shanghai Super-B&K Laboratory Animal Corp.) were randomly assigned into of two cohorts. Water and laboratory chow were provided ad libitum. Compound 6e was dissolved in sterile double-distilled water (ddH2O) and administered by i.p. injection at a dose of 40 mg/kg in 12-h intervals. Four hours after the first injection of compound 6e or mock (ddH2O) control, animals were challenged by periorbital injection of S. aureus Newman; aliquots of the inoculum were plated for enumeration of CFUs, and animals were monitored for survival over a 20-d observation period. The log-rank test was used to analyze mortality data; P < 0.05 was deemed statistically significant.
Other Procedures.
Detailed procedures are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by National Science and Technology Major Project “Key New Drug Creation and Manufacturing Program” Grant 2013ZX09507-004, National Natural Science Foundation of China Grants 91313303, 20972173, and 81230076, and Hi-Tech Research and Development Program of China Grants 2012AA020302 and 2012AA020301. Work on sortase in the laboratory of O.S. is supported by National Institute of Allergy and Infectious Diseases Grant AI038897. Computation resources were partially supported by the Computer Network Information Center, Chinese Academy of Sciences, and Shanghai Supercomputing Center.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. H.L.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1408601111/-/DCSupplemental.
References
- 1.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339(8):520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7(9):629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klevens RM, et al. Active Bacterial Core surveillance (ABCs) MRSA Investigators Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 4.Daum RS, Spellberg B. Progress toward a Staphylococcus aureus vaccine. Clin Infect Dis. 2012;54(4):560–567. doi: 10.1093/cid/cir828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fry DE. The continued challenge of Staphylococcus aureus in the surgical patient. Am Surg. 2013;79(1):1–10. [PubMed] [Google Scholar]
- 6.Johnson S, et al. Epidemics of diarrhea caused by a clindamycin-resistant strain of Clostridium difficile in four hospitals. N Engl J Med. 1999;341(22):1645–1651. doi: 10.1056/NEJM199911253412203. [DOI] [PubMed] [Google Scholar]
- 7.Thomas C, Stevenson M, Riley TV. Antibiotics and hospital-acquired Clostridium difficile-associated diarrhoea: A systematic review. J Antimicrob Chemother. 2003;51(6):1339–1350. doi: 10.1093/jac/dkg254. [DOI] [PubMed] [Google Scholar]
- 8.Mazmanian SK, Liu G, Ton-That H, Schneewind O. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science. 1999;285(5428):760–763. doi: 10.1126/science.285.5428.760. [DOI] [PubMed] [Google Scholar]
- 9.Mazmanian SK, Liu G, Jensen ER, Lenoy E, Schneewind O. Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc Natl Acad Sci USA. 2000;97(10):5510–5515. doi: 10.1073/pnas.080520697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ton-That H, Liu G, Mazmanian SK, Faull KF, Schneewind O. Purification and characterization of sortase, the transpeptidase that cleaves surface proteins of Staphylococcus aureus at the LPXTG motif. Proc Natl Acad Sci USA. 1999;96(22):12424–12429. doi: 10.1073/pnas.96.22.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazmanian SK, Ton-That H, Schneewind O. Sortase-catalysed anchoring of surface proteins to the cell wall of Staphylococcus aureus. Mol Microbiol. 2001;40(5):1049–1057. doi: 10.1046/j.1365-2958.2001.02411.x. [DOI] [PubMed] [Google Scholar]
- 12.Cheng AG, et al. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009;23(10):3393–3404. doi: 10.1096/fj.09-135467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McAdow M, et al. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog. 2011;7(10):e1002307. doi: 10.1371/journal.ppat.1002307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suree N, et al. The structure of the Staphylococcus aureus sortase-substrate complex reveals how the universally conserved LPXTG sorting signal is recognized. J Biol Chem. 2009;284(36):24465–24477. doi: 10.1074/jbc.M109.022624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh KB, et al. Bis(indole) alkaloids as sortase A inhibitors from the sponge Spongosorites sp. Bioorg Med Chem Lett. 2005;15(22):4927–4931. doi: 10.1016/j.bmcl.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 16.Mathew V, Keshavayya J, Vaidya VP, Giles D. Studies on synthesis and pharmacological activities of 3,6-disubstituted-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles and their dihydro analogues. Eur J Med Chem. 2007;42(6):823–840. doi: 10.1016/j.ejmech.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Schneewind O, Fowler A, Faull KF. Structure of the cell wall anchor of surface proteins in Staphylococcus aureus. Science. 1995;268(5207):103–106. doi: 10.1126/science.7701329. [DOI] [PubMed] [Google Scholar]
- 18.Becker S, Frankel MB, Schneewind O, Missiakas D. Release of protein A from the cell wall of Staphylococcus aureus. Proc Natl Acad Sci USA. 2014;111(4):1574–1579. doi: 10.1073/pnas.1317181111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ton-That H, Mazmanian SK, Faull KF, Schneewind O. Anchoring of surface proteins to the cell wall of Staphylococcus aureus. Sortase catalyzed in vitro transpeptidation reaction using LPXTG peptide and NH(2)-Gly(3) substrates. J Biol Chem. 2000;275(13):9876–9881. doi: 10.1074/jbc.275.13.9876. [DOI] [PubMed] [Google Scholar]
- 20.Li M, et al. MRSA epidemic linked to a quickly spreading colonization and virulence determinant. Nat Med. 2012;18(5):816–819. doi: 10.1038/nm.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ton-That H, Schneewind O. Anchor structure of staphylococcal surface proteins. IV. Inhibitors of the cell wall sorting reaction. J Biol Chem. 1999;274(34):24316–24320. doi: 10.1074/jbc.274.34.24316. [DOI] [PubMed] [Google Scholar]
- 22.Coan KE, Maltby DA, Burlingame AL, Shoichet BK. Promiscuous aggregate-based inhibitors promote enzyme unfolding. J Med Chem. 2009;52(7):2067–2075. doi: 10.1021/jm801605r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ilangovan U, Ton-That H, Iwahara J, Schneewind O, Clubb RT. Structure of sortase, the transpeptidase that anchors proteins to the cell wall of Staphylococcus aureus. Proc Natl Acad Sci USA. 2001;98(11):6056–6061. doi: 10.1073/pnas.101064198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zong Y, Bice TW, Ton-That H, Schneewind O, Narayana SV. Crystal structures of Staphylococcus aureus sortase A and its substrate complex. J Biol Chem. 2004;279(30):31383–31389. doi: 10.1074/jbc.M401374200. [DOI] [PubMed] [Google Scholar]
- 25.DeDent A, Bae T, Missiakas DM, Schneewind O. Signal peptides direct surface proteins to two distinct envelope locations of Staphylococcus aureus. EMBO J. 2008;27(20):2656–2668. doi: 10.1038/emboj.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneewind O, Model P, Fischetti VA. Sorting of protein A to the staphylococcal cell wall. Cell. 1992;70(2):267–281. doi: 10.1016/0092-8674(92)90101-h. [DOI] [PubMed] [Google Scholar]
- 27.Mazmanian SK, Ton-That H, Su K, Schneewind O. An iron-regulated sortase anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc Natl Acad Sci USA. 2002;99(4):2293–2298. doi: 10.1073/pnas.032523999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falugi F, Kim HK, Missiakas DM, Schneewind O. Role of protein A in the evasion of host adaptive immune responses by Staphylococcus aureus. MBio. 2013;4(5):e00575–e13. doi: 10.1128/mBio.00575-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDevitt D, Francois P, Vaudaux P, Foster TJ. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol. 1994;11(2):237–248. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 30.Ganesh VK, et al. Structural and biochemical characterization of Staphylococcus aureus clumping factor B/ligand interactions. J Biol Chem. 2011;286(29):25963–25972. doi: 10.1074/jbc.M110.217414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duthie ES, Lorenz LL. Staphylococcal coagulase; mode of action and antigenicity. J Gen Microbiol. 1952;6(1-2):95–107. doi: 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]
- 32.Kreiswirth BN, et al. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;305(5936):709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 33.Cheng AG, et al. Contribution of coagulases towards Staphylococcus aureus disease and protective immunity. PLoS Pathog. 2010;6(8):e1001036. doi: 10.1371/journal.ppat.1001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marraffini LA, Dedent AC, Schneewind O. Sortases and the art of anchoring proteins to the envelopes of gram-positive bacteria. Microbiol Mol Biol Rev. 2006;70(1):192–221. doi: 10.1128/MMBR.70.1.192-221.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navarre WW, Schneewind O. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev. 1999;63(1):174–229. doi: 10.1128/mmbr.63.1.174-229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schleifer KH, Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang R, et al. Structures of sortase B from Staphylococcus aureus and Bacillus anthracis reveal catalytic amino acid triad in the active site. Structure. 2004;12(7):1147–1156. doi: 10.1016/j.str.2004.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiner EM, Robson S, Marohn M, Clubb RT. The Sortase A enzyme that attaches proteins to the cell wall of Bacillus anthracis contains an unusual active site architecture. J Biol Chem. 2010;285(30):23433–23443. doi: 10.1074/jbc.M110.135434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Misra A, et al. Crystallization and preliminary X-ray diffraction studies of sortase A from Streptococcus pneumoniae. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011;67(Pt 10):1195–1198. doi: 10.1107/S1744309111029952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Race PR, et al. Crystal structure of Streptococcus pyogenes sortase A: Implications for sortase mechanism. J Biol Chem. 2009;284(11):6924–6933. doi: 10.1074/jbc.M805406200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klevens RM, Edwards JR, Gaynes RP, System NNIS. National Nosocomial Infections Surveillance System The impact of antimicrobial-resistant, health care-associated infections on mortality in the United States. Clin Infect Dis. 2008;47(7):927–930. doi: 10.1086/591698. [DOI] [PubMed] [Google Scholar]
- 42.Rossi F, et al. Transferable vancomycin resistance in a community-associated MRSA lineage. N Engl J Med. 2014;370(16):1524–1531. doi: 10.1056/NEJMoa1303359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gordon CP, Williams P, Chan WC. Attenuating Staphylococcus aureus virulence gene regulation: A medicinal chemistry perspective. J Med Chem. 2013;56(4):1389–1404. doi: 10.1021/jm3014635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji G, Beavis RC, Novick RP. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci USA. 1995;92(26):12055–12059. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Novick RP, Geisinger E. Quorum sensing in staphylococci. Annu Rev Genet. 2008;42:541–564. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 46.Lyon GJ, Mayville P, Muir TW, Novick RP. Rational design of a global inhibitor of the virulence response in Staphylococcus aureus, based in part on localization of the site of inhibition to the receptor-histidine kinase, AgrC. Proc Natl Acad Sci USA. 2000;97(24):13330–13335. doi: 10.1073/pnas.97.24.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peterson MM, et al. Apolipoprotein B Is an innate barrier against invasive Staphylococcus aureus infection. Cell Host Microbe. 2008;4(6):555–566. doi: 10.1016/j.chom.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rothfork JM, et al. Inactivation of a bacterial virulence pheromone by phagocyte-derived oxidants: New role for the NADPH oxidase in host defense. Proc Natl Acad Sci USA. 2004;101(38):13867–13872. doi: 10.1073/pnas.0402996101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hall PR, et al. Nox2 modification of LDL is essential for optimal apolipoprotein B-mediated control of agr type III Staphylococcus aureus quorum-sensing. PLoS Pathog. 2013;9(2):e1003166. doi: 10.1371/journal.ppat.1003166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim HK, Kim HY, Schneewind O, Missiakas D. Identifying protective antigens of Staphylococcus aureus, a pathogen that suppresses host immune responses. FASEB J. 2011;25(10):3605–3612. doi: 10.1096/fj.11-187963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murray EJ, et al. Targeting Staphylococcus aureus quorum sensing with nonpeptidic small molecule inhibitors. J Med Chem. 2014;57(6):2813–2819. doi: 10.1021/jm500215s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim SH, et al. Inhibition of sortase, a bacterial surface protein anchoring transpeptidase, by beta-sitosterol-3-O-glucopyranoside from Fritillaria verticillata. Biosci Biotechnol Biochem. 2003;67(11):2477–2479. doi: 10.1271/bbb.67.2477. [DOI] [PubMed] [Google Scholar]
- 53.Oh KB, et al. Discovery of diarylacrylonitriles as a novel series of small molecule sortase A inhibitors. J Med Chem. 2004;47(10):2418–2421. doi: 10.1021/jm0498708. [DOI] [PubMed] [Google Scholar]
- 54.Frankel BA, Bentley M, Kruger RG, McCafferty DG. Vinyl sulfones: Inhibitors of SrtA, a transpeptidase required for cell wall protein anchoring and virulence in Staphylococcus aureus. J Am Chem Soc. 2004;126(11):3404–3405. doi: 10.1021/ja0390294. [DOI] [PubMed] [Google Scholar]
- 55.Chan AH, et al. Discovery of Staphylococcus aureus sortase A inhibitors using virtual screening and the relaxed complex scheme. Chem Biol Drug Des. 2013;82(4):418–428. doi: 10.1111/cbdd.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Won TH, et al. Brominated aromatic furanones and related esters from the ascidian Synoicum sp. J Nat Prod. 2012;75(12):2055–2061. doi: 10.1021/np3005562. [DOI] [PubMed] [Google Scholar]
- 57.Scott CJ, et al. Irreversible inhibition of the bacterial cysteine protease-transpeptidase sortase (SrtA) by substrate-derived affinity labels. Biochem J. 2002;366(Pt 3):953–958. doi: 10.1042/BJ20020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Connolly KM, et al. Sortase from Staphylococcus aureus does not contain a thiolate-imidazolium ion pair in its active site. J Biol Chem. 2003;278(36):34061–34065. doi: 10.1074/jbc.M305245200. [DOI] [PubMed] [Google Scholar]
- 59.Kruger RG, Barkallah S, Frankel BA, McCafferty DG. Inhibition of the Staphylococcus aureus sortase transpeptidase SrtA by phosphinic peptidomimetics. Bioorg Med Chem. 2004;12(13):3723–3729. doi: 10.1016/j.bmc.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 60.Maresso AW, et al. Activation of inhibitors by sortase triggers irreversible modification of the active site. J Biol Chem. 2007;282(32):23129–23139. doi: 10.1074/jbc.M701857200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oh KB, Oh MN, Kim JG, Shin DS, Shin J. Inhibition of sortase-mediated Staphylococcus aureus adhesion to fibronectin via fibronectin-binding protein by sortase inhibitors. Appl Microbiol Biotechnol. 2006;70(1):102–106. doi: 10.1007/s00253-005-0040-8. [DOI] [PubMed] [Google Scholar]
- 62.Oh KB, et al. Therapeutic effect of (Z)-3-(2,5-dimethoxyphenyl)-2-(4-methoxyphenyl) acrylonitrile (DMMA) against Staphylococcus aureus infection in a murine model. Biochem Biophys Res Commun. 2010;396(2):440–444. doi: 10.1016/j.bbrc.2010.04.113. [DOI] [PubMed] [Google Scholar]
- 63.Kahlon AK, et al. Identification of 1-chloro-2-formyl indenes and tetralenes as novel antistaphylococcal agents exhibiting sortase A inhibition. Appl Microbiol Biotechnol. 2014;98(5):2041–2051. doi: 10.1007/s00253-013-5036-1. [DOI] [PubMed] [Google Scholar]
- 64.Oh I, et al. In vitro sortase A inhibitory and antimicrobial activity of flavonoids isolated from the roots of Sophora flavescens. Arch Pharm Res. 2011;34(2):217–222. doi: 10.1007/s12272-011-0206-0. [DOI] [PubMed] [Google Scholar]
- 65.Maresso AW, Schneewind O. Sortase as a target of anti-infective therapy. Pharmacol Rev. 2008;60(1):128–141. doi: 10.1124/pr.107.07110. [DOI] [PubMed] [Google Scholar]
- 66.Kline KA, et al. Mechanism for sortase localization and the role of sortase localization in efficient pilus assembly in Enterococcus faecalis. J Bacteriol. 2009;191(10):3237–3247. doi: 10.1128/JB.01837-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sillanpää J, et al. Characterization of the ebp(fm) pilus-encoding operon of Enterococcus faecium and its role in biofilm formation and virulence in a murine model of urinary tract infection. Virulence. 2010;1(4):236–246. doi: 10.4161/viru.1.4.11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spirig T, Weiner EM, Clubb RT. Sortase enzymes in Gram-positive bacteria. Mol Microbiol. 2011;82(5):1044–1059. doi: 10.1111/j.1365-2958.2011.07887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nallapareddy SR, et al. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J Clin Invest. 2006;116(10):2799–2807. doi: 10.1172/JCI29021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nielsen HV, et al. Pilin and sortase residues critical for endocarditis- and biofilm-associated pilus biogenesis in Enterococcus faecalis. J Bacteriol. 2013;195(19):4484–4495. doi: 10.1128/JB.00451-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.