Abstract
Women are at higher risk than men for bleeding and vascular complications after percutaneous coronary intervention (PCI). Compared with femoral access, radial access reduces these complications but may be more challenging in women because of higher rates of radial artery spasm, tortuosity, and occlusion as well as lower rates of procedure success. Whether the safety advantages of radial versus femoral access in women undergoing PCI are outweighed by reduced effectiveness has not been studied. The Study of Access site For Enhancement of PCI for Women is a prospective, randomized clinical trial comparing radial with femoral arterial access in women undergoing PCI. In conjunction with the US Food and Drug Administration‧s Critical Path Cardiac Safety Research Consortium, this study embeds the randomized clinical trial into the existing infrastructure of the National Cardiovascular Data Registry™ CathPCI Registry™ through the National Institute of Health‧s National Cardiovascular Research Infrastructure. The primary efficacy end point is a composite of bleeding (Bleeding Academic Research Consortium types 2, 3, or 5) or vascular complication requiring intervention occurring at 72 hours after PCI or by hospital discharge. The primary feasibility end point is procedure success. Secondary end points include procedure duration, contrast volume, radiation dose, quality of life, and a composite of 30-day death, vascular complication, or unplanned revascularization.
Bleeding associated with percutaneous coronary intervention (PCI) limits the use of adjunctive antithrombotic agents important for procedural success and is associated with morbidity and mortality.1 Post-PCI bleeding commonly involves the vascular access site.2,3 Compared with transfemoral intervention (TFI), transradial intervention (TRI) has been associated with significant reductions in access site bleeding, vascular complications, transfusions, and mortality.4–6 However, a recent international multicenter randomized clinical trial (RCT) of radial versus femoral artery access found no significant difference in ischemic or bleeding outcomes among patients presenting with acute coronary syndromes (ACS), emphasizing the importance of further randomized investigations.7
Despite the potential benefits of radial access, TRI use in the United States (U.S.) remains low, increasing from 1.4% to 11.4% between 2007 and 2011.5,8 Low uptake may be related to limited availability of systematic radial training and low overall per-operator PCI volumes,9 limiting the ability of low-volume operators to overcome the TRI learning curve.10 Lack of convincing randomized clinical data supporting short- and long-term benefits of TRI over TFI may also affect uptake.
To address drug-related PCI bleeding safety and low U.S. TRI adoption, the Cardiac Safety Research Consortium (CSRC), a partnership between the Duke Clinical Research Institute (DCRI) and the U.S. Food and Drug Administration (FDA), sponsored several think-tank meetings involving government, academia, and industry representatives.11 Challenges of designing an RCT of TRI versus TFI, the need for randomized investigation of the optimal PCI access strategy for women, and the cost and complexity of U.S. RCTs were discussed. Three key logistical challenges to designing a U.S.-based RCT of vascular access for PCI were identified: (1) convincing operators to randomize to radial or femoral access, (2) executing such a trial with a limited number of experienced radial operators in the United States (ie, limited TRI expertise), and (3) designing a multicenter, prospective RCT with efficiencies for expedited patient enrollment and data accrual to reduce operational expenses as a novel proof-of-concept for conducting RCTs in the U.S.
Addressing the challenge of randomization
The success of an RCT of vascular access depends on the unbiased willingness of operators to randomize. Logistically, femoral operators unfamiliar with radial access are unable to randomize to radial access, whereas experienced radial operators may be unwilling to randomize to femoral access. Therefore, the study population would need to include patients for whom equipoise for radial operators to randomize exists. This might include patients whose risk for femoral bleeding or vascular complications is balanced by a risk for procedural failure with TRI or those at risk for vascular complications from both approaches.
The CSRC discussions identified women as the highest priority population meeting such criteria. Rates of post-PCI vascular bleeding and complications are higher for women than for men,12 and female sex is an independent predictor of these events.13 Even with use of radial access, women remain at higher risk for vascular complications and transfusion than men,14 and the smaller diameter of female radial arteries may render them more prone to spasm and unable to accommodate larger catheters, possibly causing procedure failure. These factors may contribute to the observed lower rate of radial use among women.5,8 Whether favorable bleeding trends or procedural outcomes with TRI are maintained in women is uncertain. Moreover, heart disease is the leading cause of death among women in industrialized countries, and women remain an understudied population. For these reasons, women were identified as the subgroup in whom clinical equipoise to conduct an RCT of vascular access exists. The SAFE-PCI for Women (ClinicalTrials.gov NCT01406236) trial will address this equipoise by randomizing women undergoing PCI to either radial or femoral artery access.
Identification of sites with sufficient radial experience
The success of a radial versus femoral access RCT assumes operator proficiency in both techniques. Identifying sites and operators for such a trial typically involves sending surveys to site investigators to deter-mine whether their patient volume meets the criteria for acceptable experience and potential for enrollment. Such surveys frequently include inaccuracies that lead to setbacks during actual trial execution. For SAFE-PCI for Women, a more efficient and objective approach to site and operator profiles—for overall PCI volume, PCI volume in women, and total TRI volume—was developed using data from the National Cardiovascular Data Registry (NCDR) CathPCI Registry, an initiative of the American College of Cardiology Foundation (ACCF) and the Society for Cardiovascular Angiography and Interventions. CathPCI Registry includes data from all PCI records at participating sites, and follow-up surveys regarding individual radial volumes are then sent to candidate operators within each potential site.
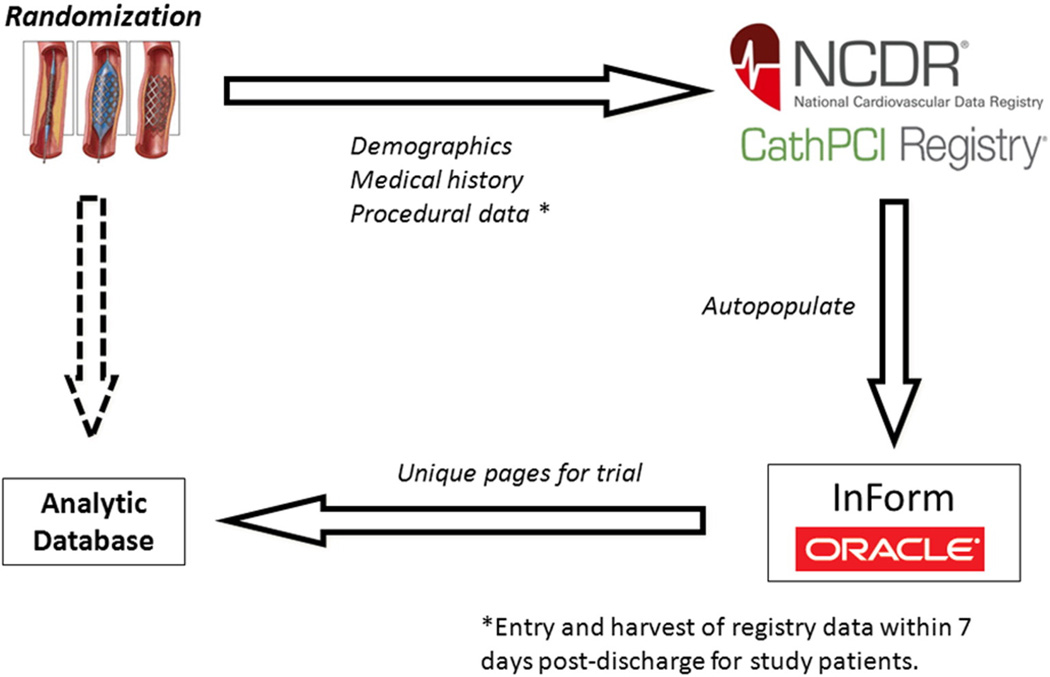
Increasing trial efficiency and reducing costs
Collaboration between Duke University and the ACCF was further leveraged to overcome challenges related to data capture and costs traditionally encountered in multicenter RCTs. The instrument to leverage the CathPCI Registry for SAFE-PCI for Women is built through the National Cardiovascular Research Infrastructure (NCRI).15 This NIH-funded Duke University–ACCF collaboration allows for the creation of an investigator research network to improve the efficiency of prospective RCT design and execution by leveraging the data collection capabilities of the CathPCI Registry. Under this construct, the ongoing workflow of registry-participating sites is accessed electronically to populate a clinical trial database for consenting patients (Figure 1). Patient demographics, medical history, concomitant medications, procedural details, and index hospitalization clinical outcomes routinely coded into the registry‧s data collection form using standardized data elements are electronically captured without study site coordinator effort. For this trial, additional information specific to vascular access and bleeding definitions not in CathPCI Registry is obtained using additional electronic case report form (CRF) pages. Overall, this approach reduces the site coordinator workload by ~65% per patient compared with the traditional “start-to-finish” dedicated study CRFs. SAFE-PCI for Women’s NCDR-NCRI infrastructure operationalizes a prospective RCT embedded into an ongoing national registry, provides for an objective means to profile and identify ideal sites with high radial volumes, and reduces site-level study coordinator workload, thus improving trial efficiency.
Figure 1.
Data workflow. CathPCI Registry data auto-populates InForm (Oracle Corporation), the study electronic case report form. Trial-specific data are directly entered into InForm and integrated with CathPCI data into the analytic database.
Another important feature of the NCDR-NCRI operational construct is that the accrued RCT data are moved from the “flat” data architecture of the NCDR to a Title 21 Code of Federal Regulations Part 11-compliant database as the final RCT data repository.16 In accordance with FDA guidelines on electronic records, this change in database architecture allows for the monitoring of electronic data through processes such as audit trails and validation systems and helps to ensure data reliability. Consistent with the mission of the Critical Path CSRC program, this more efficient RCT infrastructure may thus be suitable for new FDA Investigational Device Exemption and Investigational New Drug studies.
Study objectives
SAFE-PCI for Women is a multicenter, randomized, open-label, active-controlled trial designed to evaluate radial versus femoral access in women undergoing PCI. The primary study objective is to compare the efficacy and feasibility of both vascular approaches for PCI. We hypothesize that TRI will significantly reduce bleeding and vascular complications while maintaining similar rates of procedural success compared with TFI. Secondary objectives include assessments of vascular access and procedural metrics, as well as patient quality of life.
The primary end point for SAFE-PCI for Women represents a unique step forward for clinical trials measuring bleeding complications. Multiple bleeding definitions have been implemented in previous cardiovascular RCTs, making comparisons of bleeding outcomes across trials difficult. To address this heterogeneity and allow for meaningful interpretation of results, the Bleeding Academic Research Consortium (BARC) bleeding definitions were developed.17 SAFE-PCI for Women will be the first RCT to use these standardized BARC definitions as a primary end point.
Methods
Study population
The study population will be ~3000 U.S. women undergoing coronary angiography randomized to obtain a cohort of 1,800 women undergoing PCI. Percutaneous coronary intervention is defined as a procedure during which a coronary guide wire exits the coronary guide catheter, and systemic anticoagulation is given to achieve therapeutic levels of anticoagulation (including intravascular ultrasound, optical coherence tomography, and fractional flow reserve procedures).
Eligibility criteria
Eligibility criteria are listed in Table I. Women undergoing diagnostic angiography to evaluate ischemic symptoms with the possibility of elective or urgent PCI or those undergoing planned PCI are eligible for enrollment. Key exclusion criteria include bilateral abnormal Barbeau tests, planned right heart catheterization, presence of bilateral internal mammary artery coronary bypass grafts, and primary PCI for ST-segment elevation myocardial infarction (STEMI). We excluded patients with STEMI because of low U.S. radial volumes and concerns that lower-volume operators may be uncomfortable performing TRI for STEMI and potentially compromising door-to-balloon times and patient outcomes. Including patients with STEMI might also restrict the number of participating operators to only expert radialists who are unwilling to randomize to femoral, especially given new data published since the planning of SAFE-PCI for Women that suggest a mortality benefit for TRI in patients with STEMI.7,18 Patients are also excluded for an international normalized ratio ≥1.5 if treated with oral vitamin K antagonists (ie, warfarin), receiving oral factor Xa or IIa inhibitors ≤24 hours before procedure, or planned staged PCI within 30 days after index procedure. Investigators are encouraged to select PCI-eligible patients with ischemic clinical scenarios; based on this clinical discretion, ~60% of ad hoc patients taken for diagnostic angiography are expected to undergo PCI.
Table I.
Eligibility criteria
| Inclusion criteria |
|
| Exclusion criteria |
|
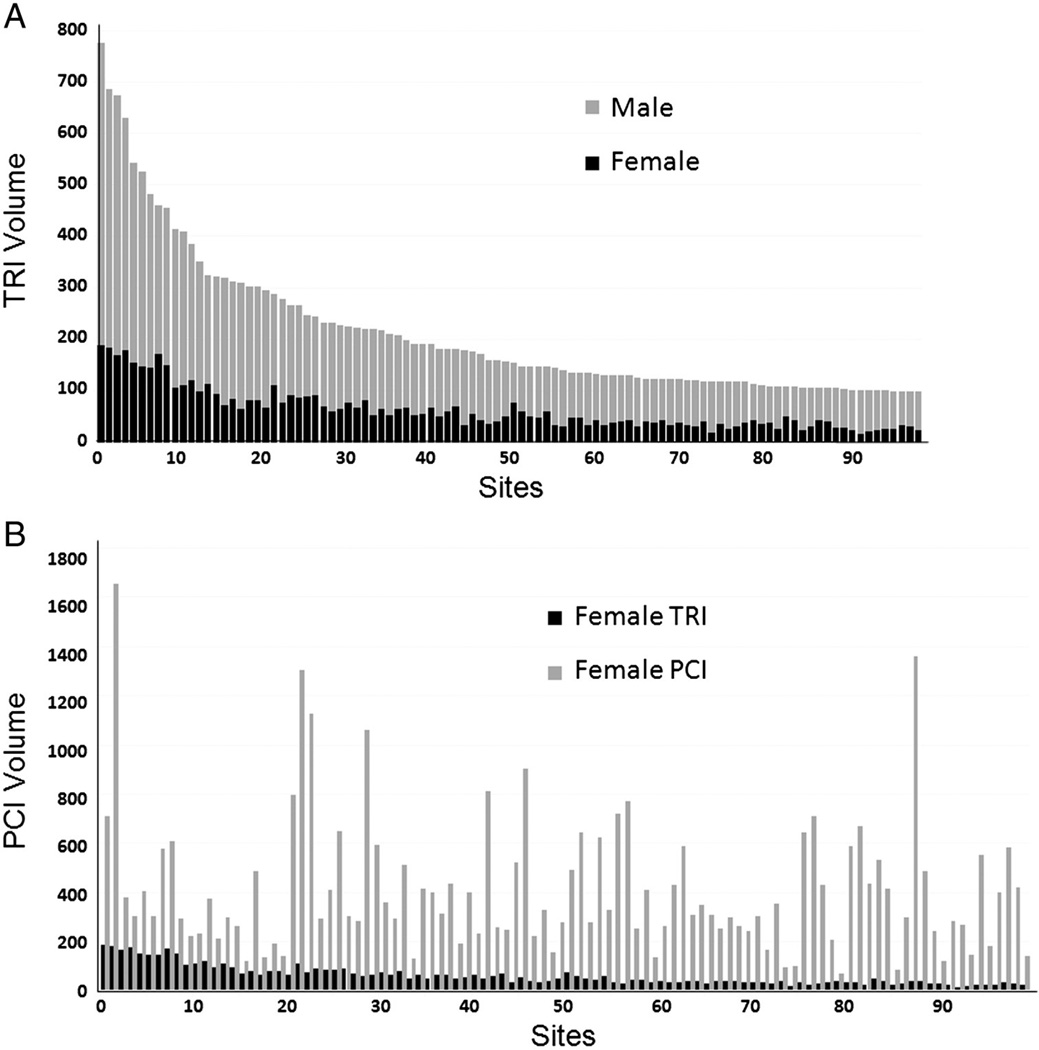
Site selection
To avoid the radial learning curve, experienced radial sites and operators will be identified using CathPCI Registry data. Sites will be profiled based on their overall CathPCI Registry TRI volume and the proportion of TRIs performed in women (Figure 2). Owing to the frequent mismatch of site-versus-operator radial volumes, potential sites will also be sent surveys regarding their site- and individual operator-level radial volumes. Although formal inclusion criteria do not exist, participating sites and principal investigators will generally be expected to meet both of the following minimum criteria: >10% of sitewide PCI volume performed radially and >30 TRI cases per operator in the last year. Sites will also be queried regarding current CathPCI Registry participation and their ability to modify workflow timelines for the trial. Data entry timelines for SAFE-PCI for Women are shorter than the typical quarterly NCDR data harvest. To ensure timely collection of trial data, sites must commit to entering registry data within 7 days of randomization for study patients.
Figure 2.
Site identification using CathPCI Registry radial PCI data. Shown are examples of CathPCI Registry data (September 2009–January 2011) used for site profiling based on TRI volume by sex (A) and proportion of TRIs among women undergoing PCI per site (B).
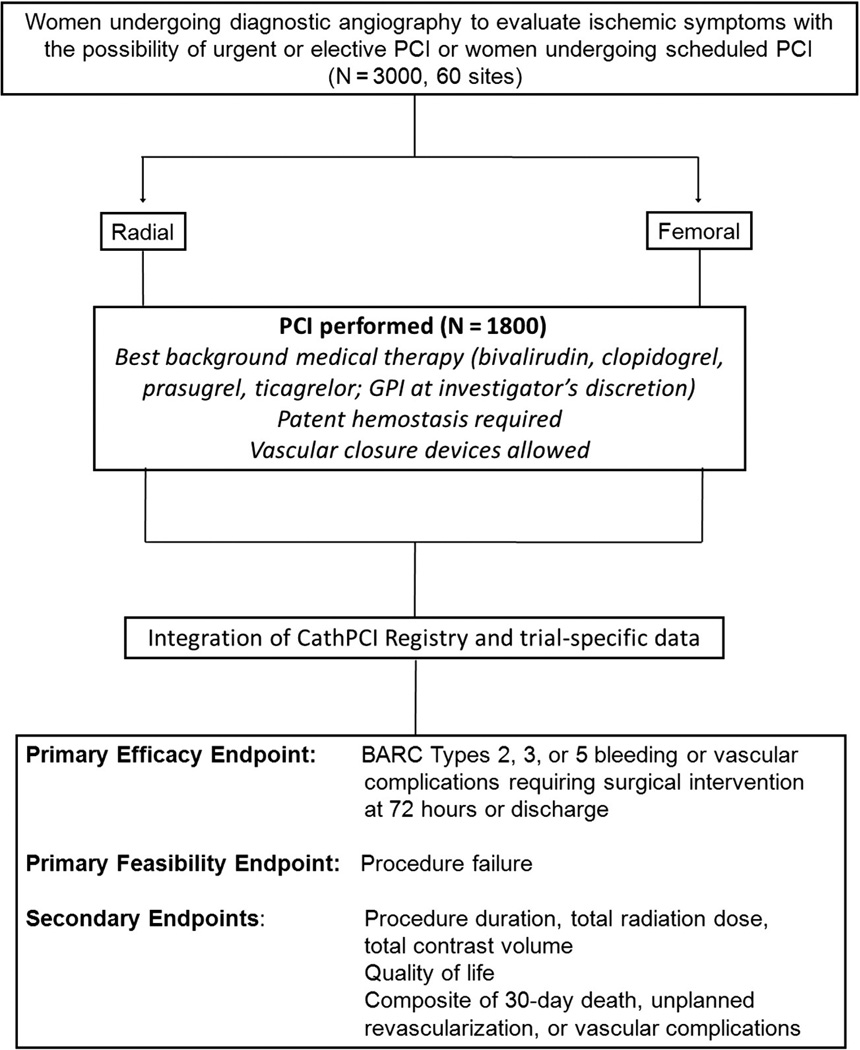
Randomization and treatment regimens
After informed consent is obtained, but before obtaining arterial access for the procedure, patients will be randomly assigned to radial or femoral access (Figure 3). The allocation ratio for randomization is 1:1 between treatment arms, and Web-based randomization will be performed using a DCRI proprietary Simple Internal Randomization Engine in block fashion within sites. Patients enrolled at sites performing ad hoc PCI will be randomized before diagnostic angiography.
Figure 3.
Study design for randomization and treatment.
Antithrombotic therapy during PCI
Antiplatelet and anticoagulant regimens for PCI may differ between operators, sites, and vascular access techniques. Consequently, use of specific agents is not mandated in the protocol, but general and access-specific recommendations are included. Patients should receive aspirin at the discretion of the investigator. At the time of trial protocol development, the choice of P2Y12 inhibitor was also per operator discretion, with recommended consideration of on-label prasugrel for patients with ACS and, otherwise, use of clopidogrel according to standard practice. When ticagrelor became available, it was added to the protocol for use by site investigators according to labeled indications and dosage. For all PCI procedures, bivalirudin is recommended at doses consistent with package labeling, in particular to leverage reported reductions of arteriotomy site bleeding. Although glycoprotein IIb/IIIa inhibitors (GPIs) are allowed on a discretionary basis, up-front intention to use GPI must be specified before randomization, whereas provisional use of GPI during the procedure is allowed per operator discretion. Finally, postdischarge duration of dualantiplatelet therapy is left to the operator but must at least 30 days in stented patients.
Radial access
Radial access may be obtained using counterpuncture (“through and through”) or anterior wall puncture techniques. In case of failure to gain access on one side, an attempt on the contralateral radial artery is recommended, if the Barbeau test on that side is normal. For patients undergoing ad hoc PCI randomized before diagnostic angiography, unfractionated heparin (minimum dose of 40 units/kg, maximum of 5,000 units) administered intravenously or intraarterially through the vascular access sheath or the first diagnostic catheter placed in the ascending aorta is recommended for anticoagulation during diagnostic angiography. For TRI patients, the patent hemostasis technique is required for post-procedure arteriotomy management.19
Femoral access
Use of femoral head fluoroscopy or ultrasound guidance is recommended when obtaining femoral access. Choice of anticoagulation during diagnostic angiography is left to the operator‧s discretion. After TFI, vascular sheaths should be removed ≥2 hours after bivalirudin is discontinued, when the activated clotting time is <150 seconds if unfractionated heparin is used, or 6 hours after the last enoxaparin dose for patients receiving enoxaparin for procedural anticoagulation. The duration of manual pressure for hemostasis, timing of ambulation after TFI, and use of vascular closure devices should be in accordance with local practice.
Data management
To improve trial efficiency, a large proportion of data will be obtained through the existing CathPCI Registry data capture. Patient files will be matched through cross-linking of registry- and trial-specific patient identification numbers and matching based on other unique patient identifiers. Data from CathPCI Registry will be transferred to the DCRI, the trial Clinical and Data coordinating center. CathPCI Registry data will auto-populate InForm (Oracle Corporation, Redwood Shores, CA), the study electronic CRF (Figure 1). Due to existing CathPCI Registry data quality checks and query processes, transfer of “clean” data via NCRI further promotes operational trial efficiency. Remaining trial-specific data will be directly entered into InForm by the site coordinator. Non-NCDR sites may enter trial data into a completely InForm-based CRF. Data will be managed by the DCRI blinded to treatment arm; DCRI statisticians responsible for data and safety monitoring committee (DSMC) report preparation will have access to unblinded event rates before database locking.
Study end points
Primary end points
Since the intervention being studied is access site in the context of intensive anticoagulant use for PCI, the primary “efficacy” end point of interest is bleeding; in particular, clinically relevant moderate-to-severe bleeding that has been associated with adverse clinical outcomes. 1 The primary end point is the composite of BARC type 2, 3, or 5 bleeding or vascular complications occurring from the first arterial access after randomization through 72 hours or hospital discharge, whichever occurs first (Table II). Vascular complications are defined as any of the following that require surgical intervention, including thrombin injection: arteriovenous fistula, arterial pseudoaneurysm, or arterial occlusion. The primary feasibility end point is procedural failure, defined as the inability to complete the procedure from the assigned vascular access site. All primary end point events will be adjudicated by an independent Clinical Events Committee.
Table II.
Study definitions
| PCI | A procedure during which a coronary guide wire exits the coronary guide catheter and systemic anticoagulation is given to achieve therapeutic anticoagulation levels (including fractional flow reserve, intravascular ultrasound, and optical coherence tomography) Inability to complete procedure using assigned access site |
| Procedural failure BARC type 2, 3, or 5 bleeding | Type 2
|
Type 3
|
|
Type 5
|
|
| Vascular complication | Any of the following complications requiring surgical intervention (including thrombin injection)
|
Secondary end points
Secondary end points include procedural metrics of procedure duration, patient radiation dose (air kerma), and contrast volume, as well as the composite of death, vascular complications, or unplanned revascularization at 30 days in patients undergoing PCI. Rates of ischemic threat to the hand and hematomas will be assessed according to the EArly discharge after Stenting of coronarY arteries (EASY) trial hematoma scale (online Appendix).20 A separate quality of life substudy for 300 randomized patients will include assessments at baseline, discharge, and, for PCI patients, at 30 days after discharge.
Statistical considerations
Sample size
SAFE-PCI for Women is designed to detect clinically important differences in the composite rate of BARC type 2, 3, and 5 bleeding or vascular complications from first arterial access after randomization through 72 hours or hospital discharge. CathPCI Registry was examined to determine the approximate rates of BARC type 2, 3, and 5 bleeding among women undergoing PCI; depending on the use of bleeding avoidance strategies, rates ranged from 6.2% to 12.5%.21 Based on these data, a post-procedure bleeding rate of 8.0% in the control (femoral) arm was assumed. At 90% power with a 2-sided α of .05, a sample size of 1,576 women undergoing PCI is necessary to detect a 50% relative risk reduction in bleeding or vascular complications with radial access. The sample size was increased to 1,800 women undergoing PCI owing to uncertainty in event rates. Given the prevalence of ad hoc PCI, ~3,000 women from at least 50 US sites are expected to be randomized for 28 months to obtain this PCI cohort.
Data analysis
Two analysis populations are defined for the study: all patients undergoing PCI (“PCI cohort”) and all patients randomized in the study (“randomized cohort”). Primary efficacy and feasibility analyses will follow modified intention-to-treat principles (“modified” through analysis of only the PCI cohort). Analysis of primary end points will be performed using logistic regression with reporting of odds ratios and corresponding 95% CIs. Primary results will be adjusted for randomized access site, planned use of GPI during PCI, and elective versus ACS indication for PCI. All treatment group comparisons will be 2-sided with a P value of .05 considered statistically significant. There will be no imputation for missing data.
Secondary efficacy analyses will also follow modified intention-to-treat principles and will be conducted within the PCI cohort. Secondary analysis of categorical end points will be conducted using logistic regression before and after adjustment for randomized access site, planned use of GPI during PCI, and elective PCI versus ACS status. Linear models with a binary indicator variable for randomization cohort will be used to assess secondary continuous outcomes.
Sensitivity and exploratory analyses have been prespecified. The primary efficacy analysis will be repeated in the randomized cohort, and both primary efficacy and feasibility analyses will be performed in the as-treated PCI cohort in which subjects are included in the treatment group according to actual treatment received. Exploratory analyses will assess bleeding events according to Thrombolysis in Myocardial Infarction (TIMI), Acute Catheterization and Urgent Intervention Triage strategY (ACUITY), and CathPCI Registry bleeding definitions (online Appendix).22,23 Definitions of TIMI and ACUITY will be modified because of the limitations of CathPCI Registry data elements. Exploratory analyses will also evaluate for interactions of presentation (elective PCI vs ACS) and site radial procedure volume on the primary efficacy and composite secondary end points and examinations of non–BARC-defined bleeding events, CathPCI-defined vascular complications, and EASY trial hematoma types by treatment group among randomized, PCI, and non-PCI subgroups through 72 hours or hospital discharge and 30 days, when applicable.
There will be a planned interim efficacy analysis when 72-hour data are available for approximately 50% of the planned PCI cohort (approximately 900 subjects).
Safety monitoring
An independent DSMC made up of 3 clinicians and a statistician will monitor the trial conduct. The DSMC will review overall primary efficacy data and the proportion of randomized subjects proceeding to PCI on at least a quarterly basis. The DSMC will notify SAFE-PCI for Women Study Leadership of unexpected rates of any of the following: BARC types 2, 3, or 5 bleeding; vascular complications; or procedure failure.
Conclusions
SAFE-PCI for Women was developed through CSRC think tank meetings to investigate whether the benefits of TRI are maintained without sacrificing procedural success in a cohort for whom there is equipoise for randomization— women. As one of the only U.S. female-specific interventional trials to date, SAFE-PCI for Women constitutes a unique public health initiative, and in its science, this is the first RCT to use consensus BARC bleeding definitions as a primary end point. Given the historically low participation of women in clinical research, this study represents an important effort to learn about an understudied population using uniquely informative end points. In addition, SAFE-PCI for Women represents a novel intellectual collaboration among multiple stakeholders and an application of partnered NCRI-NCDR-DCRI operational components to improve operational efficiency through objective site selection and reduced site coordinator workload. Importantly, these features may translate into faster trial enrollment, better site participation, and lower costs. Finally, the database architecture is part 11 compliant and will allow the NCDR-NCRI infrastructure paradigm to support not only public health studies but also FDA Investigational Device Exemption and Investigational New Drug studies.
Acknowledgements
Funding
SAFE-PCI for Women receives unrestricted funding from multiple industry sponsors (Terumo Medical, Abbott Cardiovascular Systems, Medtronic Vascular, The Medicines Company, Lilly USA, Guerbet, and ACIST Medical Systems) and the FDA Office of Women’s Health. The NCRI activity was funded by the National Institutes of Health. As a public health initiative, experts from all funding sources were all actively involved in considerations for the final study design but had no role in study conduct, operations, data management, or analysis. The authors are solely responsible for the design and conduct of this study, the drafting and editing of the manuscript, and its final contents.
Appendix
Bleeding and vascular complication definitions used for exploratory analyses
| Modified TIMI | Modified ACUITY | CathPCI Registry | EASY Hematoma Scale |
|---|---|---|---|
Major bleeding (any of the following):
|
Any of the following:
|
Bleeding (any of the following):
|
Type I: ≤5 cm diameter
|
Minor bleeding (any of the following):
|
|
Vascular complication (including but not limited to the following):
|
|
References
- 1.Doyle BJ, Rihal CS, Gastineau DA, et al. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Amer Coll Card. 2009;53:2019–2027. doi: 10.1016/j.jacc.2008.12.073. [DOI] [PubMed] [Google Scholar]
- 2.Kinnaird TD, Stabile E, Mintz GS, et al. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Amer J Card. 2003;92:930–935. doi: 10.1016/s0002-9149(03)00972-x. [DOI] [PubMed] [Google Scholar]
- 3.Verheugt FW, Steinhubl SR, Hamon M, et al. Incidence, prognostic impact, and influence of antithrombotic therapy on access and nonaccess site bleeding in percutaneous coronary intervention. JACC Cardiovasc Interv. 2011;4:191–197. doi: 10.1016/j.jcin.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Jolly SS, Amlani S, Hamon M, et al. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Amer Heart J. 2009;157:132–140. doi: 10.1016/j.ahj.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 5.Rao SV, Ou FS, Wang TY, et al. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv. 2008;1:379–386. doi: 10.1016/j.jcin.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Chase AJ, Fretz EB, Warburton WP, et al. Association of the arterial access site at angioplasty with transfusion and mortality: the m.O.R.T.A.L study (mortality benefit of reduced transfusion after percutaneous coronary intervention via the arm or leg) Heart. 2008;94:1019–1025. doi: 10.1136/hrt.2007.136390. [DOI] [PubMed] [Google Scholar]
- 7.Jolly SS, Yusuf S, Cairns J, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (rival): a randomised, parallel group, multicentre trial. Lancet. 2011;377:1409–1420. doi: 10.1016/S0140-6736(11)60404-2. [DOI] [PubMed] [Google Scholar]
- 8.Feldman DN, Swaminathan RV, Kaltenbach L, et al. Adoption of radial access and comparison of outcomes to femoral approach in percutaneous coronary intervention: an updated report from the NCDR® (2007–2011) Circulation. doi: 10.1161/CIRCULATIONAHA.112.000536. in press. [DOI] [PubMed] [Google Scholar]
- 9.Maroney J, Khan S, Powell W, et al. Current operator volumes of invasive coronary procedures in medicare patients: implications for future manpower needs in the catheterization laboratory. Catheter Cardiovasc Interv. 2013;81:34–39. doi: 10.1002/ccd.24366. [DOI] [PubMed] [Google Scholar]
- 10.Louvard Y, Lefevre T, Morice MC. Radial approach: what about the learning curve? Catheter Cardiovasc Diag. 1997;42:467–468. doi: 10.1002/(sici)1097-0304(199712)42:4<467::aid-ccd30>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 11.Hess CN, Rao SV, Kong DF, et al. Transradial education and therapeutics (treat): shifting the balance of safety and efficacy of antithrombotic agents in percutaneous coronary intervention. A report from the cardiac safety reserach consortium. Amer Heart J. 2013;165:344–353. doi: 10.1016/j.ahj.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed B, Piper WD, Malenka D, et al. Significantly improved vascular complications among women undergoing percutaneous coronary intervention: a report from the Northern New England Percutaneous Coronary Intervention Registry. Circ Cardiovasc Interv. 2009;2:423–429. doi: 10.1161/CIRCINTERVENTIONS.109.860494. [DOI] [PubMed] [Google Scholar]
- 13.Doyle BJ, Ting HH, Bell MR, et al. Major femoral bleeding complications after percutaneous coronary intervention: incidence, predictors, and impact on long-term survival among 17,901 patients treated at the Mayo Clinic from 1994 to 2005. JACC Cardiovasc Interv. 2008;1:202–209. doi: 10.1016/j.jcin.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Tizon-Marcos H, Bertrand OF, Rodes-Cabau J. Impact of female gender and transradial coronary stenting with maximal antiplatelet therapy on bleeding and ischemic outcomes. Amer Heart J. 2009;157:740–745. doi: 10.1016/j.ahj.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 15.McCourt B, Barham W, Melton L, et al. Transforming research through a National Cardiovascular Research Infrastructure. AMIA Clinical Research Informatics Summit. 2010 [Google Scholar]
- 16. http://www.fda.gov/regulatoryinformation/guidances/ucm125067.htm.
- 17.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–47. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 18.Romagnoli E, Biondi-Zoccai G, Sciahbasi A, et al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: the RIFLE-STEACS study. J Amer Coll Card. 2012;60:2481–2489. doi: 10.1016/j.jacc.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Pancholy S, Coppola J, Patel T, et al. Prevention of radial artery occlusion-patent hemostasis evaluation trial (PROPHET study): a randomized comparison of traditional versus patency documented hemostasis after transradial catheterization. Catheter Cardiovasc Interv. 2008;72:335–340. doi: 10.1002/ccd.21639. [DOI] [PubMed] [Google Scholar]
- 20.Bertrand OF, De Larochelliere R, Rodes-Cabau J, et al. Early Discharge After Transradial Stenting of Coronary Arteries Study. A randomized study comparing same-day home discharge and abciximab bolus only to overnight hospitalization and abciximab bolus and infusion after transradial coronary stent implantation. Circulation. 2006;114:2636–2643. doi: 10.1161/CIRCULATIONAHA.106.638627. [DOI] [PubMed] [Google Scholar]
- 21.Daugherty SL, Thompson LE, Kim S, et al. Patterns of use and comparative effectiveness of bleeding avoidance strategies in men and women following percutaneous coronary interventions: an observational study from the National Cardiovascular Data Registry. J Amer Coll Cardiol. 2013;61:2070–2078. doi: 10.1016/j.jacc.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis In Myocardial Infarction (TIMI) trial phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase Clinical findings through hospital discharge. Circulation. 1987;76:142–154. doi: 10.1161/01.cir.76.1.142. [DOI] [PubMed] [Google Scholar]
- 23.Manoukian SV, Feit F, Mehran R. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY trial. J Amer Coll Cardiol. 2007;49:1362–1368. doi: 10.1016/j.jacc.2007.02.027. [DOI] [PubMed] [Google Scholar]