Abstract
Because oxidative metabolism is the primary form of energy production in the brain, the amount of oxygen consumed by the brain, denoted by a physiological parameter termed cerebral metabolic rate of oxygen (CMRO2), represents a key marker for tissue viability and brain function. Quantitative assessment of cerebral oxygen metabolism in the neonate may provide an important marker in better understanding normal brain development and in making diagnosis and treatment decisions in neonatal brain injuries. Measurement of CMRO2 in human has been a challenging task, particularly in neonates. Recently, several promising techniques have been proposed to quantify neonatal CMRO2 and the purpose of this article is to provide a technical review of these techniques. Among these, we will focus the review on the NIRS optic based methods and MRI methods which are non-invasive, have been applied in normal and sick newborns and show great potentials. Potential clinical prospects of CMRO2 techniques are discussed in the context of their advantages, challenges and limitations.
Keywords: Cerebral oxygen metabolism, cerebral metabolic rate of oxygen, neonates, near-infrared spectroscopy, magnetic resonance imaging
Introduction
Brain is a big energy consumer and most of its energy is generated by oxidative metabolism, because anaerobic metabolism is inefficient and the produced lactate can cause further injury[1]. In neonates, cerebral oxidative metabolism is thought to play a particularly critical role in the early development of the brain. Starting from the third trimester and continuing until several months after birth, the energy source of the human brain shifts from anaerobic glycolysis to the more energy-efficient aerobic metabolism, in order to meet the escalating cerebral energy demands for the complex structural and functional maturational processes[2]. Consequently, disruption of oxygen supply and metabolism at this stage is highly detrimental. Several cerebral injuries have been associated with abnormal cerebral oxidative metabolism, such as hypoxic-ischemic encephalopathy, stroke, and metabolic disorders, all of which may lead to long-term neurologic deficits[3–5]. Therefore, quantitative assessment of cerebral oxygen metabolism in the neonate may provide a much needed tool to diagnose brain injuries, to provide mechanistic insights into the disease course, and to guide therapy on an individual basis.
However, measurement of cerebral oxygen metabolism, denoted by cerebral metabolic rate of oxygen (CMRO2), is particularly challenging in neonates, compared to other physiologic parameters such as perfusion and diffusion. Several CMRO2 measurement techniques have been developed in adults, but so far only a few of them have been shown to be feasible in neonates.
General principle underlying CMRO2 measurement techniques
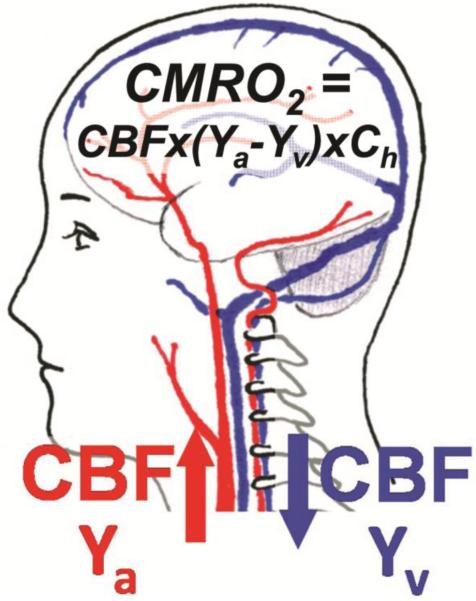
Most CMRO2 measurement techniques are based on a simple principle called the Fick's principle. Basically, the amount of O2 consumed by the brain equals the difference between the amount delivered on the arterial side and the amount drained on the venous side. As illustrated in Fig. 1, arterial blood has an oxygenation level of Ya and delivers oxygen to the brain. The flow rate is indicated by CBF. When the blood reaches brain tissue, a portion of the carried oxygen is extracted by the tissue for its metabolism, and this rate is referred to as CMRO2. The blood leaving the tissue is venous blood and has an oxygenation level of Yv. The flow rate of the venous blood is the same as that of the arterial blood, CBF. Thus, CMRO2 (in unit of μmol/100g/min) can be quantified from arterio-venous difference in oxygen content according to the Fick Principle[6]:
| Eq. [1] |
where Ch is the amount of O2 molecules that a unit volume of blood can carry and is proportional to hematocrit (8.97μmol O2/100 ml blood at Hct=0.44)[7]. The ratio of arterio-venous difference to the artery oxygenation is known as oxygen extraction fraction (OEF), i.e., OEF=(Ya-Yv)/Ya.
Figure 1.
Illustration of the relationship among different physiologic parameters associated with oxygen demand and supply of the brain.
Thus, once Ya, Yv and CBF are experimentally determined, CMRO2 can be calculated. Different modalities and techniques can be used to measure these parameters for CMRO2 quantification.
Available CMRO2 techniques
Positron Emission Tomography (PET) is considered the gold standard method to measure brain metabolism in adults[8]. In this technique, CBF, OEF and CMRO2 are measured with the infusion and inhaling of 15O- labeled radiotracers (i.e., H 152O, C15O and 15O2). In addition, repeated arterial blood sampling and on-site cyclotron for the production of 15O tracers are required. The need of ionized radiation is the primary impediment when applying this technique in pediatric population. Additional issues include complexity of the procedure and the need of special equipment in 15O-PET. To date, there were few studies that reported CMRO2 measurement in neonates using this technique[9], despite much broader applications in adults.
Near-infrared spectroscopy (NIRS) as a bed-side tool has been used to measure CMRO2 in adults[10]. It estimates oxyhemoglobin and deoxyhemoglobin concentrations (and thus Ya and Yv) by detecting the absorption and attenuation of NIR lights in brain tissue. Different techniques (both optical and non-optical) have been proposed to measure CBF[11–13]. Because of its low-cost and bed-side access, there have been increasing number of reports that used NIRS methods to measure CMRO2 in the neonate[12–16] (see more details below).
Magnetic resonance imaging (MRI) techniques that do not involve exogenous tracer have been developed more recently to measure CMRO2 in adults[17–21]. CBF is usually measured by phase-contrast MRI[20, 22–26] or arterial spin labeling (ASL) MRI[27–32]. Arterial oxygenation, Ya, is usually measured by pulse oximetry[20, 24, 32], or assigned an assumed value given the highly oxygen content and small variation in arterial blood[18, 19]. The main difference among these MRI-based CMRO2 techniques is the approach by which venous oxygenation, Yv, is determined. Based on the Yv measurement methods, these techniques can be divided into four categories: susceptibility effect in extravascular tissue[17], phase angle in intravascular blood signal[18], gas-inhalation modulated fMRI signal[21], and transverse relaxation time (T2) of blood signal[20, 32]. Among these four categories, two blood T2-based CMRO2 method[24, 32] and a phase angle-based method[33] have been shown to be feasible to apply in the neonate, which will be discussed later.
Other techniques, such as nuclear magnetic resonance (NMR) methods using 13C and 17O as exogenous tracers[34, 35], have been developed to measure CMRO2 in adults, but have not been applied to neonatal brain yet.
NIRS measurement of CMRO2 in the neonate
In NIRS measurement, the optical probes are placed on the scalp at the region of interest. The transmitted NIR light in the brain is absorbed mainly by oxyhemoglobin, deoxyhemoglobin and water while it is scattered mainly due to red blood cells. The light absorption rates of oxyhemoglobin, deoxyhemoglobin and water varies at different wavelengths. Therefore, by measuring the differential changes of the received light intensity at multiple wavelengths, the concentrations of oxyhemoglobin and deoxyhemoglobin can be estimated.
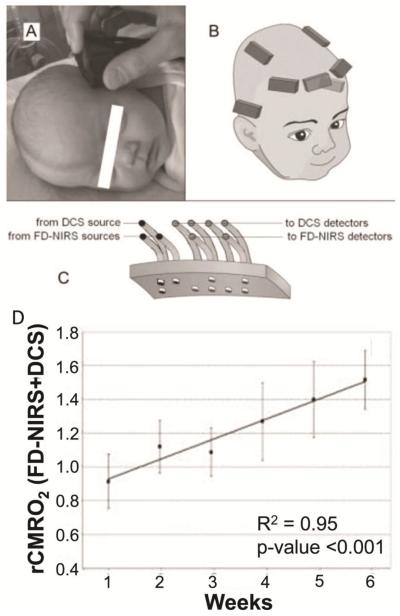
Oxygenation measurements using NIRS are particularly successful in neonates because of their thin skulls. Early studies used continuous wave NIRS to measure oxyhemoglobin and deoxyhemoglobin concentrations, which give relative oxygen saturation[12–14]. In order to obtain absolute values of venous oxygenation, ratio of arterial and venous cerebral blood volume (CBV) is either assumed[12], or estimated from blood volume changes induced by either head-down tilt maneuver[13] or partial jugular venous compression[14]. Optical imaging technologies are continuingly evolving. A recent technique called frequency domain NIRS (FDNIRS) has shown great promises in absolute quantification of oxygenation saturation and CBV[15, 16] (Fig. 2).
Figure 2.
Illustration of relative CMRO2 measurement using FD-NIRS and DCS[16]. (A) Picture of a subject during a measurement. (B) Locations of optical probes on a subject. (C) Schema of the optical probe. (D) Resulted relative CMRO2 (rCMRO2) increases quickly with age after birth.
Another challenging part for the optical methods is the quantitative measurement of cerebral blood flow (CBF). Some studies used non-optical methods as alternative for CBF quantification, such as the 133Xe clearance technique[13]. Other studies used the diffuse correlation spectroscopy (DCS) to measure microvascular blood flow non-invasively without exogenous tracers[15, 16, 36]. DCS provides measurement of an index of cerebral blood flow, and in combination with oxygen saturation, provides an index of CMRO2 (CMRO2i, [mol/dl·mm2/second])[15, 16].
In 1992, using NIRS combined with 133Xe injection and head tilting, Skov et al. reported a mean CMRO2 of 44.7±17.9 μmol/100g/min from 9 preterm neonates with respiratory distress syndrome and a mean CMRO2 of 62.6±35.8 μmol/100g/min from 10 asphyxiated, term neonates, but noted a 59% success rate using their technique[13]. Later in 1998, Yoxall et al. used NIRS with partial jugular venous occlusion for CBV estimation, and reported CMRO2 values varied between 23.2 and 78.7 μmol/100g/min from 20 neonates under intensive care aged between 24 and 41 gestational weeks, with 8 neonates under sedation during measurement, and 3 taking medication for seizure treatment[14]. More recently, Elwell et al. reported CMRO2 of 30.8 to 68.4 μmol/100g/min from 9 sick neonates between 23 to 37 gestational weeks using NIRS with assumed venous CBV and modeling[12]. Comparison of the NIRS-measured CMRO2 and other modalities are listed in Table 1.
Table 1.
CMRO2 values reported in literature.
| Study | Method | Number of subjects |
Gestational age (weeks) |
Subject condition |
Yv (%) | CBF (ml/100g/min) |
CMRO2 (μmol/100g/min) |
|---|---|---|---|---|---|---|---|
| Skov et al, 1993 [13] | NIRS | 32 | 26 – 39 | Asphyxiated; RDS | 53.44±15.36 (preterm) | 12.6±6.4 (preterm) | 44.7±17.9 (preterm) |
| 67.30±9.38 (term) | 26.5±17.9 (term) | 62.6±35.8 (term) | |||||
| Altman et al, 1993 [9] | PET | 11 | 26 – 41 | HIE and other conditions | - | 21.6±21.0 | 21.4±16.4 |
| Yoxall and weindling, 1998 [14] | NIRS | 20 | 24 – 41 | Seizure and other conditions | 64.6 (76.1 – 46.8) | 9.3 (4.5 – 28.3) | 23.1 (8.6 – 78.5) |
| Elwell et al, 2005 [12] | NIRS | 9 | 23 – 37 | Ventilatory support | - | - | 45.9±12.3 |
| Liu et al, 2014[24] | MRI | 12 | 35 – 42 | Healthy | 62.6±8.3 | 13.4±4.2 | 38.3±17.7 |
| Jain et al, 2014[33] | MRI | 33 | 38 – 40 | CHD | 55.2 (49.3 – 60.2) | 9.7 (7.5 – 15.1) | 23.2 (17.8 – 35.2) |
| De Vis et al, 2014[32] | MRI | 51 | 28 – 52 | Healthy, HIE and other diagnosis | 52±12 (healthy) | 14±3 (healthy) | 30±6 (healthy) |
| 65±13 (HIE) | 12±4 (HIE) | 24±12 (HIE) |
NIRS, near infrared spectroscopy; PET, positron emission tomography; MRJ, magnetic resonance imaging; RDS, Respiratory distress syndrome; HIE, Hypoxic-ischemic encephalopathy; CHD, Congenital heart disease.
It has been showed that relative CMRO2, or index of CMRO2 (CMRO2i), measured by FDNIRS and DCS, could also be an effective indicator to monitor brain metabolism. Roche-Labarbe et al demonstrated the CMRO2i increases by 40% during the first six weeks of life (Fig. 2D)[16]. Dehaes et al reported that neonates with hypoxic ischemic encephalopathy (HIE) has lower CMRO2i during hypothermia treatment comparing to post-treatment (p<0.01) and healthy controls (p<0.00001)[15].
NIRS approaches of CMRO2 assessment have the advantages of low-cost and bedside access. However, absolute quantification of CMRO2 with this method is not yet straightforward, primarily because of the need to make assumptions on arterio-venous volume ratio and the difficulty in determining penetration depth.
MRI measurements of CMRO2 in the neonate
With the advances in technologies that are originally developed in adults, MRI methods for absolute CMRO2 quantification in the neonates are emerging[15, 24, 32]. Techniques for quantitative and non-invasive measurements of CBF, including phase-contrast MRI and ASL MRI, are being adapted and optimized in neonates[22, 24, 32]. The feasibility of measuring oxygenation non-invasively using MRI has also been demonstrated by recent studies[15, 24, 32]. Two of such techniques measure venous blood oxygenation based on the transverse relaxation time of blood spins in magnetic field (i.e., T2 of blood)[24, 32]. The other technique is named as MR susceptometry, which measures the phase-angle induced by the blood's susceptibility[33].
T2-based CMRO2 measurement
T2-based measurements of Yv rely on the principle that T2 relaxation time of the blood has a well-known and calibratable relationship with Yv, thus one can measure pure blood T2 and then convert T2 to Yv using a calibration plot[37].
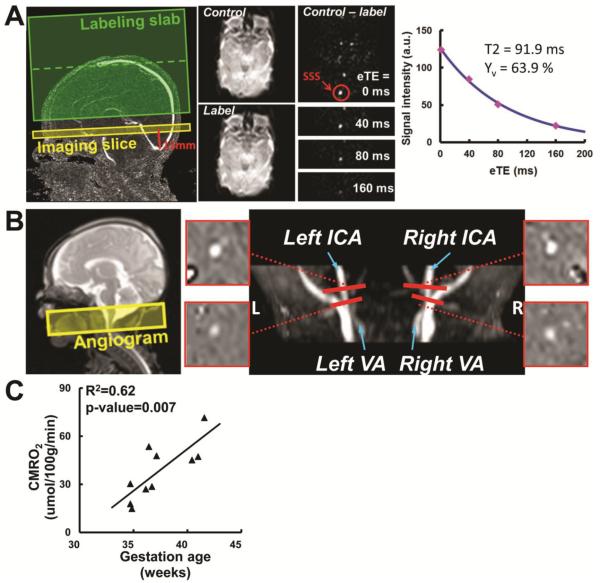
One recently developed technique that can determine Yv from blood T2 is T2-Relaxation-Under-Spin-Tagging (TRUST) MRI[37–39]. A unique aspect of TRUST MRI is that this method isolates pure venous blood by magnetically labeling the venous blood (using the principle similar to that in ASL MRI), thereby effectively minimizing confounding effects due to partial voluming of surrounding tissue and cerebrospinal fluid[38]. For T2 determination, the sequence applies various numbers of flow-insensitive T2-preparation pulses, thus the blood signal is modulate with different T2 weightings. The monoexponential fitting of the blood signal to the T2-preparation duration then gives the T2 value of the venous blood, which is then converted to Yv via T2-Yv calibration plot (Fig. 3A). The combination of Yv measurement by TRUST at the large draining vein (superior sagittal sinus, Fig. 3A) and CBF measurement by phase-contrast MRI at the brain's feeding arteries (Fig. 3B), provides a whole-brain CMRO2 measurement technique to quantify global CMRO2[24, 39]. This technique is completely non-invasive (no need for any exogenous agents), rapid (<5 min scan time), and reliable (coefficient of variation, CoV<4% in adults)[19]. It has been validated in adults[37]. More recently, Liu et al. successfully applied this method to neonatal population, and reported an average CMRO2 of 38.3±17.7 μmol/100g/min in 10 healthy and non-sedated neonates aged between 35–42 gestational weeks[24]. It was found that CMRO2 increased rapidly during this period, as manifested by a positively correlation with age (p=0.007, slope 5.2 μmol/100g/min per week) (Fig. 3C). Test-retest studies showed a coefficient of variation of 5.8±2.2 % between repeated CMRO2 measurements using the technique[24].
Figure 3.
TRUST-based CMRO2 measurement on a representative neonate. (A) Measurement of venous oxygenation (Yv) using T2-Relaxation-Under-Spin-Tagging (TRUST) MRI. SSS, superior sagittal sinus. (B) Measurement of cerebral blood flow (CBF) using Phase-Contrast (PC) MRI on the feeding arteries. ICA, internal carotid artery. VA, vertebral artery. (C) Resulted CMRO2 increases quickly with gestational age (at the time of scan).
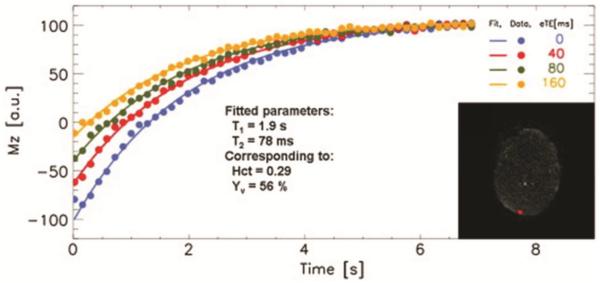
More recently, another technique was reported to measure T2-based Yv in neonates using a sequence called “T2 prepared tissue relaxation inversion recovery” (T2-TRIR)[32]. In this method, blood signal in superior sagittal sinus was detected by suppressing the static surrounding tissue. Similar to TRUST, the blood signal was modulated with different T2 weightings and then fitted for its T2 value (Fig. 4). ASL MRI was used to obtain whole brain CBF. The whole brain CMRO2 was then calculated according to Eq. [1]. Using this method, De Vis et al. reported the averaged CMRO2 of 30±12 μmol/100g/min in 10 healthy neonates (38–40 gestational weeks), which is significantly higher (p<0.01) than that of 24 μmol/100g/min measured in 9 HIE neonates(34–41 gestational weeks)[32].
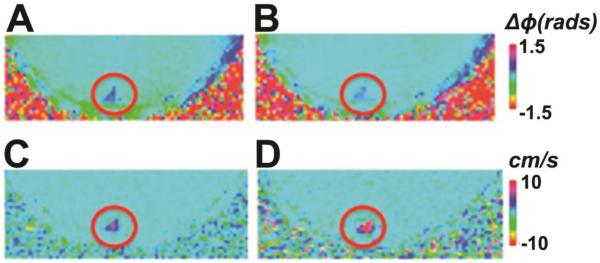
Figure 4.
T2-TRIR measurement of Yv at superior sagittal sinus[32]. Four inversion recovery curves of blood signal from sagittal sinus with different T2-weighting are shown. Insert: The brain tissue is saturated, while only inflowing blood within the sagittal sinus is visible.
MR susceptometry-based CMRO2 measurement
MR susceptometry measurement of Yv relies on the relative magnetic susceptibility difference between intravascular blood and surrounding tissue[18]. This susceptibility difference affects the intravascular water protons, which results in a phase-shift of the MR signal that is correlated with the intravascular blood oxygenation (Fig. 5A and B). In combination with phase-contrast MRI for CBF measurement (Fig. 5C and D), this technique also provides rapid whole brain CMRO2 estimation with 5 minutes[18].
Figure 5.
MR susceptometry-based CMRO2 measurement in a typical subject[33]. MR images during room air breathing (A, C) and during hypercapnia breathing (B,D) are shown. (A, B) Phase-difference images used for calculating Yv. (C, D) Velocity maps used for calculating CBF. Both acquired in the superior sagittal sinus (SSS, red circle).
Jain et al demonstrated the feasibility of applying this technique in neonates with congenital heart disease (CHD)[33]. In 32 anesthetized full term neonates with CHD, they found a median CMRO2 of 23.2 μmol/100g/min, which is lower than the CMRO2 of healthy neonates in other literature reports at this age (Table 1). They also compared the MRI-measured CMRO2 with that measured by optics method (diffuse optical spectroscopy and diffuse correlation spectroscopy). Their results demonstrated a strong linear correlation between the MRI-method and optics method[33].
Clinical applications of CMRO2 measurement
Due to the increasing demand of energy in early brain development after birth, CMRO2 as a measure of oxygen supply and metabolism is a particularly important biomarker of neonatal brain health. Measurement of CMRO2 may be used to diagnose brain injuries, to provide mechanistic insights into the disease course, and to guide therapy on an individual basis.
Studies in normal healthy neonates using both NIRS and MRI methods revealed that CMRO2 increases quickly with age[16, 24], demonstrated the escalating cerebral energy demands for the complex structural and functional development at this stage. Using NIRS measurements, Roche-Labarbe et al reported an up-to-40% increase in relative CMRO2 during the first six weeks of life[16]. Using the MRI method with absolute CMRO2 quantification, Liu et al found that CMRO2 increases with gestational age at a rate of 5.2 μmol/100g/min per week, although the highest CMRO2 value in this age range was still less than half of the adult level[24]. This rate of increase during early brain development is in line with previous PET literature which showed that cerebral metabolism continues to increase from the third trimester until 4 years old, and then gradually reduces with age afterwards[40, 41].
Abnormal CMRO2 values are expected in neonates with brain injuries and other diseases. For instance, Jain et al. reported that CMRO2 in full-term newborn infants diagnosed with cyanotic heart disease was lower than literature reports for healthy term neonates while similar to the previously reported values for premature infants[33].
Biological plausibility supports that disruption of oxygen supply and metabolism would be detrimental in newborns. A specific example in newborns is HIE, caused by a hypoxic ischemic injury, resulting in acute mortality and chronic neurological disability in survivors. A very recent study this year reported that HIE neonates have lower CMRO2 comparing to healthy neonates (24 vs. 30 μmol/100g/min)[32], suggesting decreased neural activity as a result of brain tissue damage in HIE. Previous literature also showed that the cerebral oxidative metabolism derangement at the first week of life in neonates with birth asphyxia might predict their neurodevelopmental outcome at 4 years old[42]. Thus, monitoring CMRO2 in neonatal patients and along their growth could improve the assessment of disease course and treatment outcomes.
Hypothermia is the only currently available neuroprotective therapy for HIE. One of the hypothesized mechanism by which hypothermia works is that it reduces brain metabolic rate and thus preserves high-energy phosphate compound (e.g. ATP) and prevent lactate production and tissue acidosis. Dehaes et al. using FDNIRS and DCS systems recently showed that cerebral oxygen metabolism (CMRO2i) in HIE neonates during hypothermia treatment was significantly lower than post-hypothermia and age-matched healthy controls[15]. Since it is now practical to transport to the MRI scanner[43] with ongoing hypothermia therapy, quantification of CMRO2 with MR techniques may provide in situ valuable information.
Measuring CMRO2 in real time during hypothermia will allow to confirm mechanisms of neuroprotection and to establish likelihood of therapeutic responses in real time, rather than current status of knowledge where one needs to wait 18–24 months. Similarly, future randomized neuroprotective trials [44–46] could benefit from an insight into mechanisms of therapeutic responses via measurement of CMRO2.
Conclusion
Disruptions in oxygen supply and metabolism has been associated with several cerebral injuries, such as HIE, stroke and metabolic disorders, all of which may lead to long-term neurologic deficits. Application of CMRO2 measurements in these diseases would provide a better understanding of the etiology of these neonatal brain injuries, as well as for the diagnosis and treatment assessment of these injuries on an individual basis. Fortunately, several emerging CMRO2 techniques that are suitable for neonates may be able to fill this gap by providing an quantitative index of brain function.
Research Directions.
The above mentioned techniques are still research based and not ready for clinical implementation into practice.
Further development of accurate and reliable techniques to measure CMRO2 in neonates non-invasively.
Clinical applications of CMRO2 measurement in neonatal brain injuries.
Acknowledgement
The present study was supported by National Institutes of Health (NIH) R01 MH084021 (to HL), NIH R01 NS067015 (to HL), NIH R01 AG042753 (to HL) and K23HD069521-01A11 (to LFC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement The authors declare that there are no conflicts of interest.
References
- [1].Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–45. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- [2].du Plessis AJ. Cerebral blood flow and metabolism in the developing fetus. Clin Perinatol. 2009;36:531–48. doi: 10.1016/j.clp.2009.07.002. [DOI] [PubMed] [Google Scholar]
- [3].Kiechl-Kohlendorfer U, Ralser E, Pupp Peglow U, Reiter G, Trawoger R. Adverse neurodevelopmental outcome in preterm infants: risk factor profiles for different gestational ages. Acta paediatrica. 2009;98:792–6. doi: 10.1111/j.1651-2227.2009.01219.x. [DOI] [PubMed] [Google Scholar]
- [4].Pape KE, Wigglesworth JS. Haemorrhage, Ischaemia and the Perinatal Brain. Mac Keith Press; 1993. pp. 1–196. [Google Scholar]
- [5].Trescher WH, Lehman RA, Vannucci RC. The influence of growth retardation on perinatal hypoxic-ischemic brain damage. Early human development. 1990;21:165–73. doi: 10.1016/0378-3782(90)90115-y. [DOI] [PubMed] [Google Scholar]
- [6].Kety SS, Schmidt CF. The Effects of Altered Arterial Tensions of Carbon Dioxide and Oxygen on Cerebral Blood Flow and Cerebral Oxygen Consumption of Normal Young Men. J Clin Invest. 1948;27:484–92. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Guyton AC, Hall JE. Respiration. In: Guyton AC, Hall JE, editors. Textbook of medical physiology. 11th ed Saunders/Elsevier; Philadelphia: 2005. [Google Scholar]
- [8].Mintun MA, Raichle ME, Martin WR, Herscovitch P. Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1984;25:177–87. [PubMed] [Google Scholar]
- [9].Altman DI, Perlman JM, Volpe JJ, Powers WJ. Cerebral oxygen metabolism in newborns. Pediatrics. 1993;92:99–104. [PubMed] [Google Scholar]
- [10].Obrig H, Neufang M, Wenzel R, Kohl M, Steinbrink J, Einhaupl K, et al. Spontaneous low frequency oscillations of cerebral hemodynamics and metabolism in human adults. Neuroimage. 2000;12:623–39. doi: 10.1006/nimg.2000.0657. [DOI] [PubMed] [Google Scholar]
- [11].Edwards AD, Wyatt JS, Richardson C, Delpy DT, Cope M, Reynolds EO. Cotside measurement of cerebral blood flow in ill newborn infants by near infrared spectroscopy. Lancet. 1988;2:770–1. doi: 10.1016/s0140-6736(88)92418-x. [DOI] [PubMed] [Google Scholar]
- [12].Elwell CE, Henty JR, Leung TS, Austin T, Meek JH, Delpy DT, et al. Measurement of CMRO2 in neonates undergoing intensive care using near infrared spectroscopy. Adv Exp Med Biol. 2005;566:263–8. doi: 10.1007/0-387-26206-7_35. [DOI] [PubMed] [Google Scholar]
- [13].Skov L, Pryds O, Greisen G, Lou H. Estimation of cerebral venous saturation in newborn infants by near infrared spectroscopy. Pediatr Res. 1993;33:52–5. doi: 10.1203/00006450-199301000-00011. [DOI] [PubMed] [Google Scholar]
- [14].Yoxall CW, Weindling AM. Measurement of cerebral oxygen consumption in the human neonate using near infrared spectroscopy: cerebral oxygen consumption increases with advancing gestational age. Pediatr Res. 1998;44:283–90. doi: 10.1203/00006450-199809000-00004. [DOI] [PubMed] [Google Scholar]
- [15].Dehaes M, Aggarwal A, Lin PY, Rosa Fortuno C, Fenoglio A, Roche-Labarbe N, et al. Cerebral oxygen metabolism in neonatal hypoxic ischemic encephalopathy during and after therapeutic hypothermia. J Cereb Blood Flow Metab. 2014;34:87–94. doi: 10.1038/jcbfm.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Roche-Labarbe N, Carp SA, Surova A, Patel M, Boas DA, Grant PE, et al. Noninvasive optical measures of CBV, StO(2), CBF index, and rCMRO(2) in human premature neonates' brains in the first six weeks of life. Hum Brain Mapp. 2010;31:341–52. doi: 10.1002/hbm.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bolar DS, Rosen BR, Sorensen AG, Adalsteinsson E. QUantitative Imaging of eXtraction of oxygen and TIssue consumption (QUIXOTIC) using venular-targeted velocity-selective spin labeling. Magn Reson Med. 2011;66:1550–62. doi: 10.1002/mrm.22946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jain V, Langham MC, Wehrli FW. MRI estimation of global brain oxygen consumption rate. J Cereb Blood Flow Metab. 2010;30:1598–607. doi: 10.1038/jcbfm.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu P, Xu F, Lu H. Test-retest reproducibility of a rapid method to measure brain oxygen metabolism. Magn Reson Med. 2013;69:675–81. doi: 10.1002/mrm.24295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xu F, Ge Y, Lu H. Non-invasive Quantification of Whole-brain Cerebral Metabolic Rate of Oxygen by MRI. Magn Reson Med. 2009;62:141–8. doi: 10.1002/mrm.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wise RG, Harris AD, Stone AJ, Murphy K. Measurement of OEF and absolute CMRO2: MRI-based methods using interleaved and combined hypercapnia and hyperoxia. Neuroimage. 2013;83:135–47. doi: 10.1016/j.neuroimage.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Varela M, Groves AM, Arichi T, Hajnal JV. Mean cerebral blood flow measurements using phase contrast MRI in the first year of life. NMR Biomed. 2012;25:1063–72. doi: 10.1002/nbm.2771. [DOI] [PubMed] [Google Scholar]
- [23].Haacke EM, Lai S, Reichenbach JR, Kuppusamy K, Hoogenraad FG, Takeichi H, et al. In vivo measurement of blood oxygen saturation using magnetic resonance imaging: a direct validation of the blood oxygen level dependent concept in functional brain imaging. Human Brain Mapping. 1997;5:341–6. doi: 10.1002/(SICI)1097-0193(1997)5:5<341::AID-HBM2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- [24].Liu P, Huang H, Rollins N, Chalak LF, Jeon T, Halovanic C, et al. Quantitative assessment of global cerebral metabolic rate of oxygen (CMRO2) in neonates using MRI. NMR Biomed. 2014;27:332–40. doi: 10.1002/nbm.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Aslan S, Xu F, Wang PL, Uh J, Yezhuvath US, van Osch M, et al. Estimation of labeling efficiency in pseudocontinuous arterial spin labeling. Magn Reson Med. 2010;63:765–71. doi: 10.1002/mrm.22245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bonekamp D, Degaonkar M, Barker PB. Quantitative cerebral blood flow in dynamic susceptibility contrast MRI using total cerebral flow from phase contrast magnetic resonance angiography. Magn Reson Med. 2011;66:57–66. doi: 10.1002/mrm.22776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60:1488–97. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Detre JA, Alsop DC. Perfusion magnetic resonance imaging with continuous arterial spin labeling: methods and clinical applications in the central nervous system. Eur J Radiol. 1999;30:115–24. doi: 10.1016/s0720-048x(99)00050-9. [DOI] [PubMed] [Google Scholar]
- [29].Edelman RR, Siewert B, Darby DG, Thangaraj V, Nobre AC, Mesulam MM, et al. Qualitative mapping of cerebral blood flow and functional localization with echo-planar MR imaging and signal targeting with alternating radio frequency. Radiology. 1994;192:513–20. doi: 10.1148/radiology.192.2.8029425. [DOI] [PubMed] [Google Scholar]
- [30].Kim SG. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn Reson Med. 1995;34:293–301. doi: 10.1002/mrm.1910340303. [DOI] [PubMed] [Google Scholar]
- [31].Wong EC. Vessel-encoded arterial spin-labeling using pseudocontinuous tagging. Magn Reson Med. 2007;58:1086–91. doi: 10.1002/mrm.21293. [DOI] [PubMed] [Google Scholar]
- [32].De Vis JB, Petersen ET, Alderliesten T, Groenendaal F, de Vries LS, van Bel F, et al. Non-invasive MRI measurements of venous oxygenation, oxygen extraction fraction and oxygen consumption in neonates. Neuroimage. 2014;95C:185–92. doi: 10.1016/j.neuroimage.2014.03.060. [DOI] [PubMed] [Google Scholar]
- [33].Jain V, Buckley EM, Licht DJ, Lynch JM, Schwab PJ, Naim MY, et al. Cerebral oxygen metabolism in neonates with congenital heart disease quantified by MRI and optics. J Cereb Blood Flow Metab. 2014;34:380–8. doi: 10.1038/jcbfm.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hyder F, Chase JR, Behar KL, Mason GF, Siddeek M, Rothman DL, et al. Increased tricarboxylic acid cycle flux in rat brain during forepaw stimulation detected with 1H[13C]NMR. Proc Natl Acad Sci U S A. 1996;93:7612–7. doi: 10.1073/pnas.93.15.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhu XH, Zhang N, Zhang Y, Ugurbil K, Chen W. New insights into central roles of cerebral oxygen metabolism in the resting and stimulus-evoked brain. J Cereb Blood Flow Metab. 2009;29:10–8. doi: 10.1038/jcbfm.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Durduran T, Zhou C, Buckley EM, Kim MN, Yu G, Choe R, et al. Optical measurement of cerebral hemodynamics and oxygen metabolism in neonates with congenital heart defects. Journal of biomedical optics. 2010;15:037004. doi: 10.1117/1.3425884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lu H, Xu F, Grgac K, Liu P, Qin Q, van Zijl P. Calibration and validation of TRUST MRI for the estimation of cerebral blood oxygenation. Magn Reson Med. 2012;67:42–9. doi: 10.1002/mrm.22970. PMCID3158970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lu H, Ge Y. Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation-Under-Spin-Tagging MRI. Magn Reson Med. 2008;60:357–63. doi: 10.1002/mrm.21627. PMCID2587050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Xu F, Uh J, Liu P, Lu H. On improving the speed and reliability of T2-relaxation-under-spin-tagging (TRUST) MRI. Magn Reson Med. 2012;68:198–204. doi: 10.1002/mrm.23207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chugani HT. A critical period of brain development: studies of cerebral glucose utilization with PET. Preventive medicine. 1998;27:184–8. doi: 10.1006/pmed.1998.0274. [DOI] [PubMed] [Google Scholar]
- [41].Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell metabolism. 2014;19:49–57. doi: 10.1016/j.cmet.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Roth SC, Baudin J, Cady E, Johal K, Townsend JP, Wyatt JS, et al. Relation of deranged neonatal cerebral oxidative metabolism with neurodevelopmental outcome and head circumference at 4 years. Developmental medicine and child neurology. 1997;39:718–25. doi: 10.1111/j.1469-8749.1997.tb07372.x. [DOI] [PubMed] [Google Scholar]
- [43].Wintermark P, Labrecque M, Warfield SK, DeHart S, Hansen A. Can induced hypothermia be assured during brain MRI in neonates with hypoxic-ischemic encephalopathy? Pediatric radiology. 2010;40:1950–4. doi: 10.1007/s00247-010-1816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA, et al. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatric neurology. 2005;32:11–7. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- [45].Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- [46].Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]