Significance
Invariant natural killer T (iNKT) cells are a specialized subset of T cells that recognizes lipids, rather than peptides, as antigens. Recognition of both endogenous and exogenous lipids by iNKT cells contributes to immune responses during infection, cancer, autoimmune disease, and allergic disease. The endogenous lipids recognized by iNKT cells in most contexts, however, remain unclear. In this report, we characterize the lipid antigen activity found in mammalian milk and tissues. Our data suggest that activity is related to a minor component of the glucosylceramide fraction. Whether contributed from endogenous sources or from the diet, this rare, yet potent lipid activity may play an important role in driving immune responses.
Keywords: antigen presentation, CD1d, self-reactive, dietary antigen, anomer
Abstract
Invariant natural killer T (iNKT) cells are a specialized T-cell subset that recognizes lipids as antigens, contributing to immune responses in diverse disease processes. Experimental data suggests that iNKT cells can recognize both microbial and endogenous lipid antigens. Several candidate endogenous lipid antigens have been proposed, although the contextual role of specific antigens during immune responses remains largely unknown. We have previously reported that mammalian glucosylceramides (GlcCers) activate iNKT cells. GlcCers are found in most mammalian tissues, and exist in variable molecular forms that differ mainly in N-acyl fatty acid chain use. In this report, we purified, characterized, and tested the GlcCer fractions from multiple animal species. Although activity was broadly identified in these GlcCer fractions from mammalian sources, we also found activity properties that could not be reconciled by differences in fatty acid chain use. Enzymatic digestion of β-GlcCer and a chromatographic separation method demonstrated that the activity in the GlcCer fraction was limited to a rare component of this fraction, and was not contained within the bulk of β-GlcCer molecular species. Our data suggest that a minor lipid species that copurifies with β-GlcCer in mammals functions as a lipid self antigen for iNKT cells.
The lines that distinguish innate from adaptive immunity are less clear than once believed. It is now appreciated that innate T cells, including invariant natural killer T (iNKT) cells, mucosal associated invariant T (MAIT) cells, γδ T cells, and some CD1a/b/c restricted T cells, constitute a substantial portion, perhaps 10–20% of the normal human T-cell repertoire (1). Innate T cells use the machinery of the adaptive immune system to generate antigen receptors with limited diversity and conserved antigenic specificity. Identification of the antigenic targets recognized by these cells is a fundamental step in understanding their biology in health and disease.
As the best studied member of the innate T-cell family, iNKT cells have been shown to play a role in a variety of immune contexts, including inflammatory processes such as autoimmunity and allergic disease, cancer surveillance, and prominently, in host defense to bacteria, fungi, and viruses (2). In most situations, the activation of iNKT cells requires the recognition of stimulatory lipid antigens presented by a specialized antigen-presenting molecule named CD1d. The first lipid antigen discovered, α-galactosylceramide, was purified from a marine sponge, and the α-galactose headgroup was determined to be critical for antigenicity (3). Mammals have not been shown to produce gluco- or galactolipids where the headgroup is attached with α stereochemistry. Following the discovery of α-galactosylceramide, several microbes, including Sphingomonas species (4–6), Borrelia burgdorferi (7), and Streptococcus pneumoniae (8), were shown to produce α-anomeric lipid antigens, suggesting that α-glycolipids could be a microbial signature.
Although microbe-derived lipids are likely to contribute to iNKT cell activation during bacterial and fungal infections, iNKT cells also play an important role in multiple diseases where foreign lipids are not expected to be present, such as viral infection, autoimmunity, and cancer, or in response to Toll-like receptor agonists (9). These observations suggested that endogenous lipid antigens play a central role in activating iNKT cells during many immune responses. Several candidate endogenous lipid antigens have been identified, including isoglobotrihexosylceramide (iGb3) (10), lyso-phosphatidylcholine (11), and plasmalogen lyso-phosphatidylethanolamine (12). We have reported activity in mammalian glucosylceramides (GlcCers), and we implicated β-glucosylceramides that are widely found in mammalian tissues (13). Of note, none of these endogenous antigens were described to contain an α-anomeric glycolipid.
In this report, we characterized the structures and activities present in GlcCer-enriched lipid fractions from multiple sources, including those from the milk and serum of multiple animal species. Although the GlcCer fractions from multiple origins activated iNKT cells, we identified a GlcCer-enriched lipid fraction from a human source that was unable to activate iNKT cells despite containing GlcCers with similar molecular composition to activating GlcCers from other sources. This led us to consider the possibility that activity in the naturally occurring mammalian GlcCer fractions was contributed by a rare component of the total GlcCer species. We used multiple enrichment strategies to purify the activity in the GlcCer fraction to a rare component of the starting material. The functional, mass spectrometry (MS), and NMR spectroscopy characterization of this activity is reported herein.
Results
iNKT Cells Recognize GlcCers from Diverse Sources.
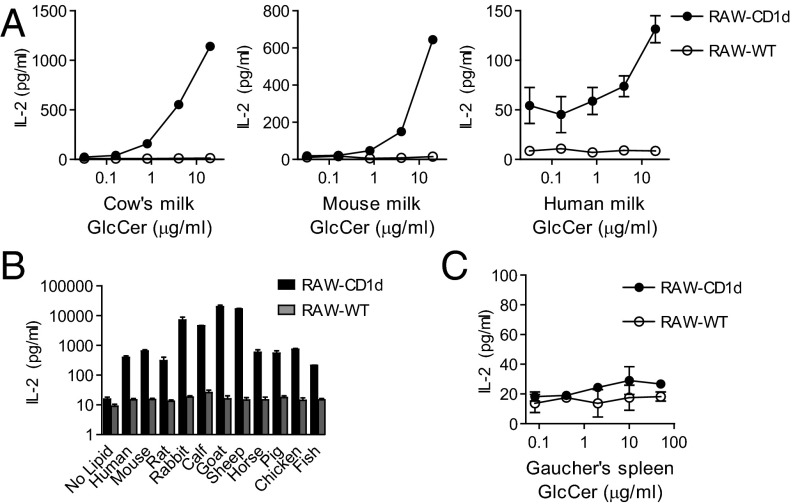
We previously reported that GlcCers from mammalian sources activate iNKT cells in a CD1d-dependent manner (13). We hypothesized that GlcCers from both endogenous and exogenous sources might broadly contain antigenic activity for iNKT cells. Having detected lipid antigenic activity with purified cow’s milk GlcCer, we asked whether milk from other sources also contained antigenic activity for iNKT cells. To address this question, we extracted polar lipids and compared lipid profiles from whole-fat cow’s milk, cow’s skim milk, human milk, mouse milk, soy milk, and cow’s milk-based infant formula. Each of these milks contained diverse polar lipid species when analyzed by normal phase TLC, and each included a density with a similar retention time to a GlcCer standard (Fig. S1A). We tested these polar lipid extracts for their ability to elicit IL-2 release from the DN32 mouse iNKT cell hybridoma when cocultured with CD1d-transfected (RAW-CD1d) or untransfected (RAW-WT) RAW-264.7 cells. Whole-fat cow’s milk, cow’s skim milk, and cow’s milk-based infant formula all activated IL-2 release from the DN32 iNKT cell hybridoma, whereas activity was not detected in this assay for the polar lipids from other milk sources (Fig. S1B). We considered the possibility that GlcCer from milk sources other than cows might be able to activate iNKT cells, but that this activity was not detectable when using whole polar lipids for assay. To test this possibility, we purified the GlcCer fractions from whole-fat cow’s milk, human milk, mouse milk, and soy milk. Using DN32 cells as responder T cells, dose-dependent activity was detected in the cow’s milk GlcCer fraction. Activity was also detected for the GlcCer fractions purified from mouse milk and human milk (Fig. 1A), but no activity was detected in the GlcCer fraction from soy milk in this mouse-based assay system.
Fig. 1.
Activity of glucosylceramide (GlcCer) fractions on mouse iNKT cells. (A) Purified GlcCer from cow’s milk, mouse milk, or human milk was cocultured with the DN32 iNKT cell hybridoma and RAW-CD1d or RAW-WT cells. Response to α-GlcCer d18:1/24:1 is shown for comparison in Fig. S1C. Please note the different scales of the axes between panels, reflecting different response magnitudes. (B) The GlcCer retention time fractions were purified from 500 μg of the indicated serum polar lipids and cocultured with DN32 and RAW-CD1d or RAW-WT cells. (C) GlcCer from the spleen of a human with Gaucher’s disease was cocultured with DN32 and RAW-CD1d or RAW-WT cells. Data presented are release of IL-2 by DN32 as measured by ELISA. Cow’s milk GlcCer was from three independent sources. Human milk GlcCer was tested from two individual donors and 10 pooled donors. Experiments were repeated at least twice, and a representative experiment is shown.
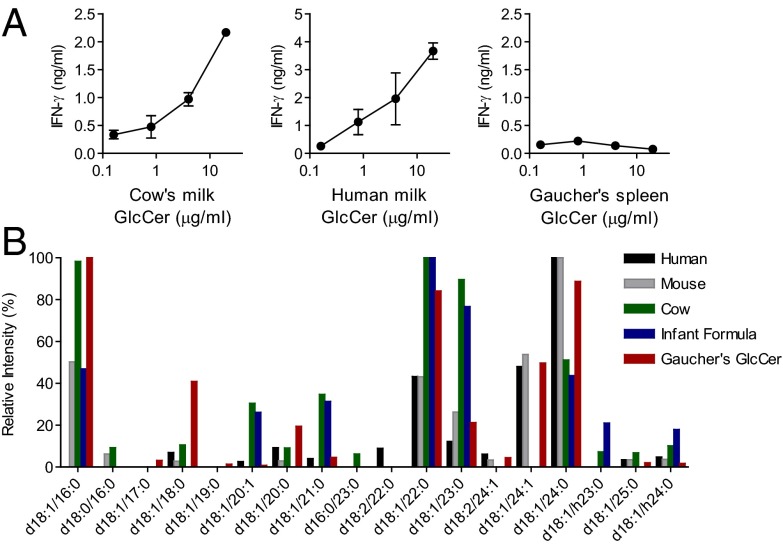
Although iNKT cells are highly conserved, and even function to some degree across species, differences in the recognition of lipid antigens by mouse and human iNKT cells have been reported (11, 14, 15). To investigate the antigenicity of GlcCer lipid fractions on human iNKT cells, we tested the GlcCer fractions from different milk sources using human iNKT cells and human antigen-presenting cells. A primary human iNKT cell line was activated by cow’s milk GlcCer and human milk GlcCer, but not by soy GlcCer (Fig. 2A and Fig. S1D). Interestingly, the human iNKT cell clone J3N.5 was activated by soy GlcCer more strongly than by cow’s milk GlcCer, but only when presented by human CD1d (Fig. S1 E and F). We concluded that milk GlcCer fractions could activate human iNKT cells, but that this activity might be T-cell clone or donor-dependent.
Fig. 2.
Activity of glucosylceramides (GlcCers) on human iNKT cells. (A) Purified GlcCer from cow’s milk, human milk, and Gaucher’s spleen were cocultured with a primary human iNKT cell line and human GM-CSF/IL-4-induced antigen-presenting cells. The activity of α-GlcCer d18:1/24:1 is shown for comparison in Fig. S1D. Data are ELISA for IFN-γ release. Three independent experiments were performed and a representative experiment is shown. (B) GlcCers from various mammalian milk sources (human, mouse, cow, infant formula) and Gaucher’s spleen were analyzed by collision-induced dissociation mass spectrometry (CDI-MSn). GlcCer molecular species identified present at more than 5% of the total are on the x axis, and relative intensity of ions corresponding to each molecular species identified is shown on the y axis.
We next asked whether cow’s milk polar lipids might contain additional fractions with iNKT cell activity. To address this possibility, we fractionated the polar lipids from cow’s milk by normal phase preparative TLC and tested these fractions for activity. Activity was only detected in the GlcCer-containing fraction, with no detectable activity in the longer or shorter retention time fractions (Fig. S2A). Comparable levels of iNKT cell activity were detected in the polar lipids from whole-fat cow’s milk and skim cow’s milk, suggesting that the activity might be protein-associated rather than triglyceride-associated (Fig. S1B). Polar lipids were extracted from cow’s milk whey and cow’s milk casein. Analysis of polar lipids from both of these sources revealed a density with a similar retention time to GlcCer, and polar lipids from both casein and whey were able to activate iNKT cells (Fig. S2B). These experiments provided a likely explanation for the activity observed with infant formulas, which are made with nonmammalian triglycerides added to skim cow’s milk.
Mammalian serum contains a diversity of lipids, including GlcCers and other glycolipids (16–18). We next investigated the polar lipids from the sera of human, mouse, rat, rabbit, calf, goat, sheep, horse, pig, chicken, and fish. Polar lipids from each of these species were extracted and visualized by normal phase TLC. Density at the GlcCer retention time was detected in each serum analyzed (Fig. S3). Using preparative TLC, we purified the GlcCer retention time material from the polar lipids of each species and tested this fraction for iNKT cell activity using the DN32/RAW coculture system described above. iNKT cell activity was detected in the GlcCer fraction from each species’ serum. The GlcCer fractions from calf serum, goat serum, sheep serum, and rabbit serum were ∼10-fold more active than those from the other species tested (Fig. 1B). We found these results intriguing, because the vast majority of in vitro functional testing of iNKT cells has been performed in the presence of calf serum. From these studies of milk and serum polar lipids from multiple different sources, we concluded GlcCer-enriched lipid fractions from diverse sources are able to activate iNKT cells.
A Human β-GlcCer 24:1 Does Not Activate iNKT Cells.
Gaucher’s disease is a genetic lipid storage disorder that occurs in humans who are unable to metabolize GlcCer, leading to GlcCer accumulation in tissues. GlcCer purified from the spleen of a patient with Gaucher’s disease was tested for its ability to activate iNKT cells. Even at concentrations in excess of 20 μg/mL, we did not detect activity for Gaucher’s spleen GlcCer in the DN32/RAW mouse iNKT cell assay system (Fig. 1C). We tested this lipid for the ability to activate a primary human iNKT cell line in the presence of human antigen-presenting cells, and again detected no activity (Fig. 2A). The lack of activity for Gaucher’s spleen GlcCer was unexpected, and we undertook MS analyses to determine whether the fatty acid chains in this inactive purified lipid differed substantially from those found in active GlcCer species.
Collision-induced dissociation tandem mass spectrometry (CID-MSn) was used to interrogate the fatty acid compositions of the inactive Gaucher’s spleen GlcCer and antigenic mammalian milk GlcCers. The structure of soy GlcCer has been reported, and revealed a different fatty acid structure compared with mammalian GlcCer (19). Gaucher’s spleen GlcCer contained 16:0, 24:0, 22:0, 24:1, 18:0, 23:0, and 20:0 N-acyl chains paired with a d18:1 sphingosine base as the most abundant ions (Fig. S4). The five most abundant GlcCer ions in cow’s milk were d18:1/22:0, d18:1/16:0, d18:1/23:0, d18:1/24:0, and d18:1/21:0, all of which were also present in Gaucher’s spleen GlcCer. A large degree of overlap in the structures of GlcCer from mammalian milks was observed, although the relative abundance of some ions varied between species (Fig. 2B). The large degree of similarity between active milk GlcCers and inactive Gaucher’s spleen GlcCer led us to question whether the activity of the naturally occurring GlcCer species might be due to a highly active, yet rare component of the GlcCer-containing lipid fractions.
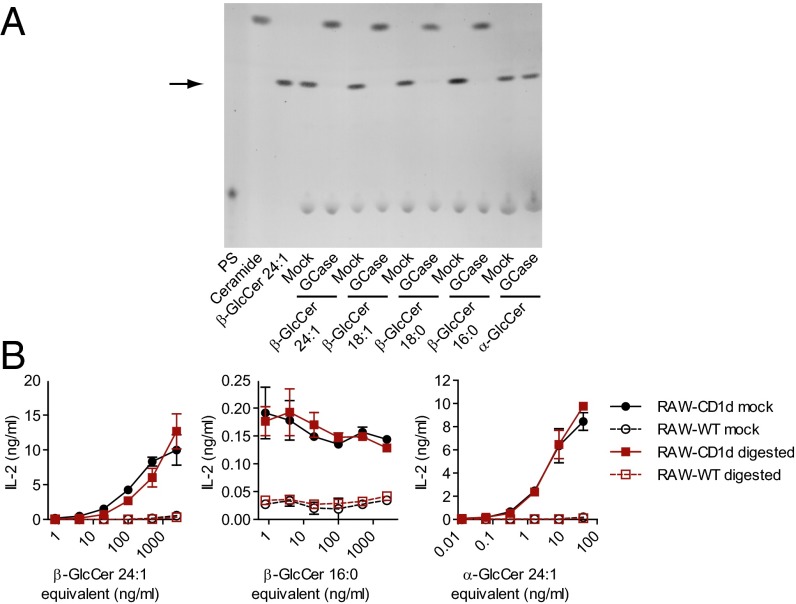
We have previously reported iNKT cell activity with a synthetic β-GlcCer with a 24:1 N-acyl chain (13). Because Gaucher’s spleen GlcCer contained appreciable GlcCer d18:1/24:1 (Fig. S4), yet did not activate iNKT cells, we questioned whether the synthetic β-GlcCer d18:1/24:1 that we have previously studied might contain an activating contaminant from the synthetic process. To test the possibility that the β-GlcCer 24:1 synthetic compound was contaminated with α-GlcCer, we used human recombinant lysosomal glucocerebrosidase (GCase) to remove the β-GlcCer component. Digestion was confirmed by reduction in density at the GlcCer retention time along with the appearance of a density comigrating with free ceramide by TLC. α-GlcCer was not cleaved by the enzyme used for these studies (Fig. 3A). After digestion, the GlcCer retention time fraction was purified by preparative TLC and tested for activity. Although >95% of the β-GlcCer 24:1 synthetic compound was removed by GCase treatment, activity was not reduced. An inactive β-GlcCer d18:1/16:0 remained inactive after GCase digestion. The activity of a synthetic α-GlcCer d18:1/24:1 was unaltered by treatment with GCase (Fig. 3B). We had previously analyzed synthetic β-GlcCer d18:1/24:1 by NMR, and were unable to detect α-GlcCer. Analysis of the current commercially available β-GlcCer d18:1/24:1 lot with a more sensitive methodology using 1D-proton and 1D-TOCSY 900 MHz NMR and acquiring numerous scans, we were able to detect α-glucosyl linkages contaminating the β-GlcCer synthetic product at 0.5–1% (Fig. S5A). To determine whether a small contamination with α-GlcCer could lead to the degree of activity that we had observed for the β-GlcCer d18:1/24:1 synthetic compound, we titrated α-GlcCer with an inactive β-GlcCer synthetic compound bearing a 16:0 carbon N-acyl chain. Activity of the β-GlcCer d18:1/24:1 synthetic compound was less than a 1% α-GlcCer contaminant, but more than that from a 0.1% α-GlcCer contaminant (Fig. S5B). We concluded from these studies that the activity of the β-GlcCer d18:1/24:1 synthetic compound studies was largely attributable to a minor component of this product, and that α-GlcCer contamination was most likely the major source of this activity.
Fig. 3.
Glucocerebrosidase (GCase) digestion of synthetic GlcCer compounds. (A) Normal phase TLC of synthetic GlcCers either GCase digested, or mock-digested (no enzyme). For each sample, 2 μg of lipid (or the equivalent amount of lipid added to the enzymatic digestion) was loaded and visualized with α-naphthol stain. An arrow marks the retention time of a GlcCer standard. Phosphatidylserine (PS) is a component of the digestion assay and does not comigrate with GlcCer. Ceramide d18:1/24:1 (Ceramide) shows the migration of free ceramide in this TLC system. Note liberated ceramide densities near the top edge of the plate. (B) Activity of antigenic β-GlcCer d18:1/24:1, nonantigenic β-GlcCer d18:1/16:0, or α-GlcCer d18:1/24:1, either digested or mock-digested was measured by coculture with DN32 and RAW-CD1d or RAW-WT cells after repurification by preparative TLC. Data presented are release of IL-2 by DN32 as measured by ELISA. Experiments were repeated at least twice and a representative experiment is shown.
Activity of Natural GlcCer Is Due To a Rare Active Component.
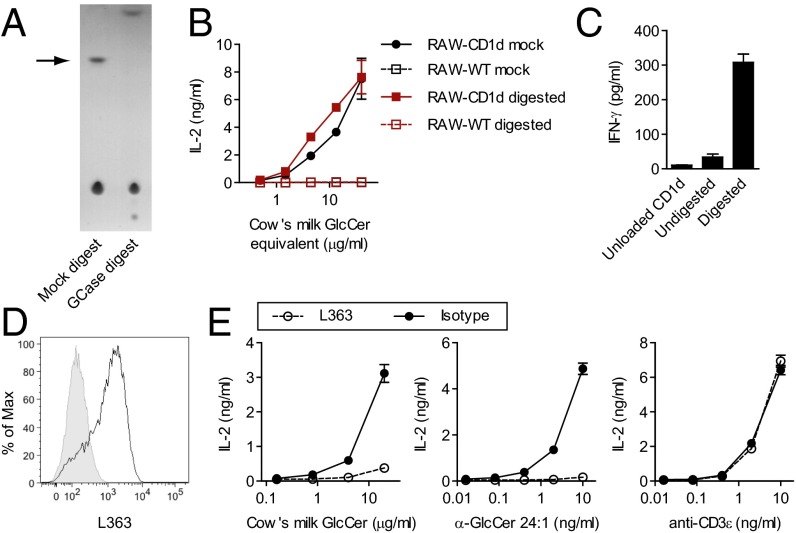
In light of the newly appreciated contamination of the synthetic β-GlcCer d18:1/24:1 compound, we next asked whether the activity observed in the naturally occurring β-GlcCer compounds might also be due to a highly-active minor constituent. We used cow’s milk GlcCer as an abundant, readily available, and naturally occurring antigenic source of GlcCer. Cow’s milk GlcCer was digested by GCase treatment (Fig. 4A), and we recovered the remaining GlcCer retention time fraction by preparative TLC for assay. Despite nearly complete digestion of cow’s milk GlcCer, no decrease in activity was observed using the DN32/RAW coculture system (Fig. 4B). We confirmed these results using GlcCer purified from calf thymus and from mouse thymus. Similar to what was observed with cow’s milk GlcCer, despite nearly complete digestion of the calf and mouse thymus GlcCers, activity of the GlcCer fraction was not diminished (Fig. S6). The digested cow’s milk GlcCer fraction was also tested in an antigen-presenting cell-free assay using plate-bound, lipid-loaded CD1d. In this plate-bound CD1d assay system, increased activity was observed in the digested GlcCer fraction (Fig. 4C). This result is consistent with a rare, GCase-resistant activating lipid with decreased competition for CD1d binding sites following digestion.
Fig. 4.
GCase digestion of cow’s milk GlcCer. (A) Normal phase TLC of cow’s milk GlcCer, either GCase digested, or mock-digested (no enzyme). Lipid (2 μg; or the equivalent amount based on lipid added to the initial enzymatic digestion) was loaded and visualized with α-naphthol stain. An arrow marks the retention time of a GlcCer standard. (B) Antigenic activity of cow’s milk GlcCer, either digested or mock-digested was measured by coculture with DN32 and RAW-CD1d or RAW-WT cells after repurification by preparative TLC. (C) Cow’s milk GlcCer, either digested or mock-digested, was loaded into biotintylated CD1d and subsequently bound to a streptavidin-coated plate. Activity of loaded lipids was assayed by culturing a primary mouse iNKT cell line with the plate-bound, lipid-loaded CD1d. (D) RAW-CD1d cells were loaded with 10 μg/mL α-GlcCer d18:1/24:1 overnight and then stained with L363, an antibody that is known to stain α-GalCer-loaded CD1d. The black tracing shows lipid-loaded RAW-CD1d cells, shaded tracing is from an unloaded control. (E) L363 was used for functional blocking in a DN32 and RAW-CD1d coculture assay. Data presented in B and E are release of IL-2, and data presented in C are release of IFN-γ (IFN-γ) as measured by ELISA. Experiments were repeated at least twice, and a representative experiment is shown.
The L363 monoclonal antibody was developed to recognize α-galactosylceramide bound to CD1d, and has proved a valuable tool for tracking α-galactosylceramide and other α-galactosphingolipids bound to CD1d (20, 21). α-GlcCer recognition by L363 has not been previously reported, and we were able to demonstrate binding of this reagent to CD1d-transfected RAW 246.7 cells loaded with α-GlcCer (Fig. 4D). We next used L363 for in vitro blocking studies using the DN32/RAW-CD1d coculture assay system. Addition of L363 to the coculture diminished the activity of α-GlcCer d18:1/24:1 and cow’s milk GlcCer, but had no effect on activation by anti-CD3ε (Fig. 4E). These results are consistent with the presence of an α-anomeric glycolipid antigen in the cow’s milk GlcCer fraction.
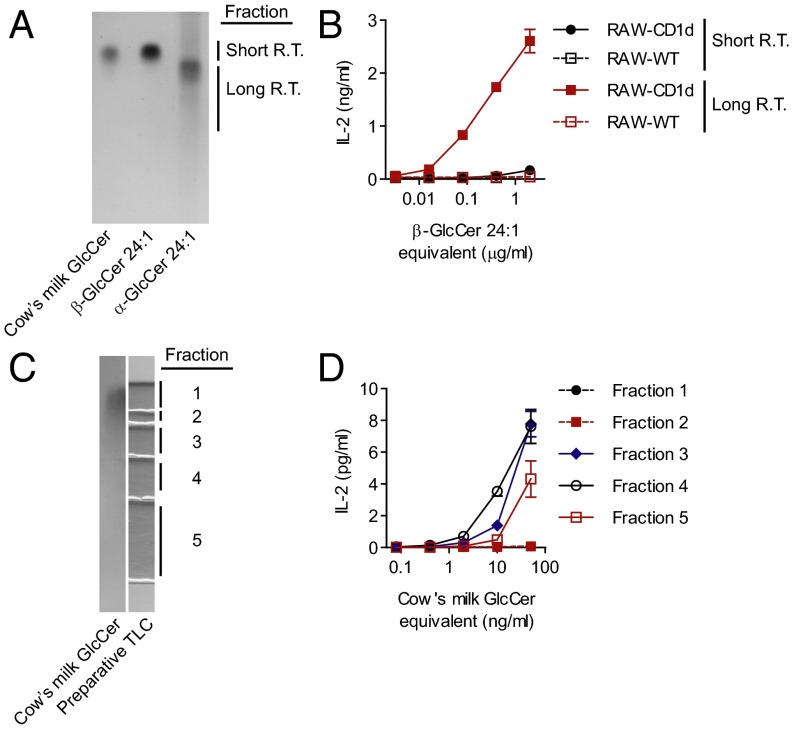
Next, we sought to develop a method for separation of α-GlcCer from β-GlcCer that did not require enzymatic digestion or incubation at low pH. Separation of underivatized synthetic GlcCer anomers was achieved with a TLC solvent system (“HICMW” comprised by hexane, isopropanol, chloroform, methanol, and water) modified from Hölzl et al. (22). In this system, α-GlcCer had a longer retention time than β-GlcCer with the same fatty acid composition (Fig. 5A). When the HICMW system was used for preparative TLC of the β-GlcCer d18:1/24:1 synthetic known to be contaminated with α-GlcCer, most of the activity was contained in the longer retention time fraction that comigrated with α-GlcCer d18:1/24:1 (Fig. 5B and Fig. S7A). Separation of an inactive synthetic β-GlcCer d18:1/16:0 using the HICMW did not reveal activity in the longer retention time fraction (Fig. S7B). We next fractionated cow’s milk GlcCer with our TLC system, and as with β-GlcCer 24:1, activity was contained in the longer retention time fractions, despite that no clear density could be visualized in this region by TLC (Fig. 5 C and D). We concluded that the activity in cow’s milk GlcCer was due to a minor component of the total GlcCer fraction, and that the active molecule purified in our HICMW TLC system had a retention time similar to that of α-GlcCer.
Fig. 5.
A TLC system for the separation of GlcCer anomers. (A) Cow’s milk GlcCer, a β-GlcCer d18:1/24:1 synthetic, or an α-GlcCer d18:1/24:1 synthetic were resolved on a silica TLC plate using a novel solvent system (HICMW, see Materials and Methods) and visualized with α-naphthol stain. R.T., retention time. (B) A β-GlcCer d18:1/24:1 synthetic known to be contaminated with α-GlcCer was fractionated by preparative TLC using the HICMW system, and the indicated fractions were tested for activity by coculture with the DN32 and RAW-CD1d or RAW-WT cells. (C) Cow’s milk GlcCer was fractionated by preparative TLC using the HICMW system. (D) The indicated fractions were tested for activity by coculture with DN32 and RAW-CD1d or RAW-WT cells. Data presented in B and D are release of IL-2 by DN32 as measured by ELISA. Experiments were repeated at least twice, and a representative experiment is shown.
Together, GCase digestion, chromatographic, and L363 studies described above suggested the presence of a minor activating component in cow’s milk GlcCer. These studies were consistent with the presence of α-GlcCer in this mammalian product, yet lipids with an α-anomeric glucose or galactose have not been demonstrated to occur in mammalian tissues. Fucosylceramide with an α-anomeric carbohydrate linage has been described (23, 24), but we did not detect fucosylceramide by MS in any of the samples analyzed. Additionally, our data did not exclude the possibility of a modified β-GlcCer or a novel activating structure comigrating with β-GlcCer by normal phase TLC. To address these possibilities, we sought additional evidence to identify the minor activating component of cow’s milk GlcCer. MS was used to identify possible modified GlcCer variants or ions corresponding to novel compounds. NMR was used to investigate the possibility of α-glycosyl moieties.
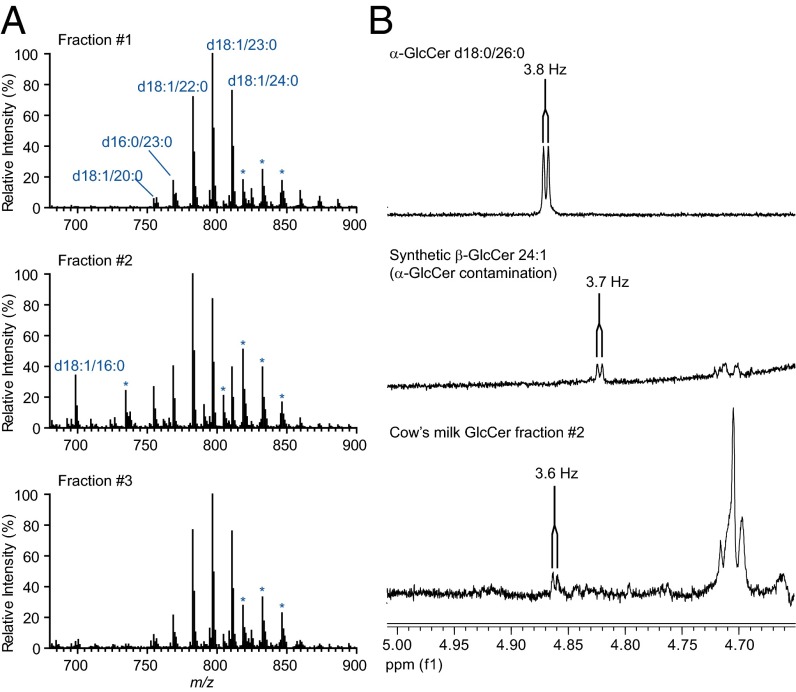
We had previously analyzed cow’s milk GlcCer by NMR and were unable to detect α-GlcCer (13). To address the possibility that our previous investigations were limited by the sensitivity of NMR, we performed a large-scale, sequential double-enrichment of the active fraction from cow’s milk GlcCer. Purified cow’s milk GlcCer (50 mg) was digested with GCase and then repurified by preparative TLC. The resulting product retained activity (Fig. S8A). The digested cow’s milk GlcCer was further fractionated using preparative HICMW TLC, and as we had previously observed, activity was limited to the longer retention time fractions (Fig. S8B). These fractions were analyzed by MS and NMR. In all fractions, MS analyses revealed only GlcCer species as major ions. The N-acyl chain complements of inactive fraction 1 and active fraction 3 showed a striking degree of similarity (Fig. 6A), despite that fraction 3 was active, whereas fraction 1 was inactive. This result is consistent with a stereochemical difference between the GlcCer species in these two fractions. One-dimensional proton NMR analysis of fraction 2 (the most active fraction) demonstrated a doublet with a chemical shift of 4.86 ppm and a coupling constant of 3.6 Hz run in 2:1 CDCl3:CD3OD (vol:vol), consistent with the H1 resonance of α-glucose (Fig. 6B). 1D-TOCSY analysis, however, was unable to definitively confirm the presence of adjacent resonant peaks from α-glucose or α-glucosylceramide in the cow’s milk GlcCer active fraction. No signal near to 4.8 ppm was detected in cow’s milk GlcCer before activity enrichment. As a control, Gaucher’s spleen GlcCer was enriched in the same manner as cow’s milk GlcCer, and a chemical shift consistent with α-glucose was not seen in this biologically inactive sample. We concluded from these studies that the cow’s milk GlcCer lipid fraction may contain trace α-GlcCer, and that α-GlcCer could be responsible for activity of this lipid fraction.
Fig. 6.
Large-scale enrichment of the active fraction in cow’s milk GlcCer. (A) CID-MSn analysis of inactive (fraction 1) and active (Fig. S8B, fractions #2 and #3) fractions, with major GlcCer ions annotated in blue. Asterisk above an ion series indicates an additional adduct (chloride) of an annotated series. (B) Fraction #2 (Fig. S8B) was analyzed by 1D-proton NMR. Signals corresponding to H1 of α-glucose are shown with the coupling constant indicated. 1D spectra from α-GlcCer d18:0/26:0 and β-GlcCer 24:1 (contains 0.5–1% α-GlcCer) are shown for comparison. Large-scale enrichment and experiments were performed twice.
Discussion
The search for endogenous iNKT cell antigens has been full of unexpected twists and turns. Lipid antigen presentation by CD1 molecules was first described in 1994 (25), CD1d restriction of iNKT cells was described in 1995 (26), and the discovery of α-galactosylceramide was reported in 1997 (3). Despite many fascinating discoveries since those initial steps, the endogenous antigenic lipids that contribute to iNKT cell activation in most situations are still not clear (9). Our description of activity with mammalian GlcCer provided a promising candidate antigen (13), and in this report, we have extended our structural characterization of antigenic glucosylceramides. Although we had previously interpreted our data to indicate that β-GlcCer was the active lipid in the glucosylceramide fraction, the refined data presented in this report demonstrate that the majority of β-GlcCer species do not potently activate iNKT cells. Possible explanations for the observed activity include a rare α-GlcCer component, another α-glycolipid such as such as α-galactosylceramide or α-glucuronic acid, an α-linked cholesterol glycoside (27, 28), a modified β-GlcCer that is GCase-resistant, or another copurifying novel structure. Although α-GlcCer remains a strong candidate, NMR analyses were not able to definitively identify α-GlcCer. Despite large-scale enrichment efforts, the activating lipid may still have been at low abundance or insufficient purity for detection by NMR. It has been thought that anomeric α-linked glycolipids are limited to select microbes and ocean-bound porifera. An α-anomeric galactolipid has recently been reported in the gastrointestinal microbial flora, although this lipid may inhibit, rather than activate iNKT cells (29, 30). Our data suggest the possibility that α-glycolipids are widely found in mammals, but only in very low abundance. Using genetic or chemical inhibition of GlcCer synthesis, we have previously demonstrated that self lipid reactivity correlates with β-GlcCer abundance (13). If the antigenic activity we have detected is indeed α-GlcCer, this raises the possibility that α-glycolipids are generated at some low rate during the synthesis of β-glycolipids, or that α-linked lipids can be generated through conversion of β-glycolipids. Enzymatic or nonenzymatic pathways by which α-linked lipids could be generated by mammals have not been described, and elucidation of these mechanisms would be an important area of investigation for the field going forward.
Our data now raises the question as to whether β-linked lipids have relevant activity for iNKT cells. In addition to our report that focused on β-glucosylceramides, several other β-linked antigens have been proposed. iGB3 has a terminal α-linked sugar. However, the anomeric glucose that is recognized by the iNKT cell T-cell receptor has been reported as β-linked (10). Trimolecular cocrystals consisting of CD1d, lipid, and T-cell receptor have been generated for both iGB3 and β-GalCer, and suggested that these lipids are “pushed” into an α-like configuration by the T-cell receptor for recognition. Additionally, a measurable affinity has been reported for β-linked ceramides by surface plasmon resonance (31, 32). A fungal β-linked lipid, Aspermide B, has also been recently reported as antigenic for iNKT cells (33). In each of these examples, it is possible that the synthetic or purified lipids tested contained trace α-glycolipids responsible for the observed activity, as our data suggests is possible for cow’s milk GlcCer. It will be important to determine whether trace α-anomeric linkages form the basis for the activity for these other proposed β-glycolipid antigens.
Our report demonstrates a large range of iNKT lipid antigen activity between the GlcCer fractions from multiple sources, and the underlying basis for these activity differences remains unclear. Several factors influence the observed activity of a lipid in assay, including the iNKT cell T-cell receptor repertoire, CD1d species and expression levels, lipid loading, and costimulatory factors. Such variables are likely to have contributed to the activity differences we observed between GlcCers from different species, and it is also possible that the content of a rare activating lipid differs between GlcCer fractions from different sources. It is notable that the GlcCer fraction from ruminants appears to be especially activating for iNKT cells. Ruminant milks are an abundant component of the human diet, and are rich in glycosphingolipids (34). The possibility that dietary lipids might contribute to iNKT cell activation is an exciting one. Supporting a possible role for oral lipid antigens, two studies have used orally-administered α-galactosylceramide as a vaccine adjuvant, and both studies reported a measureable result with oral administration (35, 36). Studies of lipid antigens from commensal bacteria also support a role for lipids antigens in the digestive system (29, 30, 37, 38). Our studies demonstrate reactivity of human iNKT cells to a component of the GlcCer-enriched lipid fraction from human milk, cow’s milk, and soy milk. In addition, milk sphingomylein has been reported as antigenic for iNKT cells from some human donors (39). Humans are the only mammal that continues to drink milk past early life, and consumption of milk or other dietary lipid antigens may play an important role in the regulation of immune responses.
An additional observation from this report has important implications for the iNKT cell field. Our studies on serum GlcCers suggest that there is great variability in the antigenicity of sera from different animal species. Interestingly, ruminants had the highest level of antigenic activity in the GlcCer fraction. Although it is not clear whether this observation has physiological relevance, it certainly has relevance to experimental design and interpretation. For every in vitro assay published, it must now be considered that exogenous antigens were provided in the calf serum that was used for cell culture, and that in vitro ‘self’ reactivity may be contributed from lipid antigens found in bovine serum.
The study of lipid antigens for iNKT cells presents numerous technical challenges. This report demonstrates that rare, nearly undetectable lipids can critically determine activity, and highlights the need for integration of immunology, synthetic chemistry, MS, and NMR approaches. Clinically important immunological outcomes for host defense, cancer, and allergic disease may be due to a set of rare microbial and endogenous lipid antigens, and defining these antigens remains a fundamental step toward understanding the biology of iNKT cells.
Materials and Methods
The lipids used in this report were purified by extraction from tissues in organic solvents, or purchased commercially. Lipid activity on iNKT cells was assessed by coculture with lipid, iNKT cells, and antigen-presenting cells for 16 h at 37 °C. iNKT cell response was measured by cytokine ELISA. CID-MSn was performed as previously described (13). Proton NMR was performed on a Varian VNMRS 900 MHz spectrometer. For details of experimental conditions and analysis, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank D. Godfrey, D. B. Moody, M. Brigl, and A. Cianferoni for thoughtful discussions. Avanti Polar Lipids and Matreya provided some materials and also offered helpful advice. L. Lynch and S. Moody assisted with mouse milk collection. Human milk samples were provided by Mothers’ Milk Bank Northeast. CD1d monomer was provided by the National Institutes of Health (NIH) Tetramer Core Facility (contract HHSN272201300006C). P.J.B. was supported by the American Academy of Asthma, Allergy and Immunology ARTrust and NIH Grants AI102945 and AI1007306. M.B.B. was supported by NIH Grants AI063428, AI1028973, and DK057521. G.S.B. was supported by J. Badrick, the Royal Society, and the Medical Research Council. F.-F.H. was supported by NIH Grants GM103422, DK56341, and DK20579.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1415357111/-/DCSupplemental.
References
- 1.Young MH, Gapin L. Group. 2011;1:CD1. doi: 10.1002/eji.201141408. [DOI] [PubMed] [Google Scholar]
- 2.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13(2):101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 3.Kawano T, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 4.Kinjo Y, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434(7032):520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 5.Mattner J, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434(7032):525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 6.Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol. 2005;35(6):1692–1701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- 7.Kinjo Y, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7(9):978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 8.Kinjo Y, et al. Invariant natural killer T cells recognize glycolipids from pathogenic Gram-positive bacteria. Nat Immunol. 2011;12(10):966–974. doi: 10.1038/ni.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gapin L. iNKT cell autoreactivity: What is ‘self’ and how is it recognized? Nat Rev Immunol. 2010;10(4):272–277. doi: 10.1038/nri2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou D, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306(5702):1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 11.Fox LM, et al. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 2009;7(10):e1000228. doi: 10.1371/journal.pbio.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Facciotti F, et al. Peroxisome-derived lipids are self antigens that stimulate invariant natural killer T cells in the thymus. Nat Immunol. 2012;13(5):474–480. doi: 10.1038/ni.2245. [DOI] [PubMed] [Google Scholar]
- 13.Brennan PJ, et al. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol. 2011;12(12):1202–1211. doi: 10.1038/ni.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanderson JP, et al. CD1d protein structure determines species-selective antigenicity of isoglobotrihexosylceramide (iGb3) to invariant NKT cells. Eur J Immunol. 2013;43(3):815–825. doi: 10.1002/eji.201242952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, et al. Lipid binding orientation within CD1d affects recognition of Borrelia burgorferi antigens by NKT cells. Proc Natl Acad Sci USA. 2010;107(4):1535–1540. doi: 10.1073/pnas.0909479107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawson G, Kruski AW, Scanu AM. Distribution of glycosphingolipids in the serum lipoproteins of normal human subjects and patients with hypo- and hyperlipidemias. J Lipid Res. 1976;17(2):125–131. [PubMed] [Google Scholar]
- 17.Ishibashi Y, Kohyama-Koganeya A, Hirabayashi Y. New insights on glucosylated lipids: Metabolism and functions. Biochim Biophys Acta. 2013;1831(9):1475–1485. doi: 10.1016/j.bbalip.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Quehenberger O, Dennis EA. The human plasma lipidome. N Engl J Med. 2011;365(19):1812–1823. doi: 10.1056/NEJMra1104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullards MC, Lynch DV, Merrill AH, Jr, Adams J. Structure determination of soybean and wheat glucosylceramides by tandem mass spectrometry. J Mass Spectrom. 2000;35(3):347–353. doi: 10.1002/(SICI)1096-9888(200003)35:3<347::AID-JMS941>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Yu ED, et al. Structural basis for the recognition of C20:2-αGalCer by the invariant natural killer T cell receptor-like antibody L363. J Biol Chem. 2012;287(2):1269–1278. doi: 10.1074/jbc.M111.308783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu KO, et al. Production and characterization of monoclonal antibodies against complexes of the NKT cell ligand alpha-galactosylceramide bound to mouse CD1d. J Immunol Methods. 2007;323(1):11–23. doi: 10.1016/j.jim.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hölzl G, et al. Processive lipid galactosyl/glucosyltransferases from Agrobacterium tumefaciens and Mesorhizobium loti display multiple specificities. Glycobiology. 2005;15(9):874–886. doi: 10.1093/glycob/cwi066. [DOI] [PubMed] [Google Scholar]
- 23.Veerapen N, Reddington F, Bricard G, Porcelli SA, Besra GS. Synthesis and biological activity of alpha-L-fucosyl ceramides, analogues of the potent agonist, alpha-D-galactosyl ceramide KRN7000. Bioorg Med Chem Lett. 2010;20(11):3223–3226. doi: 10.1016/j.bmcl.2010.04.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe K, Matsubara T, Hakomori S. alpha-L-Fucopyranosylceramide, a novel glycolipid accumulated in some of the human colon tumors. J Biol Chem. 1976;251(8):2385–2387. [PubMed] [Google Scholar]
- 25.Beckman EM, et al. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994;372(6507):691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 26.Bendelac A, et al. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268(5212):863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 27.Chang YJ, et al. Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity. J Clin Invest. 2011;121(1):57–69. doi: 10.1172/JCI44845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito Y, et al. Helicobacter pylori cholesteryl α-glucosides contribute to its pathogenicity and immune response by natural killer T cells. PLoS ONE. 2013;8(12):e78191. doi: 10.1371/journal.pone.0078191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.An D, et al. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell. 2014;156(1-2):123–133. doi: 10.1016/j.cell.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wieland Brown LC, et al. Production of α-galactosylceramide by a prominent member of the human gut microbiota. PLoS Biol. 2013;11(7):e1001610. doi: 10.1371/journal.pbio.1001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pellicci DG, et al. Recognition of β-linked self glycolipids mediated by natural killer T cell antigen receptors. Nat Immunol. 2011;12(9):827–833. doi: 10.1038/ni.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu ED, Girardi E, Wang J, Zajonc DM. Cutting edge: Structural basis for the recognition of β-linked glycolipid antigens by invariant NKT cells. J Immunol. 2011;187(5):2079–2083. doi: 10.4049/jimmunol.1101636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albacker LA, et al. Invariant natural killer T cells recognize a fungal glycosphingolipid that can induce airway hyperreactivity. Nat Med. 2013;19(10):1297–1304. doi: 10.1038/nm.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vesper H, et al. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J Nutr. 1999;129(7):1239–1250. doi: 10.1093/jn/129.7.1239. [DOI] [PubMed] [Google Scholar]
- 35.Courtney AN, et al. Alpha-galactosylceramide is an effective mucosal adjuvant for repeated intranasal or oral delivery of HIV peptide antigens. Vaccine. 2009;27(25-26):3335–3341. doi: 10.1016/j.vaccine.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silk JD, et al. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. J Clin Invest. 2004;114(12):1800–1811. doi: 10.1172/JCI22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei B, et al. Commensal microbiota and CD8+ T cells shape the formation of invariant NKT cells. J Immunol. 2010;184(3):1218–1226. doi: 10.4049/jimmunol.0902620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wingender G, et al. Intestinal microbes affect phenotypes and functions of invariant natural killer T cells in mice. Gastroenterology. 2012;143(2):418–428. doi: 10.1053/j.gastro.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jyonouchi S, et al. Invariant natural killer T cells from children with versus without food allergy exhibit differential responsiveness to milk-derived sphingomyelin. J Allergy Clin Immunol. 2011;128(1):102–109. doi: 10.1016/j.jaci.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.