Abstract
BACKGROUND & AIMS:
The oncogene MDM2, which encodes an E3 ubiquitin ligase, is overexpressed in pancreatic cancers and is therefore a therapeutic target. Current inhibitors of MDM2 target the interaction between MDM2 and P53; these would have no effect on cancer cells that do not express full-length P53, such as many pancreatic cancer cells. We searched for a compound that specifically inhibits MDM2 itself.
METHODS:
We performed a virtual screen and structure-based design to identify specific inhibitors of MDM2. We tested the activities of compounds identified on viability, proliferation, and protein levels of HPAC, Panc-1, AsPC-1, and Mia-Paca-2 pancreatic cancer cell lines. We tested whether intraperitoneal injections of one of the compounds identified affected growth of xenograft tumors from Panc-1 cells, or orthotopic tumors from Panc-1 and AsPC-1cells (injected into pancreata), in nude mice.
RESULTS:
We identified a compound, called SP141, which bound directly to MDM2, promoting its auto-ubiquitination and degradation by the proteasome. The compound reduced levels of MDM2 in pancreatic cancer cell lines, as well as their proliferation, with 50% inhibitory concentrations <0.5 μM (0.38–0.50 μM). Increasing concentrations of SP141 induced increasing levels of apoptosis and G2–M phase arrest of pancreatic cancer cell lines, whether or not they expressed functional P53. Injection of nude mice with SP141 (40 mg/kg/d) inhibited growth of xenograft tumors (by 75%, compared with control mice), and led to regression of orthotopic tumors.
CONCLUSIONS:
In a screen for specific inhibitors of MDM2, we identified a compound, called SP141, which reduces levels of MDM2 in pancreatic cancer cell lines, as well as their proliferation and ability to form tumors in nude mice. SP141 is a new class of MDM2 inhibitor that promotes MDM2 auto-ubiquitination and degradation. It might be further developed as a therapeutic agent for pancreatic cancer.
Keywords: Chemotherapy, P53-independent, P21, BCL2
Pancreatic cancer is a deadly disease associated with extremely low drug sensitivity, high mortality, and short survival times; developing an effective and safe therapy for pancreatic cancer remains an unmet medical need.1,2 It was estimated that, in 2013, more than 45,000 new cases of pancreatic cancer would be diagnosed and almost 40,000 Americans would die from this disease, largely due to the lack of effective therapeutic approaches.2 Although increasing efforts in recent years have been devoted to the early diagnosis and treatment of pancreatic cancer, little progress has been made. The current systemic therapy has produced unsatisfactory results, and only a few clinically available chemotherapeutic agents, such as gemcitabine, oxaliplatin, tarceva, and 5-fluorouracil, have resulted in some limited benefits.1,2 Recent advances in the understanding of pancreatic cancer biology have revealed some of the molecular mechanisms underlying the low responsiveness of this disease to therapeutic drugs. For instance, the loss of tumor suppressors, such as p53 and p16, and the overexpression of oncogenes, such as MDM2, KRAS, and BCL2, contribute to the poor response of this disease to treatment, 3-5 providing novel molecular targets for pancreatic cancer.
The MDM2 oncogene is an essential negative regulator of the p53 tumor suppressor.6-9 It binds to p53 and inhibits the transcriptional activity of p53.7 In addition, MDM2 functions as an E3 ligase that ubiquitinates p53 and promotes its proteasomal degradation.7-9 We and others have suggested that MDM2 is a potential cancer target.10-15 Several small molecules that activate p53 by blocking MDM2 have been discovered and are under preclinical and clinical development, such as nutlin-3,16 RITA,17 and MI219.18 However,these MDM2 inhibitors depend on the expression of wild type p53 in cancer cells to exert their anticancer activity. Therefore, they would have little or no efficacy in most cancers that have no functional wild type p53. This is particularly important, because it has been shown that more than 50% of human cancers, including pancreatic cancers, express mutant p53.19 Recent studies have indicated that MDM2 has numerous p53-independent functions that also contribute to tumorigenesis.20-23 MDM2 not only inhibits p53-dependent apoptosis, but also has p53-independent effects on apoptosis by affecting both pro-apoptotic and anti-apoptotic proteins.24,25 Other studies have shown that MDM2 overexpression in tumors is also linked to the dysregulation of cell cycle progression, DNA replication, and DNA repair.26,27 More recently, RITA, an inhibitor targeting MDM2-p53 interaction, has been shown to have both p53-dependent and –independent activity, resulting in the induction of apoptosis in neuroblastoma.28 Therefore, it has been suggested that MDM2 is a promising target for the treatment of human cancers, regardless of the p53 status in the tumor cells.10-12,15-17,21,22,29
We have been interested in the discovery and development of anti-pancreatic cancer drugs for many years.20-34 We have also proposed and evaluated various anti-MDM2 strategies, including the use of antisense oligonucleotides targeting MDM2,35-37 siRNA,38 and natural and synthetic small molecule MDM2 inhibitors.39-42 Although these molecules are effective in vitro and in vivo as MDM2 inhibitors and anticancer agents,21,29 their clinical prospects may be limited for various reasons, such as the difficulties associated with delivering them to cancer cells, their dependence on wild type p53, side effects, and low bioavailability.21,29 In our recent studies involving the performance of a high-throughput virtual screening and structure-based design, a series of 1-aryl and 1-heteroaryl pyrido[b]indole derivatives were identified as novel MDM2 inhibitors. Among more than 200 newly synthesized MDM2-interactive small molecules that were identified, 34 top candidates with excellent binding capacity to MDM2 protein were selected foe a cell-based evaluation of their in vitro anticancer activity against pancreatic cancer. Among these compounds, a novel pyrido[b]indole, termed SP141 (6-methoxy-1-(naphthalen-1-yl)-9H-pyrido[3,4-b]indole) (Figure 1A), showed both a strong binding affinity and the capacity to bind to both human and mouse MDM2 proteins in a molecular modeling study, pull-down assays and the Biacore assay (data not shown). It also showed broad spectrum cytotoxicity against human pancreatic cancer cell lines, with submicromolar IC50 values. In the present study, we investigated the in vitro activity and in vivo efficacy of SP141 against human pancreatic cancer cell lines and tumors with various p53 and MDM2 backgrounds. We also determined the effects of SP141 on the stability of the MDM2 protein and the role of MDM2 inhibition in SP141’s anticancer activity by manipulating the MDM2 expression in pancreatic cancer cells. These results provide evidence supporting the future development of SP141 for the clinical treatment of pancreatic cancer.
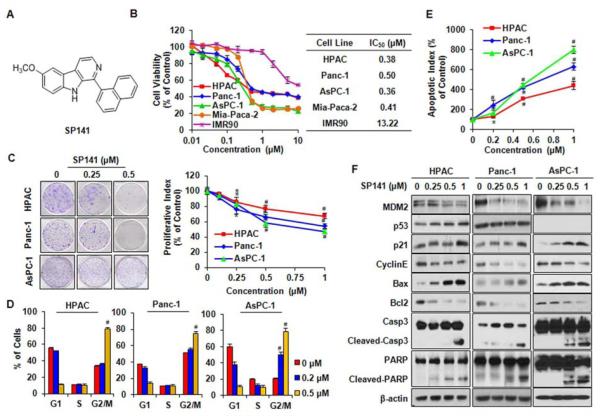
Figure 1.
SP141 induces pancreatic cancer cell death and inhibits MDM2 expression, regardless of the p53 status of the cells. (A) The chemical structure of SP141. Cells were exposed to various concentrations of SP141 for (B) 72h for cell viability studies to determine the IC50 values; (C) 24 h for the colony formation and proliferation assays; (D) 24 h for the cell cycle distribution assay; (E) 48 h for the apoptosis assay; and (F) 24 h to examine the expression of proteins related to apoptosis and cell cycle arrest in human pancreatic cancer cells. All assays were performed in triplicate. (* P < 0.05; # P < 0.01).
Materials and Methods
Chemicals, Plasmids, siRNA, and Other Reagents
SP141 was synthesized and purified by our laboratories (unpublished data), and the structure was confirmed by UV, IR, MS and NMR spectroscopy. The purity of the compound was determined to be greater than 99%. All chemicals and solvents used were of the highest analytical grade available. Antibodies, plasmids, and siRNAs were obtained commercially or were provided by other investigators; a detailed list is provided in the Supplemental Methods.
Cell Lines and Culture
Human pancreatic cancer cell lines (HPAC, Panc-1, AsPC-1, and Mia-Paca-2) were obtained from the American Type Culture Collection (Rockville, MD). Panc-1 cells stably expressing luciferase (Panc-1-Luc) were obtained from Caliper Lifesciences (Hopkinton, MA). AsPC-1 cells stably expressing luciferase (AsPC-1-Luc) were kindly provided by Dr. Debabrata Mukhopadhyay (Mayo Clinic College of Medicine, Rochester, MN). Human primary fibroblasts (IMR90) were a gift from Dr. Sam Lee (Harvard Cutaneous Biology Research Center, Charles Town, MA). The cell culture conditions were described previously29-30 and are detailed in the Supplemental Methods.
Assays for Cell Viability, Clonogenicity, Cell Proliferation, Apoptosis, and Cell Cycle Distribution
The test cells were treated with various concentrations of SP141 or vehicle controls. The methods used to determine the effects of SP141 on the cell viability (MTT assay),30-32 colony formation,39 proliferation (BrdUrd incorporation assay),30-32 apoptosis,30-32 and cell cycle distribution,30-32 were described previously. The detailed assay conditions are described in the Supplemental Methods.
Immunoblotting and Immunoprecipitation
Cells were transfected with SP141 or the indicated plasmids in the presence of Lipofectin (Invitrogen, Grand Island, NY) for various times and were lysed in NP-40 lysis buffer containing a protease inhibitor mixture from Sigma (St Louis, MO). Cell lysates were used for immunoblotting as described previously.30-32 Immunoprecipitation was performed using the indicated antibodies. The beads were then washed, and bound proteins were detected by immunoblotting as reported previously.39,42
Immunofluorescence
Cells were seeded on coverslips in 12-well plates at a density of 10,000 cells/well, allowed to attach overnight and treated with SP141 (0 and 0.5 μM) for 24 h. The cells were then fixed and immunofluorescent staining was carried out as described previously.43 Images were captured by a confocal microscope (Olympus Inc., Center Valley, PA). The detailed assay conditions are provided in the Supplemental Methods.
MDM2 Overexpression and Knockdown
Cells were transfected with control or MDM2 plasmids, or with MDM2 siRNA, according to the manufacturer’s instructions,43 followed by treatment with various concentrations of SP141. The cells were collected for cell viability and colony formation assays, or were lysed in NP40 buffer and examined for MDM2 expression by a Western blot analysis.
Xenograft and Orthotopic Models of Pancreatic Cancer and Treatment
The animal protocols were approved by the Institutional Animal Use and Care Committee (IACUC) of Texas Tech University. The animal experiments were strictly carried out according to the guidelines of the IACUC. The human pancreatic cancer xenograft model30-32 and orthotopic model34 were established as described previously (REFS). The treatment of the mice with SP141 and vehicle controls, the tumor growth and clinical monitoring, and the evaluations of the tissue pathology are detailed in the Supplemental Methods.
Statistical Analysis
All quantitative data in the present study were expressed as the means ± SD from at least three independent experiments. The statistical significance of differences was determined using a two-tailed ANOVA or Student’s t-test, with a P value < 0.05 considered to be statistically significant.
Results
SP141 Inhibits Pancreatic Cancer Cell Growth In Vitro, Regardless of p53 Status
In the initial studies, the 34 top candidate agents with excellent binding capacity for MDM2 were first tested for their cytotoxicity using the MTT assay in pancreatic cancer cells. SP141 consistently showed the most significant inhibitory effects. Subsequently, four human pancreatic cancer cell lines representing different p53 backgrounds (HPAC/p53 wild type; Panc-1 and MiaPaca-2/p53 mutant; AsPC-1/p53 null) and one “normal” (immortalized but non-malignant) fibroblast cell line (IMR90) were cultured with SP141, then the cell viability was determined. As shown in Figure 1B, SP141 inhibited human pancreatic cancer cell growth with IC50 values of less than 0.5 μM (0.38–0.50 μM) in a p53-independent manner. The IMR90 cells were much less sensitive to the compound than the pancreatic cancer cells, suggesting that SP141 has a selective cytotoxicity for cancer cells. The inhibition of colony formation and cell proliferation (Figure 1C) by SP141 was also observed, further supporting its p53-independent inhibitory effects on pancreatic cancer cells.
To investigate how SP141 suppresses pancreatic cancer cell growth, we examined the effects of SP141 on the cell cycle progression and apoptosis in pancreatic cells. As shown in Figure 1D, we found that SP141 significantly induced cell cycle arrest in the G2/M phase, with initial effects beginning at the 0.5 μM concentration in HPAC and Panc-1 cells (P < 0.01), and at the 0.2 μM concentration in AsPC-1 cells. Compared with the vehicle control, the 1-μM concentration of SP141 induced an increase in the apoptotic index by 4-fold (P < 0.01), 6-fold (P < 0.01), and 8-fold (P <0.01) in the HPAC, Panc-1and AsPC-1 cells, respectively (Figure 1E).
SP141 Inhibits MDM2 Expression, Regardless of the p53 Status
We next characterized the effects of SP141 on the expression and cellular functions of MDM2. As illustrated in Figure 1F, SP141 decreased the MDM2 protein level in a concentration-dependent manner. Consequently, in HPAC cells, the levels of wild type p53 and p21Waf1/CIP1, both of which are negatively regulated by MDM2,7 were elevated. However, no significant effect on mutant p53 was observed in the Panc-1 cells. We also found that the SP141-induced MDM2 inhibition modified the expression of multiple proteins correlated with apoptosis and cell cycle progression. In all three pancreatic cell lines, SP141 upregulated the protein levels of Bax, cleaved Caspase-3 and cleaved PARP, and decreased the expression of Cyclin E and Bcl2, which were associated with the induction of apoptosis and G2/M phase arrest.
SP141 Induces MDM2 Auto-ubiquitination and Proteasomal Degradation, a Unique Mechanism of MDM2 Inhibition
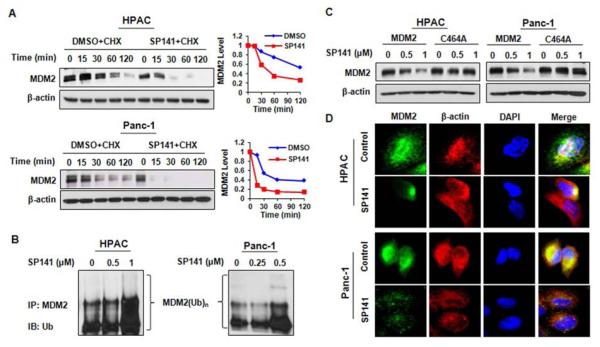
We then elucidated how SP141 reduced the MDM2 protein levels. In the presence of cycloheximide (CHX, 15 μg/mL), a protein synthesis inhibitor, SP141 increased the degradation rate of the MDM2 protein in both HPAC and Panc-1 cells (Figure 2A). To elucidate the underlying mechanism(s), the pancreatic cancer cells were co-transfected with MDM2 and ubiquitin plasmids, followed by treatment with SP141 (0.5 μM) for 24 h, and then were exposed to a proteasome inhibitor, MG132 (25 μM), for an additional 6 h. The ubiquitinated MDM2 protein in the cell lysates was immunoprecipitated using an anti-MDM2 antibody and was detected using an anti-ubiquitin antibody. As shown in Figure 2B, SP141 increased the ubiquitination of MDM2. This increase was due to increased auto-ubiquitination; an MDM2 mutant without ubiquitin E3 ligase activity (C464A) was resistant to the inhibitory effects of SP141 (Figure 2C). The downregulation of MDM2 by SP141 was further confirmed by immunofluorescence studies. The staining of MDM2 was markedly decreased in SP141-treated cells compared to control cells (Figure 2D). To determine whether SP141 affects MDM2 transcription, we analyzed the mRNA levels of MDM2 by real-time PCR, and found that there were no significant differences in the MDM2 mRNA levels observed between the SP141-treated cells and control cells in both pancreatic cell lines (Data not shown).
Figure 2.
SP141 induces MDM2 auto-ubiquitination and proteasomal degradation. (A) HPAC and Panc-1 cells were treated with DMSO or SP141 (0.5 μ M), then were exposed to a protein synthesis inhibitor, cycloheximide (CHX, 15 μ g/mL). The MDM2 expression levels were detected by immunoblotting. The relative levels of protein expression normalized to β-actin were quantitated and the results were shown in graphs on the right. (B) Cells were transfected with MDM2 and ubiquitin plasmids, followed by treatment with various concentrations of SP141 for 24 h. The cells were then lysed or further exposed to a proteasome inhibitor, MG132 (25 μM), for an additional 6 h, and the lysates were subjected to immunoprecipitation with an MDM2 antibody. The ubiquitinated MDM2 was detected using an anti-ubiquitin antibody. (C) The cells were transfected with a wild type MDM2 plasmid or mutant MDM2 plasmid without E3 ligase activity (C464A). After treatment with SP141 for 24 h, the MDM2 levels were detected by immunoblotting. (D) Representative images of MDM2 immunofluorescence in control and SP141 (0.5 μM)-treated cells.
The Effects of SP141 on Cancer Cell Growth are Dependent on MDM2 Inhibition
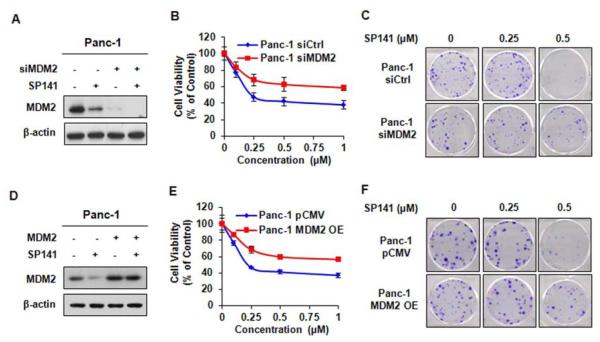
We next asked how critical the effects on MDM2 are to the anticancer activity of SP141 using specific MDM2 siRNA and MDM2 overexpression in Panc-1 cells. As shown in Figure 3A, the transient transfection of MDM2 siRNA resulted in approximately 83% knockdown of the MDM2 protein expression, and reduced the effects of SP141 on cell viability (Figure 3B) and colony formation (Figure 3C) in the Panc-1 cells, indicating that the effects on MDM2 are critical for the SP141-induced growth inhibition in pancreatic cancer cells. To further demonstrate that the effects of SP141 on MDM2 are important for its anticancer activity, we investigated the effects of SP141 on parental and MDM2 overexpressing cells. As shown in Figure 3D, the transient transfection of cells resulted in an approximately 2.9-fold increase in the expression of MDM2. The enforced MDM2 overexpression increased the cell growth, resulting in reduction of the inhibitory effects of SP141 on cell growth (Figure 3E) and colony formation (Figure 3F) in Panc-1 cells. The possible reason for this result may be related to high level of enforced MDM2 inhibition may compete for the available drug (SP141) in the assay system.
Figure 3.
MDM2 knockdown and overexpression reduce the cell response to SP141. Panc-1 cells were transfected with MDM2 siRNA for 36 h, then the cells were treated with or without 0.5 μM SP141 for 24 h prior to evaluating the MDM2 expression (A); 72 h before examining the cell viability (B); or 24 h for the colony formation assay (C). Panc-1 cells were transfected with the MDM2 overexpression plasmid for 24 h, then cells were treated with or without 0.5 μM SP141 for 24 h prior to detecting the MDM2 expression (D); 72 h before examining the cell viability (E); or (F) 24 h for the colony formation assay.
SP141 Suppresses Tumor Growth and Induces Tumor Regression in Pancreatic Xenograft and Orthotopic Mouse Models
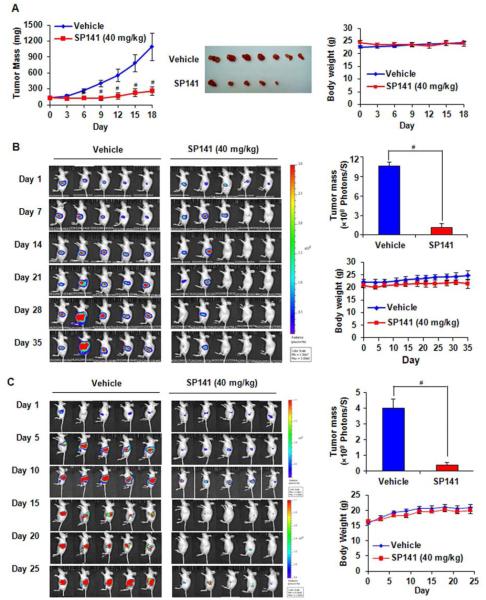
To demonstrate the anti-pancreatic cancer activity in vivo, nude mice bearing xenograft and orthotopic tumors were treated by intraperitoneal (i.p.) injections of SP141 (40 mg/kg/d). Our results showed that the SP141 treatment significantly suppressed the growth of pancreatic xenograft tumors (Figure 4A). On Day 18 of SP141 treatment, the tumor volume in the treated group was reduced by 75% compared with that in the control group (P < 0.01). There were no significant differences in the body weight (as a surrogate marker of toxicity) between the control group and the animals treated with SP141 (Figure 4A).
Figure 4.
SP141 suppresses pancreatic tumor growth in both xenograft and orthotopic mouse models. (A) The in vivo effects of SP141 administered to nude mice bearing Panc-1 xenograft tumors. SP141 was administered by i.p. injection at doses of 40 mg/kg/d, 5 d/wk for about three weeks. At the end of the experiment, representative tumors were removed and photographed. Animals were also monitored for changes in body weight as a surrogate marker for toxicity. Panc-1-Luc (B) and AsPC-1 (C) cells were implanted orthotopically into the pancreata of mice, which were treated with SP141 by i.p. injection at doses of 40 mg/kg/d, 5 d/wk for about five weeks or three weeks, respectively. The luciferase signals in the mice were detected, and the images were photographed by an IVIS in vivo imaging system twice a week. At the termination of the experiments, the average tumor volume in the SP141-treated group was compared to that in the control group. These mice were also monitored for changes in body weight as a surrogate marker for toxicity. (* P< 0.05; # P< 0.01).
In the orthotopic pancreatic tumor models, SP141 treatment led to almost complete tumor regression on Day 35 (Panc-1 model) (Figure 4B) or Day 25 (AsPC-1 model) (Figure 4C). Additionally, there were also no significant differences in the body weight between the control mice and the SP141-treated mice (Figures 4B and 4C). There were also no gross organ abnormalities at necropsy in either group, indicating that there were no remarkable side effects of SP141 at a dose that effectively induced tumor regression.
SP141 Downregulates MDM2 In Vivo
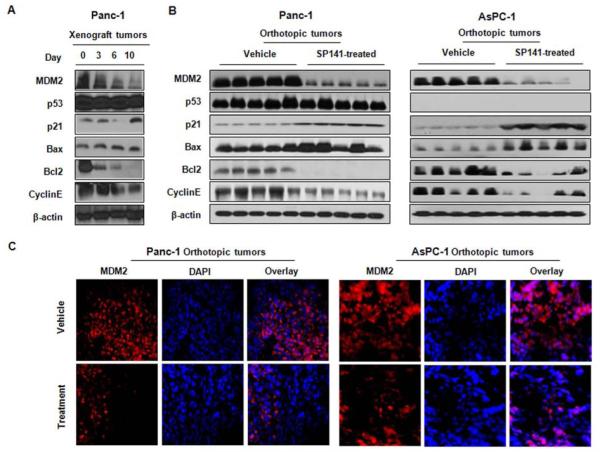
To validate the in vitro findings regarding the mechanisms of action of SP141, we assessed the in vivo expression levels of the proteins examined in vitro (Figure 5). Consistent with the in vitro data, the protein expression patterns in the treated xenograft tumors were essentially the same as those observed in vitro in the pancreatic cancer cell lines treated with SP141, showing decreased MDM2, Cyclin E, and Bcl2, and increased p21 and Bax levels in the treated tumors (Figure 5A). Similar results were seen in an orthotopic pancreatic cancer model (Figure 5B). To further confirm these observations, immunofluorescence studies were performed, providing further evidence of the in vivo inhibition of MDM2 by SP141 (Figure 5C).
Figure 5.
SP141 inhibits MDM2 in vivo. (A) The expression of proteins in tumor homogenates (on different days of the treatment) from the Panc-1 xenograft tumors was analyzed by Western blotting. (B) At the end of the experiment, orthotopic tumors were exercised and analyzed for their expression of different proteins. (C) Control and SP141-treated tumors were immunostained for MDM2, and DAPI was used as an internal reference.
Discussion
The MDM2 oncogene is a multifaceted molecule. It was initially thought that MDM2 mainly acts as a negative regulator of the tumor suppressor p53, which regulates the cell cycle, maintains the genomic integrity of cells, and controls the cellular response to DNA damage.29,44-46 More recently, increasing evidence has suggested that MDM2 also plays a critical role in carcinogenesis and cancer progression in a p53-independent manner. 20-23 The MDM2 oncogene is amplified and/or overexpressed in a number of human malignancies, including pancreatic cancer.10,12 We and others have suggested that MDM2 is a promising target for human cancer therapy and prevention.10-15 In a recent attempt to develop novel small molecule MDM2 inhibitors with drug-like properties, we performed a structure-based virtual screening, and discovered a series of pyrido[b]indole derivatives with strong binding affinity for the MDM2 protein. SP141 was selected as a lead compound for further in vitro and in vivo testing.
In the present study, we have demonstrated at least six important points: (1) SP141 is a newly designed MDM2 inhibitor that directly binds to the MDM2 protein with high affinity and high capacity; (2) SP141 decreases the viability, inhibits proliferation, and induces apoptosis and G2/M phase arrest in pancreatic cancer cells, regardless of the p53 status, which are consistent with the reported roles of MDM2 in cancer development and progression, as well as therapy; (3) the SP141-MDM2 interaction leads to increased MDM2 auto-ubiquitination and proteasomal degradation, which is a previously unknown mechanism for a MDM2 inhibitor, indicating that SP141 represents a new class of MDM2 inhibitors; (4) the SP141-induced inhibition of MDM2 is a critical and specific mechanism associated with its anticancer activities, as demonstrated by both enforced MDM2 overexpression and siRNA-induced MDM2 knockdown; (5) SP141 inhibited tumor growth and induced tumor regression in both xenograft and orthotopic models in nude mice; and (6) additional analyses of the protein expression levels of molecules associated with the MDM2 pathways revealed that similar changes occurred in vitro and in vivo after exposure to SP141, further demonstrating the specificity and the mechanism of action for SP141 as an anti-pancreatic cancer agent.
The specificity of SP141 as a novel MDM2 inhibitor was demonstrated in several assays. First, SP141 had much stronger inhibitory effects against pancreatic cancer cells in comparison with its analogs. Second, the SP141-induced anticancer activity was dependent on MDM2 inhibition, as shown in pancreatic cells with various p53 backgrounds (Fig. 2). Third, the specific mechanism underlying the SP141-induced MDM2 inhibition was shown to be the promotion of MDM2 protein degradation, not an effect on the gene expression of MDM2. It is known that MDM2 possesses E3 ubiquitin ligase activity, and that it can ubiquitinate itself, as well as other proteins.8 The present study demonstrated that SP141 causes MDM2 auto-ubiquitination and increases its degradation, a previously unrecognized mechanism of action for an MDM2 inhibitor. Finally, the changes in MDM2-related proteins seen in vitro and in vivo further corroborated the effects of MDM2 inhibition, because they were similar to those seen with other specific MDM2 inhibitors such as antisense anti-MDM2 oligonucleotides and siRNAs.
Of note, in the present study, transient siRNA knockdown and enforced overexpression of MDM2 all led to the same outcome: decreased sensitivity to SP141. Although the exact mechanisms of action are not completely understood, we speculate these results are related to our experimental conditions. In our siRNA knockdown study, the knockdown efficiency was confirmed before the cells’ exposure to SP141. The loss of sensitivity is most likely due to the loss of molecular target (MDM2). In the OE study, the enforced MDM2 expression was established and remained effective when the cells exposed to SP141. Due to the high level of MDM2 overexpression, the cell growth was increased (with high baseline of growth rate). Under this condition, MDM2 at very high level compete for the limited available compound (SP141); the effectiveness of SP141 may be decreased, compared with the control cells with normal MDM2 level. We believe that this transient OE study may not represent the situation with long-term stable MDM2 overexpression in vivo, where the cells are addictive to high level of MDM2. The current transient OE study may provide an evidence for SP141 binding to its target (MDM2).
Compared with other reported MDM2 inhibitors under preclinical and clinical development, our lead compound, SP141, is distinct in that it can exert anticancer activity in p53 wild type, p53 mutant and p53 null pancreatic cancer cells. As mentioned above, the MDM2 inhibitors that depend on blocking MDM2-p53 binding have several limitations. First, there is an MDM2-p53 feedback regulatory loop in which p53 promotes MDM2 expression. For those compounds only targeting the MDM2-p53 binding, blocking MDM2-p53 binding may release p53, but the activated p53 may in turn result in increased MDM2 expression, thus leading to an accumulation of MDM2 in the cells and diminishing the effects of those compounds. Second, more than 50% of human cancers do not express wild type 53, so the compounds only targeting MDM2-p53 binding may have little or no effects on these cancers, limiting their potential for clinical use. Third, the compounds targeting the MDM2-p53 binding would not be able to inhibit MDM2’s p53-independent activities, which have been demonstrated to be linked to carcinogenesis and cancer progression. Therefore, our newly identified MDM2 inhibitor, SP141, is expected to have broad spectrum anticancer activity, regardless of the p53 status of the cancer cells. This is important, since most patients with pancreatic cancer do not express wild type p53.
Pancreatic cancer was selected as our target disease for SP141 for several reasons. First, MDM2 is overexpressed in pancreatic cancer and has been linked to advanced disease, drug resistance and metastasis.20 Second, in our initial screening of different cancer cell lines, pancreatic cancer cells were shown to the most sensitive to SP141 and its analogs. Third, our in vitro and in vivo efficacy data for SP141 further support that it is an excellent candidate agent for pancreatic cancer therapy. Fourth, although direct toxicology data are lacking, SP141 had no obvious side effects at a dose that induced significant tumor growth inhibition and tumor regression, indicating that it may be safe to use at clinically relevant doses.
In summary, SP141 is a rationally designed small-molecule, drug-like MDM2 inhibitor that directly binds to MDM2, induces MDM2 degradation through enhanced auto-ubiquitination, and regulates the oncogenic activities of MDM2 in pancreatic cancer cells in vitro and in vivo, regardless of the p53 status of the cells. These findings provide a basis for future preclinical and clinical studies of SP141 for pancreatic cancer therapy.
Supplementary Material
Acknowledgements
We thank Dr. Xu Zhang for helpful discussions and Mrs. Subhasree Nag for excellent assistance in the preparation of this manuscript.
Funding
The contents of the paper are solely the responsibility of the authors, and do not necessarily represent the official views of the National Institutes of Health or other funding agencies. R.Z. was supported by NIH grants R01 CA112029, R01 CA121211, and R01 CA186662. J.K.B. was supported by NIH grant R15 CA100102, M.H.W. was supported by NIH grant R01 CA91980, J.Z. was supported by NSFC grants (81161120537, 30930080, 91229125, 81001231, and 81370078). H.W. was supported by NSFC grants (81125020). D.M. was supported by NIH grants R01 CA78383 and R01 CA150190 and the Bruce and Martha Atwater Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no actual or potential competing financial interests.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Paulson AS, Tran Cao HS, Tempero MA, et al. Therapeutic advances in pancreatic cancer. Gastroenterology. 2013;144:1316–1326. doi: 10.1053/j.gastro.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 3.Shah AN, Summy JM, Zhang J, et al. Development and characterization of gemcitabine- resistant pancreatic tumor cells. Ann Surg Oncol. 2007;14:3629–3637. doi: 10.1245/s10434-007-9583-5. [DOI] [PubMed] [Google Scholar]
- 4.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Li Y, Ahmad A, et al. Pancreatic cancer: understanding and overcoming chemoresistance. Nat Rev Gastroenterol Hepatol. 2011;8:27–33. doi: 10.1038/nrgastro.2010.188. [DOI] [PubMed] [Google Scholar]
- 6.Oliner JD, Pietenpol JA, Thiagalinggam S, et al. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362:857–860. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- 7.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 8.Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420:25–27. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- 9.Haupt Y, Maya R, Kazaz A, et al. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 10.Rayburn ER, Ezell SJ, Zhang R. Recent advances in validating MDM2 as a cancer target. Anticancer Agents Med Chem. 2009;9:882–903. doi: 10.2174/187152009789124628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang R, Wang H. MDM2 Oncogene as a Novel Target for Human Cancer Therapy. Curr Pharm Des. 2000;6:393–416. doi: 10.2174/1381612003400911. [DOI] [PubMed] [Google Scholar]
- 12.Rayburn E, Zhang R, He J, et al. MDM2 and Human Malignancies: Expression, Clinical Pathology, Prognostic Markers, and Implications for Chemotherapy. Curr Cancer Drug Targets. 2005;5:27–42. doi: 10.2174/1568009053332636. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Hu Y. Small molecule agents targeting the p53-MDM2 pathway for cancer therapy. Med Res Rev. 2012;32:1159–1196. doi: 10.1002/med.20236. [DOI] [PubMed] [Google Scholar]
- 14.Shangary S, Wang S. Small-molecule inhibitors of the MDM2-p53 protein-protein interaction to reactivate p53 function: a novel approach for cancer therapy. Annu Rev Pharmacol Toxicol. 2009;49:223–241. doi: 10.1146/annurev.pharmtox.48.113006.094723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q, Lozano G. Molecular pathways: targeting Mdm2 and Mdm4 in cancer therapy. Clin Cancer Res. 2013;19:34–41. doi: 10.1158/1078-0432.CCR-12-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vassilev LT, Vu BT, Graves B, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 17.Issaeva N, Bozko P, Enge M, et al. Small molecule RITA binds to p53, blocks p53- HDM-2 interaction and activates p53 function in tumors. Nat Med. 2004;10:1321–1328. doi: 10.1038/nm1146. [DOI] [PubMed] [Google Scholar]
- 18.Shangary S, Qin D, McEachern D, et al. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc Natl Acad Sci U S A. 2008;105:3933–3938. doi: 10.1073/pnas.0708917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scarpa A, Capelli P, Mukai K, et al. Pancreatic adenocarcinomas frequently show p53 gene mutations. Am J Pathol. 1993;142:1534–1543. [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z, Zhang R. p53-independent activities of MDM2 and their relevance to cancer therapy. Curr Cancer Drug Targets. 2005;5:9–20. doi: 10.2174/1568009053332618. [DOI] [PubMed] [Google Scholar]
- 21.Qin JJ, Nag S, Voruganti S, et al. Natural Product MDM2 Inhibitors: Anticancer Activity and Mechanisms of Action. Curr Med Chem. 2012;19:5705–5725. doi: 10.2174/092986712803988910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vargas DA, Takahashi S, Ronai Z. Mdm2: A regulator of cell growth and death. Adv Cancer Res. 2003;89:1–34. doi: 10.1016/s0065-230x(03)01001-7. [DOI] [PubMed] [Google Scholar]
- 23.Bouska A, Lushnikova T, Plaza S, et al. Mdm2 promotes genetic instability and transformation independent of p53. Mol Cell Biol. 2008;28:4862–4874. doi: 10.1128/MCB.01584-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu L, Zhu N, Zhang H, et al. Regulation of XIAP translation and induction by MDM2 following irradiation. Cancer Cell. 2009;15:363–375. doi: 10.1016/j.ccr.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu W, Ma Q, Chen L, et al. MDM2 acts downstream of p53 as an E3 ligase to promote FOXO ubiquitination and degradation. J Biol Chem. 2009;284:13987–14000. doi: 10.1074/jbc.M901758200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vlatkovic N, Guerrera S, Li Y, et al. MDM2 interacts with the C-terminus of the catalytic subunit of DNA polymerase epsilon. Nucleic Acids Res. 2000;28:3581–3586. doi: 10.1093/nar/28.18.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asahara H, Li Y, Fuss J, et al. Stimulation of human DNA polymerase epsilon by MDM2. Nucleic Acids Res. 2003;31:2451–2459. doi: 10.1093/nar/gkg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burmakin M, Shi Y, Hedström E, Kogner P, Selivanova G. Dual targeting of wild-type and mutant p53 by small molecule RITA results in the inhibition of N-Myc and key survival oncogenes and kills neuroblastoma cells in vivo and in vitro. Clin Cancer Res. 2013;19:5092–5103. doi: 10.1158/1078-0432.CCR-12-2211. [DOI] [PubMed] [Google Scholar]
- 29.Nag S, Qin J, Srivenugopal K, et al. The MDM2-p53 pathway revisited. J Biomed Res. 2013;27:254–271. doi: 10.7555/JBR.27.20130030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W, Rayburn ER, Zhao Y, et al. Novel ginsenosides 25-OH-PPD and 25-OCH3- PPD as experimental therapy for pancreatic cancer: anticancer activity and mechanisms of action. Cancer Lett. 2009;278:241–248. doi: 10.1016/j.canlet.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang XR, Xu H, Zhang X, et al. Preclinical Evaluation of Anticancer Efficacy and Pharmacological Properties of FBA-TPQ, a Novel Synthetic Makaluvamine Analog. Marine Drugs. 2012;10:1138–1155. doi: 10.3390/md10051138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang W, Ao L, Rayburn ER, et al. KCN1, a Novel Synthetic Sulfonamide Anticancer Agent: In Vitro and In Vivo Anti-Pancreatic Cancer Activities and Preclinical Pharmacology. PLoS ONE. 2012;7:e44883. doi: 10.1371/journal.pone.0044883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Padhye SS, Guin S, Yao HP, et al. Sustained expression of the RON receptor tyrosine kinase by pancreatic cancer stem cells as a potential targeting moiety for antibody- directed chemotherapeutics. Mol Pharm. 2011;8:2310–2319. doi: 10.1021/mp200193u. [DOI] [PubMed] [Google Scholar]
- 34.Muders MH, Vohra PK, Dutta SK, et al. Targeting GIPC/synectin in pancreatic cancer inhibits tumor growth. Clin Cancer Res. 2009;15:4095–4103. doi: 10.1158/1078-0432.CCR-08-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen L, Agrawal S, Zhou W, et al. Synergistic activation of p53 by inhibition of MDM2 expression and DNA damage. Proc Natl Acad Sci U S A. 1998;95:195–200. doi: 10.1073/pnas.95.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Z, Li M, Wang H, et al. Antisense therapy targeting MDM2 oncogene in prostate cancer: Effects on proliferation, apoptosis, multiple gene expression, and chemotherapy. Proc Natl Acad Sci U S A. 2003;100:11636–11641. doi: 10.1073/pnas.1934692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang R, Wang H, Agrawal S. Novel antisense anti-MDM2 mixed-backbone oligonucleotides: Proof of principle, in vitro and in vivo activities, and Mechanisms. Curr Cancer Drug Targets. 2005;5:43–50. doi: 10.2174/1568009053332663. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Wang H, Li M, et al. Novel MDM2 p53-independent functions identified through RNA silencing technologies. Ann NY Acad Sci. 2005;1058:205–214. doi: 10.1196/annals.1359.030. [DOI] [PubMed] [Google Scholar]
- 39.Li M, Zhang Z, Hill DL, et al. Genistein, a dietary isoflavone, down-regulates the MDM2 oncogene at both transcriptional and posttranslational levels. Cancer Res. 2005;65:8200–8208. doi: 10.1158/0008-5472.CAN-05-1302. [DOI] [PubMed] [Google Scholar]
- 40.Li M, Zhang Z, Hill DL, et al. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007;67:1988–1996. doi: 10.1158/0008-5472.CAN-06-3066. [DOI] [PubMed] [Google Scholar]
- 41.Wang W, Rayburn ER, Velu SE, et al. In Vitro and In Vivo Anti-cancer Activity of Novel Synthetic Makaluvamine Analogs. Clin Cancer Res. 2009;15:3511–3518. doi: 10.1158/1078-0432.CCR-08-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang W, Zhang X, Qin JJ, et al. Natural product ginsenoside 25-OCH3-PPD inhibits breast cancer growth and metastasis through down-regulating MDM2. PLoS One. 2012;7:e41586. doi: 10.1371/journal.pone.0041586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Wang W, Wang H, et al. Identification of ribosomal protein S25 (RPS25)-MDM2-p53 regulatory feedback loop. Oncogene. 2013;32:2782–2791. doi: 10.1038/onc.2012.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menendez D, Inga A, Resnick MA. The expanding universe of p53 targets. Nat Rev Cancer. 2009;9:724–737. doi: 10.1038/nrc2730. [DOI] [PubMed] [Google Scholar]
- 45.Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 46.Momand J, Jung D, Wilczynski S, et al. The MDM2 gene amplification database. Nucleic Acids Res. 1998;26:3453–3459. doi: 10.1093/nar/26.15.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Author names in bold designate shared co-first authors.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.