Abstract
Idiopathic pulmonary fibrosis (IPF) is a lethal lung disease with progressive fibrosis and death within 2–3 y of diagnosis. IPF incidence and prevalence rates are increasing annually with few effective treatments available. Inhibition of IL-6 results in the attenuation of pulmonary fibrosis in mice. It is unclear whether this is due to blockade of classical signaling, mediated by membrane-bound IL-6Rα, or trans signaling, mediated by soluble IL-6Rα (sIL-6Rα). Our study assessed the role of sIL-6Rα in IPF. We demonstrated elevations of sIL-6Rα in IPF patients and in mice during the onset and progression of fibrosis. We demonstrated that protease-mediated cleavage from lung macrophages was important in production of sIL-6Rα. In vivo neutralization of sIL-6Rα attenuated pulmonary fibrosis in mice as seen by reductions in myofibroblasts, fibronectin, and collagen in the lung. In vitro activation of IL-6 trans signaling enhanced fibroblast proliferation and extracellular matrix protein production, effects relevant in the progression of pulmonary fibrosis. Taken together, these findings demonstrate that the production of sIL-6Rα from macrophages in the diseased lung contributes to IL-6 trans signaling that in turn influences events crucial in pulmonary fibrosis.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a deadly lung disease that causes chronic, progressive, and irreversible fibrosis. IPF is characterized by abnormal wound healing processes related to epithelial cell injury and turnover, fibroblast proliferation and differentiation, and matrix deposition (1, 2). Most patients experience respiratory decline with resulting respiratory failure and death within 2–3 y of diagnosis. There are no known causes for IPF, no cure, and few effective therapies (3). The mortality rate for IPF currently surpasses that of many malignancies, and during the last 2 decades, prevalence and incidence rates have continued to increase (4–6). Thus, there is an urgent need to better understand the onset and progression of pulmonary fibrosis to develop effective therapies against this deadly disease.
The cytokine IL-6 is elevated in mice and humans with pulmonary fibrosis (7, 8). IL-6 signals through two pathways, classical and trans, via a receptor complex consisting of the ligand-binding IL-6Rα and the non–ligand binding, signal-transducing receptor gp130. Whereas gp130 is expressed constitutively as a membrane-bound receptor, membrane-bound IL-6Rα (mIL-6Rα) is expressed predominantly on hepatocytes and leukocytes (9–13). IL-6 classical signaling occurs on cells that coexpress mIL-6Rα and gp130, where IL-6 binds mIL-6Rα and associates with two molecules of gp130 to initiate the intracellular signaling cascade. In the alternative pathway, known as trans signaling, IL-6 complexes with a soluble form of IL-6Rα (sIL-6Rα) to associate with gp130 and initiate signaling (10, 11, 13, 14). In both pathways, binding of IL-6/IL-6Rα with gp130 activates associated JAKs and leads to phosphorylation of STAT3, which dimerizes and translocates to the nucleus, where it acts as a transcription factor to regulate target genes (15–18).
sIL-6Rα is generated largely through protease-mediated cleavage of membrane-bound IL-6Rα (11, 19, 20). A disintegrin and metalloprotease (ADAM) 17 is a membrane-bound protease responsible for cleaving a number of cell surface proteins (21). ADAM17 has been implicated as the major protease responsible for shedding IL-6Rα from cell membranes of hepatocytes, peripheral monocytes, neutrophils, and lymphocytes in response to various stimuli, including apoptosis, calcium mobilization, cellular cholesterol depletion, leptin induction, and lymphocyte activation (10, 22–26). Increased levels of this protease are seen in association with diseases that have reported increased sIL-6Rα levels, including colitis and arthritis (27–30). The role of ADAM17 in shedding mIL-6Rα to produce the soluble receptor in fibrotic lungs, however, has not been examined.
The presence of sIL-6Rα, mediating IL-6 trans signaling, allows for activation of cells not inherently responsive to IL-6, thus widening the spectrum of IL-6–responsive cells and amplifying IL-6 effects in the body, leading to important roles in chronic pathological states (11, 31). IL-6 trans signaling has been implicated in the pathogenesis of rheumatoid arthritis (32), asthma (33), inflammatory bowel disease (colitis) (34), and colitis-associated cancer (35). IL-6 and sIL-6Rα levels are elevated in association with these diseases, and in vivo blockade of IL-6 trans signaling using the natural inhibitor soluble gp130 (36, 37) has resulted in amelioration of disease (32–35). In terms of fibrosis, levels of IL-6 and sIL-6Rα are elevated in systemic sclerosis (38, 39) and liver cirrhosis (40), correlating with disease severity and suggesting involvement of IL-6 trans signaling. In the kidneys, heart, and skin, IL-6 induction promotes collagen production (41–43).
In the lungs, IL-6 is important in airway remodeling in asthma (44) and induces the conversion of human lung fibroblasts to myofibroblasts (45), and it promotes pancreatitis-associated lung injury (46). However, the role of IL-6 in pulmonary fibrosis was not defined until O’Donoghue et al. (47) demonstrated that IL-6 ablation attenuated fibrosis in a bleomycin-induced murine model. We previously demonstrated that IL-6 contributes to pulmonary fibrosis when we reported that genetic or pharmacologic removal of IL-6 resulted in attenuation of fibrosis (7). However, the role of IL-6 trans signaling in pulmonary fibrosis is unknown. We hypothesized that IL-6 trans signaling is crucial to the development of pulmonary fibrosis in that it acts as an amplification pathway that enhances the normal wound-healing process, resulting in a fibrotic response. Results indicate that sIL-6Rα is elevated in explanted lung tissues from IPF patients, as well as in mice during onset and progression of pulmonary fibrosis induced by chronic bleomycin exposure. We demonstrated a temporal increase in ADAM17 in fibrotic lungs that mirrored increases in sIL-6Rα and supported a role for ADAM17 in generating sIL-6Rα, largely from lung macrophages. In vivo neutralization of trans signaling using recombinant gp130Fc, a selective inhibitor, resulted in a reduction in pulmonary inflammation and fibrosis associated with improvement in respiratory function. In vitro studies revealed that activation of IL-6 trans signaling enhanced fibroblast proliferation and extracellular matrix protein production, suggesting a mechanism for the role of trans signaling in disease development. These findings highlight the importance of sIL-6Rα and trans signaling in pulmonary fibrosis. Furthermore, neutralization of trans signaling attenuates disease and represents a promising new approach to treating IPF.
Materials and Methods
Human samples
De-identified explanted lung samples from chronic obstructive pulmonary disease (COPD) and IPF patients were obtained from the Methodist Hospital J.C. Walter Jr. Transplant Center. Collection and use of these tissues for research were in accordance with the guidelines approved by the Methodist Hospital Institutional Review Board. Primary macrophages from IPF patients were obtained from the University of Texas Medical School bronchoscopy suite.
Mice
All mice used were inbred, male C57BL/6NHsd mice, 4–5 wk old, ordered from Harlan Laboratories. Maintenance and care of animals were in accordance with guidelines set by the Animal Welfare Committee at the University of Texas Health Science Center at Houston. All study designs were reviewed and approved by the Animal Welfare Committee.
Intraperitoneal bleomycin model
Male C57BL/6 mice (4–5 wk old) were treated with i.p. injections of saline or bleomycin (0.035 U/g; Teva Pharmaceutical, Petach Tikva, Israel). A total of eight injections were given twice a week for 4 wk. Mice were sacrificed and samples collected on day 33. For the time course experiment, mice were sacrificed and samples collected on days 5, 10, 15, 20, 25, and 33. Day 0 mice were wild-type mice.
In vivo neutralization of IL-6 trans signaling
In vivo neutralization of IL 6 trans signaling was performed using recombinant mouse gp130Fc chimera (R&D Systems, Minneapolis, MN). Male C57BL/6 mice (4–5 wk old) were treated with bleomycin or vehicle (saline) i.p. twice a week for 4 wk. Beginning on day 19 and continuing daily until day 32, mice were treated with saline (200 μl 1× sterile PBS) or gp130Fc (2 μg/mouse reconstituted in 200 μl sterile PBS). On treatment days that coincided with bleomycin injections, mice received gp130Fc injections 1–2 h before bleomycin. On day 32, arterial oxygen saturation levels were measured. On day 33, animals were sacrificed and samples collected to assess changes to pulmonary phenotype.
Assessment of arterial oxygen saturation
Assessment of arterial oxygen saturation was performed on shaved, conscious mice on day 32 using the pulse MouseOx software analysis (Starr Life Sciences, Oakmont, PA).
Plasma, bronchoalveolar lavage fluid, cellular differentials, and histology
Mice were anesthetized with avertin and blood was collected and centrifuged to isolate plasma. Lungs were lavaged four times with 0.3 ml PBS; 1 ml pooled bronchoalveolar lavage (BAL) fluid was recovered. Total cell counts were determined using a hemocytometer. Cellular differentials were performed by spinning BAL aliquots onto microscope slides and staining with Diff-Quick (Dade Behring, Deerfield, IL). Remaining BAL fluid was centrifuged and supernatant and cell pellet were stored for further analyses. After lavage, lungs were inflated, fixed in formalin, and paraffin-embedded. Five-micrometer sections were cut and used in immunostaining.
Immunohistochemistry and immunofluorescence
Immunohistochemistry was performed on cut lung sections as previously described (7, 48). Primary Abs used were: anti-STAT3 (p-S727) Ab (rabbit polyclonal, 1:100 dilution, 4°C overnight; Abcam, Cambridge, MA), anti-ADAM17 (rabbit polyclonal, 1:100 dilution at 37°C for 1 h, EMD Millipore), and anti-actin, α–smooth muscle Ab (1:1000 dilution, 4°C overnight, mouse monoclonal clone 1A4; Sigma-Aldrich, St. Louis, MO). A Vectastain ABC peroxidase rabbit IgG kit (Vector Laboratories) and 3,3′-diaminobenzidine (Sigma-Aldrich) were used to develop stains for p-STAT3 and ADAM17. A Vectastain ABC alkaline phosphatase standard kit (Vector Laboratories) and a Vector alkaline phosphatase substrate kit (Vector Laboratories) were used to develop stains for α–smooth muscle actin (α-SMA).
For dual immunohistochemistry staining of p-STAT3 and α-SMA, slides were stained for p-STAT3 first, then α-SMA. For dual immunofluorescence of p-STAT3 and α-SMA, slides were incubated in anti-STAT3 (p-S727) Ab (1:100 dilution, 4°C overnight), then in appropriate secondary Ab. An ABC alkaline phosphatase standard kit and Vector alkaline phosphatase substrate kit were used to develop p-STAT3 as a red fluorescence. Slides were then incubated in anti-actin, α–smooth muscle Ab (1:500 dilution, 4°C overnight). Secondary Ab incubation in Alexa Fluor 488 rabbit anti-mouse IgG (1:1000 dilution) was performed to allow detection of α-SMA as a green fluorescence. Slides were mounted with coverslips and Prolong Gold antifade medium with DAPI.
Immunofluorescence of BAL cells
BAL cells were cytospun onto microscope slides, allowed to air dry, then fixed in 3.7% paraformaldehyde for 10 min and permeabilized in cold methanol for 10 min. Blocking occurred using 1% BSA for 1 h at room temperature and then incubated in anti-human CD126 (IL-6R) Ab (1:50 dilution, 4°C overnight, mouse monoclonal; ABD Serotec, Raleigh, NC). Incubation in secondary Ab, Alexa Fluor 488 rabbit anti-mouse IgG (1:1000 dilution; Life Technologies, Grand Island, NY), occurred for 1 h at room temperature. Slides were mounted with coverslip and Prolong Gold antifade medium with DAPI (Life Technologies).
Assessment of fibrosis
Rehydrated lung sections were stained with Masson’s trichrome per the manufacturer’s protocol (EM Science, Gibbstown, NJ). Fibrosis was quantified using the Ashcroft method of scoring. Scoring was performed blinded with regard to animal treatment. All areas of the lung were scored, and an overall average score was given per lung section. The Sircol assay (Biocolor, Carrick, U.K.) was used to measure soluble collagen content in BAL fluid. Transcript levels of procollagen were quantitated using whole-lung mRNA. Fibronectin expression was determined using Western blot analysis of lung lysates.
Western blot of lung lysates and BAL fluid
Western blot was performed as previously described (7). Primary Abs used were: anti-human CD126 (IL-6R, 1:500 dilution, mouse monoclonal’ ABD Serotec), anti-STAT3 (p-S727) Ab (1:500 dilution, rabbit polyclonal; Abcam), anti-ADAM17 (1:2000, rabbit polyclonal; Millipore), anti-fibronectin, cellular Ab (1:2000, mouse monoclonal clone FN 3E2; Sigma-Aldrich), and anti–β-actin Ab (1:5000, mouse monoclonal clone AC-74; Sigma-Aldrich). For lung lysates, 50 μg protein was loaded per sample. For BAL fluid samples, equal volumes of samples were prepared in sample buffer and equal volumes were loaded per lane. Expression of proteins on Western blot was quantified using ImageJ analysis of scanned blots.
Analysis of whole-lung RNA
Total RNA was isolated from frozen lung tissue using TRIzol reagent (Invitrogen) per the manufacturer’s protocol. Samples were treated with RNAse-free DNAse 1 (Invitrogen) and used for quantitative real-time RT-PCR analysis. cDNA was made using SuperScript II reverse transcriptase (Invitrogen). Equal amounts of cDNA were analyzed for transcript levels of COL1A2 and MCP-1 with normalization to β-actin. Primers used for COL1A2 were: forward, 5′-AAGGGTGCTACTGGACTCCC-3′ and reverse, 5′-TTGTTACCGGATTCTCCTTTGG-3′. Primers for MCP-1 were: forward, 5′-AGCATCCACGTGTTGGCTC-3′ and reverse, 5′-TGGGATCATCTTGCTGGTG-3′. Data are presented as mean normalized transcript levels using the comparative Ct method.
ELISA analysis of sIL-6Rα in protein lysates, BAL fluid, and plasma
sIL-6Rα was quantified in protein lysates made from COPD and IPF lungs using a human IL-6Rα DuoSet ELISA kit (R&D Systems). Lysate (10 μl) was diluted in 90 μl reagent diluent and subjected to ELISA analysis per the manufacturer’s protocol. Murine BAL fluid and plasma samples were processed using a mouse IL-6Rα DuoSet ELISA kit (R&D Systems). For BAL fluid samples, 100 μl was used. For plasma samples, 10 μl plasma was diluted in 90 μl reagent diluent.
Bone marrow macrophage isolation and differentiation
Male C57BL/6 mice (4–5 wk old) were euthanized via cervical dislocation. Both femurs were isolated and removed, and bone marrow cavities were flushed with complete macrophage medium, that is, DMEM (Fisher Scientific) supplemented with 10% FBS and 20% L929 media supplement. Flushed cells were pelleted and resuspended in macrophage media, counted, and plated in 100-mm bacterial dishes at a density of 3–5 × 106 cells in 7.5 ml media per dish. Cells were incubated at 37°C, 5% CO2 for 4 d and then supplemented with an extra 5 ml media and cultured for another 3–4 d. On days 7–8, adherent macrophages were detached, collected, pelleted, and resuspended in macrophage media for seeding in tissue culture plates for experiments. To make L929 media supplement, L929 cells were plated at a density of 5 × 105 cells per T75 flask in 55 ml media consisting of DMEM with 1% HEPES, 1% penicillin-streptomycin, 1% l-glutamine, and 10% FBS. Cells were cultured for 7 d and then media were harvested and sterile filtered for addition to macrophage culture media. Days 7–8 bone marrow–derived macrophages were plated in six-well plates at a density of 1.5–2 × 106 cells/well and allowed to adhere 2–3 h. Nonadherent cells were removed and cells were then incubated in macrophage media containing IL-4 (20 ng/ml) and IL-13 (10 ng/ml) (PeproTech, Rocky Hill, NJ) for 72 h. Fresh reagents were added daily. After 72 h, macrophages were washed with sterile PBS and used in TNF-α protease inhibitor (TAPI)-1 experiments.
TAPI-1 inhibition of ADAM17 in macrophages
IL-4/IL-13–stimulated macrophages were incubated in serum-free media containing PMA (10 μg/ml; Enzo Life Sciences, Farmingdale, NY) for 2–3 h at 37°C, 5% CO2. Inhibition of ADAM17 activity was carried out by preincubation of macrophages with TAPI-1 (20 μM) for 1 h followed by PMA stimulation in the presence of TAPI-1. After 2–3 h exposure to media alone, media and TAPI-1, PMA alone, or PMA and TAPI-1, culture media were collected and sIL-6Rα was quantified using ELISA. Macrophages were lysed in RIPA lysis buffer containing protease inhibitors. Five to 10 μg protein per sample was subjected to Western blot analysis to determine expression of arginase 1 (rabbit polyclonal Ab, 1:200 dilution, overnight incubation, 4°C; Santa Cruz Biotechnology), IL-6R (anti-human CD126 Ab, mouse monoclonal, 1:500 dilution, 4°C overnight; ABD Serotec), and ADAM17 (rabbit polyclonal Ab, 1:500 dilution, overnight incubation, 4°C; EMD Millipore).
ADAM17 small interfering RNA silencing in macrophages
Days 7–8 bone marrow–derived macrophages were resuspended in antibiotic-free macrophage media, seeded in six-well plates, and allowed to adhere for 2–3 h. Cells were then incubated in antibiotic-free media containing IL-4, IL-13, Opti-MEM (Invitrogen) with Lipofectamine RNAiMAX (Invitrogen), and ADAM17 small interfering RNA (siRNA) or scrambled control siRNA (Sigma-Aldrich) for 24 h. Final concentration of siRNA was 100 nM. After 24 h, media were changed and a second transfection was performed with fresh reagents. Macrophages were incubated in the second transfection medium for 48 h. Fresh IL-4 and IL-13 were added to culture media daily. After 72 h, nontransfected macrophages, macrophages transfected with control siRNA, and macrophages transfected with ADAM17 siRNA were stimulated with PMA in serum-free conditions for 2–3 h. Culture media were collected and sIL-6Rα levels were quantified using ELISA. Cells were lysed in RIPA lysis buffer containing protease inhibitors. Five to 10 μg protein per sample was subjected to Western blot analysis to determine expression of arginase 1, IL-6R, and ADAM17.
Shedding of mIL-6Rα from primary lung macrophages
BAL fluid was collected from day 33 male C57BL/6 mice treated with PBS or bleomycin. Lungs were lavaged 10 times with 0.5 ml sterile PBS each; ∼5 ml pooled lavage fluid per mouse was recovered. BAL fluid from all PBS mice or all bleomycin mice were pooled and spun at 1200 rpm for 5 min at 4°C. The supernatant was removed and cell pellets were resuspended in complete macrophage media (RPMI 1640 containing 10% FBS and 1% penicillin-streptomycin). Cells were counted by hemocytometer, plated in six-well plates at 1 × 106 cells/well, and allowed to adhere 4 h at 37°C, 5% CO2. Culture media and nonadherent cells were removed after 4 h by washing with serum-free RPMI 1640. Cells were then incubated in macrophage media containing PMA (10 μg/ml; Enzo Life Sciences, Farmingdale, NY) for 2 h at 37°C, 5% CO2 to activate ADAM17 and induce mIL-6Rα shedding. Inhibition of ADAM17 activity was carried out by preincubation of macrophages with TAPI-1 (20 μM) for 1 h followed by PMA stimulation in the presence of TAPI-1. After 2 h exposure to media alone, PMA alone, or PMA and TAPI-1, culture media were collected and sIL-6Rα was quantified using ELISA.
Control and IPF fibroblast cell lines
CCD8Lu and LL97A (AlMy) are control and IPF fibroblast cell lines, respectively, purchased from the American Type Culture Collection (Manassas, VA). CCD8Lu was cultured in American Type Culture Collection–formulated Eagle’s MEM supplemented with 10% FBS and 1% penicillin-streptomycin-amphotericin B mixture. LL97A was cultured in Ham’s F12K medium (American Type Culture Collection) supplemented with 15% FBS and 1% penicillin-streptomycin-amphotericin B mixture. For stimulation experiments, cells were used between passages four and eight.
In vitro stimulation of IL-6 trans signaling
To assess the effects of IL-6 trans signaling on fibroblast proliferation, CCD8Lu and LL97A cells were plated in 96-well plates at a density of 3–5 × 103 cells/well and cultured to reach 70–80% confluency. Cells were serum starved for 24 h and then stimulated for 48 h in serum-free media with recombinant human TGF-β1 (10 ng/ml), recombinant human IL-6 (50 ng/ml), or IL-6 (50 ng/ml) plus recombinant human IL-6Rα (100 ng/ml) (R&D Systems). Fresh reagents were added daily. Proliferation was assessed using and ApoTox-Glo triplex assay kit (Promega, Madison, WI). Viability reagent was added and fluorescence was measured after 30 min incubation at 37°C. To assess effects of IL-6 trans signaling on extracellular matrix protein production, CCD8Lu and LL97A cells were seeded in six-well plates at a density of 1 × 105 cells/well and cultured to reach 70–80% confluency. Cells were serum starved and stimulated as mentioned above. After 48 h of stimulation, cells were lysed in RIPA lysis buffer supplemented with protease inhibitor mixture. Protein lysates were subjected to Western blot analysis to detect collagen and fibronectin production. Five to 10 μg protein per sample was loaded. Incubation was performed in primary Abs for collagen 1 (rabbit polyclonal, 1:500 dilution, 48 h at 4°C; Abcam) and fibronectin EDA (mouse monoclonal clone FN 3E2, 1:4000 dilution, overnight at 4°C; Sigma-Aldrich).
Statistical analysis
Experimental results are reported as means ± SEM. One-way ANOVA was used for comparisons among groups, and comparisons between groups were completed with a two-tailed Student t test. Statistical significance for all comparisons is presented as p values. A p value of <0.05 was considered to be significant.
Results
sIL-6Rα is elevated in IPF
As an initial assessment of whether sIL-6Rα is elevated in IPF, we measured its levels in the lungs of IPF patients. Protein lysates were prepared from explanted COPD and IPF lung tissues, and ELISA measurement of sIL-6Rα revealed a significant, 4-fold increase in IPF lungs versus COPD lungs (Fig. 1A). Secondary confirmation was achieved with Western blot analysis of these samples, probing for expression of sIL-6Rα, which revealed increased sIL-6Rα in IPF lungs (Fig. 1B). These findings demonstrate, to our knowledge for the first time, increased sIL-6Rα in IPF and suggest that IL-6 trans signaling may play a role in this disease.
FIGURE 1.
sIL-6Rα in IPF and a chronic bleomycin mouse model of pulmonary fibrosis. sIL-6Rα expression was assessed in humans and mice with pulmonary fibrosis. (A and B) ELISA measurement and Western blot analysis of sIL-6Rα in lung lysates from patients with COPD and IPF. (C) Western blot analysis of sIL-6Rα in lung lysates and (D) ELISA measurement of sIL-6Rα in BAL fluid from wild-type C57BL/6 mice given saline or bleomycin, day 33. (E) Sircol analysis of soluble collagen in BAL fluid during development and progression of pulmonary fibrosis in a chronic bleomycin mouse model. Western blot analysis (F) and ELISA quantification (G) of sIL-6Rα in BAL fluid throughout the model. All data are presented as means ± SEM, n ≥ 4. *p < 0.05, **0.001 < p < 0.01, ***p < 0.001 for difference from COPD or PBS-treated cohort.
sIL-6Rα is elevated in bleomycin-induced pulmonary fibrosis
To further evaluate the role of sIL-6Rα in IPF, we turned to a chronic model of fibrosis, the IPB model. In this model, mice are given i.p. injections of bleomycin twice weekly for four weeks, which lead to progressive development of pulmonary fibrosis (48–50). This model of chronic bleomycin exposure has been demonstrated to recapitulate features of IPF, such as the presence of hyperplastic airway epithelial cells and fibrosis that radiates inward from the pleural surfaces and is progressive, irreversible, and lethal (51, 52).
To assess levels of sIL-6Rα in this model, protein lysates were made from day 33 lungs and Western blot was performed. sIL-6Rα was absent in PBS-injected, nonfibrotic lungs but present in bleomycin-exposed, fibrotic lungs (Fig. 1C). BAL fluid was collected and analyzed for presence of the soluble receptor in the airways and airspaces of the lung. ELISA measurement of sIL-6Rα in BAL fluid demonstrated a significant, 3-fold increase in bleomycin samples in comparison with PBS samples (Fig. 1D). Furthermore, to better understand how sIL-6Rα levels change over the course of development and progression of pulmonary fibrosis, BAL fluid was collected at various time points during the course of the model (days 5, 10, 15, 20, 25, and 33). Analysis of soluble collagen in BAL fluid revealed progressive development of fibrosis in the model, with significant increases in collagen already evident in bleomycin lungs by day 5 and the most prominent increase present in day 20 samples (Fig. 1E). Western blot analysis and ELISA quantification of the samples showed a temporal increase in sIL-6Rα (Fig. 1F, 1G) that mirrors the changes in collagen. Collectively, these findings demonstrate an association between increases in sIL-6Rα and pulmonary fibrosis and suggest a role for the soluble receptor in disease onset and progression.
ADAM17 is increased in bleomycin-induced pulmonary fibrosis
To assess the underlying mechanism of sIL-6Rα accumulation in fibrotic lungs, we explored the role of the proteases ADAM17 and ADAM10 in cleaving mIL-6Rα to produce the soluble receptor. To determine whether these proteases are elevated in pulmonary fibrosis, we evaluated their expression in the IPB model. Protein lysates were made from day 33 lungs and Western blot was performed. Immature and mature forms of ADAM17 were increased in bleomycin-exposed, fibrotic lungs (Fig. 2A). No difference in ADAM10 expression was seen between bleomycin and PBS lungs (Fig. 2B). Thus, to further characterize ADAM17 expression, BAL fluid was collected and analyzed for the presence of ADAM17 in the airways and airspaces of the lung. Western blot analysis detected increases in ADAM17 in bleomycin samples (Fig. 2C). Immunostaining of sections from these lungs revealed the most prominent increase of ADAM17 in alveolar macrophages (Fig. 2D). When these macrophages were washed out of the lungs during BAL, collected and cytospun onto slides, and then subjected to immunofluorescence staining for mIL-6Rα, it was observed that macrophages from bleomycin lungs expressed more mIL-6Rα than did those from PBS lungs (Fig. 2E). This finding was confirmed using Western blot analysis of BAL cell pellet protein lysates to the detect presence of mIL-6Rα. Cell pellets from fibrotic lungs expressed more mIL-6Rα than did samples from nonfibrotic lungs (Fig. 2F). The fact that there was an increase in ADAM17 in cells that expressed more mIL-6Rα suggested a role for ADAM17 in shedding mIL-6Rα from these cells to form sIL-6Rα. Analysis of alveolar macrophages from IPF lungs also revealed abundant expression of mIL-6Rα (Supplemental Fig. 1A), further validating the potential of these cells in serving as the source of sIL-6Rα generation in human patients.
FIGURE 2.
ADAM17 expression in a mouse model of chronic bleomycin-induced pulmonary fibrosis. Expression of the protease ADAM17 was evaluated in mice with pulmonary fibrosis. Western blot analysis of (A) ADAM17 and (B) ADAM10 in lung lysates from wild-type C57BL/6 mice given saline or bleomycin, day 33. (C) Western blot analysis of ADAM17 in BAL fluid from day 33 mice. (D) Immunostaining for ADAM17 (brown) in the lungs of day 33 mice and (E) immunofluorescence staining for mIL-6R α (green) and DAPI (blue) on BAL fluid cells, day 33. Arrows denote positive cells. Images are representative of n ≥ 4 animals from each group. Scale bars, 50 μm (×100 oil immersion). (F) Western blot analysis of mIL-6Rα in protein lysates made from BAL fluid cell pellets, day 33. (G) Western blot analysis of ADAM17 in BAL fluid as pulmonary fibrosis develops and progresses in a chronic bleomycin-induced mouse model. (H) BAL macrophages throughout the model. Data are presented as means ± SEM, n ≥ 4. *p < 0.05 for difference from PBS-treated cohort.
ADAM17 is increased in association with increasing sIL-6Rα and alveolar macrophages in fibrotic lungs
Further characterization of ADAM17 expression during onset and development of fibrosis was achieved via Western blot analysis of BAL fluid samples collected throughout the duration of the IPB model. Results indicated a temporal increase in ADAM17 expression that mirrored the increase in sIL-6Rα as pulmonary fibrosis developed and progressed (Fig. 2G). These results further implicated ADAM17 in the generation of the sIL-6R in fibrotic lungs. Because our data suggested macrophages as a potential source of shedding of IL-6Rα, we sought to determine whether there was a change in alveolar macrophages as pulmonary fibrosis develops and progresses. Using BAL fluid samples collected throughout the duration of the IPB model, cellular differentials were performed and the number of BAL macrophages was determined. BAL macrophages in bleomycin-exposed lungs accumulated in a pattern similar to the change in ADAM17 and sIL-6Rα (Fig. 2H). Collectively, these findings demonstrate an association between increases in alveolar macrophages and ADAM17 and increases in sIL-6Rα in fibrotic lungs, suggesting that ADAM17 activation in alveolar macrophages of fibrotic lungs induces shedding of mIL-6Rα to produce sIL-6Rα.
ADAM17 promotes shedding of IL-6Rα from membranes of activated macrophages
Given the previous data showing increases in ADAM17 in alveolar macrophages that express higher levels of mIL-6Rα, we hypothesized that this protease is activated in these cells to cleave mIL-6Rα to produce sIL-6Rα. Alveolar macrophages found in the lungs of IPF patients and mice with bleomycin-induced pulmonary fibrosis are primarily M2 in phenotype and are reported to drive progression of disease (8, 53–57). We asked whether we could replicate the in vivo conditions using an in vitro cell system and show that activation of ADAM17 in M2 macrophages induces shedding of mIL-6Rα to increase production of sIL-6Rα.
To generate M2 macrophages in vitro, we stimulated bone marrow–derived murine macrophages with the cytokines IL-4 and IL-13 to polarize their differentiation into M2 (58–61). These cytokines were reported to be important in the lung, and their use in various studies has led to successful differentiation of macrophages to the M2 phenotype (58–61). Arginase 1 is a marker of M2 macrophages, and its expression after stimulation has been reported to be an indicator of successful differentiation (54, 58, 60, 61). Western blot analysis of protein lysates from IL-4/IL-13–stimulated macrophages revealed more arginase 1 expression than in unstimulated macrophages (Fig. 3A), suggesting they are M2 in phenotype. We next evaluated mIL-6Rα expression in the bone marrow–derived M2 macrophages to see whether the membrane receptor is present to be cleaved. Western blot analysis showed more mIL-6Rα in IL-4/IL-13–stimulated macrophages than in unstimulated macrophages (Fig. 3A). The characteristics of augmented ADAM17 and mIL-6Rα expression in our bone marrow–derived M2 macrophages mimicked those seen in alveolar macrophages isolated from fibrotic murine and human lungs. Thus, we proceeded to manipulate ADAM17 activity in these cells.
FIGURE 3.
Pharmacologic neutralization and siRNA-mediated silencing of ADAM17 activity in macrophages. (A) Western blot analysis of mIL-6Rα and arginase 1 expression in bone marrow–derived macrophages stimulated with IL-4 and IL-13. (B) ELISA measurement of sIL-6Rα in culture media of bone marrow macrophages stimulated with PMA, with and without TAPI-1. (C) Western blot analysis of ADAM17 in protein lysates of bone marrow macrophages transfected with control or ADAM17 siRNA. (D) ELISA measurement of sIL-6Rα in culture media of bone marrow macrophages transfected with control or ADAM17 siRNA and then stimulated with PMA. (E) ELISA measurement of sIL-6Rα in culture media of primary lung macrophages stimulated with PMA, with and without TAPI-1. All data are presented as means ± SEM, n ≥ 6 for (B) and (D), n = 1 or 3 for PBS or bleomycin cohorts in (E). ***p < 0.001 for difference from media only cohort, ###p < 0.001 for difference from PMA-stimulated cohort.
The reagent PMA is known to activate ADAM17-mediated shedding of mIL-6Rα (21, 62). To demonstrate that ADAM17 can shed IL-6Rα from the membrane of bone marrow–derived M2 macrophages, IL-4/IL-13–stimulated macrophages were incubated with PMA to induce shedding. To assess shedding efficiency, culture media were collected and sIL-6Rα was quantified using ELISA. PMA activation of ADAM17 led to a significant, 4-fold increase in sIL-6Rα in the culture media (Fig. 3B), suggesting that ADAM17 is responsible for the increase in shedding.
To further support the role of ADAM17 in this process, we assessed whether blocking ADAM17 would alter the extent of shedding. We first attempted to block ADAM17 activity pharmacologically using TAPI-1, a nonselective inhibitor of ADAM proteases (23, 62). Addition of TAPI-1 to macrophages without PMA stimulation was able to significantly suppress baseline shedding; the presence of TAPI-1 in the setting of PMA stimulation resulted in inhibition of sIL-6Rα release into the media (Fig. 3B). These results were confirmed using a second, more specific method of neutralizing ADAM17 activity. IL-4/IL-13–stimulated macrophages were transfected with ADAM17 siRNA (30) to silence ADAM17 and then subjected to PMA stimulation. Successful silencing was confirmed by Western blot analysis showing reduced expression of ADAM17 (Fig. 3C). ADAM17-transfected macrophages released less sIL-6Rα into the culture media than did macrophages transfected with control siRNA (Fig. 3D).
To more accurately reflect what happens in active pulmonary fibrosis, we repeated the above experiments using primary alveolar macrophages isolated from day 33 mice treated with PBS or bleomycin. Lungs were lavaged and cells collected in BAL fluid were then cultured to isolate alveolar macrophages. PMA-induced activation of ADAM17 in primary alveolar macrophages from PBS lungs induced an increase in sIL-6Rα in culture media (Fig. 3E). Preincubation with TAPI-1 reduced levels of released sIL-6Rα to below detection threshold. PMA stimulation of macrophages from bleomycin lungs resulted in a 2-fold increase in sIL-6Rα in culture media (Fig. 3E). Preincubation with TAPI-1 reduced levels of released sIL-6Rα to baseline levels. Overall, the results of our shedding experiments, both in bone marrow–derived M2 macrophages and in primary alveolar macrophages from fibrotic mouse lungs, have supported a role for ADAM17 in shedding mIL-6Rα to generate sIL-6Rα in fibrotic lungs.
In vivo neutralization of IL-6 trans signaling reduced pulmonary inflammation
Having demonstrated that sIL-6Rα was elevated in association with pulmonary fibrosis as a result of protease-mediated cleavage of the membrane receptor, we next investigated whether neutralization of this soluble receptor would result in therapeutic benefit in a mouse model of pulmonary fibrosis. In vivo neutralization of sIL-6Rα was performed in the IPB model using mouse recombinant gp130Fc, a reagent shown in previous studies to be an effective and selective inhibitor of IL-6 trans signaling (32–35). Treatment with gp130Fc was initiated late in the disease process, on day 19, when pulmonary fibrosis was established, so as to determine the therapeutic rather than preventative benefits of antagonizing trans signaling. The neutralization protocol (Supplemental Fig. 1B) was sufficient to significantly lower levels of sIL-6Rα in BAL fluid (Supplemental Fig. 1C, 1D). IL-6 protein levels in BAL fluid were not different between bleomycin and bleomycin plus gp130Fc groups (Supplemental Fig. 1E). These findings revealed that recombinant gp130Fc is able to alter levels of sIL-6Rα in the lung microenvironment, a feature that supports the notion of local sIL-6Rα generation from macrophages during active disease.
In the IPB model, mice develop extensive pulmonary fibrosis as well as pulmonary inflammation (48, 50); therefore, to assess changes in pulmonary phenotype, we first examined the effects of recombinant gp130Fc administration on pulmonary inflammation. Treatment with gp130Fc was associated with decreased inflammation, as evident by a significant reduction in total inflammatory cells recovered in BAL fluid on day 33 (Fig. 4A). Cell differential analysis of BAL fluid revealed a reduction in all cell types examined, including macrophages (Fig. 4B), lymphocytes, neutrophils, and eosinophils (Fig. 4C). The ability of gp130Fc to dampen pulmonary inflammation led us to evaluate changes to relevant inflammatory mediators, including MCP-1. Whole-lung RNA analysis revealed a significant reduction in MCP-1 in mice treated with soluble gp130 (Fig. 4D). These findings demonstrate that gp130Fc-mediated inhibition of sIL-6Rα in the lungs can attenuate pulmonary inflammation in this model.
FIGURE 4.
Pulmonary inflammation following chronic bleomycin exposure in mice treated with recombinant gp130Fc. Wild-type C57BL/6 male mice were injected i.p. with saline or bleomycin twice weekly for 4 wk. Beginning on day 19, when pulmonary fibrosis has been established, daily treatment with vehicle (saline) or recombinant gp130Fc was performed. Mice were sacrificed and samples collected on day 33. (A–C) Total cell count and cell differential from BAL fluid of wild-type C57BL/6 mice given saline or bleomycin, with and without gp130Fc. (D) Expression of MCP-1 transcript in whole-lung RNA. Data are presented as means ± SEM, n ≥ 6. *p < 0.05, **0.001 < p < 0.01, ***p < 0.001 for difference from PBS-treated cohort. #p < 0.05, ###p < 0.001 for difference from bleomycin-exposed mice.
In vivo neutralization of IL-6 trans signaling attenuated pulmonary fibrosis
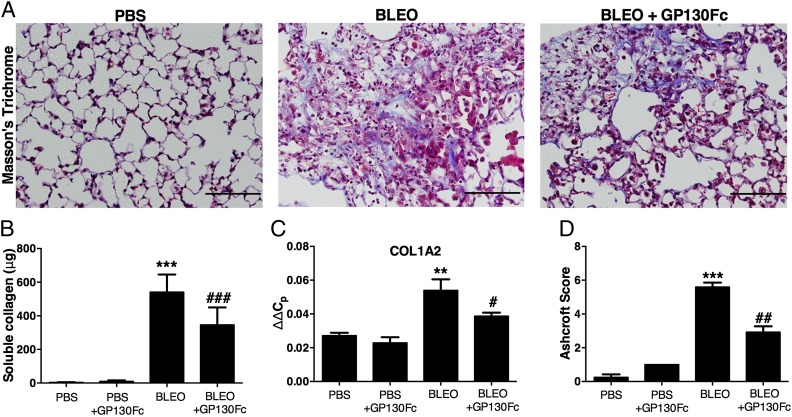
Aberrant fibroblast activation, differentiation into myofibroblasts, and excessive collagen and fibronectin production and deposition in the lungs are hallmarks of pulmonary fibrosis (1). Therefore, we determined whether treatment with recombinant gp130Fc affected these indices of pulmonary fibrosis. To assess collagen production and deposition, lung sections from day 33 mice were stained with Masson’s trichrome to visualize collagen deposition (blue). Untreated bleomycin-injected lungs were fibrotic and had extensive collagen deposition, whereas gp130Fc-treated lungs had diminished collagen deposition (Fig. 5A). Soluble collagen in BAL fluid was measured using a Sircol assay. gp130Fc treatment resulted in a significant reduction in collagen protein in the lungs (Fig. 5B). Quantification of collagen 1A2 transcript levels in whole-lung RNA revealed significantly higher transcript levels in bleomycin lungs compared with PBS lungs, and recombinant gp130Fc administration was able to suppress collagen 1A2 transcript levels (Fig. 5C). Ashcroft scoring to quantify morphologic fibrosis was performed, and gp130Fc treatment improved overall scores by almost 50% (Fig. 5D).
FIGURE 5.
Changes in pulmonary fibrosis following chronic bleomycin exposure in mice treated with recombinant gp130Fc. Wild-type C57BL/6 male mice were injected i.p. with saline or bleomycin twice weekly for 4 wk. Beginning on day 19, when pulmonary fibrosis had been established, daily treatment with vehicle (saline) or recombinant gp130Fc was performed. Mice were sacrificed and samples collected on day 33. Lung sections from day 33 mice were stained with (A) Masson’s trichrome for visualization of collagen deposition (blue). Sections are representative of n ≥ 6 mice from each group. Scale bars, 200 μm. (B) Sircol measurement of soluble collagen protein levels in BAL fluid from day 33 mice. (C) Collagen 1A2 transcripts were measured in whole-lung RNA. (D) Pulmonary fibrosis was quantified by the Ashcroft method. All data are presented as mean ± SEM, n ≥ 6. **0.001 < p < 0.01, ***p < 0.001 for difference from PBS-treated cohort. #p < 0.05, ##p < 0.01, ###p < 0.001 for difference from bleomycin-exposed mice.
To further evaluate changes to pulmonary fibrosis, we assessed myofibroblast accumulation in gp130Fc-treated lungs. Lung sections from day 33 mice were stained with an Ab against α-SMA for detection of myofibroblasts. Whereas fibrotic lungs exhibited prominent red α-SMA staining that is indicative of extensive myofibroblast accumulation, gp130Fc-treated lungs presented with diminished myofibroblast accumulation (Fig. 6A). Comparisons between bleomycin versus gp130Fc-treated lung sections visually emphasized the reduction in pulmonary fibrosis seen with gp130Fc treatment. We also analyzed the expression of fibronectin, an extracellular matrix protein found to be elevated in IPF lungs and an additional indicator of fibrosis (63–66). Protein lysates were made from day 33 lungs and Western blot analysis was performed to detect changes to fibronectin content. In comparison with bleomycin lungs, which have increased expression of fibronectin, lungs treated with gp130Fc exhibit less fibronectin (Fig. 6B). PBS-injected lungs treated with gp130Fc also experienced a reduction in fibronectin expression.
FIGURE 6.
Changes in pulmonary fibrosis following chronic bleomycin exposure in mice treated with recombinant gp130Fc. Wild-type C57BL/6 male mice were injected i.p. with saline or bleomycin twice weekly for 4 wk. Beginning on day 19, when pulmonary fibrosis has been established, daily treatment with vehicle (saline) or recombinant gp130Fc was performed. Mice were sacrificed and samples collected on day 33. Lung sections from day 33 mice were stained with (A) an Ab against α-SMA for detection of myofibroblast accumulation (red). Sections are representative of n ≥ 6 mice from each group. Scale bars, 200 μm. (B) Western blot analysis of fibronectin expression in whole-lung lysates. (C) Arterial oxygen saturation was measured using a neck collar. All data are presented as means ± SEM, n ≥ 6. ***p < 0.001 for difference from PBS-treated cohort, ###p < 0.001 for difference from bleomycin-exposed mice.
In vivo neutralization of IL-6 trans signaling improved oxygen saturation
Hypoxia is a feature of IPF, and oxygen saturation measurements are often used clinically to evaluate presence and severity of hypoxia (3). We evaluated changes to hypoxia in mice treated with gp130Fc by measuring oxygen saturation. We observed a decrease in oxygen saturation in untreated, bleomycin-injected mice that was inhibited by gp130Fc treatment (Fig. 6C). Collectively, these findings demonstrate that gp130Fc treatment attenuates pulmonary fibrosis, leading to physiologic improvement in mice.
In vivo neutralization of IL-6 trans signaling decreased STAT3 activation in myofibroblasts
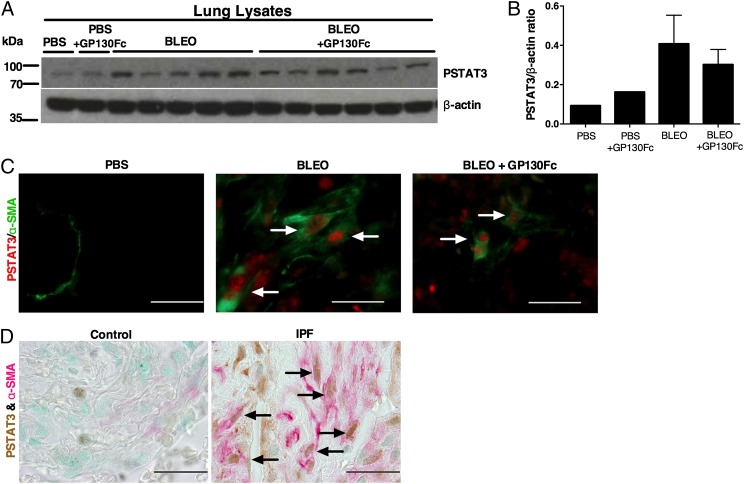
IL-6 signaling phosphorylates and activates STAT3, a transcription factor important in the pathogenesis of liver, skin, and kidney fibrosis (67, 68). Increased STAT3 activation has been reported in IPF (47, 69). Its role in pulmonary fibrosis was evaluated by O’Donoghue et al. (47) who demonstrated that IL-6–mediated STAT3 activation increased bleomycin-induced fibrosis in a mouse model. Thus, we evaluated changes to STAT3 activation in mice treated with gp130Fc. Western blot analysis of protein lysates made from day 33 lungs revealed that p-STAT3 was increased in bleomycin lungs compared with PBS lungs, and treatment with gp130Fc was associated in a trend for reduced STAT3 activation (Fig. 7A). The difference was quantified using ImageJ analysis of scanned Western blots (Fig. 7B). More specifically, there was a decrease in p-STAT3–positive myofibroblasts in gp130Fc-treated lungs (Fig. 7C). We confirmed this finding in human samples, where we saw an abundant presence of p-STAT3–positive myofibroblasts in IPF lung sections in comparison with control sections (Fig. 7D). These results suggest that IL-6 trans signaling in this population of cells could be crucial to the fibrotic process. We therefore focused our efforts on examining IL-6 trans signaling in fibroblast biology to understand its contribution to pulmonary fibrosis.
FIGURE 7.
STAT3 activation following chronic bleomycin exposure in mice treated with mouse recombinant gp130Fc and in IPF lungs. Wild-type C57BL/6 male mice were injected i.p. with saline or bleomycin twice weekly for 4 wk. Beginning on day 19, when pulmonary fibrosis had been established, daily treatment with vehicle (saline) or recombinant gp130Fc was performed. Mice were sacrificed and samples collected on day 33. (A) Expression of p-STAT3 was examined by Western blot analysis of lung lysates and (B) quantified by ImageJ analysis of Western blot images. Data are presented as mean ± SEM. (C) p-STAT3 immunofluorescence (red) in myofibroblasts (green) in lung sections. (D) p-STAT3 immunopositivity (brown) in myofibroblasts (red) in control and IPF lungs. Arrows denote positive cells. Images are representative of n ≥ 4 from each group. Scale bars, 50 μm (×100 oil immersion).
IL-6 trans signaling promoted fibroblast proliferation and extracellular matrix protein production
Using normal and IPF fibroblast cell lines, we examined changes to proliferation rate and production of collagen and fibronectin subsequent to stimulation of trans signaling in these cells. With regard to cellular proliferation, baseline proliferation rates of IPF fibroblasts were already higher than those of normal fibroblasts (Fig. 8A). Stimulation with IL-6 and sIL-6Rα resulted in increased proliferation rates in both normal and IPF fibroblasts. In normal fibroblasts, IL-6 stimulation alone did not significantly alter proliferation rates, but IL-6 in combination with sIL-6Rα resulted in a significant 1.2-fold difference in proliferation. In IPF fibroblasts, IL-6 stimulation alone was sufficient to induce a significant 1.4-fold increase in proliferation, and IL-6 in combination with sIL-6Rα resulted in a 1.5-fold increase in proliferation. The difference in response to trans signaling may be due to baseline expression of mIL-6Rα in control versus IPF fibroblasts (Fig. 8B).
FIGURE 8.
Effect of IL-6 trans signaling on proliferation rates and extracellular matrix protein production in control and IPF fibroblasts. Control and IPF fibroblasts were serum-starved for 24 h and then stimulated for 48 h with IL-6 alone or IL-6 and sIL-6Rα. Proliferation rates and collagen and fibronectin production were assessed. (A) Changes to proliferation rate in response to IL-6 and sIL-6Rα. (B) Western blot analysis of mIL-6Rα in control and IPF fibroblasts at baseline. (C) Western blot analysis of collagen and fibronectin production in normal and IPF fibroblasts in response to IL-6 and sIL-6Rα. (D and E) Collagen and fibronectin production as quantified by ImageJ analysis of Western blot images. All data are presented as means ± SEM, n ≥ 6 for (A), n = 1 or 2 for (D) and (E). **0.001 < p < 0.01, ***p < 0.001 for difference from media only cohort. ###p < 0.001 for difference from IL-6 only group.
In terms of production of extracellular matrix proteins, stimulation of IL-6 trans signaling induced production of collagen 1 and fibronectin in normal and IPF lung fibroblasts (Fig. 8C). In normal fibroblasts, IL-6 alone increased collagen 1 and fibronectin expression slightly. IL-6 plus sIL-6Rα induced a pronounced increase in collagen and fibronectin, at comparable levels to those seen with TGF-β stimulation (positive control). In IPF fibroblasts, IL-6 alone was able to increase collagen expression, whereas IL-6 plus sIL-6Rα was still able to induce collagen expression, but not to the extent of IL-6 alone. As for fibronectin, IL-6 stimulation in IPF fibroblasts led to an increase in fibronectin, whereas IL-6 plus sIL-6Rα led to an increase that was even greater than that induced by TGF-β. The differences in expression were quantified using ImageJ analysis of scanned Western blots (Fig. 8D, 8E). These results suggest that although IL-6 trans signaling increases protein production in both normal and IPF fibroblasts, the effect on collagen and fibronectin production is similar in normal fibroblasts, whereas in IPF fibroblasts its effect on fibronectin production may be greater than on collagen production. In all, the findings from these in vitro experiments support a role for IL-6 trans signaling in enhancing fibroblast proliferation and protein production that could lead to the overpopulation of the lung parenchyma and excessive protein deposition observed in pulmonary fibrosis.
Discussion
IL-6 is a cytokine with extensive effects in various physiological systems. IL-6 signaling through the trans pathway, mediated by sIL-6Rα, has been implicated in the pathogenesis of a number of chronic diseases, including rheumatoid arthritis (32), asthma (33), inflammatory bowel disease (colitis) (34), and colitis-associated cancer (35). Its role in pulmonary fibrosis, however, is unknown. In this study, we demonstrated elevations in sIL-6Rα in IPF patients and mice during the onset and progression of pulmonary fibrosis. We demonstrated a role for ADAM17 in cleaving mIL-6Rα from macrophage membranes to produce sIL-6Rα. Furthermore, our findings show that neutralization of the soluble receptor and antagonism of IL-6 trans signaling attenuate pulmonary fibrosis, emphasizing the importance of sIL-6Rα and IL-6 trans signaling in this condition. Our data also suggests that the mechanisms leading to development of pulmonary fibrosis involve enhancement of IL-6 trans signaling in pulmonary fibroblasts, leading to excessive proliferation and extracellular matrix protein production and deposition in the lungs. This study has important clinical significance in that it supports the use of gp130Fc as a promising, novel means of targeting IL-6 trans signaling in the treatment of IPF.
sIL-6Rα is a key feature of IL-6 signaling and a crucial component in the regulation of IL-6 responses. sIL-6Rα acts agonistically in vivo to either enhance IL-6 signaling on cells already expressing mIL-6Rα or to render cells lacking mIL-6Rα susceptible to the effects of IL-6 signaling (11, 31). Elevations of sIL-6Rα have previously been demonstrated in a number of human diseases (32–35) but not in IPF. A major observation in this study was that sIL-6Rα is elevated in IPF lungs and in mice with pulmonary fibrosis, suggesting that its production and presence may play a role in the disease process. Furthermore, to our knowledge, we showed for the first time that sIL-6Rα levels in the lungs were increased in a temporal pattern associated with the development and progression of pulmonary fibrosis, raising the possibility of sIL-6Rα serving as a marker of disease progression. These results illustrate the existence of IL-6 trans signaling in fibrotic lungs and support the need to further assess the role of this pathway in IPF. Interestingly, there is also evidence that the increase in sIL-6Rα in IPF is a local occurrence, only seen in a fibrotic area of the lung. ELISA analysis of sIL-6Rα in lavage fluid samples from different lobes of a single IPF lung revealed elevated sIL-6Rα in the sample from a fibrotic lobe (IPF LLL) but not a nonfibrotic lobe (IPF RML) (Supplemental Fig. 2A). These data suggest that sIL-6Rα could be used as a diagnostic and/or prognostic marker for in IPF. Further investigation of sIL-6Rα in more IPF lavage samples from fibrotic and nonfibrotic lobes is needed to validate these findings.
Another significant finding of our study is that production of sIL-6Rα in pulmonary fibrosis is due to cleavage of mIL-6Rα from alveolar macrophages via the action of the protease ADAM17. To our knowledge, this is the first study to report an increase in ADAM17 expression as pulmonary fibrosis develops and progresses, as well as the first to suggest that ADAM17 cleaves mIL-6Rα from M2 macrophages to produce the soluble receptor in fibrotic lungs. These findings suggest that ADAM17-targeted therapies may be useful in alleviating lung fibrosis. They also suggest a mechanism for the contribution of activated macrophages to pulmonary fibrosis and raise the possibility of targeting this potentially pivotal player in disease.
ADAM17 could also be used as an important indicator of the progression of pulmonary fibrosis. Note, however, that Garbers et al. (70) have argued that ADAM17 is the main protease responsible for cleavage of mIL-6Rα in humans, but that in mice, ADAM10 is primarily responsible. We acknowledge that ADAM10 may be able to cleave mIL-6Rα; however, we have not been able to demonstrate an increase in ADAM10 in fibrotic murine lungs. Our investigations have led us to conclude that ADAM17 is responsible for the generation of sIL-6Rα in our mouse model of chronic bleomycin exposure.
In support of a role for IL-6 trans signaling in pulmonary fibrosis, we have demonstrated ADAM17-mediated increases in sIL-6Rα in association with disease. To determine whether there were therapeutic benefits to in vivo neutralization of sIL-6Rα and resulting antagonism of IL-6 trans signaling in disease, we used recombinant gp130Fc to neutralize sIL-6Rα and selectively block IL-6 trans signaling in our chronic bleomycin model and evaluated the effects on pulmonary inflammation and fibrosis. In the IPB model, bleomycin-exposed mice developed significant pulmonary inflammation. In mice challenged with bleomycin and treated with gp130Fc, there was a marked reduction in pulmonary inflammation, as evident by a reduction in the number of inflammatory cells recovered in BAL fluid, including reductions in macrophages, lymphocytes, neutrophils, and eosinophils. The reduction in pulmonary inflammation with gp130Fc treatment is consistent with previous studies demonstrating that inhibition of IL-6 trans signaling resulted in improvement in chronic inflammatory conditions such as colitis (34), arthritis (32), and colitis-associated premalignant cancer (35). These results suggest that IL-6 trans signaling is responsible for the proinflammatory property of IL-6 in a model of pulmonary fibrosis. Of particular interest is the decrease in macrophages and lymphocytes, which could point to mechanisms for reduced severity of disease with gp130Fc administration. Both macrophages and lymphocytes have been suggested to play roles in the development of pulmonary fibrosis (53–57, 71, 72), but their exact contribution to IPF has not been examined. Further evaluation of these cell types and their role in disease is needed.
The compelling finding of this study was the novel observation that neutralization of IL-6 trans signaling resulted in attenuation of pulmonary fibrosis in our chronic bleomycin model. A hallmark feature of IPF is excessive fibroblast activation and differentiation and excessive production and deposition of matrix proteins, including collagen and fibronectin. Accumulation of α-SMA–positive myofibroblasts, the main effector cells of fibrosis, results in further production and deposition of extracellular matrix proteins in the lungs (1). Our study demonstrated that neutralization of IL-6 trans signaling resulted in a reduction in fibroblasts (data not shown) and myofibroblast accumulation and extracellular matrix protein production and deposition in the lungs, which translated to a reduction in pulmonary fibrosis and improvement in pulmonary oxygenation. Previous studies have suggested a role for IL-6 trans signaling in liver fibrosis (73), renal fibrosis (41), and myocardial fibrosis (42), although none has demonstrated improvement in fibrosis with blockade of trans signaling. To our knowledge, this is the first study to examine the role of IL-6 trans signaling in pulmonary fibrosis, and we demonstrated a therapeutic benefit to antagonism of this pathway in vivo.
In search of the underlying mechanism that would explain the improvement in fibrosis seen with gp130Fc treatment, we performed in vitro mechanistic studies that revealed the ability of IL-6 trans signaling to promote proliferation and collagen and fibronectin production in control and IPF fibroblasts, events crucial to disease progression. Findings from our in vitro studies of IL-6 trans signaling in fibroblasts are consistent with previous studies that have demonstrated that trans signaling can induce hepatocyte proliferation and intracellular signaling (73), promote collagen production in cardiac fibroblasts (42), and induce proliferation and extracellular matrix protein production in fibroblasts from hypertrophic scars (74). Note, however, that Moodley et al. (75) examined the effect of IL-6 on fibroblast proliferation and concluded that IL-6 inhibits proliferation in normal fibroblasts but enhanced proliferation in IPF fibroblasts. Differences between these findings may be related to the source of cells or conditions used. For example, the starvation and stimulation times were different between our studies. Both studies agree that stimulation of normal fibroblasts with IL-6 at ≤50 ng/ml does not result in inhibition of proliferation. Moodley et al. reported growth inhibition with IL-6 stimulation ≥100 ng/ml, whereas in our hands, even higher concentrations of IL-6 were not antiproliferative in normal fibroblasts (Supplemental Fig. 2B). The reason for this difference in observations is unclear. However, Moodley et al. did not examine the effect of trans signaling on fibroblast proliferation, whereas our study did. Overall, our in vivo data suggest that IL-6 trans signaling mediates proproliferative, profibrotic effects in the lungs. We acknowledge that IL-6 trans signaling likely impacts other cells types in the fibrotic lung, and additional studies are needed to fully understand the impact of this pathway on the progression of pulmonary fibrosis.
Although our study mainly focused on the role of IL-6 trans signaling in pulmonary fibrosis, note that classical signaling can contribute to disease as well, as evident in studies in colitis, where it was demonstrated that IL-6 classical signaling was pathogenic but trans signaling amplified those effects and contributed to the propagation of disease (34). We suggest that the impact of IL-6 trans signaling in pulmonary fibrosis may be similar to the situation in colitis. We have shown that pulmonary fibroblasts and myofibroblasts do express mIL-6Rα whereas type II pneumocytes do not (Supplemental Fig. 2C). This would suggest that only type II pneumocytes are susceptible to trans signaling, whereas the other pulmonary cell types are capable of responding to both classical and trans signaling. There is evidence to indicate that classical signaling is enhanced in fibrotic lungs because bleomycin-injected mouse lungs show elevated mIL-6Rα in whole-lung protein lysates in comparison with PBS lungs (Supplemental Fig. 3A). mIL-6Rα increased in association with increasing pulmonary fibrosis in the IPB model (Supplemental Fig. 3B). There is also reason to think that increased IL-6 trans signaling can promote a feedback loop that enhances classical signaling. Stimulation of trans signaling in fibroblasts resulted in increased mIL-6Rα (Supplemental Fig. 3C), and in vivo treatment with gp130Fc resulted in a reduction in mIL-6Rα in whole-lung lysates as well as in BAL cell pellet lysates (Supplemental Fig. 3D, 3E). These results support the hypothesis that, in the lungs, sIL-6Rα contributes to disease by enhancing classical signaling and overamplifying the effects of IL-6. Interestingly, we also observed that TGF-β stimulation of normal and IPF fibroblasts led to an increase in mIL-6Rα, and because TGF-β is the iconic mediator of fibrosis, perhaps part of the reported TGF-β–induced profibrotic effects in the lungs are mediated through IL-6 classical signaling.
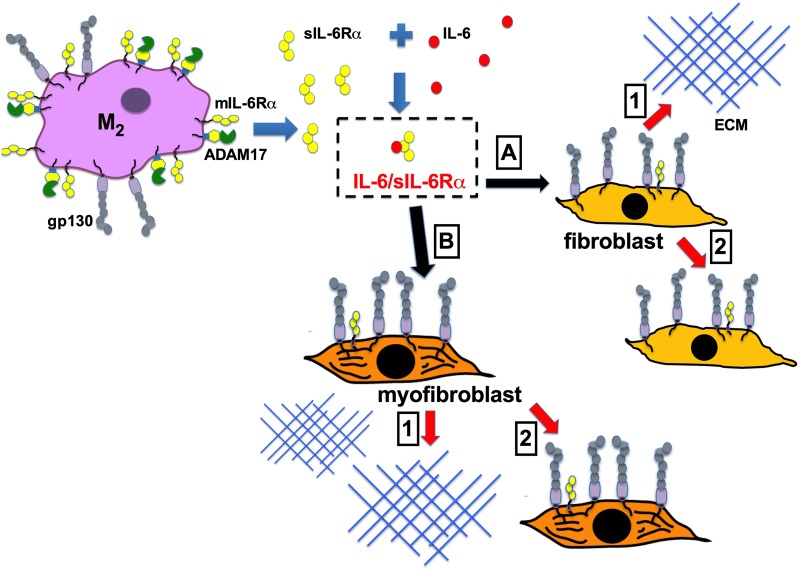
In conclusion, results from the present study demonstrate that, in response to injury, ADAM17 generates sIL-6Rα in the lungs, which accumulates and contributes to the development and progression of pulmonary fibrosis. Neutralization of sIL-6Rα and resulting antagonism of IL-6 trans signaling attenuates pulmonary inflammation and fibrosis in a mouse model of pulmonary fibrosis. In vitro studies suggest activation of trans signaling results in effects that are relevant to the progression of lung fibrosis. These findings are consistent with the hypothesis that IL-6 trans signaling is essential in mediating proinflammatory and profibrotic effects in the lungs. Although our temporal analyses demonstrate that there is an association between elevations of sIL-6Rα and the progression of pulmonary fibrosis, it is not possible from these studies to determine whether activation of this pathway is a cause or effect of pulmonary fibrosis. It is likely that the accumulation of macrophages expressing high levels of ADAM17 and the IL-6Rα in response to yet unknown mechanisms results in the production of sIL-6Rα that in turn promotes the further progression of fibrosis by impacting key effector cells such as fibroblasts (see Fig. 9). Thus, whether this pathway causes fibrosis, it is active in patients with established disease and may represent a novel pathway for disease amplification. Additional studies are necessary to validate these observations in additional human IPF tissues and samples. However, this study presents, to our knowledge, the first in vivo preclinical evidence that blockade of IL-6 trans signaling may be of significant therapeutic value to the management of IPF.
FIGURE 9.
Model of IL-6 trans signaling in pulmonary fibrosis. In fibrotic lungs, elevated ADAM17 expression in M2 macrophages leads to cleavage of mIL-6Rα to produce sIL-6Rα. sIL-6Rα binds IL-6. The IL-6/sIL-6Rα complex can then activate various cells in the lung in a paracrine manner. (A and B) Stimulation of IL-6 trans signaling in fibroblasts results in 1) increased extracellular matrix protein production and 2) increased proliferation.
Supplementary Material
Acknowledgments
We acknowledge Dr. Kelly Volcik for feedback during manuscript preparation.
This work was supported by National Institutes of Health Grants R01-HL070952 and P01-HL114457 (to M.R.B.) and a Burroughs Wellcome Fund–Brown Foundation Institute of Molecular Medicine training fellowship in gene–environment interactions (Burroughs Wellcome Fund Grant 1008200; to Thanh-Thuy T. Le).
The online version of this article contains supplemental material.
- ADAM
- a disintegrin and metalloprotease
- BAL
- bronchoalveolar lavage
- COPD
- chronic obstructive pulmonary disease
- IPB
- i.p. bleomycin
- IPF
- idiopathic pulmonary fibrosis
- mIL-6Rα
- membrane-bound IL-6Rα
- sIL-6Rα
- soluble IL-6Rα
- siRNA
- small interfering RNA
- α-SMA
- α–smooth muscle actin
- TAPI
- TNF-α protease inhibitor.
Disclosures
The authors have no conflicts of interest.
References
- 1.King T. E., Jr., Pardo A., Selman M.. 2011. Idiopathic pulmonary fibrosis. Lancet 378: 1949–1961 [DOI] [PubMed] [Google Scholar]
- 2.Wynn T. A. 2011. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 208: 1339–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raghu G., Collard H. R., Egan J. J., Martinez F. J., Behr J., Brown K. K., Colby T. V., Cordier J. F., Flaherty K. R., Lasky J. A., et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis . 2011. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 183: 788–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altekruse S. F., Krapcho M., Neyman N., Aminou R., Waldron W., Ruhl J., Howlader N., Tatalovich Z., Cho H., Mariotto A., et al. 2010. SEER Cancer Statistics Review, 1975–2007. National Cancer Institute, Bethesda, MD [Google Scholar]
- 5.Olson A. L., Swigris J. J., Lezotte D. C., Norris J. M., Wilson C. G., Brown K. K.. 2007. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am. J. Respir. Crit. Care Med. 176: 277–284 [DOI] [PubMed] [Google Scholar]
- 6.Raghu G., Weycker D., Edelsberg J., Bradford W. Z., Oster G.. 2006. Incidence and prevalence of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 174: 810–816 [DOI] [PubMed] [Google Scholar]
- 7.Pedroza M., Schneider D. J., Karmouty-Quintana H., Coote J., Shaw S., Corrigan R., Molina J. G., Alcorn J. L., Galas D., Gelinas R., Blackburn M. R.. 2011. Interleukin-6 contributes to inflammation and remodeling in a model of adenosine mediated lung injury. PLoS ONE 6: e22667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Y., Murthy J. N., Zeng D., Belardinelli L., Blackburn M. R.. 2010. Alterations in adenosine metabolism and signaling in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. PLoS ONE 5: e9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer J., Bauer T. M., Kalb T., Taga T., Lengyel G., Hirano T., Kishimoto T., Acs G., Mayer L., Gerok W.. 1989. Regulation of interleukin 6 receptor expression in human monocytes and monocyte-derived macrophages. Comparison with the expression in human hepatocytes. J. Exp. Med. 170: 1537–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalaris A., Garbers C., Rabe B., Rose-John S., Scheller J.. 2011. The soluble Interleukin 6 receptor: generation and role in inflammation and cancer. Eur. J. Cell Biol. 90: 484–494 [DOI] [PubMed] [Google Scholar]
- 11.Jones S. A., Scheller J., Rose-John S.. 2011. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J. Clin. Invest. 121: 3375–3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taga T., Kawanishi Y., Hardy R. R., Hirano T., Kishimoto T.. 1987. Receptors for B cell stimulatory factor 2. Quantitation, specificity, distribution, and regulation of their expression. J. Exp. Med. 166: 967–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S.. 2011. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 1813: 878–888 [DOI] [PubMed] [Google Scholar]
- 14.Taga T., Hibi M., Hirata Y., Yamasaki K., Yasukawa K., Matsuda T., Hirano T., Kishimoto T.. 1989. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell 58: 573–581 [DOI] [PubMed] [Google Scholar]
- 15.Heinrich P. C., Behrmann I., Müller-Newen G., Schaper F., Graeve L.. 1998. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 334: 297–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murakami M., Hibi M., Nakagawa N., Nakagawa T., Yasukawa K., Yamanishi K., Taga T., Kishimoto T.. 1993. IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase. Science 260: 1808–1810 [DOI] [PubMed] [Google Scholar]
- 17.Stahl N., Boulton T. G., Farruggella T., Ip N. Y., Davis S., Witthuhn B. A., Quelle F. W., Silvennoinen O., Barbieri G., Pellegrini S., et al. 1994. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science 263: 92–95 [DOI] [PubMed] [Google Scholar]
- 18.Zhong Z., Wen Z., Darnell J. E., Jr. 1994. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 264: 95–98 [DOI] [PubMed] [Google Scholar]
- 19.Müllberg J., Schooltink H., Stoyan T., Heinrich P. C., Rose-John S.. 1992. Protein kinase C activity is rate limiting for shedding of the interleukin-6 receptor. Biochem. Biophys. Res. Commun. 189: 794–800 [DOI] [PubMed] [Google Scholar]
- 20.Peters M., Meyer zum Büschenfelde K. H., Rose-John S.. 1996. The function of the soluble IL-6 receptor in vivo. Immunol. Lett. 54: 177–184 [DOI] [PubMed] [Google Scholar]
- 21.Scheller J., Chalaris A., Garbers C., Rose-John S.. 2011. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 32: 380–387 [DOI] [PubMed] [Google Scholar]
- 22.Briso E. M., Dienz O., Rincon M.. 2008. Cutting edge: soluble IL-6R is produced by IL-6R ectodomain shedding in activated CD4 T cells. J. Immunol. 180: 7102–7106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalaris A., Rabe B., Paliga K., Lange H., Laskay T., Fielding C. A., Jones S. A., Rose-John S., Scheller J.. 2007. Apoptosis is a natural stimulus of IL6R shedding and contributes to the proinflammatory trans-signaling function of neutrophils. Blood 110: 1748–1755 [DOI] [PubMed] [Google Scholar]
- 24.Fenton J. I., Hursting S. D., Perkins S. N., Hord N. G.. 2006. Interleukin-6 production induced by leptin treatment promotes cell proliferation in an ApcMin/+ colon epithelial cell line. Carcinogenesis 27: 1507–1515 [DOI] [PubMed] [Google Scholar]
- 25.Jones S. A., Horiuchi S., Novick D., Yamamoto N., Fuller G. M.. 1998. Shedding of the soluble IL-6 receptor is triggered by Ca2+ mobilization, while basal release is predominantly the product of differential mRNA splicing in THP-1 cells. Eur. J. Immunol. 28: 3514–3522 [DOI] [PubMed] [Google Scholar]
- 26.Matthews V., Schuster B., Schütze S., Bussmeyer I., Ludwig A., Hundhausen C., Sadowski T., Saftig P., Hartmann D., Kallen K. J., Rose-John S.. 2003. Cellular cholesterol depletion triggers shedding of the human interleukin-6 receptor by ADAM10 and ADAM17 (TACE). J. Biol. Chem. 278: 38829–38839 [DOI] [PubMed] [Google Scholar]
- 27.Booth B. W., Sandifer T., Martin E. L., Martin L. D.. 2007. IL-13-induced proliferation of airway epithelial cells: mediation by intracellular growth factor mobilization and ADAM17. Respir. Res. 8: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cesaro A., Abakar-Mahamat A., Brest P., Lassalle S., Selva E., Filippi J., Hébuterne X., Hugot J. P., Doglio A., Galland F., et al. 2009. Differential expression and regulation of ADAM17 and TIMP3 in acute inflamed intestinal epithelia. Am. J. Physiol. Gastrointest. Liver Physiol. 296: G1332–G1343 [DOI] [PubMed] [Google Scholar]
- 29.Charbonneau M., Harper K., Grondin F., Pelmus M., McDonald P. P., Dubois C. M.. 2007. Hypoxia-inducible factor mediates hypoxic and tumor necrosis factor α-induced increases in tumor necrosis factor-α converting enzyme/ADAM17 expression by synovial cells. J. Biol. Chem. 282: 33714–33724 [DOI] [PubMed] [Google Scholar]
- 30.Franchimont N., Lambert C., Huynen P., Ribbens C., Relic B., Chariot A., Bours V., Piette J., Merville M. P., Malaise M.. 2005. Interleukin-6 receptor shedding is enhanced by interleukin-1beta and tumor necrosis factor α and is partially mediated by tumor necrosis factor α-converting enzyme in osteoblast-like cells. Arthritis Rheum. 52: 84–93 [DOI] [PubMed] [Google Scholar]
- 31.Neurath M. F., Finotto S.. 2011. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 22: 83–89 [DOI] [PubMed] [Google Scholar]
- 32.Cronstein B. N. 2007. Interleukin-6: a key mediator of systemic and local symptoms in rheumatoid arthritis. Bull. NYU Hosp. Jt. Dis. 65(Suppl. 1): S11–S15 [PubMed] [Google Scholar]
- 33.Finotto S., Eigenbrod T., Karwot R., Boross I., Doganci A., Ito H., Nishimoto N., Yoshizaki K., Kishimoto T., Rose-John S., et al. 2007. Local blockade of IL-6R signaling induces lung CD4+ T cell apoptosis in a murine model of asthma via regulatory T cells. Int. Immunol. 19: 685–693 [DOI] [PubMed] [Google Scholar]
- 34.Atreya R., Mudter J., Finotto S., Müllberg J., Jostock T., Wirtz S., Schütz M., Bartsch B., Holtmann M., Becker C., et al. 2000. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat. Med. 6: 583–588 [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto S., Hara T., Mitsuyama K., Yamamoto M., Tsuruta O., Sata M., Scheller J., Rose-John S., Kado S., Takada T.. 2010. Essential roles of IL-6 trans-signaling in colonic epithelial cells, induced by the IL-6/soluble-IL-6 receptor derived from lamina propria macrophages, on the development of colitis-associated premalignant cancer in a murine model. J. Immunol. 184: 1543–1551 [DOI] [PubMed] [Google Scholar]
- 36.Müller-Newen G., Küster A., Hemmann U., Keul R., Horsten U., Martens A., Graeve L., Wijdenes J., Heinrich P. C.. 1998. Soluble IL-6 receptor potentiates the antagonistic activity of soluble gp130 on IL-6 responses. J. Immunol. 161: 6347–6355 [PubMed] [Google Scholar]
- 37.Jostock T., Müllberg J., Ozbek S., Atreya R., Blinn G., Voltz N., Fischer M., Neurath M. F., Rose-John S.. 2001. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur. J. Biochem. 268: 160–167 [DOI] [PubMed] [Google Scholar]
- 38.Hasegawa M., Sato S., Fujimoto M., Ihn H., Kikuchi K., Takehara K.. 1998. Serum levels of interleukin 6 (IL-6), oncostatin M, soluble IL-6 receptor, and soluble gp130 in patients with systemic sclerosis. J. Rheumatol. 25: 308–313 [PubMed] [Google Scholar]
- 39.Sato S., Hasegawa M., Takehara K.. 2001. Serum levels of interleukin-6 and interleukin-10 correlate with total skin thickness score in patients with systemic sclerosis. J. Dermatol. Sci. 27: 140–146 [DOI] [PubMed] [Google Scholar]
- 40.Migita K., Abiru S., Maeda Y., Daikoku M., Ohata K., Nakamura M., Komori A., Yano K., Yatsuhashi H., Eguchi K., Ishibashi H.. 2006. Serum levels of interleukin-6 and its soluble receptors in patients with hepatitis C virus infection. Hum. Immunol. 67: 27–32 [DOI] [PubMed] [Google Scholar]
- 41.Dai Y., Zhang W., Wen J., Zhang Y., Kellems R. E., Xia Y.. 2011. A2B adenosine receptor-mediated induction of IL-6 promotes CKD. J. Am. Soc. Nephrol. 22: 890–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meléndez G. C., McLarty J. L., Levick S. P., Du Y., Janicki J. S., Brower G. L.. 2010. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension 56: 225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka T., Narazaki M., Kishimoto T.. 2012. Therapeutic targeting of the interleukin-6 receptor. Annu. Rev. Pharmacol. Toxicol. 52: 199–219 [DOI] [PubMed] [Google Scholar]
- 44.Ammit A. J., Moir L. M., Oliver B. G., Hughes J. M., Alkhouri H., Ge Q., Burgess J. K., Black J. L., Roth M.. 2007. Effect of IL-6 trans-signaling on the pro-remodeling phenotype of airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 292: L199–L206 [DOI] [PubMed] [Google Scholar]
- 45.Zhong H., Belardinelli L., Maa T., Zeng D.. 2005. Synergy between A2B adenosine receptors and hypoxia in activating human lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 32: 2–8 [DOI] [PubMed] [Google Scholar]
- 46.Zhang H., Neuhöfer P., Song L., Rabe B., Lesina M., Kurkowski M. U., Treiber M., Wartmann T., Regnér S., Thorlacius H., et al. 2013. IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality. J. Clin. Invest. 123: 1019–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Donoghue R. J., Knight D. A., Richards C. D., Prêle C. M., Lau H. L., Jarnicki A. G., Jones J., Bozinovski S., Vlahos R., Thiem S., et al. 2012. Genetic partitioning of interleukin-6 signalling in mice dissociates Stat3 from Smad3-mediated lung fibrosis. EMBO Mol. Med. 4: 939–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou Y., Schneider D. J., Morschl E., Song L., Pedroza M., Karmouty-Quintana H., Le T., Sun C. X., Blackburn M. R.. 2011. Distinct roles for the A2B adenosine receptor in acute and chronic stages of bleomycin-induced lung injury. J. Immunol. 186: 1097–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swiderski R. E., Dencoff J. E., Floerchinger C. S., Shapiro S. D., Hunninghake G. W.. 1998. Differential expression of extracellular matrix remodeling genes in a murine model of bleomycin-induced pulmonary fibrosis. Am. J. Pathol. 152: 821–828 [PMC free article] [PubMed] [Google Scholar]
- 50.Karmouty-Quintana H., Zhong H., Acero L., Weng T., Melicoff E., West J. D., Hemnes A., Grenz A., Eltzschig H. K., Blackwell T. S., et al. 2012. The A2B adenosine receptor modulates pulmonary hypertension associated with interstitial lung disease. FASEB J. 26: 2546–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baran C. P., Opalek J. M., McMaken S., Newland C. A., O’Brien J. M., Jr., Hunter M. G., Bringardner B. D., Monick M. M., Brigstock D. R., Stromberg P. C., et al. 2007. Important roles for macrophage colony-stimulating factor, CC chemokine ligand 2, and mononuclear phagocytes in the pathogenesis of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 176: 78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chua F., Gauldie J., Laurent G. J.. 2005. Pulmonary fibrosis: searching for model answers. Am. J. Respir. Cell Mol. Biol. 33: 9–13 [DOI] [PubMed] [Google Scholar]
- 53.Murray L. A., Rosada R., Moreira A. P., Joshi A., Kramer M. S., Hesson D. P., Argentieri R. L., Mathai S., Gulati M., Herzog E. L., Hogaboam C. M.. 2010. Serum amyloid P therapeutically attenuates murine bleomycin-induced pulmonary fibrosis via its effects on macrophages. PLoS ONE 5: e9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trujillo G., O’Connor E. C., Kunkel S. L., Hogaboam C. M.. 2008. A novel mechanism for CCR4 in the regulation of macrophage activation in bleomycin-induced pulmonary fibrosis. Am. J. Pathol. 172: 1209–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pechkovsky D. V., Prasse A., Kollert F., Engel K. M., Dentler J., Luttmann W., Friedrich K., Müller-Quernheim J., Zissel G.. 2010. Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction. Clin. Immunol. 137: 89–101 [DOI] [PubMed] [Google Scholar]
- 56.Murray L. A., Chen Q., Kramer M. S., Hesson D. P., Argentieri R. L., Peng X., Gulati M., Homer R. J., Russell T., van Rooijen N., et al. 2011. TGF-β driven lung fibrosis is macrophage dependent and blocked by serum amyloid P. Int. J. Biochem. Cell Biol. 43: 154–162 [DOI] [PubMed] [Google Scholar]
- 57.Collins S. L., Chan-Li Y., Hallowell R. W., Powell J. D., Horton M. R.. 2012. Pulmonary vaccination as a novel treatment for lung fibrosis. PLoS ONE 7: e31299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Modolell M., Corraliza I. M., Link F., Soler G., Eichmann K.. 1995. Reciprocal regulation of the nitric oxide synthase/arginase balance in mouse bone marrow-derived macrophages by TH1 and TH2 cytokines. Eur. J. Immunol. 25: 1101–1104 [DOI] [PubMed] [Google Scholar]
- 59.Stein M., Keshav S., Harris N., Gordon S.. 1992. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 176: 287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mosser D. M., Zhang X.. 2008. Activation of murine macrophages. Curr. Protoc. Immunol. 83: 14.2.1–14.2.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wynn T. A. 2003. IL-13 effector functions. Annu. Rev. Immunol. 21: 425–456 [DOI] [PubMed] [Google Scholar]
- 62.Althoff K., Reddy P., Voltz N., Rose-John S., Müllberg J.. 2000. Shedding of interleukin-6 receptor and tumor necrosis factor α. Contribution of the stalk sequence to the cleavage pattern of transmembrane proteins. Eur. J. Biochem. 267: 2624–2631 [DOI] [PubMed] [Google Scholar]
- 63.Rennard S. I., Crystal R. G.. 1982. Fibronectin in human bronchopulmonary lavage fluid. Elevation in patients with interstitial lung disease. J. Clin. Invest. 69: 113–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crosby L. M., Waters C. M.. 2010. Epithelial repair mechanisms in the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 298: L715–L731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.White E. S., Baralle F. E., Muro A. F.. 2008. New insights into form and function of fibronectin splice variants. J. Pathol. 216: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.White E. S., Muro A. F.. 2011. Fibronectin splice variants: understanding their multiple roles in health and disease using engineered mouse models. IUBMB Life 63: 538–546 [DOI] [PubMed] [Google Scholar]
- 67.Knight D., Mutsaers S. E., Prêle C. M.. 2011. STAT3 in tissue fibrosis: is there a role in the lung? Pulm. Pharmacol. Ther. 24: 193–198 [DOI] [PubMed] [Google Scholar]
- 68.Prêle C. M., Yao E., O’Donoghue R. J., Mutsaers S. E., Knight D. A.. 2012. STAT3: a central mediator of pulmonary fibrosis? Proc. Am. Thorac. Soc. 9: 177–182 [DOI] [PubMed] [Google Scholar]
- 69.Pechkovsky D. V., Prêle C. M., Wong J., Hogaboam C. M., McAnulty R. J., Laurent G. J., Zhang S. S., Selman M., Mutsaers S. E., Knight D. A.. 2012. STAT3-mediated signaling dysregulates lung fibroblast-myofibroblast activation and differentiation in UIP/IPF. Am. J. Pathol. 180: 1398–1412 [DOI] [PubMed] [Google Scholar]
- 70.Garbers C., Jänner N., Chalaris A., Moss M. L., Floss D. M., Meyer D., Koch-Nolte F., Rose-John S., Scheller J.. 2011. Species specificity of ADAM10 and ADAM17 proteins in interleukin-6 (IL-6) trans-signaling and novel role of ADAM10 in inducible IL-6 receptor shedding. J. Biol. Chem. 286: 14804–14811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lo Re S., Lison D., Huaux F.. 2013. CD4+ T lymphocytes in lung fibrosis: diverse subsets, diverse functions. J. Leukoc. Biol. 93: 499–510 [DOI] [PubMed] [Google Scholar]
- 72.Nuovo G. J., Hagood J. S., Magro C. M., Chin N., Kapil R., Davis L., Marsh C. B., Folcik V. A.. 2012. The distribution of immunomodulatory cells in the lungs of patients with idiopathic pulmonary fibrosis. Mod. Pathol. 25: 416–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Drucker C., Rabe B., Chalaris A., Schulz E., Scheller J., Rose-John S.. 2009. Interleukin-6 trans-signaling regulates glycogen consumption after d-galactosamine-induced liver damage. J. Interferon Cytokine Res. 29: 711–718 [DOI] [PubMed] [Google Scholar]
- 74.Ray S., Ju X., Sun H., Finnerty C. C., Herndon D. N., Brasier A. R.. 2013. The IL-6 trans-signaling-STAT3 pathway mediates ECM and cellular proliferation in fibroblasts from hypertrophic scar. J. Invest. Dermatol. 133: 1212–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moodley Y. P., Scaffidi A. K., Misso N. L., Keerthisingam C., McAnulty R. J., Laurent G. J., Mutsaers S. E., Thompson P. J., Knight D. A.. 2003. Fibroblasts isolated from normal lungs and those with idiopathic pulmonary fibrosis differ in interleukin-6/gp130-mediated cell signaling and proliferation. Am. J. Pathol. 163: 345–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.