Abstract
Objective
We develop a new diabetes CHD risk estimator using traditional risk factors plus coronary artery calcium (CAC), ankle-brachial index (ABI), high sensitivity C-reactive protein, family history of CHD, and carotid intima-media thickness and compared it with United Kingdom Prospective Diabetes study (UKPDS), Framingham risk and the NCEP/ATP III risk scores in type 2 diabetes mellitus (T2DM).
Methods and Results
We combined data from T2DM without clinical CVD in the Multi-Ethnic Study of Atherosclerosis (MESA) and the Heinz Nixdorf Recall Study (N=1343). After a mean follow-up of 8.5 years, 85(6.3%) participants had incident CHD. Among the novel risk markers, CAC best predicted CHD independent of the FRS [hazard ratio: HR (95% CI): log (CAC +25):1.69(1.45 – 1.97), p<0.0001; CAC categories: CAC ≤ 25 as reference, >25 and ≤ 125:2.29(0.87 – 5.95), >125 and ≤ 400: 3.87(1.57– 9.57), >400: 5.97(2.57– 13.84), respectively). The MESA-HNR diabetes CHD risk score has better accuracy for the main outcome versus the FRS or UKPDS [area under curve (AUC) of 0.76 vs. 0.70 and 0.69, respectively; all p<0.05]. The MESA-HNR risk score improved risk classification versus the FRS (net reclassification improvement (NRI) = 0.19 and integrated discrimination improvement (IDI) =0.046, p<0.05) and UKPDS (NRI=0.215 and IDI = 0.046, p<0.05). Compared with the ATP III guidelines, the MESA-HNR score has an NRI of 0.74 for the main outcome.
Conclusions
This new CHD risk estimator has better discriminative ability for incident CHD than the FRS, UKPDS, and the ATP III/NCEP recommendations in a multi-ethnic cohort with T2DM.
Keywords: Diabetes mellitus, coronary calcium score, risk assessment, coronary heart disease
Introduction
Cardiovascular disease (CVD) preventive strategies are commonly based on an assessment of the individual patient's risk using global risk scoring tools (1, 2). However, patients with type 2 diabetes mellitus (T2DM), are typically excluded from global risk scoring tools because they are considered “coronary heart disease risk equivalents” and recommended to receive the same preventive interventions as patients with known coronary heart disease (3, 4).
For example, the Adult Treatment Panel (ATP) III Guidelines exclude patients with T2DM from the risk estimation tool because they assume that diabetes automatically confers a high risk for future cardiovascular events. However, some existing risk estimation tools have attempted to stratify patients with T2DM more precisely (5, 6). Specifically, one version of the Framingham risk score (FRS) and the United Kingdom Prospective Diabetes Study (UKPDS) score classify patients with T2DM as being at low, intermediate, and high risk of CVD based on calculated scores (2,3). It is unclear how often these alternative risk estimation tools are used in clinical practice and whether they achieve reasonable discrimination and calibration for risk estimation in patients with T2DM from other race/ethnic backgrounds.
Recently, cardiovascular risk estimation tools have begun to include measures of subclinical atherosclerosis and newer markers of risk to improve risk discrimination and classification (7–9). In the Multi-Ethnic Study of Atherosclerosis (MESA) (7), the Rotterdam Study (8), and the Heinz Nixdorf Recall (HNR) study (9), the strongest measures of risk were coronary artery calcium (CAC) score and brain natriuretic peptide (BNP), two “novel” markers excluded from the Framingham Risk Score and the UKPDS. It therefore seems reasonable to explore whether some newer markers of risk could better discriminate risk among patients with T2DM.
MESA (United States) and the HNR Study (Germany) are prospective observational studies that included patients with T2DM in their enrollment and follow-up. The goals of this report are to (a) describe a newly developed “MESA-HNR” CHD risk estimator for patients with T2DM, based on a broad array of conventional and novel clinical risk factors and biomarkers, and (b) compare its prediction performance with the FRS, UKPDS and ATP III/NCEP recommendations (1–3).
Methods
Study Population and Data Collection
The MESA study design was published previously (10). In brief, MESA is a cohort study begun in July 2000 to investigate the prevalence, correlates, and progression of subclinical CVD in individuals without known CVD at baseline. The cohort includes 6814 women and men aged 45–84 years old recruited from 6 US communities (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; northern Manhattan, New York; and St. Paul, Minnesota). In MESA, 38% of participants were white (n = 2624), 28% black (n = 1895), 22% Hispanic (n = 1492) and 12% Chinese (n = 803). Individuals with a history of physician-diagnosed myocardial infarction, angina, heart failure, stroke or transient ischemic attack, or who had undergone an invasive procedure for CVD (coronary artery bypass graft, angioplasty, valve replacement, pacemaker placement or other vascular surgeries) were excluded. However, patients with diabetes mellitus were included if they met other enrollment criteria.
The HNR recruited a total of 4814 Caucasians between 45 and 75 years of age from three neighboring cities in the metropolitan Ruhr area of Germany between 2000 and 2003 in a single center with a response rate of 55.8%(11). Participants were a random sample derived from mandatory citizen registries and provided to the study center. The study was certified according to DIN EN ISO 9001:2000, and re-certified in 2006. Both studies were approved by the appropriate Institutional Review Boards of each study site, and written informed consent was obtained from all participants.
Clinical Data
Demographics, medical history, anthropometric and laboratory data for this analysis were obtained in each study during the baseline examination. Body mass index (BMI; kg/m2) was computed based on direct measurements of height and weight. All medication utilization was based on participants' self-report. Standard enzymatic methods were used to measure total cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglycerides. Low-density lipoprotein cholesterol (LDL-C) was calculated with the Friedewald equation in MESA (12) and measured directly in HNR (14). Blood samples were obtained after a 12 h fasting in MESA. In HNR, participants were fasting 9.7+4.9 h (median 12 h) before blood sampling. In both studies, blood pressure was measured using an oscillographic method with two different systems (Dynamap, Johnson & Johnson, USA and HEM-705CP, Omron, Hoofddorp, NL). The mean values of the second and third of three measurements taken at least 2 min apart were used. Hypertension was defined in both studies as a blood pressure measurement of 140/90 mmHg or use of antihypertensive medication. In both studies, participants were considered to have diabetes mellitus if they were taking anti-diabetic medication or had a fasting glucose concentration of ≥126 mg/dl. Duration of diabetes was self –reported. Smoking history was categorized as (i) currently smoking, (ii) former, defined as not smoking within the past 30 days in MESA and as stopped smoking (a) within the past year or (b) more than 1 year ago in the HNR, and (iii) never(8,13). The use of lipid-lowering medication was documented. This included HMG CoA reductase inhibitors (`statins'), fibrates, bile acid sequestrants, and nicotinic acid derivatives. High sensitivity C- reactive protein (Hs-CRP) was measured using the BNII nephelometer (N High Sensitivity CRP; Dade Behring Inc., Deerfield, Illinois). Analytical intra-assay coefficients of variation ranged from 2.3% to 4.4%, and inter-assay coefficients of variation ranged from 2.1% to 5.7% with a detection level of 0.18 mg/L. Family history of CHD was obtained by asking participants whether any member in their immediate family (first-degree relatives: parents, siblings, and children) experienced fatal or nonfatal myocardial infarction.
Ankle-Brachial Index
The protocol and quality control of ankle brachial index (ABI) measurement in both the MESA and HNR studies were previously published (13, 14). Briefly, ABI was measured in supine participants with systolic blood pressures measured in both arms and legs with appropriately sized cuffs. For both legs (when possible), the systolic blood pressure was measured in each posterior tibial and dorsalis pedis artery. All pressures were detected using a continuous-wave Doppler ultrasound probe. The ABI was calculated as the higher systolic blood pressure in the posterior tibial or dorsalis pedis artery divided by the higher of the arm systolic blood pressure values.
Carotid Intima-Media Thickness
The protocol and quality control for carotid intima-media thickness (CIMT) measurement in the MESA and HNR studies were previously published (15, 16). CIMT images were obtained using B-mode sonography at the right and left common carotid artery and measured 1 cm starting from the bulb. The mean of maximum intima-media thickness of the common carotid artery was used. The protocols for CIMT measurement were similar in both MESA and the HNR studies.
Coronary Artery Calcium (CAC) Score by Computed Tomography (CT)
Details of the MESA and HNR CT scanning and interpretation methods have been reported by Carr et al (17) and Schmermund et al (18). Briefly, for MESA sites, scanning centers assessed CAC by chest CT with either a cardiac-gated electron-beam CT scanner (Chicago, Los Angeles, and New York) or a multidetector CT system (Baltimore, Forsyth County, and St. Paul). Certified technologists scanned all participants twice over phantoms of known physical calcium concentration. A radiologist or cardiologist read all CT scans at a central reading center (Los Angeles Biomedical Research Institute at Harbor-UCLA, Torrance, California). In the HNR study, two radiology departments scanned and analyzed the CAC score in a blinded, independent way (19). In both studies, CAC was defined as a hyper-attenuating focus of at least four contiguous pixels with a CT density of >130 Hounsfield units. The area of each focus was measured and the CAC score was determined using the methods of Agatston et al (20). The total (Agatston) CAC score was computed by summing up the CAC scores of all foci in the epicardial coronary system without phantom adjustment. Agreement with regard to the presence of CAC was high in MESA (k =0.90–0.93) and interclass correlation coefficient for CAC scoring of 0.99. In the HNR study, inter-scan variability was 5–8%, and for inter-institutional readings of the two EBCT centers, a k-value of 0.94 in 250 scans was found (21).
Composite Outcome Definition
For these prediction models, we used “hard coronary heart disease” as the composite endpoint. Hard coronary event was defined as adjudicated fatal or non-fatal myocardial infarction. The adjudication processes for both studies were published previously (8, 18). Other CHD outcomes such as revascularization and angina were excluded from our composite outcome because the two studies used significantly different definitions of angina pectoris and revascularization rates.
Statistical Analysis
Based on the similarity of procedures, data from both cohorts were pooled into a single sample for analysis. Descriptive statistics were provided showing means and standard deviations on all of the candidate predictors of hard coronary heart disease (CHD) events. We then explored whether any candidate novel risk factors (CAC, Hs-CRP, ABI, CIMT, pack-years of smoking, or family history of CHD) were associated with the outcome using a Cox model conditional on the FRS. To improve variable selection, we then used Bayesian model averaging to estimate the posterior probability of a predictor of CHD events being included in a statistical model with the highest likelihood (22, 23). This approach has been used (24, 25) to select the best set of predictors of a cardiovascular outcome when predictors are correlated. Traditionally, a posterior probability of 50% or more is used to determine which variables are included in the statistical model; here, we set a more inclusive threshold of 20% to capture as many potential predictors as possible.
All missing data (<4% of a variable) were handled using multiple imputation methods, because excluding participants with missing data would induce more bias in estimation of risk scores than using an appropriate technique for missing data. Areas under the curve (AUCs) were computed for the FRS, the UKPDS, and the best-fitting model using the HNR-MESA data. The FRS and UKPDS models were refit onto the HNR-MESA data because the raw scores tended to greatly overpredict (more than double) the rate of CHD events in this relatively healthy population. By refitting, the comparison between the prediction rates of FRS and UKPDS scores with a HNR-MESA score is more directly comparable in performance.
The possibility of overfit for this analysis was considered in two separate ways. One approach was to bootstrap the sample 100 times, imputing the missing data for each bootstrap (26). We measured the mean absolute deviation of the bootstrap models fit on the main data sample as being 0.02 AUC. The second approach was to divide the data into training and validation samples. We fit the best model on the training sample and then estimated the AUC on the validation sample.
To fully demonstrate the relative fit of the different models, we computed the net reclassification improvement (NRI) and integrated discrimination improvement (IDI) for the refit FRS, refit UKPDS, and the HNR-MESA model. Because there were very few missing data in the final set of predictors for any model, and it was unclear how to integrate these approaches with multiple imputations, we calculated the NRI and IDI on a complete case sample of the combined data. Finally, as a sensitivity analysis, we computed an alternate HNR-MESA score when CT scans were not available, testing all novel predictors for this model also. Analyses were done using a combination of SAS, STATA, and R with replication of the key parts of the analysis in multiple programming languages.
Results
Out of 1343 MESA and HNR participants with T2DM but without clinical CVD at baseline, 85(6.3%) had hard CHD after an average of 8.5 years of follow-up. Table 1 shows the demographic characteristics, CVD risk factors, and other risk markers in this combined cohort. Most participants were males and were taking antihypertensive medications during the baseline examination. Only 23% of the cohort was taking statins. Supplemental Table 1 shows the predictive value of diabetes-specific markers for hard CHD. After multiple modeling approaches, duration of diabetes and insulin use emerged as independent predictors of incident hard CHD after adjusting for the FRS variables age and sex. Log (CAC+ 25) and ankle-brachial index were independent predictors of incident hard CHD after adjusting the FRS variables in the combined cohort (Table 2).
Table 1.
Baseline Characteristics of the study cohorts. Values as mean[standard deviation] or percentage.
| Characteristics | Subjects in HNR | Subjects in MESA | All Subjects |
|---|---|---|---|
| Number of Subjects | 551 | 792 | 1343 |
| Age(years) | 61[8] | 64[9] | 63[9] |
| Male | 61% | 53% | 56% |
| Race - Chinese American | 0% | 12% | 7% |
| African American | 0% | 38% | 22% |
| Hispanic | 0% | 30% | 18% |
| Total Cholesterol(mg/dL) | 226[39] | 189[40] | 204[44] |
| HDL Cholesterol(mg/dL) | 52[16] | 47[13] | 49[14] |
| LDL Cholesterol(mg/dL) | 144[36] | 112[34] | 125[38] |
| Triglycerides(mg/dL) | 189[152] | 160[134] | 172[143] |
| Systolic Blood Pressure(mmHg) | 141[22] | 133[22] | 136[22] |
| Diastolic Blood Pressure(mmHg) | 83[11] | 72[10] | 77[12] |
| Baseline glucose (mg/dL) | 156[51] | 148[54] | 151[53] |
| Current smoker | 23% | 12% | 16% |
| Pack years of cigarette smoking | 32[30] | 12[22] | 18[26] |
| Family history | 9% | 39% | 27% |
| Unknown family history | 17% | 0% | 7% |
| Coronary Artery Calcium(mean agatston score) | 309[664] | 247[554] | 272[601] |
| Duration of diabetes (years) | 5.4[9.2] | 5.6[8.0] | 5.5[8.5] |
| HbA1c (%) | 6.6%[1.4] | 7.3%[1.7] | 7.0%[1.6] |
| Metformin use | 23% | 37% | 31% |
| Sulfonylurea use | 15% | 43% | 32% |
| Insulin use | 12% | 13% | 13% |
| Glitazone use | 6% | 10% | 9% |
| Hypertension medication use | 54% | 63% | 59% |
| Statin use | 15% | 28% | 23% |
| C-Reactive Protein (mg/L) | 4.1[8.0] | 4.4[5.8] | 4.2[6.8] |
| Ankle-brachial index | 1.1[0.2] | 1.1[0.2] | 1.1[0.2] |
| Common Carotid Intima Media Thickness (mm) | 0.71[0.13] | 0.93[0.19] | 0.85[0.20] |
| Hard Coronary Heart Disease Event | 7.4% | 5.6% | 6.3% |
HbA1C: hemoglobin A1C
Table 2.
Novel Risk Factors individually modeled and adjusted for Framingham risk score (FRS) in the HNR/MESA diabetes risk group (outcome=hard coronary heart disease (CHD) events)
| Parameter | Hazard Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
|
| |||
| Log (CAC +25) | 1.69 | 1.45 to 1.97 | <.0001 |
|
| |||
| CAC Categories | |||
| Less than 25 | 1.00 | Reference | |
| > 25 and ≤ 125 | 2.29 | 0.87 to 5.95 | 0.09 |
| > 125 and ≤ 400 | 3.87 | 1.57 to 9.57 | 0.003 |
| >400 | 5.97 | 2.57 to 13.84 | <0.0001 |
|
| |||
| C-Reactive Protein (mg/L) | 1.02 | 0.98 to 1.06 | 0.38 |
|
| |||
| Ankle-Brachial index < 0.90 | 1.78 | 1.04 to 3.06 | 0.04 |
|
| |||
| CCA intimal medial thickness (mm) | 2.10 | 0.74 to 5.97 | 0.17 |
|
| |||
| Pack-years of smoking (years) | 1.00 | 0.99 to 1.01 | 0.63 |
|
| |||
| Family History | 1.11 | 0.66 to 1.86 | 0.69 |
|
| |||
| Unknown Family History | 1.49 | 0.71 to 3.14 | 0.29 |
CAC: coronary artery calcium score; CCA: common carotid artery
MESA-HNR Diabetes CHD Risk Score
After Bayesian model averaging (posterior probability of selection cut-off of 20%), age, sex, systolic blood pressure, log (CAC + 25), and duration of diabetes were candidate variables for inclusion into the risk score (Supplemental Table 2). With the exception of Hispanic ethnicity (posterior probability of 40%), all other race/ethnicity had insufficient posterior probabilities for inclusion in our final model. The significant posterior probability for Hispanics was shown in further analysis to be a socioeconomic effect and hence was not included in the final model. The mean absolute deviation of the bootstrap model fit on the main data sample was 0.02 AUC, suggesting that the AUC for our primary model should be 0.74 instead of 0.76. This cross-validation approach estimated the AUC of the final risk model as 0.75. The cross-validation approach estimated the AUC of the final risk model as being 0.01 AUC lower than the estimate on the complete sample.
Neither approach suggested any important level of overfit. That is, both sensitivity analyses suggested degrees of overfit within the 95% confidence intervals of the AUC estimate.
Table 3 shows the discriminative ability of the candidate variables selected using the Bayesian model averaging (as shown in Table 4) and the improvement in discrimination from adding log (CAC +25) to the FRS and the UKPDS for incident hard CHD events. Log (CAC + 25) had better discriminative ability compared with either the refit FRS (AUC of 0.74 vs. 0.70) or the refit UKPDS (AUC of 0.74 vs.0.69). The addition of Log (CAC + 25) to the refit FRS or the refit UKPDS also improved discrimination. A model containing duration of diabetes and Log (CAC +25) had an AUC of 0.75 for incident CHD, whereas a model containing age, sex, systolic blood pressure, duration of diabetes and log (CAC + 25) had an AUC of 0.76.
Table 3.
Discrimination, as measured by area under the receiver operating characteristic (ROC) curves for various different predictive models for incident coronary heart disease event in T2DM
| AUC | ||
|---|---|---|
| Model: | Imputation | |
| A | Duration of diabetes, log(CAC+25) | 0.7547 |
| B | Age, sex, systolic bp, duration of diabetes, log(CAC+25) | 0.7637 |
| C | Framingham risk score (as reported) | 0.6797 |
| D | Framingham risk score refit* | 0.6964 |
| E | Framingham score refit* plus log(CAC+25) | 0.7575 |
| F | UKPDS score refit** | 0.6878 |
| G | UKPDS score refit** plus log(CAC+25) | 0.7575 |
| H | Log(CAC+25) | 0.7412 |
AUC = Area under the curve, UKPDS = United Kingdom Prospective Diabetes Study, CAC = coronary artery calcium, bp= blood pressure,
Comparing MESA-HNR, Refit FRS and UKPDS Models, and ATP III /NCEP
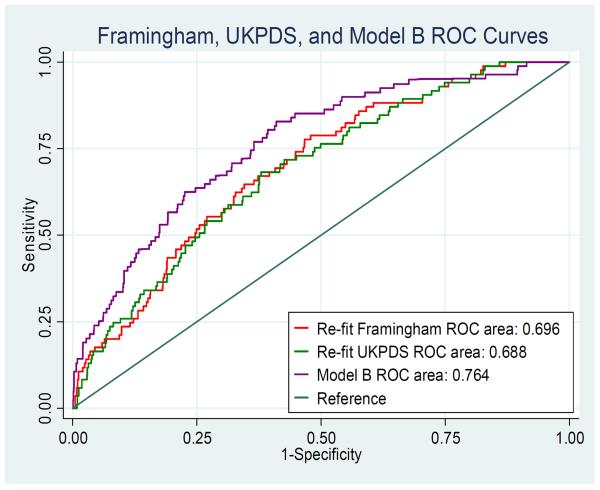
Figure 1 shows ROC curves comparing the discriminative ability of the MESA-HNR diabetes CHD risk score (model B) with the refit models from FRS and UKPDS for incident CHD events in this cohort. The MESA-HNR score had significantly higher discriminative ability compared with either the FRS [AUC (95%CI) 0.76(0.71–0.81) vs. 0.70(0.64–0.75), P<0.05] or the UKPDS [AUC (95%CI) 0.76(0.71–0.81)] vs. 0.69(0.63–0.74), P<0.05]. The FRS and UKPDS had similar discriminative ability for incident CHD [AUC (95%CI) 0.70(0.64– 0.75) vs. 0.69(0.63–0.74), P>0.05]. Log (CAC + 25). Model A [duration of diabetes and log (CAC +25)] and model B [age, sex, systolic blood pressure, duration of diabetes and log (CAC + 25)] had similar discriminative abilities for incident CHD. As shown in supplemental Figure 2, there were no differences in AUC for incident CHD when all the CAC based models are compared.
Figure 1.
ROC curves comparing the discriminative ability(AUC) of the Framingham risk score, the United Kingdom Prospective Diabetes Study (UKPDS) score and the HNR-MESA Diabetes CHD score (model B) for incident hard CHD in this cohort.
Tables 4(A and B) show the net reclassification indices when the MESA-HNR diabetes CHD risk score is compared with the refit FRS (Table 4A) and refit UKPDS (Table 4B). The MESA-HNR diabetes risk score (model B) provides an improvement in discrimination over the FRS with an NRI of 0.19 and IDI of 0.046. The MESA-HNR diabetes risk score (model B) also provides an improvement in discrimination over the refit UKPDS with an NRI of 0.22 and IDI of 0.046. The ATP III/NCEP is not a risk estimator and so cannot be used to assess the individual CHD risk for each individual in this study. However, compared with the ATP III/NCEP (assuming all T2DM individuals are at high risk for CHD), the MESA-HNR CHD risk score has an NRI of 0.74.
Table 4a.
Net Reclassmcation Improvement (NRI) table comparing the MESA-HNR score versus the Framingham Risk Score for incident coronary heart disease events.
| Risk in MESA-HNR Score | ||||||
|---|---|---|---|---|---|---|
| Risk in Refit FRS Score | <6% | 6–20% | ≥20% | Overall | Reclassified as Higher Risk | Reclassified as Lower Risk |
| <6% | ||||||
| No. of participants | 623 | 131 | 12 | 766 | ||
| No. of events | 16 | 7 | 2 | 25 | 9 | NA |
| No. of non-events | 607 | 124 | 10 | 741 | 134 | NA |
| 6–20% | ||||||
| No. of participants | 272 | 241 | 54 | 567 | ||
| No. of events | 15 | 28 | 16 | 59 | 16 | 15 |
| No. of non-events | 257 | 213 | 38 | 508 | 38 | 257 |
| ≥20% | ||||||
| No. of participants | 1 | 5 | 4 | 10 | ||
| No. of events | 0 | 0 | 1 | 1 | NA | 0 |
| No. of non-events | 1 | 5 | 3 | 9 | NA | 6 |
| Overall | ||||||
| No. of participants | 896 | 377 | 70 | 1343 | ||
| No. of events | 31 | 35 | 19 | 85 | 25 | 15 |
| No. of non-events | 865 | 342 | 51 | 1258 | 172 | 263 |
| n | NRI* | Standard Error NRI | P-value NRI |
|---|---|---|---|
| 1343 | 0.190 | 0.076 | 0.013 |
NRI= Net Reclassification Index
Table 4b.
Net Reclassification Improvement (NRI) table comparing the MESA-HNR score versus the United Kingdom Prospective Diabetes Study (UKPDS) score for incident coronary heart disease events.
| Risk in MESA-HNR Score | ||||||
|---|---|---|---|---|---|---|
| Risk in Refit UKPDS Score | <6% | 6–20% | ≥20% | Overall | Reclassified as Higher Risk | Reclassified as Lower Risk |
| <6% | ||||||
| No. of participants | 629 | 126 | 15 | 770 | ||
| No. of events | 18 | 7 | 2 | 27 | 9 | NA |
| No. of non-events | 611 | 119 | 13 | 743 | 132 | NA |
| 6–20% | ||||||
| No. of participants | 267 | 245 | 51 | 563 | ||
| No. of events | 13 | 28 | 16 | 57 | 16 | 13 |
| No. of non-events | 254 | 217 | 35 | 506 | 35 | 254 |
| ≥20% | ||||||
| No. of participants | 0 | 6 | 4 | 10 | ||
| No. of events | 0 | 0 | 1 | 1 | NA | 0 |
| No. of non-events | 0 | 6 | 3 | 9 | NA | 6 |
| Overall | ||||||
| No. of participants | 896 | 377 | 70 | 1343 | ||
| No. of events | 31 | 35 | 19 | 85 | 25 | 13 |
| No. of non-events | 865 | 342 | 51 | 1258 | 167 | 260 |
| n | NRI* | Standard Error NRI | P-value NRI |
|---|---|---|---|
| 1343 | 0.215 | 0.074 | 0.004 |
NRI= Net Reclassification Index
MESA- HNR Diabetes CHD Risk Score
Supplemental Table 3 show the parameter estimate of variables in the MESA-HNR risk score (model B) for predicting incident CHD events, using a Cox proportional hazard model. Supplemental Table 4 and Table 5 show the 8-year risk estimates and a point-based 8-year risk score developed from this cohort (model B) to assess the CHD risk of individuals with T2DM.
Table 5.
Points-based 8 year MESA-HNR diabetes coronary heart disease risk score.
| 8 Year Risk: Add points from the 5 categories | |
|---|---|
| Age: | |
|
| |
| >=65 years | +4 points |
|
| |
| Sex: | |
|
| |
| Male | +4 points |
|
| |
| Systolic bp: | |
|
| |
| >=135 | +2 points |
|
| |
| Duration of Diabetes: | |
|
| |
| >0 years | +3 points |
|
| |
| CAC: | |
|
| |
| <25 | −2 points |
|
| |
| 25 to <125 | 0 points |
|
| |
| 125 to <400 | +4 points |
|
| |
| >=400 | +16 points |
*less than 1 point indicates less than 1% risk, otherwise 1 point is ~ 1 % risk of 8 year coronary heart disease event
New Pooled Equations (ASCVD risk Estimator)
We also assessed the discriminative ability of the new pooled equations (PCE) for incident atherosclerotic cardiovascular events (ASCVD) defined as a composite of CHD and stroke (excluding TIA's) in this type 2 diabetes cohort (27). The AUC of the PCE for incident ASCVD in this cohort was 0.637. The MESA –HNR risk score had an NRI of 0.204 when compared with the PCE or ASCVD risk estimator for incident ASCVD events (Supplemental Table 5).
Discussion
The goal of this study is to develop a new diabetes CHD risk estimator with better discriminative ability using a combination of traditional risk factors and novel cardiovascular risk markers in a multi ethnic adult T2DM population. The MESA-HNR diabetes CHD risk score had better discrimination for incident CHD than the FRS, UKPDS, or the ATP III/NCEP recommendation. All three of these well-recognized risk tools (1–3) have shown only modest discriminative abilities for incident coronary heart disease in T2DM populations (28–31). Even though the ATPIII/NCEP classification of all T2DM is supported by studies such as Collaborative Atorvastatin Diabetes Study (CARDS) (32) trial, other studies, such as the Action to Control Cardiovascular Risk in Diabetes (ACCORD) (33) trial, indicate that classifying all T2DM patients as being at high risk for CVD is not justified. The present study shows that a risk estimator incorporating subclinical measures of atherosclerosis, such as CAC, may help identify which patients with T2DM could benefit from statin therapy to reduce their risk of incident CHD.
The FRS was developed exclusively in Caucasians, and the elevated cardiovascular risk in people with T2DM was not recognized during the development of the FRS (2). The UKPDS (1), a risk assessment tool uniquely developed for T2DM, includes too many variables, making it too cumbersome for routine clinical use. Some have even questioned the relevance of the UKPDS, since the derivative cohort was studied over 30 years ago when most current diabetes therapies were unavailable.
Novel CVD risk markers such as CAC and ABI predicted incident hard CHD independent of the traditional risk factors in the FRS or the UKPDS in our cohort. In addition, a risk estimator incorporating CAC has better discriminative ability compared with the FRS or the UKPDS. Only one case (event) in our cohort was classified as high risk by either the FRS or the UKPDS, compared with 19 cases using the MESA-HNR score. However, even with this improved discriminative ability, 77% of cases (events) would have been misclassified as either intermediate/low risk and these individuals would not have been offered statin therapy. Using the ATP III/NCEP recommendations, all participants in the current study would have been treated with statins, without incurring the cost of a cardiac CT scan or exposure to ionizing radiation. However, using the ATPIII/NCEP guideline would have misclassified all non- cases (93% of the total cohort) as high risk and would have recommended statin therapy for them. A formal cost-effectiveness analysis is needed to definitively investigate the best approach. But given the cost of obtaining CAC; the current cost, pleiotropic effects, and safety profile of statins; and the public health impact of CHD; the ATP III/NCEP recommendation may be the favored approach for CHD risk assessment in patients with T2DM compared with FRS, UKPDS or MESA-HNR diabetes CHD risk score.
Despite the undisputed role of modifiable risk factors such as cigarette smoking and serum lipids in the pathogenesis of atherosclerosis and hence CHD events, their posterior probability for incident CHD in this T2DM population was insufficient for inclusion in the final risk model. It appears that coronary atherosclerosis burden (such as CAC) may represent an aggregate of the risk associated with these modifiable CV risk factors. Thus irrespective of an individual's risk, smoking cessation should always be recommended. However, recommendations for lipid therapy and the use of aspirin for primary prevention should be based in an individual's global CV risk as suggested by current guidelines (3, 4).
The current study has some limitations. To generate adequate numbers for this analysis, the current cohort combined data from the MESA and HNR studies. Nonetheless, the study design, data collection, the duration of follow-up, and event adjudication process of these 2 studies were very similar. Missing data, although rare, was handled by imputation in these analyses. Sensitivity analysis showed that the imputation had no effect on the point estimates and the associations reported in this study. External validation of this MESA-HNR diabetes CHD risk estimator is still needed before it can applied for CHD risk assessment to the 366 million people worldwide with T2DM.
In conclusion, the MESA-HNR diabetes CHD risk score, which incorporates CAC, had better discriminative ability for incident CHD in T2DM compared with the FRS, UKPDS, or the ATPIII/NCEP risk approach. However, a significant proportion of T2DM participants who had CHD events were misclassified by the MESA-HNR diabetes CHD risk score. This misclassification, coupled with the current cost and safety profile of statins, may make the ATP III/NCEP recommendation a more attractive and cost-effective approach to CHD risk assessment in T2DM. This question requires additional study.
Supplementary Material
Acknowledgement
The authors thank the investigators, the staff, and the participants of MESA and Heinz Nixdorf Recall studies for their valuable contributions. We also want to thank Karen P. Klein MS for editing this paper. This research was supported by contracts N01-HC-95159 through N01-HC-95167 and Diversity Supplement R01HL098445 (PI: J. Jeffrey Carr). The Heinz Nixdorf Foundation and a grant from the German Foundation of Research (DFG). Dr. Delaney and Ms. Nance were also supported by R01 HL 103729-01A1. A full list of participating MESA and HNR investigators and institutions can be found at http://www.mesa-nhlbi.org and http://www.uk-essen.de/recall-studie/.
Joseph Yeboah Joseph A Delaney, Robin Nance and Raimund Erbel had complete access to the data.
Joseph Yeboah: writing of MESA proposal, acting as the principal investigator, statistical analysis and manuscript preparation
Joseph A Delaney: Statistical analysis and manuscript preparation
Alain Bertoni: critical expertise, data gathering, editing
David Herrington: data gathering, statistical analysis, manuscript preparation and scientific content.
Raimund Erbel: critical expertise, data gathering, manuscript preparation
Mathew Budoff: critical expertise, data gathering, editing
Susanne Moebus: data gathering, manuscript preparation
Karl-Heinz Jöckel: data gathering, manuscript preparation
Gregory L Burke: data gathering, manuscript preparation
Nathan D Wong: data gathering, manuscript preparation
Nils Lehmann: data gathering, critical expertise
Stefan Möhlenkamp: critical expertise, data gathering, editing
Philip Greenland: data gathering, manuscript preparation and scientific content.
Role of the Sponsors: The NHLBI participated in the design and conduct of MESA. A member of the NIH staff served as a coauthor and had input into the collection, management, analysis, and interpretation of the data and in preparation of the manuscript, as did the other coauthors. Although members of the NHLBI staff were able to view the manuscript prior to submission, they did not participate in the decision to submit the manuscript or approve it prior to publication. The National Center for Research Resources had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure: None
References
- 1.Stevens RJ, Kothari V, Adler AI, Stratton IM. The UKPDS Risk Engine: a model for the risk of coronary heart disease in type 2 diabetes: United Kingdom Prospective Diabetes Study (UKPDS) Group. Clinical Science. 2001;101:671–679. [PubMed] [Google Scholar]
- 2.D'Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P, CHD Risk Prediction Group Validation of the Framingham coronary heart disease prediction scores. Results of a multiple ethnic group investigation. JAMA. 2001;286:180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 3.National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 4.The fifth joint task force of the European society of cardiology and the other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). European Guidelines of Cardiovascular disease prevention in clinical practice (version 2012) Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 5.Chamnan P, Simmons RK, Sharp SJ, Griffin SJ, Wareham NJ. Cardiovascular risk assessment scores for people with diabetes: a systemic review. Diabetologia. 2009;52:2001–2004. doi: 10.1007/s00125-009-1454-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Dieren S, Beulens JW, Kengne AP, Peelen LM, Rutten GE, Woodward M, Van der Schouw YT, Moons KG. Prediction models for the risk of cardiovascular disease in patients with type 2 diabetes: a systemic review. Heart. 2012;98:360–9. doi: 10.1136/heartjnl-2011-300734. [DOI] [PubMed] [Google Scholar]
- 7.Yeboah J, McClleland RL, Polonsky TS, Burke GL, Sibley CT, O'Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate risk individuals. JAMA. 2012;308:788–95. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kavousi M, Elias-Smale S, Rutten JH, Leening MJ, Vliegenthart R, Verwoert GC, Krestin GP, Oudkerk M, de Maat MP, Leebeek FW, Mattace-Raso FU, Lindemans J, Hofman A, Steyerberg EW, van der Lugt A, van den Meiracker AH, Witteman JC. Evaluation of newer risk markers for coronary heart disease risk classification: a cohort study. Ann Intern Med. 2012;156:438–44. doi: 10.7326/0003-4819-156-6-201203200-00006. [DOI] [PubMed] [Google Scholar]
- 9.Erbel R, Möhlenkamp S, Moebus S, Schmermund A, Lehmann N, Stang A, Dragano N, Grönemeyer D, Seibel R, Kälsch H, Bröcker-Preuss M, Mann K, Siegrist J, Jöckel KH, Heinz Nixdorf Recall study investigative group Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis. J Am Coll Cardiol. 2010;56:1397–406. doi: 10.1016/j.jacc.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 10.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 11.Stang A, Moebus S, Dragano N, Beck EM, Möhlenkamp S, Schmermund A, Siegrist J, Erbel R, Jöckel KH, on behalf of the Heinz Nixdorf Study Investigative Group Baseline recruitment and analyses of non-response of the Heinz Nixdorf Recall Study: Identifiability of phone numbers as the major determinant of response. Eur J Epidemiol. 2005;20:489–496. doi: 10.1007/s10654-005-5529-z. [DOI] [PubMed] [Google Scholar]
- 12.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 13.Erbel R, Möhlenkamp S, Lehmann N, Schmermund A, Moebus S, Stang A, Gronemeyer D, Seibel R, Mann K, Volbracht L, Dragano N, Siegrist J, Jöckel KH, on behalf of the Heinz Nixdorf Recall Study Investigative Group Sex related cardiovascular risk stratification based on quantification of atherosclerosis and inflammation. Atherosclerosis. 2008;197:662–672. doi: 10.1016/j.atherosclerosis.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 14.Criqui MH, McClelland RL, McDermott MM, Allison MA, Blumenthal RS, Aboyans V, Ix JH, Burke GL, Liu K, Shea S. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010;56:1506–12. doi: 10.1016/j.jacc.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukamal KJ, Moebus S, Polak JF, Dragano N, Budoff MJ, Erbel R, McClelland RL, Multi-Ethnic Study of Atherosclerosis and the Investigator Group of the Heinz Nixdorf Recall Study Comparison of Factors Associated with Carotid Intima-Media Thickness in the Multi-Ethnic Study of Atherosclerosis (MESA) and the Heinz Nixdorf Recall Study (HNR) J Am Soc Echocardiogr. 2013;26:667–73. doi: 10.1016/j.echo.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polak JF, Pencina MJ, O'Leary DH, D'Agostino RB. Common carotid artery intima-media thickness progression as a predictor of stroke in multi-ethnic study of atherosclerosis. Stroke. 2011;42:3017–21. doi: 10.1161/STROKEAHA.111.625186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr JJ, Nelson JC, Wong ND, McNitt- Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 18.Schmermund A, Möhlenkamp S, Stang A, Gronemeyer D, Seibel R, Hirche H, Mann K, Siffert W, Lauterbach K, Siegrist J, Jöckel KH, Erbel R. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: Rationale and design of the Heinz Nixdorf RECALL Study. Am Heart J. 2002;144:212–218. doi: 10.1067/mhj.2002.123579. [DOI] [PubMed] [Google Scholar]
- 19.Schmermund A, Möhlenkamp S, Berenbein S, Pump H, Moebus S, Roggenbuck U, Stang A, Seibel R, Gronemeyer D, Jöckel KH, Erbel R. Population-based assessment of subclinical coronary atherosclerosis using electron-beam computed tomography. Atherosclerosis. 2006;185:177–182. doi: 10.1016/j.atherosclerosis.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 21.Erbel R, Delaney JA, Lehmann N, McClelland RL, Möhlenkamp S, Kronmal RA, Schmermund A, Moebus S, Dragano N, Stang A, Jöckel KH, Budoff MJ, Multi-Ethnic Study of Atherosclerosis. Investigator Group of the Heinz Nixdorf Recall Study Signs of subclinical coronary atherosclerosis in relation to risk factor distribution in the Multi-Ethnic Study of Atherosclerosis (MESA) and the Heinz Nixdorf Recall Study (HNR) Eur Heart J. 2008;29:2782–91. doi: 10.1093/eurheartj/ehn439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raftery AE. Bayesian model selection in social research (with Discussion) Sociol Methodol. 1995;25:111–196. [Google Scholar]
- 23.Raftery AE, Madigan D, Hoeting JA. Bayesian model averaging for regression models. J Am Stat Assoc. 1997;92:179–191. [Google Scholar]
- 24.Volinsky CT, Madigan D, Raftery AE, Kronmal RA. Bayesian model averaging in proportional hazard models: assessing stroke risk. J R Stat Soc C. 1997;46:433–448. [Google Scholar]
- 25.Delaney JAC, Kronmal R, Wanke C, Currier J, Scherzer R, Biggs ML, Shlipak M, Polak J, O'Leary D, Bacchetti P, Grunfeld C. Predictors of increased Carotid Intima-Medial Thickness in HIV infected patients: the Study of Fat Redistribution and Metabolic Change in HIV Infection. AIDS. 2010;24:2201–9. [Google Scholar]
- 26.Moons KG, Kengne AP, Woodward M, Royston P, Vergouwe Y, Altman DG, Grobbee DE. Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart. 2012;98:683–90. doi: 10.1136/heartjnl-2011-301246. [DOI] [PubMed] [Google Scholar]
- 27.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson J, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW. ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;2013 doi: 10.1016/j.jacc.2013.11.005. S0735-1097(13)06031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guzder RN, Gatling W, Mullee MA, Mehta RL, Byrne CD. Prognostic valve of Framingham cardiovascular risk equation and the UKPDS risk engine for coronary heart disease in newly diagnosed type 2 diabetes: results from UK study. Diabet Med. 2005;22:554–62. doi: 10.1111/j.1464-5491.2005.01494.x. [DOI] [PubMed] [Google Scholar]
- 29.Davis WA, Colaquiri S, Davis TM. Comparison of the Framingham and the UKPDS cardiovascular risk equations in Australian patients with type 2 diabetes mellitus from the Frementle diabetes study. Med. J. Aust. 2009;190:180–4. doi: 10.5694/j.1326-5377.2009.tb02684.x. [DOI] [PubMed] [Google Scholar]
- 30.Van Dieren S, Peelen LM, Noltlings U, Van der Schouw YT, Rutten GEHM, Spijkerman AMW, Van der AL, Sluik D, Boeing H, Moons KGM, Beulens JWJ. External validation of the UK. Prospective Diabetes Study (UKDPS) risk engine in patients with type 2 diabetes. Diabetologia. 2011;54:264–270. doi: 10.1007/s00125-010-1960-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kengne AP, Patel A, Colagiuri S, Heller S, Hamet P, Marre M, Pan CY, Zoungas S, Grobbee DE, Neal B, Chalmers J, Woodward M, ADVANCE collaborative Group The Framingham and UK Prospective Diabetes Study (UKPDS) risk equation do not reliably estimate the probability of cardiovascular events in large ethnically diverse sample of patients: the Action in Diabetes and Vascular Disease: Preterax and Diamicron –MR controlled Evaluation (ADVANCE) study. Diabetologia. 2010;53:821–31. doi: 10.1007/s00125-010-1681-4. [DOI] [PubMed] [Google Scholar]
- 32.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH, CARDS investigators Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes mellitus in the Collaborative Atorvastatin Diabetes Study (CARDS): multicenter randomized placebo-control trial. Lancet. 2004;364:685–96. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 33.The ACCORD study group: Effects of combination Lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.