Abstract
Load-bearing, mechanically active tissues are routinely subjected to non-linear mechanical deformations. Consequently, these tissues exhibit complex mechanical properties and unique tissue organizations. Successful engineering of mechanically active tissues relies on the integration of the mechanical sensing mechanism found in the native tissues into polymeric scaffolds. Intelligent biomaterials that closely mimic the structural organizations and multi-scale responsiveness of the natural extracellular matrices (ECM), when strategically combined with multipotent cells and dynamic culture devices that generate physiologically relevant physical forces, will lead to the creation of artificial tissues that are mechanically robust and biologically functional.
Keywords: Tissue Engineering, Mechanically Active, Mechanoresponsive, Biomaterials, Scaffolds, Bioreactor
1. Background and Introduction
Although the field of tissue engineering has witnessed great progress over the past few decades, only a few engineered products (skin,1 tracheal cartilage,2 bone3 and bladders4) have had clinical success. Many tissues/organs in the human body are mechanically active, sustaining repetitive and non-linear deformations throughout their lifetime. For example, cartilage, tendons and ligaments are exposed to compression, tension, shear and torsion associated with normal joint movement. The human heart contracts more than three billion times in an average human lifespan5 and blood vessels are subjected to mechanical forces in a form of radial distention, encompassing cyclic mechanical strain due to the pulsatile nature of blood flow.6 Human vocal folds, perhaps one of the most mechanically active tissues in the body, oscillate regularly at a frequency of 100 to 1000 Hz with a maximum strain of 30% during normal phonation.7
The demanding biomechanical functions load-bearing tissues must fulfill render them susceptible to damage. Cells in these active tissues are terminally differentiated and have minimal replicative capacity. This is further compounded by the avascularity and low celluarity of the tissues (with the exception of cardiac tissues). The stringent mechanical environment makes it especially challenging for tissue repair and regeneration. Materials-based approaches8 that yield tissue-engineered substitutes offer compelling alternatives in the treatment of diseases associated with these tissues/organs. Materials9 currently utilized for the repair and regeneration of mechanically active, soft connective tissues include hydrophobic scaffolds derived from polyesters, polyurethanes, and polycarbonates,10 hydrophilic matrices based on alginate, poly(ethylene glycol) (PEG), collagen/gelatin, hyaluronic acid (HA), fibrin, dextran, agarose, chitosan, Matrigel and self-assembled peptide fibrils, 11 and decellularized extracellular matrices (ECMs) derived from native porcine tissues, such as urinary bladder and small intestine submucosa.12
In this Prospective article, we summarize recent endeavors in design and development of synthetic biomaterials with robust mechanical properties, complex structural organizations, and defining biological features that are essential for the successful engineering of mechanically active tissues. The advantages and disadvantages of each type of material and approach are discussed. We provide a forward looking view on the importance of incorporating mechano-responsive elements in the scaffolding materials so that the materials’ properties and the cellular functions can be dynamically controlled by physiologically relevant mechanical stimuli. We believe that the strategic combination of bioactive and mechano-responsive materials with mulitpotent cells will result in controlled cell differentiation, enhanced cell growth and accelerated tissue morphogenesis.
2. Mechanoresponsive materials with elastomeric properties
Successful scaffolding materials for the engineering of mechanically active soft tissues should, first and foremost, exhibit mechanical responses comparable to the target tissues. Materials capable of complete recovery from cyclic deformations can effectively transmit the external mechanical stimulations to the cultured cells in vitro, and at the same time, fulfill the mechanical functions of the target tissues in vivo without inducing mechanical irradiations to the host tissue.13 Elastomers, by definition, are mechanoresponsive materials because they can undergo large and reversible deformations at relatively low stresses. This is accomplished via force-induced alteration of polymer conformation from random coils to extended chains. The entropic recoil, reinforced by the covalent or physical crosslinking, is the major mechanism for recovery upon cessation of the external forces. 14 Both thermoplastic (e.g. polyurethanes) and thermoset elastomers (e.g. chemically crosslinked polyesters) have been extensively explored for tissue engineering purposes. 10, 13 By varying the polymer composition, molecular weight and the crosslinking density, the mechanical properties of these materials can be readily tuned.
Elastomeric materials do not have to be hydrophobic in nature. In fact, hydrogel matrices with elastomeric properties are more desirable for tissue engineering purposes.15 Unfortunately, traditional hydrogels usually exhibit slow responses and inferior mechanical properties owing to the presence of network defects, uncontrolled heterogeneity, high water content, and small friction between the chains.16 By carefully balancing the hydrophobicity/hydrophilicity of the hydrogel network, as well as the molecular weight between crosslinks, mechanically tough and biodegradable hydrogels were developed.17 Alternatively, a loosely crosslinked network entangled around a densely crosslinked first network can effectively absorb the energy by viscous dissipation or by large deformation of the polymer chains, giving rise to double network (DN) gels that are mechanically tough.18 Finally, elastomeric gels can be obtained by careful compositing hydrophilic polymers with inorganic nanoparticles.19 Although these hydrogels exhibit unprecedented mechanical strength, their utility in tissue engineering has not been realized yet owing to the incompatible chemistries.
The natural ECM consists of less than 1% solid materials, yet they are mechanically robust and functionally diverse. 20 Among these materials is a network of elastic fibers mainly composed of elastin that is capable of withstanding significant deformations without rupture, and recovers to its original state when the stress is removed. Elastin is composed largely of two types of short segments that alternate along the polypeptide chain: highly flexible hydrophobic segments, with many transient structures that can easily change their conformation when stretched; and alanine- and lysine-rich α-helical segments that form covalent cross-links between adjacent molecules.21 Over the past decade, much work has been devoted to the development of genetically engineered artificial elastin-like polypeptides (ELPs),22 including a small number of ELPs with a multiblock molecular architecture similar to the natural protein.23 Taking advantage of controlled polymerization and orthogonal coupling chemistries, Jia and colleagues have created elastin-mimetic hybrid polymers (EMHPs) that contain PEG alternating with an alanine-rich, lysine-containing peptide that is abundant in the crosslinking regions of the natural elastin. The modular nature of our synthesis allows for ready adjustment of the peptide sequence to achieve the desired biological functions. The resultant multiblock copolymers (Figure 1), when covalently crosslinked, exhibit mechanical properties similar to natural elastin and are capable of supporting the attachment and proliferation of fibroblasts.24-26 Intrigued by the superior resilience and excellent high-frequency responsiveness of resilin found in specialized compartments of most arthropods, researchers have produced recombinant resilin-like polypeptide bearing both mechanically active 27, 28 and biologically active domains. These materials are being used for vocal fold tissue engineering.29 Peptide or protein-based materials, however, may induce immunogenicity in vivo and their production in large quantity with consistent properties may be challenging.
Figure 1.
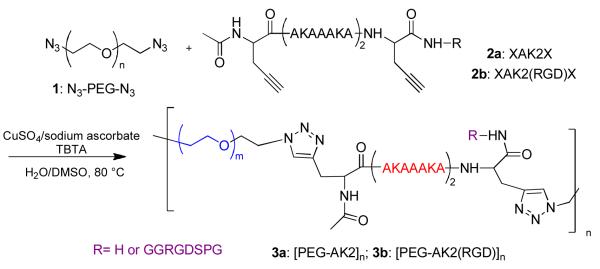
Synthesis of elastin-mimetic hybrid polymer (EMHPs), with or without the cell-adhesive RGD sequence, via the condensation polymerization of azide-functionalized PEG (1) and alkyne-terminated peptide (2a or 2b) employing copper (I)-catalyzed alkyne-azide cycloaddtion reaction.
The polymeric networks discussed above are connected by strong covalent bonds. While covalent networks can be designed to exhibit outstanding mechanical properties, they do not have self-repair mechanisms once failure occurs. Moreover, if scaffold degradation and matrix secretion by the resident cells are not perfectly matched,30 the mechanical properties of the constructs will deteriorate over time. Therefore, a reversible self-repair mechanism is desirable for the maintenance of mechanical integrity; it can be further manipulated to introduce adaptive features to the scaffolds. Recently, several groups revisited the old concept of reversible covalent networks that have the capacity for responding to an externally applied stimulus, either photochemical or thermal stimuli, for self-repair, reconstruction and adaptation. This concept explores the “living” nature of certain reactive species,31 the availability of rapid exchange reactions,32 or the reversible nature of many chemical reactions.33 These approaches, although attractive, have not been adapted to tissue engineering applications where the repair and rearrangement of the polymeric networks must be achieved in situ at body temperature in the presence of cells.
3. Mechanoresponsive materials based on supramolecular interactions
Biological assemblies are dynamic entities whose transitions may include the disassembly and re-assembly of some of the subunits to accomplish local and global conformation changes. Such dynamic responsiveness is achieved through the concerted action of weak and non-covalent interactions, a process referred to as self-assembly. Molecular self-assembly is emerging as a promising new route to engineer hydrogel matrices that provide cells with more physiologically relevant microenvironments.34 The majority of self-assembled hydrogels are constructed by the physical entanglement of β-sheet-rich nanofibrils derived from short peptidic building blocks, such as (Fmoc)-dipeptides, 35 peptide amphiphiles 36, 37 and β-hairpin peptide.38 Genetically engineered protein polymers composed of tandem repeats of silk-like and elastin-like amino acid blocks can also self-assemble to form elastic gels through the crystallization of the silk-like segments.39 Hierarchical α-helical structures, such as coiled-coils, have also been exploited for gelation purposes. 40
Although interesting from the assembly perspective, molecularly-assembled hydrogels are mechanically weak. In fact, passing the β-hairpin gel through a syringe needle can fracture the pre-assembled structure and convert it into a rheological fluid. Intriguingly, the fragmented gels can readily reform and regain their initial mechanical strength upon removal of the stress, presumably through the immediate percolation of large hydrogel domains >200 nm. This type of mechanoresponsive gels is interesting in that they exhibit shear-thinning and self-healing properties that are attractive for tissue engineering applications.41 If covalent reinforcement can be introduced without compromising the self-assembling ability, elastomeric hydrogels that are conducive to cell growth can be obtained.
Studies using nanotools revealed a diverse set of structural motifs that could change conformation over a range of mechanical forces. These motifs include the force-induced exposure of otherwise cryptic peptide sequences, the opening of mechanosensitive ion channels, and receptor–ligand interactions that strengthen or weaken when strained.42 Such force-induced changes ultimately produce changes at the biochemical level that effectively direct cellular behaviors. Nature uses non-covalent interactions with high specificity to form well-defined structures that allow them to perform distinctive functions.43 The presence of folded domains, sacrificial bonds (weak non-covalent interaction) and hidden length (entropy reduction and enthalpy increase as molecular segments are stretched) held together by secondary forces in natural ECM proteins gives rise to composite matrices with combined strength, toughness and elasticity.44, 45
Intrigued by Nature’s design, several groups have explored the use of reversible supramolecular interactions to construct dynamic mechanoresponsive biomaterials that not only capture the modular structures of natural polymers but also exhibit more improved mechanical properties than the existing synthetic biomateirals. 46-50 The synthetic, reversible folding modules are commonly based on H-bonding and hydrophobic interactions. When properly designed, these supramolecular polymers can self-heal at room temperature.51 Meijer and colleagues devised reversible self-assembling polymer systems by introducing 2-ureido-4-pyrimidone (UPy) that dimerize strongly in a self-complementary array of four cooperative hydrogen bonds.47 Linear polymers and reversible networks were formed from monomers with two and three binding sites, respectively. The thermal and environmental control over lifetime and bond strength makes many properties, such as viscosity, chain length, and composition, tunable in a way not accessible to traditional polymers. Although cell-adhesive materials were obtained by simply mixing UPy-functionalized polymers with UPy-modified cell adhesive peptides, 52 these interesting materials have not yet been applied in tissue engineering applications. A question that remains to be answered is how stable these H-bonded dimers are in an aqueous condition at 37 °C.
Guan and coworkers constructed a crosslinked poly(n-butyl acrylate) network employing a modular, cyclic crosslinker based on the UPy dimer motif. 49 (Figure 2) Mechanical testing showed increasing crosslinking density led to a simultaneous increase in both modulus andtensile strength without sacrificing the extensibility. These advanced properties are attributed to the reversible unfolding of the UPy modules, providing efficient energy dissipation and self-repair mechanisms. Using recombinant DNA techniques, Lv et al 53 produced artificial elastomeric proteins that mimic the molecular architecture of titin, with the added features bestowed by resilin. When photochemically crosslinked, these materials are exceptionally tough and resilient, owing to the presence of an efficient energy dissipating mechanism. The combination of supramolecular chemistry and external forces offers the advantages of engineering mechanically active tissue with unprecedented control.
Figure 2.
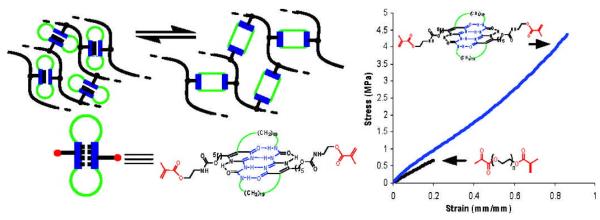
Poly(n-butyl acrylate) crosslinked by a UPy dimer-based crosslinker exhibit significantly enhanced mechanical properties over the control samples, with the combined extensibility and strength.49 Reprint with permission from American Chemical Society.
4. Scaffolding materials with heterogeneity and anisotropy
In addition to matrix stiffness and elasticity, the microstructure of the scaffolding materials determines whether these materials are conducive to cell growth and tissue assembly. The natural ECMs exhibit features at all length scales to allow cells to respond, maintain and remodel their environment as they go through various cell cycles and different stages of development.54 Mechanically active tissues, in particular, exhibit unique composition, organization and viscoelasticity that are evolutionarily adapted to the particular functions they perform. The ventricular myocardium is a highly vascularized, hierarchically organized quasi-lamellar tissue, composed of functional cardiomyocytes interwoven within collagen fibers in a honeycomb-like network where cardiomyocytes are coupled by functional gap junctions to enable the propagation of electrical impulses.55, 56 Articular cartilage, on the other hand, exhibits unique zonal organizations with distinct cellular phenotype and orientation, as well as ECM organizations depending on the relative distance from the articulating surface.57 Similarly, human vocal fold lamina propria (LP) is a trilayer structure with varying cell density and ECM composition and viscoelasticity that facilitate the free flow of the mucosa wave during phonation.7, 58
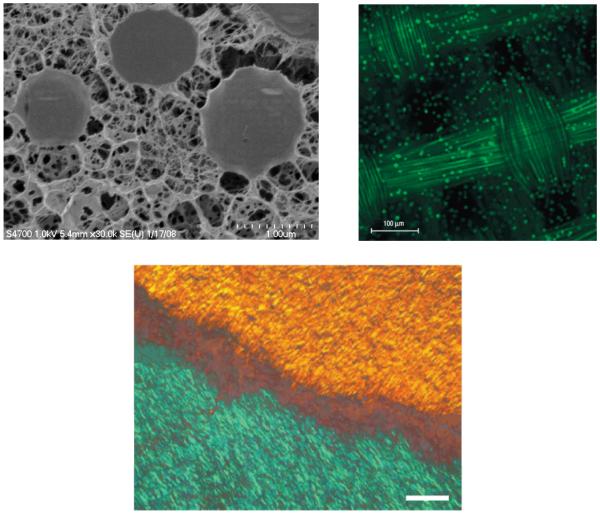
Intrigued by the multiple levels of matrix organization in the natural biological systems, we developed hyaluronic acid (HA)-based doubly crosslinked networks (DXNs) consisting of densely crosslinked HA hydrogel particles (HGPs) covalently integrated or physically entrapped in a second network that is also HA-derived.59-61 (Figure 3A) The HA DXNs discussed here are not to be confused with the aforementioned double network (DN) gels. The HA DXNs are macroscopic porous hydrogel matrices containing microscopic HA HGPs. The HGPs can be engineered to present therapeutic factors in a controlled fashion.62, 63 Through the covalent linkage, the secondary network can exert mechanical constraints on the hydrogel particles, leading to the deformation of HGPs as evidenced by the diffuse interphase between individual HGPs and the secondary matrix in the cryogenic scanning electron microscope (cryoSEM) image (Figure 3A).60, 64 Bioactive motifs (gelatin or collagen like peptide) immobilized on HGPs within the DXNs foster cell-matrix interactions65 and facilitate lineage-specific differentiation of mesenchymal stem cells (MSCs) in the absence of soluble inducers. 66
Figure 3.
(A) cryogenic scanning electron microscopy image of HA DXN,60 Reprint with permission from American Chemical Society; (B) Porcine articular chondrocytes in a fiber-reinforced 2% agarose gel,67 Reprint with permission from Nature Publishing Group, 2007; (C) Polarized light microscopy image of engineered annulus fibrous replicating the gross fiber orientation of the native tissue, 68 Scale bars: 200 μm, Reprint with permission from Nature Publishing Group.
Applying established microfabrication methods, researchers have created composite materials with tissue-like viscoelasticity and anisotropy. For example, porous, poly(glycolic acid) (PGA) scaffolds that resemble the native articular cartilage structurally and mechanically were created using a 3D woven technique. Cartilage tissue constructs were generated by infiltrating the anisotropic structures with a chondrocyte-agarose gel mixture (Figure 3B). The composite constructs show mechanical properties on par with the native cartilage tissue. It is speculated that such scaffolds will be capable of load-bearing immediately after implantation and will provide biological support for cell-based tissue regeneration in vivo.67 Nanofibrous laminates that replicate the hierarchy and anisotropy of the native annulus fibrosus were fabricated by electrospinning. MSCs seeded into these scaffolds were found to deposit an organized, collagen-rich extracellular matrix that mimicked the angle-ply, multi-lamellar architecture (Figure 3C) and the cell/scaffold constructs achieved mechanical parity with native tissue after 10 weeks of in vitro culture.68 Alternatively, an elastomeric scaffold with an accordion-likehoneycomb microstructure and tissue-like mechanical properties was prepared using poly(glycerol sebacate) (PGS) via laser microablation. Neonatal rat heart cells residing in the scaffolds can be induced to contract by electric field stimulation and exhibit a greater cell alignment than isotropic control scaffolds. Prototype bilaminar scaffolds with 3D interconnected pore networks yielded electrically excitable grafts with multi-layered neonatal rat heart cells.69 These studies represent a step forward towards the goal of providing off-the-shelf tissue-engineered products. However, it is not clear whether these constructs will be able to integrate into the host tissues and provide long-term mechanical functions in vivo.
5. Force-induced release of therapeutic factors
While the scaffold mechanics and morphology play important roles in guiding tissue regeneration, soluble factors are more potent modulators of cellular growth, development, angiogenesis, and tissue regeneration. If biomimetic, mechanoresponsive mechanisms can be introduced to the scaffolding materials, physical forces can be readily converted into biochemical cues to guide cells through various developmental stages, ultimately leading to the assembly of functional tissues. In the native ECM, growth factors are stored as an intact, latent complex through their specific binding to ECM molecules including heparan sulfate proteoglycans (HSPG) such as perlecan.70, 71 This growth factor sequestration mechanism has been introduced to HA-based hydrogel particles 62 and electrospun fibrous scaffolds 72 to improve the growth factor binding and release capability of the synthetic materials. The sequestered, latent growth factors can be activated by enzymes.70 Recent investigations demonstrate that mechanical forces, either cell generated or externally applied, can result in shedding or release of active growth factors, leading to increased local ligand concentrations that trigger cellular signaling.73 Therefore, mechanical forces can be utilized to regulate the equilibrium between storage and release of a host of matrix-bound growth factors.
Polymeric matrices that release growth factors in response to mechanical signals might provide a new approach to guide tissue formation in mechanically stressed environments. This concept has been demonstrated in a model system based on alginate hydrogel and vascular endothelial growth factor (VEGF).74 Our group has exploited the potential of self-assembled block copolymer micelles with a rubbery poly(n-butylacrylate) (PnBA) core and a hydrophilic, acrylate-decorated, poly(acrylic acid) (PAA) shell as microscopic crosslinkers for the formation of macroscopic, mechanoresponsive hydrogels.75 A model drug molecule encapsulated in the PnBA core was released from the elastomeric hydrogels in a step-wise fashion in response to external mechanical stress through the reversible deformation and unfolding of the PnBA core held together by hydrophobic interactions.76
6. Force-induced modulation of cellular function
Physical forces are not only indispensable for the proper function of these tissues; they play a crucial role in controlling tissue/organ development as well. This is most convincingly demonstrated in the vocal fold development and maturation.77 Contrary to the layered structure seen in mature vocal fold lamina propria, newborn and unphonated vocal fold LP is structurally and compositionally uniform, consisting of only one layer rich in ground substances, with sparse, immature, fibrous components homogeneously distributed throughout the LP.78-80 While fibroblasts and chondrocytes passively respond to externally imposed forces, the cardiomyocytes maintain their ability to self-beat through the tight interplay between its force generation activity and concurrent cytoarchitectural remodeling.81
Cells within mechanically active tissues are highly mechanoresponsive, sensing and responding to mechanical signals at the molecular level through a process known as cellular mechanotransduction. Cells have sophisticated environmental sensing mechanisms that modulate and regulate cellular molecular machinery and its structural organization.82 Biological behaviors regulated by mechanical signals include growth, differentiation, apoptosis, motility, and gene expression.83, 84 The unifying framework guiding our understanding of cellular mechanosensing is that cells use tensegrity (tensional integrity)85 architecture to stabilize their shape and sense mechanical signals. The presence of isometric tension at all levels of the multiscale networks ensures that various molecular scale mechanochemical transduction mechanisms proceed simultaneously and produce a concerted response.86 Transmembrane proteins (e.g. integrins, ion channels and ligand receptors) and the cytoskeleton play defining roles in mechanosensing and mechanochemical conversion.42 Understanding how the highly interactive mechanical signaling can give rise to phenotypic changes will facilitate the design of engineered microenvironments to foster tissue development, function and remodeling.87
Various tissue engineering bioreactors have been developed to simulate the mechanical environment of the target tissues, at the same time, enhancing nutrient diffusion and waste disposal. 88 Depending on the target tissues, different modes of mechanical stimulations are introduced. For example, pulsatile radial stress was introduced to the engineered blood vessels. 89 Dynamic deformational loading or shear is necessary to enhance the mechanical properties of the resultant engineered cartilage.90 Cyclic tensile culture is needed to differentiate marrow stromal cells to ligament like fibroblasts. 91
The vocal fold is one of Nature’s most mechanically versatile devices, experiencing compression, tension and shear during normal phonation. 92 The dynamic vibratory forces are not only indispensable for sound production but also contribute to the development and maturation of the tissue. Titze et al. designed a vocal fold bioreactor93, 94 composed of a low frequency (or static) actuator coupled to a series of connectors and levers to provide axial substrate elongation. The high frequency vibratory stimulus was generated using a voice coil actuator connected to a lever, which was attached to a vibrational bar. This bar moved the four vibrator arms of the flask that drive the cell-encapsulated scaffolds to oscillate at frequencies of 20-200 Hz.
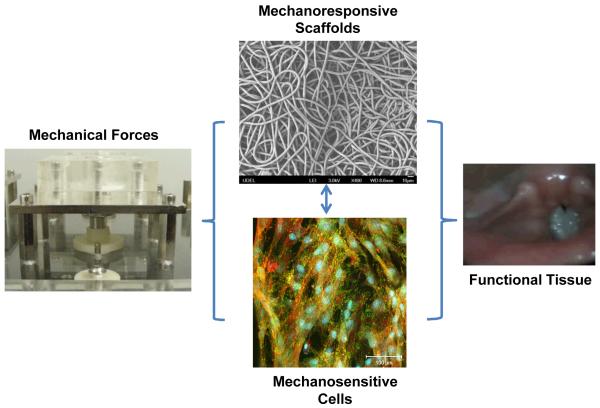
We have designed, constructed and characterized several prototypes of vocal fold bioreactors capable of generating physiologically relevant vibratory stimulations at human phonation frequencies. 95, 96 The major components of these devices are a function generator, a power amplifier, a loudspeaker and a vibration chamber containing a circumferentially-anchored silicone rubber membrane. In all cases, the vibration signals are translated to the membrane aerodynamically by the oscillating air pressure underneath the membrane. The current vibration device provides the most user-friendly features, allowing dynamic cell culture studies to be conducted in a high throughput and reproducible fashion. MSCs residing in/on a fibrous polyester scaffold were subjected to various physiologically relevant vibratory stimulations. Quantitative PCR analyses revealed a profound effect of vibratory stimulations on the expression of genes encoding important ECM proteins, pro-inflammatory cytokines and membrane-associated markers and proteins.(unpublished results) The ability to modulate the gene expression of ECM proteins underscores the role of high frequency vibration in defining the tissue structure and viscoelasticity. We believe that the engineered scaffold, when combined with physiologically relevant mechanical stimulations, will provide a dynamic environment that guides MSCs through various development stages (Figure 4). The in vivo performance of these constructs remains to be determined.
Figure 4.
Materials-based strategy for the engineering of mechanically active tissues. Vocal fold tissue engineering is shown as an example.
Collectively, we believe that successful development of mechanoresponsive biomaterials that can be fabricated into versatile scaffolds will lead to significant progress in the engineering of mechanically active tissues. An inevitable consequence of mechanoresponsiveness is the tunability of materials’ properties, such as elasticity, porosity, bioactivity and bioavailability, in response to the applied forces. Rapidly recoverable biomaterials can act as scaffolds for tissue engineering as they can accommodate cell growth while still maintaining their structure.97 These matrices will provide cells with dynamic guidance cues that are important for tissue development. Dynamic in vitro culture devices that not only enhance nutrient diffusion and waste disposal but also impose physiologically relevant biomechanical and biophysical stimulations will further increase the chance for success.
Conclusion
Although remarkable progress has been made in tissue engineering, the fabrication of mechanically active tissues remains elusive. The lack of progress can be partially attributed to the unavailability of intelligent materials that not only possess complex viscoelastic, anisotropic and non-linear mechanical properties but also encode the essential biomechanical and biochemical cues for cell fate determination and tissue maturation. Cell differentiation and tissue assembly can be controlled by the cooperative effects of forces, immobilized ligands and soluble factors. Therefore, manipulating the mechanosensing mechanism can lead to development of novel scaffolding materials that are conducive to tissue growth. We expect that advancements in biomaterials development will greatly contribute to the realization of the promise of tissue engineering in the not too distant future.
Acknowledgments
Work in the authors’ laboratory has been funded by grants from the National Institutes of Health (R01 DC008965, P20 RR017716), the National Science Foundation (Biomaterials Program, DMR 0643226), the DuPont Company and the University of Delaware Research Foundation.
References
- 1.Ozerdem OR, Wolfe SA, Marshall D. Use of skin substitutes in pediatric patients. J. Craniofac. Surg. 2003;14:517. doi: 10.1097/00001665-200307000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, Dickinson SC, Hollander AP, Mantero S, Conconi MT, Birchall MA. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 3.Gkioni K, Leeuwenburgh SCG, Douglas TEL, Mikos AG, Jansen JA. Mineralization of hydrogels for bone regeneration. Tissue Eng. Part B. 2010;16:577. doi: 10.1089/ten.TEB.2010.0462. [DOI] [PubMed] [Google Scholar]
- 4.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 5.Venugopal JR, Prabhakaran MP, Mukherjee S, Ravichandran R, Dan K, Ramakrishna S. Biomaterial strategies for alleviation of myocardial infarction. J. R. Soc. Interface. 2012;9:1. doi: 10.1098/rsif.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kakisis JD, Liapis CD, Breuer C, Sumpio BE. Artificial blood vessel: The holy grail of peripheral vascular surgery. J. Vasc. Surg. 2005;41:349. doi: 10.1016/j.jvs.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 7.Gray SD. Cellular physiology of the vocal folds. Otolaryngol. Clin. N. Am. 2000;33:679. doi: 10.1016/s0030-6665(05)70237-1. [DOI] [PubMed] [Google Scholar]
- 8.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 9.Burdick JA, Mauck RL, editors. Biomaterials for tissue engineering: A review of the past and future trends. Springer; New York, NY: 2011. [Google Scholar]
- 10.Serrano MC, Chung EJ, Ameer GA. Advances and applications of biodegradable elastomers in regenerative medicine. Adv. Funct. Mater. 2010;20:192. [Google Scholar]
- 11.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 12.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amsden B. Curable, biodegradable elastomers: Emerging biomaterials for drug delivery and tissue engineering. Soft Matter. 2007;3:1335. doi: 10.1039/b707472g. [DOI] [PubMed] [Google Scholar]
- 14.Lal J, Mark JE. Advances in elastomers and rubber elasticity. Springer; 1987. [Google Scholar]
- 15.Grieshaber SE, Jha AK, farran AJE, Jia X. In: Biomaterials for tissue engineering: A review of the past and future trends. Burdick JA, Mauck RL, editors. Springer; New York: 2011. p. 9. [Google Scholar]
- 16.Kopecek J. Hydrogels: From soft contact lenses and implants to self-assembled nanomaterials. J. Polym. Sci. Pol. Chem. 2009;47:5929. doi: 10.1002/pola.23607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang C, Aung A, Liao LQ, Varghese S. A novel single precursor-based biodegradable hydrogel with enhanced mechanical properties. Soft Matter. 2009;5:3831. [Google Scholar]
- 18.Gong JP. Why are double network hydrogels so tough? Soft Matter. 2010;6:2583. [Google Scholar]
- 19.Haraguchi K, Farnworth R, Ohbayashi A, Takehisa T. Compositional effects on mechanical properties of nanocomposite hydrogels composed of poly(n,n-dimethylacrylamide) and clay. Macromolecules. 2003;36:5732. [Google Scholar]
- 20.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular biology of the cell. Garland Science; New York: 2002. [Google Scholar]
- 21.Debelle L, Tamburro AM. Elastin: Molecular description and function. Int. J. Biochem. Cell Biol. 1999;31:261. doi: 10.1016/s1357-2725(98)00098-3. [DOI] [PubMed] [Google Scholar]
- 22.Almine JF, Bax DV, Mithieux SM, Nivison-Smith L, Rnjak J, Waterhouse A, Wise SG, Weiss AS. Elastin-based materials. Chemical Society Reviews. 2010;39:3371. doi: 10.1039/b919452p. [DOI] [PubMed] [Google Scholar]
- 23.Bellingham CM, Lillie MA, Gosline JM, Wright GM, Starcher BC, Bailey AJ, Woodhouse KA, Keeley FW. Recombinant human elastin polypeptides self-assemble into biomaterials with elastin-like properties. Biopolymers. 2003;70:445. doi: 10.1002/bip.10512. [DOI] [PubMed] [Google Scholar]
- 24.Grieshaber SE, Farran AJE, Lin-Gibson S, Kiick KL, Jia XQ. Synthesis and characterization of elastin-mimetic hybrid polymers with multiblock, alternating molecular architecture and elastomeric properties. Macromolecules. 2009;42:2532. doi: 10.1021/ma802791z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia XQ, Kiick KL. Hybrid multicomponent hydrogels for tissue engineering. Macromol. Biosci. 2009;9:140. doi: 10.1002/mabi.200800284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grieshaber SE, Farran AJE, Bai S, Kiick KL, Jia XQ. Tuning the properties of elastin mimetic hybrid copolymers via a modular polymerization method. Biomacromolecules, submitted. 2012 doi: 10.1021/bm3002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elvin CM, Carr AG, Huson MG, Maxwell JM, Pearson RD, Vuocolo T, Liyou NE, Wong DCC, Merritt DJ, Dixon NE. Synthesis and properties of crosslinked recombinant pro-resilin. Nature. 2005;437:999. doi: 10.1038/nature04085. [DOI] [PubMed] [Google Scholar]
- 28.Qin GK, Rivkin A, Lapidot S, Hu X, Preis I, Arinus SB, Dgany O, Shoseyov O, Kaplan DL. Recombinant exon-encoded resilins for elastomeric biomaterials. Biomaterials. 2011;32:9231. doi: 10.1016/j.biomaterials.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li LQ, Teller S, Clifton RJ, Jia XQ, Kiick KL. Tunable mechanical stability and deformation response of a resilin-based elastomer. Biomacromolecules. 2011;12:2302. doi: 10.1021/bm200373p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 31.Zheng P, McCarthy TJ. A surprise from 1954: Siloxane equilibration is a simple, robust, and obvious polymer self-healing mechanism. J. Am. Chem. Soc. 2012;134:2024. doi: 10.1021/ja2113257. [DOI] [PubMed] [Google Scholar]
- 32.Montarnal D, Capelot M, Tournilhac F, Leibler L. Silica-like malleable materials from permanent organic networks. Science. 2011;334:965. doi: 10.1126/science.1212648. [DOI] [PubMed] [Google Scholar]
- 33.Kloxin CJ, Scott TF, Adzima BJ, Bowman CN. Covalent adaptable networks (cans): A unique paradigm in cross-linked polymers. Macromolecules. 2010;43:2643. doi: 10.1021/ma902596s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ulijn RV, Smith AM. Designing peptide based nanomaterials. Chem. Soc. Rev. 2008;37:664. doi: 10.1039/b609047h. [DOI] [PubMed] [Google Scholar]
- 35.Orbach R, Adler-Abramovich L, Zigerson S, Mironi-Harpaz I, Seliktar D, Gazit E. Self-assembled fmoc-peptides as a platform for the formation of nanostructures and hydrogels. Biomacromolecules. 2009;10:2646. doi: 10.1021/bm900584m. [DOI] [PubMed] [Google Scholar]
- 36.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 37.Zhang SG. Emerging biological materials through molecular self-assembly. Biotechnol. Adv. 2002;20:321. doi: 10.1016/s0734-9750(02)00026-5. [DOI] [PubMed] [Google Scholar]
- 38.Schneider JP, Pochan DJ, Ozbas B, Rajagopal K, Pakstis L, Kretsinger J. Responsive hydrogels from the intramolecular folding and self-assembly of a designed peptide. J. Am. Chem. Soc. 2002;124:15030. doi: 10.1021/ja027993g. [DOI] [PubMed] [Google Scholar]
- 39.Megeed Z, Cappello J, Ghandehari H. Genetically engineered silk-elastinlike protein polymers for controlled drug delivery. Adv. Drug Deliv. Rev. 2002;54:1075. doi: 10.1016/s0169-409x(02)00063-7. [DOI] [PubMed] [Google Scholar]
- 40.Shen W, Zhang KC, Kornfield JA, Tirrell DA. Tuning the erosion rate of artificial protein hydrogels through control of network topology. Nat. Mater. 2006;5:153. doi: 10.1038/nmat1573. [DOI] [PubMed] [Google Scholar]
- 41.Yan CQ, Altunbas A, Yucel T, Nagarkar RP, Schneider JP, Pochan DJ. Injectable solid hydrogel: Mechanism of shear-thinning and immediate recovery of injectable beta-hairpin peptide hydrogels. Soft Matter. 2010;6:5143. doi: 10.1039/C0SM00642D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoffman BD, Grashoff C, Schwartz MA. Dynamic molecular processes mediate cellular mechanotransduction. Nature. 2011;475:316. doi: 10.1038/nature10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fratzl P. Biomimetic materials research: What can we really learn from nature’s structural materials? J. R. Soc. Interface. 2007;4:637. doi: 10.1098/rsif.2007.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fantner GE, Hassenkam T, Kindt JH, Weaver JC, Birkedal H, Pechenik L, Cutroni JA, Cidade GAG, Stucky GD, Morse DE, Hansma PK. Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat. Mater. 2005;4:612. doi: 10.1038/nmat1428. [DOI] [PubMed] [Google Scholar]
- 45.Becker N, Oroudjev E, Mutz S, Cleveland JP, Hansma PK, Hayashi CY, Makarov DE, Hansma HG. Molecular nanosprings in spider capture-silk threads. Nat. Mater. 2003;2:278. doi: 10.1038/nmat858. [DOI] [PubMed] [Google Scholar]
- 46.Wisse E, Govaert LE, Meijer HEH, Meijer EW. Unusual tuning of mechanical properties of thermoplastic elastomers using supramolecular fillers. Macromolecules. 2006;39:7425. [Google Scholar]
- 47.Sijbesma RP, Beijer FH, Brunsveld L, Folmer BJB, Hirschberg J, Lange RFM, Lowe JKL, Meijer EW. Reversible polymers formed from self-complementary monomers using quadruple hydrogen bonding. Science. 1997;278:1601. doi: 10.1126/science.278.5343.1601. [DOI] [PubMed] [Google Scholar]
- 48.Greef TFA, Meijer EW. Materials science - supramolecular polymers. Nature. 2008;453:171. doi: 10.1038/453171a. [DOI] [PubMed] [Google Scholar]
- 49.Kushner AM, Gabuchian V, Johnson EG, Guan ZB. Biomimetic design of reversibly unfolding cross-linker to enhance mechanical properties of 3d network polymers. J. Am. Chem. Soc. 2007;129:14110. doi: 10.1021/ja0742176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kushner AM, Guan ZB. Modular design in natural and biomimetic soft materials. Angew. Chem.-Int. Edit. 2011;50:9026. doi: 10.1002/anie.201006496. [DOI] [PubMed] [Google Scholar]
- 51.Cordier P, Tournilhac F, Soulie-Ziakovic C, Leibler L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature. 2008;451:977. doi: 10.1038/nature06669. [DOI] [PubMed] [Google Scholar]
- 52.Dankers PYW, Harmsen MC, Brouwer LA, Van Luyn MJA, Meijer EW. A modular and supramolecular approach to bioactive scaffolds for tissue engineering. Nat. Mater. 2005;4:568. doi: 10.1038/nmat1418. [DOI] [PubMed] [Google Scholar]
- 53.Lv S, Dudek DM, Cao Y, Balamurali MM, Gosline J, Li HB. Designed biomaterials to mimic the mechanical properties of muscles. Nature. 2010;465:69. doi: 10.1038/nature09024. [DOI] [PubMed] [Google Scholar]
- 54.Stevens MM, George JH. Exploring and engineering the cell surface interface. Science. 2005;310:1135. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 55.Torrent-Guasp F, Kocica MJ, Corno AF, Komeda M, Carreras-Costa F, Flotats A, Cosin-Aguillar J, Wen H. Towards new understanding of the heart structure and function. Eur. J. Cardio-Thorac. Surg. 2005;27:191. doi: 10.1016/j.ejcts.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 56.Holmes JW, Borg TK, Covell JW. Annual review of biomedical engineering, Annual Reviews, Palo Alto. 2005;7:223. doi: 10.1146/annurev.bioeng.7.060804.100453. [DOI] [PubMed] [Google Scholar]
- 57.Cohen NP, Foster RJ, Mow VC. Composition and dynamics of articular cartilage: Structure, function, and maintaining healthy state. J. Orthop. Sports Phys. Ther. 1998;28:203. doi: 10.2519/jospt.1998.28.4.203. [DOI] [PubMed] [Google Scholar]
- 58.Hirano M. Structure of the vocal fold in normal and diesease states: Anatomical and physical studies. ASHA Rep. 1981;11:11. [Google Scholar]
- 59.Jia XQ, Yeo Y, Clifton RJ, Jiao T, Kohane DS, Kobler JB, Zeitels SM, Langer R. Hyaluronic acid-based microgels and microgel networks for vocal fold regeneration. Biomacromolecules. 2006;7:3336. doi: 10.1021/bm0604956. [DOI] [PubMed] [Google Scholar]
- 60.Jha AK, Hule RA, Jiao T, Teller SS, Clifton RJ, Duncan RL, Pochan DJ, Jia XQ. Structural analysis and mechanical characterization of hyaluronic acid-based doubly cross-linked networks. Macromolecules. 2009;42:537. doi: 10.1021/ma8019442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jha AK, Malik MS, Farach-Carson MC, Duncan RL, Jia XQ. Hierarchically structured, hyaluronic acid-based hydrogel matrices via the covalent integration of microgels into macroscopic networks. Soft Matter. 2010;6:5045. doi: 10.1039/C0SM00101E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jha AK, Yang WD, Kirn-Safran CB, Farach-Carson MC, Jia XQ. Perlecan domain i-conjugated, hyaluronic acid-based hydrogel particles for enhanced chondrogenic differentiation via bmp-2 release. Biomaterials. 2009;30:6964. doi: 10.1016/j.biomaterials.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu X, Jha AK, Duncan RL, Jia XQ. Heparin-decorated, hyaluronic acid-based hydrogel particles for the controlled release of bone morphogenetic protein 2. Acta Biomater. 2011;7:3050. doi: 10.1016/j.actbio.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu X, Jha AK, Harrington DA, Farach-Carson MC, Jia XQ. Hyaluronic acid-based hydrogels: From a natural polysaccharide to complex networks. Soft Matter. 2012;8:3280. doi: 10.1039/C2SM06463D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krishna OD, Jha AK, Jia XQ, Kiick KL. Integrin-mediated adhesion and proliferation of human mscs elicited by a hydroxyproline-lacking, collagen-like peptide. Biomaterials. 2011;32:6412. doi: 10.1016/j.biomaterials.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jha AK, Xu XA, Duncan RL, Jia XQ. Controlling the adhesion and differentiation of mesenchymal stem cells using hyaluronic acid-based, doubly crosslinked networks. Biomaterials. 2011;32:2466. doi: 10.1016/j.biomaterials.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moutos FT, Freed LE, Guilak F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat. Mater. 2007;6:162. doi: 10.1038/nmat1822. [DOI] [PubMed] [Google Scholar]
- 68.Nerurkar NL, Baker BM, Sen S, Wible EE, Elliott DM, Mauck RL. Nanofibrous biologic laminates replicate the form and function of the annulus fibrosus. Nat. Mater. 2009;8:986. doi: 10.1038/nmat2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Engelmayr GC, Cheng MY, Bettinger CJ, Borenstein JT, Langer R, Freed LE. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat. Mater. 2008;7:1003. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Farach-Carson MC, Hecht JT, Carson DD. Heparan sulfate proteoglycans: Key players in cartilage biology. Crit. Rev. Eukaryot. Gene Expr. 2005;15:29. doi: 10.1615/critreveukaryotgeneexpr.v15.i1.30. [DOI] [PubMed] [Google Scholar]
- 71.Farach-Carson MC, Carson DD. Perlecan - a multifunctional extracellular proteoglycan scaffold. Glycobiology. 2007;17:897. doi: 10.1093/glycob/cwm043. [DOI] [PubMed] [Google Scholar]
- 72.Casper CL, Yang WD, Farach-Carson MC, Rabolt JF. Coating electrospun collagen and gelatin fibers with perlecan domain i for increased growth factor binding. Biomacromolecules. 2007;8:1116. doi: 10.1021/bm061003s. [DOI] [PubMed] [Google Scholar]
- 73.Tschumperlin DJ, Dai GH, Maly IV, Kikuchi T, Laiho LH, McVittie AK, Haley KJ, Lilly CM, So PTC, Lauffenburger DA, Kamm RD, Drazen JM. Mechanotransduction through growth-factor shedding into the extracellular space. Nature. 2004;429:83. doi: 10.1038/nature02543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee KY, Peters MC, Anderson KW, Mooney DJ. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408:998. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- 75.Xiao LX, Liu C, Zhu JH, Pochan DJ, Jia XQ. Hybrid, elastomeric hydrogels crosslinked by multifunctional block copolymer micelles. Soft Matter. 2010;6:5293. doi: 10.1039/C0SM00511H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiao LX, Zhu JH, Pochan DJ, Londono JD, Jia XQ. Mechanoresponsive hydrogels via the covalent integration of block copolymer micelles in macroscopic matrices. Soft Matter. 2011 Manuscript in preparation. [Google Scholar]
- 77.Hartnick CJ, Rehbar R, Prasad V. Development and maturation of the pediatric human vocal fold lamina propria. Laryngoscope. 2005;115:4. doi: 10.1097/01.mlg.0000150685.54893.e9. [DOI] [PubMed] [Google Scholar]
- 78.Sato K, Hirano M, Nakashima T. Fine structure of the human newborn and infant vocal fold mucosae. Ann. Oto. Rhinol. Laryn. 2001;110:417. doi: 10.1177/000348940111000505. [DOI] [PubMed] [Google Scholar]
- 79.Sato K, Hirano M, Nakashima T. Age-related changes of collagenous fibers in the human vocal fold mucosa. 2002. [DOI] [PubMed]
- 80.Sato K, Nakashima T, Nonaka S, Harabuchi Y. Histopathologic investigations of the unphonated human vocal fold mucosa. Acta Oto-Laryngol. 2008;128:694. doi: 10.1080/00016480701675643. [DOI] [PubMed] [Google Scholar]
- 81.Kresh JY, Chopra A. Intercellular and extracellular mechanotransduction in cardiac myocytes. Pflugers Arch. 2011;462:75. doi: 10.1007/s00424-011-0954-1. [DOI] [PubMed] [Google Scholar]
- 82.Kung C. A possible unifying principle for mechanosensation. Nature. 2005;436:647. doi: 10.1038/nature03896. [DOI] [PubMed] [Google Scholar]
- 83.Chiquet M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol. 1999;18:417. doi: 10.1016/s0945-053x(99)00039-6. [DOI] [PubMed] [Google Scholar]
- 84.Chicurel ME, Chen CS, Ingber DE. Cellular control lies in the balance of forces. Curr. Opin. Cell. Biol. 1998;10:232. doi: 10.1016/s0955-0674(98)80145-2. [DOI] [PubMed] [Google Scholar]
- 85.Ingber DE. Tensegrity-based mechanosensing from macro to micro. Prog. Biophys. Mol. Biol. 2008;97:163. doi: 10.1016/j.pbiomolbio.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ingber DE. Cellular mechanotransduction: Putting all the pieces together again. Faseb J. 2006;20:811. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 87.Fletcher DA, Mullins D. Cell mechanics and the cytoskeleton. Nature. 2010;463:485. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen HC, Hu YC. Bioreactors for tissue engineering. Biotechnol. Lett. 2006;28:1415. doi: 10.1007/s10529-006-9111-x. [DOI] [PubMed] [Google Scholar]
- 89.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 90.Davisson T, Kunig S, Chen A, Sah R, Ratcliffe A. Static and dynamic compression modulate matrix metabolism in tissue engineered cartilage. J. Orthop. Res. 2002;20:842. doi: 10.1016/S0736-0266(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 91.Doroski DM, Levenston ME, Temenoff JS. Cyclic tensile culture promotes f ibroblastic differentiation of marrow stromal cells encapsulated in poly(ethylene glycol)-based hydrogels. Tissue Eng. Part A. 2010;16:3457. doi: 10.1089/ten.tea.2010.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Titze IR. Mechanical stress in phonation. J. Voice. 1994;8:99. doi: 10.1016/s0892-1997(05)80302-9. [DOI] [PubMed] [Google Scholar]
- 93.Titze IR, Hitchcock RW, Broadhead K, Webb K, Li W, Gray SD, Tresco PA. Design and validation of a bioreactor for engineering vocal fold tissues under combined tensile and vibrational stresses. J Biomech. 2004;37:1521. doi: 10.1016/j.jbiomech.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 94.Kutty JK, Webb K. Vibration stimulates vocal mucosa-like matrix expression by hydrogel-encapsulated fibroblasts. J Tissue Eng Regen M. 2010;4:62. doi: 10.1002/term.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.USA Pat. 2010
- 96.Farran AJE, Teller SS, Jia F, Clifton RJ, Duncan RL, Jia X. Design and characterization of a dynamic vibrational culture system. J. Tissue Eng Regen. M. 2011 doi: 10.1002/term.514. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ramachandran S, Tseng Y, Yu YB. Repeated rapid shear-responsiveness of peptide hydrogels with tunable shear modulus. Biomacromolecules. 2005;6:1316. doi: 10.1021/bm049284w. [DOI] [PMC free article] [PubMed] [Google Scholar]