Abstract
Despite improvements in obstetrical and neonatal care, and introduction of hypothermia as a neuroprotective therapy, perinatal brain injury remains a frequent cause of cerebral palsy, mental retardation and epilepsy. The recognition of dysfunction of cerebral autoregulation is essential for a real time measure of efficacy to identify those who are at highest risk for brain injury.
This article will focus on the “neurovascular unit” approach to the care of asphyxiated neonates to review 1) potential mechanisms of dysfunctional cerebral blood flow (CBF) regulation, 2) optimal monitoring methodology such as NIRS (near infrared spectroscopy), and TCD (transcutaneous Doppler), and 3) clinical implications of monitoring in the neonatal intensive care setting in asphyxiated newborns undergoing hypothermia and rewarming.
Critical knowledge of the functional regulation of the neurovascular unit may lead to improved ability to predict outcomes in real time during hypothermia, as well as differentiate nonresponders who might benefit from additional therapies.
Keywords: HIE, hypothermia, rewarming, neurodevelopmental outcomes
Neonatal hypoxic-ischemic encephalopathy (HIE) secondary to birth asphyxia remains a major public health problem that afflicts millions of newborns worldwide and may result in cerebral palsy, mental retardation, learning disabilities, and even death.1,2 Impaired cerebral blood flow is the principal culprit leading to brain injury and is likely to occur as a consequence of interruption of placental blood flow and gas exchange; a state that we will refer to as asphyxia to avoid the terminology debates of hypoxic ischemic injury (HIE) and neonatal encephalopathy (NE) . The first section of the review will focus on the physiology of cerebral autoregulation in the infant born at term in health and in the presence of HIE. The second section of the review covers the methodology, as well as clinical applications of neuromonitoring in relation to autoregulation and outcomes.
I. Mechanisms of cerebral autoregulation in the neurovascular unit
A successful transition from the fetal to the neonatal environment
CBF increases with postnatal age in parallel with increased cerebral metabolic rates and energy demands in the growing brain. There is tight coupling of brain function, metabolism and blood flow during transition from fetal to neonatal life to support normal development.13 Autoregulation of CBF refers to maintenance of constant flow over a broad range of cerebral perfusion pressures (CPP) ranging from 25-50 mmHg in newborns.14
At term neonatal CBF is 10-20ml/100gm/min and represents approximately 40% of adult values. The fetus has low oxygen tension but compensatory elevations in Hgl F, cardiac output (CO), hematocrits and 2-3DPG which promotes release of oxygen to tissue and offset the low PO2 of the fetus. The most dramatic change in CBF occurs at the time of birth. In the lamb, three fold decrease in CBF occurs in the first 24 hours after birth correlating with increased oxygen content 13.
What regulates cerebral blood flow?
The “neurovascular unit” consists of specialized endothelial cells interconnected by an elaborate network of complex tight junctions surrounded by basal lamina, astrocytes and neurons. The astrocytes that surround the microvasculature provide the cellular link to the neurons and play an active role in signal transduction pathways and regulating the blood brain barrier. 15
The endothelium produces several vasoactive factors that regulate CBF, including nitric oxide (NO: endothelium-dependent hyperpolarization factor), eicosanoids, and endothelins. Cerebral autoregulation occurs through arteriolar vasoconstriction with increases, and vasodilatation with decreases in CPP.16,17 The stimulus for autoregulatory change in vascular diameter appears to be mediated by endothelial derived signals through NO and/or prostaglandins, and calcium activated K+ channels vasodilation, and endothelin-1 promoting vasoconstriction.14,18,19
The control of CBF is complex and requires involvement of every single component of the neurovascular unit to accomplish the following:
Cerebral autoregulation which maintains constant flow in response to change in CPP.
Flow-metabolism coupling which regulates blood flow to match metabolic activity.
Neurogenic regulation. 20
Initial adaptive responses to an asphyxia injury
Asphyxia starts with a fetal insult due to impaired CBF as a consequence of a substantial interruption of maternal and/or fetal placental blood flow and gas exchange. The timing, severity, pattern and duration of the fetal insult as well as the degree of recovery via fetal adaptive mechanisms determine the spectrum of disease, outcomes and possibly the responses to therapy. 14 The fetal circulatory response to hypoxemia and asphyxia is a rapid centralization and autoregulation of blood flow in favor of the vital organs: brain, heart and adrenals, at the expense of almost all other peripheral organs. The latter contributes to multiple organ dysfunction which is considered an integral part of HIE and likely precedes significant brain effects.21-23 At the cellular level, the initial reduction in CBF and oxygen delivery initiates a cascade of deleterious biochemical events resulting in a switch to anaerobic metabolism, and ultimately energy failure with depletion of high energy phosphorylated compounds such as ATP and phosphcreatinine.24-16 The immediate reperfusion period is demarcated with return of CBF and is characterized by a normal BP and acid base status.25 More importantly, reperfusion injury occurs with a later increase in CBF between 12 and 24 h, which can last hours to days and is associated with secondary energy failure and final cell death occurs.
Mechanisms of Autoregulation with perinatal asphyxia
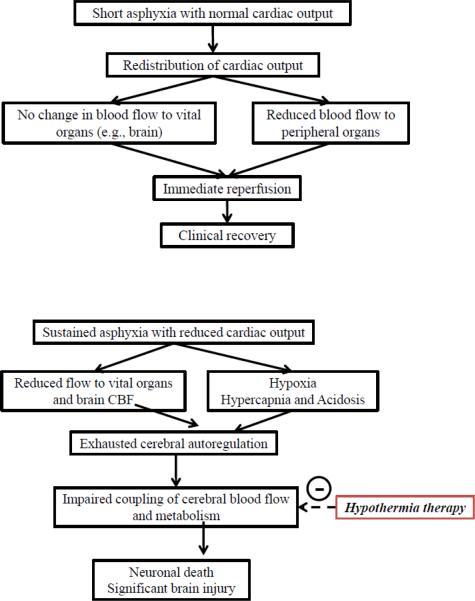
The adaptive redistribution of blood flow assures a larger proportion of cardiac output to the brain leading to an initial increase in CBF. This is subsequently followed by exhausted cerebral autoregulation due to final decrease in cardiac output and CPP. Figure.1 presents the hypothetical mechanism by which ensuing hypoxia, hypercapnia and acidosis could lead to loss of autoregulation and possible improvement following hypothermia therapy in patients with good outcomes.
Figure 1.
Hypothetical diagram illustrating 1a: the response of cerebral and systemic hemodynamics to short asphyxia with adaptive responses ensuring a stable cerebral blood flow and 1b: the response of cerebral and systemic hemodynamics to sustained asphyxia with both reduced cardiac output and cerebral blood flow and the possible effect of hypothermia.
Impaired autoregulation and reperfusion injury in HIE
Neonatal animal studies have confirmed impaired cerebral autoregulation in the face of hypoxia26, hypercarbia27 and acidosis28, all of which are integral components of neonatal HIE. Indeed, a linear relationship between CBF and systolic blood pressure was first reported in asphyxiated newborns using Xenon clearance studies.29 This suggests that changes in arterial pressure are passively transmitted into the maximally dilated cerebral circulation. Specifically, asphyxiated newborns with the most severe brain damage (death and isoelectric EEG) had the highest CBF, loss of autoregulation in response to blood pressure, and impaired vasomotor reactivity to carbon dioxide stimuli.11-30 These observations of cerebrovascular dysfunction associated with hyperperfusion have been attributed to lactate accumulation during secondary energy failure causing maximum vasodilation.31,32
Hypothermia therapy and the re-warming challenge
Hypothermia therapy provides neuroprotection via multiple pathways, including the reduction in cerebral metabolism and CBF. 33-35 The need for a better understanding of cerebrovascular function during re-warming is underscored by studies demonstrating that exposure to hyperthermia increases neuronal injury.36,37 Moreover, brain injury may occur even with modest, brief increases in brain temperature 38-40,35,41,42
Rapid re-warming can result in transient uncoupling of cerebral circulation and metabolism with a transient increase in extracellular glutamate and lactate as demonstrated by cardiac bypass studies with 1 hour duration of hypothermia.43 Temperature increments are associated with increased heart rate, cardiac output and altered hemoglobin-oxygen affinity that could result in a mismatch between oxygen delivery and consumption44 and between CBF and metabolic requirements during re-warming.45 For instance, rapid re-warming can exacerbate traumatic axonal injury and impair the cerebrovascular autoregulatory response.46,47 Notably, increased metabolic demands and seizures have been described in hypothermic cardiac bypass surgery.48 Neonatal clinical trials have avoided rapid rewarming described in surgical and cardiac patients, and have all adopted an empirical 0.5C/hour rate of rewarming. It is still possible however, that an impaired autoregulatory capacity induced by the severity of the primary insult could also cause more injury at a later stage due to hemodynamic changes in CBF and metabolic demands leading to seizures during the re-warming phase.
In summary, the regulation of CBF is complex and relies on the integrity the neurovascular unit to provide cerebral pressure autoregulation and blood flow-metabolism. The asphyxial injury with acidosis, hypoxia and hypercarbia, as well as the increased metabolic demands during rewarming, which all lead to a varying disturbances in this homeostatic system.
II. Methodology of Monitoring the “Neuro-Vascular Unit” in an NICU setting
Despite an era of medical and technological advances, fundamental gaps of knowledge remain such as lack of accessible tools for clinicians to easily determine which infants have intact CBF regulation. In fact, most of the technology reviewed below is limited to research settings.
Transcranial Doppler Ultrasonography (TCD)
TCD provides continuous measurements of CBF velocity in the basal cerebral arteries including the middle cerebral artery (MCA), anterior cerebral artery (ACA) and the posterior cerebral artery (PCA). Changes in CBF velocity represent changes in volumetric CBF if the diameters of the insonated arteries remain relatively constant. CBF velocity waveforms are displayed in real-time and can be used to obtain beat-to-beat changes in systolic, diastolic, and mean CBF velocity. TCD is non-invasive and therefore can be a useful tool to monitor cerebral hemodynamics in newborns with HIE undergoing hypothermia and re-warming.
Using this approach, cerebral autoregulation can be assessed during spontaneous changes in arterial pressure and is referred to as dynamic cerebral autoregulation. 49 Typically, dynamic cerebral autoregulation is quantified using a transfer function method between changes in arterial pressure and CBF velocity. 50-52 The metrics of the estimated transfer function gain (the amplitude relationship between changes in arterial pressure and CBF velocity), phase (the temporal relationship between changes in arterial pressure and CBF velocity), and the coherence (the linear correlation between changes in arterial pressure and CBF velocity) have been used to quantify cerebral autoregulation. More specifically, increases in transfer function gain and reduction in phase have been suggested to indicate impaired autoregulation and vice versa. It also has been shown that dynamic autoregulation is likely - most effective at the low frequencies of changes in arterial pressure in the range between 0.002 to 0.20 Hz50. Dynamic autoregulation also can be assessed in the time domain using correlation analysis between changes in arterial pressure and CBF velocity .53 Impaired autoregulation has been observed in the newborns with HIE using invasive PET studies. 11
One of the major limitations of using Doppler CBF velocity as a proxy of CBF is when the arterial cross sectional area is dynamically regulated such as during seizures when changes in blood pressure are associated with enhanced sympathetic reactivity. 54 In addition, assessment of dynamic cerebral autoregulation using the transfer function method may be appropriate only if changes cerebral hemodynamics are stationary (i.e., time-invariant) under steady-state conditions, while physiologic changes will lead to changes in vascular diameter in most clinical scenarios. Furthermore, the intrinsic nonlinear properties of the cerebral circulation may challenge the validity of transfer function methods. 54
Near Infrared Spectroscopy (NIRS)
NIRS is a non-invasive tool that can be used to measure changes in oxygenated (HbO2), deoxygenated (Hb), and total hemoglobin (HbT) of brain tissue. 55 Recently developed spatially resolved near infrared spectroscopy also can be used for bedside monitoring brain tissue oxygenation index (TOI) or regional tissue O2 saturation (rSO2). 55 TOI or rSO2 measures the mixed arterial, capillary, and venous O2 saturation. This constitutes an estimation of changes in cerebral tissue oxygenation (venous 75%, capillary 5%, arterial 20%).56 The value under physiological conditions, range between 65- 80% in newborns. 56 The measurement of TOI or rSO2 provides indirect measures of CBF under conditions of stable arterial oxygen saturation (SaO2).57 In addition, NIRS measurement of HbT can be used to estimate changes in cerebral blood volume (ΔCBV = ΔHbO2+ ΔHb) 58. Good agreement has been reported between NIRS and TCD autoregulation.59
Fractional tissue O2 extraction (FTOE = (SaO2 − TOI)/SaO2) can be used to reflect brain tissue oxygen utilization which is influenced by CBF and SaO2 and normal reference ranges between 0.2–0.3 in newborns58. A normal FTOE suggests an intact coupling between CBF and brain metabolic needs. During restricted blood flow, increases in FTOE are expected to occur to compensate for potential reduction in TOI. Conversely in the presence of constant oxygen delivery, a decrease of FTOE suggests decreased oxygen extraction due to less utilization as seen with cell death.60 In asphyxiated newborns, before the hypothermia era, high rSO2 and lower FTOE at 24h reflected secondary energy failure and poor outcomes.32,61-63,64
TOI or rSO2 can be affected by arterial saturation, CBF, cerebral blood volume and cerebral oxygen consumption.65 Therefore, these variables need to be stable in order to for TOI to reflect accurate steady-state measurements24 tissue oxygenation or CBF. Furthermore, changes in skin blood flow may contaminate NIRS signal for assessment of brain tissue oxygenation.
Amplitude-integrated (aEEG) as a measure of neuronal integrity
aEEG is a simple, non-invasive bedside tool that permits continuous evaluation of cortical electrical activity widely used since 1969 66and has been extensively reviewed in the neonatal literature. 9 Studies of aEEG by our group 67 as well as by others have reported the usefulness of using aEEG to predict outcomes 68-70and detection of subclinical seizures in newborns with HIE71. The aEEG therefore can provide quantitative as well as qualitative assessment of brain electrical activity during different phases of hypothermia or rewarming. Together with the TCD and NIRS monitoring of cerebral hemodynamics, aEEG can be used as a useful marker to examine the integrity of the neurovascular unit in newborns with HIE. Complete absence of background cortical electrical activity has been reported when CBF falls below 7ml/100g/min (i.e., ≈50% of normal resting CBF). 11
III. Clinical applications in newborns undergoing hypothermia & re-warming
A continuous neuromonitoring protocol for newborns with HIE started in 2010 at Parkland hospital in Dallas to determine dynamic autoregulation and to evaluate responses to hypothermia and re-warming. This protocol includes monitoring with a digital data acquisition system (Vital Sync System, Somanetics) that allows input from all bedside monitoring tools, including pulse oximetry, blood pressure, heart rate and NIRS neuromonitoring as well as aEEG qualitative and quantitative analysis using the Brain AnalyZe Research software, which exports the raw data and calculates minute to minute average values for the maximum and minimum electrical activity. Illustrative cases are presented below in Figures 2 and 3 depicting clinical scenarios where neuromonitoring provided insights into mechanism of injury and associated outcomes.
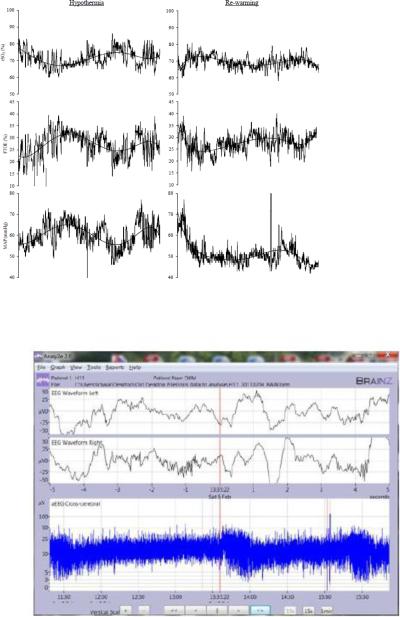
Figure 2.
Normal regulation of 2a: cerebral oxygen saturation (rSO2), cerebral fractional tissue oxygen extraction (FTOE), mean arterial pressure (MAP) and 2b: amplitude EEG (aEEG) during 6 hours of hypothermia (left) and re-warming (right). Polynomial line fit was used to show the trend of cerebral and systemic hemodynamic variables over time. Patient maintains normal reference ranges of rSO2 (70-85%) and FTOE (25-35%) as well as continuous aEEG with sleep wake cycles during hypothermia and re-warming. His clinical condition was stable with oxygen saturation of 100% on room air, HR (80 bpm), and MAP (35-45 mmHg). This patient had a normal Bayley III outcome >85 at 24 months.
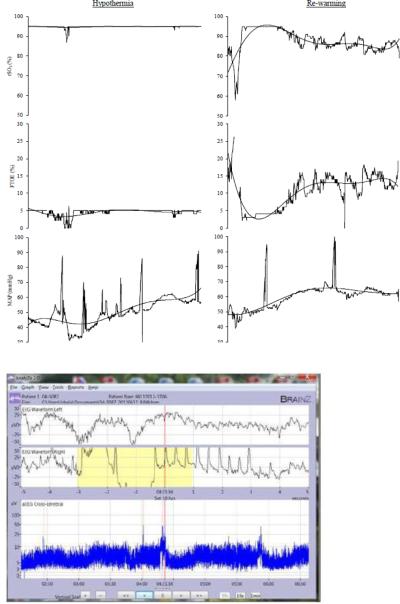
Figure 3.
Impaired regulation of 3a: cerebral oxygen saturation (rSO2), cerebral fractional tissue oxygen extraction (FTOE), mean arterial pressure (MAP) and 3b: amplitude EEG (aEEG) during 6 hours of hypothermia (left) and re-warming (right) . Polynomial line fit was used to show the trend of cerebral and systemic hemodynamic variables over time. Note the persistently increased rSO2 of 95% with a concurrent FTOE of <5%. aEEG revealed a pattern of low voltage discontinuous activity with no sleep wake cycles. Infant had seizures four hours after initiation of re-warming (red arrow). This infant had an abnormal outcome (Bayley III at 24 months: cognitive score 65, motor score 82).
Conclusions
Despite improvements in obstetrical and neonatal care and introduction of hypothermia as a neuroprotective therapy, perinatal cerebral hypoxic-ischemic injury remains a frequent cause of cerebral palsy and mental retardation. The recognition of dysfunctional cerebral autoregulation in asphyxiated neonates may identify those who are at highest risk for brain injury and seizures.
Key guidelines.
- CBF increases with postnatal age in parallel with increased cerebral metabolic rates and energy demands in the growing brain.
- The control of CBF is complex and requires involvement of every single component of the neurovascular unit. There is tight coupling of brain function, metabolism and blood flow during transition from fetal to neonatal life to support normal development.
- The asphyxial injury with acidosis, hypoxia and hypercarbia, as well as higher metabolic demands of rewarming after hypothermia can all lead to disturbances in CBF autoregulation.
- In high risk HIE infants, ongoing neurological monitoring of the neurovascular unit with NIRS, Doppler and AEEG could provide useful prognostic information.
Research directions:
- Fundamental gaps of knowledge remain with respect to development of clinical tools to determine an intact CBF regulation or how to quantify the extent of impaired autoregulation.
- We lack evidence as to whether modulation of autoregulation measures can affect outcomes.
- Future studies to identify sensitive biomarkers of cerebrovascular integrity are highly needed and may guide future targeted neuroprotective therapies to optimize outcomes of neonatal care.
Acknowledgments
Funding Dr. Chalak is supported by NIH Grant K23HD069521 and a Gerber Foundation grant.
Abbreviations
- HIE
Hypoxic-ischemic encephalopathy
- MRI
magnetic resonance imaging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Levene ML, Kornberg J, Williams TH. The incidence and severity of post-asphyxial encephalopathy in full-term infants. Early Hum Dev. 1985 May;11(1):21–26. doi: 10.1016/0378-3782(85)90115-x. [DOI] [PubMed] [Google Scholar]
- 2.Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet. 2005 Mar 26 1;Apr 26 1;365(9465):1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs SE, Morley CJ, Inder TE, et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011 Aug;165(8):692–700. doi: 10.1001/archpediatrics.2011.43. [DOI] [PubMed] [Google Scholar]
- 4.Zhou WH, Cheng GQ, Shao XM, et al. Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr. 2010 Sep;157(3):367–372. 372, e361–363. doi: 10.1016/j.jpeds.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 5.Simbruner G, Mittal RA, Rohlmann F, Muche R. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics. 2010 Oct;126(4):e771–778. doi: 10.1542/peds.2009-2441. [DOI] [PubMed] [Google Scholar]
- 6.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009 Oct 1;361(14):1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 7.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005 Feb 19-25;365(9460):663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 8.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005 Oct 13;353(15):1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 9.Spitzmiller RE, Phillips T, Meinzen-Derr J, Hoath SB. Amplitude-integrated EEG is useful in predicting neurodevelopmental outcome in full-term infants with hypoxicischemic encephalopathy: a meta-analysis. J Child Neurol. 2007 Sep;22(9):1069–1078. doi: 10.1177/0883073807306258. [DOI] [PubMed] [Google Scholar]
- 10.del Zoppo GJ. The neurovascular unit in the setting of stroke. J Intern Med. 2010 Feb;267(2):156–171. doi: 10.1111/j.1365-2796.2009.02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pryds O, Greisen G, Lou H, Friis-Hansen B. Vasoparalysis associated with brain damage in asphyxiated term infants. J Pediatr. 1990 Jul;117(1 Pt 1):119–125. doi: 10.1016/s0022-3476(05)72459-8. [DOI] [PubMed] [Google Scholar]
- 12.Chalak LF, Rouse DJ. Neuroprotective approaches: before and after delivery. Clin Perinatol. 2011 Sep;38(3):455–470. doi: 10.1016/j.clp.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Richardson BS, Carmichael L, Homan J, Tanswell K, Webster AC. Regional blood flow change in the lamb during the perinatal period. Am J Obstet Gynecol. 1989 Apr;160(4):919–925. doi: 10.1016/0002-9378(89)90311-6. [DOI] [PubMed] [Google Scholar]
- 14.Volpe JJ. Neurology of the newborn. Major Probl Clin Pediatr. 1981;22:1–648. [PubMed] [Google Scholar]
- 15.Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol. 2009 Nov;31(4):497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- 16.MacKenzie ET, Strandgaard S, Graham DI, Jones JV, Harper AM, Farrar JK. Effects of acutely induced hypertension in cats on pial arteriolar caliber, local cerebral blood flow, and the blood-brain barrier. Circ Res. 1976 Jul;39(1):33–41. doi: 10.1161/01.res.39.1.33. [DOI] [PubMed] [Google Scholar]
- 17.Laptook AR. The effects of sodium bicarbonate on brain blood flow and O2 delivery during hypoxemia and acidemia in the piglet. Pediatr Res. 1985 Aug;19(8):815–819. doi: 10.1203/00006450-198508000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Meadow W, Rudinsky B, Bell A, Lozon M, Randle C, Hipps R. The role of prostaglandins and endothelium-derived relaxation factor in the regulation of cerebral blood flow and cerebral oxygen utilization in the piglet: operationalizing the concept of an essential circulation. Pediatr Res. 1994 Jun;35(6):649–656. doi: 10.1203/00006450-199406000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Chemtob S, Li DY, Abran D, Hardy P, Peri K, Varma DR. The role of prostaglandin receptors in regulating cerebral blood flow in the perinatal period. Acta Paediatr. 1996 May;85(5):517–524. doi: 10.1111/j.1651-2227.1996.tb14077.x. [DOI] [PubMed] [Google Scholar]
- 20.Peterson EC, Wang Z, Britz G. Regulation of cerebral blood flow. International journal of vascular medicine. 2011;2011:823525. doi: 10.1155/2011/823525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah P, Riphagen S, Beyene J, Perlman M. Multiorgan dysfunction in infants with post-asphyxial hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2004 Mar;89(2):F152–155. doi: 10.1136/adc.2002.023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perlman JM, Tack ED, Martin T, Shackelford G, Amon E. Acute systemic organ injury in term infants after asphyxia. Am J Dis Child. 1989 May;143(5):617–620. doi: 10.1001/archpedi.1989.02150170119037. [DOI] [PubMed] [Google Scholar]
- 23.Martin-Ancel A, Garcia-Alix A, Gaya F, Cabanas F, Burgueros M, Quero J. Multiple organ involvement in perinatal asphyxia. J Pediatr. 1995 Nov;127(5):786–793. doi: 10.1016/s0022-3476(95)70174-5. [DOI] [PubMed] [Google Scholar]
- 24.Shalak L, Perlman JM. Hypoxic-ischemic brain injury in the term infant-current concepts. Early Hum Dev. 2004 Nov;80(2):125–141. doi: 10.1016/j.earlhumdev.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Lorek A, Takei Y, Cady EB, et al. Delayed (“secondary”) cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatr Res. 1994 Dec;36(6):699–706. doi: 10.1203/00006450-199412000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Tweed A, Cote J, Lou H, Gregory G, Wade J. Impairment of cerebral blood flow autoregulation in the newborn lamb by hypoxia. Pediatr Res. 1986 Jun;20(6):516–519. doi: 10.1203/00006450-198606000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2(2):161–192. Summer. [PubMed] [Google Scholar]
- 28.Ong BY, Greengrass R, Bose D, Gregory G, Palahniuk RJ. Acidemia impairs autoregulation of cerebral blood flow in newborn lambs. Can Anaesth Soc J. 1986 Jan;33(1):5–9. doi: 10.1007/BF03010901. [DOI] [PubMed] [Google Scholar]
- 29.Lou HC, Lassen NA, Friis-Hansen B. Impaired autoregulation of cerebral blood flow in the distressed newborn infant. J Pediatr. 1979 Jan;94(1):118–121. doi: 10.1016/s0022-3476(79)80373-x. [DOI] [PubMed] [Google Scholar]
- 30.Wyatt JS. Near-infrared spectroscopy in asphyxial brain injury. Clin Perinatol. 1993 Jun;20(2):369–378. [PubMed] [Google Scholar]
- 31.Soul JS, Hammer PE, Tsuji M, et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res. 2007 Apr;61(4):467–473. doi: 10.1203/pdr.0b013e31803237f6. [DOI] [PubMed] [Google Scholar]
- 32.Meek JH, Elwell CE, McCormick DC, et al. Abnormal cerebral haemodynamics in perinatally asphyxiated neonates related to outcome. Arch Dis Child Fetal Neonatal Ed. 1999 Sep;81(2):F110–115. doi: 10.1136/fn.81.2.f110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laptook AR. Use of therapeutic hypothermia for term infants with hypoxic-ischemic encephalopathy. Pediatr Clin North Am. 2009 Jun;56(3):601–616. doi: 10.1016/j.pcl.2009.03.007. Table of Contents. [DOI] [PubMed] [Google Scholar]
- 34.Laptook AR, Corbett RJ, Burns DK, Sterett R. A limited interval of delayed modest hypothermia for ischemic brain resuscitation is not beneficial in neonatal swine. Pediatr Res. 1999 Oct;46(4):383–389. doi: 10.1203/00006450-199910000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Laptook AR, Shalak L, Corbett RJ. Differences in brain temperature and cerebral blood flow during selective head versus whole-body cooling. Pediatrics. 2001 Nov;108(5):1103–1110. doi: 10.1542/peds.108.5.1103. [DOI] [PubMed] [Google Scholar]
- 36.Lundgren J, Smith ML, Blennow G, Siesjo BK. Hyperthermia aggravates and hypothermia ameliorates epileptic brain damage. Exp Brain Res. 1994;99(1):43–55. doi: 10.1007/BF00241411. [DOI] [PubMed] [Google Scholar]
- 37.Tooley JR, Satas S, Porter H, Silver IA, Thoresen M. Head cooling with mild systemic hypothermia in anesthetized piglets is neuroprotective. Ann Neurol. 2003 Jan;53(1):65–72. doi: 10.1002/ana.10402. [DOI] [PubMed] [Google Scholar]
- 38.Lieberman E, O'Donoghue C. Unintended effects of epidural analgesia during labor: a systematic review. Am J Obstet Gynecol. 2002 May;186(5 Suppl Nature):S31–68. doi: 10.1067/mob.2002.122522. [DOI] [PubMed] [Google Scholar]
- 39.Laptook A, Tyson J, Shankaran S, et al. Elevated temperature after hypoxic-ischemic encephalopathy: risk factor for adverse outcomes. Pediatrics. 2008 Sep;122(3):491–499. doi: 10.1542/peds.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wyatt JS, Gluckman PD, Liu PY, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007 May;119(5):912–921. doi: 10.1542/peds.2006-2839. [DOI] [PubMed] [Google Scholar]
- 41.Schwab M, Bauer R, Zwiener U. Physiological effects and brain protection by hypothermia and cerebrolysin after moderate forebrain ischemia in rats. Exp Toxicol Pathol. 1997 Feb;49(1-2):105–116. doi: 10.1016/S0940-2993(97)80078-4. [DOI] [PubMed] [Google Scholar]
- 42.Gunn AJ, Gunn TR. Effect of radiant heat on head temperature gradient in term infants. Arch Dis Child Fetal Neonatal Ed. 1996 May;74(3):F200–203. doi: 10.1136/fn.74.3.f200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakamura T, Miyamoto O, Sumitani K, Negi T, Itano T, Nagao S. Do rapid systemic changes of brain temperature have an influence on the brain? Acta Neurochir (Wien) 2003 Apr;145(4):301–307. doi: 10.1007/s00701-002-1065-8. [DOI] [PubMed] [Google Scholar]
- 44.Gebauer CM, Knuepfer M, Robel-Tillig E, Pulzer F, Vogtmann C. Hemodynamics among neonates with hypoxic-ischemic encephalopathy during whole-body hypothermia and passive rewarming. Pediatrics. 2006 Mar;117(3):843–850. doi: 10.1542/peds.2004-1587. [DOI] [PubMed] [Google Scholar]
- 45.Enomoto S, Hindman BJ, Dexter F, Smith T, Cutkomp J. Rapid rewarming causes an increase in the cerebral metabolic rate for oxygen that is temporarily unmatched by cerebral blood flow. A study during cardiopulmonary bypass in rabbits. Anesthesiology. 1996 Jun;84(6):1392–1400. doi: 10.1097/00000542-199606000-00016. [DOI] [PubMed] [Google Scholar]
- 46.Suehiro E, Povlishock JT. Exacerbation of traumatically induced axonal injury by rapid posthypothermic rewarming and attenuation of axonal change by cyclosporin A. J Neurosurg. 2001 Mar;94(3):493–498. doi: 10.3171/jns.2001.94.3.0493. [DOI] [PubMed] [Google Scholar]
- 47.Ueda Y, Suehiro E, Wei EP, Kontos HA, Povlishock JT. Uncomplicated rapid posthypothermic rewarming alters cerebrovascular responsiveness. Stroke. 2004 Feb;35(2):601–606. doi: 10.1161/01.STR.0000113693.56783.73. [DOI] [PubMed] [Google Scholar]
- 48.van der Linden J, Ekroth R, Lincoln C, Pugsley W, Scallan M, Tyden H. Is cerebral blood flow/metabolic mismatch during rewarming a risk factor after profound hypothermic procedures in small children? Eur J Cardiothorac Surg. 1989;3(3):209–215. doi: 10.1016/1010-7940(89)90068-7. [DOI] [PubMed] [Google Scholar]
- 49.Panerai RB. Cerebral autoregulation: from models to clinical applications. Cardiovasc Eng. 2008 Mar;8(1):42–59. doi: 10.1007/s10558-007-9044-6. [DOI] [PubMed] [Google Scholar]
- 50.Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol. 1998 Jan;274(1 Pt 2):H233–241. doi: 10.1152/ajpheart.1998.274.1.h233. [DOI] [PubMed] [Google Scholar]
- 51.Birch AA, Dirnhuber MJ, Hartley-Davies R, Iannotti F, Neil-Dwyer G. Assessment of autoregulation by means of periodic changes in blood pressure. Stroke. 1995 May;26(5):834–837. doi: 10.1161/01.str.26.5.834. [DOI] [PubMed] [Google Scholar]
- 52.Diehl RR, Linden D, Lucke D, Berlit P. Phase relationship between cerebral blood flow velocity and blood pressure. A clinical test of autoregulation. Stroke. 1995 Oct;26(10):1801–1804. doi: 10.1161/01.str.26.10.1801. [DOI] [PubMed] [Google Scholar]
- 53.Czosnyka M, Brady K, Reinhard M, Smielewski P, Steiner LA. Monitoring of cerebrovascular autoregulation: facts, myths, and missing links. Neurocrit Care. 2009;10(3):373–386. doi: 10.1007/s12028-008-9175-7. [DOI] [PubMed] [Google Scholar]
- 54.Greisen G. Analysis of cerebroarterial Doppler flow velocity waveforms in newborn infants: towards an index of cerebrovascular resistance. J Perinat Med. 1986;14(3):181–187. doi: 10.1515/jpme.1986.14.3.181. [DOI] [PubMed] [Google Scholar]
- 55.Greisen G. Is near-infrared spectroscopy living up to its promises? Semin Fetal Neonatal Med. 2006 Dec;11(6):498–502. doi: 10.1016/j.siny.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 56.Edmonds HL, Jr., Ganzel BL, Austin EH., 3rd. Cerebral oximetry for cardiac and vascular surgery. Semin Cardiothorac Vasc Anesth. 2004 Jun;8(2):147–166. doi: 10.1177/108925320400800208. [DOI] [PubMed] [Google Scholar]
- 57.Caicedo A, De Smet D, Naulaers G, et al. Cerebral tissue oxygenation and regional oxygen saturation can be used to study cerebral autoregulation in prematurely born infants. Pediatr Res. 2011 Jun;69(6):548–553. doi: 10.1203/PDR.0b013e3182176d85. [DOI] [PubMed] [Google Scholar]
- 58.Liem KD, Greisen G. Monitoring of cerebral haemodynamics in newborn infants. Early Hum Dev. 2010 Mar;86(3):155–158. doi: 10.1016/j.earlhumdev.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 59.Steiner LA, Pfister D, Strebel SP, Radolovich D, Smielewski P, Czosnyka M. Near-infrared spectroscopy can monitor dynamic cerebral autoregulation in adults. Neurocrit Care. 2009;10(1):122–128. doi: 10.1007/s12028-008-9140-5. [DOI] [PubMed] [Google Scholar]
- 60.Naulaers G, Morren G, Van Huffel S, Casaer P, Devlieger H. Cerebral tissue oxygenation index in very premature infants. Arch Dis Child Fetal Neonatal Ed. 2002 Nov;87(3):F189–192. doi: 10.1136/fn.87.3.F189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toet MC, Flinterman A, Laar I, et al. Cerebral oxygen saturation and electrical brain activity before, during, and up to 36 hours after arterial switch procedure in neonates without pre-existing brain damage: its relationship to neurodevelopmental outcome. Exp Brain Res. 2005 Sep;165(3):343–350. doi: 10.1007/s00221-005-2300-3. [DOI] [PubMed] [Google Scholar]
- 62.Lemmers PM, Toet M, van Schelven LJ, van Bel F. Cerebral oxygenation and cerebral oxygen extraction in the preterm infant: the impact of respiratory distress syndrome. Exp Brain Res. 2006 Aug;173(3):458–467. doi: 10.1007/s00221-006-0388-8. [DOI] [PubMed] [Google Scholar]
- 63.Toet MC, Lemmers PM, van Schelven LJ, van Bel F. Cerebral oxygenation and electrical activity after birth asphyxia: their relation to outcome. Pediatrics. 2006 Feb;117(2):333–339. doi: 10.1542/peds.2005-0987. [DOI] [PubMed] [Google Scholar]
- 64.Thavasothy M, Broadhead M, Elwell C, Peters M, Smith M. A comparison of cerebral oxygenation as measured by the NIRO 300 and the INVOS 5100 Near-Infrared Spectrophotometers. Anaesthesia. 2002 Oct;57(10):999–1006. doi: 10.1046/j.1365-2044.2002.02826.x. [DOI] [PubMed] [Google Scholar]
- 65.van Bel F, Lemmers P, Naulaers G. Monitoring neonatal regional cerebral oxygen saturation in clinical practice: value and pitfalls. Neonatology. 2008;94(4):237–244. doi: 10.1159/000151642. [DOI] [PubMed] [Google Scholar]
- 66.Maynard D, Prior PF, Scott DF. Device for continuous monitoring of cerebral activity in resuscitated patients. Br Med J. 1969 Nov 29;4(5682):545–546. doi: 10.1136/bmj.4.5682.545-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shalak LF, Laptook AR, Velaphi SC, Perlman JM. Amplitude-integrated electroencephalography coupled with an early neurologic examination enhances prediction of term infants at risk for persistent encephalopathy. Pediatrics. 2003 Feb;111(2):351–357. doi: 10.1542/peds.111.2.351. [DOI] [PubMed] [Google Scholar]
- 68.Bjerre I, Hellstrom-Westas L, Rosen I, Svenningsen N. Monitoring of cerebral function after severe asphyxia in infancy. Arch Dis Child. 1983 Dec;58(12):997–1002. doi: 10.1136/adc.58.12.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hellstrom-Westas L. Amplitude-integrated EEG--useful for early outcome prediction after birth asphyxia? Nat Clin Pract Neurol. 2008 Feb;4(2):74–75. doi: 10.1038/ncpneuro0710. [DOI] [PubMed] [Google Scholar]
- 70.Hellstrom-Westas L, Rosen I. Continuous brain-function monitoring: state of the art in clinical practice. Semin Fetal Neonatal Med. 2006 Dec;11(6):503–511. doi: 10.1016/j.siny.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 71.Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics. 2007 Oct;120(4):770–777. doi: 10.1542/peds.2007-0514. [DOI] [PubMed] [Google Scholar]