Abstract
Although influenza vaccination is recommended for all adults annually, the incidence of vaccine failure, defined as weak or absent increase in neutralizing antibody titers, is increased in the elderly compared to young adults. The T follicular helper subset of CD4 T cells (Tfh) provides B cell help in germinal centers and is necessary for class-switched antibody responses. Previous studies suggested a role for circulating T follicular helper cells (cTfh) following influenza vaccination in adults, but cTfh have not been studied in elderly adults where weak vaccine responses are often observed. Here, we studied cTfh expressing CXCR5 and Programmed Death 1 (PD-1). cTfh from elderly adults were present at reduced frequency, had decreased in vitro B cell help ability, and greater expression of inducible costimulator (ICOS) compared to young adults. At seven days after inactivated influenza vaccination, cTfh correlated with influenza vaccine-specific IgM and IgG responses in young adults but not in elderly adults. In sum, we have identified aging-related changes in cTfh that correlated with reduced influenza vaccine responses. Future rational vaccine design efforts should incorporate Tfh measurement as an immune correlate of protection, particularly in the setting of aging.
Keywords: T follicular helper, geriatric, influenza vaccine, immunosenescence
Introduction
Annual vaccination against influenza A is recommended for all individuals over six months of age, but vaccine efficacy for the inactivated vaccine in young, healthy adults is only 60%, depending on the year (1). For adults over age 65, vaccine efficacy may be 0–60% (2–4). Moreover, 90% of annual influenza-related deaths occur among adults over age 65 (5). Thus, there is a strong rationale for improved influenza vaccines for use in the elderly. The etiology of poorer vaccine effectiveness in the elderly as compared to younger adults is multifactorial (6–8). For influenza vaccine, the primary correlate of protection is the development of strain-specific neutralizing antibodies (9–11). A variety of defects in antibody production have been identified in the elderly including reduced antibody diversity (12), reduced numbers of vaccine-induced plasmablasts (13), and abnormal activity of antigen-presenting cells (14). These factors together result in weak vaccine responses in a population already at high risk for morbidity and mortality.
To produce high-affinity, class-switched antibodies, B cells in germinal centers require help from the lymphoid T follicular helper (Tfh) subset of CD4 T cells (15). This subset was first identified in human tonsillar tissue and peripheral blood based on the chemokine receptor CXCR5 (16). Expression of CXCR5 increases after stimulation of naïve cells which likely permits trafficking of these cells to the B cell zones of lymphoid tissue (17, 18). Tfh interact with other cells via a number of different mechanisms including Programmed Death-1 (PD-1), SLAM-associated protein (SAP), cytokine receptors, costimulatory molecules, and others (19–25). Absence of Tfh leads to immune dysregulation and a failure to develop memory B cells (19, 26–28). In the setting of HIV infection, increased Tfh activity leads to dysregulated antibody production (29) and contributes to poor vaccine-induced IgG responses (30). Since nearly all clinically available vaccines rely on antibody production for maximal effect, generation of balanced Tfh responses will be critical to improved vaccines.
Recent efforts have focused on evaluating circulating Tfh-like cells (cTfh). A subset of circulating memory CD4 T cells was identified that expressed CXCR5, had the ability to provide B cell help in vitro, and was expanded in the setting of chronic inflammatory conditions (31–35). However, circulating CD4+CXCR5+ cells lacked increased Bcl-6 expression, a marker of germinal center Tfh, and were different from lymphoid Tfh in microarray analyses (31, 33). A subset of these, identified as CD4+CXCR5+CXCR3+CCR6−, expressed PD-1 but not Bcl-6, responded to influenza vaccination, and correlated with neutralizing antibody responses in adults but not children (36). Recently, Locci et al. identified CXCR5+CXCR3−PD-1+ cells as being highly similar to lymphoid Tfh; these cells correlated with neutralizing antibody production against HIV (37). In contrast, Spensieri et al. identified an expanded subset of CD4+ cells 21 days post-influenza vaccination that expressed IL-21 and PD-1 after in vitro stimulation, lacked CXCR5 expression, and correlated with neutralizing antibody titers (38). Thus, the precise definition of cTfh continues to evolve in human studies. Understanding the relationship of these cells to different vaccine outcomes between young and elderly individuals holds promise for improving vaccines against pathogens such as influenza.
Given the deficiencies in influenza vaccine responses observed in the setting of aging, we hypothesized that abnormal cTfh responses in the elderly may underlie poor vaccine responses when compared to young adults. Here, we found that the elderly have 35% fewer cTfh and these cells expressed greater levels of ICOS compared to cells from young adults. Moreover, we observed that cTfh from the elderly had a per-cell decrease in functional ability to help B cells, compared to cTfh from young adults. Influenza vaccination induces a clear increase in ICOS expression in cTfh from young adults but only a weak increase in the elderly, suggesting defects in vaccine-induced Tfh activation. This change in ICOS is predictive of vaccine-induced IgM and IgG responses in young adults but not elderly adults. In summary, we identify aging-related changes in cTfh that are associated with reduced influenza vaccine-induced antibody responses. Future studies of cTfh as a T cell-based immunological correlate of protection are warranted.
Materials and Methods
Human subjects for Cohort 1
In the Fall of 2012, study subjects were recruited and consented at the Clinical Research Unit at Duke University Medical Center (Durham, NC, USA), in accordance with the Institutional Review Boards of both Duke University and the University of Pennsylvania (Philadelphia, PA, USA). Subjects were classified as young (30–40 years of age) or elderly (65 years of age or older). Subjects were excluded if they had contraindications to influenza vaccine, active substance abuse, HIV/AIDS, clinically active malignancy, immunomodulatory medication need (i.e. chemotherapy, corticosteroids), active intercurrent illness (i.e. active respiratory tract infections), or were homebound. All participants gave written informed consent prior to enrollment. Demographic data was collected as part of the study. Seasonal trivalent influenza vaccine (Fluvarix, GlaxoSmithKline) was administered and peripheral venous blood was drawn on days 0, 7, and 14 after vaccination. Blood was collected into heparinized tubes and shipped overnight to Philadelphia, PA.
Human subjects for Cohort 2
Study subjects were recruited and consented at the Louis Stokes Veterans Affairs Medical Center medical clinics or Case Western Reserve University in Cleveland, Ohio, in accordance with the Institutional Review Boards of the Veterans Affairs and Case Western Reserve University. Subjects were excluded if they had contraindications to influenza vaccination, HIV/AIDS, recent or current illness requiring hospitalization, immunomodulatory medication need (i.e. chemotherapy, corticosteroids), or active intercurrent illness. Demographic data was collected at baseline on all subjects. Peripheral venous blood was drawn into heparinized tubes for peripheral blood mononuclear cell (PBMC) isolation.
Flow cytometry
PBMC and plasma were isolated using Ficoll-Paque PLUS (GE Healthcare), rested overnight at 37°C in RPMI 1640 containing 10% FCS, and stained for surface and intracellular markers. The following antibody conjugates were used in Cohort 1: Violet and Aqua LIVE/DEAD (Invitrogen); CCR7-Pacific Blue (G043H7, Biolegend); CD14-V500, CD16-V500, CD19-V500 and CD19-APC-Cy7 (BD Biosciences); CD4-Qdot 655 (BD Biosciences); CD4-eFluor650 (eBiosciences); PD-1-BV785 (EH12.2H7, Biolegend); CD126-PE (BD Biosciences); CD62L-PE-TexasRed (BD Biosciences); CD38-PE-Cy5 (HIT2, BD Biosciences); CXCR4-PE-Cy5 (12G5, Biolegend); CD4-PE-Cy5.5 (Invitrogen); CD45RA-PE-Cy5.5 (Invitrogen); ICOS-PE-Cy7 (C398.4a, Biolegend); CXCR5-Alexa Fluor 647 (RF8B2, BD Biosciences); CD8-APC-eFluor780 (eBiosciences); and CD3-Qdot 585 (custom conjugated). Permeabilization was performed using the Foxp3 Fixation/Permeabilization Concentrate and Diluent kit (eBioscience) and intracellular stains were done with Bcl6-PerCP-eFluor710 (BCL-UP, eBioscience); and Ki67-FITC (BD Bioscience). For Cohort 2, additional conjugates used included PD-1-BV421 (Biolegend); CCR7-BV711 (Biolegend); CD3-BV570 (Biolegend); CD8-Qdot 605 (Invitrogen); CD27-Qdot 655 (Invitrogen); CXCR5-Alexa Fluor 488 (BD Biosciences); CD45RO-PE-TexasRed (Beckman Coulter); CD14-PE-Cy5 (Invitrogen); and CD16-PE-Cy5 (Biolegend). Permeabilization was performed using the Cytofix/Cytoperm kit (BD Biosciences). ICOS clone comparison was performed against ICOS-APC-eFluor 780 (ISA-3, eBioscience). Cells were resuspended in 1% para-formaldehyde until acquisition on a BD Biosciences LSR II cytometer and analyzed using FlowJo (Tree Star). Fluorescence-minus-one controls were performed in pilot studies.
IL-21 by flow cytometry
PBMC were stimulated for six hours in the presence of 1 μg/mL Staphylococcal enterotoxin B (SEB) or left unstimulated as a control. Brefeldin A was added for the final five hours of stimulation. Staining was performed at 37°C in the stimulation medium followed by fixation with 1% para-formaldehyde for 10 minutes at room temperature. Cells were then permeabilized with the Foxp3 Fixation/Permeabilization Concentrate and Diluent kit and intracellular staining with IL-21 (3A3-N2, eBioscience) was performed for one hour at room temperature.
RT-qPCR
Sorted PBMC were lysed and RNA isolated using the RNeasy Mini Kit (Qiagen) followed by reverse transcription (Applied Biosystems). Quantitative PCR was performed using the Taqman Universal PCR Master Mix (Roche) and predesigned primers and hydrolysis probes from IDT (Coralville, IA) for BCL6, BTLA, CD200, SH2D1A, and IL21. Data were normalized against the reference gene β2M and were expressed as fold-change compared to naïve.
B cell co-culture assays
Sorted CD4 T cell subsets were plated with either autologous (sorted as CD3−CD19+IgD+CD27−CD38−) or allogenic naïve B cells (Miltenyi Naïve B cell Isolation Kit) in a 1:1 ratio and co-cultured for 7 days in the presence or absence of 0.1 μg/mL Staphylococcal enterotoxin B or F (Toxin Technology). Cells were analyzed by flow cytometry as above. Supernatants were analyzed by ELISA. Briefly, Nunc Maxisorp™ plates were coated with goat anti-human IgG+IgM (Jackson Immunoresearch) or goat anti-human IgM or IgG (Abcam and Bethyl Labs, respectively). Bound antibodies were detected by biotinylated anti-human IgM or IgG (Jackson Immunoresearch) or horseradish peroxidase-conjugated mouse anti-human IgM or IgG (Southern Biotech and Bethyl Labs, respectively).
Antibody responses
The two influenza A vaccine strains of the 2012/2013 seasonal influenza vaccine, A/California/7/2009 (H1N1)pdm09-like virus and A/Victoria/361/2011 (H3N2)-like virus, were obtained from the Centers for Disease Control (Atlanta, GA). Assays to detect hemagglutinin inhibitory titers and microneutralization titers were performed (39). Infectious virus was used for neutralizing antibody assays or inactivated by β-propionolactone for H1N1/California- and H3N2/Victoria-specific binding antibody ELISA assays. Nunc Maxisorp™ plates were coated with 10 μg/mL of influenza A/H1N1/California and A/H3N2/Victoria virus along with isotype standards for IgA1, IgG and IgM (Athens Research & Technology) in bicarbonate buffer overnight at 4°C. Plates were blocked with 3% BSA in PBS and incubated with heat-inactivated sera of young and elderly subjects. Antibodies were detected using alkaline phosphatase-conjugated mouse anti-human IgA1, IgG, and IgM (Southern Biotech).
Statistics
Statistical analyses were performed with Excel (Microsoft) and Prism (GraphPad). Data was compared using Student’s t test, paired t test, or one-way Analysis of Variance (ANOVA) with Tukey post-hoc analysis.
Results
Circulating Tfh-like cells are similar to lymphoid Tfh cells
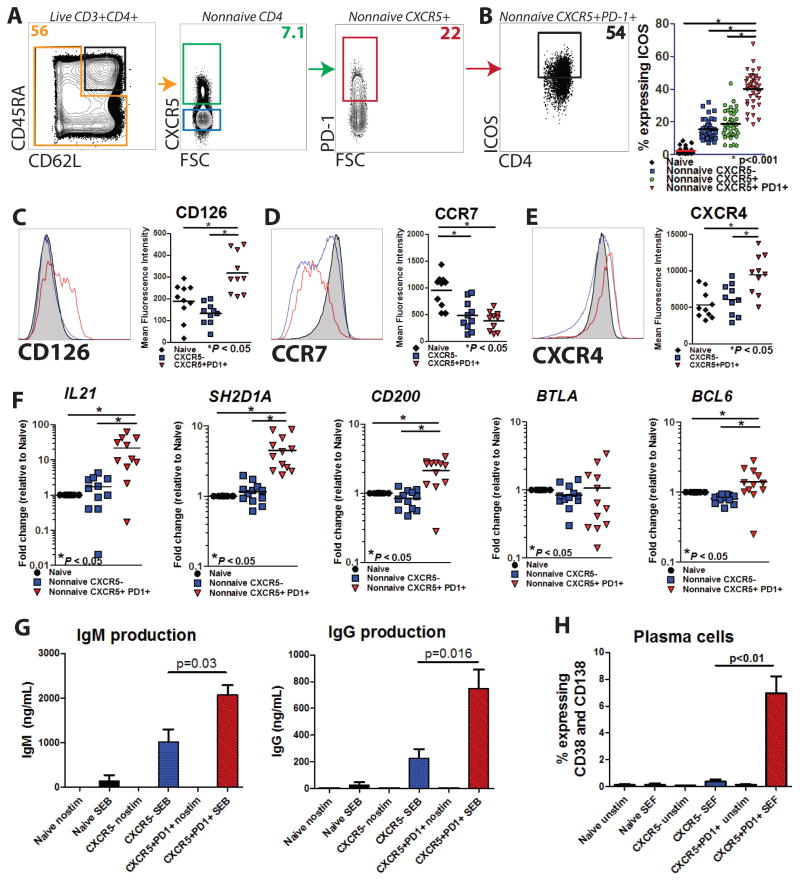
Lymphoid T follicular helper cells express many markers including CXCR5, ICOS, PD-1, SAP, Bcl-6, and others (15, 25). Given the importance of ICOS expression for Tfh function (27, 28, 33, 40), we defined cTfh cells as non-naive CD4+CXCR5+PD-1+ based on previous work (41, 42) and observed increased expression of ICOS compared to other subsets (Figure 1A–B and Supplementary Figure 1A–F). To further probe the relationship between cTfh cells and previously described lymphoid Tfh (25), we undertook extended phenotyping for additional Tfh markers. cTfh cells demonstrated increased expression of CD126 (IL-6Rα, Figure 1C), decreased expression of CCR7 (Figure 1D), and increased expression of CXCR4 (Figure 1E) compared to naïve cells, all of which were consistent with prior studies of lymphoid Tfh (25). Furthermore, cTfh cells demonstrated greater expression of CD126 and CXCR4 than non-naive CXCR5− cells. These data suggested high phenotypic similarity between lymphoid Tfh and cTfh cells.
Figure 1. Circulating Tfh cells resemble lymphoid Tfh by phenotype and function.
A. Circulating Tfh (cTfh) were identified among non-naive CD4+ cells (orange box, gated on live CD3+CD4+ cells) among PBMCs from young and elderly subjects as CXCR5+PD-1+ (red box). Naïve cells (black box, left panel) and non-naive CXCR5− cells (blue box, second panel) are also indicated. B. ICOS expression of cTfh cells was measured for different subsets of CD4+ cells (n=63). Expression of IL-6Rα(CD126; C), CCR7 (CD197; D), and CXCR4 (CD184; E) in the CD4+ subsets were measured by flow cytometry (n=10–11). F. Analysis of mRNA of additional Tfh genes as measured by RT-qPCR for the genes indicated (n=10–11) for sorted naïve (black), non-naive CXCR5− (blue), and cTfh (red) cells for combined young and elderly subjects are shown. G. Autologous naïve B cells were sorted and co-cultured with sorted naïve CD4+ cells (gated as CD45RA+CD62L+), non-naive memory CXCR5− cells, or cTfh cells from young subjects in the presence of SEB for seven days, followed by analysis of total IgM and IgG levels by ELISA. H. Plasma cell (CD19+CD38+CD138+) differentiation after co-culture with naïve (black), non-naïve memory CXCR5−(blue), or cTfh (red) and autologous naïve B cells in the presence of SEF for seven days was assessed by flow cytometry. Data are shown as frequency of CD38-CD138 co-expression among all live cells. Mean and SEM from 3 independent experiments are shown. Not all statistically significant findings are shown.
To further interrogate this relationship, transcriptional analysis was performed on sorted CD4 T cell subsets. Analysis by RT-qPCR demonstrated increased transcripts for IL21, SH2D1A (SAP), and CD200, but not BTLA compared to naïve CD4 cells (Figure 1F). We further confirmed that CD200 protein expression was higher in cTfh than in non-naïve CXCR5− cells (p<0.001) at baseline, and that BTLA protein expression was not different between CD4 subsets (data not shown). Interestingly, Bcl6 protein expression was not different between cTfh cells and CXCR5− non-naive cells by flow cytometry (data not shown) but cTfh had two-fold higher BCL6 mRNA expression than naïve CD4+ cells.
cTfh have an intrinsic ability to provide B cell help in vitro, despite lower Bcl6 protein expression than their lymphoid counterparts (31, 32, 36, 37). Co-culture of sorted autologous naïve B cells with sorted cTfh, non-naive CXCR5−, or naive cells for 7 days was performed in the presence of superantigen. Co-culture with cTfh resulted in greater production of IgM and IgG in the supernatant (Figure 1G) and greater B cell differentiation to plasma cells (Figure 1H and Supplementary Figure 1G) compared to co-culture with naïve or non-naive CXCR5− CD4+ cells. Together, these data suggest a strong resemblance between circulating cTfh cells and lymphoid Tfh cells based on phenotype, transcriptional analysis, and functional ability.
cTfh from elderly subjects demonstrate reduced circulating frequency and increased expression of ICOS
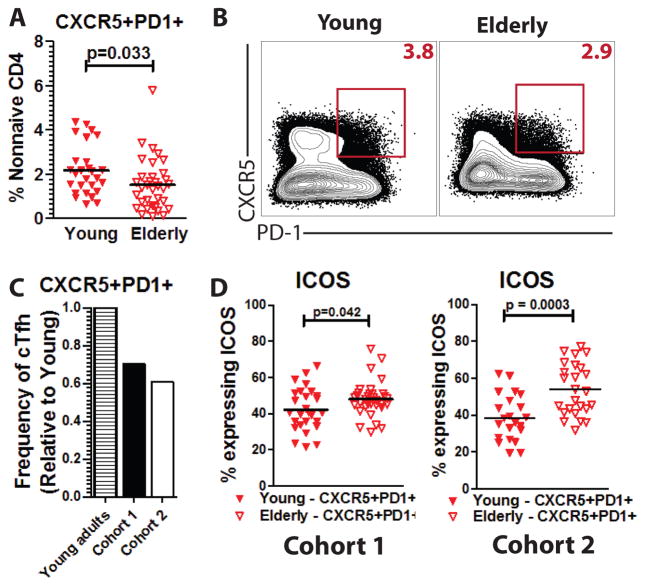
We hypothesized that differences in cTfh between young and elderly adults might contribute to weaker vaccine responses often seen in the elderly. To test this, we assessed cTfh frequency at baseline in a cohort (Cohort 1) of young (ages 30–40, n=28) and elderly (ages 66–88, n=35) adults (Supplementary Table 1). An average of 2.18% of non-naive CD4+ T cells were CXCR5+PD-1+ in the young adult cohort whereas only 1.12% were CXCR5+PD-1+ in the non-naive CD4+ T cells of the elderly (p=0.033) (Figure 2A–B).
Figure 2. cTfh from elderly are reduced in frequency and express more ICOS than cTfh from young adults.
A. Frequency of cTfh among the total non-naive CD4+ pool at baseline is shown for young (n=28, filled triangles) and elderly adults (n=35, open triangles). B. Representative plots are shown for cTfh from one young and one elderly adult (red box). Frequency of cTfh in non-naïve CD4+ pool (red number) is shown. C. A ratio of elderly-to-young adult cTfh frequency is shown for Cohort 1 (elderly, n=35) and Cohort 2 (elderly, n=26). D. Expression of ICOS was measured by flow cytometry for cTfh cells from young (filled triangles) and elderly adults (open triangles) in Cohort 1 and Cohort 2.
We then examined cTfh in a second, independently-recruited cohort (Cohort 2) of young (n=21, ages 22–54) and elderly (n=26, ages 63–90) adults (Supplementary Table 1). Once again, there were fewer circulating cTfh in the elderly compared to young adults (p=0.016, Supplementary Figure 2A). Based on the combined cohorts, elderly adults had a 35% reduction in cTfh frequency compared to young adults (Figure 2C).
To determine whether there might be other differences between young and elderly adults, we assessed expression of ICOS in cTfh. Interestingly, cTfh from elderly adults had greater baseline expression of ICOS in both Cohorts 1 and 2 (p=0.042 and p=0.0003, respectively). Thus, we found fewer circulating cTfh in the elderly compared to young adults, but cTfh from the elderly expressed more ICOS.
cTfh strongly co-express Ki67 and CD38 but have reduced functional ability in the elderly
To address whether reduced proliferation could explain the reduced frequency of circulating Tfh in elderly adults, we measured co-expression of Ki67 and CD38, which have been previously used to identify proliferating cells after vaccination (43–45). Non-naive CXCR5− cells had weak co-expression of these markers, whereas cTfh showed much greater co-expression of Ki67 and CD38 (Figure 3A and 3B, Supplementary Figure 2C). Furthermore, ICOS+-cTfh had even greater expression of Ki67 and CD38. There was no apparent difference in expression of Ki67 and CD38 in cTfh or ICOS+-cTfh cells between young and elderly adults in the steady state, however (Figure 3B).
Figure 3. cTfh express Ki67 and CD38 but demonstrate reduced B cell help ability with aging.
A. From PBMCs, co-expression of Ki67 and CD38hi for one representative subject for non-naïve CXCR5−(blue box), cTfh (red box), or ICOS+ cTfh cells (purple box) is shown. B. Summary plots for Ki67+CD38hi co-expression among all PBMCs for combined young and elderly adults (left panel, n=23) are shown. Data are further stratified by young (n=8, filled squares) and elderly adults (n=15, open squares) for cTfh (middle panel) and ICOS+ cTfh (right panel). C. Allogeneic naïve B cells from a young adult subject were sorted and co-cultured with sorted naïve or cTfh cells from young (n=16, filled squares) and elderly (n=17, open squares) adults in the presence of SEB for 7 days. IgM and IgG production was measured in the supernatant by ELISA. D. IL-21 production in cTfh is shown for one representative young and elderly donor following 6-hour stimulation with SEB in vitro. E. IL-21 production in CD4 T cell subsets is shown for young (n=6) and elderly (n=5) subjects. Not all statistically significant findings are shown.
To determine whether functional ability of cTfh differed in young and elderly adults, sorted allogeneic naïve B cells (>90% IgD+IgM+) from a young subject were co-cultured with sorted cTfh cells from young or elderly subjects in the presence of SEB for 7 days. Measurement of IgM and IgG production in the supernatant by ELISA revealed similar IgM levels in cultures containing cTfh from either young or elderly subjects; however, IgG levels were decreased in cultures containing cTfh from the elderly, compared to cTfh from young subjects (Figure 3C). In the absence of SEB, IgG production in cultures with cTfh from the young compared to the elderly was substantially lower, but not different between the groups (112 ng/mL and 251 ng/mL, respectively; p=0.31, data not shown). Thus, cTfh from the elderly demonstrated reduced B cell help ability on a per-cell basis, compared to cTfh from young adults.
Since IL-21 production is a key property of Tfh, we asked whether cTfh from the young and elderly were equally capable of making IL-21 protein. After six hours of SEB stimulation, cTfh produced more IL-21 than memory CXCR5− CD4 cells (Figures 3D–E). However, we did not observe any difference between cTfh from young or elderly subjects in IL-21 expression (p=0.54) or in mean fluorescence intensity (Supplementary Figure 2B) in a representative subgroup of subjects. This result suggests that despite a numerical reduction, cTfh from the elderly retain the ability to produce this important B cell help cytokine.
To determine whether alteration of CD4 T cell responses to SEB with age (e.g. perhaps due to age-related TCR repertoire changes) could explain the reduced B cell help provided by cTfh from elderly subjects, PBMC were stimulated with SEB, SEF, or PMA/ionomycin in vitro for 6 hours and assayed by flow cytometry. No difference in the frequency of CD4+IFNγ+ cells was observed between young and elderly subjects for any stimulation condition (data not shown), suggesting an equal proportion of CD4+ T cells in young and elderly are capable of responding to superantigen stimulation by producing IFNγ.
Influenza vaccine induces increased ICOS expression in cTfh in the young but not elderly
We were next interested in whether the differences in cTfh frequency and functional ability described above would result in altered cTfh responses to influenza vaccination. Subjects from Cohort 1 (28 young and 35 elderly subjects) were given seasonal trivalent inactivated influenza vaccine and followed by serial phlebotomy on days 0, 7, and 14. Samples were analyzed by flow cytometry for cTfh responses (Figure 4A–B).
Figure 4. After influenza vaccination, cTfh show increased ICOS expression in young but not elderly adults.
A–B. Influenza vaccine was administered to young (n=28) and elderly (n=35) adults followed by serial phlebotomy. cTfh were measured by flow cytometry. Plots indicate CD38 vs ICOS expression of cTfh for one young (A) and one elderly (B) subject at days 0 and 7 after vaccination. Red numbers indicate frequency of ICOS+CD38hi-cTfh among non-naive CD4+ T cells for each time-point. Frequency of cTfh after influenza vaccination in young adults (n=28; C) and elderly adults (n=35; F) are shown. ICOS expression in cTfh cells from young adults (D) and elderly adults (G) are shown. Co-expression of ICOS and CD38 in cTfh cells from young adults (E) and elderly adults (H) are shown. Co-expression of Ki67 and CD38 in cTfh is shown for one representative young (I) and elderly (J) subject at days 0 and 7. Summary plots of co-expression of Ki67 and CD38 in cTfh (K) and in ICOS+ cTfh (L) cells are shown. p values indicate results of paired t test analyses comparing days 0 and 7.
Similar to a prior study (36), there was no change in circulating cTfh frequency after vaccination in young or elderly adults (Figure 4C and 4F). However, ICOS expression on cTfh increased in the young (p=0.0031) (Figure 4A and 4D), but only showed a trend towards increase in the elderly (p=0.094, Figure 4B and 4G). In a subset of subjects, there was a stronger trend toward increased ICOS+CD38hi in the young than in the elderly (Figure 4A, 4B, 4E, 4H). We then assessed whether there was a difference between young and elderly in proliferation following vaccination based on Ki67+CD38hi co-expression. We observed increased co-expression of both markers after vaccination when analyzed across the whole cohort (Figure 4I–L). Surprisingly, this increase was predominantly due to expression of Ki67+CD38hi in the elderly cTfh (Figure 4K). Analysis of ICOS+ cTfh (Figure 4L) revealed a small but statistically significant increase in co-expression of these markers in cTfh from the young (p=0.04) and a larger increase in cTfh from the elderly (p=0.011). These data suggest that cTfh from the elderly, and in particular the ICOS+ subset, had recently entered cell cycle to a greater extent than in young subjects, but this proliferation did not result in increased cTfh circulating frequency overall or in ICOS expression. In sum, cTfh from young but not elderly adults showed an increased expression of ICOS following influenza vaccination.
Change in ICOS expression in cTfh correlated with antibody production in the young but not elderly
We hypothesized that the cTfh response would predict the vaccine-induced antibody response in young but not elderly adults. Prior studies have correlated neutralizing antibodies with the Tfh response in adults (36, 46). Others have evaluated total IgM and IgG responses to influenza vaccine and observed variability depending on vaccine year (47–49). Nevertheless, evaluation of the total humoral response is important for understanding the overall impact of the cTfh response, as neutralizing antibodies likely represent a very narrow slice of the overall antibody response to vaccination.
To assess whether Tfh correlated with antibody responses, we evaluated titers of the influenza vaccine-induced IgM and IgG response in all subjects. The fold-change for influenza A strains (H1N1/Cal and H3N2/Vic) were averaged. At baseline, young and elderly adults had similar circulating levels of influenza-specific IgM and IgG antibodies (p=0.18 and p=0.58 respectively, Figure 5A–D). Following influenza vaccination, total influenza-specific IgM and IgG responses were 3.5-fold and 1.2-fold greater in young adults at day 7 compared to day 0, respectively (Figure 5A and 5B), which was similar in range to that previously reported (47, 49). Both isotypes were still slightly higher (3.0-fold and 1.2-fold, respectively) at day 14 compared to day 0. Elderly adults had a 2.38-fold increase of IgM at day 7 and 2.44-fold increase at day 14 over day 0 (Figure 5C). However, IgG in the elderly was only 1.05-fold greater at day 7 and day 14 compared to day 0 (Figure 5D). Thus, the influenza vaccine led to a robust IgM response in the young adults and a slightly weaker response in the elderly, whereas the vaccine led to a weak IgG response in the young and a nearly absent IgG response in the elderly.
Figure 5. After influenza vaccination, cTfh show increased ICOS expression in young but not elderly adults.
A–D. Total binding IgM and IgG against influenza vaccine was measured by ELISA from serum from young (n=28) and elderly (n=35) subjects at days 0, 7, and 14 after vaccination. All statistical comparisons are performed against day 0 (*p<0.05, **p<0.01, ***p<0.001). E–H. Change in total binding IgM or IgG at day 7 was plotted as a fold-change against day 0 to day 7 fold-change in ICOS expression of cTfh cells for young and elderly subjects. Pearson r and p value are shown.
We then assessed whether the peak cTfh response would correlate with the influenza-specific antibody response. Prior studies have identified a correlation between cTfh expression of ICOS and neutralizing antibody titers at day 28 (36). Microneutralization (MN) at day 14 showed a correlation between the ICOS response in cTfh in young (r=0.51, p=0.009) but not elderly (r=0.13, p=0.47) subjects (Supplementary Figure 3A), whereas hemagglutinin inhibition assays (HAI) at day 14 did not correlate with the ICOS response in cTfh in young or elderly subjects (Supplementary Figure 3B).
However, we reasoned that HAI and MN only identify a small proportion of the total antibody pool generated by influenza vaccination. We assessed whether the cTfh response would correlate with the total influenza-specific IgM and IgG response. There was no correlation between the change in circulating frequency of cTfh and the total IgM or IgG response of either cohort (data not shown) (36). However, there was a significant correlation between the change in the ICOS+-cTfh population and the total IgM and IgG responses in the young adults (Figure 5E and 5F). In contrast, there was only a weak, non-significant trend observed with IgM responses in the elderly and no correlation at all with IgG responses (Figure 5G and 5H). To exclude a later antibody response, we compared day 7 cTfh responses to day 14 antibody responses but found weaker associations in young subjects and no association in elderly subjects (Supplementary Figure 3C). Finally, we found no correlation between changes in Ki67 and CD38 co-expression and IgM or IgG antibody production in subsets of young and elderly subjects (data not shown). Together, these data suggest that deficient immune responses in the elderly, as manifested by reduced cTfh activation, could underlie decreased antibody responses.
Discussion
Understanding Tfh cells is key to developing strategies to optimize vaccine responses. We have identified clear differences in cTfh cells between young and elderly adults. There was no age-related difference in expression of CD126, CXCR4, or CCR7, nor was there any age-related difference in transcripts for IL21, SH2D1A, CD200, or BTLA (data not shown). However, at baseline, elderly adults had a 35% reduction in the circulating frequency of cTfh compared to young adults. The etiology for this decrease was not clear but does not appear to be due to reduced proliferation, based on the analysis of CD38 and Ki67 expression. While reduced in frequency, cTfh in elderly adults had higher expression of ICOS and coexpression of Ki67 and CD38 after influenza vaccination, compared to young adults. However, increased activation did not lead to strong vaccine-induced IgG antibody responses in the elderly. Moreover, direct analyses of cTfh helper function revealed a per-cell decrease in B cell help ability. That, coupled with the reduced circulating frequency in non-naïve CD4 in the elderly, could have an impact on vaccine responsiveness. In addition to reduced vaccine-induced plasmablasts (13) and abnormal antigen-presenting cell activity (14), poor cTfh activity should be considered in the multifactorial picture of vaccine nonresponses in the elderly.
ICOS is important for Tfh-B cell interactions (27, 40); increased ICOS expression coupled with reduced function suggest there may be broader, more fundamental age-related changes in cTfh. Of note, inflammation has been strongly correlated with, and may be a direct cause of, immune senescence (7). The increased ICOS expression at baseline in the elderly may also reflect chronic, nonspecific inflammation. Furthermore, IL-6, which is highly relevant to Tfh (23, 24), has been implicated as a direct inducer of senescence in other settings (50). The age-related changes in cTfh are unlikely to be gender or ethnicity-related, given the demographically different cohorts in the present study.
Despite increased expression of activation markers at baseline, cTfh from the elderly were less efficient at helping B cells in vitro on a per-cell basis. Aging-related B cell dysfunction has been described (51). To control for this issue, we used a single source of allogeneic naïve B cells from a young subject. Therefore, these B cell help assay results focus attention on changes in cTfh with age. Although the reduced ability to produce IL-21 was implicated in reduced B cell help ability of cTfh in the setting of HIV (52), we did not find the same to be true in the elderly compared to young adults. However, the kinetics and/or efficiency with which IL-21 protein or other help-related proteins such as CD40L are produced locally and made available to B cells, or the ability to form cTfh-B cell conjugates during prolonged stimulation, should be important areas for future investigation. In sum, we have identified age-related changes in circulating frequency, ICOS expression, and B cell help ability in cTfh that may have important implications for antibody generation.
In our study, we observed higher expression of ICOS at baseline using the C398.4A clone than others have previously reported using the ISA-3 clone (37, 53). The C398.4A clone is a cross-reactive clone that binds a different epitope than ISA-3 (54, 55) and is known to exhibit stronger baseline staining (Supplementary Figure 1E–F). Young and elderly samples were treated the same in all experiments. Furthermore, we did not observe differential nonspecific activation effects from the overnight rest prior to staining (Supplementary Figure 1D). Nonetheless, the ICOS clone and appropriate controls should be reported in future studies to facilitate comparisons.
In the current study, cTfh have phenotypic and functional properties that strongly resemble those of lymphoid Tfh. At baseline, cTfh demonstrated expression of CD126, ICOS, PD-1, and CXCR4, had increased mRNA transcripts for IL21, SH2D1A, and CD200, and provided B cell help activity in vitro, which are all consistent with prior studies (25, 31, 32, 36). There were, however, notable discrepancies between lymphoid Tfh and the cTfh subset. In our study, cTfh had increased mRNA transcripts for BCL6 over naive cells but not increased protein expression. Discordance between BCL6 mRNA and Bcl-6 protein expression has been previously reported (56, 57). Moreover, prior studies have not detected elevated Bcl-6 protein expression in PBMCs expressing CXCR5+ over other CD4 subsets (31, 33, 34, 36). In addition, BTLA has been reported as a lymphoid Tfh marker (25), but we did not detect elevated BTLA mRNA in cTfh cells. In a recent study, Locci et al. identified CXCR5+PD-1+CXCR3− cells as being highly similar to germinal center-Tfh based on microarray analyses; this particular subset was better than other CXCR5+ subsets in providing in vitro B cell help (37). Our studies are consistent with recent data (36, 37). We further extend the emerging definition of cTfh and provide strong evidence that cTfh defined as CD4+CXCR5+PD-1+ possess B cell help capacity. However, the precise relationship of these cTfh to lymphoid Tfh during germinal center responses has not been fully elucidated in humans. Nonetheless, our results demonstrate appropriate cTfh responses can be a useful predictor of vaccine-induced antibody responses.
New questions arise from our findings. First, prior reports demonstrated a correlation between changes in ICOS expression at day 7 in CD4+CXCR5+CXCR3+CCR6− cells with both hemagglutinin inhibition (HAI) titer and microneutralization (MN) titers measured at day 28 (36). Vaccine responses are typically assessed using day 28 titers. Less is known about HAI titers before day 28, although neutralizing antibody production was higher at 14 days after influenza vaccination compared to 7 days (58). We assessed HAI and MN titers but did not find consistent correlations with changes in ICOS expression in cTfh or circulating frequency early after vaccination, although MN titers did correlate at day 14 after vaccination. Our measurements of influenza-specific IgM and IgG are similar to a previous report at day 7 after vaccination, but these values vary depending on the vaccine strain and by year (47, 49).
Second, the importance of antigen-specificity is unknown in the cTfh response. cTfh in other studies produced few cytokines, making this approach to identify antigen-specific cells problematic (36, 37). Future studies to address antigen specificity of cTfh may require tetramer reagents or phospho-flow studies. Given the observation of preferential boosting of neutralizing antibody titers over many years (59), seasonal influenza vaccination may also preferentially boost only certain cTfh responses. However, CD4 responses may be directed against conserved internal proteins (60) whereas strong HAI responses could require hemagglutinin-specific cTfh.
Third, our data identified increased Ki67 expression in cTfh from the elderly compared to the young after vaccination. The reasons for the increased proliferation of cTfh in the elderly are unclear. Lymphoid Tfh have a limited proliferation capacity (33, 41, 61, 62), which may be due to expression of inhibitory receptors such as PD-1 (63). Paradoxically, excess proliferation in cTfh in the elderly may be evidence of dysfunction, reflecting compensation for reduced frequency, reduced diversity in the T cell receptor repertoire with aging (64), or reduced ability to provide B cell help on a per cell basis, as compared to young adults. Although differences in cTfh between young and elderly adults were observed here, it will be important to extend these observations to lymphoid tissues where help to B cells is actually delivered and neutralizing antibodies are generated.
Successful influenza vaccines are predicated on increased production of high-avidity, neutralizing antibodies. The generation of such antibodies requires the efficient cooperation of several arms of the immune response including antigen-specific B cells and Tfh. In addition to other age-related immunological changes, the ability of CD4 T cells to provide help to B cells and induce robust antibody responses may decline in older humans. These data, therefore, suggest that future vaccine studies should evaluate cTfh responses as an additional correlate of protection. Our findings highlight the importance of developing vaccine strategies that boost Tfh responses in order to improve vaccine responsiveness in the elderly.
Supplementary Material
Acknowledgments
Funding: R.S.H. and M.A.R. were supported by the National Institutes of Health (5T32 AI007632). K.E.S. was supported in part by the NIH/NIA (AG028716) Pepper OAIC. D.H.C. was supported by CSR&D VA Merit, AI36219, and AI080313. This research was supported by NIH (R01 AI076066) and U.S. BAA grant HHSN272201100018C to EJW.
Footnotes
Authorship contributions
R.S.H., D.V.D., and K.D.M. designed and performed experiments on Cohort 1. Patient accrual was performed by K.E.S. and D.H.C. M.R.B, O.Z.B, H.A., M.A.R., and D.H.C. conceived and performed experiments related to Cohort 2. R.K.K., S.K., and H.E. performed antibody assays. R.S.H. and E.J.W. conceived the overall study design and wrote the paper. All authors analyzed and interpreted data, discussed the results, and commented on the manuscript.
Disclosures of conflicts of interest
The authors declare no competing financial interests.
References
- 1.Osterholm MT, Kelley NS, Sommer A, Belongia Ea. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–69. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 3.Jackson L, Jackson ML, Phillips CH, Benoit J, Cole D, Koepel TAK, Mclean HQ, Meece JK, Strey SK, Maria E, Vandermause M, Song J, Clipper L, Kjar D, Robertson A. Interim adjusted estimates of seasonal influenza vaccine effectiveness - United States, February 2013. MMWR Morb Mortal Wkly Rep. 2013;62:119–23. [PMC free article] [PubMed] [Google Scholar]
- 4.Talbot HK, Griffin MR, Chen Q, Zhu Y, Williams JV, Edwards KM. Effectiveness of seasonal vaccine in preventing confirmed influenza-associated hospitalizations in community dwelling older adults. J Infect Dis. 2011;203:500–8. doi: 10.1093/infdis/jiq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson WW, Moore MR, Weintraub E, Cheng P-Y, Jin X, Bridges CB, Bresee JS, Shay DK. Estimating influenza-associated deaths in the United States. Am J Public Health. 2009;99(Suppl 2):S225–30. doi: 10.2105/AJPH.2008.151944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haynes L, Swain SL. Why aging T cells fail: implications for vaccination. Immunity. 2006;24:663–6. doi: 10.1016/j.immuni.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moro-García MA, Alonso-Arias R, López-Larrea C. When Aging Reaches CD4+ T-Cells: Phenotypic and Functional Changes. Front Immunol. 2013;4:107. doi: 10.3389/fimmu.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14:428–36. doi: 10.1038/ni.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Influenza (Seasonal) 2009:1–3. [Google Scholar]
- 10.McCullers JA, Huber VC. Correlates of vaccine protection from influenza and its complications. Hum Vaccin Immunother. 2012;8:34–44. doi: 10.4161/hv.8.1.18214. [DOI] [PubMed] [Google Scholar]
- 11.Ohmit SE, Petrie JG, Cross RT, Johnson E, Monto AS. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J Infect Dis. 2011;204:1879–85. doi: 10.1093/infdis/jir661. [DOI] [PubMed] [Google Scholar]
- 12.Jiang N, He J, Weinstein Ja, Penland L, Sasaki S, He X-S, Dekker CL, Zheng N-Y, Huang M, Sullivan M, Wilson PC, Greenberg HB, Davis MM, Fisher DS, Quake SR. Lineage structure of the human antibody repertoire in response to influenza vaccination. Sci Transl Med. 2013;5:171ra19. doi: 10.1126/scitranslmed.3004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki S, Sullivan M, Narvaez CF, Holmes TH, Furman D, Zheng N, Nishtala M, Wrammert J, Smith K, James JA, Dekker CL, Davis MM, Wilson PC, Greenberg HB, He X-S. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest. 2011;121:3109–19. doi: 10.1172/JCI57834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira LF, de Souza APD, Borges TJ, Bonorino C. Impaired in vivo CD4+ T cell expansion and differentiation in aged mice is not solely due to T cell defects: decreased stimulation by aged dendritic cells. Mech Ageing Dev. 2011;132:187–94. doi: 10.1016/j.mad.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–63. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 16.Schaerli P, Willimann K, Lang aB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–62. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaerli P, Loetscher P, Moser B. Cutting edge: induction of follicular homing precedes effector Th cell development. J Immunol. 2001;167:6082–6. doi: 10.4049/jimmunol.167.11.6082. [DOI] [PubMed] [Google Scholar]
- 18.Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, Cyster JG. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J Exp Med. 1999;190:1123–34. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Booth C, Gilmour KC, Veys P, Gennery AR, Slatter Ma, Chapel H, Heath PT, Steward CG, Smith O, O’Meara A, Kerrigan H, Mahlaoui N, Cavazzana-Calvo M, Fischer A, Moshous D, Blanche S, Pachlopnik Schmid J, Pachlopnick-Schmid J, Latour S, de Saint-Basile G, Albert M, Notheis G, Rieber N, Strahm B, Ritterbusch H, Lankester A, Hartwig NG, Meyts I, Plebani A, Soresina A, Finocchi A, Pignata C, Cirillo E, Bonanomi S, Peters C, Kalwak K, Pasic S, Sedlacek P, Jazbec J, Kanegane H, Nichols KE, Hanson IC, Kapoor N, Haddad E, Cowan M, Choo S, Smart J, Arkwright PD, Gaspar HB. X-linked lymphoproliferative disease due to SAP/SH2D1A deficiency: a multicenter study on the manifestations, management and outcome of the disease. Blood. 2011;117:53–62. doi: 10.1182/blood-2010-06-284935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu J, Havenar-Daughton C, Crotty S. Modulation of SAP dependent T:B cell interactions as a strategy to improve vaccination. Curr Opin Virol. 2013:1–8. doi: 10.1016/j.coviro.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroenke Ma, Eto D, Locci M, Cho M, Davidson T, Haddad EK, Crotty S. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J Immunol. 2012;188:3734–44. doi: 10.4049/jimmunol.1103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–9. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi YS, Eto D, Yang Ja, Lao C, Crotty S. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J Immunol. 2013;190:3049–53. doi: 10.4049/jimmunol.1203032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, Yusuf I, Crotty S. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One. 2011;6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, Deenick EK, Tangye SG. Early commitment of naïve human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol Cell Biol. 2009;87:590–600. doi: 10.1038/icb.2009.64. [DOI] [PubMed] [Google Scholar]
- 26.Ma CS, Pittaluga S, Avery DT, Hare NJ, Maric I, Klion AD, Nichols KE, Tangye SG. Selective generation of functional somatically mutated IgM+CD27+, but not Ig isotype-switched, memory B cells in X-linked lymphoproliferative disease. J Clin Invest. 2006;116:322–33. doi: 10.1172/JCI25720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akiba H, Takeda K, Kojima Y, Usui Y, Harada N, Yamazaki T, Ma J, Tezuka K, Yagita H, Okumura K. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J Immunol. 2005;175:2340–2348. doi: 10.4049/jimmunol.175.4.2340. [DOI] [PubMed] [Google Scholar]
- 28.Bossaller L, Burger J, Draeger R, Grimbacher B, Knoth R, Plebani A, Durandy A, Baumann U, Schlesier M, Welcher Aa, Peter HH, Warnatz K. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J Immunol. 2006;177:4927–32. doi: 10.4049/jimmunol.177.7.4927. [DOI] [PubMed] [Google Scholar]
- 29.Lindqvist M, vanLunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, Flanders MD, Cutler S, Yudanin N, Muller MI, Davis I, Farber D, Hartjen P, Haag F, Alter G, Schulze zur Wiesch J, Streeck H. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest. 2012;122:3271–80. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cubas Ra, Mudd JC, Savoye A-L, Perreau M, van Grevenynghe J, Metcalf T, Connick E, Meditz A, Freeman GJ, Abesada-Terk G, Jacobson JM, Brooks AD, Crotty S, Estes JD, Pantaleo G, Lederman MM, Haddad EK. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med. 2013;19:494–9. doi: 10.1038/nm.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chevalier N, Jarrossay D, Ho E, Avery DT, Ma CS, Yu D, Sallusto F, Tangye SG, Mackay CR. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J Immunol. 2011;186:5556–68. doi: 10.4049/jimmunol.1002828. [DOI] [PubMed] [Google Scholar]
- 32.Morita R, Schmitt N, Bentebibel S, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, Lavecchio EM, Punaro M, Pascual V, Banchereau J, Ueno H. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–21. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasheed A-U, Rahn H-P, Sallusto F, Lipp M, Müller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur J Immunol. 2006;36:1892–903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- 34.Simpson N, Gatenby Pa, Wilson A, Malik S, Fulcher Da, Tangye SG, Manku H, Vyse TJ, Roncador G, Huttley Ga, Goodnow CC, Vinuesa CG, Cook MC. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62:234–44. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 35.Christensen JR, Börnsen L, Ratzer R, Piehl F, Khademi M, Olsson T, Sørensen PS, Sellebjerg F, Romme Christensen J. Systemic inflammation in progressive multiple sclerosis involves follicular T-helper, Th17- and activated B-cells and correlates with progression. PLoS One. 2013;8:e57820. doi: 10.1371/journal.pone.0057820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bentebibel S, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, Flano E, Mejias A, Albrecht RA, Blankenship D, Xu H, Pascual V, Banchereau J, Garcia-Sastre A, Palucka AK, Ramilo O, Ueno H. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med. 2013;5:176ra32. doi: 10.1126/scitranslmed.3005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, Su LF, Cubas R, Davis MM, Sette A, Elias K, Protocol I, Investigators CP, Poignard P, Crotty S, Haddad EK. Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39:1–12. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spensieri F, Borgogni E, Zedda L, Bardelli M, Buricchi F, Volpini G, Fragapane E, Tavarini S, Finco O, Rappuoli R, Del Giudice G, Galli G, Castellino F. Human circulating influenza- CD4+ ICOS1+IL-21+ T cells expand after vaccination, exert helper function, and predict antibody responses. Proc Natl Acad Sci U S A. 2013;110:2–7. doi: 10.1073/pnas.1311998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Network S. WHO Global Influenza Surveillance Network. Manual for the laboratory diagnosis and virological surveillance of influenza. WHO Press; Geneva, Switzerland: 2011. [Google Scholar]
- 40.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, Crotty S. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–46. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perreau M, Savoye A-L, De Crignis E, Corpataux J-M, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. 2013;210:143–56. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lüthje K, Kallies A, Shimohakamada Y, Belz GT, Light A, Tarlinton DM, Nutt SL. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat Immunol. 2012;13:491–8. doi: 10.1038/ni.2261. [DOI] [PubMed] [Google Scholar]
- 43.Li X, Miao H, Henn A, Topham DJ, Wu H, Zand MS, Mosmann TR. Ki-67 expression reveals strong, transient influenza specific CD4 T cell responses after adult vaccination. Vaccine. 2012;30:4581–4. doi: 10.1016/j.vaccine.2012.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soares A, Govender L, Hughes J, Mavakla W, de Kock M, Barnard C, Pienaar B, Janse van Rensburg E, Jacobs G, Khomba G, Stone L, Abel B, Scriba TJ, Hanekom Wa. Novel application of Ki67 to quantify antigen-specific in vitro lymphoproliferation. J Immunol Methods. 2010;362:43–50. doi: 10.1016/j.jim.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, Murali-Krishna K, Mahar PL, Edupuganti S, Lalor S, Germon S, Del Rio C, Mulligan MJ, Staprans SI, Altman JD, Feinberg MB, Ahmed R. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710–22. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 46.Pallikkuth S, Parmigiani A, Silva SY, George VK, Fischl M, Pahwa R, Pahwa S. Impaired peripheral blood T-follicular helper cell function in HIV-infected nonresponders to the 2009 H1N1/09 vaccine. Blood. 2012;120:985–93. doi: 10.1182/blood-2011-12-396648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cox RJ, Brokstad Ka, Zuckerman Ma, Wood JM, Haaheim LR, Oxford JS. An early humoral immune response in peripheral blood following parenteral inactivated influenza vaccination. Vaccine. 1994;12:993–9. doi: 10.1016/0264-410x(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 48.el-Madhun aS, Cox RJ, Søreide a, Olofsson J, Haaheim LR. Systemic and mucosal immune responses in young children and adults after parenteral influenza vaccination. J Infect Dis. 1998;178:933–9. doi: 10.1086/515656. [DOI] [PubMed] [Google Scholar]
- 49.Remarque EJ, de Jong JM, van der Klis RJ, Masurel N, Ligthart GJ. Dose-dependent antibody response to influenza H1N1 vaccine component in elderly nursing home patients. Exp Gerontol. 1999;34:109–15. doi: 10.1016/s0531-5565(98)00060-6. [DOI] [PubMed] [Google Scholar]
- 50.Kuilman T, Michaloglou C, Vredeveld LCW, Douma S, van Doorn R, Desmet CJ, Aarden La, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–31. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 51.Kogut I, Scholz JL, Cancro MP, Cambier JC. B cell maintenance and function in aging. Semin Immunol. 2012;24:342–9. doi: 10.1016/j.smim.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Parmigiani A, Alcaide ML, Freguja R, Pallikkuth S, Frasca D, Fischl Ma, Pahwa S. Impaired antibody response to influenza vaccine in HIV-infected and uninfected aging women is associated with immune activation and inflammation. PLoS One. 2013;8:e79816. doi: 10.1371/journal.pone.0079816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, Sun X, Vandenberg K, Rockman S, Ding Y, Zhu L, Wei W, Wang C, Karnowski A, Belz GT, Ghali JR, Cook MC, Riminton DS, Veillette A, Schwartzberg PL, Mackay F, Brink R, Tangye SG, Vinuesa CG, Mackay CR, Li Z, Yu D. Circulating precursor CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39:770–81. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 54.Buonfiglio D, Bragardo M, Bonissoni S, Redoglia V, Cauda R, Zupo S, Burgio VL, Wolff H, Franssila K, Gaidano G, Carbone A, Janeway CA, Dianzani U. Characterization of a novel human surface molecule selectively expressed by mature thymocytes, activated T cells and subsets of T cell lymphomas. Eur J Immunol. 1999;29:2863–74. doi: 10.1002/(SICI)1521-4141(199909)29:09<2863::AID-IMMU2863>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 55.Buonfiglio D, Bragardo M, Redoglia V, Vaschetto R, Bottarel F, Bonissoni S, Bensi T, Mezzatesta C, Janeway CA, Jr, Dianzani U. The T cell activation molecule H4 and the CD28-like molecule ICOS are identical. Eur J Immunol. 2000;30:3463–7. doi: 10.1002/1521-4141(2000012)30:12<3463::AID-IMMU3463>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 56.Allman D, Jain A, Dent A, Maile RR, Selvaggi T, Kehry MR, Staudt LM. BCL-6 expression during B-cell activation. Blood. 1996;87:5257–68. [PubMed] [Google Scholar]
- 57.Niu H, Ye BH, Dalla-Favera R. Antigen receptor signaling induces MAP kinase-mediated phosphorylation and degradation of the BCL-6 transcription factor. Genes Dev. 1998;12:1953–61. doi: 10.1101/gad.12.13.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gross Pa, Russo C, Dran S, Cataruozolo P, Munk G, Lancey SC. Time to earliest peak serum antibody response to influenza vaccine in the elderly. Clin Diagn Lab Immunol. 1997;4:491–2. doi: 10.1128/cdli.4.4.491-492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller MS, Gardner TJ, Krammer F, Aguado LC, Tortorella D, Basler CF, Palese P. Neutralizing Antibodies Against Previously Encountered Influenza Virus Strains Increase over Time: A Longitudinal Analysis. Sci Transl Med. 2013;5:198ra107. doi: 10.1126/scitranslmed.3006637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roti M, Yang J, Berger D, Huston L, James EA, Kwok WW. Healthy human subjects have CD4+ T cells directed against H5N1 influenza virus. J Immunol. 2008;180:1758–68. doi: 10.4049/jimmunol.180.3.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weber JP, Fuhrmann F, Hutloff A. T-follicular helper cells survive as long-term memory cells. Eur J Immunol. 2012;42:1981–8. doi: 10.1002/eji.201242540. [DOI] [PubMed] [Google Scholar]
- 62.Marinova E, Han S, Zheng B. Human germinal center T cells are unique Th cells with high propensity for apoptosis induction. Int Immunol. 2006;18:1337–45. doi: 10.1093/intimm/dxl066. [DOI] [PubMed] [Google Scholar]
- 63.Trautmann L, Janbazian L, Chomont N, Said Ea, Gimmig S, Bessette B, Boulassel M-R, Delwart E, Sepulveda H, Balderas RS, Routy J-P, Haddad EK, Sekaly R-P. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 64.Blackman Ma, Woodland DL. The narrowing of the CD8 T cell repertoire in old age. Curr Opin Immunol. 2011;23:537–42. doi: 10.1016/j.coi.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.