Emerging evidence within both the animal and human literatures suggests that cortisol levels and cortisol patterning are influenced by early experience. Rodent models have shown that maternal caregiving behavior shapes rat pup cortisol reactivity to stress (1), and recent work with young children suggests that cortisol patterning across the day and cortisol responses to stress are sensitive to quality of caregiving (2–6). The current study sought to examine the associations between quality of maternal care at bedtime and cortisol levels and patterning in very young infants.
Infant Cortisol Secretion and Mental Health
Cortisol, the predominant glucocorticoid produced by humans, is secreted by the adrenal glands as the final product of the hypothalamus-pituitary-adrenal (HPA) axis (7). In typical individuals, cortisol secretion follows a particular pattern across the day. This circadian rhythm consists of a spike in cortisol approximately 30 minutes after awakening in the morning, called the cortisol awakening response (CAR), followed by decreasing levels across the day and a slight rise throughout the night to moderate levels by morning (8). Thus, cortisol levels are generally at their lowest overnight and increase across the early morning hours. Infants are born without a circadian pattern in cortisol secretion, and studies cite varying estimates for when infants first establish the typical rhythm, ranging from as early as 2-3 months (9–11) to as late as 9 months (12). The range in these estimates may be due to substantial interindividual variability in the timing of the emergence of this rhythm, as well as in its stability once established in infancy (8,13). For example, de Weerth et al. (8) found the presence of a cortisol rhythm to appear and disappear across monthly samples in infants assessed from 2 to 5 months of age.
Previous research has shown that deviations from the typical rhythm are related to both internalizing and externalizing behavior. Hyperarousal of the HPA axis, such that an individual shows elevated cortisol levels across the day, has been associated with internalizing behavior in both early and middle childhood (14–16). Hypoarousal of the HPA axis, such that an individual shows reduced cortisol secretion across the day, is generally associated with externalizing behavior (17-18). There are exceptions to these patterns, and it may be that linkages between cortisol levels and behavior depend on the child's age and developmental history (17-18). It is also unclear to what degree atypical cortisol rhythms observed in later childhood are rooted in atypical cortisol rhythms established at earlier points in development. Nevertheless, the establishment of a circadian cortisol rhythm in early infancy may be foundational for stress regulation in later childhood, and decreased risk for problem behaviors.
Early Caregiving Quality and Infant Cortisol
Consistent with theory and studies of stress reactivity in rodents, human infant cortisol regulation appears to be influenced by early maternal caregiving experiences. Albers and colleagues (2) found that newborn infants who experienced higher quality maternal care during a bath showed better cortisol regulation in response to the stress of removal from the bath, such that lower quality maternal behavior was associated with higher levels of infant cortisol 40 minutes post-bath. This suggests that infants who experienced less sensitive care during the bathtime were less able to regulate their cortisol response to the stress of removal from the bath. Similarly, Grant et al. (4) found that 7 month old infants whose mothers were rated as less sensitive in a free play context showed a more prolonged cortisol recovery from the still-face paradigm. These studies with human infants corroborate findings from rodent work suggesting that early experience influences HPA axis functioning. Furthermore, there is some evidence that specific aspects of maternal caregiving quality may be particularly relevant to infant cortisol functioning. For example, a recent study found that maternal sensitivity, cooperation, and higher quality of physical contact during infant bathtime were associated with a reduced cortisol response to the stress of a bath, whereas quality of maternal vocal contact and maternal acceptance of the infant were not (19). It is possible that these influences are context-dependent, though work is needed that compares maternal care across different contexts.
While previous studies have addressed linkages between parenting quality and infant cortisol reactivity and regulation in response to specific stressors, no work to-date has examined the association between parenting and cortisol levels and patterning in very young infants. Thus, the principal aim of the present study was to examine the relationship between quality of maternal care during infant bedtimes and cortisol levels and patterning in young infants. That quality of parental care can influence cortisol patterning was suggested in a recent study by Luijk and colleagues (5), who found attachment security to be associated with cortisol patterning in 14 month old infants. The authors found infants classified as disorganized in the Strange Situation procedure to show a flattened diurnal cortisol rhythm. Because quality of maternal care is theorized to be the primary determinant of attachment security in infancy (20), this study suggests that infants receiving lower quality caregiving may be less likely to show the typical cortisol rhythm than their peers. Additionally, previous work has found an association between maternal sensitivity and cortisol levels and patterning in older children. Murray and colleagues (6) found that maternal withdrawal measured when children were 9 months old predicted elevated mean and morning cortisol when the children were adolescents. Furthermore, Fisher and colleagues found maternal emotional support of their preschool aged children during a challenge task to be related to children's cortisol, such that children of less supportive mothers showed less of a decrease in cortisol across the day (3). Together, these studies suggest that caregiving that is stressful or that inadequately buffers children from stressful experiences is associated with elevations in cortisol and deviations from the typical cortisol rhythm. The present study extends this work by examining the association between quality of bedtime parenting and infant cortisol during the first 3 months of life.
The Present Study
The present study examined the association between maternal emotional availability (EA) during infant bedtime and infant cortisol levels and patterning at 1 and 3 months. This study contributes to the existing literature concerning parenting and infant cortisol in several ways. First, it examined parenting as a predictor of infant cortisol in the first 3 months of life, when studies suggest a circadian rhythm in cortisol is first becoming established in some infants (9–11). Additionally, this study examined maternal EA in a unique context- bedtime. Because infants may be especially tired at bedtime, they may have reduced tolerance for stress and therefore require additional help in regulating their emotions. Thus, parents’ ability to soothe their children and create a quiet, safe environment which allows them to fall asleep may be particularly relevant to infant regulatory processes such as cortisol secretion. In order to examine the relevance of the bedtime context for infant cortisol functioning, this study compared the associations between infant cortisol and maternal EA at bedtime to the associations between infant cortisol and maternal EA during face-to face play, which is more traditionally examined. Additionally, this study explored which dimensions of maternal EA were most closely related to infant cortisol levels and patterning. To our knowledge, this study represents the first examination of the association between maternal EA at bedtime and infant cortisol, and the first to assess the association between quality of parental care and cortisol levels and patterning during the first 3 months of life.
Hypotheses
The following hypotheses were proposed:
Hypothesis 1a: Because early maternal withdrawal behavior has been associated with elevations in cortisol in children (4, 6), we hypothesized that higher maternal emotional availability at bedtime would be linked to lower concurrent cortisol levels at 1 and 3 months. We examined the area under the curve with respect to ground (AUCG) and area under the curve with respect to increase (AUCI) (21) in addition to cortisol levels at each time point sampled. AUCG is a measurement of total cortisol secretion that takes into account the amount of time between samples as well as absolute cortisol levels at each time point. AUCI measures total cortisol secretion with respect to cortisol levels at initial time of assessment while also taking into account amount of time between samples. We expected the maternal EA subscales to generally mirror the findings for overall EA, with higher ratings associated with lower infant cortisol levels. Because it was not clear from previous work which subscales would be most closely related to infant cortisol, however, we did not make specific hypotheses regarding the individual subscales. Thus, these analyses were exploratory.
Hypothesis 1b: In addition to the concurrent hypotheses concerning the associations between maternal EA and cortisol levels, we also predicted that higher 1 month bedtime EA would be longitudinally associated with lower 3 month cortisol levels, as maternal EA may shape infant cortisol across time. We expected the individual EA subscales at 1 month to generally be associated with lower infant cortisol levels at 3 months.
Hypothesis 2a: We hypothesized that 3 month old infants of more emotionally available mothers at bedtime would show greater evidence of a decline in their cortisol levels across the evening, followed by an increase across the nighttime into the morning, than infants of mothers who were less emotionally available. This is consistent with previous work relating sensitive parenting to optimal cortisol patterning in childhood (2–6). However, we did not anticipate finding a link between 1 month EA and 1 month infant cortisol patterning, because infants as young as 1 month old have not been shown to demonstrate a circadian rhythm in cortisol. Additionally, we hypothesized associations that higher scores on the individual maternal EA subscales would be associated with more optimal infant nighttime cortisol patterning at 3 months.
Hypothesis 2b: In addition to the concurrent hypothesis regarding the association between maternal EA and the shape of the cortisol rhythm at 3 months, we also predicted that higher 1 month EA at bedtime would be longitudinally associated with an evening decline and overnight rise in cortisol at 3 months, as maternal EA may influence infant cortisol across time. We also predicted that higher ratings on the individual maternal EA subscales would be longitudinally associated with more optimal infant cortisol patterning.
Hypothesis 3: Finally, we hypothesized that maternal EA at bedtime would be more closely related to infant cortisol across the night than maternal daytime EA. We based this hypothesis on the expectation that infants are likely to be more tired, have reduced tolerance for stress, and require more parental assistance to regulate their emotions at bedtime than during routine play with mothers during the day. Furthermore, bedtime maternal EA is more proximal in time to nighttime cortisol levels and patterning than maternal daytime EA.
Methods
Participants
Participants in the study were part of Project SIESTA (Study of Infants’ Emergent Sleep TrAjectories), a NIH-funded, ongoing, longitudinal study of parenting, infant sleep, and infant development (R01HD052809) at a rural northeastern university in the United States. Funding was awarded to the sixth author. Mothers were approached by project staff at local hospitals when their infants were born and given information regarding the study. Study procedures were reviewed and approved by the university Institutional Review Board as well as the hospitals’ Institutional Review Boards. Two weeks following the infant's birth, mothers who expressed interest in the study were called and recruited into the project. Informed consent was obtained from parents during the first visit to the home when their infant was 1 month old.
One hundred sixty seven infants (47% male) and their families were recruited in the sample and completed the 1 month assessment. Mean infant age was 1.21 months (SD = .16) at the time data collection began. Sixty-two infants were firstborns, and 105 were later born. Eighty-one percent of mothers were married and living with their infant's father. Mothers’ average age was 29.4 years old (SD = 5.3), and they ranged in age from 18 to 43 years old. Eighty-four percent of mothers were White, 4% were Asian-American, 3% were African-American, 5% were Latino, and 4% identified themselves as “Other.” Eighty-seven percent of mothers completed some post-secondary education, and 62% were employed outside the home. Median yearly family income was $65,000 (SD = $47,000) and ranged from $9,500 to $300,000. Forty-eight percent of the infants slept in the same room as their parents but in a separate bed, 26% slept in a separate room, 12% slept in their parents’ bed, and 14% slept in a combination of places across the night. At 1 month, 26 infants were in child care. One hundred fifty-six of these dyads participated in the study when the infants were 3 months old. Mean infant age at this time point was 3.09 months (SD = .31). Twenty-eight percent of the infants slept in same the room but a separate bed from their parents at this age, 54% slept in a separate room, 7% slept in the same bed, and 11% slept in a combination of places. Fifty-eight of these infants were in child care. The 11 dyads who did not participate in the study at 3 months were not significantly different from the originally recruited sample in terms of any demographic characteristics collected.
However, complete maternal emotional availability data at bedtime were only available for subgroups of the larger SIESTA sample (N = 113 at 1 month, N = 106 at 3 months), either because families did not consent to be videorecorded (N = 3), turned on the video cameras too late during bedtime to allow a sufficient amount of parent-infant interaction time to be recorded (at least 3 consecutive minutes), or conducted bedtime in rooms other than those being videotaped. At 1 month, average bedtime length was 77 minutes (SD = 47 minutes). At 3 months, average bedtime length was 71 minutes (SD = 65 minutes). Within the subsamples, 17 infants were missing cortisol data for at least one time point at 1 month, and 18 were missing cortisol data for at least one time point at 3 months. Cortisol data were missing because parents did not remember to take a sample (N = 12 at 1 month, N = 13 at 3 months) or did not record the time the sample was taken, (N = 5 at 1 month, N = 2 at 3 months). Because cortisol values change across the day, time of sampling was necessary for all analyses. Thus, analyses were conducted on 96 one month old infants and 88 three month old infants in the current study.
Because a substantial amount of the sample was excluded from analyses, dyads who were missing bedtime EA and cortisol data were compared at each age point to the sample originally recruited at 1 month. One-way ANOVA analyses revealed that dyads missing data at 1 or 3 months were not significantly different from the full sample of 167 families in terms of mothers’ age, family income, or infant age at each of the visits. Chi-square tests also indicated that there were no significant differences between those missing data at each age point and the full sample with respect to mothers’ employment status, infant gender, infant sleep location, or whether the infant was a first or later-born. However, dyads with mothers who attended at least some college were more likely to have complete data at 1 month than families with mothers who did not attend college, χ2(1, N = 167) = 4.63, p < .05. Additionally, dyads with mothers who were married and/or living with their partner were more likely to have complete data at 3 months than mothers who were single and not living with a partner, χ 2(1, N = 156) = 5.70, p < .05, as were dyads in which the infant was not in day care χ 2(1, N = 156) = 5.39, p < .05. Dyads with mothers who identified themselves as Caucasian were also more likely to have complete data at 3 months than mothers who identified themselves as another race, χ 2(1, N = 156) = 8.88, p < .01.
Overall Procedure
Families were visited at three time points across seven days of data collection at both 1 and 3 months of infant age. At the first visit, parents were given a variety of questionnaires to complete, including one questionnaire requesting demographic information and seven sleep diaries to record the times the infant spent sleeping each of the days. When infants were 3 months old, mothers were also given a questionnaire regarding infant temperament. On one day of data collection, project staff set up video and audio recording equipment within each family's home in order to capture parents’ interactions with their infants at bedtime, as well as across the night. This same day, parents were given materials with which to collect and store their infant's saliva for cortisol assay. Project staff returned on the following day in order to collect the equipment and samples. During one day of data collection at each time point, mothers and infants were also videotaped engaging in face-to-face play for 5 minutes.
Questionnaire Measures
Demographic questionnaire
At each age point mothers reported on their demographic characteristics, including their age, level of education, whether they were breastfeeding their infant, their income, and whether their infant was regularly in child care, and if so, the type of child care the infant received.
Infant sleep diary
Because infant sleep quality has been associated with cortisol patterning (8,13, 22-23), infant daytime nap duration and nighttime waking duration across the seven days of data collection was assessed at 1 and 3 months. Because infant sleep location has also been associated with cortisol (24), sleep arrangements were measured as well. For each of the seven days of data collection, parents completed a sleep diary, adapted from Burnham et al. (25), in which they indicated the times when their infants fell asleep and woke up during both the day and night, as well as the location(s) in which their infant slept during the night.
Parents also indicated on the diary whether they fed their infant prior to putting him or her to sleep and during the night when he or she woke up. Because feeding may also affect cortisol levels, dummy variables were created reflecting whether the infant was fed prior to sleep and whether the infant was fed during the night the cortisol samples were taken.
Finally, parents noted on the diary if any special events had taken place that day, which included if their infant was ill and/or taking medication. Home visiting staff took note of this as well. Although great care was exercised in scheduling the visits for days when the infant was healthy, parents sometimes reported that their infant was not feeling well at the time of the visits. Thus, a dummy variable was created reflecting parents’ report about whether the infant was ill during the night the video was recorded and the cortisol samples were taken, and a second dummy variable was created representing whether the child was taking medication.
Infant temperament questionnaire
Because child temperamental difficulty has been associated with cortisol levels and patterning (15, 26-27), infant temperament was assessed at 3 months old. Mothers rated their infant's temperament using the Infant Behavior Questionnaire-Revised (IBQ-R) (28). The IBQ-R consists of 191 items that tap into three broad dimensions: surgency/extraversion, negative affectivity, and orienting/regulation. For each item, mothers rated how often their infant displayed a particular behavior in the past week on a 7-point Likert-type scale (i.e. When face was washed, how often did the baby fuss or cry?; 1 = Never, 7 = Always). For the current study, the negative affectivity dimension was used in analyses. Internal consistency for this subscale has been demonstrated to be high, with a Cronbach's alpha of .91. In the current study, the alpha for the negative affectivity subscale was .96.
Bedtime Videos
Video collection
Project staff set up video and audio recording equipment within each family's home on one day of data collection at both 1 and 3 months, consulting with parents about the locations in the home where the infant was taken to prepare for bed, where the infant slept, and where the infant was taken upon awakening during the night. In most homes, 2-4 cameras were required to capture the majority of parent-infant interaction across bedtime and nighttime. Video and audio recordings were made by a Bosch Divar XF 8-Channel Digital Versatile Recorder. Video was captured using infrared security cameras made by ARM Electronics (Model No. C420BCVFIR), and audio information was collected via Channel Vision microphones (Model No. 5104-MIC). Parents and project staff were able to view the recordings on an Audiovox Portable LCD Monitor and DVD Player (Model No. D9000). If the infant's bedtime and nighttime routines took place across different rooms, up to two cameras were set up wirelessly. For all families, one camera was placed above the crib or bed where the infant slept. Up to three additional cameras were placed such that they provided a view of a chair where the infant was fed or rocked, the infant's changing table, or an overview of the room, depending on what the parents communicated to project staff as the infant's bedtime and nighttime routine. Cameras were positioned using boom stands, gorilla pods, or foam pieces such that they were as unobtrusive to the families as possible. All equipment was connected to one power strip that staff asked parents to turn on one hour prior to bedtime to ensure that all of the bedtime routine was captured on camera. Parents were asked to turn off the cameras in the morning, after their infant was awake and out of bed for the day.
Maternal emotional availability coding
Maternal emotional availability during the infant's bedtime was scored using the Emotional Availability Scales (EAS) (29). The EAS is designed to measure the emotional quality of parents’ interactions with their children and consists of four scales: sensitivity, structuring, nonintrusiveness, and nonhostility. Two additional scales assessing children's emotional availability to the parent were not scored in this study because of the very young age of the infants. In previous studies, maternal EA in the context of interaction with infants has been shown to be negatively associated with maternal depression (30) and positively associated with infant attachment (31) and infant sleep quality (32). All coding of maternal EA was conducted by a reliable coder, who was certified on EAS 2007.
The present study was concerned with maternal EA at bedtime, rather than during night wakings, because the longest bouts of mother-infant interaction took place at this time. Similarly, this study concentrated on maternal rather than paternal EA because of the limited number of tapes with sufficient father-infant interaction at bedtime to code for EA. Coders scored bedtime from when the infant first appeared on camera until the end of five consecutive minutes of infant sleep, where sleep was determined by closed eyes and the absence of gross motor movement. Because EA is typically coded across parent-infant play contexts, maternal EA in this study was coded using the same adaptations to the bedtime context described by Teti et al. (32). Briefly, highly sensitive mothers were rated as such when they showed awareness of infant cues, as well as accurate interpretation and response to these signals. If mothers did not respond to distressed infant vocalizations following one minute of crying, they were scored lower for sensitivity. Mothers of infants who slept in a separate bedroom could still receive high scores for sensitivity if they did not respond to nondistressed infant vocalizations after their infants were placed in their cribs. Mothers received high scores for structuring when they used quiet, soothing routines that guided their infants toward sleep. Mothers scored highly on nonintrusiveness by keeping the volume of their voices low and quiet when talking to their infants or other family members, and reducing the number of initiated interactions with their infants as they came closer to sleep. Mothers were rated highly for nonhostility when they showed no overt or covert irritability or anger towards their infants during bedtime. Following completion of EA scoring, coders recorded the length of the bedtime in minutes.
Interrater reliability was established between two coders on the four EA dimensions based on 8 tapes when infants were 1 month old and 8 tapes when they were 3 months old. Intraclass correlations (ICC's) for maternal sensitivity, structuring, nonintrusiveness, and nonhostility were calculated at each age point and ranged from .70-.98. A composite maternal EA score was created by converting the scores for each of the four dimensions to z scores, then taking the mean for the converted scores across the standardized dimensions. The ICC for composite EA was .98 at both 1 and 3 months.
Daytime Videos
Video collection
At each time point, mothers and infants were recorded engaging in face-to-face play for 5 minutes. Mothers were asked to limit other distractions in the room during this time, but could play with their infant in any way and with any materials they wished. Project staff recorded this interaction using a handheld camera.
Maternal emotional availability coding
As with the bedtime videos, maternal emotional availability was scored for the daytime interactions using the Emotional Availability Scales (EAS) (30).
Interrater reliability was established between two coders on the four EA dimensions based on 30 tapes. Intraclass correlations (ICC's) for maternal sensitivity, structuring, nonintrusiveness, and nonhostility ranged from .84-.93. A composite daytime maternal EA score was created by converting the scores for each of the four dimensions to z scores, then taking the mean for the converted scores across the standardized dimensions. The ICC for composite EA was .82.
Cortisol Collection and Assay
Cortisol sampling
Infant cortisol was assessed via saliva samples taken the same night as the bedtime video when infants were 1 and 3 months old. Parents were provided with three pieces of filter paper to place on their infant's tongue for 2 minutes at three time points across the evening and following morning. The first sample was taken in the late afternoon, preceding dinnertime, the second sample was taken just before the infant fell asleep at bedtime, and the third sample when the infant awoke in the morning. These times were selected in order to model the change in infant cortisol across the evening and nighttime hours. In order to control for effects of feeding on cortisol levels, staff asked parents to refrain from feeding their infants for at least a half hour prior to the late afternoon sample, and to take the morning sample prior to feeding the infant. Because many infants are fed just prior to sleep, we did not think it was possible to ask parents not to feed their infants for a half hour before taking this sample. Instead, parents recorded whether the infant was fed on the sleep diaries.
Parents placed each sample in labeled plastic conical tubes following collection and stored the tubes in their freezer. They also indicated the times the samples were collected on a provided sheet. The tubes were transported in cooler bags and stored in a freezer in the laboratory until assay was conducted by project staff. The filter paper method has been shown to be a valid measure for collecting saliva and assessing cortisol levels in very young infants (33). Additionally, ranges of cortisol levels assessed via the filter paper method have been shown to be comparable to those detected using alternate methods of collecting saliva from infants.
Cortisol assay
Prior to assay, the tubes were removed from the laboratory freezer and placed in racks for 10-15 minutes at room temperature to thaw. The tubes were wiped to remove condensation and then weighed, and these post-weight values were subtracted from the pre-weights of the tubes, which were taken prior to distributing them to the families. The weight difference from pre to post saliva collection was used as a multiplicative coefficient to control for the amount of saliva absorbed into the filter paper. Next, 1500 μL of diluted (10:1) wash buffer solution was added to each tube. Tubes were then placed on a test tube rotator for 24 hours in order to extract the saliva samples from the filter paper. Following rotation, tubes were refrozen. On a separate day, the tubes were defrosted and vortexed for 15 seconds in order to remix the cortisol and wash buffer solution. Next, 600 μL of each sample were pipetted into correspondingly labeled Eppendorf tubes. The Eppendorf tubes were weighed prior to and following this step in order to determine the volumes of the saliva samples collected. These Eppendorf tubes were placed in a SpeedVac machine for 4-10 hours in order to concentrate the samples to 120 μL, then weighed again to determine the exact sample volumes following concentration.
On a separate day, the samples were assayed using the Salimetrics, LLC High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit (catalog No. 1-3002). Prior to assay, samples were placed in racks at room temperature until thawed, then centrifuged for 15 minutes at 4500 rpm. Assays were carried out by the second author, following the procedures included with the kit. Because of the number of samples to be analyzed (cortisol data are collected for mothers, fathers, and infants across seven time points in SIESTA), we were not able to analyze samples in duplicate. However, the controls were plated twice and their concentrations were used order to determine the intra- and inter-assay coefficients, which were 5% and 14%, respectively. Samples with a cortisol value greater than 3.0 μg/dL were reanalyzed.
Results
Preliminary Analyses
First, the cortisol data were examined for the presence of outliers. At 1 month, there were 10 cortisol values greater than three standard deviations above the time point mean (2 at T1, 4 at T2, 4 at T3), and at 3 months there were 9 cortisol values greater than three standard deviations above the time point mean (3 at T1, 4 at T2, 2 at T3). These outliers were winsorized by replacing them with the next highest value within the three standard deviations range (34). Outliers ranged in value from 6.17-24.27 μg/dL. Because some previous work has removed cortisol values greater than 7.25 μg/dL (35), we ran the analyses twice; once with outliers above 7.25 μg/dL removed and once with these values winsorized. The results were the same with both methods. Because any sample with a cortisol values greater than 3.0 μg/dL was assayed twice, and the results were the same whether these high values were windsorized or removed, we elected to use the windsorized values in order to use more of the data.
Next, AUCG and AUCI were computed at each age, following the steps outlined by Pruessner and colleagues (22). Computation of AUCG and AUCI required that the infants had complete cortisol data, as well as a complete record for the times the cortisol samples were taken. Descriptive information for the infant cortisol and time of sampling data is presented in Table 1.
Table I.
Descriptive information for infant Cortisol and time of sampling variables
| AUCG (ug/dL) | AUCI (ug/dL) | Late afternoon cortisol (ug/dL) | Late afternoon sample time (hr:min) | Bedtime cortisol (ug/dL) | Bedtime sample time (hr:min) | Morning cortisol (ug/dL) | Morning sample time (hr:min) | |
|---|---|---|---|---|---|---|---|---|
| 1 month | ||||||||
| Mean | 4.06 | −.47 | .32 | 6:10pm | .22 | 9:50pm | .37 | 8:10am |
| SD | 6.36 | 7.06 | .69 | 1:20 | .42 | 2:40 | .69 | 1:30 |
| 3 months | ||||||||
| Mean | 3.93 | 1.85 | .15 | 6:20pm | .23 | 9:30pm | .41 | 8:00am |
| SD | 6.97 | 6.38 | .24 | 1:10 | .64 | 1:40 | .60 | 1:30 |
Notes: N = 96 at 1 month
N = 88 at 3 months
At 1 month, composite bedtime EA ranged from -3.52 to 1.10, (M = .00, SD = .80), and at 3 months composite EA ranged from -2.93 to .91, (M = .00, SD = .77). The 1 month and 3 month maternal EA composites were moderately correlated, r(73) = .59, p < .001. At 1 month, mothers who were more emotionally available at bedtime were less likely to feed their infants at bedtime, were older, and had more years of education and higher incomes than mothers who were less emotionally available, rs = -.28, .30, .37, and .34, respectively, all ps < .05. The length of their infant's bedtime was also shorter, r = -.27. At 3 months, mothers who were more emotionally available at bedtime had fewer children, were older, and had more years of education and higher incomes, rs = -.21, .22, .41, and .26, respectively, all ps < .05. The length of their infant's bedtime was shorter, r = -.40. Maternal EA was not correlated with the times the samples were taken at either age point, all ps > .10.
Identification of covariates
With the exception of analyses involving AUCI and AUCG (which incorporates time of sampling into its calculation), time of cortisol sampling was always used as a covariate in all analyses because cortisol values are strongly time-sensitive across the day (8). Other variables, however, were examined as potential covariates, based on whether they were significantly associated with cortisol values at 1 or 3 months. Based on prior work relating socio-demographics (36), daytime and nighttime sleep (8,13, 23-24), sleep arrangements (25), child care (37) and difficult child temperament (15, 27-28) to infant cortisol values, several variables in the present study, from the demographics, sleep diary, and temperament assessments, were examined as possible additional covariates in the main analyses. These included maternal age and education, family income, whether the infant was in child care, number of children in the home, number of hours the infant spent sleeping during the day of cortisol sampling, number of hours the infant spent awake during the night, infant sleep location, infant negative affectivity, infant gender, and whether the infant was not feeling well or on medication, breastfeeding, fed prior to going to sleep, or fed during nighttime awakenings. Among these, amount of time the infant spent napping on the day of cortisol sampling was associated with cortisol values, and only at 1 month: r(86) = -.26 for cortisol AUCI, r(86) = .33 for cortisol AUCG , r(86) = .40 for afternoon cortisol levels, and r(86) = .33 for bedtime levels, all ps < 01. Attendance in child care was associated with a smaller AUCI at 1 month, F (1, 91) = 4.36, p < .05 and higher awakening cortisol at 3 months, F (1, 86) = 4.22, p = < .05. Type of child care was not associated with infant cortisol. Analyses testing study hypotheses were run with and without total nap duration and child care included (along with time of cortisol sampling). All statistically significant relationships between maternal EA and infant cortisol levels remained significant whether nap duration and child care were included or not. Thus, all analyses only included time of sampling as a covariate, and did not include nap duration and child care.
Furthermore, because of the large variability in time spent engaged in bedtime across participants, length of bedtime was also examined as a potential covariate. Bedtime length was not correlated with any of the cortisol samples at either time point. All statistically significant relationships between maternal EA and infant cortisol levels remained significant whether bedtime length was included or not. For these reasons, we felt that despite variation in the length of the bedtimes, we were able to adequately score maternal EA at bedtime for each family.
Primary Analyses
All analyses were conducted for the sample with complete maternal bedtime EA, cortisol, and time of sampling data. Complete data were available for 96 infants at 1 month and 88 infants at 3 months.
Maternal EA and Infant Cortisol Levels
To test hypotheses 1a and 1b, regression analyses were conducted using the 1 month, 3 month, and longitudinal data in order to determine if maternal EA at bedtime predicted infant AUCG and AUCI, as well as cortisol levels at each time point assessed. By measuring maternal EA across a typical infant bedtime, we believe that we were able to tap into mothers’ usual level of emotional availability in this context. This idea was supported by moderate stability in bedtime EA across time in the current study. (r = .59 from 1 to 3 months). Additionally, maternal daytime and bedtime EA were correlated at 3 months, r = .33, p < .01, suggesting that maternal EA is associated across contexts at this age point. For these reasons, we believed that the infant cortisol sample taken in the afternoon could be studied with relation to maternal EA at bedtime, though it must be noted that there was still substantial variability in maternal EA across time and context.
1 month
Regression analyses were conducted to test hypothesis 1a, that higher maternal EA at bedtime was concurrently associated with lower infant cortisol levels, at 1 month. To test this hypothesis, AUCG and AUCI as well as infant cortisol levels at late afternoon, bedtime, and morning awakening were regressed onto maternal EA. For the individual cortisol level variables, time of sampling was entered in the model as a covariate. Results indicated a trend suggesting that higher maternal EA was related to smaller AUCG, β= -.18, t(94) = -1.80, p < .10; R2 = .03, F(1, 94) = 3.25, p < .10. Maternal EA was not significantly associated with AUCI or infant cortisol at the individual time points.
To explore whether there were specific components of maternal EA at bedtime that accounted for the marginally significant association between maternal EA and infant AUCG, we correlated each of the EA dimensions (sensitivity, structuring, nonhostility, nonintrusiveness) with AUCG. These analyses revealed that higher scores for structuring and nonhostility were significantly associated with smaller AUCG for infants at 1 month, r(94) = -.20 and -.27, both p < .05.
3 months
A second set of regression analyses was conducted to test hypothesis 1a, that higher maternal EA at bedtime was concurrently associated with lower infant cortisol levels, at 3 months. These analyses indicated that infants of more emotionally available mothers had smaller AUCG and AUCI, p < .05 for both. Infants of more emotionally available mothers also had lower cortisol levels at bedtime, p < .05, and there was a trend suggesting an association between higher maternal EA and lower morning cortisol, p < .06. Maternal EA was not significantly associated with infant cortisol in the late afternoon. These results are presented in Table 2.
Table II.
Results of concurrent multiple regression analyses, 3 months
| AUCG (ug/dL) | AUCI (ug/dL) | Late afternoon cortisol (ug/dL) | Bedtime cortisol (ug/dL) | Morning cortisol (ug/dL) | |
|---|---|---|---|---|---|
| β | β | β | β | β | |
| Time of sampling (hr) | -- | -- | .07 | −.14 | −.08 |
| Maternal EA | −.24* | −.25* | .00 | −.26* | −.21† |
| R2 | .06 | .06 | .00 | .07 | .04 |
| F | 5.47* | 5.91* | .19 | 3.42* | 1.88 |
Notes: N = 88
** Significant at p ≤.01
Significant at p ≤ .05
Significant at p ≤ .06
To again explore whether there were specific components of maternal EA at bedtime that drove the associations between maternal EA and infant AUCG and AUCI as well as bedtime and morning cortisol, we correlated each of the EA dimensions with AUCG and AUCI and ran partial correlations between the EA dimensions and infant bedtime and morning cortisol at 3 months, controlling for time of sampling. Higher scores for structuring and nonintrusiveness were related to smaller AUCG, r(86) = -.21 and -.27, both p < .05, as well as smaller AUCI, r(86) = -.21 and -.21, both p < .05. Additionally, higher scores for structuring were associated with lower cortisol levels at bedtime, partial r(85) = -.22, p < .05, and higher scores for nonintrusiveness were associated with lower levels of cortisol in the morning, partial r(85) = -.29, p < .01.
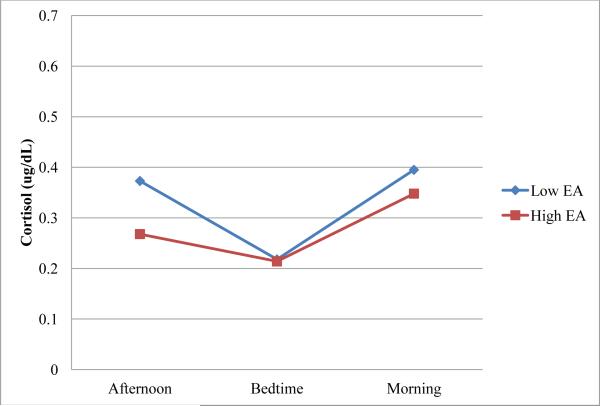
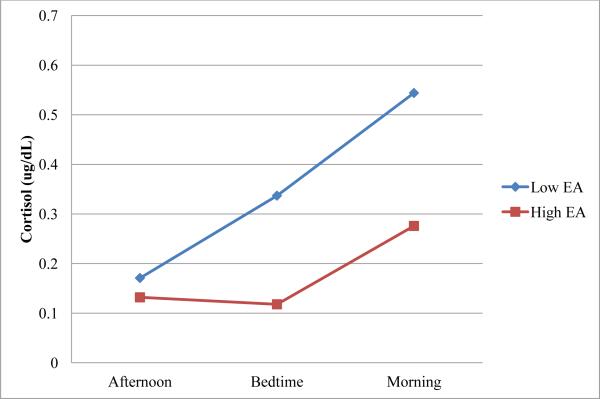
To illustrate the concurrent associations between maternal bedtime EA and infant cortisol, at each age point maternal composite EA was split at the median to create two subgroups of equal size, one subgroup consisting of infants whose mothers were above than the sample median in emotional availability and the other subgroup consisting of infants whose mothers received scores below the sample median for EA. Median EA was .28 at 1 month and .27 at 3 months. Mean cortisol levels at each time point (late afternoon, bedtime, morning) for these subgroups are plotted in Figures 1-2.
Figure 1.
Cortisol levels over time for infants at 1 month.
Figure 2.
Cortisol levels over time for infants at 3 months.
Longitudinal analyses
Lastly, regression analyses tested hypothesis 1b, that higher maternal bedtime EA at 1 month was associated with lower infant cortisol levels at 3 months. Maternal bedtime EA at 3 months was controlled for in these analyses. These analyses were not significant.
Although it was not the focus of our study, we also explored the possibility that infant cortisol levels at 1 month predicted maternal EA at 3 months. Parents may have a primary role within parent-child interactions, but bidirectionality exists within the relationship (38, 39). Thus, it is also possible that infants who show poorer cortisol regulation, in the form of higher cortisol levels and a less of a diurnal pattern in cortisol, may be more difficult to care for and therefore evoke lower quality caregiving from their parents. We tested this possibility by regressing maternal bedtime EA onto infant AUCG and cortisol levels at each time point. None of these associations was significant.
Maternal EA and Infant Cortisol Patterning
To test hypotheses 2a and 2b, multilevel modeling analyses were conducted in SAS (version 9.3) using the 1 month, 3 month, and longitudinal data in order to determine if maternal EA at bedtime was associated with infant cortisol patterning across the night. The models tested were two-level, with infant cortisol at the three time points (late afternoon, bedtime, morning awakening) as the level 1 variable and maternal EA as the level 2 variable.
1 month
Multilevel model analyses were conducted to test hypothesis 2a, that higher maternal EA at bedtime was associated with greater evidence of an evening decline and overnight increase in cortisol, at 1 month. These analyses were used to characterize cortisol secretion across the night, as well as to examine differences in cortisol patterning across time with respect to maternal emotional availability. Because cortisol levels are expected to decline from afternoon to bedtime, and then rise across the night to morning awakening (8), we expected the change in cortisol to have both linear and quadratic components. Analyses were run using maximum likelihood estimation and specifying an autoregressive covariance structure, which accounts for the dependency in cortisol samples taken a few hours apart. An unconditional model was tested first, including parameters for linear and quadratic change in cortisol over time. These parameters were created by centering the time of sampling variable, then entering it into the model for the linear component, as well as entering the time of sampling squared variable into the model for the quadratic component. At 1 month, neither the linear nor quadratic component was significant, suggesting that cortisol levels did not follow a regular linear or quadratic pattern across the night at this early age.
Next, a conditional model was specified by including variables for maternal emotional availability as well as interactions between maternal bedtime EA with linear change and with quadratic change over time. The estimates for maternal EA and for the interactions of maternal EA with time were not significant. Thus, there were no significant differences in cortisol patterning across time with respect to maternal EA at 1 month.
In order to determine if any of the infants in our sample showed evidence of patterning in their cortisol, we re-examined the data using the procedures set forth by Krieger et al. (40). They defined a diurnal rhythm in cortisol as occurring when an individual's afternoon and evening cortisol levels were less than 75% of their morning value. Using this method, 38 (out of 96, or 40%) infants were characterized as having the typical pattern in their cortisol across the night. Thus, a substantial minority of infants in our sample showed nighttime patterning in their cortisol, but the shape of the rhythm did not appear to be influenced by concurrent maternal EA at bedtime.
3 months
Identical multilevel analyses were conducted to test hypothesis 2a for data collected at 3 months, that higher maternal EA at bedtime was associated with greater evidence of an evening decline and overnight increase in cortisol. An unconditional model was tested first, including parameters for linear and quadratic change in cortisol over time. The linear change component was significant, p < .01, suggesting that cortisol values generally increased from late afternoon to the following morning across the sample at 3 months. The quadratic change was not significant, suggesting that across the sample infants did not show in a quadratic pattern in cortisol from late afternoon to the following morning.
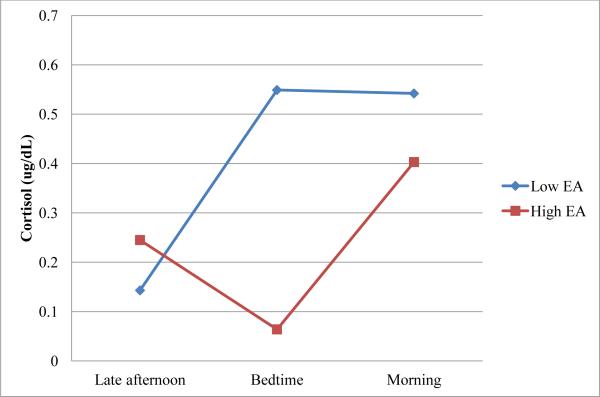
Next, a conditional model was specified by including variables for maternal EA at bedtime as well as interactions between maternal bedtime EA with linear change and with quadratic change over time. This model showed better fit to the data than the unconditional model, (χ2 (3, N = 88) = 8.0, p < .05). The two models were compared by subtracting the deviance for the conditional model, as indicated by the -2 log likelihood, from that of the unconditional model. These results are presented in Table 3. As in the unconditional model, the linear change component was significant in this model, p < .01, and the quadratic change component was not. Additionally, the maternal bedtime EA estimate was significant, p < .01, indicating that mean infant cortisol levels were lower for infants of mothers who were more emotionally available. This is shown graphically in Figure 2, which displays infant cortisol levels at each time point for mothers 1 standard deviation above and below the sample EA mean. The interaction between maternal EA and linear change approached significance, p < .10, suggesting that at higher levels of emotional availability, there was a trend for infants to show a decrease in cortisol across time. On the other hand, infants of mothers who were scored lower for EA tended to show an increase in cortisol from late afternoon to the following morning. The interaction between maternal EA and quadratic change was also significant, p = .05, suggesting that infants of more emotionally available mothers showed more of a quadratic pattern in their cortisol levels across time. Because cortisol levels are expected to follow a U-shaped quadratic pattern such that cortisol declines across the afternoon and increases over night (8), these analyses suggest that infants of more emotionally available mothers showed more of the typical diurnal patterning in their cortisol.
Table III.
Fixed effects for the unconditional and maternal EA covariate models, 3 months
| Estimate | Lower (α = .05) | Upper (α = .05) | .E. | t (df= 87) | Deviance | |
|---|---|---|---|---|---|---|
| Unconditional model | 361.1 | |||||
| Fixed effects | ||||||
| Intercept | .2456 | .1252 | .3659 | 061 | 4.06** | |
| Linear | .0152 | .0047 | .0257 | 005 | 2.87** | |
| Quadratic | .0005 | −.0018 | .0028 | 001 | .45 | |
| Random effects | ||||||
| Intercept | .1197 | 026 | ||||
| Maternal EA covariate model | 353.1 | |||||
| Fixed effects | ||||||
| Intercept | .2337 | .1131 | .3543 | 061 | 3.85** | |
| Linear | .0147 | .0004 | .0251 | 005 | 2.78** | |
| Maternal EA | −.1918 | −.3306 | −.0531 | 070 | −2.73** | |
| Maternal EA × Linear | −.0124 | −.0257 | .0008 | 007 | −1.85† | |
| Quadratic | .0009 | −.0014 | .0032 | 001 | .76 | |
| Maternal EA × Quadratic | .0022 | −.0000 | .0043 | 001 | 1.95* | |
| Random effects | ||||||
| Intercept | .1147 | 0254 |
Notes:
Significant at p ≤ .01
Significant at p ≤ .05
Significant at p ≤ .10
As in the regression analyses, we also examined whether there were specific components of bedtime EA that explained the significant interaction between maternal EA and quadratic change. Interaction terms were created for each of the dimensions of EA (sensitivity, structuring, nonhostility, and nonintrusiveness) with quadratic change. None of these interactions reached significance, though the interactions of sensitivity and nonhostility with quadratic change were marginally significant, p < .10.
Lastly, we examined how many infants showed evidence of nighttime patterning in cortisol at 3 months using the method described in the 1 month analyses. Using the Krieger et al. (38) method, 50 (out of 88, or 57%) infants were characterized as showing the typical rhythm in their cortisol across the night. Thus, over half of the infants in our sample showed patterning in their cortisol across the night at 3 months, and, as reported above, these infants were more likely to have emotionally available mothers at bedtime than infants who did not show this pattern.
Longitudinal analyses
A final set of multilevel analyses was conducted to test hypothesis 2b, that higher bedtime maternal EA at 1 month was associated with a greater evidence of an evening decline and overnight rise in cortisol at 3 months. Maternal EA at 3 months was controlled for in these analyses. However, the results indicated that bedtime EA at 1 month was not associated with cortisol patterning at 3 months.
We also explored the possibility that infant cortisol at 1 month was related to maternal bedtime EA at 3 months, supporting the theory that infants who are less well-regulated in terms of the diurnal patterning evoke lower caregiving quality. Results examining the association between 1 month cortisol patterning across the night and maternal EA were not significant, however.
Daytime Maternal EA
To test hypothesis 3, that maternal EA at bedtime would be more closely related to infant cortisol than maternal daytime EA, we conducted an identical set of analyses at both age points, replacing maternal EA at bedtime with daytime maternal EA. At 1 month, composite daytime EA ranged from -2.19 to 1.15, (M = .02, SD = .74), and at 3 months composite EA ranged from -3.29 to 1.10, (M = .01, SD = .79). The 1 month and 3 month daytime maternal EA composites were moderately correlated, r = .23, p < .05.
At 1 month, no results were significant. At 3 months, higher daytime maternal EA significantly predicted higher afternoon cortisol, b = .29, t = 2.26, p < .05; R 2= .09, F = 2.70, p < .10. There were no other significant associations between daytime maternal EA and infant cortisol.
Discussion
The findings from the current study contribute to the existing literature concerning parenting and infant cortisol. They demonstrate that maternal EA at bedtime is related to infant cortisol levels and patterning in the first 3 months of life, when studies suggest a circadian rhythm in cortisol is first becoming established in some infants (9–11). Furthermore, the results suggest that bedtime may be a particularly important context for examining the links between caregiving quality and infant physiology.
Maternal EA and Elevations in Infant Cortisol
In hypotheses 1a and 1b, we predicted that maternal EA at bedtime would be associated with infant cortisol secretion across the night as well as infant cortisol levels at each time point sampled, both concurrently and longitudinally. These hypotheses were partially supported. The results indicated a trend suggesting that higher maternal EA was associated with smaller AUCG at 1 month. Follow-up analyses revealed that mothers who scored more highly for structuring and nonhostility at bedtime had infants who showed less cortisol secretion across the night. Additionally, higher maternal EA was associated with smaller AUCG and AUCI as well as lower cortisol levels at bedtime at 3 months. There was also a marginal association between higher maternal EA and lower morning awakening cortisol when infants were 3 months old. Follow-up analyses suggested that 3 month old infants of mothers who received higher scores for structuring and nonintrusiveness showed less cortisol secretion across the night. In addition, better maternal structuring was associated with lower infant cortisol at bedtime and higher nonintrusiveness was associated with lower morning cortisol.
These results indicate that very early in infancy, quality of care experienced at bedtime is associated with infant cortisol levels. The association between maternal EA and infant AUCG was only of marginal significance at 1 month, and became statistically significant at 3 months. Maternal EA was also significantly associated with cortisol levels at bedtime and marginally associated with cortisol levels at morning awakening at 3 months, but not at 1 month. Furthermore, maternal EA was associated with infant cortisol secretion across the night at 3 months regardless of whether afternoon cortisol levels were controlled for using the AUCI measure. These findings extend those of Murray and colleagues (6), who found that maternal withdrawal at 9 months was associated with elevated mean and morning cortisol levels in older children (13 years).
Interestingly, the follow-up analyses examining specific associations between the maternal EA subscales at bedtime and infant cortisol indicated that structuring at bedtime was particularly relevant to infant cortisol levels. Bedtime may be less physiologically arousing for infants whose mothers establish quiet, soothing routines, and this may contribute to infant's total secretion across the night. Because for many infants bedtime represents a time during which they are quite tired, it may be especially stressful to them. These results, along with the findings of Teti et al. (32), suggest that mothers’ sensitivity within this context may be related to infant cortisol secretion as well to the quality of infant sleep throughout the night.
Maternal EA and Infant Cortisol Patterning
Hypotheses 2a and 2b predicted that higher maternal EA at bedtime would be associated with a greater evidence of an evening decline and overnight rise in infant cortisol, both concurrently and longitudinally. These hypotheses were partially supported. When infants were 1 month old, maternal EA was not associated with changes in cortisol across the night. This result was expected, as infants as young as 1 month old have not been demonstrated to show a circadian pattern in cortisol in previous research. When infants were 3 months old, however, maternal EA was related to change in infant cortisol over time. Infants of mothers who were more emotionally available at bedtime showed a quadratic pattern in their cortisol, such that cortisol levels tended to decline from late afternoon to bedtime, and increase from bedtime to morning awakening. This pattern conforms to the expected circadian rhythm across the night. Infants of less emotionally available mothers, on the other hand, tended to show a linear increase in cortisol across the evening from late afternoon to the following morning. These results are consistent with previous work relating sensitive parenting to cortisol patterning across the day in childhood (3,5). Furthermore, they extend previous research by demonstrating that parenting quality is related to cortisol patterning even in very young infants.
Follow-up analyses indicated that a substantial minority of infants showed evidence of a circadian rhythm in their cortisol at 1 month, and that more infants showed evidence of a circadian rhythm in cortisol at 3 months. Many infants did not yet show a circadian pattern at both ages, however, supporting de Weerth and colleagues’ (8) finding that there is substantial interindividual variability in the emergence of a diurnal rhythm in cortisol.
Daytime Maternal EA
In Hypothesis 3, we predicted that maternal bedtime EA would be more strongly associated with infant cortisol than maternal daytime EA. This hypothesis was supported. There were no associations between daytime maternal EA and infant cortisol at 1 month, and there was only one significant association at 3 months. These results suggest that bedtime may be a meaningful context for evaluating for quality of maternal caregiving behaviors towards their infants. Because bedtime can be a stressful and emotionally-laden context (21), parents’ ability to create a soothing environment for their infants during this time may be particularly related to regulatory processes. Additionally, maternal EA at bedtime occurs closer in time to nighttime cortisol patterning than daytime EA. Our results indicate that the quality of care young infants experience in bedtime context is associated with their cortisol secretion as well as cortisol patterning across the night.
Implications
Altogether, the results suggest the cumulative influence of bedtime parenting quality over time on infant cortisol regulation. Previous work as well as findings from the current study suggest that at 1 month of age, only a minority of infants are beginning to show lower cortisol levels in the afternoon and evening and higher levels in the morning (9-11). Thus, influences of early experiences on the development of this regulation may be less evident across the sample. Previous work has not found associations between parenting quality and infant cortisol at this young age. Over time, however, the cumulative effects of parenting quality may become more apparent. By 3 months, when more infants are beginning to show evidence of circadian patterning and have been exposed to environmental experiences for a longer period of time, the influence of parenting quality may be more detectable.
These results are consistent with theory suggesting that parents play an important role in buffering their infants from distress early in infancy (41). Generally, cortisol levels are expected to decrease across the evening hours (8). Emotionally available mothers, who by definition are responsive to their infant's distress and use soothing routines that guide their infants towards sleep, may allow for this downregulation, therefore helping infants to establish healthy cortisol patterning. Mothers low in emotional availability may fail to promote downregulation however, and may also cause increases in cortisol secretion, as a result of overstimulating, intrusive, or hostile behavior toward their children. As elevated cortisol levels and deviations from the typical diurnal rhythm in childhood are associated with behavior problems, these results have important implications. Elevated cortisol levels are frequently associated with internalizing (14–16), and a flattened rhythm is generally linked to externalizing (17,18). Thus, lower cortisol levels and an established circadian cortisol rhythm early in infancy may help to lay the foundation for behavioral and emotional regulation in childhood.
Interestingly, the results suggested that maternal EA was more strongly linked to concurrent, rather than longitudinal infant cortisol levels and patterning. These findings indicate that, at this early point in development, infants may be especially sensitive to concurrent parenting influences, such that changes in their cortisol are closely linked to the care they are experiencing. This idea could be relevant to early intervention work, as it may suggest that infant cortisol secretion would be immediately responsive to improvements in maternal care.
Limitations and Future Directions
There are several limitations of the current study that must be noted.
First, the marginal findings between maternal bedtime EA and infant cortisol AUCG at 1 month and between maternal EA and infant morning cortisol at 3 months must be interpreted with caution. We included these results because, along with the statistically significant findings, they suggest the cumulative influence of parenting quality on infant cortisol across time. These marginal results in particular require further study and replication, however. Similarly, because we were studying emerging associations between maternal parenting quality and infant cortisol during the very beginning of life, we did not expect large effects and were concerned about conducting conservative tests of significance that might overlook emergent trends in cortisol patterning. We acknowledge that this created the potential for identifying spurious effects. However, because the effects were in the same direction at 1 and 3 months, we believe the data reflect an interesting developmental story about the emergence of cortisol patterning in early infancy and its sensitivity to the quality of early maternal care. Furthermore, while maternal daytime and bedtime EA were correlated at 3 months, they were not associated at 1 month. Maternal EA may vary more substantially across contexts at 1 month, suggesting that the findings using the afternoon cortisol sample, which is taken prior in time to measurement of maternal EA at bedtime, must also be interpreted with caution.
In addition, the samples were only taken across one night at each time point. Because there is substantial intra-individual variability in the circadian cortisol rhythm across days in infancy (8), it would be valuable to examine data for multiple days at each age. This data could provide more information concerning intra- and inter-individual variability in cortisol levels and patterning as infants develop a circadian rhythm across the first few months of life. It would also be useful to sample infant cortisol across the day, in addition to examining cortisol levels across the night as in the current study, in order to facilitate comparisons to previous work. This would allow for further examination of the significant positive association between maternal daytime EA and infant afternoon cortisol in the current study. It may be that infants of mothers who are more emotionally available during the day have higher cortisol levels at some points during the day, but show a clear decline and therefore more optimal patterning. In addition, the late afternoon cortisol sample was taken prior in time to the assessment of maternal EA. It would be desirable for future work to examine maternal EA prior to the assessment of infant cortisol when examining the predictive association between these two variables. Also, although staff asked parents to take the late afternoon cortisol sample from their infants at least a half hour following a meal, and to take the morning sample prior to feeding the infant, we did not have information about parental compliance with these requests.
There were some limitations in the coding of maternal emotional availability. That mothers knew they were being recorded may have influenced their behavior, which of course is a potential risk associated with most home-based behavioral observations. Fortunately, we were still able to obtain variability in maternal EA scores. Additionally, a substantial amount of our larger sample of 167 families was missing data for maternal EA because parents did not turn the cameras on in time to capture bedtime. Analyses revealed that families missing EA data were more likely to be single mothers, a minority, and have fewer years of education. This suggests that our results may not apply to these families, and that extra care must be taken to ensure bedtimes for these families are recorded in future work. Potential ways to increase the likelihood of bedtimes being recorded could include placing more cameras in the families’ homes, and leaving the cameras on after setting them up so that families do not have to remember to turn them on prior to bedtime.
Our study was also limited in terms of sample homogeneity. Mothers and infants in our study were mainly White and living in a rural area. Thus, it is not clear whether the same results would be obtained for dyads of other races living in different neighborhood settings. Additionally, as most of our families included two parents, it would be valuable to replicate this work in single parent homes, for which the demands of child care may be greater.
The use of maternal report of infant daytime and nighttime sleep is another limitation of our study. It may have been difficult for mothers to record each time their young infants fell asleep and woke up across 7 days of data collection. However, we did find that infant daytime sleep was moderately associated with cortisol levels at 1 month, in accord with Watamura et al. (23). This suggests that a daily diary measure of infant sleep quality, though imperfect, was correlated with outcome variables in expected ways in the current study.
Our study was also limited in terms of causal inference. Analyses examining longitudinal linkages between maternal EA at 1 month and infant cortisol at 3 months were not significant. Thus, we cannot draw conclusions about causal direction, and we cannot rule out the possibility that infants who show poorer cortisol regulation early in life evoke lower quality caregiving from their parents. However, consistent with theory suggesting that parental behavior influences infant cortisol regulation early in life (41), we believe that parents are a greater force in this bidirectional relationship. Furthermore, it is possible that it is not maternal EA specifically that influences infant cortisol, but the evening and nighttime environment that the mother creates, which may be related to EA. For example, it could be that more emotionally available mothers create less arousing environments for their infants to fall asleep in, and that this quality of the environment is related to infant cortisol levels and patterning, rather than the specific quality of her behaviors. We believe that assessment of the environment in which infants are sleeping in relation to their cortisol will be an important avenue for future work. Additionally, because there is some evidence that mothers with higher cortisol reactivity display lower quality parenting (42-43), and mother and infant cortisol are positively correlated (44-47), it may be that the relations between maternal EA and infant cortisol detected in this study can by accounted for by the correlation between mother and infant cortisol. Teasing apart these associations is another area for further study.
Finally, it would be useful to incorporate multiple measures of both maternal and paternal EA across different contexts in future work examining cortisol levels and patterning very early in infancy. It could be the combination of caregiving experiences with both parents that influences infant cortisol, rather than experiences with only mothers. However, in our study fathers typically were not involved in infant bedtimes at this young age, as only 47 of our EA video recordings at 1 month and 37 at 3 months included scorable father data.
This study showed that maternal EA at bedtime is of particular relevance to infant cortisol during the first three months of life, although it is likely that parental EA in other caregiving contexts (i.e. changing, bathtime, feeding) may also be related to stress regulation in very early infancy. We hope that future work is designed to compare and contrast predictive linkages between parenting quality during bedtime and other contexts and cortisol rhythms in early infancy. In addition, although prior work with older children suggests that deviations from expected circadian cortisol patterns are associated with internalizing and externalizing behavior problems (14–18), it is not yet clear whether and to what extent infants with lower cortisol levels and who establish a cortisol rhythm in the first 3 months of life are advantaged, in terms of the development of self-regulatory capacities, relative to infants who do so later in the first year. We believe these are important questions for future work.
Figure 3.
Interaction between maternal EA and quadratic change over time predicting infant cortisol at 3 months.
Acknowledgments
This study was supported by a grant from NIH (R01HD052809) awarded to the sixth author. We thank Corey Whitesell for her tireless efforts collecting data and we are grateful to the families who participated in this study. We also express our appreciation to the reviewers for their thoughtful feedback and suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None declared.
References
- 1.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic responses to stress. Science. 1997;277(5332):1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 2.Albers EM, Riksen-Walraven JM, Sweep FCGJ, de Weerth C. Maternal behavior predicts infant cortisol recovery from a mild everyday stressor. J Child Psychol Psychiatry. 2008;49(1):97–103. doi: 10.1111/j.1469-7610.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- 3.Fisher DB, Serbin LA, Stack DM. Intergenerational Predictors of Diurnal Cortisol Secretion in Early Childhood. Inf Child Dev. 2007;16:151–70. [Google Scholar]
- 4.Grant K-A, McMahon C, Austin M-P, Reilly N, Leader L, Ali S. Maternal prenatal anxiety, postnatal caregiving and infants’ cortisol responses to the still-face procedure. Dev Psychobiol. 51(8):625–37. doi: 10.1002/dev.20397. 200. [DOI] [PubMed] [Google Scholar]
- 5.Luijk MPCM, Saridjan N, Tharner A, van Ijzendoorn MH, Bakermans-Kranenburg MJ, Jaddoe VWV, et al. Attachment, depression, and cortisol: Deviant patterns in insecure-resistant and disorganized infants. Dev Psychobiol. 2010;52(5):441–52. doi: 10.1002/dev.20446. [DOI] [PubMed] [Google Scholar]
- 6.Murray L, Halligan SL, Goodyer I, Herbert J. Disturbances in early parenting of depressed mothers and cortisol secretion in offspring: a preliminary study. J Affect Disord. 2010;122(3):218–23. doi: 10.1016/j.jad.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 7.Gunnar MR, Porter FL, Wolf CM, Rigatuso J, Larson MC. Neonatal Stress Reactivity: Predictions to Later Emotional Temperament. Child Dev. 1995;66:1–13. doi: 10.1111/j.1467-8624.1995.tb00851.x. [DOI] [PubMed] [Google Scholar]
- 8.de Weerth C, Zijl RH, Buitelaar JK. Development of cortisol circadian rhythm in infancy. Early Hum Dev. 2003;73:39–52. doi: 10.1016/s0378-3782(03)00074-4. [DOI] [PubMed] [Google Scholar]
- 9.Mantagos A, Moustogiannis A, Vagenakis AG. Diurnal variation of plasma cortisol levels in infancy. J Pediatr Endocrinol Metab. 1998;11(4):549–53. doi: 10.1515/jpem.1998.11.4.549. [DOI] [PubMed] [Google Scholar]
- 10.Price DA, Close GC, Fielding BA. Age of appearance of circadian rhythm in salivary cortisol values in infancy. Arch Dis Child. 1983;58(6):454–6. doi: 10.1136/adc.58.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santiago LB, Jorge SM, Moreira AC. Longitudinal evaluation of the development of salivary cortisol circadian rhythm in infancy. Clin Endocrinol. 1996;44(2):157–61. doi: 10.1046/j.1365-2265.1996.645466.x. [DOI] [PubMed] [Google Scholar]
- 12.Kiess W, Meidert A, Dressendörfer RA, Schriever K, Kessler U, König A, et al. Salivary cortisol levels throughout childhood and adolescence: relation with age, pubertal stage, and weight. Pediatr Res. 1995;37:502–6. doi: 10.1203/00006450-199504000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Spangler G. The emergence of adrenocortical circadian function in newborns and infants and its relationship to sleep, feeding and maternal adrenocortical activity. Early Hum Dev. 1991;25(3):197–208. doi: 10.1016/0378-3782(91)90116-k. [DOI] [PubMed] [Google Scholar]
- 14.Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Dev. 1987;58(6):1459–73. [PubMed] [Google Scholar]
- 15.Schmidt LA, Fox NA, Rubin KH, Sternberg EM, Gold PW, Smith CC, et al. Behavioral and Neuroendocrine Responses in Shy Children. Dev Psychobiol. 1997;30:127–140. doi: 10.1002/(sici)1098-2302(199703)30:2<127::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt LA, Santesso DL, Schulkin J, Segalowitz SJ. Shyness is a necessary but not sufficient condition for high salivary cortisol in typically developing 10 year-old children. Pers Indiv Diff. 2007;43(6):1541–51. [Google Scholar]
- 17.Ruttle PL, Shirtcliff EA, Serbin LA, Fisher DB-D, Stack DM, Schwartzman AE. Disentangling psychobiological mechanisms underlying internalizing and externalizing behaviors in youth: longitudinal and concurrent associations with cortisol. Horm Behav. 2011;59(1):123–32. doi: 10.1016/j.yhbeh.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alink LRA, van Ijzendoorn MH, Bakermans-Kranenburg MJ, Mesman J, Juffer F, Koot HM. Cortisol and externalizing behavior in children and adolescents: mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Dev Psychobiol. 2008;50(5):427–50. doi: 10.1002/dev.20300. [DOI] [PubMed] [Google Scholar]
- 19.Hane AA, Philbrook LE. Beyond licking and grooming: Maternal regulation of infant stress in the context of routine care. Parent-Sci Pract. 2012;12(2-3):144–53. doi: 10.1080/15295192.2012.683341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ainsworth MDS, Blehar MC, Waters E, Wall S. Patterns of Attachment: A Psychological Study of the Strange Solution. Lawrence Erlbaum Associates; Hillsdale, NJ: 1978. [Google Scholar]
- 21.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrino. 2003;28:916–31. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 22.Watamura SE, Donzella B, Kertes DA, Gunnar MR. Developmental changes in baseline cortisol activity in early childhood: relations with napping and effortful control. Dev Psychobiol. 2004;45:125–133. doi: 10.1002/dev.20026. [DOI] [PubMed] [Google Scholar]
- 23.White BP, Gunnar MR, Larson MC, Donzella B, Barr RG. Behavioral and physiological responsivity, sleep, and patterns of daily cortisol production in infants with and without colic. Child Dev. 2000;71(4):862–77. doi: 10.1111/1467-8624.00196. [DOI] [PubMed] [Google Scholar]
- 24.Tollenaar MS, Beijers R, Jansen J, Riksen-Walraven JMA, de Weerth C. Solitary sleeping in young infants is associated with heighted cortisol reactivity to a bathing session but not to a vaccination. Psychoneuroendocrino. 2012;37(2):167–177. doi: 10.1016/j.psyneuen.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Burnham MM, Goodlin-Jones BL, Gaylor EE, Anders TF. Nighttime sleep-wake patterns and self-soothing from birth to one year of age: a longitudinal intervention study. J Child Psychol Psychiatry. 2002;43(6):713–25. doi: 10.1111/1469-7610.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kertes DA, Donzella B, Talge NM, Garvin MC, Van Ryzin MJ, Gunnar MR. Inhibited temperament and parent emotional availability differentially predict young children's cortisol responses to novel social and nonsocial events. Dev Psychobiol. 2009;51(7):521–32. doi: 10.1002/dev.20390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talge NM, Donzella B, Gunnar MR. Fearful temperament and stress reactivity among preschool-aged children. Inf Child Dev. 2008;17(4):427–45. doi: 10.1002/icd.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gartstein MA, Rothbart MK. Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behav Dev. 2003;26(1):64–86. [Google Scholar]
- 29.Biringen Z, Robinson JL, Emde RN. Emotional availability scales. 3rd ed. Department of Human Development and Family Studies, Colorado State University; Fort Collins: 1998. [Google Scholar]
- 30.Vliegen N, Luyten P, Biringen Z. A multimethod perspective on emotional availability in the postpartum period. Parent-Sci Pract. 2009;9(3-4):228–43. [Google Scholar]
- 31.Ziv Y, Aviezer O, Gini M, Sagi A, Koren-Karie N. Emotional availability in the mother-infant dyad as related to the quality of infant-mother attachment relationship. Attach Hum Dev. 2000;2(2):149–69. doi: 10.1080/14616730050085536. [DOI] [PubMed] [Google Scholar]
- 32.Teti DM, Kim B-R, Mayer G, Countermine M. Maternal emotional availability at bedtime predicts infant sleep quality. J Fam Psychol. 2010;24(3):307–15. doi: 10.1037/a0019306. [DOI] [PubMed] [Google Scholar]
- 33.Neu M, Goldstein M, Gao D, Laudenslager ML. Salivary cortisol in preterm infants: validation of a simple method for collecting saliva for cortisol determination. Early Hum Dev. 2007;83(1):47–54. doi: 10.1016/j.earlhumdev.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Barnett V, Lewis T. Outliers in statistical data. John Wiley & Sons; Chichester: 1994. [Google Scholar]
- 35.Azak S, Murison R, Wentzel-Larsen T, Smith L, Gunnar MR. Maternal depression and infant daytime cortisol. Dev Psychobiol. 2013;55:334–351. doi: 10.1002/dev.21033. [DOI] [PubMed] [Google Scholar]
- 36.Saridjan NS, Huizink AC, Koetsier JA, Jaddoe VW, Mackenbach JP, Hofman A, et al. Do social disadvantage and early family adversity affect the diurnal cortisol rhythm in infants? The Generation R Study. Horm Behav. 2010;57(2):247–54. doi: 10.1016/j.yhbeh.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Watamura SE, Donzella B, Alwin J, Gunnar MR. Morning-to-afternoon increases in cortisol concentrations for infants and toddlers in child care: Age differences and behavioral correlates. Child Dev. 2003;74(4):1006–1020. doi: 10.1111/1467-8624.00583. [DOI] [PubMed] [Google Scholar]
- 38.Lerner RM. Theories of human development: Contemporary perspectives. In: Damon W, Lerner RM, editors. Handbook of child psychology: Vol.1. Theoretical models of human development. Wiley; New York: 1998. pp. 1–24. [Google Scholar]
- 39.Lerner RM, Rothbaum F, Boulos S, Castellino DR. Developmental systems perspective on parenting. In: Bornstein MH, editor. Handbook of parenting: Vol. 2. Biology and ecology of parenting. Erlbaum; Mahwah,NJ: 2002. pp. 315–344. [Google Scholar]
- 40.Krieger DT, Allen W, Rizzo F, Krieger HP. Characterization of the normal temporal pattern of plasma corticosteroid levels. J Clin Endocrinol Metab. 1971;32:266–84. doi: 10.1210/jcem-32-2-266. [DOI] [PubMed] [Google Scholar]
- 41.Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrino. 2002;27(1-2):199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- 42.Kiel EJ, Buss KA. Toddler inhibited temperament, maternal cortisol reactivity and embarrassment, and intrusive parenting. J Fam Psychol. 27(3):512–517. doi: 10.1037/a0032892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martorell GA, Bugental DB. Maternal variations in stress reactivity: Implications for harsh parenting practices with very young children. J Fam Psychol. 2006;20(4):641–647. doi: 10.1037/0893-3200.20.4.641. [DOI] [PubMed] [Google Scholar]
- 44.Bright MA, Granger DA, Frick JE. Do infants show a cortisol awakening response? Dev Psychobiol. 2012;54(7):736–743. doi: 10.1002/dev.20617. [DOI] [PubMed] [Google Scholar]
- 45.Granger DA, Serbin LA, Schwartzman A, Lehoux A, Cooperman J, Ikeda S. Children's salivary cortisol, internalising behaviour problems, and family environment: Results from the Concordia longitudinal risk project. Int J Behav Dev. 1998;22(4):707–728. [Google Scholar]
- 46.Hibel LC, Granger DA, Blair C, Cox MJ. Intimate partner violence moderates the association between mother-infant adrenocortical activity across an emotional challenge. J Fam Psychol. 2009;23(5):615–625. doi: 10.1037/a0016323. [DOI] [PubMed] [Google Scholar]
- 47.Sethre-Hofstad L, Stansbury K, Rice MA. Attunement of maternal and child adrenocortical response to child challenge. Psychoneuroendocrino. 2002;27(6):731–747. doi: 10.1016/s0306-4530(01)00077-4. [DOI] [PubMed] [Google Scholar]