Significance
Adaptor proteins often regulate substrate selection by AAA+ enzymes, but the molecular mechanisms of adaptor-mediated substrate delivery are poorly understood. We find that an unstructured N-terminal extension (NTE) of ClpS, the adaptor that delivers N-degron substrates to the ClpAP protease, enters the ClpA translocation pore during substrate delivery and must be actively engaged for delivery to occur. ClpA engagement of the ClpS NTE promotes delivery of substrate bound to the same adaptor molecule. These results support a model in which ClpA remodels ClpS by translocating its NTE, triggering delivery of the N-degron substrate. Active remodeling of components in delivery complexes by AAA+ unfoldases and proteases is likely a widespread mechanism.
Keywords: AAA+ ATPase adaptor, N-degron substrate selection, adaptor remodeling, AAA+ unfoldase/translocase
Abstract
The ClpS adaptor collaborates with the AAA+ ClpAP protease to recognize and degrade N-degron substrates. ClpS binds the substrate N-degron and assembles into a high-affinity ClpS-substrate-ClpA complex, but how the N-degron is transferred from ClpS to the axial pore of the AAA+ ClpA unfoldase to initiate degradation is not known. Here we demonstrate that the unstructured N-terminal extension (NTE) of ClpS enters the ClpA processing pore in the active ternary complex. We establish that ClpS promotes delivery only in cis, as demonstrated by mixing ClpS variants with distinct substrate specificity and either active or inactive NTE truncations. Importantly, we find that ClpA engagement of the ClpS NTE is crucial for ClpS-mediated substrate delivery by using ClpS variants carrying “blocking” elements that prevent the NTE from entering the pore. These results support models in which enzymatic activity of ClpA actively remodels ClpS to promote substrate transfer, and highlight how ATPase/motor activities of AAA+ proteases can be critical for substrate selection as well as protein degradation.
AAA+ molecular machines power cellular processes as diverse as protein degradation, microtubule severing, membrane fusion, and initiation of DNA replication, with the common theme that macromolecules are actively remodeled (1–3). Furthermore, protein quality control in all organisms involves deployment of ATP-dependent proteases, consisting of hexameric AAA+ rings that unfold and translocate specific substrates into an associated peptidase barrel (3, 4). Adaptor proteins are known to aid recognition and degradation of certain substrates (5–8), but how enzyme–adaptor pairs ensure proper substrate selection is poorly understood.
In prokaryotes and eukaryotes, the N-end rule pathway governs degradation of proteins with specific N-terminal amino acids (9, 10). In Escherichia coli, the primary destabilizing N-degron amino acids are Phe, Tyr, Trp, and Leu (11, 12). ClpS, a widespread bacterial adaptor, recognizes and delivers N-degron substrates to the ClpAP or ClpCP AAA+ proteases (6, 11, 13). These enzymes consist of the AAA+ ClpA or ClpC unfoldases coaxially stacked with the ClpP peptidase (14–16). In eukaryotes, a family of E3 ligases shares homology with the substrate-binding region of ClpS (17, 18). These ligases recognize N-degron substrates and promote ubiquitination, which then targets the modified protein to the 26S proteasome (17, 18).
Multiple crystal structures reveal the regions of ClpS that bind to the N-degron, as well as a patch that binds the N-terminal domain of ClpA (19–22). This bivalent binding to the substrate and the enzyme tethers N-degron substrates to ClpAP. Tethering alone is insufficient for ClpS to promote substrate delivery however, given that deletion of 12 amino acids of the ClpS unstructured N-terminal extension (NTE; residues 1–25 in E. coli ClpS) (Fig. 1A) prevents N-degron substrate degradation, but does not block formation of a high-affinity delivery ternary complex (HADC) consisting of substrate, the ClpS adaptor, and the ClpAP protease (Fig. 1B) (19). Importantly, the identity of the NTE sequence is not critical for ClpS function (23). An active delivery model has been proposed in which the translocation pore of ClpA engages the ClpS NTE with subsequent translocation that remodels the delivery complex to achieve substrate engagement (Fig. 1C) (19).
Fig. 1.
Model for the active delivery mechanism used by ClpS. (A) The adaptor protein ClpS has a long, flexible N-terminal extension (NTE; residues 1–25) and a folded core domain (ClpScore; residues 26–106). ClpScore binds N-degrons. A substrate Tyr in the binding pocket is shown in red (Protein Data Bank ID code 3O1F). Successful substrate delivery requires that the ClpS NTE be at least 14-aa long (shown in green). (B) Formation of an HADC between ClpS, substrate, and ClpA (19) involves formation of additional contacts among ClpA, ClpS, and the N-degron substrate. Assembly of this complex increases the affinity of the substrate for ClpAS by ∼100-fold (19). (C) The current model for ClpA-driven disassembly of the HADC and N-degron substrate delivery. Translocation-mediated ClpA “pulling” on the NTE remodels the ClpScore structure, weakens the interactions of ClpS with the N-degron, and facilitates its transfer to a site in the ClpA pore. Finally, because ClpS cannot be unfolded by ClpA (19), the adaptor escapes the enzyme, and the substrate is unfolded by ClpA and subsequently degraded by ClpP.
In the present work, we investigated how the ClpS NTE functions during delivery of N-degron substrates. We show that the NTE can only promote delivery of substrates that are bound to the same ClpS molecule. Furthermore, we demonstrate that the NTE enters the ClpA translocation pore and provide strong evidence that ClpA pulls on the ClpS NTE to trigger substrate delivery.
Results
The ClpS NTE Acts in Cis During Substrate Delivery.
Multiple ClpS-substrate complexes can dock on the N-domains of a single ClpA hexamer (23–25). As established previously, an NTE of at least 14 amino acids is necessary for ClpS to deliver an N-degron substrate (Fig. 1A) (23); however, whether the NTE acts in cis to deliver the substrate bound to its own ClpS molecule or in trans to activate delivery of a substrate bound to another ClpS molecule is unknown. The optimal ratio of ClpS to ClpA hexamer in the delivery complex has not been established, but many ratios yield functional complexes (6, 23, 24).
To test whether the ClpS NTE acts in cis or in trans, we monitored delivery of substrates by mixtures of ClpS variants with a full-length functional NTE or with a truncated nonfunctional NTE (ClpS∆13) and a WT or M40A (ClpSM40A) N-degron–binding pocket. The ClpSM40A variant recognizes β-branched (Val and Ile) residues, termed *N-degrons, in addition to the natural E. coli N-degrons Tyr, Leu, Phe, and Trp (Fig. 2) (20).
Fig. 2.
The ClpS NTE delivers N-degron substrates in cis. (A) Cartoon showing the protein variants for the mixing experiments performed to test cis vs. trans activation by the ClpS-NTE. Although present, the N-degron peptide (Phe-Val) is not depicted. (B) Degradation of the *N-degron substrate (vlfvqela-GFP) by ClpAP. Only when the full-length functional NTE and *N-degron–binding pocket were present on the same ClpS molecule was this substrate efficiently degraded (cis delivery experiment, blue trace). The mixing experiments contained each of the ClpS variants shown in A (1.2 µM each), 1 µM of an N-degron peptide, and 1 µM of *N-degron substrate. (C) Binding of fluorescein-labeled ClpSΔ13/M40A (1.2 µM) to ClpA6 in the presence of ATPγS (2 mM), ClpS (1.2 µM), N-degron peptide (1 µM), and N*-degron peptide (1 µM), as assayed by fluorescence anisotropy (KD = 112 ± 13 nM).
In one experiment (Fig. 2A, Left), ClpS and ClpSΔ13/M40A were mixed with ClpAP, an N-degron dipeptide [to promote formation of an HADC (19)], as well as the *N-degron substrate vlfvqela-GFP. In this experiment, the functional NTE was provided by WT ClpS, whereas the *N-degron substrate only bound ClpSΔ13/M40A (20). If engagement of the NTE can work in trans, then *N-degron substrate delivery would be observed; however, if engagement of the NTE functions only in cis, then the absence of a functional NTE in ClpSΔ13/M40A would prevent degradation of the *N-degron substrate. Upon addition of ATP, the *N-degron substrate was not efficiently degraded (Fig. 2B, pink trace). Under conditions similar to those of the degradation experiments, fluorescence-anisotropy experiments established that a fluorescent-labeled ClpSΔ13/M40A variant bound ClpA tightly (Fig. 2C). Thus, the lack of efficient degradation of the *N-degron substrate was not caused by a failure of ClpSΔ13/M40A to bind ClpAP. Rather, these data indicate that the NTE does not function in trans to trigger substrate delivery.
To ensure that ClpSM40A with a functional NTE is able to perform substrate delivery under the conditions of this assay, we mixed it with ClpSΔ13 (nonfunctional NTE), ClpAP, N-degron peptide, and *N-degron substrate (Fig. 2A, Right). In this case, the *N-degron substrate was efficiently degraded (Fig. 2B). Taken together, these experiments show that delivery requires a functional substrate-binding pocket and a functional NTE within the same ClpS molecule.
The ClpS NTE Physically Enters the ClpA Pore.
Previous studies suggested a model in which N-degron substrate delivery requires engagement of the ClpS NTE by the ClpA translocation pore (Fig. 1C) (19, 23). To test this model directly, we used Förster resonance energy transfer (FRET) between a donor fluorophore, 5-(2-aminoethylamino)-1-napthalene sulfonate (EDANS), at the entrance of the ClpP proteolytic chamber (ClpP residue 17, adjacent to the bottom of the ClpA pore; ClpPED) (25) and an acceptor fluorophore (fluorescein) placed at different positions either along the ClpS NTE or on the surface of the folded domain (Fig. 3A). The calculated Förster radius for the EDANS-fluorescein pair is ∼46 Å. Based on the dimensions of ClpC, a close relative of ClpA, a distance of ∼100 Å separates the top of the ClpA pore from the ClpP neck (16). Consequently, robust FRET would only be expected if a fluorescein dye on ClpS were able to enter the ClpA pore.
Fig. 3.
The ClpS NTE localizes inside the ClpA pore. (A) Cartoon of the protein variants used in the FRET experiments. Three single-cysteine variants of ClpS were labeled with fluorescein (acceptor fluorophore, yellow star). The labeled positions were C5 and C17, both sites in the ClpS NTE (ClpS5-Fl and ClpS17-Fl), and C96, which is in the ClpS core domain (ClpS96-Fl). Unlabeled ClpA was used with a ClpP variant in which residue 17 of each subunit was changed to cysteine and labeled with EDANS (donor fluorophore, green star; ClpPED). This ClpP variant also contains the S97A active-site mutation (45). (B) Emission spectra of the donor fluorophore in ClpPED on excitation at 336 nm in the presence of ClpA6 and ATPγS (black trace); emission spectra of the acceptor fluorophore in ClpS5-Fl on excitation at 336 nm in the presence of ATPγS (green trace); addition spectra of the two independent traces obtained from the emission of the donor and acceptor proteins (gray line); and observed emission spectra characteristic of FRET obtained in reactions containing ATPγS, ClpS5-Fl, ClpAPED, and the N-degron substrate ylfvq-titin I27 (red trace). The red arrow pointing up at ∼525 nm denotes an increase in fluorescence of the acceptor fluorophore, and the red arrow pointing down at ∼475 nm denotes the decreased signal of the donor fluorophore. (C) FRET was also observed when the experiment shown in B was repeated with ClpS17-Fl as the acceptor molecule (Left, red). In contrast, no FRET was detected when the acceptor molecule was ClpS96-Fl (Right, red). (D) ClpS5-Fl fluorescence was insensitive to the fluorescence quencher 4-amino-TEMPO when bound in a complex with ClpAPED and N-degron substrate.
When residue 5 of the ClpS NTE was labeled with fluorescein (ClpS5-Fl) and incubated with ClpAPED, N-degron substrate, and ATPγS (Fig. 3B), FRET was observed between the donor and acceptor dyes. Excitation of the donor fluorophore in ClpPED increased acceptor fluorescence (525 nm) and decreased donor fluorescence (475 nm) (Fig. 3B, red trace) compared with the sum of the spectra of each component alone (Fig. 3B, gray trace). If this signal resulted from FRET between the NTE and ClpPED, then a reduced signal would be expected if the fluorescein were placed at position 17 of the ClpS NTE, a more C-terminal location that should be farther from ClpP. Furthermore, little or no FRET would be predicted if the dye were attached to ClpS residue 96, near the N-degron–binding pocket and far from the NTE (Fig. 3A). This pattern of FRET signals was observed (Fig. 3C), supporting our hypothesis that the ClpS NTE enters the ClpA axial pore with its N-terminal residues reaching close to the ClpA-ClpP complex junction.
To further test whether FRET between ClpS5-Fl and ClpPED occurs because the NTE is located within the pore rather than on the surface of the enzyme, we repeated the experiment with donor dye at NTE position 5 in the presence of the solution quencher 4-amino-2,2,6,6-tetramethylpiperidine-1-oxyl (4-amino-TEMPO), which has an anhydrous diameter of ∼10 Å and thus should not efficiently diffuse into the ClpA pore (26). Indeed, fluorescence of free ClpS5-Fl was quenched ∼30% by 4-amino-TEMPO, whereas quenching of the fluorescence of ClpS5-Fl in complex with ClpAPED and substrate was <5% (Fig. 3D). Taken together, these results support a model in which the ClpS NTE enters the ClpA pore in the ClpAPS-substrate complex.
Increasing the Length of the ClpS NTE Results in Truncation by ClpP.
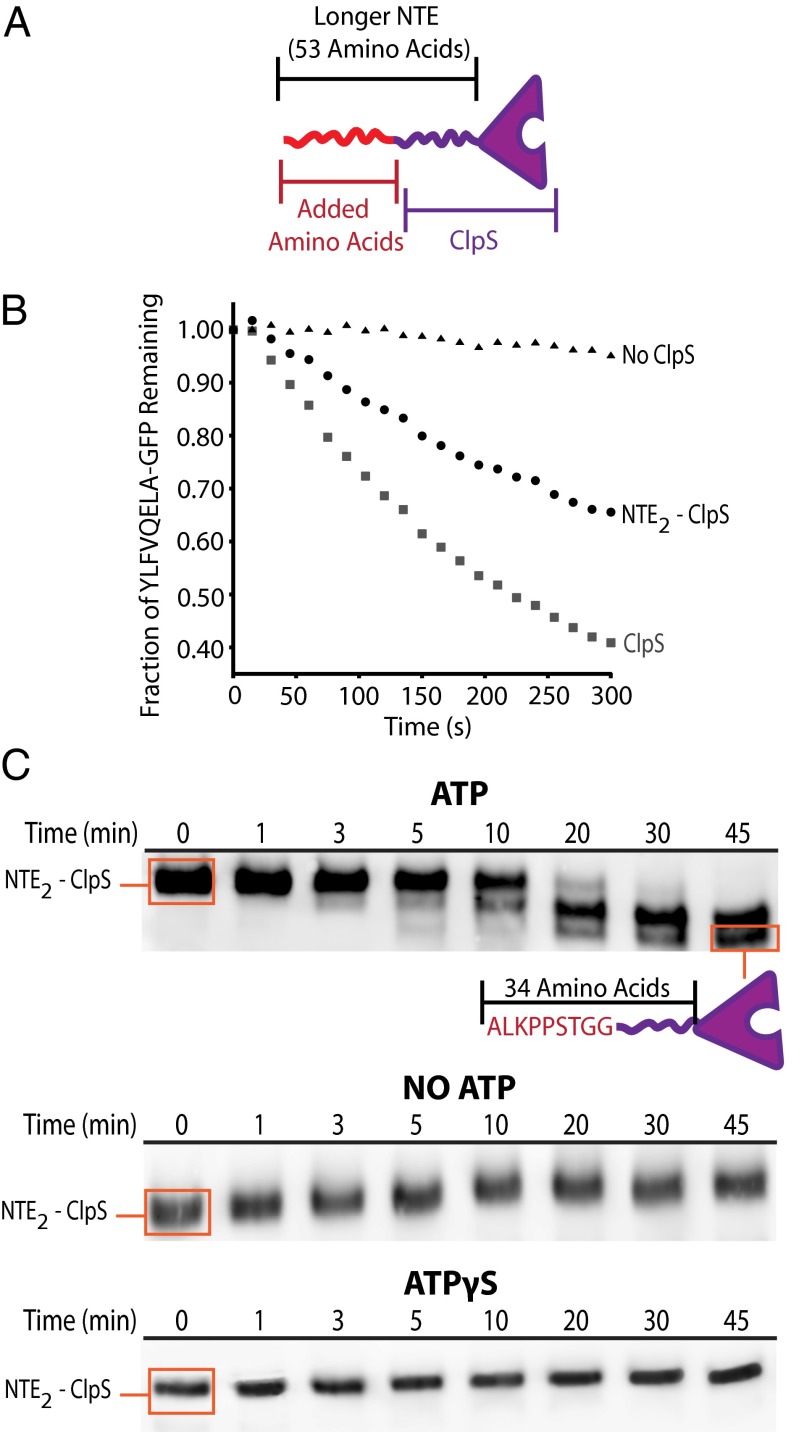
As an orthogonal method to determine whether the ClpS NTE enters the ClpA pore during N-degron delivery, we constructed an NTE2-ClpS variant with a duplicated NTE (Fig. 4A). We reasoned that if the NTE enters the ClpAP pore during N-degron delivery, then the longer NTE2 sequence would enter the ClpP chamber, where it can be cleaved by the ClpP active sites. Control experiments revealed that NTE2-ClpS delivered the N-degron substrate ylfvqela-GFP for degradation, albeit somewhat less efficiently than ClpS (Fig. 4B). Importantly, during these time-course delivery experiments, the NTE2-ClpS both delivered the N-degron substrate and was truncated by ClpP (Fig. 4 B and C). Truncation of the NTE2-ClpS depended on the ATP-driven translocation activity of ClpA, given that it did not occur either in the absence of ATP or with the poorly hydrolyzed analog, ATPγS (Fig. 4C). N-terminal sequencing of the smallest truncated ClpS species revealed removal of 19 amino acids of NTE2-ClpS, leaving the native ClpS sequence with 9 additional N-terminal residues originating from the NTE duplication (Fig. 4C). Such NTE2-ClpS cleavage by ClpP strongly supports the model in which the NTE of WT ClpS is engaged by the ClpA axial pore during delivery of N-degron substrates.
Fig. 4.
ClpAP cleaves an extended ClpS NTE. (A) Cartoon of the NTE2-ClpS variant. (B) Delivery and degradation of the N-degron substrate, ylfvqela-GFP, to ClpAP in the absence of ClpS, in the presence of NTE2-ClpS, or in the presence of WT ClpS. Degradation was monitored by the decrease in substrate fluorescence. (C) Truncation of NTE2-ClpS was observed during delivery of N-degron substrates to ClpAP in the presence of ATP (Top), but was not observed without ATP (Middle) or with ATPγS (Bottom). N-terminal sequencing of the lowest molecular weight product revealed an NTE “tail” of 34 amino acids. This “trimmed” NTE2-ClpS truncation product is depicted as a cartoon below the Top panel.
Antagonizing NTE Engagement Inhibits N-Degron Substrate Delivery.
To probe whether entry of the ClpS NTE into the ClpA pore is required for delivery, we constructed a ClpS variant with mouse dihydrofolate reductase (DHFR) attached to the N terminus of the ClpS NTE (H6-DHFR-ClpS) (Fig. 5A). In our experiment, the N-terminal H6 tag of DHFR served as a ClpA degron (19), and the DHFR domain of this substrate was unfolded and degraded by ClpAP, exposing the ClpS NTE (Fig. 5B, Left). As expected based on previous studies of DHFR degradation by AAA+ proteases (27), the addition of methotrexate stabilized DHFR and prevented truncation of the DHFR-ClpS chimera by ClpAP (Fig. 5B, Right).
Fig. 5.
Engagement of the ClpS NTE is necessary for delivery of N-degron substrates. (A) Cartoon of the H6-DHFR-ClpS fusion protein. (B) Cartoon of results obtained upon addition of ClpAP and ATP to H6-DHFR-ClpS in the absence or presence of methotrexate. Protein processing was monitored by Western blot analysis of the H6-DHFR-ClpS protein with anti-ClpS antisera. ClpAP-dependent cleavage of the fusion protein and release of a truncated ClpS adaptor (with an available NTE) was observed in the absence of methotrexate (Left), whereas no processing of the fusion protein was detected when methotrexate was present (Right). (C) Delivery and degradation of the N-degron substrate ylfvqela-GFP by ClpAP promoted by either H6-DHFR-ClpS or ClpS in the presence and absence of methotrexate. (D) Formation of an HADC by ClpS (Kapp= 35 ± 1 nM), H6-DHFR-ClpS (Kapp= 107 ± 17 nM), and H6-DHFR-ClpS in the presence of methotrexate (Kapp= 119 ± 21 nM) assayed by anisotropy using a fluorescent N-degron peptide.
Importantly, the H6-DHFR-ClpS adaptor promoted degradation of the N-degron substrate ylfvqela-GFP in the absence of, but not in the presence of, methotrexate (Fig. 5C). Interestingly, H6-DHFR-ClpS stimulated degradation of ylfvqela-GFP only after a lag of ∼100 s, suggesting that degradation of the DHFR domain is a prerequisite for NTE engagement and subsequent substrate delivery (Fig. 5C). As expected, methotrexate did not inhibit WT ClpS delivery of ylfvqela-GFP to ClpAP (Fig. 5C). Furthermore, H6-DHFR-ClpS assembled normally with ClpAP, ATPγS, and a fluorescent N-degron peptide (llyvqrsdec-fl) both in the absence and the presence of methotrexate (Fig. 5D). Thus, the degradation defect caused by blocking entry of the ClpS NTE into the ClpA pore appears to occur at a step after assembly of the initial substrate-adaptor-enzyme ternary complex.
Taken together, our experiments with the H6-DHFR-ClpS chimera demonstrate that preventing entry of the ClpS NTE into the ClpA pore inhibits delivery and degradation of ClpS-bound N-degron substrates. These results strongly support a model in which engagement and partial translocation of the ClpS NTE through the ClpA pore is an essential step in the delivery of N-degron substrates.
Discussion
Regulation of macromolecular complexes is commonly implemented by the formation of multiple weak binary interactions that synergistically stabilize the complex (19, 28). Stable complexes can serve as checkpoints in a sequential mechanism to enhance specificity, but also can make downstream steps slow or inaccessible if stabilizing interactions must be broken before the next step can occur. AAA+ enzymes play important roles in catalyzing both the remodeling and destabilizing macromolecular complexes, including the examples of severing microtubules and promoting both assembly and critical reaction transitions during RNA splicing (1, 2, 19, 28, 29).
Previous studies have established that adaptor-mediated recognition of several substrates by AAA+ proteases involves formation of a high-affinity complex between the enzyme, substrate, and adaptor (19, 30–35). The delivery complex consisting of ClpAP, N-degron substrate, and the ClpS adaptor is one such example (Fig. 1B) (19). Here we identify features of the interactions between ClpA and ClpS that are critical for releasing substrates from this high-affinity complex and thus enabling the downstream steps of unfolding and degradation. Our FRET and protein processing experiments demonstrate that the NTE enters the ClpA pore during substrate delivery. Importantly, these experiments also show that engagement of the NTE by the ClpA pore is essential for ClpS-mediated degradation of N-degron substrates. Consistent with these observations, previous experiments have established that the ClpS NTE can act as a ClpAP degradation tag when attached to other proteins (19).
Why is engagement of the ClpS NTE by the ClpA pore critical for transfer of the N-degron of the substrate from ClpS to the ClpA pore? At the simplest level, ATP-dependent translocation of the ClpS NTE through the ClpA pore pulls the folded domain of ClpS against the pore entrance, distorting the folded structure of ClpS and catalyzing release of the N-degron from the binding pocket (Fig. 1C); however, because the NTE and N-degron–binding pocket are on opposite sides of the ClpS molecule, if the substrate were released far from the entrance to the ClpA pore, it would be poorly positioned for efficient pore capture. As discussed below, one possibility is that conformational changes in ClpS, caused by ClpA pulling, place the N-degron–binding pocket close to the entrance to the ClpA pore and allow transfer of the N-degron or nearby segments of the protein substrate (Fig. 6) (36).
Fig. 6.
Model for ClpA-dependent N-degron substrate transfer. (A) HADC. (B) ClpA-dependent translocation of the ClpS NTE begins to deform the ClpScore by pulling on the middle β1 strand of the three-stranded β-sheet. (C) Extraction of the β1 strand of ClpS facilitates substrate transfer by inverting the adaptor, thereby positioning the N-degron–binding pocket close to the ClpA pore, and by weakening interactions between the substrate and the ClpS-binding pocket. (D) Subsequently, ΔβClpS resists further unfolding and thus is released from the ternary complex, allowing for refolding of the adaptor and translocation and degradation of the N-degron substrate to commence.
One speculative model posits that NTE-tugging by ClpA both distorts and inverts ClpS by at least transiently pulling out the β strand proximal to the NTE (β1 strand), which is part of a three-stranded β sheet (Fig. 6 A–C). Pulling this central strand out of the sheet and into the ClpA pore would flip the remaining ∆βClpS structure relative to ClpA (Fig. 6C), positioning the N-degron–binding pocket close to the axial pore for transfer (Fig. 6 B–D). In this model, ∆βClpS remains stably folded but has reduced N-degron affinity, facilitating transfer of the substrate to ClpA. This model also requires that ∆βClpS not be globally denatured and degraded by ClpAP, because it has been established that ClpS is not degraded during substrate delivery (19). There is precedent for this type of β strand extraction by AAA+ unfoldases; for example, we note that ClpXP initially extracts a terminal β strand from a sheet in GFP-ssrA without causing global unfolding (37, 38). Moreover, under some conditions, the extracted β strand appears to slip from the pore of the AAA+ unfoldase, allowing refolding to native GFP (38). For the ClpS-delivery model, we suggest that following transfer of the N-degron, a slipping event could also allow ∆βClpS to refold and thus restore native ClpS. This reaction would reinvert the structure and favor ClpS escape, because its affinity for ClpA is weaker without bound N-degron (19).
A strong prediction of any NTE-tugging model is that an NTE would only promote delivery of a substrate bound to the same ClpS molecule and would not influence delivery of substrates bound to different molecules of ClpS, even if they were bound to the same ClpA hexamer. Our results strongly support this cis-only aspect of ClpS NTE function, demonstrating that only N-degron substrates bound to a ClpS molecule with a functional NTE were degraded by ClpAP. These results support an NTE-pulling model and argue against models in which the NTE serves simply as an allosteric activator of ClpA (23). During substrate transfer, both the ClpS NTE and the N-terminal residues of the N-degron substrate may need to occupy the ClpA pore. We assume that these multiple polypeptide chains can be accommodated in the ClpA pore, given that experiments with the related ClpXP enzyme show that pore engagement of multiple polypeptides is possible (39).
Parallels can be drawn between our active handoff model and other protein-degradation systems. For example, the SspB adaptor delivers ssrA-tagged substrates to the ClpXP protease via the formation of a high-affinity ternary complex that involves interactions among SspB dimers, the N-domain(s) of ClpX, and a segment of the ssrA-degron (5, 32, 33). In this case, the complex is broken, and initiation of substrate degradation proceeds when the ClpX translocation pore engages the ssrA-degron (5, 32, 35, 40). Translocation of this initiation region of the substrate serves to break interactions in the ternary complex, allowing degradation to begin and the adaptor to be recycled. An unstructured initiation region is also required for unfolding and degradation by the proteasome (41–43). Proteins are targeted to the proteasome by a two-part degradation signal consisting of a disordered region within the substrate and a polyubiquitin tag. The proteasome recognizes the ubiquitin tag and initiates unfolding at the unstructured region within the substrate. Once the proteasome has engaged its substrate, the polyubiquitin tag is cleaved off by deubiquitination enzymes, allowing recycling of ubiquitin. For ClpXP-SspB degradation of ssrA-tagged proteins and degradation of substrates by the proteasome, disassembly of the complex occurs when the initiation region on the substrate is engaged. In contrast, for ClpS-mediated delivery, the unstructured region required for complex disassembly is instead provided by the adaptor, which in turn is recycled as it escapes degradation (Fig. 6); however, an initiation region in the substrate is also necessary for transfer to ClpA and substrate unfolding (36, 44). In the case of N-degron substrates, this dual-initiation active handoff allows delivery of substrates whose degron is a single N-terminal amino acid recognized with high affinity by the ClpS adaptor but only with low affinity by ClpA.
Materials and Methods
Proteins and Peptides.
Mutants were generated by the QuikChange method (Stratagene) or PCR. ClpS, ClpS mutants, and substrates were purified as described previously (19). In brief, ClpS, ClpS mutants, and substrates were initially fused to the C terminus of H6-Sumo in pet23b (Novagen). After expression, fusion proteins were purified by Ni-NTA chromatography (Qiagen) and cleaved with Ulp1 protease. The cleaved H6-Sumo fragment was removed by passage through Ni-NTA, and the protein of interest was purified by gel filtration on a Superdex 75 column and/or ion-exchange chromatography on MonoQ column (GE Healthcare). ClpS variants were concentrated and stored in 20 mM Hepes (pH 7.5), 150 mM KCl, 1 mM DTT, and 10% glycerol. ClpA, ClpP, and ClpPED were purified as described previously (19). Because ClpS variants were purified using the Sumo-fusion and Ulp1 cleavage method, the N-terminal methionine of ClpS should be present. Previous publications have reported the use of either Sumo-cleavage or native expression for ClpS variants (19, 23). The N-degron (llfvqrdskec) and N*-degron (ilyvqrdekec) peptides were synthesized by standard Fmoc techniques using an Apex 396 solid-phase instrument.
Fluorescent Labeling.
Peptides were labeled with fluorescein maleimide as described previously (20). Labeled ClpS variants and ClpPED were labeled with fluorescein maleimide and EDANS maleimide, respectively, as described previously (19). In brief, ClpS variants (50 μM) and ClpP containing a single cysteine were incubated with 50 mM DTT in 100 mM TrisCl (pH 8) for 1.5 h at 4 °C, then buffer-exchanged into 100 mM Na2PO4 (pH 8) and 1 mM EDTA. The variants were then singly labeled by the addition of 0.3 mg/mL fluorescein maleimide or EDANS maleimide (Thermo Scientific) for 2 h at room temperature in the dark. Excess reagent was removed by size-exclusion chromatography, and the modified protein was stored in 10 mM Hepes (pH 7.5), 200 mM KCl, and 1 mM DTT.
FRET Experiments.
FRET experiments were performed using a Photon Technology International fluorimeter. ClpA6 (200 nM), ClpPED (200 nM), ClpS* variants (200 nM), N-degron substrate ylfvq-titin I27 (500 nM) (44), ATPγS (2 mM), and AT-Quencher (10 μM), when necessary, were incubated for 10 min at 30 °C in reaction buffer (50 mM Hepes [pH 7.5], 300 mM NaCl, 20 mM MgCl2, 0.5 mM DTT, and 10% glycerol) at 30 °C before obtaining a spectrum. Samples were excited at 336 nm, and emission scans were obtained from 400 to 600 nm.
Degradation Assays and Western Blot Analysis.
ClpAPS degradation assays were performed as described previously (44). In brief, ClpA6 (100 nM), ClpP14 (200 nM), and ClpS variants (1 μM) were preincubated in reaction buffer (50 mM Hepes [pH 7.5], 300 mM NaCl, 20 mM MgCl2, 0.5 mM DTT, and 10% glycerol) with ylfvqela-GFP or vlfvqela-GFP (1 µM) and methotrexate (10 μM, Sigma-Aldrich), when necessary, for 3 min at 30 °C, followed by the addition of ATP regeneration mix (8 mM ATP, 50 mg/mL creatine kinase, and 5 mM creatine phosphate) or ATPγS (2 mM) to initiate the assay. GFP degradation was assayed by loss of fluorescence. Reported kinetic parameters were averages (n ≥3) ± 1 SD. Formation of ClpS truncation products was monitored by SDS/PAGE and Western blot analysis as described previously (23). In brief, samples were separated by SDS/PAGE, followed by an anti-ClpS Western blot. For cis/trans experiments, degradation assays were conducted under the same conditions but with 1.2 µM ClpS or ClpS variants and 1 µM Phe-Val dipeptide.
Binding Assays.
Binding assays, monitored by fluorescence anisotropy, were performed using a Photon Technology International fluorimeter. Data were fitted using a quadratic equation for tight binding. Reported Kapp values are averages (n ≥3), with errors calculated as SQRT([K − Kavg]2/n).
Supplementary Material
Acknowledgments
We thank A. Olivares, B. Stinson, J. Kardon, B. Stein, A. Torres-Delgado, S. Calmat, V. Baytshtok, and J. Grabenstatter for helpful discussions and advice, and E. Weber-Ban for providing the mutated active site ClpP construct. This work was supported in part by National Institutes of Health Pre-Doctoral Training Grant T32GM007287, the Howard Hughes Medical Institute, and National Institutes of Health Grants GM-49224 and Al-16892. T.A.B. is an employee of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
References
- 1.Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9(1):27–43. [PubMed] [Google Scholar]
- 2.Hanson PI, Whiteheart SW. AAA+ proteins: Have engine, will work. Nat Rev Mol Cell Biol. 2005;6(7):519–529. doi: 10.1038/nrm1684. [DOI] [PubMed] [Google Scholar]
- 3.Gottesman S, Maurizi MR. Regulation by proteolysis: Energy-dependent proteases and their targets. Microbiol Rev. 1992;56(4):592–621. doi: 10.1128/mr.56.4.592-621.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sauer RT, Baker TA. AAA+ proteases: ATP-fueled machines of protein destruction. Annu Rev Biochem. 2011;80:587–612. doi: 10.1146/annurev-biochem-060408-172623. [DOI] [PubMed] [Google Scholar]
- 5.Wah DA, Levchenko I, Baker TA, Sauer RT. Characterization of a specificity factor for an AAA+ ATPase: assembly of SspB dimers with ssrA-tagged proteins and the ClpX hexamer. Chem Biol. 2002;9(11):1237–1245. doi: 10.1016/s1074-5521(02)00268-5. [DOI] [PubMed] [Google Scholar]
- 6.Dougan DA, Reid BG, Horwich AL, Bukau B. ClpS, a substrate modulator of the ClpAP machine. Mol Cell. 2002;9(3):673–683. doi: 10.1016/s1097-2765(02)00485-9. [DOI] [PubMed] [Google Scholar]
- 7.Mogk A, et al. Broad yet high substrate specificity: The challenge of AAA+ proteins. J Struct Biol. 2004;146(1-2):90–98. doi: 10.1016/j.jsb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Baker TA, Sauer RT. ATP-dependent proteases of bacteria: Recognition logic and operating principles. Trends Biochem Sci. 2006;31(12):647–653. doi: 10.1016/j.tibs.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachmair A, Finley D, Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986;234(4773):179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- 10.Varshavsky A. Discovery of cellular regulation by protein degradation. J Biol Chem. 2008;283(50):34469–34489. doi: 10.1074/jbc.X800009200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tobias JW, Shrader TE, Rocap G, Varshavsky A. The N-end rule in bacteria. Science. 1991;254(5036):1374–1377. doi: 10.1126/science.1962196. [DOI] [PubMed] [Google Scholar]
- 12.Bachmair A, Finley D, Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986;234(4473):179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- 13.Wang KH, Sauer RT, Baker TA. ClpS modulates but is not essential for bacterial N-end rule degradation. Genes Dev. 2007;21(4):403–408. doi: 10.1101/gad.1511907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cranz-Mileva S, et al. The flexible attachment of the N-domains to the ClpA ring body allows their use on demand. J Mol Biol. 2008;378(2):412–424. doi: 10.1016/j.jmb.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 15.Effantin G, Ishikawa T, De Donatis GM, Maurizi MR, Steven AC. Local and global mobility in the ClpA AAA+ chaperone detected by cryo-electron microscopy: Functional connotations. Structure. 2010;18(5):553–562. doi: 10.1016/j.str.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang F, et al. Structure and mechanism of the hexameric MecA-ClpC molecular machine. Nature. 2011;471(7338):331–335. doi: 10.1038/nature09780. [DOI] [PubMed] [Google Scholar]
- 17.Lupas AN, Koretke KK. Bioinformatic analysis of ClpS, a protein module involved in prokaryotic and eukaryotic protein degradation. J Struct Biol. 2003;141(1):77–83. doi: 10.1016/s1047-8477(02)00582-8. [DOI] [PubMed] [Google Scholar]
- 18.Tasaki T, Kwon YT. The mammalian N-end rule pathway: New insights into its components and physiological roles. Trends Biochem Sci. 2007;32(11):520–528. doi: 10.1016/j.tibs.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Román-Hernández G, Hou JY, Grant RA, Sauer RT, Baker TA. The ClpS adaptor mediates staged delivery of N-end rule substrates to the AAA+ ClpAP protease. Mol Cell. 2011;43(2):217–228. doi: 10.1016/j.molcel.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang KH, Roman-Hernandez G, Grant RA, Sauer RT, Baker TA. The molecular basis of N-end rule recognition. Mol Cell. 2008;32(3):406–414. doi: 10.1016/j.molcel.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeth K, et al. Structural analysis of the adaptor protein ClpS in complex with the N-terminal domain of ClpA. Nat Struct Biol. 2002;9(12):906–911. doi: 10.1038/nsb869. [DOI] [PubMed] [Google Scholar]
- 22.Zeth K, Dougan DA, Cusack S, Bukau B, Ravelli RB. Crystallization and preliminary X-ray analysis of the Escherichia coli adaptor protein ClpS, free and in complex with the N-terminal domain of ClpA. Acta Crystallogr D Biol Crystallogr. 2002;58(Pt 7):1207–1210. doi: 10.1107/s0907444902006960. [DOI] [PubMed] [Google Scholar]
- 23.Hou JY, Sauer RT, Baker TA. Distinct structural elements of the adaptor ClpS are required for regulating degradation by ClpAP. Nat Struct Mol Biol. 2008;15(3):288–294. doi: 10.1038/nsmb.1392. [DOI] [PubMed] [Google Scholar]
- 24.De Donatis GM, Singh SK, Viswanathan S, Maurizi MR. A single ClpS monomer is sufficient to direct the activity of the ClpA hexamer. J Biol Chem. 2010;285(12):8771–8781. doi: 10.1074/jbc.M109.053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolygo K, et al. Studying chaperone-proteases using a real-time approach based on FRET. J Struct Biol. 2009;168(2):267–277. doi: 10.1016/j.jsb.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Hamman BD, Chen JC, Johnson EE, Johnson AE. The aqueous pore through the translocon has a diameter of 40–60 Å during cotranslational protein translocation at the ER membrane. Cell. 1997;89(4):535–544. doi: 10.1016/s0092-8674(00)80235-4. [DOI] [PubMed] [Google Scholar]
- 27.Lee C, Schwartz MP, Prakash S, Iwakura M, Matouschek A. ATP-dependent proteases degrade their substrates by processively unraveling them from the degradation signal. Mol Cell. 2001;7(3):627–637. doi: 10.1016/s1097-2765(01)00209-x. [DOI] [PubMed] [Google Scholar]
- 28.Wahl MC, Will CL, Lührmann R. The spliceosome: Design principles of a dynamic RNP machine. Cell. 2009;136(4):701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Vale RD. AAA proteins: Lords of the ring. J Cell Biol. 2000;150(1):F13–F19. doi: 10.1083/jcb.150.1.f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levchenko I, Seidel M, Sauer RT, Baker TA. A specificity-enhancing factor for the ClpXP degradation machine. Science. 2000;289(5488):2354–2356. doi: 10.1126/science.289.5488.2354. [DOI] [PubMed] [Google Scholar]
- 31.Wah DA, Levchenko I, Baker T, Sauer R. Characterization of a specificity factor for an AAA+ ATPase: Assembly of SspB dimers with ssrA-tagged proteins and the ClpX hexamer. Chem Biol. 2002;9(11):1237–1245. doi: 10.1016/s1074-5521(02)00268-5. [DOI] [PubMed] [Google Scholar]
- 32.Bolon DN, Wah DA, Hersch GL, Baker TA, Sauer RT. Bivalent tethering of SspB to ClpXP is required for efficient substrate delivery: A protein-design study. Mol Cell. 2004;13(3):443–449. doi: 10.1016/s1097-2765(04)00027-9. [DOI] [PubMed] [Google Scholar]
- 33.Levchenko I, Grant RA, Wah DA, Sauer RT, Baker TA. Structure of a delivery protein for an AAA+ protease in complex with a peptide degradation tag. Mol Cell. 2003;12(2):365–372. doi: 10.1016/j.molcel.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 34.Martin A, Baker TA, Sauer RT. Diverse pore loops of the AAA+ ClpX machine mediate unassisted and adaptor-dependent recognition of ssrA-tagged substrates. Mol Cell. 2008;29(4):441–450. doi: 10.1016/j.molcel.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolon DN, Grant RA, Baker TA, Sauer RT. Nucleotide-dependent substrate handoff from the SspB adaptor to the AAA+ ClpXP protease. Mol Cell. 2004;16(3):343–350. doi: 10.1016/j.molcel.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Erbse A, et al. ClpS is an essential component of the N-end rule pathway in Escherichia coli. Nature. 2006;439(7077):753–756. doi: 10.1038/nature04412. [DOI] [PubMed] [Google Scholar]
- 37.Martin A, Baker TA, Sauer RT. Protein unfolding by a AAA+ protease is dependent on ATP-hydrolysis rates and substrate energy landscapes. Nat Struct Mol Biol. 2008;15(2):139–145. doi: 10.1038/nsmb.1380. [DOI] [PubMed] [Google Scholar]
- 38.Nager AR, Baker TA, Sauer RT. Stepwise unfolding of a β barrel protein by the AAA+ ClpXP protease. J Mol Biol. 2011;413(1):4–16. doi: 10.1016/j.jmb.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burton RE, Siddiqui SM, Kim YI, Baker TA, Sauer RT. Effects of protein stability and structure on substrate processing by the ClpXP unfolding and degradation machine. EMBO J. 2001;20(12):3092–3100. doi: 10.1093/emboj/20.12.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGinness KE, Bolon DN, Kaganovich M, Baker TA, Sauer RT. Altered tethering of the SspB adaptor to the ClpXP protease causes changes in substrate delivery. J Biol Chem. 2007;282(15):11465–11473. doi: 10.1074/jbc.M610671200. [DOI] [PubMed] [Google Scholar]
- 41.Inobe T, Fishbain S, Prakash S, Matouschek A. Defining the geometry of the two-component proteasome degron. Nat Chem Biol. 2011;7(3):161–167. doi: 10.1038/nchembio.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inobe T, Matouschek A. Paradigms of protein degradation by the proteasome. Curr Opin Struct Biol. 2014;24:156–164. doi: 10.1016/j.sbi.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prakash S, Tian L, Ratliff KS, Lehotzky RE, Matouschek A. An unstructured initiation site is required for efficient proteasome-mediated degradation. Nat Struct Mol Biol. 2004;11(9):830–837. doi: 10.1038/nsmb814. [DOI] [PubMed] [Google Scholar]
- 44.Wang KH, Oakes ESC, Sauer RT, Baker TA. Tuning the strength of a bacterial N-end rule degradation signal. J Biol Chem. 2008;283(36):24600–24607. doi: 10.1074/jbc.M802213200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kress W, Mutschler H, Weber-Ban E. Assembly pathway of an AAA+ protein: Tracking ClpA and ClpAP complex formation in real time. Biochemistry. 2007;46(21):6183–6193. doi: 10.1021/bi602616t. [DOI] [PubMed] [Google Scholar]