Significance
Inflammatory bowel diseases (IBDs) are debilitating conditions with no known cure. Recent evidence suggests that elevated intestinal hydrogen sulfide (H2S) synthesis promotes healing and reduces inflammation. H2S is synthesized from cysteine largely via vitamin B6-dependent enzymes. People with IBD are also at increased risk of hyperhomocysteinemia, a condition that is often caused by vitamin B deficiency. Dietary induction of vitamin B deficiency markedly increased serum homocysteine levels and worsened colitis in rodents. The latter was due to the absence of the typical injury-induced elevation of H2S synthesis. Interleukin-10 plays a key role in increasing H2S synthesis, attenuating the severity of colitis, and reducing serum homocysteine levels. The H2S–interleukin 10 axis may be a viable target for therapy of IBD.
Abstract
Vitamin B deficiencies, which can lead to hyperhomocysteinemia (Hhcy), are commonly reported in patients with inflammatory bowel disease (IBD) and may be a causative underlying factor. However, the mechanism for this effect is not known. Hydrogen sulfide (H2S) is a gaseous mediator that promotes tissue repair and resolution of inflammation. In experimental colitis, a marked increase in colonic H2S synthesis drives ulcer healing and resolution of inflammation. Because H2S synthesis is in part dependent upon enzymes that require vitamin B6 as a cofactor, we tested the hypothesis that Hhcy in rodent models would increase the susceptibility to colitis. In all three models tested, diet-induced Hhcy significantly exacerbated colitis. The usual elevation of colonic H2S synthesis after induction of colitis was absent in all three models of colitis. Administration of an H2S donor to Hhcy rats significantly decreased the severity of colitis. Compared with wild-type mice, interleukin (IL) 10-deficient mice on a normal diet had decreased levels of colonic H2S synthesis, a 40% increase in serum homocysteine, and a phenotype similar to wild-type mice with Hhcy. IL-10–deficient mice fed the vitamin B-deficient diet exhibited more severe colonic inflammation, but the normal elevation of colonic H2S synthesis was absent. Administration of IL-10 to the IL-10–deficient mice restored colonic H2S synthesis and significantly decreased serum homocysteine levels. These results suggest that the exacerbation of colitis in Hhcy is due in part to impaired colonic H2S synthesis. Moreover, IL-10 plays a novel role in promoting H2S production and homocysteine metabolism, which may have therapeutic value in conditions characterized by Hhcy.
Vitamin deficiencies are commonly reported in patients with inflammatory bowel disease (IBD) and, in most cases, are a consequence of reduced intake or decreased absorption secondary to intestinal injury or surgical resection (1, 2). One of the most common deficiencies in IBD is of vitamin B6, affecting up to 30% of patients (3). Vitamin B deficiency can result in elevated blood homocysteine levels (hyperhomocysteinemia; Hhcy) (3, 4). Hhcy is associated with increased risk of thrombosis and microvascular disorders (5, 6), as well as with a significant worsening of IBD (1, 7). The mechanisms through which Hhcy exacerbates intestinal inflammation are not known.
In recent years, hydrogen sulfide (H2S) has become recognized as an important signaling molecule in many organs and tissues (8), and particularly as an anti-inflammatory and cytoprotective mediator (9). H2S is produced throughout the gastrointestinal (GI) tract, and its synthesis is markedly increased following mucosal injury (10–13). In such settings, H2S accelerates repair of damaged tissue and promotes resolution of inflammation (10, 11, 14). Conversely, inhibition of H2S synthesis leads to GI mucosal inflammation and impairment of healing of injury (10, 11, 14, 15).
There are three enzyme systems for endogenous synthesis of H2S, two of which require pyridoxal 5′-phosphate (P5P), the biologically active form of vitamin B6, as a cofactor for their activity (8). The two P5P-dependent enzymes for H2S synthesis are cystathionine γ-lyase (CSE) and cystathionine β-synthase (CBS). The Hhcy that develops during vitamin B deficiency is in part due to the insufficient conversion of homocysteine to cysteine via P5P-dependent enzymes. This is recapitulated in mice with genetic deficiencies of CSE (16) or CBS (17). These mice exhibit elevated blood levels of homocysteine (but not cysteine). During colitis, the marked elevation of H2S synthesis is primarily due to up-regulation of CSE (11, 14). The third key pathway for H2S synthesis, which is also up-regulated in colitis (14), involves a P5P-independent enzyme, 3-mercaptopyruvate transferase (3MST) (8).
It stands to reason that when levels of vitamin B6 are diminished, there may be an impairment of H2S synthesis. We hypothesized that Hhcy-related exacerbation of IBD may be a consequence of diminished intestinal production of H2S. To test this hypothesis, we examined the effects of diet-induced vitamin B deficiency in three models of colitis. One of these models was the interleukin (IL) 10–deficient mouse. In addition to choosing this model because it is a genetic model of colitis, rather than chemical, it is also relevant to the human disease because of established links between IBD and defective IL-10 signaling (18, 19). In the course of our study, we discovered an important regulatory interaction between IL-10 and H2S in modulating colonic tissue integrity that appears to be affected by, and be an influence upon, circulating homocysteine levels.
Results
Rats consuming the vitamin B-deficient (B-Def) diet for 7 wk developed significant Hhcy, with plasma homocysteine levels being approximately eightfold greater than for rats consuming the control diet (94.4 ± 15.3 μmol/L vs. 12.6 ± 1.0 μmol/L, respectively; P < 0.001). The mean body weights in the two groups were identical at the start of the study (223 g), but after 6 wk the rats on the B-Def diet exhibited significantly lower body weights (383 ± 5 g vs. 481 ± 7 g in controls; P < 0.01).
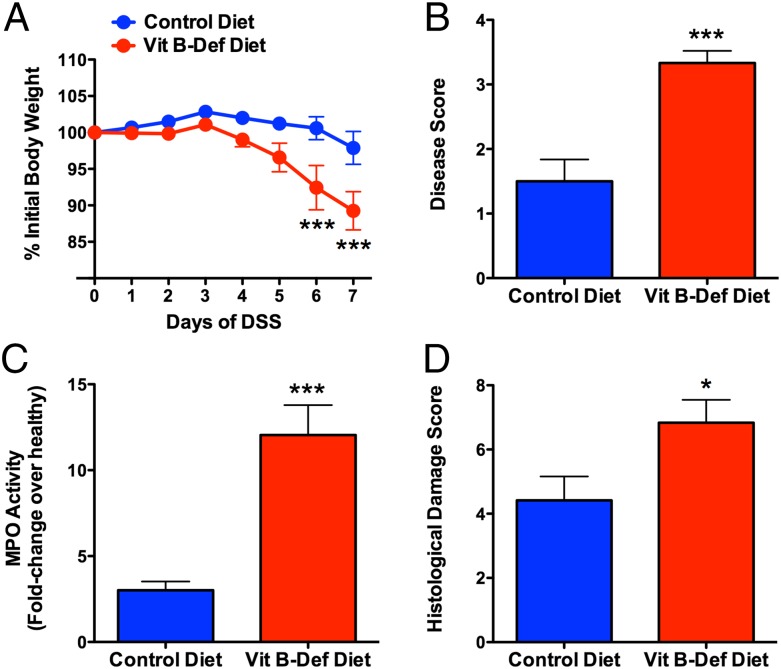
Effects of Hhcy on the severity of colitis were first examined in the dextran sodium sulfate (DSS) model (20). In the control group that received normal drinking water during the seventh week of the study, there were no differences between the two diets in terms of the macroscopic or histological appearance of the colon. However, in the rats on the B-Def diet, the basal colonic tissue levels of myeloperoxidase (MPO) were reduced by 75% (P < 0.001) compared with the rats on the control diet. The reduced tissue MPO in rats with Hhcy (Fig. 1C) is consistent with our observation of extensive neutrophil margination/extravasation in these rats and with reports of systemic neutropenia in humans with Hhcy (21, 22) and rats with vitamin B6 deficiency (23). When the drinking water was supplemented with DSS during the seventh week, significant weight loss, colonic damage, and inflammation were observed, and these changes were much more severe in the rats on the B-Def diet (Fig. 1). Colonic MPO levels increased significantly in both groups, but the extent of the increase was much greater in rats on the B-Def diet (12-fold) than in rats on the control diet (3-fold) (Fig. 1C). Consistent with the increase in the severity of colitis, there was also significantly greater expression of mRNA for tumor necrosis factor (TNF) α, IL-1β, and intercellular adhesion molecule (ICAM) 1 in colonic tissue from the rats on the B-Def diet than those on the control diet. We also observed an increase in colonic tissue expression of mRNA for IL-10, but it was a more variable response than that of the other cytokines (Fig. S1). It is noteworthy that the significantly greater severity of colitis in the rats on the B-Def diet occurred despite those rats consuming ∼36% less of the DSS-supplemented water over the final 3 d of the 7-d treatment period. The increased severity of colitis in the rats on the B-Def diet was confirmed by histology (Fig. 1D and Fig. S1A).
Fig. 1.
Diet-induced Hhcy exacerbates colitis induced in rats by DSS. When given DSS in drinking water, rats with Hhcy exhibited significant weight loss (A) and developed more severe colitis, as measured by a macroscopic colitis severity score (B), colonic tissue MPO activity (C), and a histological colitis severity score (D). Mean ± SEM with 10 rats per group. *P < 0.05, ***P < 0.001 versus the control diet group (one-way analysis of variance and Dunnett’s multiple comparison test).
Enteric bacteria contribute significantly to the pathogenesis of IBD, and can produce H2S (13, 24). To determine whether the B-Def diet altered enteric bacterial H2S production, fecal samples from rats on the two diets were collected over the 6-wk feeding period and their ability to produce H2S was determined (13). There was a transient decrease in H2S production in both groups at the end of 1 wk (Fig. S2), and thereafter fecal H2S production in the two groups was indistinguishable.
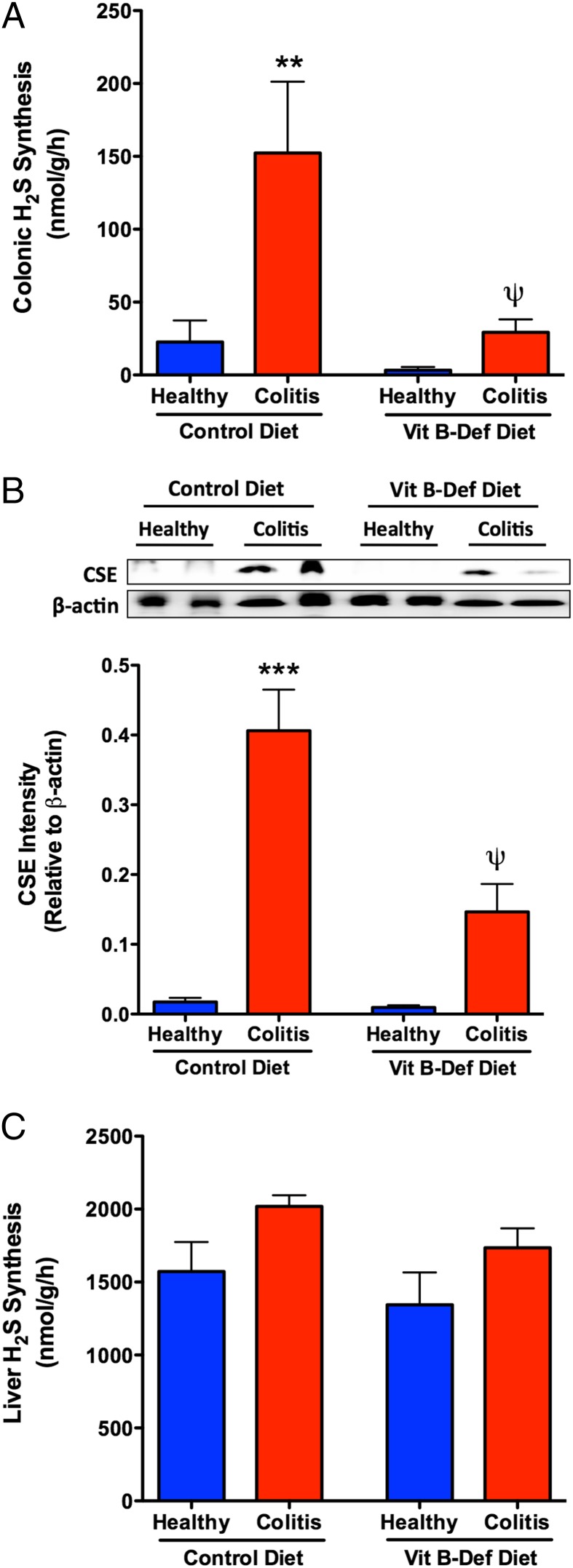
As described previously (11, 14), there was a substantial increase in colonic H2S synthesis (>7-fold; Fig. 2A) and colonic CSE expression (>12-fold; Fig. 2B) in rats on the control diet that received DSS. This increase in H2S synthesis has been shown to be crucial in limiting tissue injury and promoting resolution of colitis and healing of ulcers (11, 14). However, in rats on the B-Def diet, the usual robust increases in colonic H2S synthesis and colonic CSE expression were absent (Fig. 2 A and B). In contrast to these changes in the colon, induction of colitis and consumption of the B-Def diet had no effect on hepatic H2S synthesis (Fig. 2C). Colonic H2S synthesis via the 3MST (P5P-independent) pathway, and 3MST expression, were also elevated in the rats on the normal diet following induction of colitis. However, as was the case for CSE/CBS-derived H2S synthesis, the usual increase in colonic H2S synthesis observed in rats with colitis was absent when those rats were fed the B-Def diet (Fig. S3).
Fig. 2.
Damage-associated elevations in colonic H2S synthesis were abolished in rats with diet-induced Hhcy. Induction of colitis with DSS resulted in a marked elevation in colonic H2S synthesis (A) in rats on a control diet, but the increase in H2S synthesis was absent in the rats with diet-induced Hhcy. The increase in H2S synthesis was likely due largely to the increase in expression of CSE, a key enzyme for H2S synthesis (B), which was absent in rats with diet-induced Hhcy. Neither colitis nor Hhcy significantly changed H2S synthesis in the liver (C). Mean ± SEM with 10 rats per group. **P < 0.01, ***P < 0.001 versus the control diet group; ψP < 0.05 versus the corresponding control diet group (one-way analysis of variance and Dunnett’s multiple comparison test).
Many of the alterations observed in rats fed the B-Def diet were reversed by treatment with an H2S donor. Thus, twice-daily administration of diallyl disulfide (DADS) for 7 d during the period when the rats were receiving DSS resulted in a significant reduction in the colonic disease score and tissue MPO activity. Treatment with DADS also resulted in a marked increase in colonic CSE expression and H2S synthesis, to similar levels as observed in rats on the control diet following induction of colitis (Fig. S4). DADS administration also resulted in significant attenuation of colonic expression of mRNA for TNF-α, IL-1β, and ICAM-1, and a near-significant reduction of IL-10 expression (P = 0.07; Fig. S5). Thus, administration of the H2S donor reversed most of the changes that had been induced by feeding the rats the vitamin B-deficient diet.
Effects of Hhcy were also examined using a hapten-induced model of colitis. Intracolonic dinitrobenzene sulfonic acid (DNBS) administration resulted in severe ulceration and inflammation in rats on both diets. MPO activity in rats on the control diet increased threefold after DNBS administration, whereas in the B-Def group, DNBS provoked an approximately sixfold increase in MPO (P < 0.001; Fig. S6). As in the DSS model of colitis, there were marked changes in colonic H2S synthesis following induction of colitis with DNBS. Compared with rats fed the control diet, colonic H2S synthesis via the CSE/CBS pathways was 91% lower in the rats on the B-Def diet (P < 0.05; Fig. S6B), whereas colonic H2S synthesis via the 3MST pathway was reduced by 61% (P < 0.05; Fig. S6C). Thus, as in DSS-induced colitis, the severity of DNBS-induced colitis was increased, and the usual increase in colonic H2S synthesis was absent in rats on the B-Def diet.
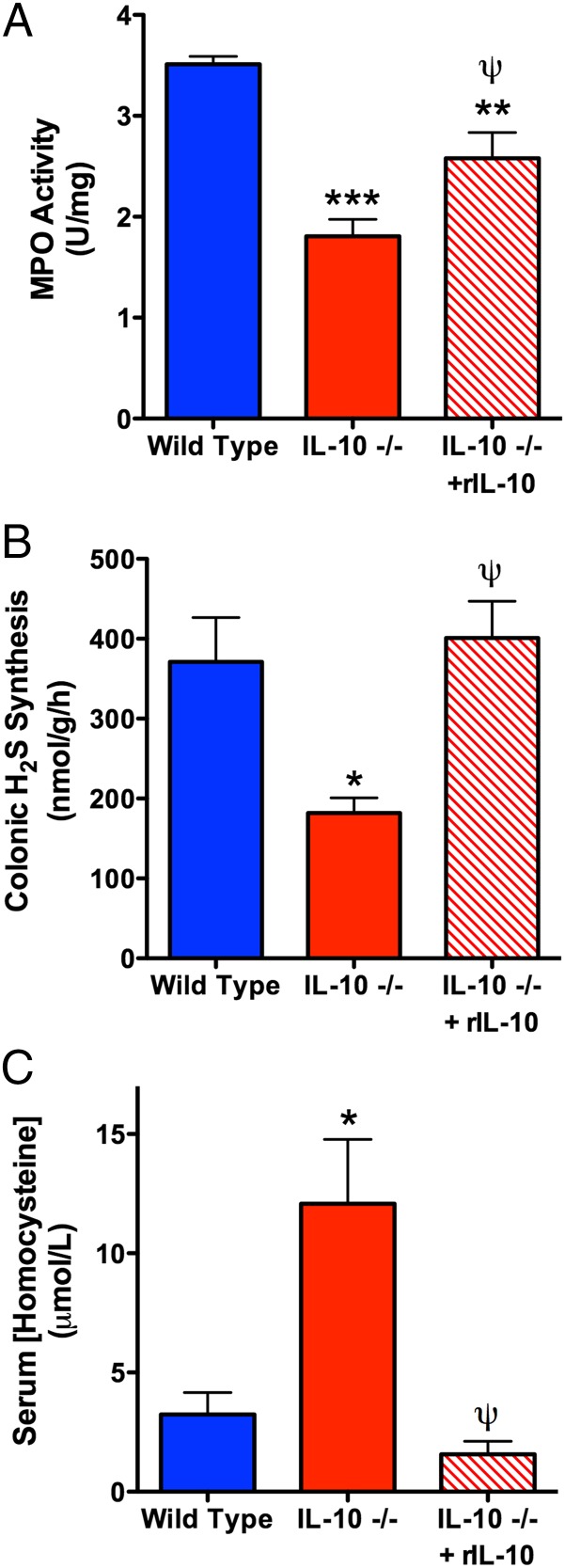
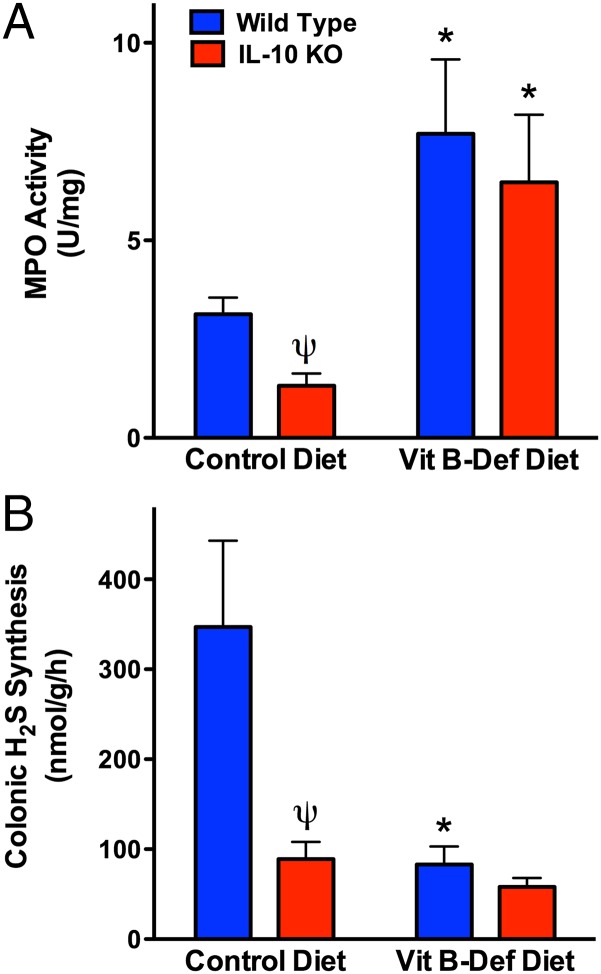
IL-10–deficient mice will spontaneously develop colitis, although the onset and severity vary depending on factors such as the relative cleanliness of animal housing facilities (22). We therefore used IL-10–deficient mice as a third model to test our hypothesis that a B-Def diet would increase the susceptibility to colitis, but the studies revealed some additional, intriguing data. IL-10–deficient mice fed the control diet for 7 wk did not exhibit overt signs of colitis (consistent with previous studies of these mice in the McMaster Animal Care Facility), and colonic MPO levels were significantly lower (∼70%) than those in wild-type littermates (see Fig. 4A). The reduced tissue granulocyte numbers (MPO) in IL-10–deficient mice are consistent with observations of neutropenia in humans with impaired IL-10 production (25). When fed the B-Def diet, significant colonic inflammation developed in both IL-10–deficient and wild-type mice, as indicated by the marked increases in colonic MPO levels (Fig. 3A). Although the absolute levels of MPO were similar in the two groups, the fold increase in MPO in the IL-10–deficient mice was more than double that in the wild-type mice, owing to the fact that the baseline levels of tissue MPO in IL-10–deficient mice were significantly reduced compared with wild-type mice.
Fig. 4.
Administration of recombinant IL-10 (rIL) to IL-10–deficient mice normalized colonic MPO levels and H2S synthesis and markedly reduced serum homocysteine levels. Mean ± SEM for six mice per group (*P < 0.05, **P < 0.01, ***P < 0.001 versus wild-type mice; ψP < 0.05 versus vehicle-treated IL-10–deficient mice; ANOVA and Dunnett’s multiple comparison test).
Fig. 3.
IL-10–deficient mice exhibit impaired colonic H2S synthesis and increased colonic inflammation in response to a vitamin B-deficient diet. IL-10–deficient mice on the control diet had significantly lower colonic levels of MPO than wild-type mice (A) and reduced colonic H2S synthesis (B). Mean ± SEM for six mice per group (*P < 0.05 versus the corresponding mice on the control diet; ψP < 0.05 versus the corresponding wild-type group; ANOVA and Dunnett’s multiple comparison test).
IL-10–deficient mice on the control diet exhibited greatly reduced colonic H2S synthesis compared with the wild-type mice (Fig. 3B). When wild-type mice were fed the B-Def diet, they demonstrated the same marked reduction in colonic H2S synthesis. Thus, the data from wild-type mice recapitulated what had been observed in the rat studies (vitamin B deficiency leading to impaired colonic H2S synthesis). On the other hand, the data from IL-10–deficient mice suggested a “baseline” impairment of colonic H2S synthesis in those mice, similar to what could be induced in wild-type mice through vitamin B deficiency/Hhcy. Because the IL-10–deficient mice were exhibiting a phenotype similar to that of rats with Hhcy with respect to colonic H2S synthesis and basal tissue MPO activity, we investigated the possibility that IL-10–deficient mice had elevated serum levels of homocysteine. Indeed, as shown in Fig. 4C, serum homocysteine levels in IL-10–deficient mice were elevated more than fourfold over those of wild-type mice (P < 0.05), achieving concentrations deemed as hyperhomocysteinemic in mice (26). In contrast, the wild-type mice had serum homocysteine levels in the normal range for that strain (26).
Administration of IL-10 to these mice twice over 24 h resulted in normalization of colonic H2S synthesis, a significant recovery of colonic MPO activity toward that of wild-type mice, and a reduction in serum homocysteine levels to those of wild-type mice (Fig. 4).
To further examine the potential regulation of IL-10 synthesis by H2S, rats (n = 5 each) were treated intraperitoneally with either an inhibitor of H2S synthesis (l-propargylglycine; 25 mg/kg) or with an H2S donor (NaHS; 100 μmol/kg), and serum IL-10 levels were measured 4 h later. Serum levels of IL-10 in vehicle-treated rats averaged 55 ± 4 pg/mL. The inhibitor of H2S synthesis significant decreased serum IL-10 levels (by 45%; P < 0.05), whereas the H2S donor significantly increased serum IL-10 levels (by 38%; P < 0.05).
Discussion
The incidence of IBD (Crohn disease and ulcerative colitis) has been steadily increasing over the past 7 decades, apparently with earlier age of onset (27, 28). The causes of IBD remain poorly understood and, as a consequence, its treatment remains a challenge. The present study was undertaken to try to gain a better understanding of the reasons for the significant association between IBD and Hhcy, with the hope of gaining insights that would facilitate development of improved treatment options. We examined the role of hydrogen sulfide in this context because its synthesis occurs largely through vitamin B6-dependent enzymes, and it has been shown to play vital roles in modulating GI mucosal defense, accelerating healing of ulcers, and promoting resolution of inflammation (10, 11, 14). Inhibition of H2S synthesis leads to mucosal inflammation, an increase in susceptibility to injury, and impaired healing of damaged tissue (10, 11, 14). Conversely, H2S donors accelerate ulcer healing and exert significant anti-inflammatory effects in the GI tract and elsewhere (10, 11, 29). In the present study, induction of Hhcy through feeding animals a diet deficient of B vitamins led to a marked impairment of colonic H2S synthesis and a significant increase in the severity of colitis in three different models. These results are consistent with clinical observations of an association between Hhcy and IBD (5, 6). Administration of an H2S donor reversed the detrimental effects of the B-def diet. Moreover, studies in IL-10–deficient mice, which can spontaneously develop colitis (30), revealed a marked impairment of colonic H2S synthesis and a modest elevation of serum homocysteine levels, both of which were reversed by administration of IL-10. Moreover, IL-10–deficient mice, like rats with Hhcy, had diminished tissue levels of neutrophils, which was also abolished by treatment with IL-10.
H2S is synthesized from cysteine through three different enzyme systems, two of which are dependent upon vitamin B6 for their activity (8). We speculated that in Hhcy, the diminished serum levels of cysteine could lead to impaired H2S synthesis, and in turn to an increased susceptibility to colitis. The robust increase in colonic H2S synthesis that normally occurs after injury was absent in the Hhcy rats, but this impairment was seen with both the vitamin B6-dependent and -independent pathways of H2S synthesis. One possible explanation is that Hhcy led to a reduced systemic availability of cysteine, as a consequence of reduced conversion of homocysteine to cysteine. However, although a decrease in H2S synthesis was clearly evident in the colon, there was no significant alteration of H2S synthesis in the liver. Another possible explanation is that Hhcy resulted in decreased expression of enzymes that convert cysteine to H2S, particularly CSE. Indeed, we observed a profound reduction in expression of CSE in the colon of rats fed the B-Def diet, and a failure of the normal, rapid up-regulation of CSE expression after administration of colitis-triggering chemicals (DSS or DNBS). Administration of an H2S donor restored the up-regulation of CSE expression and, in turn, colonic H2S synthesis.
IL-10–deficient mice were used as a third model of colitis and, as in the DSS and DNBS models, feeding these mice a vitamin B-deficient diet resulted in a significant worsening of colitis (e.g., markedly greater increase in colonic MPO activity). However, the IL-10–deficient mice, even on a normal diet, exhibited a phenotype similar to what was observed with rats on the vitamin B-deficient diet. Thus, levels of colonic H2S synthesis in the IL-10–deficient mice were significantly lower than in wild-type mice when fed either the control or the vitamin B-deficient diet. As in the rats with Hhcy, the IL-10–deficient mice exhibited marked neutropenia, as has been reported to occur in humans with Hhcy (21, 22) and in rats with vitamin B6 deficiency (23). The similarity in phenotype between the rats with diet-induced Hhcy and the IL-10–deficient mice was confirmed by measurements of serum homocysteine levels, which confirmed that these mice had mild to moderate Hhcy (31). Moreover, we confirmed that there is an interplay among IL-10, homocysteine, and H2S synthesis, because serum homocysteine levels and colonic H2S synthesis could be normalized in the IL-10–deficient mice through administration of recombinant IL-10.
A stimulatory effect of H2S on IL-10 expression/synthesis has been demonstrated in several studies. Thus, administration of H2S donors has been shown to suppress expression/synthesis of several “proinflammatory” cytokines (e.g., IL-1β, IL-6, IL-8, IL-18, TNF-α, IFN-γ, RANTES, etc.) in a variety of tissues while either sparing or stimulating expression/synthesis of IL-10 (29, 32–35). In the present study, treatment of rats with colitis with the H2S donor (DADS) resulted in significant suppression of expression of IL-1β, TNF-α, and ICAM-1. Although expression of IL-10 was also reduced, it remained ∼12-fold greater than expression in healthy rats. The ability of H2S to modulate IL-10 expression/synthesis was also evident from the observation that administration of an inhibitor of H2S synthesis increased serum IL-10 levels in rats, whereas administration of an H2S donor significantly reduced those levels.
There is substantial evidence for IL-10 playing an essential role in modulating intestinal integrity in patients with IBD. Genome-wide association studies have established strong links between defective IL-10 production/secretion and the development of IBD (18, 36, 37). IL-10 plays a critical role in promoting resolution of mucosal inflammation (38). A loss of this IL-10 signaling results in impaired resolution, which is observed clinically in “very early onset” IBD patients who have a deficiency of IL-10 receptors (18). There is also evidence for altered IL-10 signaling in patients with Hhcy, including reduced effectiveness of IL-10 in modulating release of matrix metalloprotease 9 (39). Recombinant IL-10 has shown some promise as a therapy for IBD (40).
Consistent with our findings, there are similarities in vascular responses in IL-10–deficient mice and animals with Hhcy. For example, carotid arteries from IL-10–deficient mice exhibit increased production of superoxide and impaired relaxation of arteries (41). Impaired vasodilation and endothelial dysfunction are hallmark features of Hhcy, and can be observed in mice deficient of either of the vitamin B6-dependent enzymes involved in H2S synthesis (16, 42). Indeed, H2S is one of the important endothelial-derived hyperpolarizing factors (43), raising the possibility, consistent with our observations, that impaired endothelial vasodilation in Hhcy is due, at least in part, to lack of H2S production. Homocysteine is able to induce inflammation and vascular dysfunction through its oxidative actions (44). Conversely, H2S has been shown to attenuate or abolish the oxidative injury associated with Hhcy, including that in the GI tract (45–47), and to be a potent endogenous anti-inflammatory substance (48).
In summary, our studies demonstrate that dietary induction of Hhcy results in exacerbation of colitis in three animal models, thus mimicking the clinical scenario in which IBD is more prevalent and severe in patients with elevated homocysteine levels. Furthermore, the absence of a rapid elevation of colonic H2S synthesis in animals with Hhcy appears to be a key factor in the observed exacerbation of colitis. There is significant cross-regulation of production of H2S and IL-10 by one another, which can impact resolution of inflammation in the colon as well as circulating homocysteine levels. These studies suggest that modulation of the IL-10/H2S signaling pathway may be a rational target for novel therapeutics for IBD.
Methods and Materials
Animals.
Male Wistar rats (125–150 g; Charles River Breeding Farms) and C57BL/6J homozygous IL-10–deficient mice (8 wk of age; Jackson Laboratory) were housed in plastic cages and maintained under controlled temperature (20 °C), humidity (60%–70%), and light cycle (12 h:12 h light–dark). All experimental protocols were approved by the Animal Research Ethics Board at McMaster University and adhered to the guidelines established by the Canadian Council on Animal Care. The body weights of animals were measured weekly.
Induction of Hyperhomocysteinemia.
Rats and mice were provided one of two diets (Harlan Teklad) for 6 wk. One diet (“B-Def”) lacked vitamins B6, B9, and B12 (49). The control diet was identical except that it contained the above-mentioned vitamins and folate. Both diets contained 1% sulfathiazole to inhibit folate formation by gut bacteria (49). Fecal samples were collected weekly. Serum homocysteine levels were measured by chemiluminescent microparticle immunoassay (rat) or ELISA (mouse).
Effects of Hhcy on Severity of Colitis.
Three models of colitis were used. In the first, after 6 wk on the B-Def or control diet, rats were provided either normal drinking water or drinking water supplemented with dextran sodium sulfate (5% wt/vol; 36–50 kDa) ad libitum. All rats remained on the diets while receiving DSS/water for 7 d. Consumption of drinking water (±DSS) was measured daily.
The second model involved induction of colitis, after 6 wk on the B-Def or control diet, by intracolonic administration of the hapten dinitrobenzene sulfonic acid (50). Severity of colitis was evaluated 3 d after DNBS administration as described previously (50).
IL-10–deficient mice were used as the third model of colitis (30). These mice and wild-type littermates (10 per group) were provided the B-Def or control diet for 7 wk, and severity of colitis was then evaluated.
After the animals were euthanized, the colons were removed, opened by incision along the mesenteric border, and blindly evaluated for disease severity using a modified version of a previously described “disease activity index” (20). Each animal was scored for the presence of adhesions between the colon and other visceral tissues (0, no adhesions; 1, adhesions present; 2, severe adhesions present), diarrhea (0 or 1 for absence or presence of diarrhea, respectively), and luminal blood (0, no bleeding; 1, presence of blood; 2, frank bleeding).
Histological damage scoring of the colon was performed blindly on formalin-fixed, paraffin-embedded sections stained with H&E using previously described scoring criteria (20, 51).
Tissue samples were excised from the colon of each animal for measurement of myeloperoxidase, a biochemical marker of tissue granulocyte content (52).
In some experiments, 8-wk-old IL-10 KO and wild-type mice were treated with 4 μg of recombinant mouse IL-10 (i.p.) in 100 μL of sterile PBS or with vehicle alone (53). The mice received two doses of IL-10, 12 h apart. Blood was drawn for measurement of plasma homocysteine levels 4 h after the second administration. Colonic tissue was collected for measurement of H2S synthesis, MPO activity, and Western blot analysis.
Tissue H2S Synthesis.
The ability of tissue to produce H2S was measured from homogenized tissue in the presence of exogenous substrate using a modified version (10) of a previously described zinc-trapping assay (54). The homogenates were incubated in the presence/absence of l-cysteine (4 mM) and pyroxidal-5′-phosphate (2 mM).
Effects of an H2S Donor on Colitis.
Beginning 1 h after DSS (dissolved in drinking water) was provided, groups of rats were treated twice daily, intracolonically, with diallyl disulfide (30 μmol/kg) or vehicle (1% carboxymethylcellulose) for 7 d. DADS exerts protective effects at this dose (15). The severity of colitis was blindly evaluated 2 h after the final dose. Colonic tissue samples were processed for measurement of MPO activity, histology, RT-PCR, and Western blotting. Primary antibodies for CSE (1:200), 3MST (1:200), and β-actin (1:10,000) were used.
Quantitative PCR.
RNA was extracted from colonic tissue and quantitative PCR was performed as previously described (55). Bioinformatically validated primer assays for mouse CSE, TNF-α, IL-1β, IL-10, and β-actin were used (Qiagen). In addition, a validated, custom-made primer for ICAM-1 was used (10, 11). All data were analyzed as previously described, with results expressed as fold increase relative to β-actin (55).
Materials.
Isoflurane was from Abbott Laboratories. Recombinant, carrier-free, mouse IL-10 was from Cell Signaling. The validated ICAM-1 primer set was from Integrated DNA Technologies. All other reagents were from Sigma-Aldrich.
Statistical Analysis.
All data are expressed as the mean ± SEM. Groups of data were compared using a one-way analysis of variance and the Dunnett’s multiple comparison test, or the Student t test where appropriate. An associated probability of less than 5% was considered significant.
Supplementary Material
Acknowledgments
The authors are grateful to Michael Dicay and Webb McKnight for their assistance with these studies. This work was supported by grants from the Canadian Institutes of Health Research and Crohn’s Colitis Canada.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a Prearranged Editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1413390111/-/DCSupplemental.
References
- 1.Oldenburg B, Fijnheer R, van der Griend R, vanBerge-Henegouwen GP, Koningsberger JC. Homocysteine in inflammatory bowel disease: A risk factor for thromboembolic complications? Am J Gastroenterol. 2000;95(10):2825–2830. doi: 10.1111/j.1572-0241.2000.03193.x. [DOI] [PubMed] [Google Scholar]
- 2.Vagianos K, Bernstein CN. Homocysteinemia and B vitamin status among adult patients with inflammatory bowel disease: A one-year prospective follow-up study. Inflamm Bowel Dis. 2012;18(4):718–724. doi: 10.1002/ibd.21785. [DOI] [PubMed] [Google Scholar]
- 3.Vagianos K, Bector S, McConnell J, Bernstein CN. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2007;31(4):311–319. doi: 10.1177/0148607107031004311. [DOI] [PubMed] [Google Scholar]
- 4.Jacques PF, et al. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation. 1996;93(1):7–9. doi: 10.1161/01.cir.93.1.7. [DOI] [PubMed] [Google Scholar]
- 5.den Heijer M, et al. Hyperhomocysteinemia as a risk factor for deep-vein thrombosis. N Engl J Med. 1996;334(12):759–762. doi: 10.1056/NEJM199603213341203. [DOI] [PubMed] [Google Scholar]
- 6.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ. 2002;325(7374):1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saibeni S, et al. Low vitamin B(6) plasma levels, a risk factor for thrombosis, in inflammatory bowel disease: Role of inflammation and correlation with acute phase reactants. Am J Gastroenterol. 2003;98(1):112–117. doi: 10.1111/j.1572-0241.2003.07160.x. [DOI] [PubMed] [Google Scholar]
- 8.Kimura H. Production and physiological effects of hydrogen sulfide. Antioxid Redox Signal. 2014;20(5):783–793. doi: 10.1089/ars.2013.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace JL, et al. Anti-inflammatory and cytoprotective actions of hydrogen sulfide: Translation to therapeutics. Antioxid Redox Signal. April 15, 2014 doi: 10.1089/ars.2014.5901. [DOI] [PubMed] [Google Scholar]
- 10.Wallace JL, Dicay M, McKnight W, Martin GR. Hydrogen sulfide enhances ulcer healing in rats. FASEB J. 2007;21(14):4070–4076. doi: 10.1096/fj.07-8669com. [DOI] [PubMed] [Google Scholar]
- 11.Wallace JL, Vong L, McKnight W, Dicay M, Martin GR. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology. 2009;137(2):569–578.e1. doi: 10.1053/j.gastro.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Martin GR, et al. Hydrogen sulphide synthesis in the rat and mouse gastrointestinal tract. Dig Liver Dis. 2010;42(2):103–109. doi: 10.1016/j.dld.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Flannigan KL, McCoy KD, Wallace JL. Eukaryotic and prokaryotic contributions to colonic hydrogen sulfide synthesis. Am J Physiol Gastrointest Liver Physiol. 2011;301(1):G188–G193. doi: 10.1152/ajpgi.00105.2011. [DOI] [PubMed] [Google Scholar]
- 14.Flannigan KL, Ferraz JG, Wang R, Wallace JL. Enhanced synthesis and diminished degradation of hydrogen sulfide in experimental colitis: A site-specific, pro-resolution mechanism. PLoS ONE. 2013;8(8):e71962. doi: 10.1371/journal.pone.0071962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace JL. Physiological and pathophysiological roles of hydrogen sulfide in the gastrointestinal tract. Antioxid Redox Signal. 2010;12(9):1125–1133. doi: 10.1089/ars.2009.2900. [DOI] [PubMed] [Google Scholar]
- 16.Yang G, et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine γ-lyase. Science. 2008;322(5901):587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamath AF, et al. Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood. 2006;107(2):591–593. doi: 10.1182/blood-2005-06-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noguchi E, Homma Y, Kang X, Netea MG, Ma X. A Crohn’s disease-associated NOD2 mutation suppresses transcription of human IL10 by inhibiting activity of the nuclear ribonucleoprotein hnRNP-A1. Nat Immunol. 2009;10(5):471–479. doi: 10.1038/ni.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moran CJ, et al. IL-10R polymorphisms are associated with very-early-onset ulcerative colitis. Inflamm Bowel Dis. 2013;19(1):115–123. doi: 10.1002/ibd.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69(2):238–249. [PubMed] [Google Scholar]
- 21.Watkins D, Rosenblatt DS. Inborn errors of cobalamin absorption and metabolism. Am J Med Genet C Semin Med Genet. 2011;157C(1):33–44. doi: 10.1002/ajmg.c.30288. [DOI] [PubMed] [Google Scholar]
- 22.Niyikiza C, et al. Homocysteine and methylmalonic acid: Markers to predict and avoid toxicity from pemetrexed therapy. Mol Cancer Ther. 2002;1(7):545–552. [PubMed] [Google Scholar]
- 23.Cassel S, Robson L, Rosse C. The effects of vitamin B6 deficiency on the bone marrow of the rat. Anat Rec. 1978;191(1):47–53. doi: 10.1002/ar.1091910105. [DOI] [PubMed] [Google Scholar]
- 24.Duboc H, et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013;62(4):531–539. doi: 10.1136/gutjnl-2012-302578. [DOI] [PubMed] [Google Scholar]
- 25.Madsen KL. Inflammatory bowel disease: Lessons from the IL-10 gene-deficient mouse. Clin Invest Med. 2001;24(5):250–257. [PubMed] [Google Scholar]
- 26.Koumaki V, et al. Pro-inflammatory bone marrow milieu in patients with chronic idiopathic neutropenia is associated with impaired local production of interleukin-10. Br J Haematol. 2006;135(4):570–573. doi: 10.1111/j.1365-2141.2006.06345.x. [DOI] [PubMed] [Google Scholar]
- 27.Benchimol EI, et al. Increasing incidence of paediatric inflammatory bowel disease in Ontario, Canada: Evidence from health administrative data. Gut. 2009;58(11):1490–1497. doi: 10.1136/gut.2009.188383. [DOI] [PubMed] [Google Scholar]
- 28.Molodecky NA, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54.e42. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Fiorucci S, et al. Enhanced activity of a hydrogen sulphide-releasing derivative of mesalamine (ATB-429) in a mouse model of colitis. Br J Pharmacol. 2007;150(8):996–1002. doi: 10.1038/sj.bjp.0707193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidson NJ, et al. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med. 1996;184(1):241–251. doi: 10.1084/jem.184.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ernest S, Hosack A, O’Brien WE, Rosenblatt DS, Nadeau JH. Homocysteine levels in A/J and C57BL/6J mice: Genetic, diet, gender, and parental effects. Physiol Genomics. 2005;21(3):404–410. doi: 10.1152/physiolgenomics.00199.2004. [DOI] [PubMed] [Google Scholar]
- 32.Li T, et al. Regulatory effects of hydrogen sulfide on IL-6, IL-8 and IL-10 levels in the plasma and pulmonary tissue of rats with acute lung injury. Exp Biol Med (Maywood) 2008;233(9):1081–1087. doi: 10.3181/0712-RM-354. [DOI] [PubMed] [Google Scholar]
- 33.Tokuda K, et al. Inhaled hydrogen sulfide prevents endotoxin-induced systemic inflammation and improves survival by altering sulfide metabolism in mice. Antioxid Redox Signal. 2012;17(1):11–21. doi: 10.1089/ars.2011.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng J, Lin X, Fan H, Li C. Hydrogen sulfide attenuates the inflammatory response in a mouse burn injury model. Mol Med Rep. 2013;8(4):1204–1208. doi: 10.3892/mmr.2013.1610. [DOI] [PubMed] [Google Scholar]
- 35.Wu J, et al. Inhibition of hydrogen sulfide generation contributes to lung injury after experimental orthotopic lung transplantation. J Surg Res. 2013;182(1):e25–e33. doi: 10.1016/j.jss.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 36.van der Linde K, et al. A Gly15Arg mutation in the interleukin-10 gene reduces secretion of interleukin-10 in Crohn disease. Scand J Gastroenterol. 2003;38(6):611–617. [PubMed] [Google Scholar]
- 37.Franke A, et al. IBSEN Study Group Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet. 2008;40(11):1319–1323. doi: 10.1038/ng.221. [DOI] [PubMed] [Google Scholar]
- 38.Shouval DS, et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40(5):706–719. doi: 10.1016/j.immuni.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holven KB, et al. Impaired inhibitory effect of interleukin-10 on the balance between matrix metalloproteinase-9 and its inhibitor in mononuclear cells from hyperhomocysteinemic subjects. Stroke. 2006;37(7):1731–1736. doi: 10.1161/01.STR.0000226465.84561.cb. [DOI] [PubMed] [Google Scholar]
- 40.Colombel JF, et al. Interleukin 10 (Tenovil) in the prevention of postoperative recurrence of Crohn’s disease. Gut. 2001;49(1):42–46. doi: 10.1136/gut.49.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gunnett CA, Heistad DD, Berg DJ, Faraci FM. IL-10 deficiency increases superoxide and endothelial dysfunction during inflammation. Am J Physiol Heart Circ Physiol. 2000;279(4):H1555–H1562. doi: 10.1152/ajpheart.2000.279.4.H1555. [DOI] [PubMed] [Google Scholar]
- 42.Cheng Z, et al. Hyperhomocysteinemia impairs endothelium-derived hyperpolarizing factor-mediated vasorelaxation in transgenic cystathionine beta synthase-deficient mice. Blood. 2011;118(7):1998–2006. doi: 10.1182/blood-2011-01-333310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20(21):6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ingenbleek Y. The oxidative stress of hyperhomocysteinemia results from reduced bioavailability of sulfur-containing reductants. Open Clin Chem J. 2011;4:34–44. [Google Scholar]
- 45.Elrod JW, et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA. 2007;104(39):15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang L, et al. Hydrogen sulfide inhibits myocardial injury induced by homocysteine in rats. Amino Acids. 2008;34(4):573–585. doi: 10.1007/s00726-007-0011-8. [DOI] [PubMed] [Google Scholar]
- 47.Yonezawa D, et al. A protective role of hydrogen sulfide against oxidative stress in rat gastric mucosal epithelium. Toxicology. 2007;241(1-2):11–18. doi: 10.1016/j.tox.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 48.Zanardo RC, et al. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20(12):2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 49.Troen AM, et al. B-vitamin deficiency causes hyperhomocysteinemia and vascular cognitive impairment in mice. Proc Natl Acad Sci USA. 2008;105(34):12474–12479. doi: 10.1073/pnas.0805350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wallace JL, Le T, Carter L, Appleyard CB, Beck PL. Hapten-induced chronic colitis in the rat: Alternatives to trinitrobenzene sulfonic acid. J Pharmacol Toxicol Methods. 1995;33(4):237–239. doi: 10.1016/1056-8719(95)00001-x. [DOI] [PubMed] [Google Scholar]
- 51.Kim JJ, et al. Targeted inhibition of serotonin type 7 (5-HT7) receptor function modulates immune responses and reduces the severity of intestinal inflammation. J Immunol. 2013;190(9):4795–4804. doi: 10.4049/jimmunol.1201887. [DOI] [PubMed] [Google Scholar]
- 52.Boughton-Smith NK, Wallace JL, Whittle BJR. Relationship between arachidonic acid metabolism, myeloperoxidase activity and leukocyte infiltration in a rat model of inflammatory bowel disease. Agents Actions. 1988;25(1-2):115–123. doi: 10.1007/BF01969102. [DOI] [PubMed] [Google Scholar]
- 53.Mittal R, et al. IL-10 administration reduces PGE-2 levels and promotes CR3-mediated clearance of Escherichia coli K1 by phagocytes in meningitis. J Exp Med. 2010;207(6):1307–1319. doi: 10.1084/jem.20092265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J. 1982;206(2):267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chávez-Piña AE, et al. Lack of effects of acemetacin on signalling pathways for leukocyte adherence may explain its gastrointestinal safety. Br J Pharmacol. 2008;155(6):857–864. doi: 10.1038/bjp.2008.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.