Significance
A salient feature of the immune system is its ability to discriminate self from nonself. We define the molecular mechanism governing activation of an ancient and central component: the lectin pathway of complement. The basis is the association of two proteases in distinct complexes with at least five pattern recognition molecules. Clustering of these complexes on ligand surfaces allows cross-activation of the proteases, which subsequently activate downstream factors to initiate a proteolytic cascade. This is conceptually similar to signaling by cellular receptors and could be viewed as cellular signaling turned inside out. Different pattern recognition complexes “talk to each other” to coordinate immune activation, which may impart differential activation based on recognition of simple vs. complex ligand patterns.
Keywords: innate immunity, collectin, inflammation, homeostasis
Abstract
Defining mechanisms governing translation of molecular binding events into immune activation is central to understanding immune function. In the lectin pathway of complement, the pattern recognition molecules (PRMs) mannan-binding lectin (MBL) and ficolins complexed with the MBL-associated serine proteases (MASP)-1 and MASP-2 cleave C4 and C2 to generate C3 convertase. MASP-1 was recently found to be the exclusive activator of MASP-2 under physiological conditions, yet the predominant oligomeric forms of MBL carry only a single MASP homodimer. This prompted us to investigate whether activation of MASP-2 by MASP-1 occurs through PRM-driven juxtaposition on ligand surfaces. We demonstrate that intercomplex activation occurs between discrete PRM/MASP complexes. PRM ligand binding does not directly escort the transition of MASP from zymogen to active enzyme in the PRM/MASP complex; rather, clustering of PRM/MASP complexes directly causes activation. Our results support a clustering-based mechanism of activation, fundamentally different from the conformational model suggested for the classical pathway of complement.
Complement is a central component of humoral immunity (1). Activation of the classical pathway occurs through ligand binding of complement component C1q, inducing a conformational change in the C1qC1r2C1s2 complex and causing complement component C1r to autoactivate and subsequently activate C1s, which in turn cleaves complement components C4 and C2 (2, 3). The lectin pathway proteins are similar, prompting suggestions of a similar mode of activation (4, 5). However, important differences defy this simple analogy. Five different pattern recognition molecules (PRMs)—mannan-binding lectin (MBL); H-, L- and M-ficolin (also known as Ficolin-3, -2, and -1, respectively); and collectin-kidney 1 (CL-K1)—associate with MBL-associated serine proteases (MASP)-1 and -2 to activate complement. In addition, CL-K1 and the related collectin-liver 1 (CL-L1) form heteromers that also associate with MASPs and activate complement (6). The PRMs are highly polydisperse oligomers of homotrimeric subunits, whereas C1q is a hexamer of heterotrimeric subunits. In contrast to the C1r2C1s2 tetramer, which associates with C1q to form the C1 complex, MASP homodimers independently associate with PRMs, and the predominant oligomers of MBL in serum carry only a single MASP homodimer (7, 8). Whereas C1r cleaves only C1s, MASP-1 cleaves both MASP-2 and C2, and MASP-2, like C1s, cleaves C4 and C2 (9–11).
MASP-1 and MASP-2 must be brought in close proximity during activation to cooperate. In circulation they associate with distinct oligomeric forms of MBL, indicating their spatial separation at homeostasis (7). Although MASP-2 is able to autoactivate (12), a role of MASP-1 in activating MASP-2 suggested an intercomplex activation mechanism as opposed to the intracomplex mechanism of C1 (13, 14). This potential mode of activation was never examined experimentally. The recent demonstration that MASP-1 is the exclusive activator of MASP-2 under physiological conditions (15, 16) prompted us to investigate whether activation of MASP-2 by MASP-1 occurs through juxtaposition of distinct PRM complexes on ligand surfaces. Our results support a clustering-based mechanism of activation for the lectin pathway, fundamentally different from the classical pathway.
Results
Tetrameric MBL Does Not Allow Formation of Cocomplexes of MASPs, but Supports Complement Activation.
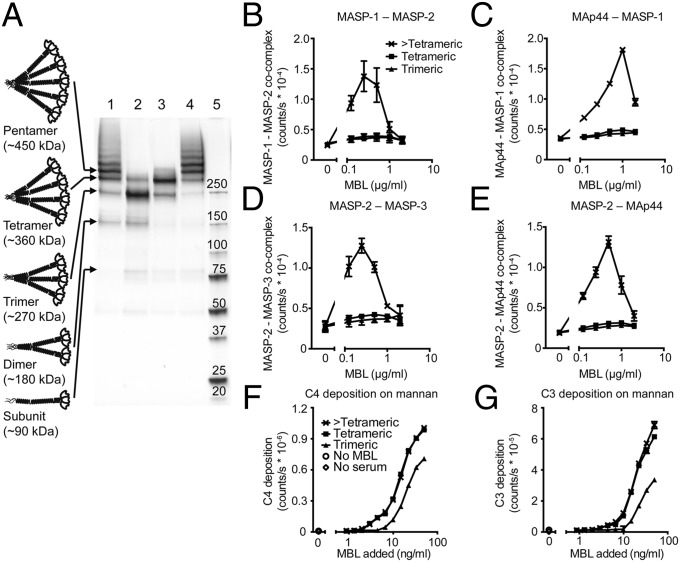
Serum MBL is a polydisperse mixture of oligomers of a trimeric subunit, the most predominant (∼70% of total) forms being trimers and tetramers (9 and 12 polypeptide chains) (7) (Fig. S1 A–C). Previous data indicated no difference in MASP binding between these two forms and suggested that they bind only a single MASP homodimer (8). If MASP-1 and MASP-2 were unable to colocalize in tetrameric or smaller PRM complexes, and this was prerequisite to complement activation, the function of the most abundant MBL forms would be left unaccounted for.
Polydisperse recombinant human MBL was fractionated into (i) mainly trimers, (ii) mainly tetramers, and (iii) higher-order oligomers (Fig. 1A and Fig. S1 D and E) and then analyzed for capacity to support formation of cocomplexes of MASP homodimers. Only higher-order oligomers had this ability (Fig. 1 B–E). The nature of these complexes was confirmed based on the requirement for calcium and sensitivity to high ionic strength (Fig. S1 F–I). We compared the complement-activating potential of the highest oligomer able to associate with only a single dimer (tetramer) and the lowest oligomer able to associate with two dimers (>tetramer), minimizing differences in ligand avidity. Mannan, a polysaccharide from the cell wall of Saccharomyces cerivisiae, is a strong ligand for MBL. The ability of tetrameric and higher-order oligomeric MBL to reconstitute complement activation in MBL-deficient human serum on mannan was assayed. Both supported C4 and C3 fragment deposition (Fig. 1 F and G), indicating that colocalization of MASP-1 and MASP-2 in the same MBL complex is not required for activation. There was a vast difference in the capacity of tetrameric and >tetrameric MBL fractions to support cocomplex formation (Fig. 1 B–E), yet minute amounts of cocomplexes formed by higher-order oligomeric MBL in the tetrameric fraction (Fig. 1A) could be initiating activation, subsequently propagating through intercomplex activation. The trimeric fraction contained mainly trimer, with a significant amount of tetramer and dimer, but undetectable pentamer and higher oligomers (Fig. S1 D and E). Although it had no capacity for cocomplex formation (Fig. 1 B–E), it supported complement activation, albeit to a somewhat lesser extent than the tetrameric fraction (Fig. 1 F and G). The lower capacity for activation could be explained by (i) the significant amount of dimer, which has no activity, and (ii) the significantly lower maximal binding capacity and higher dissociation rate constants for binding of carbohydrate ligands by trimeric MBL compared with tetrameric MBL (8).
Fig. 1.
Only higher-order oligomeric MBL supports cocomplex formation of MASPs, but both tetrameric and higher-order oligomeric forms of MBL can reconstitute the lectin pathway in MBL-deficient serum. (A) Silver stain of recombinant human MBL (lane 1) and purified fractions containing predominantly trimer (lane 2), tetramer (lane 3), and higher-order oligomeric forms (lane 4) of the trimeric subunit. Molecular size markers are indicated (lane 5), as are schematic structures of the oligomeric forms. Note that the recombinant human MBL has an oligomer distribution with predominance of higher oligomers compared with plasma-derived MBL. (B) Analysis of cocomplex formation between recombinant MASP-1 and MASP-2 afforded by trimeric, tetrameric, or >tetrameric MBL, measured by capture of complexes with anti–MASP-1 (5F5) and development with anti–MASP-2 (8B5). (C) As in B, but for MAp44 (2D5) and MASP-1 (rat 3). (D) As in B, but for MASP-2 (8B5) and MASP-3 (5F5). (E) As in B, but for MASP-2 (8B5) and MAp44 (5F5). Note that in B–E the trimeric and tetrameric symbols largely overlap. (F) C4 fragment deposition from an MBL-deficient serum as a function of reconstitution with increasing amounts of trimeric, tetrameric, or >tetrameric MBL. (G) As in F, but measuring C3 deposition. In F and G, the tetrameric and >tetrameric symbols overlap, as do “no MBL” and “no serum.” (B, C, D, and E) Mean and SD of four measurements in two experiments or (F and G) duplicates from one representative experiment of two.
Distinct MBL/MASP-1 and MBL/MASP-2 Complexes Cooperate on Ligand Surfaces.
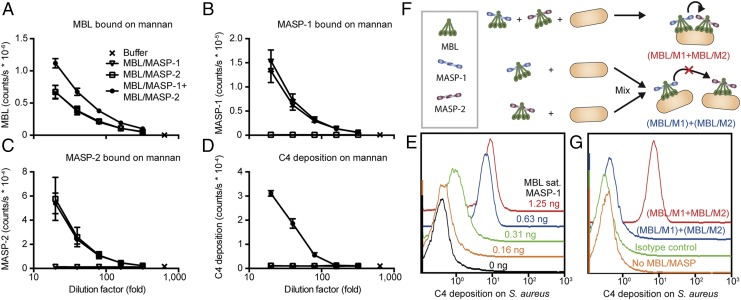
To directly examine intercomplex activation of MASPs by juxtaposition of distinct complexes of MBL/MASPs on activating surfaces, tetrameric MBL saturated with either MASP-1 or MASP-2 was assayed for C4 deposition capacity on mannan, individually or combined. Virtually no cocomplexes were formed with tetrameric MBL (Fig. 1 B–E), and presaturation of MBL with either MASP-1 or MASP-2 served to isolate each of the two MASPs on their own pool of tetrameric MBL. When both MASP-1 and MASP-2 were present, the tetrameric MBL mediated C4 activation (Fig. 2 A–D), indicating intercomplex activation. This did not preclude dynamic formation of cocomplexes on a minor contaminant of higher-order oligomeric MBL upon ligand binding, which could trigger activation.
Fig. 2.
Tetrameric MBL permits cooperation of MASP-1 and MASP-2 on a ligand surface. Complexes of tetrameric MBL with MASP-1, MASP-2, or the two combined were incubated on a mannan surface. In parallel was measured the level of MBL bound (A), MASP-1 bound (B), MASP-2 bound (C), and C4 fragments deposited after addition of purified C4 (D). Mean and SD are from four measurements in two experiments. In A and D, MBL/MASP-1 and MBL/MASP-2 overlap; in B, MBL/MASP-1 and MBL/MASP-1+MBL/MASP-2 overlap; and in C, MBL/MASP-2 and MBL/MASP-1+MBL/MASP-2 overlap. (E) Tetrameric MBL was saturated with MASP-1 or MASP-2. A fixed amount of MBL saturated with MASP-2 was added to S. aureus. MBL saturated with MASP-1 was titrated onto the bacteria. The bacteria were incubated with purified C4 and analyzed for C4 deposition by flow cytometry. Representative of three experiments. (F and G) MASP-1–saturated MBL and MASP-2–saturated MBL were incubated with S. aureus together (red curve) or separately followed by admixture (blue curve). Samples were incubated with purified C4 and analyzed by flow cytometry. Mouse IgG1k isotype control (green curve) and S. aureus without addition of MBL/MASP complexes (orange) were included. Representative of two experiments.
To approach the physiological scenario, we analyzed activation on the surface of Staphylococcus aureus, a clinically relevant human pathogen targeted by MBL (17). C4 deposition on S. aureus depended on cooperation between tetrameric MBL carrying MASP-1 and tetrameric MBL carrying MASP-2 (Fig. 2E). MBL binding and C4 deposition was inhibited by mannose or EDTA (Fig. S2). Asking whether cooperation of MASP-1 and MASP-2 was a consequence of juxtaposition of tetrameric MBL/MASP-1 and tetrameric MBL/MASP-2 on the same surface, rather than simply an effect of both MASP-1 and MASP-2 being present, we incubated S. aureus with both MBL/MASP-1 and MBL/MASP-2 or with either separately, followed by admixture of the two discrete populations (Fig. 2F). In the former setup, MBL/MASP-1 and MBL/MASP-2 are allowed to bind to the same bacteria, whereas in the latter, half the bacteria carry MBL/MASP-1 and half carry MBL/MASP-2. The latter combination was insufficient to support complement activation (Fig. 2G). We concluded that the driving force for activation was intercomplex activation because the absence of C4 deposition in the scenario with two discrete populations of bacteria with MBL/MASP-1 and MBL/MASP-2 demonstrated that no exchange of complexes or dynamic formation of cocomplexes occurred.
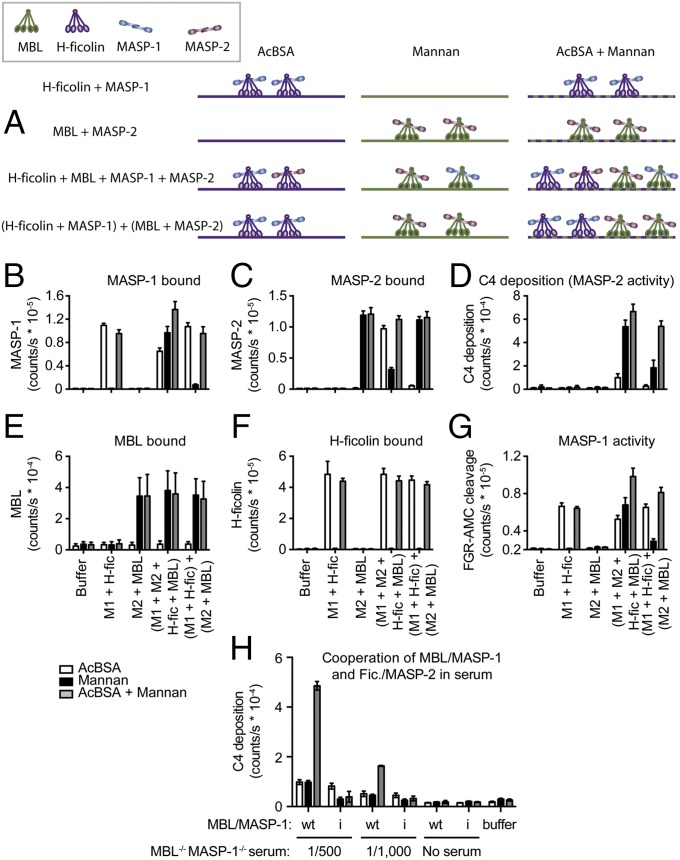
Distinct PRM/MASP Complexes Cooperate on a Mixed Ligand Surface.
The observation of inter–MBL-complex cooperation raised the question whether distinct PRM/MASP complexes in general cooperate on complex ligand surfaces. We examined cooperation of H-ficolin/MASP-1 and MBL/MASP-2 on a ligand surface presenting both acetylated groups [acetylated BSA (AcBSA)] binding H-ficolin and carbohydrate groups (mannan) binding MBL. Four types of PRM/MASP complexes were generated: H-ficolin/MASP-1; MBL/MASP-2; mixed complexes of MASP-1 and MASP-2 with H-ficolin and MBL; and, finally, pregenerated H-ficolin/MASP-1 combined with pregenerated MBL/MASP-2 (Fig. 3A). The ability of these four reagents to support C4 deposition was examined on AcBSA, mannan, or a combined AcBSA and mannan coat. At the same time, the amount of MASP-1, MASP-2, MBL, and H-ficolin bound, as well as MASP-1 activity (using a synthetic peptide substrate, methylsulfonyl-d-Phe-Gly-Arg-AMC, FGR-AMC), was measured. H-ficolin and MBL were detected only on surfaces containing their cognate ligands (Fig. 3 E and F). When MASP-1 was incubated with only H-ficolin, or preincubated with H-ficolin before mixing with preincubated MBL/MASP-2, MASP-1 was detected only on surfaces containing AcBSA (Fig. 3B). Similarly, MASP-2 was detected only on surfaces containing mannan, when incubated with MBL only or when preincubated with MBL before mixing with preincubated H-ficolin/MASP-1 (Fig. 3C). Thus, minimal exchange of MASPs occurred between preformed H-ficolin/MASP-1 and preformed MBL/MASP-2 complexes, upon PRM binding to ligand. When MASP-1, MASP-2, H-ficolin, and MBL were mixed, a blend of complexes resulted. Consequently, both MASP-1 and MASP-2 were bound on all three surfaces in this sample (Fig. 3 B and C), yielding MASP-1 enzymatic activity and C4 deposition on all three surfaces (Fig. 3 D and G ). H-ficolin/MASP-1 and MBL/MASP-2 samples served as controls; i.e., MASP-1 enzymatic activity was only on AcBSA surfaces (Fig. 3G), and neither sample yielded C4 deposition on any surface (Fig. 3D). When discrete H-ficolin/MASP-1 and MBL/MASP-2 complexes were present, full C4 activation was achieved only on the mixed ligand surface (Fig. 3D), demonstrating direct cooperation between discrete PRM/MASP complexes.
Fig. 3.
H-ficolin/MASP-1 and MBL/MASP-2 cooperate on mixed ligand surfaces, MASP-1 is able to activate MASP-2, and exogenous MBL/MASP-1 cooperates with endogenous H-ficolin/MASP-2 in human serum deficient in MBL and MASP-1. (A) MASP-1 was preincubated with H-ficolin (M1 + H-fic); MASP-2 was preincubated with MBL (M2 + MBL); or MASP-1 and MASP-2 was mixed with MBL and H-ficolin (M1 + M2 + H-fic + MBL). The H-fic + M1, MBL + M2, or these two mixed together [(M1 + H-fic) + (M2 + MBL)] and the (M1 + M2 + H-fic + MBL) sample were added to AcBSA, mannan, or AcBSA + mannan-coated wells. MASP-1 bound (B), MASP-2 bound (C), C4 fragment deposition (D), MBL bound (E), H-ficolin bound (F), and MASP-1 activity (G) were measured. Mean and SD of six measurements in three experiments. (H) Human serum deficient in MASP-1/-3/MAp44 and functional MBL was reconstituted with preformed MBL/MASP-1 or catalytically inactive MBL/MASP-1(Ser646Ala) complexes, incubated in wells coated with AcBSA, mannan, or AcBSA + mannan, and C4 deposition was measured. Mean and SD of four measurements in two experiments.
In a more physiological scenario, we tested the ability of preformed MBL/MASP-1 complexes to reconstitute C4 deposition in serum from an individual with a combined MASP1 gene defect and nonproducing MBL genotype and hence deficient in MASP-1, MASP-3, and MAp44 as well as functional MBL (15). Recent studies indicated that two collectins related to MBL, CL-K1, and CL-L1, or heteromers of these, can activate complement. However, when the serum was reconstituted with preformed MBL/MASP-1 complexes and C4 deposition was assayed, endogenous CL-K1/CL-L1/MASP-2 complexes were insufficient to cooperate with exogenous MBL/MASP-1 on the mannan surface (Fig. 3H). Thus, functionally, the serum contained only ficolin/MASP-2 complexes before reconstitution with the preformed MBL/MASP-1 complexes. On a combined mannan and AcBSA coat, the signal was markedly increased, demonstrating cooperation of exogenous MBL/MASP-1 and endogenous ficolin/MASP-2 complexes. Serum reconstituted with MBL complexed with catalytically inactive MASP-1 (rMASP-1i, Ser646Ala) and MBL/MASP-1 or MBL/MASP-1i without serum yielded no activation (Fig. 3H).
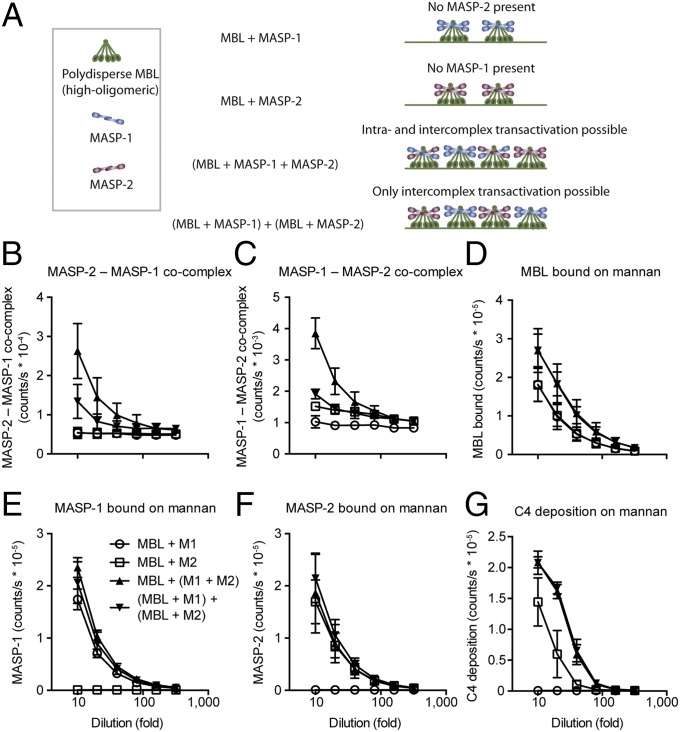
Intercomplex Cooperation Is the Main Driver of Activation.
We recently found that colocalization of MASP-1 and MASP-2 in higher-order oligomeric MBL complexes can drive activation of complement when complexes are bound on a ligand or antibody surface (18). However, in that study we neither demonstrated intracomplex activation directly nor ruled out activation between these complexes. Therefore, we sought to compare the scenario in which both intra- and intercomplex activation of MASP-2 by MASP-1 could occur with the scenario in which only intercomplex activation could occur. Four types of MBL/MASP complexes were generated: polydisperse (unfractionated) MBL saturated with MASP-1; with MASP-2; or with a mixture of MASP-1 and MASP-2; and a mixture of presaturated MBL/MASP-1 with presaturated MBL/MASP-2. The samples were added to mannan-coated wells and analyzed for C4 activation and the amounts of MASP-1, MASP-2, and MBL bound (Fig. 4A). In parallel, the samples were analyzed for formation of MASP-1–MASP-2 cocomplexes either by capture with anti–MASP-1 antibody and development with anti-MASP-2 or vice versa. Comparable amounts of MASP-1 and MASP-2 were bound on mannan for samples containing MASP-1 and MASP-2, respectively (Fig. 4 E and F). The MBL was saturated with MASP, and conditions were optimized to bind comparable amounts of each MASP, so twice as much MBL was bound for samples containing both MASPs as for samples containing either MASP (Fig. 4D). Some cocomplexes formed when preformed MBL/MASP-1 complexes were mixed with preformed MBL/MASP-2 complexes, but to lesser extent than when MBL was mixed with MASP-1 and MASP-2 (Fig. 4 B and C). C4 cleaving activity was absent for MBL/MASP-1 complexes alone, whereas it was intermediate for MBL/MASP-2 complexes alone. Of note, MASP-2 was present at unphysiologically high concentration, comparable to that of MASP-1, explaining its partial self-sufficiency. Despite the markedly lower level of MASP-1–MASP-2 cocomplexes when preformed MBL/MASP-1 complexes were mixed with preformed MBL/MASP-2 complexes, C4 activation was indistinguishable from that of MBL complexes formed with a mixture of MASP-1 and MASP-2 (Fig. 4G). This suggested that the driving force in activation is the colocalization of MASP-1 and MASP-2, whether by cocomplex formation (higher MBL oligomers) or juxtaposition on ligand surfaces (both lower and higher oligomers). This was observed despite the use of MBL containing predominantly higher-than-physiological oligomers, favoring a potential effect of intracomplex activation.
Fig. 4.
Intra- and intercomplex vs. intercomplex-only activation scenarios are equivalent. (A) Polydisperse MBL was saturated with either MASP-1 or MASP-2 or a mixture of the two. This does not allow cooperation of MASP-1 and MASP-2 (either alone with MBL), allows both intra- and intercomplex activation (MBL + MASP-1 + MASP-2), or allows intercomplex activation only [(MBL + MASP-1) + (MBL + MASP-2)]. Note that the potential for intercomplex activation between the two latter conditions is not significantly different when one considers the 3D arrangement of activating complexes on a ligand surface (see Fig. 6D). Hence comparison of these two allows dissection of the contribution of intracomplex activation. The samples were incubated in anti–MASP-2–coated (B), anti–MASP-1–coated (C), or mannan-coated (D, E, F, and G) wells. Wells were analyzed for cocomplexes of MASP-2–MASP-1 (8B5–5F5) (B), MASP-1–MASP-2 (5F5–8B5) (C), MBL bound on mannan (D), MASP-1 bound on mannan (E), MASP-2 bound on mannan (F), and C4 deposited on mannan (G). Mean and SD of six measurements in three experiments. In B, MBL + M1 and MBL + M2 overlap; in C, all curves except for MBL + M1 overlap at high dilution; in D, MBL + M1 and MBL + M2, and MBL + (M1 + M2) and (MBL + M1) + (MBL + M2), overlap pairwise; in E, all curves except for MBL + M2 overlap; in F, all curves except for MBL + M1 overlap; and in G, MBL + (M1 + M2) and (MBL + M1) + (MBL + M2) overlap.
MASP-1 Activates in a Concentration-Dependent Manner.
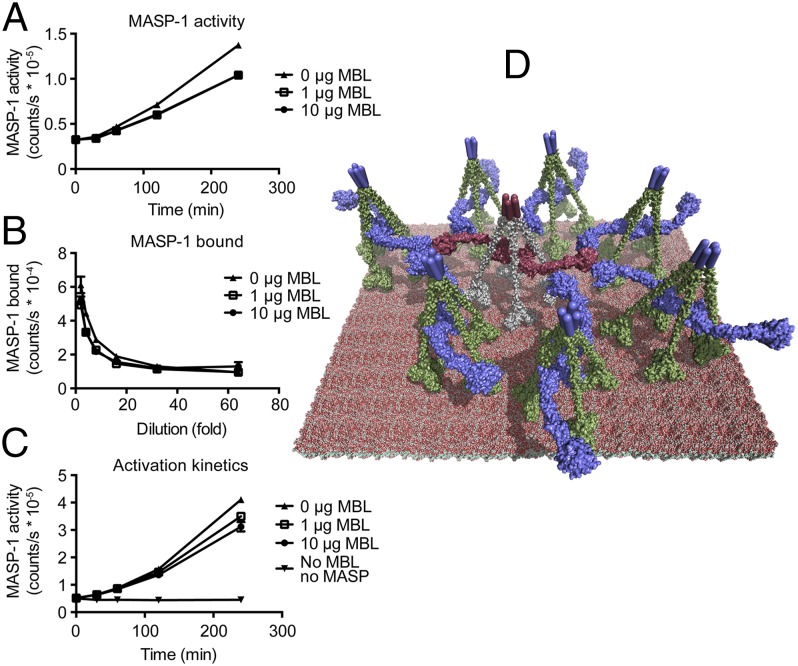
The observation of juxtapositional activation on ligand surfaces, rather than intracomplex activation, suggested that the lectin pathway activation mechanism differs from that of the classical pathway. We analyzed the autoactivating capacity of MASP-1 in the context of PRM complexes under various conditions. Our results from the experiments with activation of discrete MBL/MASP or PRM/MASP complexes indicated that the PRM/MASP complex binding and activation events could be separated in time (Fig. S3). We directly examined the importance of the sequence of binding events: preformed MBL/MASP complexes binding to mannan versus MASP binding to MBL already bound to mannan. Assuming a conformational activation mechanism driven by glycan binding, one would expect that only preformed complexes of MBL/MASP could activate. If MBL were already bound to mannan, the conformational drive and binding energy released by ligand recognition would dissipate before interaction of MASP with MBL. However, the two scenarios were equivalent (Fig. 5 A–C), indicating that PRM ligand binding does not directly escort the MASP from zymogen to active conformation.
Fig. 5.
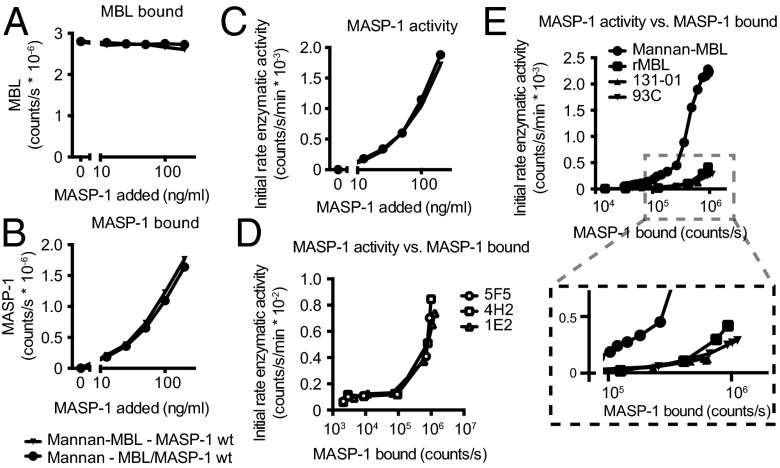
Activation of MBL/MASP-1 on mannan is independent of sequence of binding events, and MASP-1 activates in a concentration-dependent manner. MASP-1 was added to mannan-coated wells preincubated with MBL, or MASP-1 preincubated with MBL was added to mannan-coated wells. At the same time, MBL bound (A), MASP-1 bound (B), and MASP-1 activity (C) was measured. Mean and SD are from duplicate measurements in one of two experiments. Note that the curves overlap. (D) A dilution series of MASP-1 was added to wells coated with either of three anti–MASP-1 antibodies (5F5, 4H2, and 1E2), and at the same time the level of MASP-1 activity and MASP-1 bound (detecting with a fourth anti–MASP-1 antibody) was measured. Representative experiment of two, with minor variations in setup. Note that the curves overlap. (E) As in D, but for wells containing mannan-MBL, directly coated MBL (rMBL), or MBL bound to either of two anti-MBL antibodies (131-01 or 93C). Representative experiment of two, with minor differences in setup. Note that rMBL, 131–01, and 93C largely overlap.
We asked whether activation of MASP-1 could be simply driven by clustering, akin to the mechanism governing signaling through many cellular receptors. Microtiter wells were coated with different antibodies toward MASP-1 and then incubated with zymogen MASP-1, and activation of the zymogen was assayed by catalytic activity toward FGR-AMC. MASP-1 activated in a straightforward concentration-dependent manner on the various antibody capture coats (Fig. 5D). Two anti-MBL capture coats, MBL directly coated, and MBL bound to mannan also demonstrated a concentration-dependent activation of MASP-1. However, per amount of MASP-1 bound, the MBL-mannan surface proved a more potent activator (Fig. 5E). We confirmed that substrate cleavage was a function of the catalytic activity of activated MASP-1 by assaying in parallel the activity of WT MASP-1 vs. MASP-1i and a constitutive zymogen form of MASP-1 (MASP-1 Arg448Gln), and the activation on anti-MBL coats was dependent on MBL (Fig. S4A). The finding was generalized from surface clustering to cross-linking in solution, with the observation that cross-linking of MASP-1 in solution by mouse monoclonal anti–MASP-1 and a cross-linking anti-mouse Ig antibody, as well as cross-linking of MBL/MASP-1 or H-ficolin/MASP-1 by anti-MBL and anti–H-ficolin, respectively, could also drive activation (Fig. S4 B–D).
Structural Model for Activation of the Lectin Pathway.
Our results suggested a fundamentally different mechanism of activation of the lectin pathway from that inferred by analogy with the classical pathway (3–5). Although it is unlikely that directly coated MBL, MBL captured on either of two different antibody coats, as well as MBL cross-linked in solution and H-ficolin cross-linked in solution (Fig. 5 D and E and Fig. S4 A–D), could all induce a conformational change similar to ligand binding and hence drive activation; we sought to examine this possibility. A conformational model would predict that even if MASP-1 had an activity in isolation, it would be inactive when in complex with nonligand-bound MBL and would only assume an active conformation following a conformational change in the complex driven by MBL binding to ligand. In a recent model (5), MASP-1 was tucked away inside the cone created by the collagen stems of MBL and hence should not be accessible for antibody binding. To test this, we either incubated MASP-1 alone or preformed complexes of excess tetrameric MBL with MASP-1 in wells coated with anti–MASP-1 antibody directed toward the CCP1 domain. Wells were developed in parallel with MASP-1 substrate and anti–MASP-1 antibody reacting with the C-terminal part of the SP domain. MASP-1 was readily available for capture through CCP1, even when in complex with MBL, and readily activated (Fig. 6 A and B). A slightly stronger activation seen for free MASP-1 (Fig. 6A) was paralleled by a slightly higher degree of capture (Fig. 6B), likely due to absence of sterical hindrance by MBL. However, during incubation on the antibody capture coat, MASP-1 binding by anti–MASP-1 could drive a shift in equilibrium between free MASP-1 and MBL/MASP-1 or a shift in equilibrium between spontaneously dynamically exposed MASP-1 in the MBL/MASP-1 complex and masked MASP-1 in the MBL/MASP-1 complex. To assess this, we performed kinetic measurements of MASP-1 activation in absence of the preincubation step on anti–MASP-1. Activation of free MASP-1 was only marginally higher than that of preformed MBL/MASP-1 complexes, indicating that MASP-1 in complex with MBL in solution is largely exposed for antibody capture, supporting the notion that clustering directly drives activation (Fig. 6C). We ruled out any interfering preactivation or catalytic activities (Fig. S5).
Fig. 6.
Model for intercomplex activation of PRM/MASP. (A) MASP-1 enzymatic activity as a function of time following capture of MBL/MASP-1 complexes or free MASP-1 in wells coated with anti–MASP-1 CCP1. (B) Measurement of MASP-1 captured in wells in the setup presented in A. Representative experiment from two repeats with minor differences in setup. (C) MASP-1 enzymatic activity as function of time during capture of MBL/MASP-1 complexes or free MASP-1 in wells coated with anti–MASP-1 CCP1 (similar to A, but without preincubation on antibody capture coat and washing unbound MASP-1 away). Representative experiment from two repeats with minor differences in setup. Note that at early time points the curves overlap, and for B the curves largely overlap throughout. (D) Structure-based model of MBL transactivating complexes on a glycan surface. Seven MBL tetramers (green) harboring MASP-1 dimers (blue) clustered around one MBL tetramer (gray) binding a MASP-2 dimer (dark red) placed on an atomic model of the P. aeruginosa core LPS layer. Cylindrical rods at top of the MBL molecules represent their N-terminal disulfide bridged regions, for which no structural information is available.
The most parsimonious explanation for the superiority of ligand-bound MBL in activation of MASP-1 is that mannan provides a roughly planar ligand pattern, which orients MBL/MASP complexes and places the catalytic domains at a similar distance from the surface, facilitating interaction of neighboring MASPs. A complete molecular model of the core LPS layer of Pseudomonas aeruginosa has been simulated. Sugar groups recognizable by MBL are exposed terminally in a near-planar and dense glycan layer (19). This core layer can be modified by less abundant O-antigen glycosylations, lipid rafts, and embedded proteins, introducing nonplanarity, but this may be compensated by the flexibility and polydispersity of MBL. It therefore seems a reasonable assumption that neighboring MBL/MASP complexes are oriented roughly in a plane. We recently described a molecular model for tetrameric MBL in a 1:1 complex with a MASP homodimer and the corresponding MBL/MASP-2–C4 complex (20). In these models, MBL carbohydrate recognition domains are contained within a diameter of 240 Å, whereas the two catalytic sites in the protease domains of a MASP dimer are separated by 290 Å. Therefore, the catalytic sites are well accessible for their very large substrates C4, C4bC2, or a MASP protease domain on a neighboring zymogen MBL/MASP complex (Fig. 6D). Our model suggests that each MBL/MASP complex could be surrounded by several other complexes acting either as MASP activators or in C4/C4bC2 cleavage. Based on their relative abundance (Table S1), almost all C4-cleaving MASP-2 complexes will have a C4bC2-cleaving MASP-1 complex as the closest neighbor making substrate channeling between complexes efficient.
Discussion
We demonstrated here that discrete PRM/MASP complexes cooperate on ligand surfaces. Trimers and tetramers of MBL subunits associate with only one dimer of either MASP-1 or MASP-2, whereas higher oligomers associate with two dimers and may contain both MASP-1 and MASP-2. We saw no difference in activation between the situation where MASP-1 and MASP-2 were colocalized in the same MBL complex vs. the situation where they were localized in distinct complexes. The majority (∼70%) of serum MBL is trimeric or tetrameric, implying that intracomplex activation in higher-order oligomers is not a major driver of physiological activation. This agrees with observations that, in blood, MASP-1 and MAp19 are predominantly associated with trimeric MBL, whereas MASP-2 and MASP-3 associate preferentially with tetrameric MBL (7). Thus, intercomplex activation appears a prerequisite for physiological activation of the cascade, yet we cannot exclude that intracomplex activation in higher-order oligomers also plays a role. We recently demonstrated that colocalization of MASP-1 and -2 in higher-order oligomeric MBL complexes can drive activation when complexes are bound on a ligand or antibody surface (18). In light of the present observations, we conclude that activation by heterocomplexes is a specific instance of the general principle that we demonstrate here, i.e., juxtaposition- and concentration-dependent activation. The possibility remains that some physiological ligand surfaces may allow efficient binding of single PRM/MASP complexes with spacing between complexes precluding intercomplex activation.
Our findings contrast with a previous report that isolated zymogen MASP-2 can bind C4, but when zymogen MASP-2 is associated with MBL, C4 binding is diminished (11). Chen and Wallis (11) estimated the affinity of zymogen MASP-2 for C4 around 7 µM, suggesting that MASP-2 would have to be sequestered in the MBL complex to prevent spontaneous activation of complement. However, recombinant constitutive zymogen MASP-2 in complex with MBL on mannan is unable to activate C4 (15), and when MASP-1 is inhibited in serum, preventing activation of MASP-2, no activation of C4 occurs (16).
We propose a mechanistically simple mode of operation that integrates the available data and fits the primordial origins of the lectin pathway: the PRMs (i) concentrate the MASPs on ligand surfaces, (ii) orient the MASPs relative to the surface and each other, and (iii) juxtapose MASP-1 and MASP-2. This serves to (i) tip the balance of MASPs and inhibitors to favor MASP activation, (ii) allow intercomplex activation of MASP-1, and (iii) facilitate intercomplex activation of MASP-2 by MASP-1. This simple mode of activation could represent the primordial origins of the elaborate conformational mechanism proposed for higher-order oligomeric MBL/MASP complexes and the C1 complex.
Materials and Methods
To assay C4 and C3 fragment deposition based on defined oligomers of MBL, purified tetrameric and >tetrameric MBL serially diluted 1.5-fold was added to MBL-deficient serum, incubated 10 min at room temperature, then added to mannan-coated wells. After incubation for 1 h at 37 °C, wells were developed with anti-C3c or -C4c. To analyze C4 deposition by tetrameric MBL, MASP-1 or MASP-2 was incubated with tetrameric MBL overnight at 4 °C (∼0.5 µg MASP per 1 µg MBL, i.e., close to a 1:1 stoichiometry). MBL/MASP-1 and MBL/MASP-2 complexes (100 ng/mL MASP-saturated MBL), or a 1:1 mixture of the two, were serially diluted twofold and then added to mannan-coated wells. Wells were incubated for 4 h on ice to bind MBL and then received 2 µg/mL purified human C4 and were incubated for 30 min at 37 °C and then developed for C4 (mAb 162–02), MASP-1 (4H2), MASP-2 (8B5), and MBL (131-1). MBL binding and C4 deposition on S. aureus was analyzed by incubating strain WOOD for 2 h at 4 °C with 10 ng/mL tetrameric recombinant MBL (rMBL) presaturated with zymogen MASP-2 and decreasing concentrations of tetrameric rMBL presaturated with zymogen MASP-1. C4 was added, and samples were incubated for 30 min at 37 °C and then analyzed for C4 deposition and MBL binding. For mixed samples, 5 ng/mL tetrameric rMBL presaturated with zymogen MASP-2 or 5 ng/mL tetrameric rMBL presaturated with zymogen MASP-1 were added to bacteria, and these were subsequently mixed before incubation with C4. Alternatively, bacteria were incubated simultaneously with both.
For the mixed ligand assays, purified H-ficolin or rMBL was added to recombinant MASP-1 and MASP-2 supernatants (each 0.5 µg/mL) to a final concentration of 1 µg/mL. Similarly, rMBL and purified H-ficolin were added to a mixture of the two supernatants to a final concentration of 0.5 µg/mL each and then incubated overnight at 4 °C. The preformed H-ficolin/MASP-1, MBL/MASP-2, the (H-ficolin + MBL)+(MASP-1 + MASP-2), or a 1:1 mixture of H-ficolin/MASP-1 with MBL/MASP-2 were added to microtiter wells coated with mannan, AcBSA (Sigma), or both. Wells were developed with C4 and anti-C4c (162-02); with FGR-AMC substrate (methylsulfonyl-d-Phe-Gly-Arg-AMC; American Diagnostica), 0.1 mM, incubated at 37 °C and fluorescence read over time; or with anti-MBL (131-01); anti–H-ficolin (4H5); anti–MASP-1 (4H2); or anti–MASP-2 (8B5). To analyze cooperation of MBL/MASP-1 and ficolin/MASP-2 in serum, MBL presaturated with rMASP-1 or rMASP-1(S646A) was added to buffer or MBL and MASP-1/-3/MAp44–deficient serum (15). Samples were added to wells coated with mannan, AcBSA, or both and then developed for C4 deposition. For comparison of intracomplex versus intercomplex scenarios, microtiter wells were coated with mannan, 8B5, or 5F5. Recombinant MASP-1 (1 µg/mL), recombinant MASP-2 (1 µg/mL), or a 1:1 mixture of the two, were incubated overnight at 4 °C with 1 µg/mL rMBL (2:1 stoichiometry). Serial dilutions of the MBL/MASP-1, the MBL/MASP-2, or the MBL/(MASP-1+MASP-2) samples were added to wells. Alternatively, MBL/MASP-1 and MBL/MASP-2 were mixed 1:1, serially diluted, and added to wells. After incubation for 4 h on ice, mannan-coated wells were developed for MASP-1 (4H2), MASP-2 (8B5), or added C4 and developed for deposition. The 5F5-coated wells were developed with anti–MASP-2 (8B5) and the 8B5-coated wells with anti–MASP-1 (4H2).
To analyze dependence of activation on sequence of binding events, rMBL was added at 1 or 0 µg/mL to mannan-coated wells. A twofold dilution series of MASP-1 was added to wells that received MBL. MBL was added to a twofold dilution series of MASP-1 to a final concentration of 1 µg/mL and was subsequently added to wells that had not received MBL. Wells were analyzed for MASP-1 enzymatic activity, MASP-1 (4H2), or MBL (131-01). To examine the concentration dependence of activation, microtiter wells were coated with a threefold dilution series of anti–MASP-1 antibodies, 5F5, 4H2, or 1E2, and then 100 ng/mL MASP-1 was added. Parallel wells were developed for MASP-1 activity and MASP-1 bound (2B11). Wells were also coated with a twofold dilution series of mannan or rMBL or two different anti-MBL antibodies (131-01 or 93C). To the 131–01- and 93C-coated wells was added 10 µg rMBL/mL. Wells were developed for MASP-1 activity and MASP-1 bound (4H2). Additional details are in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Professor Uffe Skov Sørensen for the S. aureus. S.E.D. was supported by the Carlsberg and Lundbeck Foundations. S.T. was supported by The Danish Council for Independent Research, Medical Sciences and by the Lundbeck Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1406849111/-/DCSupplemental.
References
- 1.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: A key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venkatraman Girija U, et al. Structural basis of the C1q/C1s interaction and its central role in assembly of the C1 complex of complement activation. Proc Natl Acad Sci USA. 2013;110(34):13916–13920. doi: 10.1073/pnas.1311113110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaboriaud C, et al. Structure and activation of the C1 complex of complement: Unraveling the puzzle. Trends Immunol. 2004;25(7):368–373. doi: 10.1016/j.it.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Gingras AR, et al. Structural basis of mannan-binding lectin recognition by its associated serine protease MASP-1: Implications for complement activation. Structure. 2011;19(11):1635–1643. doi: 10.1016/j.str.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Wallis R, Mitchell DA, Schmid R, Schwaeble WJ, Keeble AH. Paths reunited: Initiation of the classical and lectin pathways of complement activation. Immunobiology. 2010;215(1):1–11. doi: 10.1016/j.imbio.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henriksen ML, et al. Heteromeric complexes of native collectin kidney 1 and collectin liver 1 are found in the circulation with MASPs and activate the complement system. J Immunol. 2013;191(12):6117–6127. doi: 10.4049/jimmunol.1302121. [DOI] [PubMed] [Google Scholar]
- 7.Dahl MR, et al. MASP-3 and its association with distinct complexes of the mannan-binding lectin complement activation pathway. Immunity. 2001;15(1):127–135. doi: 10.1016/s1074-7613(01)00161-3. [DOI] [PubMed] [Google Scholar]
- 8.Teillet F, et al. The two major oligomeric forms of human mannan-binding lectin: Chemical characterization, carbohydrate-binding properties, and interaction with MBL-associated serine proteases. J Immunol. 2005;174(5):2870–2877. doi: 10.4049/jimmunol.174.5.2870. [DOI] [PubMed] [Google Scholar]
- 9.Matsushita M, Thiel S, Jensenius JC, Terai I, Fujita T. Proteolytic activities of two types of mannose-binding lectin-associated serine protease. J Immunol. 2000;165(5):2637–2642. doi: 10.4049/jimmunol.165.5.2637. [DOI] [PubMed] [Google Scholar]
- 10.Rossi V, et al. Substrate specificities of recombinant mannan-binding lectin-associated serine proteases-1 and -2. J Biol Chem. 2001;276(44):40880–40887. doi: 10.1074/jbc.M105934200. [DOI] [PubMed] [Google Scholar]
- 11.Chen CB, Wallis R. Two mechanisms for mannose-binding protein modulation of the activity of its associated serine proteases. J Biol Chem. 2004;279(25):26058–26065. doi: 10.1074/jbc.M401318200. [DOI] [PubMed] [Google Scholar]
- 12.Gál P, et al. A true autoactivating enzyme. Structural insight into mannose-binding lectin-associated serine protease-2 activations. J Biol Chem. 2005;280(39):33435–33444. doi: 10.1074/jbc.M506051200. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi M, et al. Mannose-binding lectin (MBL)-associated serine protease (MASP)-1 contributes to activation of the lectin complement pathway. J Immunol. 2008;180(9):6132–6138. doi: 10.4049/jimmunol.180.9.6132. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi M, Mori S, Shigeta S, Fujita T. Role of MBL-associated serine protease (MASP) on activation of the lectin complement pathway. Adv Exp Med Biol. 2007;598:93–104. doi: 10.1007/978-0-387-71767-8_8. [DOI] [PubMed] [Google Scholar]
- 15.Degn SE, et al. Mannan-binding lectin-associated serine protease (MASP)-1 is crucial for lectin pathway activation in human serum, whereas neither MASP-1 nor MASP-3 is required for alternative pathway function. J Immunol. 2012;189(8):3957–3969. doi: 10.4049/jimmunol.1201736. [DOI] [PubMed] [Google Scholar]
- 16.Héja D, et al. Revised mechanism of complement lectin-pathway activation revealing the role of serine protease MASP-1 as the exclusive activator of MASP-2. Proc Natl Acad Sci USA. 2012;109(26):10498–10503. doi: 10.1073/pnas.1202588109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi L, et al. Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J Exp Med. 2004;199(10):1379–1390. doi: 10.1084/jem.20032207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Degn SE, Jensen L, Olszowski T, Jensenius JC, Thiel S. Co-complexes of MASP-1 and MASP-2 associated with the soluble pattern-recognition molecules drive lectin pathway activation in a manner inhibitable by MAp44. J Immunol. 2013;191(3):1334–1345. doi: 10.4049/jimmunol.1300780. [DOI] [PubMed] [Google Scholar]
- 19.Kirschner KN, Lins RD, Maass A, Soares TA. A glycam-based force field for simulations of lipopolysaccharide membranes: Parametrization and validation. J Chem Theory Comput. 2012;8(11):4719–4731. doi: 10.1021/ct300534j. [DOI] [PubMed] [Google Scholar]
- 20.Kjaer TR, Thiel S, Andersen GR. Toward a structure-based comprehension of the lectin pathway of complement. Mol Immunol. 2013;56(4):413–422. doi: 10.1016/j.molimm.2013.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.