Abstract
Recipient CD4 T regulatory cells inhibit the acute T cell-mediated rejection of renal allografts in wild type mice. The survival of single class II MHC-disparate H-2bm12 renal allografts was tested in B6.CCR5−/− recipients, which have defects in Treg activities constraining alloimmune responses. In contrast to wild type C57BL/6 recipients, B6.CCR5−/− recipients rejected the bm12 renal allografts. However, donor-reactive CD8 T cells rather than CD4 T cells were the primary effector T cells mediating rejection. The CD8 T cells induced to bm12 allografts in CCR5-deficient recipients were reactive to peptides spanning the 3 amino acid difference in the I-Abm12 versus I-Ab β chains presented by Kb and Db class I MHC molecules. Allograft-primed CD8 T cells from CCR5-deficient allograft recipients were activated during culture either with pro-inflammatory cytokine stimulated wild type endothelial cells pulsed with the I-Abm12 peptides or with proinflammatory cytokine simulated bm12 endothelial cells, indicating their presentation of the I-Abm12 β chain peptide/class I MHC complexes. In addition to induction by bm12 renal allografts, the I-Abm12 β chain-reactive CD8 T cells were induced in CCR5-deficient, but not wild type C57BL/6, mice by immunization with the peptides. These results reveal novel alloreactive CD8 T cell specificities in CCR5-deficient recipients of single class II MHC renal allografts that mediate rejection of the allografts.
Keywords: CD8 T cells, allogeneic class II MHC reactivity, cell mediated graft rejection
Introduction
Vigorous donor-reactive T cell responses are generated in response to complete MHC-mismatched skin and solid organ allografts (1–3). At early times after transplant, the majority of donor-reactive T cells are primed to allogeneic class I and class II MHC molecules presented by graft-derived antigen presenting cells (4–6). At later times post-transplant, T cells are primed to recipient-derived antigen-presenting cells that acquire and present donor allogeneic peptides in complexes with self MHC molecules (4–6). This indirect pathway is particularly important for the priming of allo-peptide/self class II MHC reactive CD4 T cells that provide helper signals to generate donor-specific antibody responses and allogeneic class I MHC-reactive cytolytic CD8 T cells (7–10). Although effector CD8 T cells primed to viral peptide/self class I MHC complexes can cross react with allogeneic class II MHC molecules (11, 12), the impact of CD8 T cells with direct or indirect specificities for allogeneic class II MHC on allograft injury and/or rejection is unknown.
The B6.H-2bm12 (bm12) strain arose through spontaneous mutation of three amino acids within the β chain of the class II MHC I-Ab molecule (13, 14). In C57BL/6 recipients, bm12 skin grafts rapidly induce donor-reactive CD4 T cells producing IFN-γ that reject the grafts, whereas vascularized bm12 heart allografts induce a low level CD4 T cell response of short duration that is incapable of provoking acute rejection (15). A critical mechanism restricting the magnitude and duration of the alloreactive CD4 T cell response in C57BL/6 recipients of bm12 heart allografts is the activity of CD4+CD25+ T regulatory cells (Tregs) (15, 16). This regulation is absent when bm12 heart allografts are placed in CCR5-deficient C67BL/6 recipients and these recipients acutely reject the allografts, indicating that the effective Treg cell activity during responses to bm12 cardiac allografts is directly dependent on CCR5 expression (17).
Several studies have indicated the rapid infiltration and activity of Treg cells into complete MHC-mismatched kidney grafts in many donor to recipient mouse strain combinations and that the infiltrating Tregs inhibit acute rejection and promote long-term allograft survival (18–20). Based on our previous studies indicating defective Treg activity during alloimmune responses in CCR5-deficient mice, we predicted that B6.CCR5−/− recipients would reject bm12 renal allografts. While this prediction proved to be correct, rejection of the single class II MHC disparate allografts was mediated by bm12-reactive CD8 T cells rather than by reactive CD4 T cells. The results demonstrate unique allogeneic specificities of CD8 T cells capable of mediating rejection of class II MHC disparate renal allografts that arise in the absence of CCR5 expression.
Materials and Methods
Mice
C57BL/6 (H-2b) and B6.H-2bm12 (bm12) mice were obtained through Charles River Laboratories (Wilmington, MA). B6.CCR5−/− and C57BL/6-Igh-6tm1Cgn (µMT−/−) mice were obtained from Jackson Laboratory (Bar Harbor, ME) and crossed to generate CCR5−/−/µMT−/− mice. Males 8–12 weeks of age were used and all animal procedures were approved by the Cleveland Clinic Institutional Animal Care and Use Committee.
Transplantation
Murine kidney transplantation and urinary reconstruction was performed as previously detailed (21, 22). On day 4 post-transplant the native (left) kidney was removed rendering the recipient dependent on the function of the graft. Graft function and survival was followed by daily examination of animal health and monitoring serum creatinine levels. Whole blood creatinine levels were determined using an I-Stat portable clinical analyzer (Heska Corp., Fort Collins, CO). Conventional units (mg/dl) were converted to SI units by multiplying the conventional units by 88.4. The concentration of creatinine is expressed in mol/L. Allograft rejection was deemed ongoing when the recipient showed signs of illness and the creatinine level was elevated to ≥100 mol/L and at this time recipients were sacrificed and grafts harvested for histopathology analysis.
Heterotopic, intra-abdominal cardiac transplantation was performed as previously detailed (23, 24). Graft survival was monitored by daily abdominal palpation and cessation of detectable beating indicating rejection was confirmed by laparotomy.
Full thickness trunk skin transplants were performed as previous detailed (24). After bandages were removed on day 7 post-transplant, the B6.H-2bm12 skin allografts were examined daily and were considered rejected when 80% or more of the graft tissue was destroyed as assessed by visual examination.
Antibodies
The following antibodies were used: rat anti-mouse CD4 (GK 1.5) mAb and rat anti-mouse CD8α (53-6.7) mAb (BD Pharmingen, San Jose, CA); rat anti-mouse macrophage (F4/80) mAb (Serotec, Raleigh, NC); purified rat anti-mouse Gr-1 (RB6.8C5), anti-Kb (HB-176, anti-Db (HB-51) and anti-I-Ab (MKD-6) mAb were purified from hybridoma culture supernatants. For depletion of CD4 or CD8 T cells, graft recipients were treated with 100 µg each GK1.5 plus YTS 191 or TIB 105 plus YTS 169 (200 µg total per day) on days −3, −2, −1 prior to the transplant. Sentinel mice were treated with mAb to ensure the efficiency of the depleting regimen, which was always > 95% on day 14 post-transplant.
RNA purification and qRT-PCR
Snap-frozen grafts were crushed, homogenized, and RNA was isolated using fibrous tissue kits (Qiagen, Valencia, CA). Reverse transcription and real-time PCR were performed using commercially available reagents, probes, and a 7500 fast real-time thermocycler, all from Applied Biosystems (Foster City, CA).
bm12 peptide synthesis
Based on the I-Abm12 β chain sequence comprising the 3 amino acid differences with the I-Ab β chain, peptide octamers and nonamers spanning eight residues upstream and eight residues downstream of the sequence (total sequence span = EYWNSQPEFLEQKRAELDTVC, with differences bolded) were generated using web-based software (www.syfpeithi.de/Scripts/MHCServer.dll/EpitopePrediction.htm). Octamers restricted to H2-Kb binding and nonamers restricted to H2-Db binding were chosen based on predicted binding score and binding affinity (determined from www.cbs.dtu.dk/services/NetMHCpan). Purified peptides were produced at the Cleveland Clinic Molecular Biotechnology Core facility with purity and quality control being confirmed by HPLC and MALDI-TOF. Peptide sequences, MHC restriction, and binding affinities are shown in Table 1.
Table I.
Characteristics of control and I-Abm12 β chain peptides
| Peptide | Sequencea | MHC Restriction |
Binding Scoreb |
Binding Affinity (nM)c |
|---|---|---|---|---|
| Control | SIINFEKL | Kb | 25 | 1252.65 |
| 1 | SQPEFLEQ | Kb | 13 | 18524.27 |
| 2 | WNSQPEFL | Kb | 12 | 8001.27 |
| 3 | LEQKRAEL | Kb | 12 | 11541.13 |
| 4 | YWNSQPEFL | Db | 14 | 8792.23 |
| 5 | FLEQKRAEL | Db | 14 | 17837.99 |
| 6 | SQPEFLEQK | Db | 12 | 33517.94 |
| 7 | QKRAELDTV | Db | 11 | 29102.53 |
Bold letters indicate mutation residues in I-Abm12 β chain vs. I-Ab β chain: EYWNSQPEFLEQKRAELDTVC
Predicted based on 14-0 scale generated using SYFPEITHI web soft
Predicted using NETMHCpan Server web software.
Flow Cytometry
Analysis and quantitation of graft-infiltrating leukocytes was performed using modifications to the method reported by Afanasyev and colleagues (25, 26). Total numbers of each leukocyte population were calculated by: (the total number of leukocytes in the sample counted using an hemocytometer) x (% of the leukocyte population in the CD45+ cells by flow cytometry)/100. The data are reported as number of each leukocyte population/mg graft tissue.
Immunohistochemistry
Renal grafts were excised and fixed in 10% buffered formalin and embedded in paraffin. For histological analysis, 5 µM sections were mounted on glass slides and stained with hematoxylin and eosin (H&E). Cross-sections of the graft center were frozen at –80°C in OCT compound (Sakura Finetek, Torrance, CA) and 5 µM sections placed onto slides for immunohistochemical staining. Images were captured and analyzed with Image-Pro Plus (Media Cybernetics, Silver Springs, MD).
ELISPOT assays
Donor-specific T cell priming was assessed by enumerating IFN-γ producing T cells in response to irradiated donor vs. recipient spleen cells using ELISPOT assay (24, 26). For in vitro positive selection of CD4+ and CD8+ cells, recipient spleen cells were incubated with anti-CD4 or anti-CD8 antibody coated magnetic beads, run through a magnetic column (MACS Beads, Miltenyi Biotec, Auburn, CA), and after thoroughly washing out the unbound cells, the magnet was removed and the positively selected cells collected. In some assays, test stimulator cells were C57BL/6 spleen cells depleted of T cells using magnetic beads (Invitrogen) pulsed with 20 µg individual class I MHC binding peptides for 3 h at 37°C. In all experiments, T cell depleted bm12 splenocytes were used as a positive control stimulator and the Kb-binding SIINFEKL peptide (a generous gift from Dr. B. Min, Cleveland Clinic) was used as a non-specific control peptide. Spots were counted on an ImmunoSpot Series 3 analyzer (Cellular Technology, Shaker Heights OH).
Aortic endothelial cells
Endothelial cells were prepared from mouse aortas by microdissection, cultured on a collagen matrix, and maintained in culture for up to five passages as previously described (27, 28). After two weeks, ECs expressed typical morphological characteristics and were used as antigen presenting cells following activation with 1 ng/ml TNF-α (R&D Systems), 1 ng/ml IL-1 (R&D Systems), and 100 U/ml IFN-γ (R&D Systems) for 12 hours. The wells with adherent endothelial cells were washed three times prior to the addition of responder CD8 T cells.
Statistics
Data analysis was performed using GraphPad Prism Pro (GraphPad Software Inc., San Diego, CA). Unpaired Students’ t-test was used to determine significance throughout. Kaplan-Meyer survival curves were generated and log-rank testing was used to determine significance with P < 0.05 being considered significant.
Results
B6.H-2bm12 kidney rejection by B6.CCR5−/− recipients
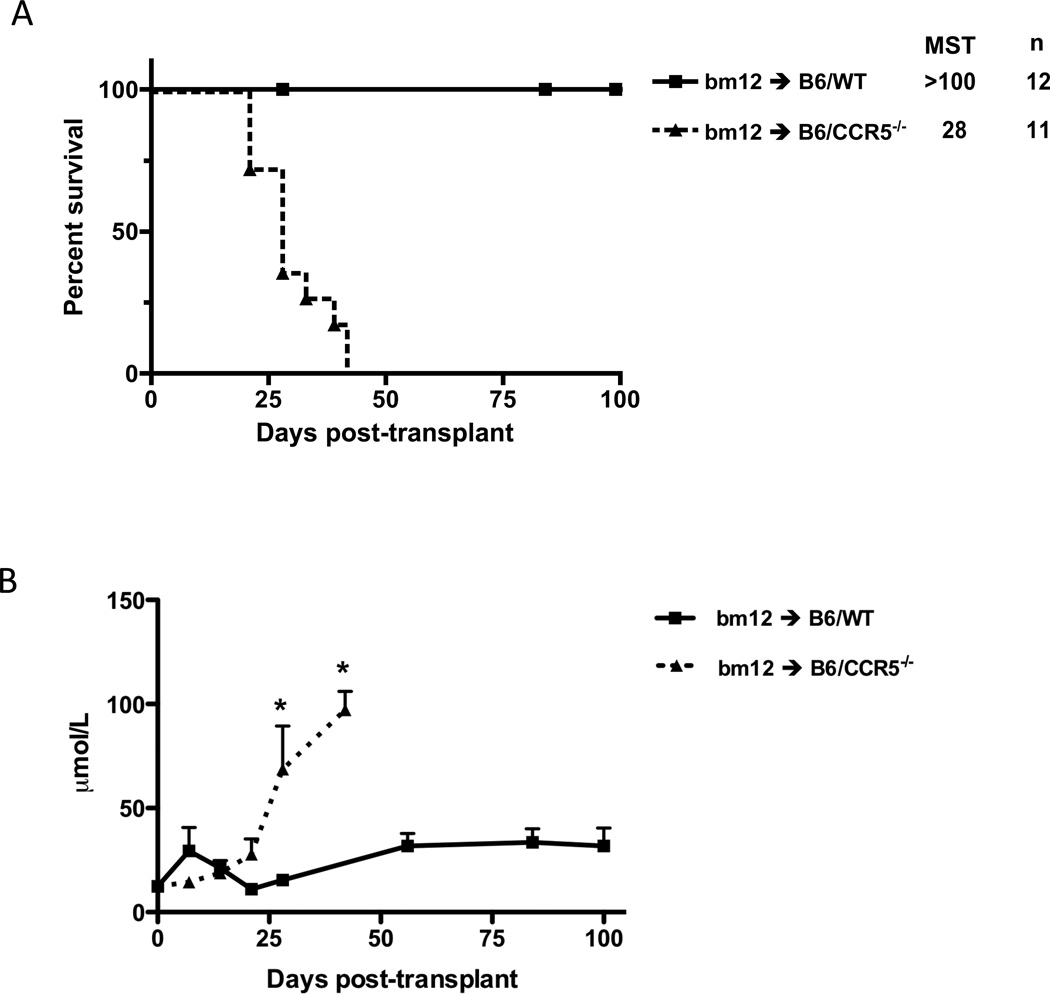
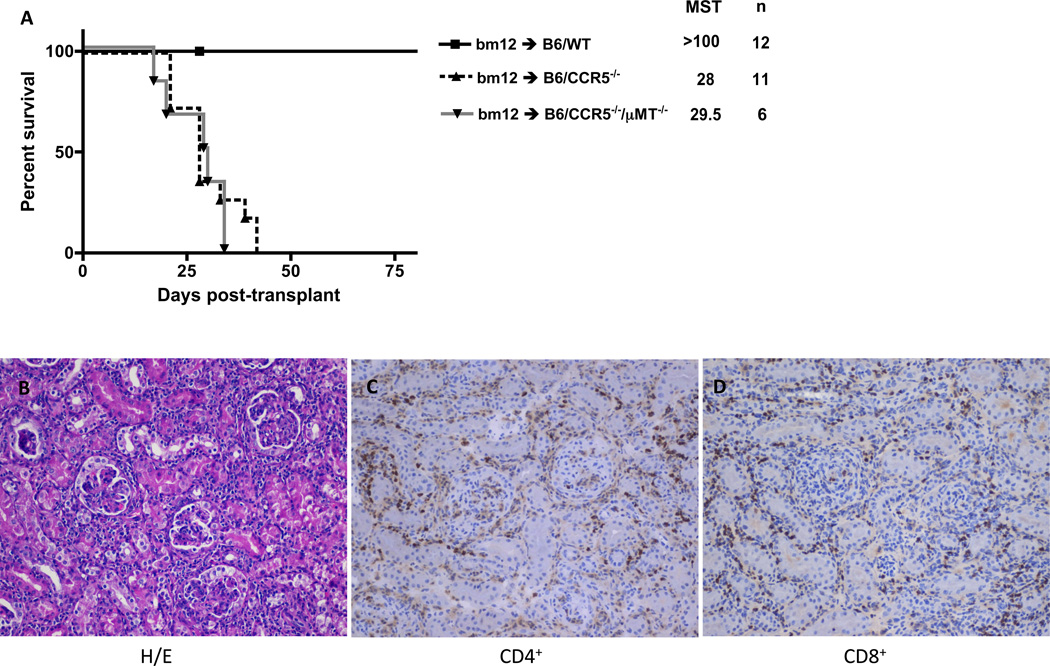
Single class II MHC disparate B6.H-2bm12 (bm12) renal allograft rejection by C57BL/6 (n = 12) vs. B6.CCR5−/− (n = 11) recipients was compared. Consistent with the inability of C57BL/6 mice to reject complete MHC-mismatched A/J renal allografts (21), all bm12 renal allografts survived longer than 100 days in wild type recipients. In contrast, CCR5-deficient recipients rejected all bm12 renal allografts by day 42 post-transplant (Figure 1A). Neither C57BL/6 nor B6.CCR5−/− recipients rejected syngeneic renal grafts (data not shown and (21)). Acute injury and dysfunction of bm12 renal allografts in CCR5−/− recipients was indicated by sharp rises in serum creatinine levels beginning on day 25 post-transplant (Figure 1B). C57BL/6 bm12 renal allograft recipients had a modest rise in serum creatinine levels, first detected at day 56 post-transplant and maintained through day 100 post-transplant.
Figure 1.
Single class II MHC-disparate renal allograft rejection by CCR5-deficient, but not wild type C567BL/6, recipients. Groups of wild type C57BL/6 and B6.CCR5−/− mice received single class II MHC disparate renal grafts from B6.H-2bm12 donors. (A) B6.CCR5−/− mice rejected the allografts within 40 days post-transplant (MST=28, n=11) whereas wild type recipients did not reject the allografts. (B) Serum creatinine levels in the renal allograft recipients were measured weekly after transplant and the mean serum concentration for each group is shown at each time point ± SEM. C57BL/6 kidney isografts were maintained by both wild type C57BL/6 and B6.CCR5−/− recipients long-term without any rise in serum creatinine levels (data not shown). *p < 0.05.
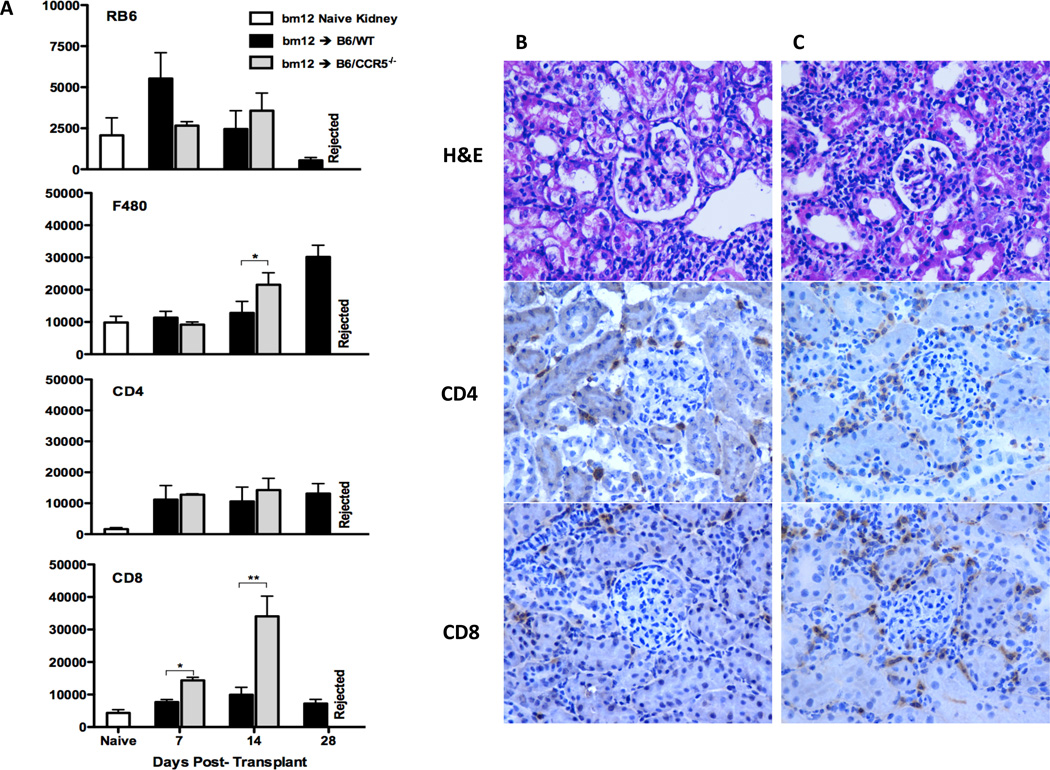
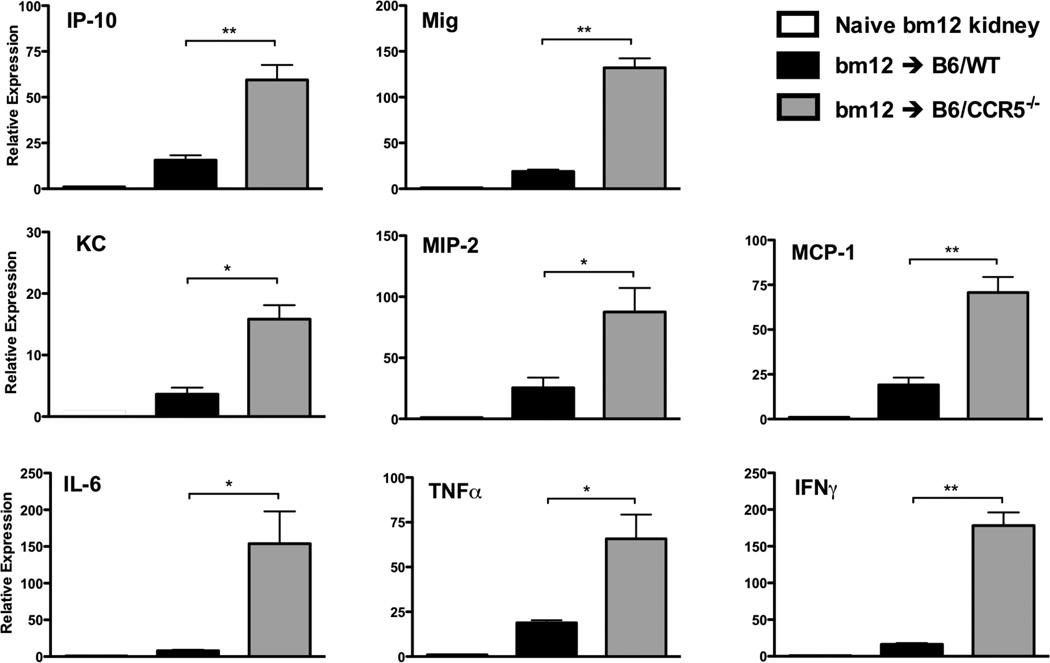
Histological evaluation of bm12 renal allografts at day 14 post-transplant indicated intense mononuclear infiltration in grafts from B6.CCR5−/−, but not C57BL/6, recipients (Figure 2C vs. B, respectively). Neutrophil, macrophage and T cell infiltration into bm12 renal allografts was detected as early as day 7 post-transplant (Figure 2A). Numbers of CD4 T cells infiltrating bm12 allografts were nearly identical in C57BL/6 and B6.CCR5−/− recipients. Surprisingly, compared to the low CD8 T cell infiltration into bm12 renal allografts in wild type recipients, intense CD8 T cell infiltration into allografts in B6.CCR5−/− recipients was observed as early as day 7 post-transplant and increased markedly thereafter (Figure 2A, B and C). The intense CD8 T cell infiltration into the bm12 renal allografts in B6.CCR5−/− recipients was accompanied by high mRNA levels of all proinflammatory cytokine genes tested, including TNFα and IFN-γ when assessed on day 14 post-transplant where as expression of these proinflammatory mediators was low-absent in allografts from wild type recipients (Figure 3).
Figure 2.
Leukocyte infiltration into single class II MHC-disparate renal allografts in CCR5-deficient and wild type C57BL/6 recipients. (A) bm12 renal allografts were harvested from groups of wild type C57BL/6 and B6.CCR5−/− recipients on the indicated days post-transplant. Following digestion of the allografts and bm12 kidneys from non-transplanted B6.H-2bm12 mice, aliquots of prepared single cells suspensions were stained with antibody and analyzed by flow cytometry to determine the numbers of graft infiltrating leukocyte populations, expressed as numbers/mg graft tissue ± SEM. *p < 0.05; **p < 0.01. Renal allografts from (B) C57BL/6 and (C) B6.CCR5−/− recipients were harvested on day 14 post-transplant and frozen sections were prepared and stained with hematoxylin and eosin or with anti-CD4 or anti-CD8 antibodies. Magnification ×400.
Figure 3.
Proinflammatory cytokine mRNA expression in single class II MHC-disparate renal allografts in CCR5-deficient and wild type C57BL/6 recipients. bm12 renal allografts were harvested from groups of C57BL/6 and B6.CCR5−/− recipients on day 14 post-transplant and whole cell RNA was isolated from grafts and from native bm12 kidneys and analyzed by qPCR for expression levels of the indicated inflammatory molecules. Results shown indicate mean expression level of each cytokine in 4 allograft samples per group ± SEM. *p < 0.05; **p < 0.01.
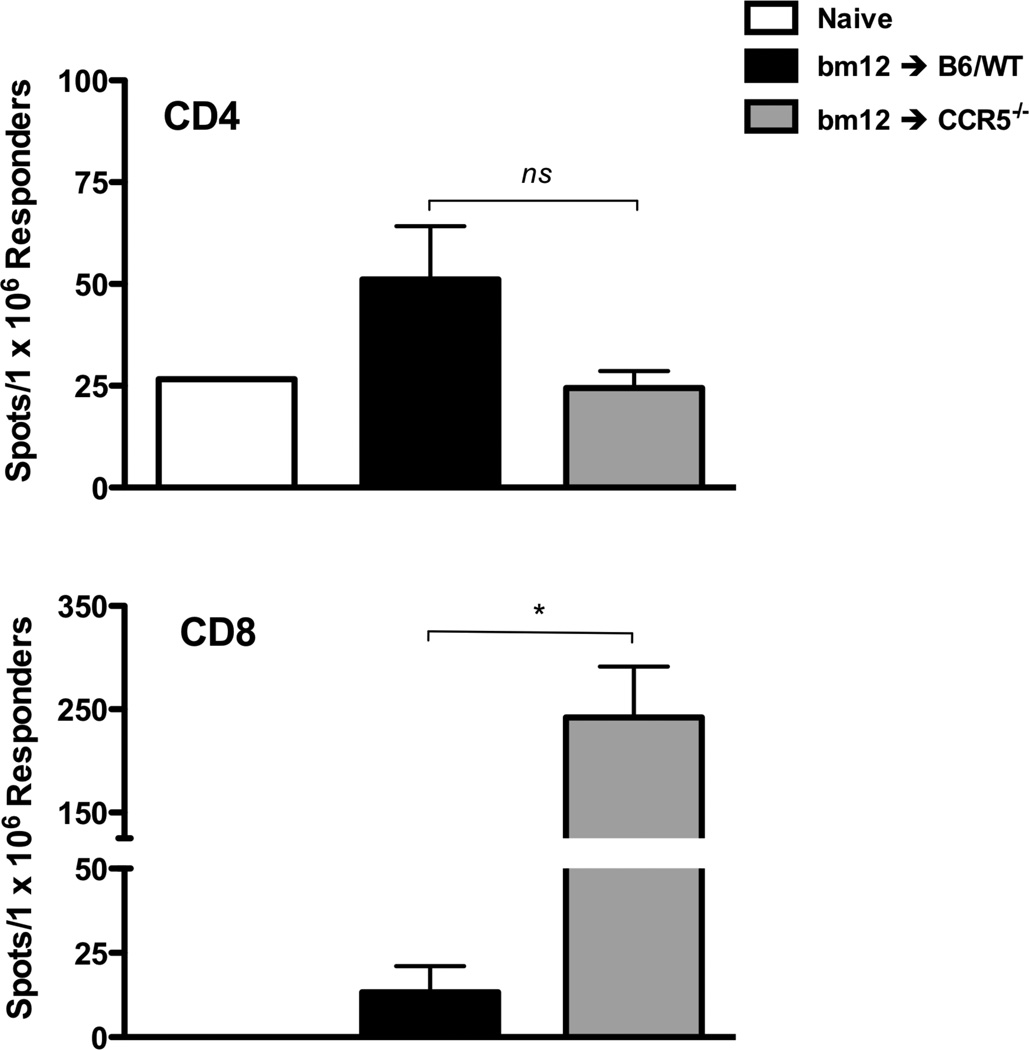
The CD8 T cell infiltration into the bm12 renal allografts in B6.CCR5−/− recipients led us to test evidence of bm12-reactive CD8 T cell priming in the recipients spleen. On day 14 post-transplant, CD4 and CD8 T cells were isolated from the spleens of C57BL/6 and B6.CCR5−/− recipients of bm12 renal allografts and enumerated for cells producing IFN-γ during culture with bm12 stimulator cells by ELISPOT assay (Figure 4). In wild type C57BL/6 recipients, bm12 renal allografts induced low numbers of bm12-reactive CD4 and CD8 T cells producing IFN-γ. In B6.CCR5−/− allograft recipients the numbers of donor-reactive CD4 T cells producing IFN-γ were similar to those in the spleens of naïve C57BL/6 mice, but extremely high numbers of bm12-reactive CD8 T cells producing IFN-γ were induced.
Figure 4.
Absence of recipient CCR5 results in exaggerated CD8 T cell responses to single class II MHC-disparate bm12 renal allografts. Groups of wild type C57BL/6 and B6.CCR5-deficient mice received bm12 renal allografts. On day 14 post-transplant CD4 and CD8 T cells were purified from each of the recipient spleens and the frequency of bm12 donor-reactive CD4 and CD8 T cells producing IFN-γ was quantified by ELISPOT assay. The results indicate the mean number of spots for splenic CD4 or CD8 T cells from 4 individual mice per group ± SEM.*p < 0.05
CD8 T cells mediate bm12 renal allograft rejection in B6.CCR5−/− recipients
Previous results from this laboratory demonstrated that B6.CCR5−/− rejection of complete MHC-mismatched renal allografts is mediated by donor-specific antibodies (21). Although the bm12 mutation is localized to 3 amino acids lying in a α helix of the I-A β chain in positions that are not likely to be accessible to antibody (13, 14, 29), we considered the possibility that rejection of bm12 renal allografts was mediated by donor-specific antibody. However, CCR5−/−/µMT−/− recipients that were unable to make antibody rejected bm12 renal allografts as rapidly as CCR5−/− recipients with robust infiltration of CD4 and CD8 T cells at the time of rejection, indicating that the rejection was not mediated by donor-specific antibody (Figure 5).
Figure 5.
Rejection of single class II MHC-disparate bm12 renal allografts by B6.CCR5-deficient recipients is not mediated by antibody. (A) Groups of wild type C57Bl/6, B6.CCR5−/− and B6.CCR5−/−µMT−/− mice received single class II MHC disparate renal grafts from B6.H-2bm12 donors and allograft survival was monitored. (B) Grafts were harvested from B6.CCR5−/−µMT−/− recipients at the time of rejection and formalin-fixed/paraffin embedded sections were stained with hematoxylin and eosin and frozen sections were stained with antibodies to detect CD4 and CD8 T cells. Magnification x400.
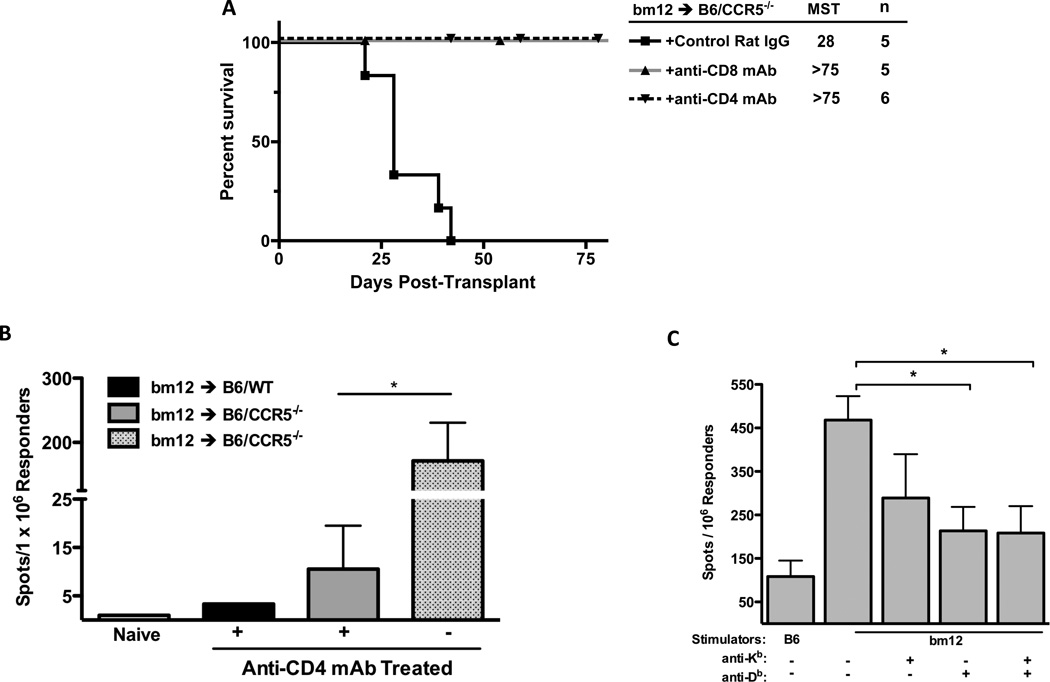
The requirement for CD4 and CD8 T cells in the rejection of bm12 renal allografts by B6.CCR5−/− recipients was then tested by treating recipients with specific monoclonal antibody to deplete CD4 or CD8 T cells or with control IgG before transplant. Depletion of either CD4 or CD8 T cells abrogated the ability of B6.CCR5−/− recipients to reject the bm12 allografts, indicating both T cell populations were required for rejection (Figure 6A).
Figure 6.
Rejection of single class II MHC-disparate bm12 renal allografts in B6.CCR5-deficient recipients is dependent on CD4 and CD8 T cells. (A) B6.CCR5−/− mice were treated with 200 µg control rat IgG, anti-CD4 mAb or anti-CD8 mAb on three consecutive days prior to transplantation with a B6.H-2bm12 kidney and allograft survival was monitored. (B) Groups of wild type C57Bl/6 and B6.CCR5-deficient mice received bm12 renal allografts, with the indicated groups depleted of CD4 T cells by treating with anti-CD4 mAb prior to the transplant. On day 14 post-transplant, CD8 T cells were purified from each of the recipient spleens and the frequency of bm12 donor-reactive CD8 T cells producing IFN-γ was quantified by ELISPOT assay. The results indicate the mean number of spots for splenic CD8 T cells from 4 individual mice per group ± SEM. *p < 0.05. (C) Groups of B6.CCR5−/− mice received bm12 renal allografts. On day 20 post-transplant, CD8 T cells were purified from each of the recipient spleens and the frequency of CD8 T cells producing IFN-γ during culture with syngeneic C57BL/6 or bm12 stimulator cells was quantified by ELISPOT assay. In the indicated cultures, 20 µg of anti-Kb and/or anti-Db mAb was added at the initiation of the ELISPOT culture. The results indicate the mean number of spots for splenic CD8 T cells from 4 individual mice per group in each of the stimulation cultures ± SEM. *p < 0.05.
To test the possible requirement for CD4 T cells in the induction of bm12-reactive CD8 T cells in B6.CCR5−/− recipients, priming of donor-reactive CD8 T cells was tested in recipients treated with control IgG or with CD4 T cell-depleting antibodies. On day 14 post-transplant, CD8 T cells were purified from recipient spleens and numbers of donor-reactive CD8 T cells producing IFN-γ were enumerated by ELISPOT assay (Figure 6B). In control Ig-treated bm12 renal allograft recipients, high numbers of donor-reactive CD8 T cells producing IFN-γ were induced. In contrast, depletion of CD4 T cells reduced this number more than 10-fold indicating that CD4 T cells are required for the priming of the CD8 T cells that mediate rejection of the single class II MHC disparate renal allografts.
The ability of bm12-reactive CD8 T cells from B6.CCR5−/− mice to directly reject B6.H-2bm12 allografts was tested in a model in which CD4 T cells are not required for donor-reactive CD8 T cell priming. Groups of wild type C57BL/6 and B6.CCR5−/− mice were treated with control IgG or anti-CD4 or anti-CD8 mAb to deplete CD4 or CD8 T cells and then received full thickness B6.H-2bm12 trunk skin allografts. Depletion of CD4 T cells in both groups treated with anti-CD4 mAb was greater than 98% and depletion of CD8 T cells was > 90% in B6.CCR5−/− recipients and > 80% in wild type C57BL/6 recipients treated with anti-CD8 mAb, when assessed on day 15 post-transplant (not shown). Rejection of B6.H-2bm12 skin allografts was observed by day 19 in wild type recipients treated with control IgG or with anti-CD8 mAb but depletion of CD4 T cells abrogated bm12 skin allograft rejection (Table II). In contrast, B6.CCR5-deficient recipients depleted of either CD4 or CD8 T cells rejected the bm12 skin allografts by day 13 post-transplant. Thus, rejection of bm12 skin allografts is mediated by CD4, but not CD8, T cells in wild type recipients whereas either CD4 or CD8 T cells can mediate bm12 skin allograft rejection by B6.CCR5−/− recipients.
Table II.
CD8 T cell-mediated rejection of B6.H-2bm12 skin allografts by B6.CCR5−/− recipients
| Allograft Recipient |
Recipient Treatment |
Day of Graft Rejection |
|---|---|---|
| C57Bl/6 | Control IgG | 13, 15, 19, 19, 23, 1 × >23 |
| C57Bl/6 | Anti-CD4 mAb | 19, 5 × >23 |
| C57Bl/6 | Anti-CD8 mAb | 13, 16, 20, 21, 23, 1 × >25 |
| B6.CCR5−/− | Anti-CD4 mAb | 10, 10, 11, 12 |
| B6.CCR5−/− | Anti-CD8 mAb | 10, 10, 12, 13 |
Groups of 4–5 wild type C57Bl/6 and B6.CCR5−/− mice received full thickness trunk skin allografts from B6.H-2bm12 donors. Graft status was monitored daily and the day of rejection indicated by >80% graft destruction. Recipients received 200 µg of control IgG, anti-CD4 mAb or anti-CD8 mAb on days −3, −2, −1, +5 and +10.
CD8 T cell recognize class II MHC peptides presented by class I MHC molecules
We considered two potential mechanisms to account for the induction of a CD8 T cell response to the bm12 allografts in B6.CCR5−/− recipients. First, the bm12-reactive CD8 T cells could react directly to the allogeneic class II MHC molecules. Second, the CD8 T cells could recognize a bm12 β chain-derived peptide presented by Kb or Db. Since an antibody specific for the B6.H-2bm12 class II MHC molecule was not available, we tested the ability of anti-Kb and anti-Db antibodies to block the activation of the donor-primed CD8 T cells. On day 20 post-transplant, CD8 T cells were purified from the spleens and tested for numbers of bm12-reactive T cells producing IFN-γ by ELISPOT assay in the presence of the anti-Kb and anti-Db antibodies (Figure 6C). Addition of either anti-Kb or anti-Db antibodies as well as both antibodies together resulted in significant decreases in donor-reactive CD8 T cells producing IFN-γ, suggesting that activation of the CD8 T cells requires engagement of self class I MHC, raising the possibility that the class I MHC molecules were presenting I-Abm12-derived peptides.
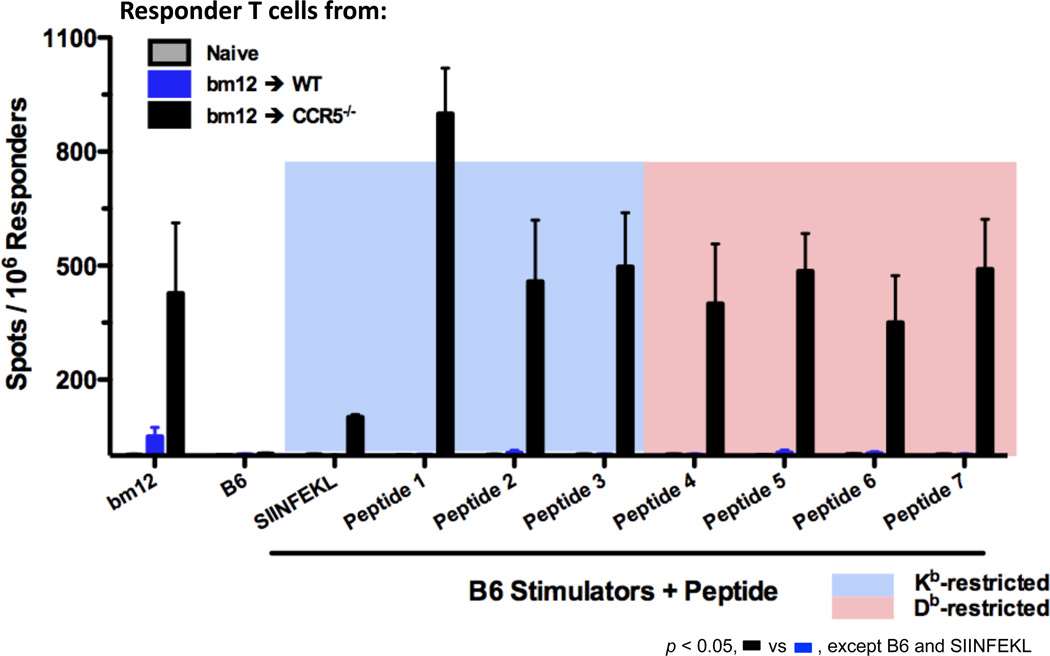
A series of different I-Abm12 β chain peptides spanning the sequence containing the three amino acid differences from I-Ab were designed and their binding restriction and calculated affinity to Kb or to Db were determined (Table 1). The peptides were synthesized and T cell depleted B6 splenocytes cells pulsed with the different peptides were tested for activation of CD8 T cells isolated from the spleens of C57BL/6 and B6.CCR5−/− bm12 renal allograft recipients (Figure 7). CD8 T cells from B6.CCR5−/− recipients were activated during culture with bm12 stimulators and during culture with B6 splenocytes pulsed with the Kb- and Db-presented I-Abm12 β chain-derived peptides but not during culture with C57BL/6 cells alone or with C57BL/6 cells pulsed with the Kb-binding SIINFEKL peptide. In contrast, neither bm12 cells nor C57BL/6 cells pulsed with any of the peptides stimulated CD8 T cells from C57BL/6 allograft recipients.
Figure 7.
Donor reactive CD8 T cells induced in response to single class II MHC-disparate bm12 renal allografts in B6.CCR5-deficient recipients react to I-Abm12 β chain peptides. Groups of wild type C57BL/6 and B6.CCR5-deficient mice received bm12 renal allografts. On day 14 post-transplant, CD8 T cells were purified from each of the recipient spleens and from the spleens of naïve mice and the frequency of CD8 T cells producing IFN-γ during culture with syngeneic C57Bl/6 or bm12 stimulator cells was quantified by ELISPOT assay. In the indicated cultures, 20 µg of the indicated control (SIINFEKL) or I-Abm12 β chain peptides were added at the initiation of the ELISPOT cultures. The results indicate the mean number of spots for splenic CD8 T cells from 4 individual mice per group ± SEM. p < 0.05 for all CCR5−/− recipients compared to wild type recipients with either bm12 or C57BL/6 plus bm12 peptide stimulator cells.
CD8 T cells are activated by I-Abm12-derived peptides presented by endothelial cells
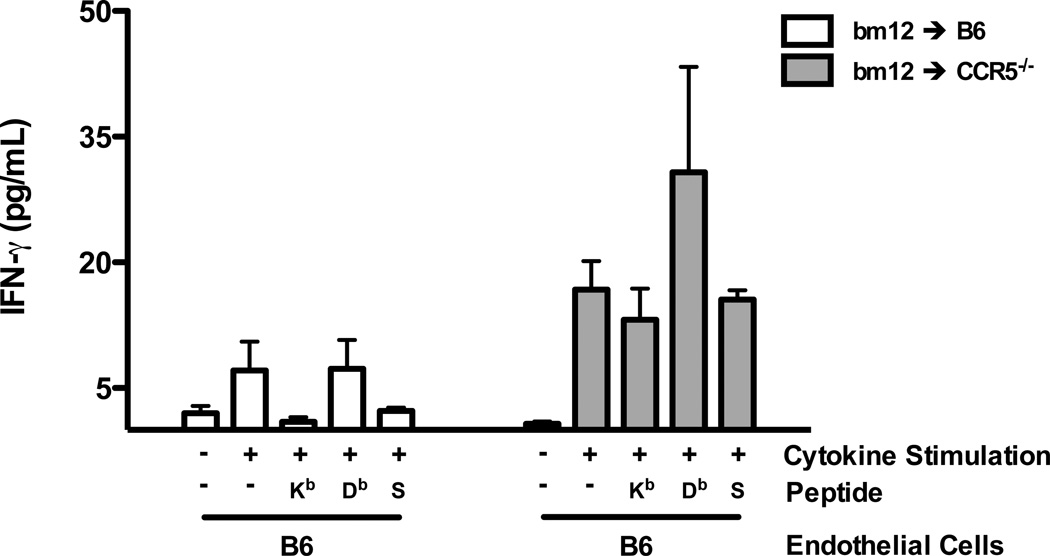
We postulated that the initial activation of the bm12-reactive CD8 T cells would occur at the vascular endothelium of the allograft followed by their infiltration into the graft parenchymal tissue (30). To test the presentation of I-Abm12 β chain-derived peptides by allograft endothelial cells, aortic endothelial cells from bm12 mice and from C57BL/6 mice, as a negative control, were generated and grown in culture. CD8 T cells were purified from the spleens of C57BL/6 and B6.CCR5−/− recipients of bm12 heart allografts on day 10 post-transplant. Aliquots of C57BL/6 or bm12 aortic endothelial cells were stimulated with a cocktail of proinflammatory cytokines or not stimulated and cultured with aliquots of the purified CD8 T cells from the heart allograft recipients to assess activation of the CD8 T cells to produce IFN-γ (Figure 8). CD8 T cells from C57BL/6 recipients did not produce IFN-γ during culture with unstimulated bm12 endothelial cells but produced low levels of IFN-γ when cultured with cytokine stimulated endothelial cells. Addition of Kb or Db restricted I-Abm12 peptides to cytokine stimulated C57BL/6 endothelial cells only provoked low levels of IFN-γ production by CD8 T cells from C57BL/6 recipients. In contrast, CD8 T cells from B6.CCR5−/− bm12 heart allograft recipients were activated to produce high levels of IFN-γ during culture with cytokine stimulated, but not unstimulated, bm12 endothelial cells. In addition, the CD8 T cells from CCR5-deficient recipients of bm12 heart allografts produced high levels of IFN-γ when cultured with cytokine stimulated C57BL/6 endothelial cells pulsed with the Db-restricted, but not Kd-restricted, peptides. These data suggest that CD8 T cells primed to bm12 cardiac allografts are activated to produce IFN-γ in response to I-Abm12-derived peptides presented in complex with self class I MHC on graft endothelial cells.
Figure 8.
Donor reactive CD8 T cells induced in response to single class II MHC-disparate bm12 cardiac allografts in B6.CCR5-deficient recipients react to cytokine-stimulated bm12 aortic endothelial cells. Groups of 4 wild type C57BL/6 and B6.CCR5-deficient mice received bm12 heart allografts. On day 10 post-transplant, CD8 T cells were purified from each of the recipient spleens and cultured with aortic endothelial cells from syngeneic C57BL/6 or bm12 mice that had been pre-activated with cytokines as detailed in the Materials and Methods. The activation of the CD8 T to produce IFN-γ after 24 hours of culture was quantified by ELISA of the culture supernatants. In the indicated cultures, 20 µg of Kb- or Db-restricted peptides (#1 and #4 in Table 1) or control SIINFEKL peptide (S) were added at the initiation of the cultures. The results indicate the mean concentration of IFN-γ produced in CD8 T cell stimulation cultures from 4 individual allograft recipients per group ± SEM. *p < 0.05
Inability to induce bm12 peptide/class I MHC-reactive CD8 T cells in C57BL/6 mice
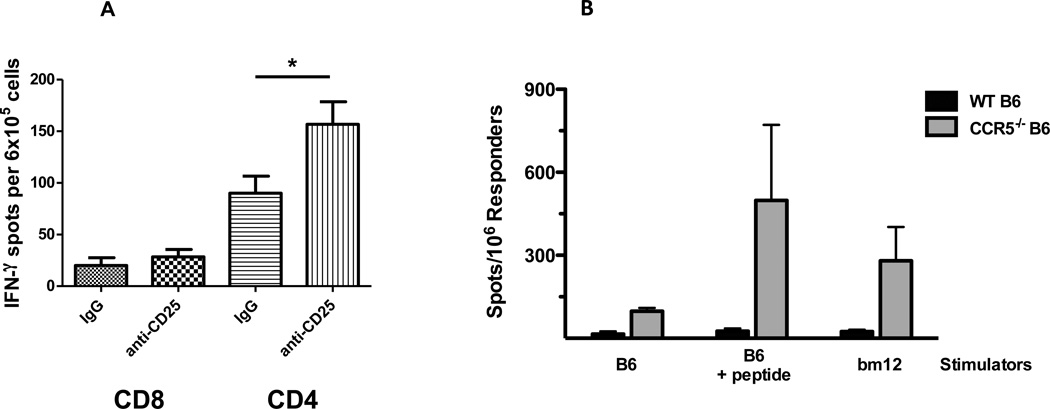
We have previously shown that Treg inhibit the magnitude of the bm12-reactive CD4 T cell response in wild type C57BL/6 recipients of bm12 heart allografts (15). Consistent with these previous studies, C57BL/6 bm12 cardiac allograft recipients treated with anti-CD25 mAb had marked increases in CD4 T cells producing IFN-γ compared to control-treated recipients, but continued to have low-absent numbers of bm12-reactive CD8 T cells (Figure 9A). Therefore, we tested an alternative approach to testing the generation of bm12-reactive CD8 T cells in wild type C57BL/6 mice by immunizing groups of C57BL/6 and B6.CCR5−/− mice with a mixture of four Kb- and Db-restricted bm12 peptides emulsified in Complete Freund’s Adjuvant. After 12 days, splenic CD8 T cells were isolated and the number of bm12-reactive or peptide-reactive CD8 T cells producing IFN-γ was tested by ELISPOT. Peptide immunization of CCR5-deficient mice induced CD8 T cells that were activated to produce IFN-γ during culture with either bm12 spleen cells or autologous C57BL/6 spleen cells pulsed with the immunizing peptides (Figure 9B). In contrast, CD8 T cells from immunized C57BL/6 mice did not produce IFN-γ during culture with either bm12 stimulator cells or autologous cells pulsed with the immunizing peptides.
Figure 9.
Absence of bm12-reactive CD8 T cell priming in wild type C57BL/6 recipients of bm12 heart allografts. (A) Groups of C57BL/6 mice received B6.H-2bm12 heart allografts and were treated with 250 µg anti-CD25 mAb on days −1, +1, +3, +5, +7 and +9 post-transplant. On day 18 post-transplant, CD4 and CD8 T cells were purified from each recipient spleen and the frequency of bm12 donor-reactive CD4 and CD8 T cells producing IFN-γ was quantified by ELISPOT assay. The results indicate the mean number of spots for the splenic T cell populations from 4 individual mice per group ± SEM. *p < 0.05. (B) Groups of wild type C57Bl/6 and B6.CCR5-deficient mice were immunized subcutaneously with a mixture of 2 Kb- plus 2 Db-restricted I-Abm12 β chain peptides emulsified in Complete Freund’s Adjuvant and 12 days later the CD8 T cells were purified from each of the spleens. The frequency of CD8 T cells producing IFN-γ during culture with syngeneic C57Bl/6 or bm12 stimulator cells was quantified by ELISPOT assay. In the indicated cultures, 20 µg of the I-Abm12 β chain peptides were added at the initiation of the ELISPOT cultures with the C57Bl/6 stimulator cells. The results indicate the mean number of spots for splenic CD8 T cells from 4 individual mice per group ± SEM. *p < 0.05.
Discussion
The response to single class II MHC-disparate, B6.H-2bm12 heart grafts in C57BL/6 recipients is comprised almost entirely of bm12-reactive CD4 T cells that is restricted by CD4+ Tregs (15). Treatment of bm12 heart allograft recipients with anti-CD25 mAb to deplete the Tregs markedly increases the bm12-reactive CD4 T cell response and provokes acute rejection of the allografts within 20–40 days post-transplant. In contrast to wild type C57BL/6 recipients, B6.CCR5−/− recipients of bm12 heart allografts have a Treg dysfunction and the unregulated bm12-reactive effector T cell response in these recipients rapidly provokes acute rejection (17). Colvin and colleagues identified the rapid infiltration of CD4+FoxP3+ cells into MHC mismatched renal allografts as the mechanism underlying the long-term survival of complete MHC-mismatched renal allografts in many donor → recipient strain combinations of mice (19), prompting us to test the rejection of renal allografts with a single class II MHC disparity in recipients with compromised immunoregulatory function.
B6.CCR5−/− recipients did reject bm12 renal allografts, but donor-reactive CD8 T cells rather than CD4 T cells were the primary effector cells mediating the rejection. Several studies have reported data either suggesting or documenting class II MHC-reactive CD8 T cells. C57BL/6 nude mice reconstituted with purified CD8 T cells and transplanted with bm12 heart allografts developed an IFN-γ dependent vasculopathy suggesting CD8 T cells reactive to I-Abm12 as a possible mechanism of the chronic pathology (31). With regards to CD8 T cells expressing receptors cross-reactive with allogeneic class II MHC molecules, human CD8 T cells induced to cytomegalovirus (CMV) peptide/HLA Cw complexes were also reactive to a B cell derived peptide presented by HLA-DR on allogeneic B cells (12). In contrast to direct cross-reactivity with allogeneic class II MHC, in vitro activation of the bm12-reactive CD8 T cells to produce IFN-γ was inhibited by antibodies to Kb or Db class I MHC molecules, indicating restriction to presentation by syngeneic class I MHC. Moreover, bm12 renal and cardiac allografts in B6.CCR5-deficient, but not C57Bl/6, recipients induced CD8 T cells reactive to peptides spanning the 3 amino acid difference in the I-Abm12 vs. I-Ab β chains presented by the class I MHC molecules shared by the graft donor and recipient. It is important to note that the bm12 allograft induced CD8 T cells were responsive to a wide range of Kb and Db binding peptides from the I-Abm12 β chain, but it is also worth considering that these peptides were chosen for high affinity binding to Kb or Db. These results are reminiscent of CD8 T cells induced by vaccination with rhesus monkey CMV expressing SIV genes that had reactivity to a broad range of SIV peptides presented by syngeneic and allogeneic class II MHC molecules versus the CD8 T cells with restricted peptide/class I MHC specificities induced by vaccination with SIV constructs alone (11).
The first graft cells that alloantigen-primed T cells will encounter are the vascular endothelial cells. The I-Abm12 peptide/class I MHC-reactive CD8 T cells from renal and heart allograft recipients were activated to produce IFN-γ during culture with cytokine stimulated aortic bm12 endothelial cells or endothelial cells from C57BL/6 mice presenting exogenously added I-Abm12 peptides. These results implicate this presentation and the allograft endothelial cells as major targets in the acute rejection of the bm12 renal allografts by B6.CCR5−/− recipients. The targeting of allograft endothelial cells presenting allogeneic complexes is well established and their apoptosis is a key event during acute injury as well as during the development of chronic allograft injury (30, 32–34). Similarly, transgenic CD8 T cells reactive to the male antigen H-Y peptide presented by Db molecules rejected skin allografts from male C3H (H-2k) donors on female RAG-1−/− recipients if the endothelium revascularizing the allograft expressed the appropriate class I MHC molecule (28). It is worth noting that our results indicated that the bm12-reactive CD8 T cells were activated by endothelial cells presenting the Db-restricted peptides but not by presentation of the Kb-restricted peptides. Whether this restricted presentation reflects a function of endothelial cells is unknown at this time but certainly warrants further investigation. Also, other class II MHC expressing cells, such as kidney interstitial dendritic cells and resident macrophages may also present the complexes to activate the bm12-reactive CD8 T cells as they infiltrate the kidney parenchymal tissue and be required to complete rejection. Since the allograft donor and recipient share class I MHC alleles, recipient-derived monocytes and dendritic cell populations may acquire the bm12 class II MHC molecules as they infiltrate the renal allografts and following processing, cross-present the class II MHC/class I MHC complexes to activate the infiltrating CD8 T cells.
The results of this study clearly indicate that the renal graft injury imposed by the bm12-reactve CD8 T cells is very inefficient and delays the progression to allograft failure. The donor-reactive CD8 response was apparent at high numbers in the recipient spleen as early as day 14 post-transplant and the allograft is heavily infiltrated with the CD8 T cells at this time. However, increases in serum creatinine were not apparent until day 25 post-transplant and the allografts did not begin to reject until this time and several survived until day 45. One possible mechanism to account for this delay is that efficient rejection of renal allografts requires the synergistic activities of donor-reactive T cells and antibody (35, 36). The recipient and donor class II MHC molecules (I-Ab and I-Abm12) are closely homologous, reflected by the reactivity of most anti-I-Ab antibodies with I-Abm12, so any induction of an anti-donor antibody response to the bm12 renal allografts would be likely to mediate autoreactive pathology in the recipient (13, 14, 29).
Our previous studies had indicated that depletion or absence of the Tregs dysregulated the bm12-reactive CD4 T cell response and provoked acute rejection of bm12 heart allografts (15). Treatment of wild type C57BL/6 recipients of bm12 renal allografts with anti-CD25 mAb prior to and following the transplant did not result in the appearance of the CD8 T cells reactive to I-Abm12 β chain peptides presented by Kb or Db. Furthermore, the reactive CD8 T cells were also induced by immunizing CCR5-deficient mice with I-Abm12 β chain peptides emulsified in CFA but this strategy failed to induce a similar CD8 T cell response in the wild type C57BL/6 mice. These findings suggest that the CCR5 deficiency uncovers or allows for the development of bm12-reactive CD8 T cell clones not present in wild type mice implicating an important role for chemokines and chemokine receptors in early T cell repertoire development and selection.
The results of this study reveal novel specificities of alloreactive CD8 T cells in CCR5-deficient recipients of single class II MHC renal and cardiac allografts. These CD8 T cells are activated through recognition of the peptide/class I MHC complexes on graft or recipient presenting cells through the direct (donor antigen-presenting cells) and indirect (host antigen-presenting cells) presentation pathways, respectively. To our knowledge these are the first observations of CD8 T cells reactive to class II MHC peptides presented by class I MHC. The response elicited is sufficient to provoke rejection of the renal allograft though not with the efficiency observed in the rejection of class I MHC-mismatched allografts that induce both donor-reactive CD8 T cell and antibody responses. It is important to note that about 1% of Caucasians express a homozygous mutation, Δ32, rendering CCR5 non-functional and renal transplantation has been performed in small numbers of Δ32 patients (37). Such Δ32 patients would have received renal grafts with greater disparity than a single class II MHC difference. Nevertheless, the available repertoire of alloreactive CD8 T cells in Δ32 patients is likely to be more diverse and of a higher frequency than that of a patient expressing functional CCR5 and may put such patients at increased risk of acute and/or chronic graft injury. Overall, the current studies in CCR5-deficient mice predict similar novel T cell specificities in these patients and warrant further studies.
Acknowledgements
We thank the staff of the Cleveland Clinic Biological Resources Unit for excellent care of the animals used in this study.
Footnotes
This work was supported by grants from the NIH, RO1 AI40459 to RLF and PO1 AI087586 to WMB, AV, and RLF.
References
- 1.Rosenberg AS, Singer A. Cellular basis of skin allograft rejecion: an in vivo model of immune-mediated tissue destruction. Annu. Rev. Immunol. 1993;10:333–358. doi: 10.1146/annurev.iy.10.040192.002001. [DOI] [PubMed] [Google Scholar]
- 2.Sho M, Yamada A, Najafian N, Salama AD, Harada H, Sandner SE, Sanchez-Fueyo A, Zheng XX, Strom TB, Sayegh MH. Physiological mechanisms of regulating alloimmunity: cytokines, CTLA-4, CD25+ cells, and the allorecctive T cell clone size. J. Immunol. 2002;169:3744–3751. doi: 10.4049/jimmunol.169.7.3744. [DOI] [PubMed] [Google Scholar]
- 3.Wood KJ, Goto R. Mechanisms of rejection: current perspectives. Transplantation. 2012;93:1–10. doi: 10.1097/TP.0b013e31823cab44. [DOI] [PubMed] [Google Scholar]
- 4.Afzall B, Lombari G, Lechler RI. Pathways of major histocompatibility complex allorecognition. Curr. Opin. Organ Transplant. 2008;13:438–444. doi: 10.1097/MOT.0b013e328309ee31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J. Immunol. 1999;162:352–358. [PubMed] [Google Scholar]
- 6.Heeger PS. T-cell allorecognition and transplant rejection: a summary and update. Am. J. Transplant. 2003;3:525–533. doi: 10.1034/j.1600-6143.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q, Chen Y, Fairchild RL, Heeger PS, Valujskikh A. Lymphoid sequestration of alloreactive memory CD4 T cells promotes cardiac allograft survival. J. Immunol. 2006;176:770–777. doi: 10.4049/jimmunol.176.2.770. [DOI] [PubMed] [Google Scholar]
- 8.Lee RS, Grusby MJ, Glimcher LH, Winn HJ, Auchincloss H., Jr Indirect recognition by helper cells can induce donor-specific cytotoxic T lymphocytes in vivo. J. Exp. Med. 1994;179:865–872. doi: 10.1084/jem.179.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pettigrew GJ, Lovegrove E, Bradley JA, Maclean J, Bolton EM. Indirect T cell allorecognition and alloantibody-mediated rejection of MHC class I-disparate heart grafts. J. Immunol. 1998;161:1292–1298. [PubMed] [Google Scholar]
- 10.Steele DJ, Laufer TM, Smiley ST, Ando Y, Grusby MJ, Glimcher LH, Auchincloss H., Jr Two levels of help for B cell alloantibody production. J. Exp. Med. 1996;183:699–703. doi: 10.1084/jem.183.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I, Gilbride RM, Lewis MS, Gilliam AN, Ventura AB, Malouli D, Xu G, Richards R, Whizin N, Reed JS, Hammond KB, Fischer M, Turner JM, Legasse AW, Axthelm MK, Edlefsen PT, Nelson JA, Lifson JD, Fruh K, Picker LJ. Cytomegalovirus vector violate CD8+ T cell epitope recognition paradigms. Science. 2013;340 doi: 10.1126/science.1237874. 1237874-1237871-1237817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rist M, Smith C, Bell MJ, Burrows SR, Khanna R. Cross-recognition of HLA DR4 alloantigen by virus-specific CD8+ T cells: a new paradigm for self-/nonself-recognition. Blood. 2009;114:2244–2253. doi: 10.1182/blood-2009-05-222596. [DOI] [PubMed] [Google Scholar]
- 13.McKenzie IF, Morgan GM, Sandrin MS, Michaelides MM, Melvold RW, Kohn HI. B6.C-H-2bm12. A new H-2 mutation in the I region of the mouse. J. Exp. Med. 1979;150:1323–1338. doi: 10.1084/jem.150.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mengle-Gaw L, Conner S, McDevitt HO, Fathman CG. Gene conversion between murine class II major histocompatibility complex loci. Functional and molecular evidence from the bm12 mutant. J. Exp. Med. 1984;160:1184–1194. doi: 10.1084/jem.160.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schenk S, Kish DD, He C, El-Sawy T, Chiffoleau E, Chen C, Wu Z, Sandner S, Gorbachev AV, Fukamachi K, Heeger PS, Sayegh M, Turka LA, Fairchild RL. Alloreactive T cell responses and acute rejection of single class II MHC-disparate heart allografts are under strict regulation by CD4+CD25+ T cells. J. Immunol. 2005;174:3741–3748. doi: 10.4049/jimmunol.174.6.3741. [DOI] [PubMed] [Google Scholar]
- 16.Yun JJ, Fischbein MP, Whiting D, Irie Y, Fishbein MC, Burdick MD, Belperio J, Strieter RM, Laks H, Berliner JA, Ardehali A. The role of MIG/CXCL9 in cardiac allograft vasculopathy. Am. J. Pathol. 2002;161:1307–1313. doi: 10.1016/S0002-9440(10)64407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nozaki T, Rosenblum JM, Schenk AD, Ishii D, Fairchild RL. CCR5 is required for regulatio of alloreactive T-cell respnses to single class II MHC-mismatched murine cardiac grafts. Am. J. Transplant. 2009;9:2251–2261. doi: 10.1111/j.1600-6143.2009.02786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bickerstaff AA, Wang JJ, Pelletier RP, Orosz CG. Murine renal allografts: spontaneous acceptance is associated with regulated T cell-mediated immunity. J. Immunol. 2001;167:4821–4827. doi: 10.4049/jimmunol.167.9.4821. [DOI] [PubMed] [Google Scholar]
- 19.Miyajima M, Chase CM, Alessandrini A, Farkash EA, Pelle PDella, Benichou G, Graham JA, Madsen JC, Russell PS, Colvin RB. Early acceptance of renal allograts in mice is dependent on FoxP3+ cells. Am. J. Pathol. 2011;178:1635–1645. doi: 10.1016/j.ajpath.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell PS, Chase CM, Colvin RB, Plate JM. Kidney transplants in mice: an analysis of the immune status of mice bearin long-term, H-2 incompatible transplants. J. Exp. Med. 1978;147:1449–1468. doi: 10.1084/jem.147.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bickerstaff A, Nozaki T, Wang J-J, Pelletier R, Hadley G, Nadasdy G, Nadasdy T, Fairchild RL. Acute humoral rejection of renal allografts in CCR5−/− recipients. Am. J. Transplant. 2008;8:557–566. doi: 10.1111/j.1600-6143.2007.02125.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Schlachta C, Duff J, Stiller C, Grant D, Zhong R. Improved techniques for kidney transplantation in mice. Microsurgery. 1995;16:103–109. doi: 10.1002/micr.1920160212. [DOI] [PubMed] [Google Scholar]
- 23.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. Transplantation. 1973;16:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Schenk AD, Nozaki T, Rabant M, Valujskikh A, Fairchild RL. Donor-reactive CD8 memory T cells infiltrate cardiac allografts within 24-h posttransplant in naive recipients. Am. J. Transplant. 2008;8:1652–1661. doi: 10.1111/j.1600-6143.2008.02302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afanasyeva M, Georgakopoulos D, Belardi DF, Ramsundar AC, Barin JG, Kass DA, Rose NR. Quantitative analysis of myocardial inflammation by flow cytometry in murine autoimmune myocarditis: correlation with cardiac function. Am. J. Pathol. 2004;164:807–815. doi: 10.1016/S0002-9440(10)63169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Setoguchi K, Schenk AD, Ishii D, Hattori Y, Baldwin WM, III, Tanabe K, Fairchild RL. LFA-1 antagonism inhibits early infiltration of endgenous memory CD8 T cells into cardiac allografts and donor-reactive T cell priming. Am. J. Transplant. 2011;11:923–935. doi: 10.1111/j.1600-6143.2011.03492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreisel D, Krupnick AS, Szeto WY, Popma SH, Sankaran D, Krasinskas AM, Amin KM, Rosengard BR. A simple method fo culturing mouse vascular endothelium. J. Immunol. Methods. 2001;254:31–45. doi: 10.1016/s0022-1759(01)00371-4. [DOI] [PubMed] [Google Scholar]
- 28.Valujskikh A, Lantz O, Celli S, Matzinger P, Heeger PS. Cross-primed CD8 T cells mediate graft rejection via a distinct effector pathway. Nat. Immunol. 2002;3:844–851. doi: 10.1038/ni831. [DOI] [PubMed] [Google Scholar]
- 29.Melino MR, Epstein SL, Sachs DH, Hansen TH. Idiotypic and fluorometric analysis of the antibodies that distinguish the lesion of the I-A mutant B6.C-H-2bm12 . J. Immunol. 1983;131:359–364. [PubMed] [Google Scholar]
- 30.Kreisel D, Krupnick AS, Gelman AE, Engels FH, Popma SH, Krasinskas AM, Balsara Kr, Szeto WY, Turka LA, Rosengard BR. Non-hematopoietic allograft cells directly activate CD8+ T cells and trigger acute rejection: an alternaitve mechanism of allorecognition. Nat. Med. 2002;8:233–239. doi: 10.1038/nm0302-233. [DOI] [PubMed] [Google Scholar]
- 31.Schnickel GT, Whiting D, Hsieh GR, Yun JJ, Fischbein MP, Fishbein MC, Yao W, Shfizadeh A, Ardehali A. CD8 lymphocytes are sufficient for the development of chronic rejection. Transplantation. 2004;78:1634–1639. doi: 10.1097/01.tp.0000141362.33931.40. [DOI] [PubMed] [Google Scholar]
- 32.Al-Lamkis RS, Bradley JR, Pober JS. Endothelial cells in allograft rejection. Transplantation. 2008;86:1340–1349. doi: 10.1097/TP.0b013e3181891d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skaro AI, Liwski RS, Zhou J, Vessie EL, Lee TDG, Hirsch GM. CD8+ T cells mediate aortic allograft vasculopathy by direct killing and an interferon-γ-dependent indirect pathway. Cardiovasc. Res. 2005;65:283–291. doi: 10.1016/j.cardiores.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 34.Valujskikh A, Heeger PS. Emerging roles of endothelial cells in transplant rejection. Curr. Opin. Immunol. 2003;15:493–498. doi: 10.1016/s0952-7915(03)00110-9. [DOI] [PubMed] [Google Scholar]
- 35.Bickerstaff A, Pelletier R, Wang J-J, Nadasdy G, DiPaola N, Orosz C, Satoskar A, Hadley G, Nadasdy T. An experimental model of acute humoral rejection of renal allografts associated with concomitant cellular rejection. Am. J. Pathol. 2008;173:347–357. doi: 10.2353/ajpath.2008.070391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun Q, Liu ZH, Cheng Z, Chen J, Ji S, Zeng C, Li LS. Treatment of early mixed cellular and humoral renal allograft rejection with tacrolimus and mycophenolate mofetil. Kidney Int. 2007;71:24–30. doi: 10.1038/sj.ki.5001870. [DOI] [PubMed] [Google Scholar]
- 37.Fischereder M, Luckow B, Hocher B, Wuthrich RP, Rothenpieler W, Schneeberger H, Panzer U, Stahl RAK, Hauser IA, Budde K, Neumayer H-H, Kramer BK, Land W, Schlondorff D. CC chemokine receptor 5 and renal-transplant survival. Lancet. 2001;357:1758–1761. doi: 10.1016/s0140-6736(00)04898-4. [DOI] [PubMed] [Google Scholar]