Abstract
Peripheral tolerance mechanisms are in place to prevent T cells from mediating aberrant immune responses directed against self and environmental antigens. Mechanisms involved in the induction of peripheral tolerance include T cell intrinsic pathways, such as anergy or deletion, or exogenous tolerance mediated by regulatory T cells. We have previously shown that the density of peptide-MHC class I recognized by the TCR determines whether CD8+ T cells undergo anergy or deletion. Specifically, using a TCR-transgenic CD8+ T cell model we demonstrated that persistent peripheral exposure to low or high dose peptides in the absence of inflammatory signals resulted in clonal deletion or anergy of the T cell, respectively. Here, by altering the affinity of the peptide-MHC toleragen for TCR, we have confirmed this mechanism is absolutely dependent upon the level of T cell receptor signaling the CD8+ T cell receives. Using altered peptide ligands (APL) displaying high TCR affinities, we show that increasing the TCR signaling favors anergy induction. Conversely, using APLs displaying a decreased TCR affinity tilted our system in the direction of deletional tolerance. Thus, we demonstrate how differential peripheral CD8+ T cell tolerance mechanisms are controlled by both the potency and density of MHC class I-peptide toleragen.
Introduction
The mechanism of negative selection blocks T cells displaying TCRs with high affinity for self-peptide-MHC complexes from escaping the thymus(1). However, this process is not absolute, and significant numbers of T cells that recognize and respond to peripheral self-peptide-MHC molecules emigrate from the thymus (2). Fortunately peripheral tolerance mechanisms, such as anergy, deletion and T regulatory cells, are in place to protect against dangerous auto-reactive T cell responses(3-5). The identification of the factors controlling the induction of such mechanisms is the focus of much research, as once harnessed these mechanisms can be exploited to prevent or treat a myriad of immune disorders.
In a steady-state system where T cell activation through the TCR (signal 1) occurs in the absence of co-stimulation (signal 2) and pro-inflammatory cytokines (signal 3) we, and others(6,7), have observed that the mechanism of peripheral tolerance induced was dependent upon the level of antigen in the milieu. Following exposure of CD8+ T cells to a persistent low dose of antigen, they initially expand, but soon undergo apoptosis through a BIM-dependent mechanism (6). On the other hand, a high antigen dose rendered the CD8+ T cells unresponsive (anergic) to TCR signals but cells survived. Upon removal of antigen, these cells became a pool of memory cells that could later respond to antigen (6).
The ability to induce differential tolerance mechanisms by varying the level of antigen exposure suggests the strength of TCR signalling as a controlling factor. In these previous studies the density of antigen was varied, rather than the affinity of the TCR for MHC-peptide. As both antigen affinity and antigen concentration can contribute to the overall strength of TCR signal, here we have evaluated whether affinity differences are also able to determine whether tolerance occurs through deletion or anergy. We hypothesized that by using altered versions of the tolerizing peptide, we could control whether we induced CD8+ T cell tolerance through deletion or anergy. Furthermore, all of our initial conclusions were based on studies of a single TCR exhibited by CL4 CD8 cells. Here we generalize our findings by extending these studies to other TCRs expressed by CD8 cells.
Materials and Methods
Mice
B10.D2, C57/BL6, Bim-/- and NOD mice were purchased from The Jackson Laboratory or the Animal Breeding Facility at the Scripps Research Institute (La Jolla, CA). B10.D2 CL4 and CL1 TCR transgenic mice have been previously described (8, 12). OT-1 TCR Transgenic mice were kindly provided by Dr. Charlie Suhr (TSRI, La Jolla, CA). All animals were bred at our facilities and housed under specific pathogen-free conditions. All animal experiments were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Scripps Research Institute.
Peptides and Immunizations
Influenza HA518-526 peptides (native and variant sequences) were synthesized by GenScript (Piscataway, NJ). OVA257-264 (SIINFEKL) and A2 variant were a kind gift from Dr. N. Gascoigne (TSRI, La Jolla, CA). Influenza virus A/PR/8/34 H1N1 (PR8) was grown in the allantoic cavity of 10- to 11-day-old hen's eggs. Upon isolation, the allantoic fluid was titrated for HA activity using chicken RBC and stored at −70°C. Mice were infected i.p. with 500 HA units of PR8 virus. Recombinant Listeria Monocytogenes expressing OVA (rLM-OVA) was provided by Dr. C Suhr (TSRI, La Jolla, CA). Mice were infected i.v. with 1 × 105 CFU rLM-OVA.
Preparation and Adoptive Transfer of Naïve TCR Transgenic T cells
CD8+ T cells were isolated from the lymph nodes of CL1, CL4 or OT-1 TCR mice (ages 6–8 weeks) by negative selection using the CD8+ T cell isolation kit (BD Bioscience). T cell purity was >90% with no contaminating CD4+ cells. For adoptive transfer experiments, the indicated number of cells was injected i.v. in 100 μl of HBSS.
Flow Cytometry
Suspensions of spleen cells were stained for FACS analysis in HBSS containing 1% FCS and 2 mM EDTA with the following mAbs (from BD Biosciences unless otherwise stated): CD8α-PE (53–6.7), CD44-FITC (IM7), NKG2a-FITC (20d5), Thy1.1-PerCP.Cy5 (OX-7), and PD-1-APC (29F.1A12; Biolegend).
For ex vivo detection of Erk phosphorylation, splenocytes were harvested and processed to single cell suspensions, placed in a 96 well plate at 2 × 106 cells/well, then restimulated with 1 μg/ml Kd HA peptide, or PMA (200 ng/ml) and ionomycin (12.5 μg/ml) in RPMI 1640 medium containing 10% heat-inactivated FCS for 15 min at 37°C, in 96 well plate. Immediately following Ag restimulation, cells were fixed in fresh 2% paraformaldehyde for 10 min at room temperature, then permeabilized with ice-cold 90% methanol for 1 h at 4°C. After washing, cells were stained with Thy1.1-PerCp.Cy5 and CD8α-PE (BD Bioscience) for 30 min at 4°C. After washing, phospho-Erk1/2-APC (pT202/pY204; BD Biosciences) was added and cells stained for 1 h at room temperature. Cells were washed twice with HBSS containing 0.1% w/v BSA (Sigma-Aldrich) and 0.02% w/v sodium azide, then immediately analyzed with a FACSCalibur and FlowJo software (Becton Dickinson).
Loading of spleen cells with ILA-peptide
ILA peptide-loaded spleen cells were prepared by osmotic shock using the method described by Steinman and coworkers (27). Briefly, 150 × 106 splenocytes from B10.D2 mice were washed twice in RPMI 1640 medium and resuspended in 1 ml of hypertonic medium (0.5 M sucrose, 10% w/v polyethylene glycol 1000, and 10 mM HEPES in RPMI 1640 (pH 7.2)), containing peptides, for 10 min at 37°C. Then 13 ml of pre-warmed hypotonic medium (40% H2O, 60% RPMI 1640) was added, and the cells were incubated for an additional 2 min at 37°C. Immediately thereafter, the cells were pelleted by centrifugation and washed twice with ice-cold HBSS, and 30 × 106 in 0.2 ml of ILA-loaded splenocytes was injected i.v. into each recipient mouse as a source of dying cells.
Statistical analysis
Data are expressed as mean + standard deviation (SD) for each group. Statistical differences between groups were evaluated using a Student's t-test using GraphPad Prism 5.0b software. P<0.05 was considered statistically significant.
Results
Peripheral tolerance induction using high affinity peptides promotes CL4 CD8+ cell unresponsiveness
Previous studies performed in our laboratory used CD8+ T cells from CL4 transgenic mice, expressing a TCR that recognize the Kd-restricted HA peptide (IYSTVASSL) derived from the influenza HA virus (8). We demonstrated that daily i.v. injections of low dose (0.1 or 1 μg) of KdHA for 3 days resulted in the deletion CL4 CD8+ T cells (6). On the other hand, daily i.v. injections of high dose (10 or 100 μg) KdHA for 3 days induced a state of non-responsiveness (anergy) in the CL4 CD8+ T cell population (6). Here, to determine whether the mechanism of peripheral tolerance induced by cognate peptide-MHC was dependent upon TCR affinity, we designed experiments incorporating altered peptide ligands (APLs) containing an amino acid substitution at a TCR contact position within the Kd-HA sequence. To test our first hypothesis, that increasing the strength of TCR-signaling would promote the acquisition of an anergic phenotype, we used the Kd-HA super agonist A517G as the tolerizing peptide antigen. A517G had previously been shown to activate CL4 cells at a lower concentration (factor of 10 - 30) than native peptide sequence (9). Importantly, the binding affinity of A517G for Kd was the same as the native KdHA peptide. Therefore, the substitution of a glycine for alanine at position 517 increased the TCR signaling strength directly. We found that A517G induced CL4 proliferation at approximately 1 log lower concentration than native KdHA peptide (data not shown).
B10.D2 mice harboring 3 × 106 adoptively transferred CL4 cells were treated with 3 daily i.v. injections of a dose (1 μg) of native peptide antigen shown previously to delete all CL4 cells, or the same amount of A517G peptide. On the third day, groups of mice were sacrificed and the transferred CL4 cells analyzed. CD44 was equally up-regulated on all CL4 cells in both antigen treatment groups, indicating all transferred CL4 cells had been exposed to antigen (Fig. 1a.). Previous experiments in which CL4 cells undergoing anergy vs. deletion were examined for expression of markers known to be associated with exhaustion revealed greater up-regulation of the negative co-stimulatory molecules NKG2a and PD-1 on CL4 anergic cells (Verdeil and Smith, unpublished observation). Treatment with the super agonist A517G promoted the acquisition of the anergy-associated phenotype, with increased levels of NKG2a and PD-1 expressed on the CL4 cells as compared with these same cells activated with native HA peptide (Fig. 1a). T cell anergy has been associated with reduced phosphorylation of TCR-mediated signaling molecules such as ERK and JNK protein kinases (10, 11). We previously reported that treatment of CL4 with a low dose of HA peptide that resulted in their deletion was associated with attenuated ERK signaling as compared to untreated cells while treatment with a higher dose completely abrogated activation of ERK as assessed upon re-stimulation through the TCR (6). Similarly, we found that treatment with 1 μg A517G abrogated ERK activation upon re-stimulation of CL4 cells with Kd-HA (Fig. 1b). ERK signaling was still observed in CL4 cells after treatment with 1 μg of native Kd-HA, however it was attenuated when compared to naïve CL4 cells, consistent with a partial anergic phenotype prior to deletion.
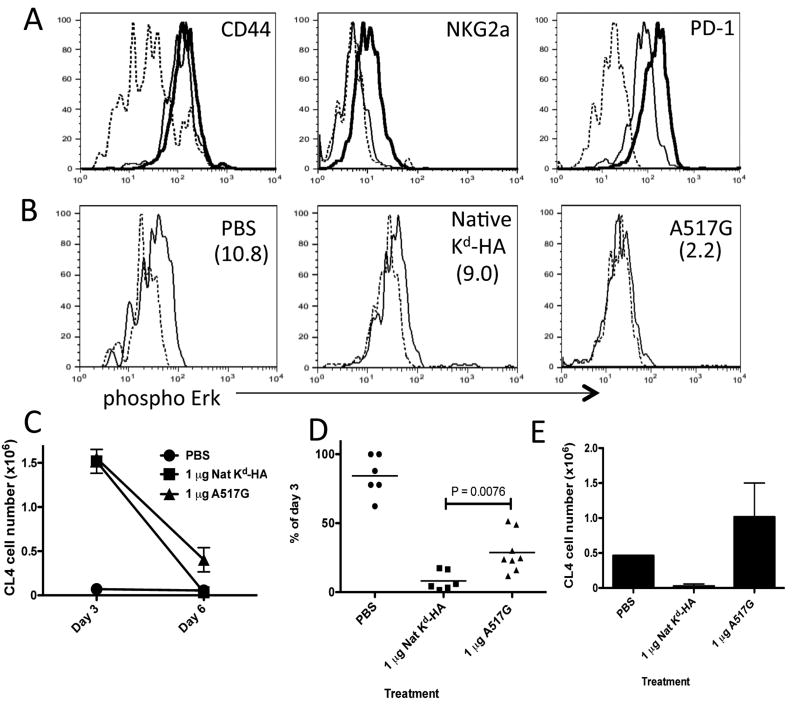
Figure 1. Use of high TCR affinity peptide in peripheral tolerance protocol promotes CD8+ T cell anergy.
B10.D2 mice containing donor Thy1.1+ CL4 CD8+ T cells were treated daily with PBS, 1 μg of native Kd-HA or A517G peptide between days 0-2. (a) Day 3 flow cytometric analysis of spleen-derived CL4 cells stained with antibody specific for the indicated molecules. Lines; Dashed - PBS, thin - native Kd-HA and thick - A517G treatments. (b) Day 3 ex vivo phosphorylation of Erk in CL4 cells re-stimulated with Kd-HA peptide (thin line) and unstimulated (dashed line). Treatment condition indicated in top right corner of plots and the mean MFI increase from unstimulated to stimulated cells in parenthesizes. Survival of CL4 cells in the spleen. Expressed as the absolute number (c) or percentage (d) of CL4 cells detected by FACS on day 6 compared to day 3 for each treatment condition. (e) On day 30 mice were challenge with PR8 influenza virus (i.p. 500 HA units) and six days later donor CL4 cells were detected by FACS in the spleen. Each graph is representative of 3 to 5 experiments using age and sex matched mice. In the experiments depicted in c and d there were between 4 to 6 mice per treatment group.
As previously stated, low dose antigen treatment initiates a mechanism that results in cell death, while high doses of antigen induced unresponsiveness. We measured CL4 cell survival after treatment with native peptide or the super-agonist. Kinetic studies revealed that the size of the splenic CL4 cell pool expands during the first 3 days of treatment with a deleting dose of KdHA, before contraction begins on day 4, and by day 6 only a fraction of the CL4 population can be detected (Verdeil and Smith, unpublished observation). We therefore defined the efficiency of deletion as a percentage of CL4 cells remaining on day 6 in comparison to day 3. Treatment with 1 μg native KdHA results in efficient deletion of the CL4 cell population, whereas A517G treatment results in a significantly higher level of survival (Fig. 1c&d). To confirm that all the cells treated with native peptide were deleted and that memory cells had not been formed, we measured the influenza virus recall response of any residual CL4 cells 30 days later (Fig.1e). A robust expansion to virus challenge occurred in the mice that received PBS or 1 μg A517G treatment, but this response was negligible in mice that received 1 μg native KdHA treatment. These results indicate that treatment with a toleragen with strong TCR signaling properties favors the induction of the anergic form of peripheral tolerance over deletion.
Peripheral tolerance induction using low TCR affinity peptides promotes CL4 CD8+ T cell deletion
Our original hypothesis predicted that peptides with low TCR affinity would favor deletion. We compared tolerance induction mechanisms induced in CL4 cells treated with equal doses of native KdHA or an APL (I512V) displaying weaker TCR affinity. I512V had previous been shown to be a weak TCR agonist, despite equivalent binding to Kd-HA (9). Thus, its weak agonistic characteristic was deemed to be due to weaker TCR interactions due to an isoleucine to valine substitution at the 512 position. We chose to determine the tolerance phenotype induced at a dose of peptide (10 μg) that had been shown to cause anergy in previous experiments using native peptide (6). We first ascertained that 10 μg treatment with I512V induced activation (CD44 up-regulation) of all transferred CL4 cells (Fig. 2a). In comparison to native KdHA peptide, I512V treatment up-regulated NKG2a to a similar level, but PD-1 expression was lower (Fig. 2a). The ability of CL4 cells to phosphorylate ERK was completely lost after treatment with 10 μg native KdHA, but some remained, although greatly attenuated, in I512V treated cells (Fig. 2b). Analysis of cell survival showed a dramatic difference between the two peptides. 10 μg native KdHA treatment resulted in only a slight contraction in the CL4 pool between days 3 and 6 (Fig. 2c), and influenza virus recall at day 30 induced a robust response (Fig. 2d). However, 10 μg I512V treatment resulted in a severe loss of the CL4 cell pool between days 3 and 6 (Fig. 2c), and a negligible recall response to day 30 viral challenge (Fig. 2d). Thus, signalling through the TCR with a lower affinity ligand skews the mechanism of tolerance towards deletion.
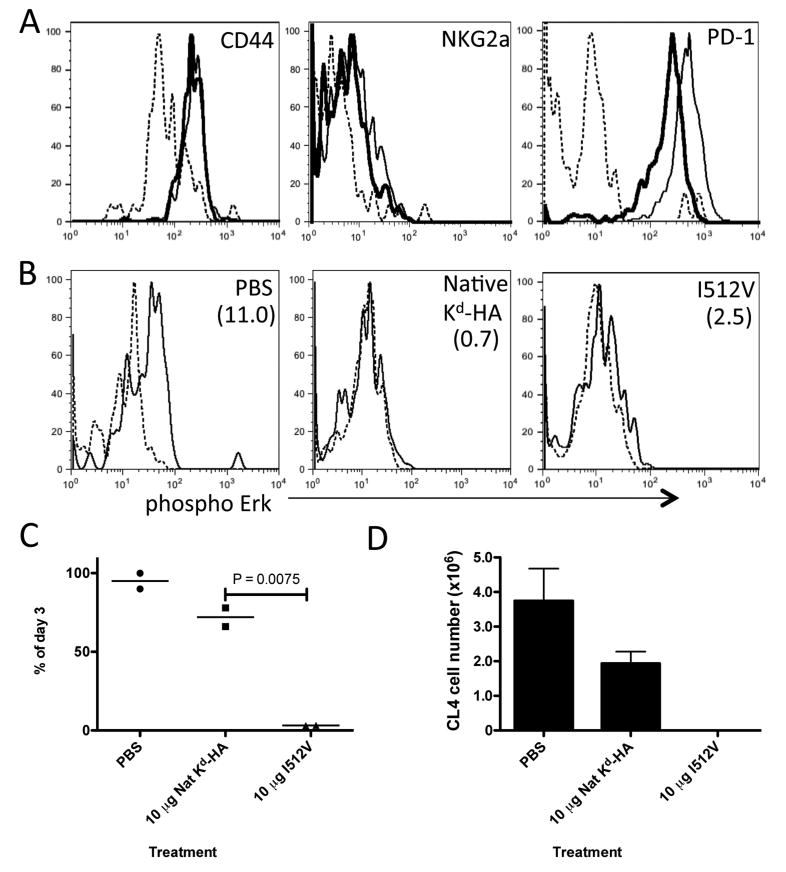
Figure 2. Use of low TCR affinity peptide in peripheral tolerance protocol promotes CD8+ T cell deletion.
B10.D2 mice containing donor Thy1.1+ CL4 CD8+ T cells were treated daily with PBS, 10 μg of native Kd-HA or I512V peptide between days 0-2. (a) Day 3 flow cytometric analysis of donor CL4 cells in the spleen stained with antibody specific for the indicated molecule. Lines; Dashed - PBS, thin - native Kd-HA and thick – I512V treatments. (b) Day 3 ex vivo phosphorylation of Erk in CL4 cells re-stimulated with Kd-HA peptide (thin line) or unstimulated (dashed line). Treatment condition indicated in top right corner of plots and the mean MFI increase from unstimulated to stimulated cells in parenthesizes. (c) Survival of CL4 cells in the spleen. Expressed as the percentage of CL4 cells detected by FACS on day 6 compared to day 3 for each treatment condition. (d) On day 30 mice were challenge with PR8 influenza virus (I.P 500 HA units) and six days later donor CL4 cells were detected by FACS in the spleen. Each graph is representative of 2 to 3 experiments using age and sex matched mice.
Anergy induction is impaired in CD8+ T cells expressing a low affinity TCR
The previous experiments suggest that anergy of peripheral CD8+ T cells may only be induced by potent signaling through the TCR. Therefore, if a T cell expressed a TCR displaying weak affinity for self antigen one would expect a deletional tolerance mechanism to be favored upon antigen recognition in the absence of signals 2 and 3. We tested this hypothesis in a system containing T cells expressing a TCR that recognized the native KdHA peptide with low affinity, CL1 (12). CL1 was originally derived from a mouse expressing HA as a self antigen, and therefore represents an example of a T cell that escapes thymic and peripheral tolerance due to its low affinity for self antigen. When compared with CL4 cells, CL1 cells displayed weak Kd-HA tetramer binding, and a 10-100 fold increased concentration of Kd-HA peptide was needed to induce comparable levels of proliferation (18). To determine the susceptibility of CL1 to peripheral tolerance mechanisms, experiments were designed in which CL1 cells were subjected to the peptide tolerance treatment protocol with escalating doses (1, 10 and 100 μg) of native Kd-HA peptide, and peripheral tolerance parameters measured. We first confirmed that CD44 up-regulation was induced on transferred CL1 cells after the third daily injection of native Kd-HA, though its expression on cells in the group receiving 1 μg of peptide was more variable than the other conditions (Fig. 3a). NKG2a expression was not significantly up-regulated by any dose of native Kd-HA, but the level of PD-1 increased with escalating dose (Fig. 3a). The ability to phosphorylate ERK upon re-stimulation through the TCR decreased with an increase of treatment dosage (Fig. 3b). However CL1 cells still displayed a higher level of ERK phosphorylation upon re-stimulation (Fig. 3b) than day 3 CL4 cells that had been treated with 10 μg doses of Kd-HA peptide (Fig. 2b). Next we analyzed the survival of CL1 cells. By day 6 the majority of CL1 were deleted in all treatment groups (Fig.3c). However, significant differences were observed between groups. The incomplete deletion associated with the 1 μg treatment group could be due to the presence of a significant number of cells that had not received sufficient exposure to antigen to induce deletion as suggested by the varying levels of expression of CD44 on the CL1 cells in the 1 μg treatment group (Fig. 3a). The slight increase in CL1 cell survival in the 100 μg group compared to the 10 μg group may indicate a small proportion of the cells attained an anergic phenotype by the high concentration of peptide. However, analysis of the day 30 viral recall response failed to reveal a robust response in any treatment group (Fig. 3d). Thus, T cells expressing low affinity TCRs are significantly more susceptible to deletion than anergy.
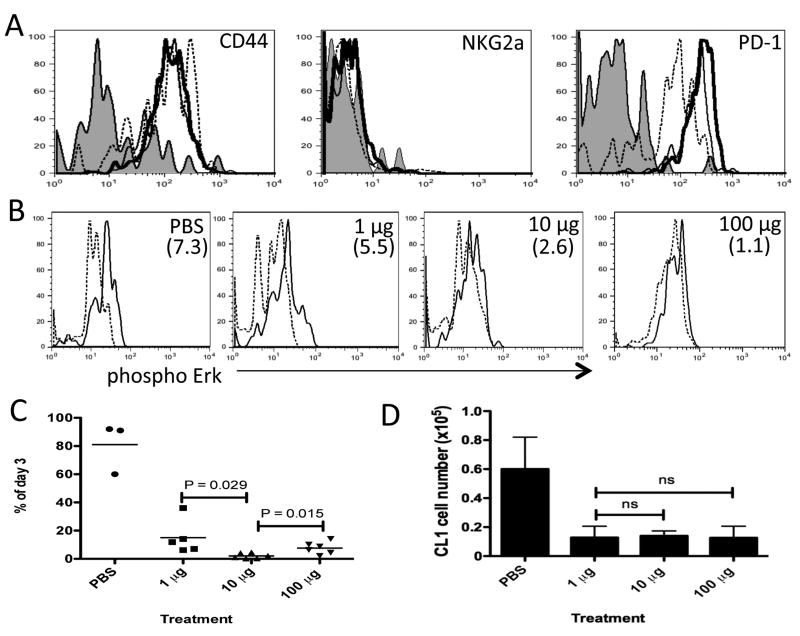
Figure 3. CD8+ T cells expressing a low affinity TCR for tolerizing antigen are not efficiently anergized.
B10.D2 mice containing donor Thy1.1+ CL1 CD8+ T cells were treated daily with PBS, 1, 10 or 100 μg of native Kd-HA peptide between days 0-2. (a) Day 3 flow cytometric analysis of donor CL1 cells in the spleen stained with antibody specific for indicated molecule. Lines; Shade –PBS, dashed – 1 μg, thin – 10 μg and thick – 100 μg treatments. (b) Day 3 ex vivo phosphorylation of Erk in CL1 cells re-stimulated with Kd-HA peptide (thin line) or unstimulated (dashed line). Treatment condition indicated in top right hand corner of plots and the mean MFI increase from unstimulated to stimulated cells in parenthesizes. (c) Survival of CL1 cells in the spleen. Expressed as the percentage of CL1 cells detected by FACS on day 6 compared to day 3 for each treatment condition. (d) On day 30 mice were challenge with PR8 influenza virus (I.P 500 HA units) and six days later donor CL1 cells were detected by FACS in the spleen. Each graph is representative of 3 experiments using age and sex matched mice. In the experiments depicted in c and d there were between 3 to 6 mice per treatment group.
Differential tolerance induction in OVA-reactive CD8+ T cells is also dependent upon dose of antigen and strength of signalling
The experiments demonstrating that differential tolerance mechanisms can be induced by alterations in dose and TCR affinity have been limited to Kd-HA reactive CD8+ T cells on the B10.D2 background. To assess the generality of these findings, we designed experiments using a different antigenic system. C57BL/6 OT-1 CD8+ T cells recognize the ovalbumin-derived peptide SIINFEKL in the context of Kb. Using OT-1 CD8+ T cells we first analyzed the induction of tolerance mechanisms after a multiple dose treatment protocol and the effect of altering the dose of tolerizing peptide (SIINFEKL). We studied the effect of tolerizing doses of 0.01, 0.1, 1 and 10 μg SIINFEKL on the induction of anergy or deletion mechanisms. Daily exposure to 1 or 10 μg doses favoured the induction of anergy, and we observed up-regulation of NKG2a and PD-1 (Fig. 4a), and failure to phosphorylate ERK after re-stimulation through the TCR (Fig. 4b). Furthermore, there was an increase in survival of OT-1 cells between day 3 and 6, compared to 0.01 or 0.1 μg treatments (Fig. 4c). Treatment with a 0.01 or 0.1 μg dose caused the efficient deletion of the OT-1 cells (Fig. 4c). Therefore, as found with CL4 CD8+ T cells, OT-1 cells are differentially tolerized depending on the dose of toleragen. However, the phosphorylation of ERK in response to TCR stimulation on day 3 was not strictly associated with deletion. ERK activation was turned off in 0.1 μg-treated OT-1's undergoing deletion, but present in 0.01 μg treated OT-1's.
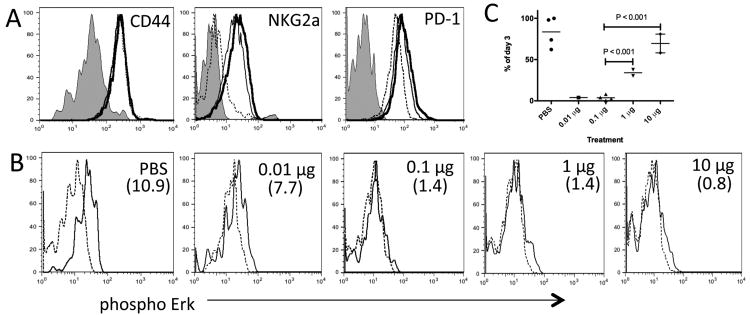
Figure 4. Type of peripheral tolerance induced in OVA-reactive CD8+ T cells is dependent on dose of peptide.
C57BL/6 mice containing donor Thy1.1+ OT-1 CD8+ T cells were treated daily with PBS, 0.01, 0.1, 1 or 10 μg of native SIINFEKL peptide between days 0-2. (a) Day 3 flow cytometric analysis of donor OT-1 cells in the spleen stained with antibody specific for indicated molecule. Lines; Shade – PBS, dashed – 0.1 μg, thin – 1 μg and thick – 10 μg treatments. (b) Day 3 ex vivo phosphorylation of Erk in OT-1 cells re-stimulated with SIINFEKL peptide (thin line) or unstimulated (dashed line). Treatment condition indicated in top right hand corner of plots and the mean MFI increase from unstimulated to stimulated cells in parenthesizes. (c) Survival of OT-1 cells in the spleen. Expressed as the percentage of OT-1 cells detected by FACS on day 6 compared to day 3 for each treatment condition. Each graph is representative of 2 to 3 independent experiments using age and sex matched mice.
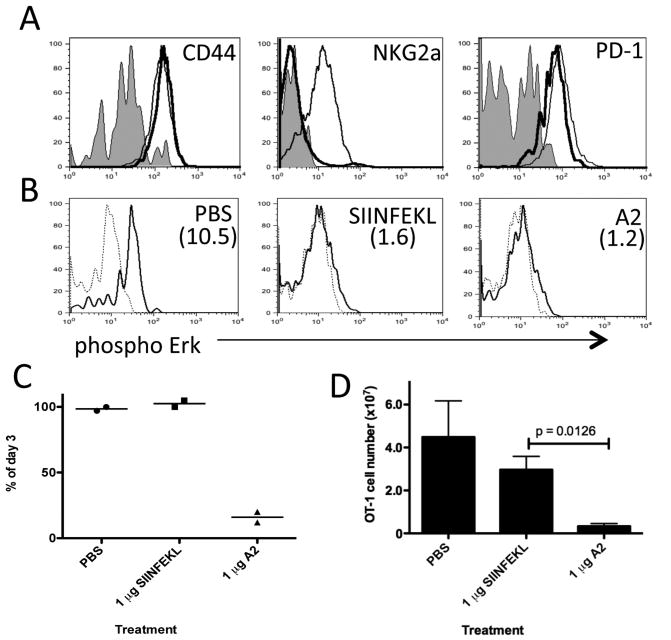
We proceeded to determine whether the induction of anergy or deletion was dependent on the agonistic strength displayed by the tolerizing antigen using the A2 altered variant of SIINFEKL. Of interest, A2 (SAINFEKL) has been described to bind to Kb equally and to possess similar TCR affinity, but displays a 10 fold lower agonist ability than the native SIINFEKL (13, 14). It has been proposed that the weaker agonistic potency of A2 is due to its weaker ability to promote interactions between CD8β and CD3ζ within the immunological synapse (15). Because APL A2 possesses 10 % of the potency of native SIINFEKL, we hypothesized that treatment with 1 μg of A2 would favour the induction of deletion. Figure 5a shows equal expression of CD44, but failure to upregulate NKG2a, and lower levels of PD-1 on OT-1 cells treated with APL A2 compared to native SIINFEKL. Day 3 after TCR stimulation phosphorylation of ERK was turned off by both APL and native peptide treatments (Fig. 5b). Analysis of OT-1 survival at day 6 and 30 days after List-OVA challenge revealed efficient deletion of OT-1 cells by 1 μg APL A2, but not 1 μg native SIINFEKL treatment protocol (Fig. 5c and d). In conclusion, treatment with a toleragen that induces a weaker TCR signal, in this case due to inefficient CD8-TCR interaction, favours the induction of deletion.
Figure 5. Tolerance inducing treatment using an APL displaying weaker agonist properties than native peptide favors the induction of OT-1 CD8+ T cell deletion.
C57BL/6 mice containing donor Thy1.1+ OT-1 CD8+ T cells were treated daily with PBS, 1 μg of native SIINFEKL or 1 μg A2 variant peptide between days 0-2. (a) Day 3 flow cytometric analysis of donor OT-1 cells in the spleen stained with antibody specific for indicated molecule. Lines; Shade – PBS, thin – 1 μg SIINFEKL and thick – 1 μg A2 treatments. (b) Day 3 ex vivo phosphorylation of Erk in OT-1 cells re-stimulated with SIINFEKL peptide (thin line) or unstimulated (dashed line) and the mean MFI increase from unstimulated to stimulated cells in parenthesizes. (c) Survival of OT-1 cells in the spleen. Expressed as the percentage of OT-1 cells detected by FACS on day 6 compared to day 3 for each treatment condition. (d) On day 30 mice were challenge with recombinant Listeria-OVA (i.v. 1 x 105 CFU). Five days later donor OT-1 cells were detected by FACS in the spleen. Each graph is representative of 2-3 independent experiments using age and sex matched mice.
Deletional tolerance induced after activation by cross-presented antigen is dose-dependent
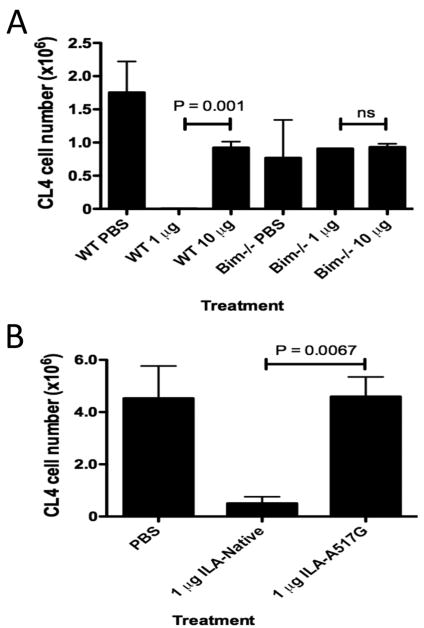
We have previously described an efficient method to introduce nominal CD8+ T cell epitopes into the cross-presentation pathway (16). This method is based on in vitro pulsing of spleen cells that are undergoing apoptosis with a three amino acid (ILA) N-terminal extended version of the nominal Kd-HA peptide. Upon injection, these dying cells are captured by DCs that cross-present associated Kd-HA peptide to CL4 T cells. We utilized this model to determine whether different levels of cross-presented peptide controlled the form of peripheral tolerance induced. We treated animals with multiple doses of apoptotic cells loaded with 1 or 10 μg ILA-Native HA or PBS. Upon day 30 virus recall we observed that low level (1 μg) cross-presentation of apoptotic cell-derived peptide lead to efficient deletion (Fig 6a), whereas cross-presentation of a high level of peptide (10 μg) failed to efficiently delete CL4 cells. We had previously shown deletional tolerance induced by peptide delivery in InsHA transgenic mice to be BIM-dependent (17). Here we confirmed that deletion of CL4 cells associated with low dose of cross-presented HA antigen was also BIM-dependent, as BIM deficient CL4 cells were not deleted (Fig. 6a).
Figure 6. Efficient deletion after constant exposure to cross-presented antigen is dependent upon bim expression, and the dose and TCR affinity of antigen.
B10.D2 mice containing donor WT or Bim-/- Thy1.1+ CL4 CD8+ T cells were treated on days 0, 3 and 6 with apoptotic cells carrying various concentrations of ILA-Native HA (a) or ILA-A517G (b) peptide. On day 30 mice were challenge with PR8 influenza virus (i.p. 500 HA units). Six days later, donor CL4 cells were detected by FACS in the spleen. Each graph is representative of 2 experiments using age and sex matched mice.
To determine whether deletion associated with a low dose of cross-presented peptide was dependent on the affinity of TCR signaling we designed experiments using the ILA N-terminal extension associated with the super agonist variant A517G or native peptide. Figure 6b demonstrates that a low dose (1 μg) of ILA-A517G does not induce efficient deletion of CL4 cells, whereas ILA-HA (native peptide) is highly efficient at deletion. Thus, the deletion of CD8+ T cells upon exposure to cross-presented antigen is dependent on the level of TCR signaling.
Discussion
Previous studies in our laboratory using CL4 CD8+ T cells revealed that the mechanism of peripheral tolerance, either deletion or anergy, was dependent on the dose of peptide and duration of exposure (3, 6). Induction of BIM-dependent deletion occurred after persistent exposure to low dose antigen, while anergy was induced upon persistent exposure to a high dose of antigen. Here we aimed to determine whether TCR signal strength, as determined by TCR affinity rather than dose of antigen, also controlled the path of tolerance chosen by the CD8+ T cells. We also sought to determine the generality of these findings by extending them to CD8 cells expressing different TCRs.
Several different methods were selected for altering the strength of TCR signal. First we used altered peptide ligands that possessed higher (A517G) or lower affinity (I512V) than the native KdHA peptide for CL4 TCR expressing cells. These results indicated that the strength of signal, in this case due to altered TCR affinity rather than density of peptide antigen, also determined whether tolerance occurred through deletion or anergy. Whereas the administration of 1 μg of native peptide resulted in deletion and 10 μg caused anergy of CL4 cell, as little as 1 μg of the super-agonist was sufficient to induce anergy. 10 μg of the low-affinity agonist still resulted in deletion. We demonstrated this in the context of absolute cell numbers and percentage change in CL4 cells between days 3 and 6. Furthermore, we showed upon systemic virus recall challenge at day 30 there was no CL4 cell expansion, ruling out the possibility of reservoirs of non-deleted cells residing in peripheral tissues. Thus, both the density of antigen and TCR affinity for MHC-peptide contribute to the mechanism of tolerance.
Next, we used the CL1 TCR that has inherently low affinity for its ligand. This HA-specific TCR has the identical specificity as CL4, except it was obtained from a mouse that expressed HA as a self-antigen and required over 30-fold more antigen than CL4 to exhibit a comparable response (18). As assessed by expression of CD44, 1 μg of HA peptide was sufficient to obtain stimulation of the cells in vivo, however, even when we used 100-fold more peptide, we still could not achieve anergy. The cells appeared to be obligatorily deleted. This suggests that there may be an affinity threshold that must be crossed before the cells can become anergic. CL1 was obtained from a TCR transgenic mouse that expressed HA in the pancreatic islets but also express low levels in the thymus (19). It is tempting to speculate that the reason CL1 cannot become anergic is because this threshold is higher than what is permitted in order to escape thymic deletion in vivo. Obligatory deletion may be a safer route for the host than anergy if cells encounter a higher concentration of a self-antigen in the periphery. It is also of interest that even the highest amount of peptide used in vivo was insufficient to induce expression of NKG2A by CL1 cells, whereas there was an increase in expression of PD-1 with increasing amount of peptide, suggesting the strength of signal required for NKG2A expression is greater than for PD-1.
We also extended these studies to OT-1 cells for which many altered peptide ligands have been defined (15, 20). We were particularly interested in the A2 peptide as it binds to Kb as efficiently as SIINFEKL, and the TCR on OT-1 cells also binds as well to A2-Kb as to SIINFEKL-Kb. Yet A2 is a 5-10 fold weaker agonist than SIINFEKL due to the weaker interaction between CD8 and the CD3ζ chain when binding the A2-Kb complex (15). CD8 is critical to responsiveness both through stabilization of TCR- pMHC interaction and by assisting in the delivery of Lck to the TCR-CD3 complex (21). Our results indicate the weak agonist activity of A2 peptide extends to signaling for tolerance, as OT-1 cells were deleted rather than anergized by A2. It is of interest that non-depleting anti-CD8 antibodies have long been known to have beneficial effects in transplantation tolerance yet without full understanding of mechanism (22, 23). Promoting deletion of allospecific CD8 cells by reducing TCR signal transduction may be one such mechanism.
We sought to determine whether cross-presented antigen was also able to induce anergy and deletion. We had previously reported a method that allowed us to alter the concentration of cross-presented antigen in vivo (16). This relied upon N-terminal extension of cognate peptide by the 3 residues, ILA. This was sufficient to prevent recognition of peptide in vivo unless it was first processed by proteasomes in antigen presenting cells (24). This method was used to compare the fate of CL4 cells exposed to the same concentration of either native HA or super-agonist, A517G. Indeed, the cells were deleted in response to ILA-HA yet underwent anergy in response to ILA-A517G. Thus, the antigen delivered to CD8 cells by professional presenting cells in vivo follows these same rules of tolerance.
Our observations suggest that the potency and the density of the ligand are interchangeable factors in determining the induction of peripheral tolerance mechanisms, so long as a threshold of TCR affinity is reached. These findings are in line with a report concerning CD4+ T cell tolerance induction by Gottschalk et al. (25). They found weak TCR signaling associated with either low affinity or low density ligands resulted in comparable induction of FoxP3. However, a recent study by the same group has observed distinctive molecular outcomes after an equal cumulative TCR signal is reached by ligand density in comparison to ligand potency (26). Using CD4+ TCR transgenic cells they reported distinct influences of peptide ligand quantity when compared to ligand quality at the level of regulation of the IL-2 pathway after TCR activation, but not in the induction of proliferation. Gene expression analysis by the authors suggested that the responses mediated by TCR signals are segregated into those that are sensitive to the potency of the TCR ligand independent of ligand density, and those that are controlled by ligand density and cumulative levels of TCR signals. While outside the scope of this study, it would be of interest to determine if there are distinct gene expression profiles associated with the anergy phenotype after a TCR signaling threshold is reached by increased ligand density as compared with ligand potency.
In conclusion, our results suggest that, under tolerizing conditions, anergy and deletion of CD8+ T cells can be manipulated by simply altering the strength of signal delivered through the TCR. Both changes in the density of MHC-peptide or affinity of the TCR can deliver the signals required for each of these tolerance mechanisms, although there appears to be threshold of affinity that must be reached in order for anergy to occur. These results should prove of value in devising methods that are most effective in eliminating self-reactive T cells.
Acknowledgments
We thank Dr. Nicolas Gascoigne for the kind donation OT-1 altered peptide ligands, and Dr. Charles Surh for the OT-1 Tg mice. We acknowledge Ms. Jocelyn Chang for mouse breeding and technical assistance.
Grant support: This work was supported by the National Institutes of Health Grant R0I DK050824, awarded to L.A.S.
References
- 1.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 2.Yan J, Mamula MJ. Autoreactive T cells revealed in the normal repertoire: escape from negative selection and peripheral tolerance. J Immunol. 2002;168:3188–3194. doi: 10.4049/jimmunol.168.7.3188. [DOI] [PubMed] [Google Scholar]
- 3.Redmond WL, Sherman LA. Peripheral tolerance of CD8 T lymphocytes. Immunity. 2005;22:275–284. doi: 10.1016/j.immuni.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Wing K, Yamaguchi T. Dynamics of peripheral tolerance and immune regulation mediated by Treg. Eur J Immunol. 2009;39:2331–2336. doi: 10.1002/eji.200939688. [DOI] [PubMed] [Google Scholar]
- 6.Redmond WL, Marincek BC, Sherman LA. Distinct requirements for deletion versus anergy during CD8 T cell peripheral tolerance in vivo. J Immunol. 2005;174:2046–2053. doi: 10.4049/jimmunol.174.4.2046. [DOI] [PubMed] [Google Scholar]
- 7.Rocha B, Grandien A, Freitas AA. Anergy and exhaustion are independent mechanisms of peripheral T cell tolerance. J Exp Med. 1995;181:993–1003. doi: 10.1084/jem.181.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo D, Freedman J, Hesse S, Palmiter RD, Brinster RL, Sherman LA. Peripheral tolerance to an islet cell-specific hemagglutinin transgene affects both CD4+ and CD8+ T cells. Eur J Immunol. 1992;22:1013–1022. doi: 10.1002/eji.1830220421. [DOI] [PubMed] [Google Scholar]
- 9.Hartemann-Heurtier A, Mars LT, Bercovici N, Desbois S, Cambouris C, Piaggio E, Zappulla J, Saoudi A, Liblau RS. An altered self-peptide with superagonist activity blocks a CD8-mediated mouse model of type 1 diabetes. J Immunol. 2004;172:915–922. doi: 10.4049/jimmunol.172.2.915. [DOI] [PubMed] [Google Scholar]
- 10.DeSilva DR, Feeser WS, Tancula EJ, Scherle PA. Anergic T cells are defective in both jun NH2-terminal kinase and mitogen-activated protein kinase signaling pathways. J Exp Med. 1996;183:2017–2023. doi: 10.1084/jem.183.5.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Whaley CD, Mondino A, Mueller DL. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science. 1996;271:1272–1276. doi: 10.1126/science.271.5253.1272. [DOI] [PubMed] [Google Scholar]
- 12.Lyman MA, Nugent CT, Marquardt KL, Biggs JA, Pamer EG, Sherman LA. The fate of low affinity tumor-specific CD8+ T cells in tumor-bearing mice. J Immunol. 2005;174:2563–2572. doi: 10.4049/jimmunol.174.5.2563. [DOI] [PubMed] [Google Scholar]
- 13.Alam SM, Davies GM, Lin CM, Zal T, Nasholds W, Jameson SC, Hogquist KA, Gascoigne NR, Travers PJ. Qualitative and quantitative differences in T cell receptor binding of agonist and antagonist ligands. Immunity. 1999;10:227–237. doi: 10.1016/s1074-7613(00)80023-0. [DOI] [PubMed] [Google Scholar]
- 14.Hogquist KA, Jameson SC, Bevan MJ. Strong agonist ligands for the T cell receptor do not mediate positive selection of functional CD8+ T cells. Immunity. 1995;3:79–86. doi: 10.1016/1074-7613(95)90160-4. [DOI] [PubMed] [Google Scholar]
- 15.Yachi PP, Ampudia J, Zal T, Gascoigne NR. Altered peptide ligands induce delayed CD8-T cell receptor interaction--a role for CD8 in distinguishing antigen quality. Immunity. 2006;25:203–211. doi: 10.1016/j.immuni.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Wei CH, Sherman LA. N-terminal trimer extension of nominal CD8 T cell epitopes is sufficient to promote cross-presentation to cognate CD8 T cells in vivo. J Immunol. 2007;179:8280–8286. doi: 10.4049/jimmunol.179.12.8280. [DOI] [PubMed] [Google Scholar]
- 17.Redmond WL, Wei CH, Kreuwel HT, Sherman LA. The apoptotic pathway contributing to the deletion of naive CD8 T cells during the induction of peripheral tolerance to a cross-presented self-antigen. J Immunol. 2008;180:5275–5282. doi: 10.4049/jimmunol.180.8.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nugent CT, Morgan DJ, Biggs JA, Ko A, Pilip IM, Pamer EG, Sherman LA. Characterization of CD8+ T lymphocytes that persist after peripheral tolerance to a self antigen expressed in the pancreas. J Immunol. 2000;164:191–200. doi: 10.4049/jimmunol.164.1.191. [DOI] [PubMed] [Google Scholar]
- 19.Morgan DJ, Nugent CT, Raveney BJ, Sherman LA. In a transgenic model of spontaneous autoimmune diabetes, expression of a protective class II MHC molecule results in thymic deletion of diabetogenic CD8+ T cells. J Immunol. 2004;172:1000–1008. doi: 10.4049/jimmunol.172.2.1000. [DOI] [PubMed] [Google Scholar]
- 20.Koniaras C, Carbone FR, Heath WR, Lew AM. Inhibition of naive class I-restricted T cells by altered peptide ligands. Immunol Cell Biol. 1999;77:318–323. doi: 10.1046/j.1440-1711.1999.00828.x. [DOI] [PubMed] [Google Scholar]
- 21.Artyomov MN, Lis M, Devadas S, Davis MM, Chakraborty AK. CD4 and CD8 binding to MHC molecules primarily acts to enhance Lck delivery. Proc Natl Acad Sci U S A. 2010;107:16916–16921. doi: 10.1073/pnas.1010568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cobbold SP. T cell tolerance induced by therapeutic antibodies. Philos Trans R Soc Lond B Biol Sci. 2005;360:1695–1705. doi: 10.1098/rstb.2005.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cobbold SP, Martin G, Waldmann H. The induction of skin graft tolerance in major histocompatibility complex-mismatched or primed recipients: primed T cells can be tolerized in the periphery with anti-CD4 and anti-CD8 antibodies. Eur J Immunol. 1990;20:2747–2755. doi: 10.1002/eji.1830201232. [DOI] [PubMed] [Google Scholar]
- 24.Rock KL, Shen L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol Rev. 2005;207:166–183. doi: 10.1111/j.0105-2896.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 25.Gottschalk RA, Corse E, Allison JP. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J Exp Med. 2010;207:1701–1711. doi: 10.1084/jem.20091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottschalk RA, Hathorn MM, Beuneu H, Corse E, Dustin ML, Altan-Bonnet G, Allison JP. Distinct influences of peptide-MHC quality and quantity on in vivo T-cell responses. Proc Natl Acad Sci U S A. 2012;109:881–886. doi: 10.1073/pnas.1119763109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu K, Iyoda T, Saternus M, Kimura Y, Inaba K, Steinman RM. Immune tolerance after delivery of dying cells to dendritic cells in situ. J Exp Med. 2002;196:1091–1097. doi: 10.1084/jem.20021215. [DOI] [PMC free article] [PubMed] [Google Scholar]