Abstract
Background
Brachial blood pressure (BP) reactivity to stress predicts large artery damage and future cardiovascular (CV) events. Central BP is an emerging risk factor associated with target organ damage (TOD). Currently, little is known about the central BP response to mental stress and its association to TOD.
Methods and Results
Twenty-five healthy, non-obese adults completed a computerized mental stress test. Brachial and carotid systolic (S)BP reactivity to stress were calculated as SBP during stress minus resting SBP. Resting carotid intima-media thickness (IMT) was also measured. Carotid SBP reactivity to stress was significantly associated with carotid IMT, independent of age, sex, body mass index, non-high density lipoprotein cholesterol and brachial SBP reactivity to stress (r=0.386, p<0.05).
Conclusion
The relationship between carotid SBP reactivity and carotid IMT suggests that the central BP response to stress may prove to be an early risk marker for potential subclinical TOD.
Keywords: Central blood pressure reactivity, carotid intima-media thickness, mental stress
1. Introduction
The brachial systolic blood pressure (SBP) response to mental stress (SBP reactivity) is associated with an increased risk for future cardiovascular (CV) events, independent of resting BP level (1). Repeated episodes of stress-induced elevations in SBP over an individual’s lifetime may contribute to target organ damage (TOD), mediated by damage to the large central arteries such as the aorta or carotid arteries (2, 3).
Carotid intima-media thickness (IMT) is an established measure of subclinical atherosclerosis and TOD (4, 5). Although there is an association between brachial BP and carotid IMT (6), mounting evidence suggests that central BP is more tightly coupled pathophysiologically to regional TOD (6, 7), and as such may be a stronger predictor of CV outcomes than brachial BP (8, 9). This raises the intriguing possibility that the carotid SBP response to stress may be a stronger correlate of regional vascular TOD than traditional measures of CV reactivity to stress such as brachial SBP reactivity. The aim of the present study was to examine the relationship between carotid SBP stress reactivity and carotid IMT, independent of brachial SBP reactivity.
2. Methods
Twenty five healthy, non-obese (body mass index [BMI] < 30 kg/m2) men and women between the ages of 18-58 years participated in this study. Subjects were excluded if they self-reported daily cigarette smoking, history of hyperlipidemia, cardiovascular disease (CVD), diabetes mellitus, or if taking vasoactive medications. All subjects signed a written informed consent approved by the Institutional Review Board of Syracuse University.
This study required 2 laboratory visits. For the first visit, participants arrived after a 12 hour (overnight) fast to provide a fasting blood sample and complete questionnaires. For the second visit, participants were instructed to arrive at least 3 hours after their last meal for CV data acquisition at rest and during the mental stress protocol. For both study visits, participants refrained from caffeine or alcohol ingestion and strenuous physical activity at least 12 hours prior to testing. Blood samples were obtained via finger lancet for measurement of blood lipids and glucose using a validated point-of-care device (Alere Cholestech LDX, San Diego, CA) (10).
2.1. Brachial and Carotid Blood Pressure
Brachial SBP was measured in duplicate at baseline (after 10 minutes rest in the supine position) and in triplicate during the mental stress protocol using a validated, oscillometric cuff (EW3109, Panasonic Electric Works, Secaucus NJ). Carotid pressure waveforms were obtained from a 10 second epoch using applanation tonometry (SphygmoCor, AtCor Medical, Syndey, Australia) and calibrated to brachial mean arterial pressure and diastolic (D)BP at baseline and during mental stress. Brachial and carotid BP were measured simultaneously.
2.2. Carotid Doppler Ultrasonography
Images of the common carotid artery were obtained using Doppler ultrasound (ProSound α7, Aloka, Tokyo, Japan) and 7.5-10.0 mHz linear-array probe. The carotid artery was imaged using a longitudinal view of the near wall, distal to the carotid bulb as the distance from the lumenintima interface to the media-adventitia interface across a 5 mm region of interest via semiautomated digital calipers (11).
2.3. Mental Stress Protocol
Following acquisition of resting measures, participants remained in a supine position and completed a 4-minute customized incongruent Stroop color-word interference task (12) (E-Prime, Psychology Software Tools Inc, Sharpsburg PA). Target words were identified using a remote response clicker so that participants did not speak during carotid measures.
2.4. Statistical Analysis
Results are reported as mean ± standard deviation (SD). The CV response to the mental stress task and comparisons by sex were examined using paired t tests. Absolute changes in SBP were calculated as reactivity scores. Pearson’s correlation coefficients were used to examine associations of interest between baseline measures and SBP reactivity scores. Partial correlations were used to adjust for possible confounders including age, sex, BMI and non-high density lipoprotein-cholesterol (HDL-C). All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS, version 20, IBM, Chicago, IL).
3. Results
Descriptive subject characteristics are presented in Table 1. Brachial SBP increased from 116 (±9) mmHg to 126 (±11) mmHg in response to stress (p<0.001). The increase in carotid SBP from 109 (±8) to 115 (±9) mmHg (p<0.001), was lower than the brachial SBP response by an average difference of 4 mmHg (p=0.001). Brachial and carotid SBP reactivity were not significantly different between men and women (p=0.476 and p=0.664, respectively). Age was associated with carotid IMT (r=0.661, p<0.001), brachial SBP reactivity (r=0.480, p=0.008) and carotid SBP reactivity (r=0.499, p=0.006). Common CVD risk factors, such as sex, BMI, total cholesterol (TC), HDL-C, non-HDL-C, and fasting glucose were not associated with brachial or carotid BP reactivity to stress or carotid IMT (data not shown).
Table 1.
Baseline Characteristics and Systolic Blood Pressure Reactivity in Men and Women (mean ±SD)
| All n=25 |
Men n=12 |
Women n=13 |
p-value | |
|---|---|---|---|---|
| Age (years) | 38 (±13) | 39 (±13) | 37 (±13) | 0.617 |
| Body Mass Index (kg/m2) | 23.6 (±2.8) | 24.4 (±2.8) | 22.8 (±2.6) | 0.138 |
| Total Cholesterol (mg/dL) | 186 (±34) | 182 (±37) | 190 (±32) | 0.613 |
| HDL-Cholesterol (mg/dL) | 69 (±21) | 61 (±20) | 77 (±20) | 0.048 |
| Non-HDL-Cholesterol (mg/dL) | 117 (±28) | 122 (±33) | 112 (±23) | 0.422 |
| Glucose (mg/dL) | 88.3 (±9.1) | 91.8 (±9.5) | 85.1 (±7.8) | 0.066 |
| Carotid-IMT (mm) | 0.48 (±0.09) | .49 (±.10) | .46 (±.09) | 0.494 |
| Resting Brachial SBP (mmHg) | 116 (±9) | 119 (±11) | 113 (±5) | 0.170 |
| Resting Brachial DBP (mmHg) | 73 (±5) | 73 (±5) | 74 (±4) | 0.778 |
| Resting Carotid SBP (mmHg) | 109 (±8) | 112 (±9) | 106 (±5) | 0.052 |
| Brachial SBP Reactivity (mmHg) | 10 (±7) | 11 (±5) | 9 (±8) | 0.476 |
| Carotid SBP Reactivity (mmHg) | 6 (±6) | 6 (±5) | 5 (±7) | 0.664 |
HDL: high density lipoprotein, IMT: intima-media thickness; SBP: systolic blood pressure, DBP: diastolic blood pressure. p<0.05 indicates significant difference between men and women.
Before adjustment for covariates, carotid IMT was associated with brachial (r=0.457, p=0.011) and carotid (r=0.605, p=0.001) SBP reactivity to stress, but not with resting brachial SBP (r=−0.042, p=0.422) or carotid SBP (r=−0.107, p=0.306) (Table 2). After adjustment for age, sex, BMI and non-HDL-C, carotid SBP reactivity to stress remained strongly associated with carotid IMT (r=0.528, p=0.007), but brachial SBP reactivity to stress was not associated with carotid IMT (r=0.194, p=0.200) (Table 2). Although brachial and carotid SBP reactivity are strongly associated (r=0.726, p<0.001), the association between the carotid SBP response to stress and carotid IMT remained significant after additional adjustment for brachial BP reactivity (r=0.386, p=0.046). In contrast, after adjustment for age, sex, BMI, non-HDL-C and carotid SBP reactivity, there was no association between brachial SBP reactivity and carotid IMT (r=−0.103, p=0.333).
Table 2.
Univariate and partial correlations between systolic blood pressure and carotid intima-media thickness
|
Carotid IMT
|
||||||
|---|---|---|---|---|---|---|
| Zero-order |
Adjusted for age, sex, non-HDL-C and BMI |
Adjusted for age, sex, BMI, non-HDL-C and brachial SBP reactivity |
||||
| Variables | r | p | r | p | r | p |
| Resting Brachial SBP | −0.042 | 0.422 | −0.176 | 0.222 | −0.170 | 0.237 |
| Resting Carotid SBP | −0.107 | 0.306 | −0.212 | 0.178 | −0.220 | 0.176 |
| Brachial SBP reactivity | 0.457 | 0.011 | 0.194 | 0.200 | -- | -- |
| Carotid SBP reactivity | 0.605 | 0.001 | 0.528 | 0.007 | 0.386 | 0.046 |
Significant correlations (p < 0.05) are highlighted in bold font.
SBP: systolic blood pressure, DBP: diastolic blood pressure, IMT: intima-media thickness, BMI: body mass index, non-high density lipoprotein-cholesterol (non-HDL-C).
4. Discussion
This study revealed an association between carotid SBP reactivity to stress and carotid IMT, which was independent of age, sex, BMI, non-HDL-C and brachial SBP reactivity. Over time, an exaggerated CV response to stress increases the hemodynamic load on the vasculature and may contribute to subclinical vascular damage, manifesting as an increase in arterial wall IMT (2). The present study confirmed the established associations between traditional measurements of brachial SBP reactivity to stress and carotid IMT (2). However, this relationship did not retain significance after standard adjustments for confounders such as age, sex, BMI, and non-HDL-C which may be attributed to our healthy study population, as discussed later. These results support the concept that brachial BP may not offer a complete picture of the hemodynamic load on the vasculature and target organs.
Central arteries, such as the carotid, naturally have a buffering capacity which dissipates pressure and prevents damage to sensitive high-flow target organs (13). Despite our finding that the carotid SBP response to stress was more blunted than the brachial SBP response, change in pressure in central arteries may still have more damaging consequences to target organs. Our findings on the relationship between carotid SBP reactivity and carotid IMT suggest that carotid SBP reactivity may be a more robust predictor of carotid TOD than brachial SBP reactivity. Additionally, these results support the concept that the relationship between brachial SBP reactivity and carotid TOD may be due to its association with carotid SBP reactivity. In older adults, brachial pressures are more predictive of cardiovascular outcomes because, with aging, the amplification of BP from central to peripheral arteries diminishes, such that brachial pressures begin to approximate central pressures more closely (14). In contrast, in young, healthy adults with more elastic arteries, there can be large differences in central and brachial pressures, which may explain our finding that the central SBP response to stress is more predictive of carotid TOD in this setting.
Exposure of the regional carotid artery to high levels of BP may contribute to endothelial damage, leading to fibrosis and scarring (15). It has been posited that at lower levels of carotid IMT, as observed in the present study, gradation in IMT size may reflect medial vessel hypertrophy (15), rather than being representative of atherosclerosis development. Our observations support the hypothesis that below the level at which IMT interferes with lumen diameter, carotid IMT may be an adaptive response to exposure to changes in local transmural pressure (16). Our results suggest that individuals who exhibit an elevated central SBP response to stress may be at greater risk for carotid TOD. Regardless of whether carotid IMT represents atherosclerosis, IMT measurement may still reflect total burden of atherosclerosis at other sites in the arterial system (17) and remains a marker for cardiovascular risk (4, 5).
The discrepancies between results from this study compared to prior studies may be explained by the low range/variability of carotid IMT in our healthy study population. Another limitation of this study is our inability to identify the directionality of the observed cross-sectional relationship between carotid BP reactivity and carotid IMT. An alternative interpretation exists, that increased carotid IMT may directly impact the carotid BP response to stress via alterations in regional baroreceptor sensitivity (18).
In conclusion, this study demonstrated that carotid SBP reactivity is significantly associated with carotid IMT after adjustment for age, sex, BMI, non-HDL-C and brachial SBP reactivity. Several studies have identified a relationship between exaggerated CV response to stress and increased risk of CVD using the more traditional assessment of brachial BP (1). The central BP response to stress may prove to be an earlier or more sensitive predictor of future subclinical TOD. This notion requires further investigation. Future studies should examine whether interventions that target central pressure reactivity to stress, such as physical activity, have a favorable effect on vascular TOD.
Figure 1. The association between systolic blood pressure (BP) reactivity and carotid intima-media thickness (IMT).
( ) represents the correlation between brachial systolic BP reactivity and carotid IMT (r=0.457, p=0.011). (
) represents the correlation between brachial systolic BP reactivity and carotid IMT (r=0.457, p=0.011). (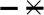 ) represents the correlation between carotid systolic BP and carotid IMT (r=0.605, p=0.001).
) represents the correlation between carotid systolic BP and carotid IMT (r=0.605, p=0.001).
Acknowledgments
Sources of funding
This work was supported by the Dairy Research Institute (Dairy Management Inc.) Grant1154 (KSH), NIH NIA P30 AG0344645 05 (KSH), NIH NIEHS R01 ES023252 02 (BBG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None.
References
- 1.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55(4):1026–32. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- 2.Jennings JR, Kamarck TW, Everson-Rose SA, Kaplan GA, Manuck SB, Salonen JT. Exaggerated blood pressure responses during mental stress are prospectively related to enhanced carotid atherosclerosis in middle-aged Finnish men. Circulation. 2004;110(15):2198–203. doi: 10.1161/01.CIR.0000143840.77061.E9. [DOI] [PubMed] [Google Scholar]
- 3.Matthews KA, Zhu S, Tucker DC, Whooley MA. Blood pressure reactivity to psychological stress and coronary calcification in the Coronary Artery Risk Development in Young Adults Study. Hypertension. 2006;47(3):391–5. doi: 10.1161/01.HYP.0000200713.44895.38. [DOI] [PubMed] [Google Scholar]
- 4.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115(4):459–67. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 5.Liu L, Zhao F, Yang Y, Qi LT, Zhang BW, Chen F, et al. The clinical significance of carotid intima-media thickness in cardiovascular diseases: a survey in Beijing. J Hum Hypertens. 2008;22(4):259–65. doi: 10.1038/sj.jhh.1002301. [DOI] [PubMed] [Google Scholar]
- 6.Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50(1):197–203. doi: 10.1161/HYPERTENSIONAHA.107.089078. [DOI] [PubMed] [Google Scholar]
- 7.Waddell TK, Dart AM, Medley TL, Cameron JD, Kingwell BA. Carotid pressure is a better predictor of coronary artery disease severity than brachial pressure. Hypertension. 2001;38(4):927–31. doi: 10.1161/hy1001.096107. [DOI] [PubMed] [Google Scholar]
- 8.Vlachopoulos C, Aznaouridis K, O'Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010 Aug;31(15):1865–71. doi: 10.1093/eurheartj/ehq024. [DOI] [PubMed] [Google Scholar]
- 9.Roman MJ, Devereux RB, Kizer JR, Okin PM, Lee ET, Wang W, et al. High central pulse pressure is independently associated with adverse cardiovascular outcome the strong heart study. J Am Coll Cardiol. 2009;54(18):1730–4. doi: 10.1016/j.jacc.2009.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carey M, Markham C, Gaffney P, Boran C, Maher V. Validation of a point of care lipid analyser using a hospital based reference laboratory. Ir J Med Sci. 2006;175(4):30–5. doi: 10.1007/BF03167964. [DOI] [PubMed] [Google Scholar]
- 11.Thijssen DH, Scholten RR, van den Munckhof IC, Benda N, Green DJ, Hopman MT. Acute change in vascular tone alters intima-media thickness. Hypertension. 2011 Aug;58(2):240–6. doi: 10.1161/HYPERTENSIONAHA.111.173583. [DOI] [PubMed] [Google Scholar]
- 12.Sheu LK, Jennings JR, Gianaros PJ. Test-retest reliability of an fMRI paradigm for studies of cardiovascular reactivity. Psychophysiology. 2012 Jul;49(7):873–84. doi: 10.1111/j.1469-8986.2012.01382.x. Research Support, N.I.H., Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility--Reykjavik study. Brain. 2011;134:3398–407. doi: 10.1093/brain/awr253. Pt 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilkinson IB, Franklin SS, Hall IR, Tyrrell S, Cockcroft JR. Pressure amplification explains why pulse pressure is unrelated to risk in young subjects. Hypertension. 2001;38(6):1461–6. doi: 10.1161/hy1201.097723. [DOI] [PubMed] [Google Scholar]
- 15.Bots ML, Hofman A, Grobbee DE. Increased common carotid intima-media thickness. Adaptive response or a reflection of atherosclerosis? Findings from the Rotterdam Study. Stroke. 1997;28(12):2442–7. doi: 10.1161/01.str.28.12.2442. [DOI] [PubMed] [Google Scholar]
- 16.Pries AR, Secomb TW, Gaehtgens P. Design principles of vascular beds. Circ Res. 1995;77(5):1017–23. doi: 10.1161/01.res.77.5.1017. [DOI] [PubMed] [Google Scholar]
- 17.Bots ML, Hofman A, De Jong PT, Grobbee DE. Common carotid intima-media thickness as an indicator of atherosclerosis at other sites of the carotid artery. The Rotterdam Study. Ann Epidemiol. 1996;6(2):147–53. doi: 10.1016/1047-2797(96)00001-4. [DOI] [PubMed] [Google Scholar]
- 18.Mukai S, Gagnon M, Iloputaife I, Hamner JW, Lipsitz LA. Effect of systolic blood pressure and carotid stiffness on baroreflex gain in elderly subjects. J Gerontol A Biol Sci Med Sci. 2003;58(7):626–30. doi: 10.1093/gerona/58.7.m626. [DOI] [PubMed] [Google Scholar]