Abstract
GATA-3 expression is crucial for T cell development and peaks during commitment to the T-cell lineage, midway through the CD4−CD8− (DN) 1-3 stages. We used RNA interference and conditional deletion to reduce GATA-3 protein acutely at specific points during T-cell differentiation in vitro. Even moderate GATA-3 reduction killed DN1 cells, delayed progression to DN2 stage, skewed DN2 gene regulation, and blocked appearance of DN3 phenotype. Although a Bcl-2 transgene rescued DN1 survival and improved DN2 cell generation, it did not restore DN3 differentiation. Gene expression analyses (qPCR, RNA-seq) showed that GATA-3-deficient DN2 cells quickly upregulated genes including Spi1 (PU.1) and Bcl11a and downregulated genes including Cpa3, Ets1, Zfpm1, Bcl11b, Il9r and Il17rb, with gene-specific kinetics and dose-dependencies. These targets could mediate two distinct roles played by GATA-3 in lineage commitment, as revealed by removing wildtype or GATA-3-deficient early T-lineage cells from environmental Notch signals. GATA-3 worked as a potent repressor of B-cell potential even at low expression levels, so that only full deletion of GATA-3 enabled pro-T cells to reveal B-cell potential. The ability of GATA-3 to block B-cell development did not require T-lineage commitment factor Bcl11b. In prethymic multipotent precursors, however, titration of GATA-3 activity using tamoxifen-inducible GATA-3 showed that GATA-3 inhibits B and myeloid developmental alternatives at different threshold doses. Furthermore, differential impacts of a GATA-3 obligate repressor construct imply that B and myeloid development are inhibited through distinct transcriptional mechanisms. Thus, the pattern of GATA-3 expression sequentially produces B-lineage exclusion, T-lineage progression, and myeloid-lineage exclusion for commitment.
INTRODUCTION
Gata3 is required for T cell development, but the mechanisms through which it works are only partially understood. Gata3 is expressed in a number of embryonic tissues as well as in the thymus, and the germline knockout generates an embryonic lethal phenotype before the earliest T development stages, between E11 and E12 in Gata3 knockout pups (1). Rag2−/− blastocyst complementation experiments show that Gata3 null ES cells can contribute to all hematopoietic lineages except the T lineage (2-5). Experiments showing that antisense oligonucleotides to Gata3 could block appearance of CD3+ cells in fetal thymic organ culture provided initial evidence that GATA-3 acts after thymic entry (6). GATA-3 is also required for generation of the earliest intrathymic precursors (7), and in some conditions regulates self-renewal behavior of prethymic stem cells as well (8, 9). Poor viability of the earliest T-cell precursors when GATA-3 is removed prethymically(7) has limited exploration of the role GATA-3 plays in T cell specification and commitment, and Lck-Cre deletes a conditional allele too late to probe a role in lineage commitment as such (10). However, recent work has linked GATA-3 to the important decision of T-cell precursors to eliminate B-cell potential in the DN1 and DN2 stages (11). The present study was undertaken to introduce stage-specific, acute, early perturbations of GATA-3 that could shed light on its actions between thymic entry and commitment.
Ideally, GATA-3's roles could be inferred from its target genes. GATA-3 binding sites have been mapped across the genome in CD4+ CD8+ thymocytes and earlier CD4− CD8− (DN) precursors (12, 13). However, the distribution of sites detected has turned out to be variable according to stage, implying that GATA-3 regulates different target genes at different points in development. Complementing GATA-3-deficient cells with retroviral GATA-3 is also challenging because GATA-3 overexpression is as toxic for early T-cell precursors as loss of GATA-3 (14). In this study, therefore, we have retrovirally introduced shRNA into precursors undergoing T-lineage differentiation in vitro (15, 16), to impose loss of function at specific precommitment, pro-T cell stages, and we have examined the effects of acute Gata3 deletion at short time scales. We show that a critical level of GATA-3 activity is needed to progress through commitment, and demonstrate that GATA-3 contributes directly and uniquely to T-lineage commitment through two different mechanisms.
MATERIALS AND METHODS
Mice
C57BL/6 (B6), B6D2 F2, or Eμ-Bcl-2-25 (Bcl-2-tg) (17) were used. C57BL/6 (B6) or Eμ-Bcl2-25 (Bcl2-tg) fetal mice were maintained in our colony, and C57BL/6 × DBA/2 (B6D2) F2 embryos were obtained from the California Institute of Technology Genetically Engineered Mouse service. ROSA26R-EYFP reporter mice for Cre-mediated excision (18) were bred from stock generously donated by Dr. Frank Costantini (Columbia University). Gata-3fl/fl mice (10) were bred from stock kindly provided by Dr. I-Cheng Ho. Spi1fl/fl (PU.1 floxed) mice were kindly provided by Dr. Stephen Nutt (19). Bcl11bfl/fl (Bcl11b floxed) mice were previously described (20). ROSA26-Cre-ERT2 mice were generated in our colony by crossing PLBD (Bcl11bfl/fl; ROSA26-Cre-ERT2) mice, kindly provided by Dr. Pentao Liu (21), to B6 mice. To control the timing of Gata3 deletion, these mice were further crossed to Gata-3fl/fl mice to generate Gata-3fl/fl; ROSA26-Cre-ERT2 mice. Note that unlike the ROSA26R-EYFP Cre recombinase reporter strains, these mice express Cre-ERT2 constitutively, but the nuclear localization and activity of the Cre protein depend on tamoxifen treatment. All animals were bred, maintained under specific pathogen-free conditions, genotyped, and euthanized using IACUC approved protocols.
Cell lines
Adh.2C2
The pro-T cell-like Adh.2C2 cell line, originally subcloned from the SCID.adh cell line (22), was generated and maintained as previously described (23). The murine macrophage-like cell line RAW264.7 was used as a negative control for specificity of GATA-3 intracellular staining. OP9 stromal cell lines which either did (OP9-DL1, OP9-DL4) or did not (OP9-control) express Delta-like ligands were obtained from J.C. Zúñiga-Pflücker and cultured as previously described (15, 16, 24).
Fetal liver derived double negative cells (FLDN)
E13.5-14 fetal liver was obtained from timed pregnancies of mice of the indicated genotypes. Differentiated cells were depleted using a cocktail of biotinylated antibodies specific for Ter119, Gr-1, F4/80, and CD19 (Lin cocktail) using tube adherence to a block magnet followed by a further depletion step on a MACS Miltenyi LS column. They were then sorted for c-Kit+/CD27+/Lin−/7AAD− phenotype (Lin=depletion cocktail above). These cells are referred to as Fetal Liver-derived Precursors, or “FLP”. To generate FLDN, sorted precursors (15-100 × 103 per plate) were added to 10 cm confluent plates of OP9-DL1 or OP9-control stroma and cultured for 6-7 days (OP9-DL1 condition). Cells were recovered from these cultures and infected overnight with retroviral vectors (performed as previously described (25)). From these transduced pools, ETP, DN2,and DN3 FLDN cells were sorted, based on expression of GFP, c-Kit, CD25, and CD44, with CD45 to exclude stromal cells, prior to subsequent culture. For comparison, “in vivo” ETP, DN2, DN3, and DN4 cells were sorted from E14.5 fetal thymus (FT) or from the thymus of 4-6 week old adult mice after magnetic bead depletion with Lin cocktail supplemented with antibodies against TCRβ, TCR-γδ, CD8, NK1.1, and CD11b (Mac1), or using antibodies against CD3, CD4, ,CD8, CD19 and Ter119 for experiments shown in Fig. 1F and Fig. S1.
Figure 1. Expression patterns of GATA-3 in early T lineage cells.
A. Four color intracellular detection of GATA-3 in early DN thymocyte subsets from C57BL/6 adult mice. Top panels: Subsets defined by expression of Kit, CD 44, and CD25. Lower panels: GATA-3 protein levels. Upper histogram: levels in stages from ETP-DN2b; lower histogram: levels in stages from DN2b-DN4 (DN2b is included in both for orientation).
B. Gata3 RNA expression in DN1-DN4 cells. Gata3 RNA levels determined by qPCR analysis of samples from fetal thymocytes (FT) and FLDN generated as shown in Fig. 1E. Gata3 expression levels are shown relative to β-actin for each sample. From 2 (FT) or 4 (FLDN) independently sorted sample sets of DN1-4.
C. Intracellular staining of GATA-3 in cells from Rag-2−/− weanling thymocytes and wild type E14 fetal thymocytes.
D. Intracellular flow cytometric detection of GATA-3 protein in FLDN subsets derived as in 1E, and gated as indicated (top). Histogram color coding as in 1A.
E. Schematic of FLDN generation: fetal liver precursors in OP9-DL1 coculture for 4-7 days (top) differentiate to DN1-3 stage pro-T cells (middle, d7 initial culture). These are then sorted as pure subsets of DN1, DN2, and DN3 and re-plated on OP9-DL1 for 4-7 days more. Phenotypes shown are descended from the indicated sorted subsets after 7 more days of co-culture (lower panels).
F. Gene expression comparison of sorted thymic T-cell precursors with in vitro generated FLDN subsets. Early DN thymocyte subsets from adult and fetal murine thymus were depleted of mature T and non-T lineage markers by magnetic bead binding and column selection and sorted into DN1,2,3,4 subsets. Two independent biological samples of each series were generated for this analysis. AT (adult thymus) samples were composed of two adult mouse thymi per sorted biological sample. Fetal thymus was obtained from E14/E14.5 fetuses from timed mated C57/BL6 mice. FLDN were OP9- DL1 cultured cells generated from c-Kit+Lin−27+ E13.5/E14 fetal liver precursors cultured on OP9 -DL1 for 6 days, then sorted as shown into pure DN1, DN2, DN3, DN4 developmental populations for analysis. Red/pink bars, Fetal thymic DN1-4; Green bars, FLDN1-4, Blue bars, Adult thymic DN1-DN4.
Flow cytometry
Flow cytometric analysis was performed on a Becton-Dickinson (BDIS, Mountain View, CA) FACSCalibur or Miltenyi MACSQuant; sorts were done using either the BDIS FACSVantage SE, or the BDIS FACSAria IIu. CD45 was used in all cases to discriminate between infected developing hematopoietic cells and OP9-derived stromal cells. The following antibody conjugates (BD Pharmingen, eBioscience) were used: c-Kit-PE, CD27-APC, Gr-1-biotin, Ter119-biotin, F4/80 biotin, CD19-biotin, for sorting of FL precursors; c-Kit-PE, CD44-PE-Cy5.5, CD45-APC, CD25-Alexa750 or CD25-APC-Cy7, and streptavidin-Alexa 405, for sorting fetal thymus and FLDN precursors.
Four color staining with intracellular GATA-3 and PU.1
Intracellular staining of transcription factors was performed using a modified version of the BD Cytofix/Cytoperm protocol. Antibodies used were BD Pharmingen clone L50-823 for GATA-3 and Cell Signaling clone 9G7 for PU.1. Three color surface staining was performed as normal, cells were washed once to remove antibody, then pelleted and aspirated. Cells were then re-suspended to disrupt the cell pellet, prior to addition of 100μl cold BDIS Cytofix/Cytoperm and followed by a 20min incubation on ice. Subsequently, 1ml/tube of 1X Permwash was added, cells were pelleted and resuspended in 100μl 1X Permwash/tube +intracellular staining antibody. For both Gata-3 and PU.1, 20 μl Ab per 1 million cells was used, and the equivalent microgram concentration was used for staining with isotype control antibody from the same supplier (BD Pharmingen or Cell Signaling). The cells were incubated with intracellular staining antibody for 30 min at room temperature. Following this, 1ml 1X Permwash/tube was added to wash. Cells were then resuspended in 2% formaldehyde in PBS and data acquired by flow cytometry within 1-2days. Critically, for four color staining that included staining for intracellular GATA-3 or PU.1, fully stained three-color +intracellular isotype controls were used to determine accurate, population-specific background levels for GATA-3 and PU.1.
Retroviral vectors and infection of cells
The original Banshee retrovirus was provided by Dr. John Rossi (Beckman Institute of the City of Hope). Dr. Gabriela Hernandez-Hoyos constructed the Banshee-GATA-3 shRNA expressing virus (called Banshee G3 3W), a derivative of a previously published GATA-3 shRNA construct (26) that incorporates two wobble mutations to prevent self inactivation of the viral transcript (27) and a slightly altered loop sequence (ttcgtagc rather than tagcttcg) (27). The sequence targeted is in exon 2, the first translated exon, immediately after the second in-frame Met codon. Controls were empty Banshee vector or Banshee driving expression of a scrambled shRNA insert.
LZRS retrovirus (Lz) based construct Lz-GATA-3 was previously described (26, 28). Other constructs were made by members of the Rothenberg lab as follows. Lz-G3-ENG and a related GATA-2 construct, Lz-G2-ENG, were constructed (by Sanket Acharya and Mark Zarnegar) using fusion/overlap PCR to join the first 298 aa of Drosophila Engrailed protein to the DNA binding domains of GATA-2 (aa 250-437) and GATA-3 (aa 251-443), to yield products with intact Engrailed N-termini and intact GATA factor C-termini. PCR products were cloned into pGEM-T Easy, transferred to pEF1-Myc-His A to screen for orientation, then excised by EcoRI/XhoI digestion and cloned into Lz. Lz(ER) was constructed by Elizabeth-Sharon David-Fung by cloning the tamoxifen-dependent ER from STAT6-ER (provided by Naoko Arai, DNAX (29)) into Lz. Lz-GATA-3(ER) was then constructed by cloning full-length GATA-3 into Lz(ER), retaining the 5-aa linker SNSDP between the GATA-3 C-terminus and the estrogen receptor sequence. Lz-CRE-NGFR was constructed by Dr. Tom Taghon (Ghent University, Ghent, BE). Bcl-xL cDNA cloned into the MigR1 vector was purchased from Addgene (Cambridge, MA).
Retroviral infections of FLP in most experiments were carried out overnight with virus bound to plates coated with 50 ng/ml Retronectin™ (Takara, Tokyo, Japan). Conditions were essentially as previously described (25), except that unbound virus was not removed from the Retronectin coated wells prior to addition of cells and fresh media. Infections of fetal thymocytes and cells beginning T-cell development by coculture with OP9-DL cells were carried out as described for individual experimental protocols.
Nucleofection
Direct nucleofection of Lin-depleted FL precursors was done using the Amaxa nucleofection system (Lonza) with a “T cell” kit and the X-001 program. Control siRNA (“RISC-free”, Dharmacon RNAi Technologies, Thermo Scientific) was transfected in parallel with a Gata-3 targeted siRNA. Amaxa's pMAX GFP construct was transfected simultaneously to monitor transfection efficiency in some experiments. Post transfection, cells were recovered and transferred to OP9-DL1 layers for recovery overnight, then sorted to purity using the same depletion protocol as described for derivation of FLDN. Adh.2C2 and RAW264.7 cells were transfected as described previously (23, 30).
Gene expression comparison of FLDN with DN subsets from primary fetal thymus (FT) and adult thymus (AT)
FLDN cells, obtained after 6 days of coculture with OP9-DL1, were sorted based on CD45, CD25, c-Kit and CD44 for purification of total cell RNA, and equivalent DN populations were sorted by CD25, c-Kit and CD44 expression from Lin-depleted fetal thymus (E14.5) and adult thymic DN cells, in two independent biological replicates for each type of sample. cDNAs from these three types of DN1, 2, 3, and 4 populations were compared by qPCR for the genes shown in Figs. 1F and S1, showing levels expressed relative to β-actin.
Quantitative real-time reverse transcriptase-dependent PCR (qPCR) and Primers
Primer sequences, RNA preparation methods, and qPCR conditions have been reported previously (14, 20, 25, 31). Additional primers are listed in Table 1.
TABLE 1.
New primers for qPCR: RNA expression analysis and genotyping
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| RNA analysis | ||
| Bcl11a | GTCTGCACACGGAGCTCTAA | CACTGGTGAATGGCTGTTTG |
| Bcl11b exons 2-4* | AGGTCACCCCTGATGAAGAT | CATGTGTGTTCTGTGCGTGCT |
| Bcl11b exon 4* | GGACAGCAATCCTTTCAACC | ATGACTCGGTCGAAGGCACT |
| CD44 | GCAGATCGATTTGAATGTAACC | GTCCGGGAGATACTGTAGC |
| Spi1 (PU.1) alternate | CTCAGTCACCAGGTTTCC | TCCAAGCCATCAGCTTCTC |
| Thy1 | GGGCGACTACTTTTGTGAGC | AGGGCTCCTGTTTCTCCTTG |
| TCRbC2 | GGGTTCTGTCTGCAACCATC | CTATGGCCAGGGTGAAGAAC |
| TCRg Cluster1 | GCTGTGAGCACTGTGATAGCTC | TCAGCAACAGAAGGAAGGAAA |
| TCR Vγ3(5)-Cγ1 | ATCGGATGAAGCCACGTACT | GAAGGAAAATAGTGGGCTTGG |
| TCR Vγ2(4)-Cγ1 | CGAAGCTATCTACTACTGTTCC | GAAGGAAAATAGTGGGCTTGG |
| TCRd | TACGACTGCTGTTTGCCAAG | TCTGAAGCACTGAGAAGTTGGA |
| Tcf7 (TCF-1) | CGAGAAGAGCAGGCCAAGTA | CCGAATGCATTTCTTTTTCC |
| Tcf7 alternate | TCCCCATGCCAATACTTCTT | GTTGGTGCCAAGGTTGAAAG |
| Zfpm1 (FOG1) alternate | CCAAGATGTCCGAGTTGGTG | GCGCTTGTGCACATAGAAGT |
| EBF-1 | CCATCCGAGTTCAGACACCT | ATGCCGAGGAATGACCTTCT |
| Pax5 E2/E3 | GAACTTGCCCATCAAGGTGT | GAGTGGCAACCTTTGGTTTG |
| Pax5 E5/E6 | GGGCTCCTCATACTCCATCA | CTGCTGCTGTGTGAACAGGT |
| Genotyping | ||
| GATA-3 Floxed | GGCATTCTCGCACGCTTCAAA (G3-F) | GGGCCGGTTCTGCCCATT (G3-R1) |
| GATA-3 Deleted | GGCATTCTCGCACGCTTCAAA (G3-F) | GGATGGGCACCACCCCGGTGAA (G3-R2) |
| Bcl11b Deleted | ACGCCGGACCTAGTAAATGCA (B-F) | GTTAGGCTGGACTGCCGCCTC (B-R) |
Single cell cultures with Gata3 shRNA
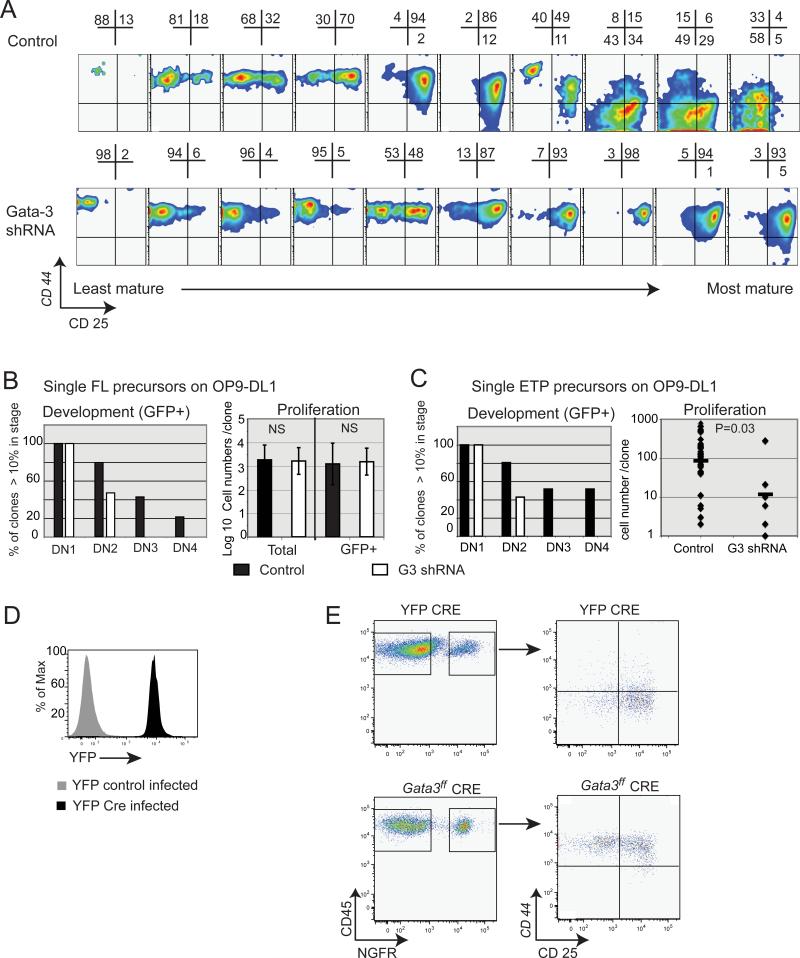
Retrovirus-transduced FL precursors were sorted at 1 cell/well directly into 96 well plates preseeded with OP9-DL1 or OP9 control cells. Wells were assessed for growth by microscopy and equal numbers of wells of Gata-3 shRNA expressing and control infected cells were harvested at 8 days, then stained for developmental markers. All remaining wells were harvested on day 12, and the additional identified expanding clones, while not included in Fig. 4A, were analyzed and included in the progression quantitation shown in Fig. 4B. Similarly, single infected ETPs were directly sorted from wildtype or Bcl-2 transgenic mouse thymocytes, into individual OP9-DL1 co-culture wells and cultured either 7 (wildtype) or 9 (Bcl-2 transgenic) days prior to analysis. Descendants of single plated cells were scored for developmental progression (left) by tallying the stages through which each clone's descendants had passed (using a 10% frequency cut off to determine what counts as progression). Thus a clone in which 40% of cells are in DN1, 49% in DN2, and 11% in DN3, and 0% in DN4, would be scored as having “achieved” progression to DN1, DN2, and DN3, but not DN4. Input cells were sorted initially as GFP+, but survival pressure on the cells frequently resulted in outgrowth of some GFP- cells in the colonies. Although these were also usually inhibited in progression past DN2 when the G3 shRNA was present, developmental progression scoring (e.g. Fig. 4B) was based only on the cells remaining GFP+.
Figure 4. Clonal development phenotypes and Cre-mediated deletion phenotypes of GATA-3 loss.
A. B6D2F2 FL precursor cells were transduced with empty vector (top) or G3-3W (bottom), sorted as single cells onto OP9-DL1 monolayers in microtiter wells, and cultured for 8 or 12 days. Phenotype of GFP+ descendants is shown, with clones ordered from least development to most (left to right).
B. Quantitation of developmental progression and proliferation of FLDN clones shown in A. Development bar graph (left) shows the frequency of clones deriving either from control infected or G3 3W infected single cells, scored for progression of at least 10% of the GFP+ clonal descendants through DN1, DN2, DN3, and DN4 stages, as described in Materials and Methods. Total CD45+ cell numbers/clone after 8 days culture are shown (left) and those remaining GFP+ (right).
C. E14-14.5 B6D2F2 fetal thymic ETP were infected with G3-3W or control and sorted clonally as described. Quantitation of ETP developmental progression (left) and proliferation (right) in clonal progeny are shown as in B.
D. FL precursors from Gata3+/+;ROSA26R-EYFP (Cre excision reporter) mice were infected with a Cre expressing retrovirus. Histogram shows efficient Cre excision to activate the EYFP reporter after 3 days in OP9-control preculture.
E. Gata3fl/fl and ROSA26R-EYFP;Gata3+/+ FL precursors were induced to express Cre by retroviral transduction during preculture on OP9 control layers as in D, then sorted for the linked retroviral marker NGFR or for YFP, respectively, and transferred to OP9-DL1 culture for 7 days. Progression is shown for Gata3fl/fl and Gata3+/+ ROSA26R-EYFP CRE expressing cells. Cells in the second column are NGFR gated (representative of 2 similar experiments; two additional related experiments analyzed at later timepoints).
Switch cultures of DN2 cells from Gata3, Bcl11b and Bcl11b;Gata3 homozygously floxed mice
Cells were taken from mice with homozygously floxed alleles of the indicated genes or control +/+ genotypes, all crossed onto the ROSA26R-EYFP background. For experiments shown in Fig. 7, Cre retroviral infection of Ter119-, Gr-1-, and CD19-depleted FL precursors was carried out in the presence of 5 ng/ml each Flt3L, IL-7, and SCF (Kit ligand). The next day, Lin−Kit+ CD27+ YFP+ cells were sorted preparatively and stored in frozen aliquots at 5-6 × 103 cells/vial or used to seed cultures immediately. Thus, the controls as well as the conditional deletion samples were Cre-treated and validated to have expressed active Cre. In these experiments, OP9-DL4 stroma were used to support T-cell development. The cells were cultured on OP9-DL4 for an initial 7-9 days with 5 ng/ml each Flt3L, SCF and IL-7 before preparative sorting of DN2 cells. SCF was added to the primary cultures in an attempt to preserve viability of GATA-3 deficient DN1 cells by providing stronger Kit signaling. Some apparent violation of the DN2 block in the primary cultures of Cre+ Gata3fl/fl cells was occasionally seen in these experiments, but this was a result of the overpowering selection (Fig. 7B) for cells that had retained one undeleted Gata3fl allele during the ETP stage, and were thus heterozygotes, as revealed in Fig. 7G. Only sorted Kit+ CD44+ CD25+ CD45+ CD19− DN2 cells from the primary cultures were transferred to secondary cultures, where 1% were moved to fresh OP9-DL4 cells and the rest were challenged by shifting to the OP9-control environment. Thus, any CD19+ cells that emerged later came from cells that had undergone at least a week of strong Notch signaling in culture and had then been sorted for a T-lineage, CD19-negative phenotype. For limiting cell-number cultures of DN2 cells from the FLDN primary cultures, 25 cells/well were deposited by cell sorting into microtiter wells pre-seeded with OP9-control cells, then cultured with 5 ng/ml each IL-7 and Flt3L. These cultures were harvested for analysis after two weeks.
Figure 7. Deletion of Gata3 unleashes B-cell potential in fetal liver precursor-derived DN2 cells in the presence or absence of Bcl11b.
A. Experimental design. Retroviral-Cre infected FLP from ROSA26R-EYFP (YFP), ROSA26R-EYFP;Bcl11bfl/fl (Bcl11bfl/fl), ROSA26R-EYFP;Gata3fl/fl (GATA-3fl/fl), and ROSA26R-EYFP;Bcl11bfl/fl;Gata3fl/fl (Bcl11bfl/fl;GATA-3fl/fl) were all sorted for successful activation of the YFP Cre reporter and cultured on OP9-DL4 for 7-8 days. YFP+ DN2 cells were then sorted and split to secondary cultures on OP9-DL4 or OP9-control (OP9-Mig) for 7-14 days more before analysis.
B. Cell proliferation of primary cultures on OP9-DL4 in bulk culture. Proliferation Index was calculated by dividing the cell numbers of Day 7 cultures by the input FLP cell numbers.
(C and D) Primary culture phenotype and secondary culture outputs of control, Bcl11b-deficient, and/or Gata3-deficient DN2 cells after treatment with Cre. Deletion of Gata3 confers gain of alternative function (B-cell potential) in Bcl11b-knockout context. DN2 cells were sorted from 7-day primary cultures as indicated in (C) and analyzed after 7-day secondary cultures on OP9-control stroma. As shown in (D), cells with NK markers developed from Gata3 positive cells, whereas CD19+, CD45intermediate B-lineage cells developed only from Gata3 single or double knockout DN2 cells on OP9-control stroma.
E. Colony size of CD19+ cells developed from both double knockout and single GATA-3 knockout DN2 cells in limiting dilution (see examples shown in Fig. S3A-D): when detected, B cell colonies (red symbols) proliferated more than NK cell colonies (blue colonies).
F. Efficiency of Gata3 deletion from NK (blue symbols) and B (red symbols) cells growing out in limiting dilution colonies from different Gata3, Bcl11b genotypes (see Fig. S3A-D). qPCR reactions were performed on genomic DNA with primer sets to quantitate deleted-Gata3 and still-intact floxed-Gata3 (see G), in samples generated from Gata3fl/fl precursors. Plots show the ratio of the “deleted” allele concentration divided by the “floxed” allele concentration. A ratio of 1, as in the NK colonies, indicates that as many alleles are undeleted as deleted, i.e. that the cells are mostly heterozygous. A ratio >102 indicates that more than 99% of alleles are deleted. DL4: cultured on OP9-DL4. “Control”: cultured on OP9-control. G3: ROSA26R-EYFP;Gata3fl/fl. BxG: ROSA26R-EYFP;Bcl11bfl/fl;Gata3fl/fl (Bcl11bfl/fl;GATA-3fl/fl).
G. qPCR primer sets used to measure the deletion of Gata3 locus used in (F). G3-F/G3-R1 primer set detects the floxed Gata3 alleles. G3-F/G3-R2 primer set detects deleted Gata3 alleles.
H. Deletion of Bcl11b in the samples was measured as in (F), except showing ratio of “deleted” allele to GAPDH gene signal. The Bcl11b deleted product can only be generated from genotypes containing a Bcl11b floxed gene (here, BxG), but importantly deletion (Bcl11b deleted:GAPDH ratio>0.1) shows no correlation with NK or B fate of the cells.
I. qPCR primer sets used to measure the deletion of Bcl11b locus in (H).
Switch cultures of adult bone marrow derived DN2 cells from Gata-3fl/fl; ROSA26-Cre-ERT2 mice
Bone marrow stem cells from control ROSA26-Cre-ERT2 and Gata3fl/fl; ROSA26-Cre-ERT2 mice were sorted as Lin− Sca-1+ Kit+ (LSK) and cultured on OP9-DL4 with 5 ng/ml Flt3L and IL7 for 11 days. 4-hydroxytamoxifen was added into the culture at 0.1 μM for 16 hours. DN2 cells were then sorted and shifted to OP9-control stromal cocultures with Flt3L and IL7 at 5 ng/ml, but without tamoxifen, for 10 days before FACS analysis. For the colony assay experiment shown in Fig. 8G, the DN2 cells were sorted and cultured in retroviral infection conditions with Bcl-xL or empty vector virus, in the presence of 4-hydroxytamoxifen (4OHT) to induce Cre activity. The cells were sorted again to purify GFP+ Bcl-xL or empty vector virus infected cells. The sorted cells were then transferred to 96-well plates at inputs of 25 cells/well, for two weeks with OP9-control stroma, Flt3L and IL7 to permit B cell development.
Figure 8. Acute deletion of Gata3 in adult bone marrow LSK-derived DN2 T cells allows B-cell development from DN2 T cells.
A. Strategy for analysis of B-cell potential in pro-T cells when Gata3 is conditionally deleted only once cells reach DN2 stage, using Cre-ERT2 activation by 4-hydroxytamoxifen (4OHT) 16 hrs prior to sorting.
B. Adult bone marrow LSK-derived DN2 cells were sorted right after 16 hrs treatment with 4OHT.
C. CD19+ CD45int B-cells developed from GATA-3 deleted DN2 cells in secondary culture even if deletion was delayed until DN2 stage.
D. Strategy for analysis of the combined effects of Gata3-deletion and Bcl-xL overexpression in DN2 cells on the emergence of B cells. T-lineage differentiation cultures of Cre-ERT2 and Gata3fl/fl bone marrow Lin− Sca-1+ Kit+ cells were followed with acute Gata3 deletion at DN2 stage by 4OHT-activated Cre, and transduction with Bcl-xL or an empty vector. B-cell potential of sorted, transduced cells was then assayed by limiting dilution culture on OP9-control stroma.
E. Phenotypes and sort gates for DN2 cells before Cre activation.
F. Sorting for cells transduced (GFP+) with empty vector or Bcl-xL after tamoxifen treatment and retroviral transduction.
G. Bcl-xL enhancement of recovery of B cell colonies from converted DN2 cells after acute Gata3 deletion.
Acute homozygous and heterozygous Gata3 deletion
To examine short-term impact of deleting Gata3, Gata3fl/fl or Gata3fl/+ embryos with a ROSA26R-EYFP homozygous or heterozygous genetic background were obtained at E13.5 as a source of FLP and started into T-cell development by a 4-d preculture on OP9-DL1. They were then transduced with retroviral Cre for 4 hr, returned to OP9-DL1 overnight (18 ±2 hr), and sorted the next day to purify YFP+ (successfully Cre-transduced) cells. These were then either analyzed immediately or returned to OP9-DL1 coculture. To analyze the effect of deleting a single copy of Gata3, YFP+ DN1 cells were sorted, returned to secondary culture for 7 days, and finally the DN2a, DN2b, and DN3 YFP+ cells generated were analyzed and/or sorted for RNA expression analysis, in comparison with control Cre-transduced YFP+ cells derived from ROSA26R-EYFP Gata3+/+ mice. Retroviral marker expression (NGFR) was highly concordant with activation of the ROSA26R-EYFP reporter gene by Cre-mediated excision of the stop cassette. To avoid confounding effects due to cell death, gene expression responses to deleting both copies of Gata3 were analyzed only after shorter times. Cycloheximide chase experiments in 2C2 cells, analyzed by intracellular staining and western blotting (S. Qin, George Freedman and S. S. Damle, data not shown), had verified that GATA-3 protein has a t1/2 of only ~2hr. Therefore, after Cre transduction and overnight coculture with OP9-DL1, YFP+ DN1, YFP+ DN2a, and sometimes also YFP+ DN2b cells were sorted for immediate RNA analysis by qPCR (i.e. within <1 day of Cre introduction). To generate homozygously Gata3-deleted DN2 samples for whole genome surveys using RNA-seq, a similar protocol was used.
Pooling of multiple data sets, statistics
Means shown for RNA expression and cell numbers are geometric means, and error bars represent one standard deviation from the means. Statistical significance was calculated using a two tailed t-test and p<0.05 was considered significant. Alternatively, for sample sets with low n and non-normal distributions, the Mann-Whitney Wilcoxon U test (two-tailed) was used to calculate p, using the web site <http://elegans.som.vcu.edu/~leon/stats/utest.html>.
RNA-seq analysis
Two RNA-seq comparisons were done. In each, ROSA26R-EYFP+ Gata3f/f:cells were transduced with Cre retrovirus to generate putatively Gata3-deleted cells, identified as EYFP+ DN2 cells. One experiment compared Cre+ Gata3f/f cells with Cre retrovirus-transfected B6 DN2 cells (Gata3+/+, DN2 pool approximately 50% transduced), and the other compared Cre+ Gata3f/f cells with control virus-transduced Gata3f/f cells (same genetic background but without deletion). Libraries for RNA-seq analysis were generated by the Jacobs Genetics and Genomics Center at Caltech essentially as described previously (13) with minor modifications, using NEBNeXT multiplex reagents (New England Biolabs) and determining single-end sequences on an Illumina HiSeq 2500 high-throughput sequencer. Raw sequence reads as fastq files were processed using Tophat (v2.0.6) to map to the mm9 mouse genome assembly, using Cufflinks (v2.1.1) to generate .BAM files, bamToBed to generate BED files (BedTools v2.14.2), and then Bedtools genomecov to generate bedgraph files that were normalized by the total number of mapped reads (RPM). For visualization in the UCSC genome browser (genome.ucsc.edu), we converted the bedgraphs to BigWig files. Data were deposited in Gene Expression Omnibus (accession number GSE59215) (www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE59215).
Differential expression analysis was carried out as follows. First, results were filtered to remove small gene models (Snor, Mir, Rpl, and Rps) and genes with low expression values (<5 fpkm). Then the remaining values (9580 gene models) were log2 transformed (floor of 0.01), and a Pearson correlation matrix was computed for the four datasets. All four datasets cross-correlated with r≥0.9. The developmental staging of the samples was assessed by using an index set of 173 developmentally regulated genes annotated as transcription regulators, from our RNA expression data reported previously for wildtype FLDN cells and adult thymocytes (13). Values for these index genes in each experimental sample were compared with the values in our reference samples using principal component analysis. This analysis verified that the samples fall into the DN2a-DN2b interval as expected by differentiation time and surface phenotype (see previous section). Then, two statistical approaches were applied to identify new candidates for particularly GATA-3-sensitive target genes. Cuffdiff (32) was used to identify differentially expressed genes in the two comparisons pooled together, and those with p values of <0.01 were considered. In parallel, genes with substantial differential expression within each pair were identified by rank product and reverse rank product in the two comparisons pooled together (downregulated and upregulated genes), with p values for the replicate comparisons calculated based on minimizing (rank product)/(ranksum) (p values <0.005)(33). Additional differential expression criteria were used for transcription factor genes as described in the text. The early timepoint chosen and the dynamic developmental context meant that only strongly and rapidly affected genes could be detected above background. However, this survey of limited statistical power was designed as a search tool to identify new candidates for rapidly GATA-3-sensitive genes that could be tested for validation in additional experiments, i.e. the GATA-3 knockdown or deletion tests assayed by qPCR in independent experiments as presented in the text.
RESULTS
Expression pattern of GATA-3 in T development
Gata3 is expressed in multipotent prethymic precursors for T development (Lin− Kithi Sca-1hi CD27+ fetal liver or bone marrow cells) (34). However, much higher Gata3 expression is seen even in the most immature cells within the thymus(35) with a little further increase (~ 3 fold) in Gata3 RNA during T-lineage commitment (11, 13, 28, 31, 36). However, GATA-3 protein levels can be regulated by post-transcriptional mechanisms as well (37-41). We therefore measured GATA-3 protein content by direct intracellular staining in each of the early DN stages (Fig. 1A; top panels show gating strategy).
In adult C57BL/6 mice, GATA-3 protein is initially low in the Kithigh CD44+ CD25− (Early T-cell precursor) population, the subset of CD44+ CD25− “DN1” thymocytes that includes T-cell precursor activity. GATA-3 levels barely change as the cells initiate expression of CD25 (“DN1 25lo”), but rise ≥3 fold higher as they turn on CD25 to become DN2a (Kithigh CD44+ CD25+)(Fig. 1A, middle). Dissecting the DN2 stage by Kit expression levels to separate uncommitted from committed cells (42), the DN2b (Kit lower) newly-committed cells have the highest levels of GATA-3 protein and RNA (Fig 1A). DN3 cells (Kitlow CD44low CD25+) before β-selection or γδ-selection express slightly less, with the lowest expression in a distinct subset of DN3 cells with no detectable Kit expression (DN3 Kitneg). In cells beginning β-selection (DN3 25lo) and through the DN4 stage (Kitlow CD44low CD25low), GATA-3 protein levels rise again (Fig. 1A, bottom), but then subside as the cells reach CD4+ CD8+ (DP) stage (26). RNA levels, like protein levels, rise slightly in the DN2 and DN3 stages (Fig. 1B). This pattern is seen also in both Rag2−/− adult thymocytes and normal C57BL/6 fetal thymocytes, confirming that the increase does not depend on TCR gene rearrangement (Fig 1C). These results agree well with the expression reported for Gata3 knock-in alleles (3, 7, 11).
GATA-3 follows a similar pattern during T-cell differentiation in vitro when Kit+ CD27+ Lin− precursors from E13.5 fetal liver (FL) are cultured on OP9-DL1 stroma to generate DN pro-T cells (Fig 1D, E). As shown by detailed expression comparisons of >55 genes in Fig. S1, FLDN subsets prepared in this way correspond closely to normal primary fetal thymocyte DN subsets. Where the gene expression patterns differ, as illustrated for 25 transcripts in Fig. 1F, gene expression patterns in FLDN subsets are intermediate between those of E14.5 fetal thymocyte subsets and those of corresponding adult DN thymocyte subsets. Thus, small variations in program dynamics reflect the ontogeny of the precursors more than the conditions of differentiation. Like fresh fetal thymocytes, FLDNs have slightly higher Gata3 RNA expression (Fig. 1B, F) and a slightly accelerated GATA-3 protein increase relative to adult thymocytes, peaking at DN2a and DN2b, then subsiding slightly from DN2b to DN3 (Fig. 1C, D). In all three sources of developing T cells, the timing of peak GATA-3 expression at DN2 suggests a role between thymic entry or initiation of T-cell development and commitment.
Reduction of GATA-3 inhibits viability and population expansion
To test the effect of acutely downregulating GATA-3, we used Banshee-G3-3W (Fig. 2A), a bicistronic retroviral shRNA expression construct with a GFP marker designed to block translation from Gata3 transcripts specifically (26, 27). The magnitude, speed, and stability of knockdown using this construct were assayed by intracellular GATA-3 staining in the DN3-like Adh.2C2 cell line (23), using Raw264 myeloid cells, which do not express GATA-3, as a specificity control (Fig 2B). Retroviral expression of Gata3 specific shRNA reduced GATA-3 protein to ~50% within 20 hr, stabilizing at ~45% of control levels for at least 6 days (Fig. 2C, D). Similarly, FLDN cultured for 6 days post infection with Gata3 shRNA had GATA-3 protein levels reduced to ~50% of control in cells with DN2 and DN3 phenotypes and reduced to isotype control background levels in cells retaining a DN1 phenotype (Fig. 2E). For more extensive confirmation of the effects of this shRNA on Gata3 RNA and GATA-3 protein in the Adh.2C2 system, also see our recent report (43). Gata3 shRNA thus consistently lowered GATA-3 protein levels, despite its generally mild effect on RNA levels.
Figure 2. Specificity and efficiency of GATA-3 knockdown.
A. Map of G3-3W cloned in Banshee, showing Gata3-targeting shRNA sequences.
B. Specificity of intracellular flow cytometric staining in Adh.2C2 pro-T cells, in comparison with RAW264.7 myeloid cells (negative control). For additional independent experiments on Adh.2C2 cells see ref. (43).
C. Stable GATA-3 knockdown in Adh.2C2 cells after infection with G3-3W or control vector at 20h and 136h (upper panels). (Lower panels) Correlation of GFP reporter with GATA-3 knockdown: Intracellular GATA-3 staining of Adh.2C2 cells at 65h post infection with G3-3W or control vector (also see ref. (43)).
D. Time courses of shRNA effects on Gata3 RNA levels (left graph, qPCR) and GATA-3 protein levels in Adh.2C2 cells (right graph, intracellular staining). Values from GFP+ G3-3W transduced cells, compared to GFP+ empty vector controls at the same timepoints, are shown. RNA levels are normalized based on β-actin. For another independent experiment see panel F (representative of at least 3 experiments); similar results for primary cells are shown in Fig. 5C,D.
E. Subset-specific effects of G3-3W shRNA on GATA-3 protein in FLDN cells. FLDN1, DN2 and DN3 subsets from OP9-DL1 culture were infected with G3-3W or control vector, then returned to culture, and intracellular flow cytometric staining was used to measure GATA-3 at 6 days post transduction.
F. Effects of GATA-3 knockdown on Adh.2C2 cells after 6 days. (Top) Gata3 RNA levels in GFP+ cells. (Middle) Viabilities, measured by 7AAD exclusion, of GFP− and GFP+ fractions of G3-3W infected or control infected cells. (Bottom) Fold changes in cell numbers (same cell populations used in viability analysis; value of 1 = no change). Results of one experiment with Adh.2C2 cells.
G. Comparison of GATA-3 knockdown effects on cell recovery in T-lineage and non-T lineage cells. Maintenance of GFP+ populations (as % of total) after transduction with G3-3W (Gata-3 shRNA) or control constructs is shown for Adh.2C2 cells tracked over 238h culture (top), in primary E13.5 FL precursors (FLP) cultured for up to 112 hr in T-cell conditions on OP9-DL1 (middle), and in FLP cultured in non-T cell conditions on OP9-control stroma (lower). Inhibitory effects shown in 2F and 2G were also representative of results in primary FL-derived precursors growing in T-cell conditions (7 experiments) and FLDNs recultured (7 experiments), although the 2C2 results shown are from a single experiment.
Even this modest reduction in protein expression had pronounced functional consequences. Six days post infection, Banshee-G3-3W infected Adh.2C2 cells were profoundly inhibited in proliferation, viability, and the ability to sustain vector GFP expression as compared to cells infected with control virus, implying that cells with most severe GATA-3 loss were being eliminated (Fig. 2F & 2G, top panels). When fetal liver precursors (FLP) were infected with G3-3W and cultured with OP9-DL1 stroma (T-cell conditions), a steep, progressive loss of virus-transduced GFP+ cells was seen after 48 hr (Fig. 2G, middle; note time scale; also see Fig. S2D). Yet when the same precursors were cultured on OP9-control to promote B and myeloid development, the percentage of GFP+ cells continued to increase (Fig 2G, lower; and see Fig. S2D). Thus, the effects of Gata3-specific shRNA expression faithfully reflect the T-lineage specific requirement for GATA-3.
The strength of these effects was surprising because no evident phenotype had been reported after Cre treatment of a simple Gata3fl/+ heterozygote. We also failed to detect substantial alterations in FLDN development in vitro from Gata3fl/+;ROSA26R-EYFP cells that were transduced with Cre retrovirus and sorted for Cre-induced EYFP expression (Fig. S2A). The normal cell surface phenotypes of these Gata3 heterozygous cells were validated by their normal expression of a panel of developmentally regulated genes in the cells (Fig. S2B). It was not possible to use GATA-3 intracellular staining to measure protein loss from the deleted allele because the targeted exons do not affect expression of the epitope detected. However, single-copy deletion of Gata3 also consistently showed 60-80% of normal levels of Gata3 RNA including the floxed exons, implying some compensation mechanism (Fig. S2C, and see below). Thus the shRNA reduces GATA-3 expression more than the heterozygous deletion.
Reduced GATA-3 levels impair the DN2/DN3 transition
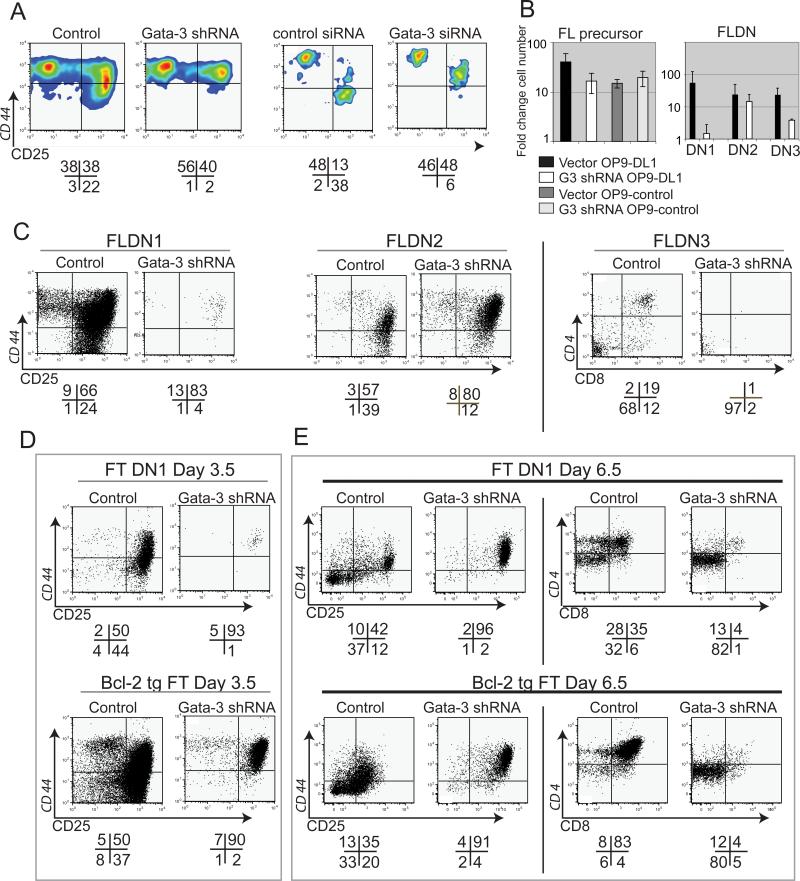
Reduced GATA-3 protein levels caused a stage-specific developmental block as well as reduced proliferation, as shown for sorted populations of Banshee-G3-3W transduced GFP+ fetal liver precursors after 3-4 days of differentiation on OP9-DL1 (Fig. 3A, B, left panels). Direct nucleofection of a commercial anti-GATA-3 siRNA into such precursors gave similar results (Fig 3A, right panels “siRNA”). In both cases, the control cells were transitioning to DN3 stage at harvest, but the GATA-3 knockdown samples were blocked with a DN2 phenotype (Fig. 3A). A similar phenotype using this shRNA was also recently seen by Kee and coworkers (44).
Figure 3. Developmental effects of GATA-3 knockdown.
A. Sorted FL precursors were transduced with G3-3W or control (left panels) or nucleofected with Gata-3 targeted or control siRNAs (right panels), and cultured on OP9- DL1 for 3.5 days. Numbers below plots: quadrant percentages. Data shown are representative of two retroviral infection and two nucleofection experiments.
B. Fold change in cell numbers for FL or FLDN subsets over a 3.5 day culture period is shown. (Left panel) FL precursors treated as in Fig. 3A and cocultured with OP9-DL1 or OP9-control as in Fig. 2G. No significant differences in recovery were seen between G3-3W transduced and control cells (two independent experiments). (Right panel) Cell yields from FLDN generated as in Fig. 1E, then infected with control or G3-3W, sorted to pure vector-positive DN1, DN2, or DN3 subsets, then re-plated on OP9-DL1 for 5 to 7 more days. In three experiments, fold differences in yield of G3-3W vs. controls were significant for FLDN1 (p=0.007) and FLDN3 (p=0.007). In three similar experiments with Bcl2-transgenic FLDN, G3-3W transduced samples yielded only 20±3% of the number of cells from control transduced samples of FLDN1, DN2, and DN3 (p=0.004, 0.006, and 0.02 respectively).C. Developmental progression in FLDN subsets. FLDN cells were infected with control or G3-3W vectors, then sorted into purified subsets, returned to OP9-DL1 stroma for 5 to 7 days, then analyzed. Numbers below plots are quadrant percentages. Data shown for DN2 precursors are representative of three experiments in which some “breakthrough” was seen, albeit in DN2 only; in three experiments there was no breakthrough.
D. Bcl-2 transgenic fetal thymic E14-14.5 ETP (“Bcl-2 tg FT”) and nontransgenic ETP (“FT DN1”) were sorted post infection with G3-3W or empty vector and cultured on OP9-DL1 for 3.5 days (representative of two experiments, in which Bcl-2 transgenic and non-transgenic cells were cultured in parallel). Bcl-2 consistently enhanced yields but not progression.
E. A separate experiment from D, but in this case cells were cultured 6.5 days on OP9 DL-1 (representative of two experiments).
The observed block was consistent with two possibilities. First, GATA-3 might need to act during DN2 stage to drive progression to DN3. Second, because of earlier roles of GATA-3 (7), the knockdown cells might simply be delayed in development. To distinguish between these possibilities, we allowed FL derived precursors to develop normally to DN1, DN2 and DN3 stages in vitro, and only then transduced them overnight with control or Gata3 shRNA expressing retrovirus. Each infected subset was then sorted and returned to culture for further differentiation (Fig. 3C). FL-derived DN1 cells transduced with Gata3 shRNA halted at DN2 stage in OP9-DL1 culture (Fig 3C, FLDN1), and catastrophic failure of cell expansion accompanied this block (Fig. 3B, right). Even after cells had already developed to the DN2 stage, GATA-3 reduction delayed progression to DN3 (Fig 3C, FLDN2) and often blocked it altogether (3/6 experiments, not shown), implying that the DN2 to DN3 transition was specifically vulnerable to loss of GATA-3. Notably, while decreased GATA-3 substantially inhibited FLDN1 and FLDN3 population expansion, the FLDN2 population was more resistant (Fig 3B).
Loss of GATA-3 from FL-derived DN3 cells caused a delay in progression to the DP stage as well as severe loss of cellularity, as reported for conditional Gata3 knockouts with Lck-Cre in vivo (10) (Fig 3C, FLDN3). However, surviving cells from some of these GATA-3 knockdown samples displayed a “retrograde” CD44+ DN2-like phenotype (50% of DN3 knockdown experiments, data not shown). Thus, at a minimum, survival in the DN3 stage appears less tolerant of reduced levels of GATA-3 than in the DN2 stage.
E14-14.5 fetal thymocytes develop more rapidly than FLDN in OP9-DL1 culture and pass more efficiently through DN2 to DN3 (Fig. 3D, E). By day 6.5 ~50% of control vector transduced fetal thymocytes had acquired CD4 and/or CD8. However, if transduced with G3-3W, the great majority of fetal thymic DN1/ETP cells were blocked at DN2 at both early (3.5 days: Fig. 3D, top) and late (6.5 days: Fig. 3E, top) time points. Thus, the DN2 block is robust under both fast and slow development kinetics.
The DN2 stage block caused by GATA-3 knockdown is separable from growth inhibition
Severe cell loss raised the possibility that the DN2-DN3 developmental block might simply reflect apoptosis at DN3. To test whether viable cells are developmentally blocked, we knocked down expression of GATA-3 in Bcl-2 transgenic fetal thymic ETP. Bcl-2 transgenic E14-14.5 ETP survived ~3x better than wildtype cells after knockdown of GATA-3, yet these cells went on to be as severely DN2 arrested as their non-transgenic counterparts (Fig 3D, lower panels, and data not shown). Even when the Bcl-2 transgenic fetal thymic ETP were cultured 6.5 days, Gata3 shRNA expressing ETP remained blocked at DN2, while control cells reached the DP stage (Fig. 3E, lower). Thus the loss of the viability-promoting role of GATA-3 is not the sole cause for the observed arrest at DN2.
Clonal analysis of differentiation from single-cell precursors provided additional evidence that reduced GATA-3 could inhibit developmental progression separately from its role in survival and proliferation. Individual FL precursors infected with a control vector, sorted as GFP+ onto OP9-DL1 layers in 96 well plates, generated a range of descendants spanning all the early stages of T development after 8-12 days (Fig. 4A, top). The clonal descendants of Gata3 shRNA expressing FL precursors, in contrast, again were unable to progress beyond DN2 (Fig. 4A, lower). We quantified developmental progression by assuming that cells pass through DN1, 2, 3, and 4 in order, and scoring individual clones as “positive” for every stage that at least 10% of the cells in the clone have reached or are assumed to have passed through. Using this scale to score progression in each clone, the developmental distributions of the control and GATA-3 knockdown clones are shown (Fig. 4B left). No significant progression beyond DN2 was seen in any clones which grew from Gata3 shRNA transduced FL precursors (only 1/17 had >1% DN3 cells). In contrast, >40% of empty vector transduced clones had reached DN3 or beyond (Fig. 4A, top, Fig. 4B, and data not shown). Importantly, the Gata3 shRNA-expressing clones could not surmount the DN2 block even in the cases where individual precursors had generated cell numbers comparable to controls (Fig. 4B, right). DN2 arrest was also observed with sorted single fetal thymocyte ETPs expressing Gata3 shRNA (Fig. 4C, left), and these ETP clones showed reduced expansion, consistent with behavior in bulk cultures (Fig. 4C, right and data not shown). Thus T-cell precursors with reduced GATA-3 levels are intrinsically inhibited from developmental progression to DN3.
GATA-3 conditional knockout confirms a requirement for GATA-3 for DN2/3 transition
To exclude off-target shRNA responses (45) as the cause of the DN2/3 block, we also tested the early GATA-3 role by inducing conditional deletion of the gene in vitro, infecting Gata3fl/fl FLP (10) with a Cre expressing retrovirus before initiating T-cell differentiation. More severe effects were expected in cells with complete loss of Gata3 than with shRNA-induced dosage reduction (7, 11), but the question was whether any cells that did reach DN2 would be able to proceed to DN3. To minimize the number of cells in which Gata3 could escape deletion, we sorted all cells for Cre vector expression after initial culture period on OP9-control stroma, and only then was T development triggered by transfer to OP9-DL1. As positive controls for Cre activity, cells from Gata3+/+ ROSA26R-EYFP fetal mice were transduced in parallel (Fig. 4D). Cre treatment of cells with a floxed Gata3 allele showed that as with shRNA, development was blocked at DN2 (Fig. 4E), while Cre-treated control precursors progressed to DN4. Thus this deletion model also demonstrated that GATA-3 was needed for transit from DN2 to DN3.
GATA-3 reduction causes derangement of the DN2-DN3 transitional program
The DN2 to DN3 transition is defined not only by downregulation of CD44 but also by downregulation of c-Kit, and intracellularly, the downregulation of PU.1. Despite the strong effect on DN2-DN3 progression seen by criterion of CD44 expression, the G3-3W shRNA treated cells continued to downregulate c-Kit expression almost like controls, implying that CD44 and c-Kit regulation had become divergent (Fig. 5A). The Gata3 shRNA-transduced cells also appeared to increase their CD25 levels as a consequence of increased average cell size (data not shown), unlike normally progressing pro-T cells which shrink on entering DN3a stage. This implied a more substantial defect in regulation of the DN2 to DN3 program as a whole when GATA-3 levels are reduced.
Figure 5. Disjunct DN2-DN3 phenotype in GATA-3 knockdown cells.
A. Phenotype of DN cells arrested by GATA-3 shRNA is discordant for c-Kit and CD44 expression. Bcl2-transgenic FLPs were transduced with G3-3W or Banshee vector expressing a randomized shRNA sequence and then cocultured with OP9-DL1 for 7 days. Analysis of CD45+ GFP+ cells (top panels) after 7 days showed a reduced yield of GFP+ and especially GFP-bright cells from the G3-3W sample (cf. Fig. 2G, Fig. 3). Middle panels show phenotypic analysis of gated GFP “low/negative” cells and the brightest subset of GFP+ cells from each sample, using CD25 vs. c-Kit (second row) and CD25 vs. CD44 (third row). The fourth row contour plots show that GATA-3 knockdown caused an elevation of CD44 levels relative to Kit levels for all subsets of cells present in the cultures at this timepoint. Although the DN2-like arrest is evident in terms of CD44 expression, Kit expression profiles are much less affected. Numbers: percentages of cells in the indicated gates. Gates used to define “GFP lo/-“, total GFP+, and “GFP-hi” cells are indicated in top left panel; gates used to define “DN1”, “DN2a” and “DN2b” subsets (sorted for panel B analysis) are indicated in second row left panel.
B. Abnormal gene expression in FLDN cells with GATA-3 shRNA in DN2-like arrest. Cells from A were sorted into DN1, DN2a, and DN2b-like fractions based on CD25 and c-Kit expression, to test the possibility that GATA-3 knockdown simply caused a skewed CD44 surface phenotype. Panel shows results from qPCR analysis with indicated genes. GATA-3 knockdown cells are well-matched with controls in Kit, CD25, and other features of gene expression, but substantially skewed toward an immature profile in terms of reduced Bcl11b and Ets1 expression and overexpressed PU.1 and Bcl11a as well as CD44. Data were compiled from two independent experiments, except for Tcf7, measured in one, which is shown for reference. Bars show geomean values, error bars indicate half-range.
C. Bcl-2 transgenic FLDN cells were transduced with Banshee or G3-3W, sorted as GFP+ DN1 and DN2 subsets, then each was recultured for 60h on OP9-DL1 prior to re-sorting DN2 and DN3 progeny for RNA analysis (blue bars: DN2 and DN3 progeny of DN1 cells; green bars: DN2 and DN3 progeny of DN2 cells). In parallel, aliquots of each parental DN1 and DN2 sample were recultured for 60 hr on OP9-control and resorted to collect all GFP+ CD45+ progeny (red bars as indicated), to examine the effect of GATA-3 on maintenance of T-lineage genes in the absence of DL1.
D. Bcl-2 transgenic E15.5 fetal thymocytes were transduced with Banshee or G3-3W overnight, transferred to OP9- DL1, then Thy1+ GFP+ cells were sorted after 24 hr more (40 hr total) for gene expression analysis. QPCR analysis was done in two batches with Spi1 (PU.1) and Gata3 in both. Gene expression normalized to β-actin is shown as log10 (G3-3W value/Banshee value). Blue shaded bars: p <0.05. Orange shading: Gata3.
E. PU.1 deficiency does not rescue the DN2 to DN3 block in Gata3 shRNA transduced cells. E14.5 FL precursors from PU.1fl/fl or C57BL/6 mice were infected with Cre retrovirus, and Cre+ Kit+ CD27+ cells were sorted for culture with OP9-DL1. On day 5, DN cells from these cultures were transduced with G3-3W or Banshee empty vector, and GFP+ ETPs were sorted the next day and then returned to culture on OP9-DL1. Staining profiles of GFP-high cells are shown after 7 further days of culture.
The discordant Kit and CD44 levels raised the question whether the cells are intrinsically more like Kit-low DN2 cells or like CD44-high DN3 cells. To resolve this, we transduced Bcl2-ttransgenic FLPs with G3 3W or scrambled shRNA, cultured them for a week on OP9-DL1, and then sorted DN1, DN2a, and DN2b subsets of the transduced GFP+ cells based on their Kit levels. Although little G3 3W effect on the protein levels of GATA-3, little effect on Gata3 RNA was seen. However, the G3-3W transduced cells that had reached DN1, DN2a, and DN2b-like phenotypic categories exhibited pronounced and specific gene expression abnormalities. Their elevated Spi1 (PU.1) and Bcl11a and reduced Bcl11b and Ets1 transcripts, in comparison to scrambled shRNA transduced controls, implied an early DN2 arrest state. However, at the same time they also showed experimentally variable, precocious expression of the Notch-dependent DN2b-DN3-specific HEBalt transcript (an alternative promoter isoform of Tcf12) and the DN3-specific SpiB transcript (Fig. 5B, C).
Gene expression tests at earlier timepoints confirmed changes in gene expression that were discordant with normal developmental patterns as well (Fig. 5C, D). At 60 hr (Fig. 5C) or 40 hr (Fig. 5D) after transduction with GATA-3 shRNA, PU.1 (Spi1) expression was repeatedly found slightly elevated in GATA-3-knockdown cells as compared to controls. At these earlier timepoints, too, SpiB and often HEBalt were found to be upregulated. The large experiment shown in Fig. 5C was carried out to measure short-term effects of GATA-3 knockdown on cells that were in precisely defined developmental stages before shRNA treatment, with RNA analysis then performed on progeny populations resorted as DN1, DN2, or DN3 after 60 hr further culture. The three experiments summarized in Fig. 5D tested earlier responses to transduction with G3-3W, in primary E15.5 fetal thymocytes from Bcl2-transgenic mice. A consistent finding among the panel of developmentally significant genes measured was that Spi1 levels were elevated relative to controls as early as two days after transduction (Fig. 5B, C, D), while onset of Bcl11b expression from ETP/DN1 precursors to DN2 was also dampened, though not maintenance of Bcl11b once already activated in late DN2 stage (Fig. 5C, also B).
HEBalt depends on Notch signaling and HEB/E2A (46), so that the HEBalt upregulation seen in several short-term experiments (Fig. 5C and data not shown) implies that these crucial T-cell regulatory inputs persist despite GATA-3 reduction (11). The normal or increased expression of Notch target genes CD25 (Il2ra) (Fig. 5B), HEBalt (Fig. 5C, D), and Dtx1 (Fig. 5D) in Gata3-deficient cells also implies that Notch signaling continues, independent of GATA-3 activity levels. At the same time, the split DN2/DN3 phenotype of GATA-3 knockdown cells is a mirror image of our previous finding that when Gata3 is overexpressed in fetal thymocytes, Kit is upregulated and PU.1 is downregulated (14) (and see below).
GATA-3 is epistatic to PU.1 in regulating DN2 to DN3 progression
PU.1 and GATA-3 act as antagonists in a network circuit in which GATA-3 restrains the ability of PU.1 to cause myeloid differentiation of DN pro-T cells, even when Spi1 expression itself is not under GATA-3 control (43). PU.1 naturally begins at high levels in ETP and DN2a cells, but falls immediately after GATA-3 reaches its peak (data not shown) (42), and our results suggested that GATA-3 might be required for this decline. CD44 that defines the “DN2” arrest also appears to be positively regulated by PU.1 (A. Champhekar, unpublished results). To test whether sustained PU.1 expression is the cause of the DN2-DN3 abnormality that occurs when GATA-3 levels are reduced, we asked whether deletion of PU.1 would restore normal development of GATA-3 knockdown fetal liver precursors. Taking fetal liver precursors from Spi1fl/fl mice and B6 controls, we treated them in parallel with retroviral Cre to delete PU.1 from the mutant precursors and placed them in T-cell differentiation culture for 5 days. Then PU.1-deficient and control cultures were harvested, infected with G3-3W or empty vector, sorted to isolate transduced ETPs, and these were returned to culture for 7 days more. Fig. 5E shows that loss of PU.1 indeed accelerated the progression from ETP to DN3 in cells with normal levels of GATA-3. However, knocking down GATA-3 still blocked development at DN2, with or without an intact PU.1 gene. Therefore the need for GATA-3 to promote DN2 to DN3 progression involves additional target genes besides PU.1.
Acute deletion of Gata3 reveals additional GATA-3 sensitive targets
Overall, the relatively modest gene expression effects seen at early timepoints raised the concern that shRNA mediated inhibition could be too slow, quantitatively mild or asynchronous to detect the whole first tier of GATA-3 regulatory targets. For closer study of early responses to GATA-3 deprivation, we therefore used retroviral Cre transduction of cells from homozygous Gata3fl/fl; ROSA26R-YFP mice and sorted the YFP+ DN1, DN2a, and DN2b cells for analysis within 18±2 hr, before viability or proliferation based selection could skew the population (Fig. 6A). These cells were compared with empty vector-infected cells or Cre-treated YFP+ cells from Gata3+/+;ROSA26R-EYFP mice as controls. The level of Gata3 RNA from the intact floxed gene was reduced by >10 fold by Cre treatment, as determined by exon 3-4-specific qPCR (Fig. 6B). In these cells, within the first day, again Spi1 (PU.1) was upregulated. However, in contrast to cells transduced with Gata3 shRNA, now Zfpm1 (FOG1) was clearly downregulated. This was significant because Zfpm1 exhibits strong binding of GATA-3 in vivo (13) and seems responsive to GATA-3 in a model cell line system (43). If incubation was continued for an additional day before harvesting the cells, detectable reductions in Tcf7, Rag1, Kit, and Zbtb16 (PLZF) were seen, as well as effects on Bcl11b and Ets1 (Fig. 6B). These effects suggest that such targets associated with particularly strong binding of GATA-3 in vivo (13) may require more than 2-3 fold reductions of GATA-3 activity berfore their expression is affected.
Figure 6. RNA-seq analysis of acute responses to Gata3 deletion.
A. Schematic of experimental strategy for generating samples to analyze acute short-term impacts of Gata3 deletion. In all experiments, Gata3-deleted cells were generated by retroviral Cre transduction of Gata3ff; ROSA26R-EYFP+ fetal liver precursors. In two of the three experiments used for graph in B, controls were Gata3++;ROSA26R-EYFP+ fetal liver cells also transduced with Cre. In a third experiment and RNA-seq samples shown in C-E, one control was B6 transduced with Cre (~50% of sorted DN2 cells were transduced), and the other control was Gata3ff transduced with empty Banshee vector and sorted for GFP+.
B. Changes in expression of key genes in first 16-40 hr after deletion of Gata3 exons 4-5. Cells sorted immediately as shown in A (“first day”) or returned to OP9-DL1 coculture for 22-24 more hours “second day” and resorted were analyzed by qPCR for the expression of the indicated genes. Levels are shown on a log2 scale relative to levels of β-actin. Averages (+/− standard deviations) of log2-transformed data from three independent experiments are shown in which values were measured separately for cells sorted as DN2a and cells sorted as DN2b. Asterisks indicate significant p values for first-day (above) and second-day (below) differences separately (two-tailed t test with unequal variances on difference values). *, p <0.05. **, p<0.005. ***, p<0.001.
C. RNA-seq tracks showing complete deletion of exons 4 and 5 from the Gata3 gene within one day of acute Cre treatment. Tracks from the UCSC genome browser view of the Gata3 gene (mm9) are shown, with the RefSeq structure of the gene (top) and the direction of transcription indicated as a blue arrow between the gene model and the sequence conservation track. Red tracks: RNA-seq from GATA-3 deleted samples from two RNA-seq experiments. Black tracks: RNA-seq from controls in two RNA-seq experiments. The structure of the floxed Gata3 gene enables Cre to excise exons 4 & 5 (magenta box).
D. Principal component analysis of overall developmental staging of GATA-3 knockout samples (G3KO1 & 2, red) compared to controls (CTRL1&2, blue), based on RNA-seq analysis of expression levels of 173 diagnostic transcription factors (see Table S1A). The axes for the principal component analysis were calculated from the expression levels of these factors in samples described in ref. (13). Overall, the experimental samples are situated between normal FLDN2a and FLDN2b, consistent with the phenotypes and lengths of culture, but the two experiments are not identical.
E. Highest scoring genes affected within 20 hr by deletion of Gata3 in two experiments: genes downregulated by loss of Gata3 at lower left, genes upregulated by loss of Gata3 at upper right. Graph shows fold changes in expression relative to controls on log2 scale with effects seen in comparison 1 on the × axis and effects seen in comparison 2 on the y axis. Genes indicated in black are clearly expressed. Names of other genes fulfilling statistical criteria but expressed at very low levels are indicated in gray.
Global analysis of GATA-3 dependent genes in DN2 cells by RNA-seq
These results suggested that additional immediate targets of Gata3 in thymocytes undergoing commitment might be found in a whole-genome screen using the acute deletion strategy. We therefore carried out exploratory genome-wide analyses of control and acutely Gata3-deleted DN2 cells, using RNA-seq. The Cre-transduced FLDN2 cells from Gata3fl/fl;ROSA26R-EYFP+ mice were sorted ~18 hr after transduction in two independent experiments, compared in each case with a different control that had been cultured in parallel: in one, Cre-treated cells from ROSA26R-EYFP+ mice (wildtype Gata3) and in the other, vector marker-positive cells from Gata3fl/fl;ROSA26R-EYFP mice transduced with an empty vector. At this very early timepoint, there were no detectable differences yet in viability, cell number or phenotype between the Gata3-deleted and control cells in either experiment (data not shown), but sequences from Gata3 exons 4 and 5 were effectively eliminated from the transcript repertoire of the Cre+ Gata3f/f cells (Fig. 6C). Transcriptome analyses were carried out using high-throughput sequencing and gene expression was mapped and quantitated using Tophat and Cufflinks software.
The data were monitored first to establish the global developmental stages that each sample best represented. Catching the cells at identical points even in a normal developmental trajectory is difficult in independent experiments. Overall, the expression patterns agreed well across the four samples (9580 transcripts scored), with Pearson's r values of 0.90-0.96 between all pairwise comparisons. To gain a finer mapping of the cells’ developmental positions, we established a reference list of 173 developmentally regulated transcription factors from our previous analysis of wildtype cells (13) to create a high-dimensional metric of any sample's advancement through the commitment process. The expression levels of these genes were then used for principal component analysis (Table S1A, Fig. 6D) of 11 samples from previously defined T-cell developmental stages in comparison with the new GATA-3 knockout samples and controls. The first two principal components explained over 85% of the total variance among the samples and established a strong canonical trajectory for normal development from FLDN1 through FLDN2a, FLDN2b, and thymic DN3a to thymic CD4+ CD8+ stage. As shown in Fig. 6D, expression values of the index genes in the new controls placed them appropriately between the FLDN2a and FLDN2b stages. The values for the two GATA-3 knockout samples showed them close to their respective controls, but displaced from the normal trajectory, in parallel. This was consistent with the interpretation that the effects of Gata3 deletion are not simply a developmental block or acceleration of the normal program, but a deviation from it.
To locate candidates for new GATA-3-sensitive genes, we ranked the genes with greatest differences between controls and experimental samples in both experiments, using both rank product and Cuffdiff analyses with a cutoff to exclude genes expressed at very low levels, as described in Materials and Methods (Table S1B-D). In general, at the time point analyzed (~18 hr of Cre exposure), most expression differences were still small, limiting statistical power. However, the limited comparison still revealed approximately 100 genes of most interest (Table S1C), and fold changes in expression values in the two comparisons for the top scoring genes are shown in Fig. 6E. Although genes detected as differentially regulated by qPCR (cf. Fig. 5B-D, Fig. 6B) also behaved consistently in this RNA-seq analysis (e.g. Ets1, Bcl11b, Zbtb16, Rag1), most did not show the strongest effects statistically. However, Spi1 (PU.1), SpiB, and Bcl11a were found among the most upregulated in these Gata3-deficient DN2 cells (Fig. 6E), supporting the interpretation that these are directly or indirectly repressed in normal DN2 cells by GATA-3.
The top candidates for GATA-3 positive regulatory targets included genes known to play roles in Th2 cell development, ILC2 development, and allergic disease, albeit not previously known to be involved in early pro-T cell specification. A highly GATA-3 dependent gene, Cpa3, had previously been shown to be upregulated when fetal thymocytes overexpressed GATA-3 (14). These comparisons showed strong effects also on Il9r, Il17rb (IL-25 receptor component), Fgf3, Tnfrsf9 (4-1BB, CD137), Cyp11a1, and germline transcripts of the TCRγ C1 cluster. Il9r, Il17rb,Tnfrsf9, Pdcd1 and Cyp11a1 are also genes recently shown to be positively regulated by GATA-3 in ILC2 and/or Th2 cells (12, 47, 48), and Il17rb, Pdcd1, and Ms4a4b in ILC3 or Th17 cells (12, 47). In contrast, the genes appearing as negative regulation targets in our cells do not generally appear GATA-3 regulated in Th2 or ILC2 cells (with the exception of Lair1), mostly because they are completely silent in those developmental contexts. While not all of the genes affected in our analysis are necessarily direct targets (see Discussion), they reveal some commonalities in GATA-3 targets between early pro-T cells and peripheral Th2 and ILC2 cells (47-49).
GATA-3 as a positive and negative regulator of other developmental regulatory factors
Because genes of most interest to explain developmental changes prominently include those encoding transcription factors, we specifically examined the impacts of Gata3 deletion on a genome-wide list of transcriptional regulatory genes (Table S1E, F). From a master list of 1640 regulators (Table S1 F1), 973 were “expressed” highly enough to evaluate (Table S1 F2, 3). To accommodate the variation in starting developmental states in the two comparisons, we used two scoring systems to identify genes of interest (Table S1E), based on consistency of the Gata3 deletion effect, minimum fold change, agreement between the controls, and overall strength of expression so as to minimize measurement noise. One list was identified using slightly less stringent criteria but adding a requirement for Cuffdiff pvalue <0.1 (Table S1E list 1), the other was identified using more stringent categorical criteria irrespective of Cuffdiff pvalue (Table S1E list 3). In both lists, Ets1 was scored as a positive target while SpiB and Spi1 were scored as negative regulatory targets. Bcl11b also crossed the threshold as a positive target in one list and Bcl11a did the same as a negative target. This screen also identified Pou2f1 (Oct1), Hmbox1, Anks1, and Hmga2 as positive regulatory targets of GATA-3 and Cbx8 and Bc13 as additional negative targets. Note that Zfpm1, Tcf7, and Myb, also genes with strong GATA-3 binding in vivo, showed weaker but repeated changes consistent with being functionally positive regulatory targets even though they did not rise to the top statistically (Table S1E list 2).
The significance of effects on Pou2f1, Hmbox1, Anks1 and Hmga2 are difficult to evaluate because so little is known about their roles in T-cell development. However, the evidence that Bcl11b and Ets1 depend on GATA-3 activity to be induced raises the possibility that these mediate GATA-3 roles in T-cell lineage commitment as well as differentiation.
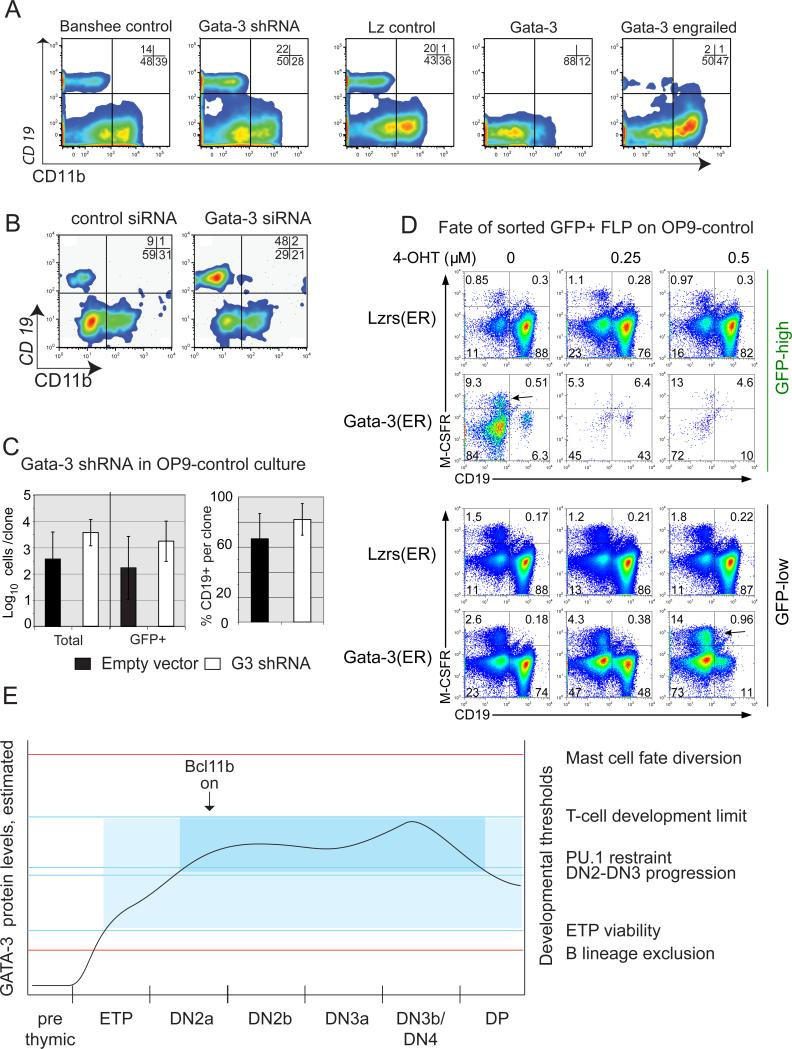
GATA-3 suppresses B cell potential: a commitment role distinct from that of Bcl11b
Full commitment as well as progression from DN2 to DN3 are known to depend on Bcl11b, the regulatory gene that is most dramatically activated between DN1 and DN2b in normal cells (20, 50). If GATA-3 mediates commitment effects through induction of Bcl11b, then Bcl11b knockouts should recapitulate some of those effects in a GATA-3+ context (11). However, the effects of Bcl11b and GATA-3 on early pro-T cells are not the same in general. Unlike Gata3-deleted cells, Bcl11b-knockout cells are highly viable. They upregulate Kit rather than downregulating it, and even after weeks of OP9-DL1 culture remain proliferative, yet are still able to generate NK and myeloid cells if shifted to OP9-control stroma (20). We therefore tested whether the specific roles of GATA-3 and Bcl11b in commitment are the same. The results of this series of experiments with fetal liver and bone-marrow precursor-derived DN cells are reported in full in Table 2.
To characterize the genetic interactions between GATA-3 and Bcl11b, we generated doubly-floxed Bcl11bfl/fl;Gata3fl/fl homozygotes. We crossed the ROSA26R-EYFP reporter into both singly and doubly floxed genotypes as a monitor for Cre efficiency. FL precursors from ROSA26R-EYFP control, Bcl11bfl/fl, Gata3fl/fl, and Bcl11bfl/fl Gata3fl/fl mice were retrovirally Cre-treated, sorted based on their activation of YFP (Fig. 7A), seeded on OP9-DL4 stroma, and compared for subsequent development (Fig. 7C, D, Fig. S3 and data not shown). With or without Bcl11b, GATA-3 loss caused a severe relative reduction in cell yield (Fig. 7B), and downregulation of Kit, with an increased expression of CD25, in contrast to the effects seen when Bcl11b is deleted alone (Fig. 7B-D, Fig. S3G and data not shown). In all these populations, lacking a Bcl2 transgene, cells surviving the 7-8 day preculture were biased toward preservation of at least one intact Gata3 allele (see below), and this made the DN2 arrest appear incomplete. However, when DN2 cells were sorted from the Cre-treated cells of all four genotypes (Fig. 7C), then transferred to OP9-control stroma, they displayed sharply different developmental potentials (Fig. 7D; Fig. S3A-D). All genotypes generated NK-like cells consistently (Fig. S3A-D, cells staining with NK1.1+DX5). However, a new phenotype also emerged from the DN2 cells of genotypes with deleted Gata3: profuse blooms of CD19+, CD45intermediate B lineage cells (Fig. 7D, E, Fig. S3H). The cells with a B-cell surface phenotype strongly expressed B-lineage transcription factors Ebf1 and Pax5 and had substantially downregulated T-cell genes such as Thy1, Tcf7, and TCRβ germline transcripts (Fig. S3I). However, in contrast to normal B cells, these cells expressed TCRγ and TCRδ transcripts, consistent with their early T-lineage origin (Fig. S3I). Generation of B cells from such OP9-DL4-cultured, sorted DN2 pro-T cells was never observed with wildtype cells, and was barely detectable in any experiments with Bcl11b single knockout cells (Table 2A).
TABLE 2A. GATA-3 deletion but not Bcl11b deletion preserves access to the B-cell developmental option.
Summary of the Bcl11bfl/fl;Gata3fl/fl Experiments
| Percentage of DN2 in Primary Culture (FLP on OP9-DL4) | Percentage of CD19+ cells in Secondary Culture (DN2 on OP9-Control) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B6 YFP | Bcl11bfl/fl | Gata3fl/fl | Bcl11bfl/fl;Gata3fl/fl | B6 YFP | Bcl11bfl/fl | Gata3fl/fl | Bcl11bfl/fl;Gata3fl/fl | Comments | ||
| E5 | Experiment 1 | 54% | 62% | 33% | 0.23% | 0.80% | 70.30% | For details, please see Fig. S3 | ||
| E6 | Experiment 2 | 64.4% | 8.03% | 1.89%; 3.17% | 97.7%; 93.1% | Two independent secondary cultures | ||||
| E7 | Experiment 3 | 71.3% | 17.7% | 0.0372% | 16.2% | |||||
| E17 (A) | Experiment 4 | 75.5% | 56.6% | 55.2% | ||||||
| E17 (B) | Experiment 5 | 49.8% | 29.7% | |||||||
| E18 | Experiment 6 | 42.5% | 27.7% | 33% | 32.6% | 0 | 0-0.0125% | 94.7% | 91.6%; 98.6% | For details, please see Fig. 7. |
| E18 | Experiment 6, continued | 0/96 | 0/96 | 1/96 | 2/96 | Frequency of CD19+ wells. | ||||
Note (1) Primary cultures: Fetal liver precursors were cultured on OP9-DL4 with 5 ng/ml IL7, Flt3L and SCF for 8-10 days. Then, DN2 cells were sorted.
(2) Secondary culture: DN2 cells were cultured on OP9-Mig with 5 ng/ml IL7 and Flt3L for 7-14 days.
(3) Bold indicates substantial yield of CD19+ cells.
B cells were generated in secondary culture from Cre-treated DN2 cells with a floxed Gata3 allele, with or without floxed Bcl11b, in 7/7 experiments (Tables 2A, B). However, this was not a uniform trait within the Cre-treated Gata3fl/fl DN2 populations. In microtiter cultures seeded with limiting numbers of sorted ROSA26R-EYFP+ DN2 precursors of different genotypes, no B cells emerged from B6 control or Bcl11b single-knockout cells (Fig. S3A,B). B cells did emerge from Gata3 single knockout or Gata3, Bcl11b double knockout cells, but only from a minority of these limiting dilution cultures (Fig. S3C,D). The colonies containing B cells proliferated strongly, outstripping those with NK cells only (Fig. 7E, red dots vs. blue dots).
Table 2B. GATA-3 deletion but not Bcl11b deletion preserves access to the B-cell developmental option.
Summary of experiments deleting Gata3 at the DN2 stage
| Percentage of DN2 in Primary Culture (BM-derived LSK on OP9-DL4) | Percentage of CD19+ in Secondary Culture (DN2 on OP9-Control) | Comments | ||||
|---|---|---|---|---|---|---|
| Cre-ERT2 | Cre-ERT2;Gata3fl/fl | Cre-ERT2 | Cre-ERT2;Gata3fl/fl | |||
| F8 | Experiment 1 | 70.4% | 91.1% | 0.07% | 40.4% | For details, please see Fig. 8A-C. |
| F11 | Experiment 2 | 78.8% | 94.3% | 0.84% | 92.6% | |
| G2 | Experiment 3 | 85.7% | 88.3% | 0/96 wells (MigR1) | 2/96 wells (MigR1 vector) | For details, please see Fig. 8D-G. |
| 0/96 wells (Bcl-xL) | 17/96 wells (+ Bcl-xL) | |||||
Note: (1) Primary cultures: Bone marrow Lin- Sca-1+ c-Kit+ (BMLSK) were cultured on OP9-DL4 with 5 ng/ml IL7 and Flt3L for 11 days. Then, 4OHT was added into the culture for 16 hours before DN2 cells were sorted.
(2) Secondary culture: DN2 cells were cultured on OP9-Mig with 5 ng/ml IL7 and Flt3L for 7-14 days.
(3) The secondary culture of G2 is a colony frequency assay.
(4) Bold indicates CD19+ cells.
One explanation for why B-cell clones emerged rarely could be that survival pressures caused many Cre+ DN2 cells to retain a Gata3 allele. This proved to be true, based on real-time PCR assays that quantitated deleted vs. intact (floxed) Gata3 alleles (Fig. 7F, G). Not only the starting populations, but also all “GATA-3 knockout” colonies that grew on OP9-DL4 stroma actually retained one floxed, undeleted Gata3 allele. Of the colonies that grew on OP9-control, those of NK cells (Fig. 7F, blue symbols) also all retained Gata3. The only colonies that had deleted both copies of Gata3 were the ones that generated B cells (Fig. 7F, red symbols). In contrast, both NK and B-cell colonies showed equal, complete deletion of Bcl11b, when these alleles were floxed (Fig. 7I, H). Thus only by loss of all Gata3, irrespective of the presence or absence of Bcl11b, could DN2 pro-T cells convert into B cells.
Deletion of Gata3 at DN2 stage can restore B-cell potential
To determine whether GATA-3 itself is needed continuously to suppress B cell potential, we allowed cells to develop to DN2 stage with Gata3 intact and then induced deletion with a tamoxifen-inducible Cre-ERT2 transgene. Bone marrow cells from these mice were cultured on OP9-DL4 to DN2 pro-T cell stage, then treated with tamoxifen, and DN2 cells were purified by sorting (Fig. 8A,B). They were then tested for B-cell potential by transfer to OP9-control cultures. Short-term activation of Cre-ERT2 did not cause disappearance of the DN3 cells already generated but changed the potential of the DN2 cells. Again, no B cells developed from control Cre-treated DN2 cells, but B cells consistently grew out from the Cre-treated Gata3fl/fl precursors, even though they had harbored an intact Gata3 gene until the DN2 stage (Fig. 8C).
Despite the strong outgrowth of converted B cells, the B-cell precursor frequency within the Gata3fl/fl Cre-treated DN2 population was low. Real potential might be masked by the harsh T-lineage effects of acute Notch signal deprivation plus the sudden loss of GATA-3, in transition before the cells switch fates. To obtain a clearer estimate of B-cell potential, we generated DN2/3 cells from Gata3fl/fl Cre-ERT2 and control Cre-ERT2 mouse bone marrow Lin− Kit+ Sca1+ cells (Fig. 8D,E), then infected them with Bcl-xL or control retroviral vectors (Fig. 8F), and only then treated them overnight with tamoxifen to activate the Cre. The transduced cells were then sorted and transferred to B-cell conditions at limiting dilution for 14 days. As shown in Fig. 8G, imposed expression of Bcl-xL enhanced the detection of B-cell precursors in the Gata3-deleted population by 5-10 fold, without unmasking any B-cell potential in the controls. Once GATA-3 is induced, therefore, its vital role in survival amplifies its effectiveness as a suppressor of alternative fates.
Dose-dependent effects of GATA-3 on B and myeloid developmental choice
The difference between full GATA-3 expression and GATA-3 dosage reduction can directly contribute to myeloid potential, through the GATA-3-sensitivity of PU.1 expression (Fig. 5, Fig. 6A) and PU.1 function (43) (data not shown). Although Gata3 deletion also unmasks B-cell potential as late as DN2 stage (Figs. 7, 8), B-cell development was never seen from G3-3W treated DN pro-T cells; and the experiments shown in Figs. 5 and 6 revealed no significant activation of Ebf1 or Pax5 when GATA-3 activity was merely reduced (data not shown). We therefore considered that B-cell potential might be particularly easily repressed by low doses of GATA-3. Indeed, FL precursors in which GATA-3 was reduced by either shRNA or siRNA (Fig. 9A, B) often generated an absolutely enhanced yield of B-lineage cells (CD19+) under B/myeloid conditions in OP9 control cultures (in 7/10 experiments)(Fig. 9C). Conversely, GATA-3 overexpressing FL precursors, even if Bcl2-transgenic, generated dramatically reduced numbers of CD19+ and CD11b+ cells in B/myeloid conditions (Fig. 9A, “Gata-3”). To test whether GATA-3 may act as a direct repressor of genes that promote B-cell fate, we designed a construct that fuses GATA-3 to the repression domain of Drosophila Engrailed in order to create an obligate repressor with GATA factor DNA binding specificity. This construct indeed dampens Kit expression in early pro-T cells, consistent with the previous evidence that this gene can be positively regulated by GATA-3 (14)(Fig. S2E). Gata-3 engrailed mimicked wildtype GATA-3 expression, however, as it inhibited B-cell development (Gata-3 engrailed, Fig. 9A; Fig. S2D). This effect was particularly striking because Gata-3 engrailed was far less antagonistic to myeloid development from the fetal liver precursors than wildtype GATA-3, implying a sharp difference in the crucial target genes for these two developmental restriction effects (Fig. 9A, right panels).
Figure 9. Expression of inducible GATA-3 and obligate repressor GATA-3 show distinct mechanisms for GATA-3 inhibition of B and myeloid development.
A. GATA-3 knockdown enhances B-cell emergence; GATA-3ENG expression blocks B-cell emergence from FLP. Bcl-2 transgenic FL precursors were infected in parallel with either G3-3W or Banshee control constructs, or with retroviral constructs overexpressing normal GATA-3 or an obligate repressor form of GATA-3 (Gata-3 Engrailed), or the corresponding vector control (Lz). Sorted FL precursors were then cultured 3.5 days with OP9 control stromal cells (B/myeloid conditions) before analysis (three independent experiments).
B. Loss of GATA-3 promotes B-cell emergence. FL precursors were nucleofected with siRNA specific for Gata3, then sorted and cultured 4-6 days under B/myeloid conditions on OP9 control stroma. Representative data shown (7/10 experiments using nucleofection or retroviral transduction of Gata-3 shRNA).
C. B-cell recovery from GATA-3 knockdown in clonal precursors. Single FL precursors transduced with Banshee or G3-3W were sorted into 96 well plates with OP9 control stroma and cultured for 12 days. Cell numbers for total cells/clone and GFP+ cells/clone are shown for G3-3W and control infected clones (left)(geomean ± s.d.). Percentages of each clone which were CD19+ are shown (right). At least as many B cells were generated from cells remaining GFP+ after transduction with 3W as with Ban; differences were not statistically significant.
D. Dose-dependent hierarchy of B-cell and myeloid inhibition by tamoxifen-enhanced GATA-3 [GATA3(ER)]. Fetal liver precursors were infected with GATA3(ER) or empty vector [Lz(ER)] and sorted the next day to purify GFP+ transductants. GFP+ cells were cultured for 6 days on OP9-control in the absence or presence of tamoxifen as indicated. Some CD45+ cells continued strong vector expression (GFP-high) while others downregulated it (GFP-low). These were analyzed for B (CD19+) and myeloid (CD115+) development (representative of two independent experiments). Concordant results were obtained in an additional experiment under different culture conditions (not shown).
E. Natural changes in GATA-3 levels cross distinct developmental dose thresholds. Schematic of effects shown here and in other work (3, 7, 11, 14, 44). Red lines: thresholds seen in absence of Notch signaling. Blue lines: thresholds seen in presence of Notch signaling. Light blue zone: permissive for T-lineage entry. Darker blue: permissive for full T-cell commitment.
To test systematically how GATA-3 dose might affect developmental alternatives, we generated a retroviral GATA-3 construct fused through a linker to an estrogen receptor hormone binding domain [GATA-3(ER)]. This construct offers two levels of dose control, the first through downregulation of vector transcription which occurs in some cells (28) and the second through titrated tamoxifen control of nuclear localization of the expressed fusion protein. We transduced FL precursors with this construct, sorted GFP+ transductants, then cultured them under B- and myeloid-permissive conditions, with and without tamoxifen. With tamoxifen, GATA-3(ER) blocked GFPhigh cells from both B and myeloid fates (Fig. 9D, and data not shown), especially M-CSF-R+ (CD115+) and F4/80+ macrophages (data not shown). However, generation of CD19+ B cells was clearly most sensitive. B cell generation could be inhibited by expression of GATA-3(ER) even without tamoxifen (Fig. 9D, GFPhigh, and data not shown), through the small amount of GATA-3 that still “leaks” to the nucleus in these conditions (A. A., unpublished data). In this case, myeloid differentiation (CD115+) was clearly less affected (Fig. 9D, arrows; and data not shown). GFPlow cells that had downregulated the GATA-3(ER) construct showed a parallel response pattern, at higher doses of tamoxifen (relative sparing of myeloid cells: arrows in Fig. 9D, GFP-high vs. GFP-low). Thus, as summarized in Fig. 9E, distinct dose thresholds for GATA-3 not only control DN2 to DN3 progression but also limit distinct alternatives to T-cell fate.
DISCUSSION
GATA-3 has long been recognized as essential for T cell development, and it is expressed with only modest quantitative changes throughout T cell development. We show here that it is profoundly dose-dependent in its action. Important qualitative effects on lineage choice, differentiation, and gene targeting can emerge from increased levels of GATA-3 expression; excess GATA-3 aborts T-cell development, even when the excess is small (12, 14, 44). Yet in its roles to promote T-lineage fates, GATA-3 has been studied exclusively in an all-or-none fashion (3, 7, 10, 11). Here we have used a variety of developmental conditions, differing in precursor sources, developmental timings, and durations of perturbation, to reduce GATA-3 dosage to show that the gentle increase in GATA-3 expression from ETP/DN1 stage to DN2b stage can have significant regulatory consequences. First, T-cell precursors need GATA-3 to rise beyond its ETP/DN1 level to generate correctly programmed DN2 and DN3 cells. These higher levels of GATA-3 also antagonize PU.1 which may limit myeloid potential. Second, even at low levels, GATA-3 is an acutely sensitive repressor of B cell developmental potential. Thus its early induction in thymic immigrants is properly timed to explain the early exclusion of B-cell potential (51) (rev. by (52-54)).
Dose reduction of GATA-3 by shRNA severely impacted normal population dynamics of DN1 cells and also prevented the development of normal DN2 and DN3 cells from prethymic or DN1/ETP precursors. These effects were initially weak but became increasingly pronounced over a period of days. The surviving “DN2” cells with reduced GATA-3 activity were abnormal in several respects, with uncoupling of CD44 and Kit regulation and loss of control of both DN1-specific genes and DN3-specific genes. Even with these effects of GATA-3 knockdown, the cells were evidently strongly selected for retention of some residual GATA-3 expression. Cre-mediated deletion of both copies of the Gata3 gene had catastrophic effects on viability as long as the cells were maintained in Notch-signaling conditions to support T-cell development. It was clearly only the cells with full deletion of Gata3 that re-acquired access to the B-cell pathway.
Explaining these effects on ETP/DN1 survival, DN2 to DN3 progression competence, and access to the B and myeloid fates at a molecular level will require further research, but our results present a wealth of provocative candidate mediators. The early T-cell program includes both positive and negative regulatory targets of GATA-3 action, and our results imply that these have different patterns of response to differing degrees of GATA-3 dose reduction. Some genes respond rapidly to any decrease in GATA-3 expression level, and many of these are genes that appear to be relieved from repression when GATA-3 doses drop. Although the magnitudes of the effects are initially modest, these include three genes encoding transcription factors of great developmental significance: the progenitor-cell and myeloid regulatory factor PU.1, the closely related factor that collaborates with PU.1 in B cell development SpiB, and the indispensable lymphoid progenitor factor Bcl11a. The repressive effect of GATA-3 on SpiB supports earlier evidence that SpiB/GATA-3 antagonism can control T-cell vs. plasmacytoid dendritic cell fate determination in human precursors (55). Likely positive regulatory targets of GATA-3 are less enriched for putative regulatory genes, but include genes encoding Ets1 and Bcl11b, two transcription factors that play prominent roles in T-lineage commitment and in the DN3 state. However, these genes responded more slowly to the decrease of GATA-3 expression than SpiB, PU.1, and Bcl11a. Many of the targets most rapidly downregulated by loss of GATA-3 were in fact genes associated with ILC2 (innate lymphocyte type 2) cells and mature GATA-3+ effector T cells (12, 47, 48), clearly expressed in the normal DN2 cells but not previously associated with early T-cell developmental functions. It will be very interesting to determine whether Il9r and Il17rb (encoding a chain of the IL-25 receptor) are functionally important in early T-lineage development.
ChIP-seq data from two groups have provided maps of GATA-3 binding sites across the genome in thymocytes (12, 13). Unfortunately, these peak patterns in themselves turn out to be poor predictors of the genes most sensitive to acute GATA-3 perturbation. Some likely positive target genes with particularly strong GATA-3 binding like Zfpm1, encoding the GATA factor partner FOG-1, were completely insensitive to the shRNA and were only affected by a complete deletion of the Gata3 gene. More sensitive targets, both positive and negative, were often only weakly bound by GATA-3 in vivo, if binding was detected at all. While some of these genes may be indirectly regulated by GATA-3, the very short timepoints we used were specifically designed to limit indirect effects. Furthermore, the very limited number of transcription factor genes affected at all, and the generally weak effects on those regulatory genes seen at 18-20 hr, makes them unlikely candidates for mediating the GATA-3 effects. Thus, although technical factors could be relevant, it is possible that sites with strong GATA-3 occupancy in fact have too high an affinity to be affected by 2-3x dose reductions. In this case, the molecular mechanisms underlying the potent developmental impact of GATA-3 knockdown must be sought among genes with weak, rapidly exchanging GATA-3 binding sites.
In agreement with our previous results of GATA-3 overexpression, GATA-3 loss of function did not have a clearcut impact on global Notch signaling; some Notch target genes including HEBalt and Dtx1 were even somewhat enhanced in expression. However, there are a number of indirect pathways through which GATA-3 could be collaborating with Notch signals. Our results confirm that GATA-3 is a restraining negative regulator of PU.1 levels in early thymocytes when expressed at its normal physiological level as well as when overexpressed. This means it can protect Notch signaling from PU.1 inhibition and also play a role in the eventual extinction of myeloid potential during commitment. The need for GATA-3 to promote accurate DN2 to DN3 progression may also be reflected through its specific importance to induce Bcl11b and Ets1 expression, as well. The impact of GATA-3 on ETP/DN1 viability could reflect its positive role in Kit gene expression, but our data show that this interpretation can be complicated by the disjunction of Kit and CD44 developmental regulation when GATA-3 is removed. Other GATA-3 regulated genes are predicted to encode PHD domain and chromobox chromatin modifiers which open a wide range of possibilities for regulating survival and self-renewal. Strikingly, however, the trio of repressed genes Bcl11a, PU.1, and SpiB, whether direct or indirect targets, suggest a pathway through which loss of GATA-3 could simultaneously relieve multiple constraints on the B-cell program. It will be of great interest to test these hypotheses.
The distinct dosage thresholds for different critical GATA-3 effects clarify the position of GATA-3 in a T-cell commitment network (Fig. 9E). Our results imply that the rising level of GATA-3 first excludes the B-cell option, then enables T-lineage genes to be turned on, then provides quantitative control over the silencing of PU.1 and myeloid options. Even before helping to activate a definitive T-cell program, low-level GATA-3 becomes essential for viability of the earliest T-cell precursors. The stage-specific effects of G3-3W shRNA on viability indicate a likely threshold mechanism: even the weak effect of shRNA may push GATA-3 below this threshold when starting from the low levels in ETP/DN1 cells, but not from higher DN2 levels. However, higher, DN2-stage levels of GATA-3 are needed in full to arm cells for competence in developmental progression. Our results confirm that GATA-3 is required to prevent B-lineage access, even after T-lineage differentiation has begun, through mechanisms that are complementary to, but distinct from, those mediated by Bcl11b (11, 20, 50, 53). In sum, GATA-3 at the DN2 stage is revealed to be a critically dose-dependent regulator of both T lineage progression and unique aspects of T-lineage commitment.
Supplementary Material
Acknowledgments
The authors thank Gabriela Hernandez-Hoyos for the Gata3 shRNA construct, I-Cheng Ho and Sung-Yun Pai (Brigham & Women’s Hospital, Harvard Medical School) for GATA-3-floxed mice and Barbara Kee (University of Chicago), James Di Santo and Roel Klein Wolterink (Institut Pasteur), and Marcos García-Ojeda (UC Merced) for stimulating discussions and for sharing their data before publication. We thank Christopher Franco and Kira Sarup-Lytzen for assistance with qPCR, Jerry Kwong and Benjamin Park for technical help, and Rochelle Diamond, Stephanie Adams, and Diana Perez for cell sorting. We also thank Frank Costantini (Columbia University), Mark Leid (Oregon State University), Pentao Liu (Cambridge University), and Stephen Nutt (Walter & Eliza Hall Institute) for ROSA26R-EYFP, two types of Bcl11bfl/fl, and Spi1fl/fl founder mice, respectively, Juan Carlos Zúñiga-Pflücker (University of Toronto) for OP9-DL4 cells, and John Rossi (City of Hope), Naoko Arai (DNAX), Tom Taghon (Ghent University), and lab members Mark Zarnegar, Sanket Acharya, and Elizabeth-Sharon David-Fung for providing and characterizing retroviral vectors.
Footnotes
Funding for this study was from National Institutes of Health (USPHS) grants R01CA98925, RC2CA148278, R01CA90233, and R01AI95943, the Louis A. Garfinkle Memorial Laboratory Fund, the Al Sherman Foundation, and from the Albert Billings Ruddock Professorship to E.V.R. Initial support for D.D.S.A. was also provided by the Elisabeth Ross Endowment at Caltech and an NIH training grant (5T32 HD07257).
Conflict of Interest Statement
The authors declare no conflict of interest.
References
- 1.Pandolfi PP, Roth ME, Karis A, Leonard MW, Dzierzak E, Grosveld FG, Engel JD, Lindenbaum MH. Targeted disruption of the GATA3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nature genetics. 1995;11:40–44. doi: 10.1038/ng0995-40. [DOI] [PubMed] [Google Scholar]
- 2.Ting CN, Olson MC, Barton KP, Leiden JM. Transcription factor GATA-3 is required for development of the T-cell lineage. Nature. 1996;384:474–478. doi: 10.1038/384474a0. [DOI] [PubMed] [Google Scholar]
- 3.Hendriks RW, Nawijn MC, Engel JD, van Doorninck H, Grosveld F, Karis A. Expression of the transcription factor GATA-3 is required for the development of the earliest T cell progenitors and correlates with stages of cellular proliferation in the thymus. European journal of immunology. 1999;29:1912–1918. doi: 10.1002/(SICI)1521-4141(199906)29:06<1912::AID-IMMU1912>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 4.Vosshenrich CA, Garcia-Ojeda ME, Samson-Villeger SI, Pasqualetto V, Enault L, Richard-Le Goff O, Corcuff E, Guy-Grand D, Rocha B, Cumano A, Rogge L, Ezine S, Di Santo JP. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol. 2006;7:1217–1224. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- 5.Samson SI, Richard O, Tavian M, Ranson T, Vosshenrich CA, Colucci F, Buer J, Grosveld F, Godin I, Di Santo JP. GATA-3 promotes maturation, IFN-γ production, and liver-specific homing of NK cells. Immunity. 2003;19:701–711. doi: 10.1016/s1074-7613(03)00294-2. [DOI] [PubMed] [Google Scholar]
- 6.Hattori N, Kawamoto H, Fujimoto S, Kuno K, Katsura Y. Involvement of transcription factors TCF-1 and GATA-3 in the initiation of the earliest step of T cell development in the thymus. The Journal of experimental medicine. 1996;184:1137–1147. doi: 10.1084/jem.184.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosoya T, Kuroha T, Moriguchi T, Cummings D, Maillard I, Lim KC, Engel JD. GATA-3 is required for early T lineage progenitor development. J Exp Med. 2009;206:2987–3000. doi: 10.1084/jem.20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ku CJ, Hosoya T, Maillard I, Engel JD. GATA-3 regulates hematopoietic stem cell maintenance and cell-cycle entry. Blood. 2012;119:2242–2251. doi: 10.1182/blood-2011-07-366070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buza-Vidas N, Duarte S, Luc S, Bouriez-Jones T, Woll PS, Jacobsen SE. GATA3 is redundant for maintenance and self-renewal of hematopoietic stem cells. Blood. 2011;118:1291–1293. doi: 10.1182/blood-2011-02-338046. [DOI] [PubMed] [Google Scholar]
- 10.Pai SY, Truitt ML, Ting CN, Leiden JM, Glimcher LH, Ho IC. Critical roles for transcription factor GATA-3 in thymocyte development. Immunity. 2003;19:863–875. doi: 10.1016/s1074-7613(03)00328-5. [DOI] [PubMed] [Google Scholar]
- 11.García-Ojeda ME, Klein Wolterink RG, Lemaître F, Richard-Le Goff O, Hasan M, Hendriks RW, Cumano A, Di Santo JP. GATA-3 promotes T cell specification by repressing B cell potential in pro-T cells in mice. Blood. 2013;121:1749–1759. doi: 10.1182/blood-2012-06-440065. [DOI] [PubMed] [Google Scholar]
- 12.Wei G, Abraham BJ, Yagi R, Jothi R, Cui K, Sharma S, Narlikar L, Northrup DL, Tang Q, Paul WE, Zhu J, Zhao K. Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity. 2011;35:299–311. doi: 10.1016/j.immuni.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang JA, Mortazavi A, Williams BA, Wold BJ, Rothenberg EV. Dynamic transformations of genome-wide epigenetic marking and transcriptional control establish T cell identity. Cell. 2012;149:467–482. doi: 10.1016/j.cell.2012.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taghon T, Yui MA, Rothenberg EV. Mast cell lineage diversion of T lineage precursors by the essential T cell transcription factor GATA-3. Nat Immunol. 2007;8:845–855. doi: 10.1038/ni1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmitt TM, Zúñiga-Pflücker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 16.Taghon TN, David ES, Zuniga-Pflucker JC, Rothenberg EV. Delayed, asynchronous, and reversible T-lineage specification induced by Notch/Delta signaling. Genes & development. 2005;19:965–978. doi: 10.1101/gad.1298305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strasser A, Harris AW, Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 18.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nutt SL, Metcalf D, D'Amico A, Polli M, Wu L. Dynamic regulation of PU.1 expression in multipotent hematopoietic progenitors. The Journal of experimental medicine. 2005;201:221–231. doi: 10.1084/jem.20041535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Leid M, Rothenberg EV. An early T cell lineage commitment checkpoint dependent on the transcription factor Bcl11b. Science. 2010;329:89–93. doi: 10.1126/science.1188989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li P, Burke S, Wang J, Chen X, Ortiz M, Lee SC, Lu D, Campos L, Goulding D, Ng BL, Dougan G, Huntly B, Gottgens B, Jenkins NA, Copeland NG, Colucci F, Liu P. Reprogramming of T cells to natural killer-like cells upon Bcl11b deletion. Science. 2010;329:85–89. doi: 10.1126/science.1188063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carleton M, Ruetsch NR, Berger MA, Rhodes M, Kaptik S, Wiest DL. Signals transduced by CD3ε, but not by surface pre-TCR complexes, are able to induce maturation of an early thymic lymphoma in vitro. J Immunol. 1999;163:2576–2585. [PubMed] [Google Scholar]
- 23.Dionne CJ, Tse KY, Weiss AH, Franco CB, Wiest DL, Anderson MK, Rothenberg EV. Subversion of T lineage commitment by PU.1 in a clonal cell line system. Dev Biol. 2005;280:448–466. doi: 10.1016/j.ydbio.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 24.Mohtashami M, Shah DK, Nakase H, Kianizad K, Petrie HT, Zúñiga-Pflücker JC. Direct comparison of Dll1- and Dll4-mediated Notch activation levels shows differential lymphomyeloid lineage commitment outcomes. J Immunol. 2010;185:867–876. doi: 10.4049/jimmunol.1000782. [DOI] [PubMed] [Google Scholar]
- 25.Franco CB, Scripture-Adams DD, Proekt I, Taghon T, Weiss AH, Yui MA, Adams SL, Diamond RA, Rothenberg EV. Notch/Delta signaling constrains reengineering of pro-T cells by PU.1. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11993–11998. doi: 10.1073/pnas.0601188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernandez-Hoyos G, Anderson MK, Wang C, Rothenberg EV, Alberola-Ila J. GATA-3 expression is controlled by TCR signals and regulates CD4/CD8 differentiation. Immunity. 2003;19:83–94. doi: 10.1016/s1074-7613(03)00176-6. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez-Hoyos G, Alberola-Ila J. Analysis of T-cell development by using short interfering RNA to knock down protein expression. Methods Enzymol. 2005;392:199–217. doi: 10.1016/S0076-6879(04)92012-5. [DOI] [PubMed] [Google Scholar]
- 28.Anderson MK, Hernandez-Hoyos G, Dionne CJ, Arias AM, Chen D, Rothenberg EV. Definition of regulatory network elements for T cell development by perturbation analysis with PU.1 and GATA-3. Dev Biol. 2002;246:103–121. doi: 10.1006/dbio.2002.0674. [DOI] [PubMed] [Google Scholar]
- 29.Kurata H, Lee HJ, O'Garra A, Arai N. Ectopic expression of activated Stat6 induces the expression of Th2-specific cytokines and transcription factors in developing Th1 cells. Immunity. 1999;11:677–688. doi: 10.1016/s1074-7613(00)80142-9. [DOI] [PubMed] [Google Scholar]
- 30.Zarnegar MA, Chen J, Rothenberg EV. Cell-type-specific activation and repression of PU.1 by a complex of discrete, functionally specialized cis-regulatory elements. Mol Cell Biol. 2010;30:4922–4939. doi: 10.1128/MCB.00354-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.David-Fung ES, Butler R, Buzi G, Yui MA, Diamond RA, Anderson MK, Rowen L, Rothenberg EV. Transcription factor expression dynamics of early T-lymphocyte specification and commitment. Dev Biol. 2009;325:444–467. doi: 10.1016/j.ydbio.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nature biotechnology. 2013;31:46–53. doi: 10.1038/nbt.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breitling R, Armengaud P, Amtmann A, Herzyk P. Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett. 2004;573:83–92. doi: 10.1016/j.febslet.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 34.Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity. 2002;17:117–130. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 35.Tydell CC, David-Fung ES, Moore JE, Rowen L, Taghon T, Rothenberg EV. Molecular dissection of prethymic progenitor entry into the T lymphocyte developmental pathway. J Immunol. 2007;179:421–438. doi: 10.4049/jimmunol.179.1.421. [DOI] [PubMed] [Google Scholar]
- 36.Tabrizifard S, Olaru A, Plotkin J, Fallahi-Sichani M, Livak F, Petrie HT. Analysis of transcription factor expression during discrete stages of postnatal thymocyte differentiation. J Immunol. 2004;173:1094–1102. doi: 10.4049/jimmunol.173.2.1094. [DOI] [PubMed] [Google Scholar]
- 37.Shinnakasu R, Yamashita M, Kuwahara M, Hosokawa H, Hasegawa A, Motohashi S, Nakayama T. Gfi1-mediated stabilization of GATA3 protein is required for Th2 cell differentiation. J Biol Chem. 2008;283:28216–28225. doi: 10.1074/jbc.M804174200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maneechotesuwan K, Xin Y, Ito K, Jazrawi E, Lee KY, Usmani OS, Barnes PJ, Adcock IM. Regulation of Th2 cytokine genes by p38 MAPK-mediated phosphorylation of GATA-3. J Immunol. 2007;178:2491–2498. doi: 10.4049/jimmunol.178.4.2491. [DOI] [PubMed] [Google Scholar]
- 39.Cook KD, Miller J. TCR-dependent translational control of GATA-3 enhances Th2 differentiation. J Immunol. 2010;185:3209–3216. doi: 10.4049/jimmunol.0902544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gimferrer I, Hu T, Simmons A, Wang C, Souabni A, Busslinger M, Bender TP, Hernandez-Hoyos G, Alberola-Ila J. Regulation of GATA-3 expression during CD4 lineage differentiation. J Immunol. 2011;186:3892–3898. doi: 10.4049/jimmunol.1003505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frelin C, Herrington R, Janmohamed S, Barbara M, Tran G, Paige CJ, Benveniste P, Zúñiga-Pflücker JC, Souabni A, Busslinger M, Iscove NN. GATA-3 regulates the self-renewal of long-term hematopoietic stem cells. Nat Immunol. 2013;14:1037–1044. doi: 10.1038/ni.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yui MA, Feng N, Rothenberg EV. Fine-scale staging of T cell lineage commitment in adult mouse thymus. J Immunol. 2010;185:284–293. doi: 10.4049/jimmunol.1000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Del Real MM, Rothenberg EV. Architecture of a lymphomyeloid developmental switch controlled by PU.1, Notch, and GATA-3. Development. 2013;140:1207–1219. doi: 10.1242/dev.088559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu W, Carr T, Ramirez K, McGregor S, Sigvardsson M, Kee BL. E2A transcription factors limit expression of Gata3 to facilitate T lymphocyte lineage commitment. Blood. 2013;121:1534–1542. doi: 10.1182/blood-2012-08-449447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim DH, Rossi JJ. Overview of gene silencing by RNA interference. Curr Protoc Nucleic Acid Chem. 2009 doi: 10.1002/0471142700.nc1601s36. Chapter 16: Unit 16 11. [DOI] [PubMed] [Google Scholar]
- 46.Wang D, Claus CL, Vaccarelli G, Braunstein M, Schmitt TM, Zuniga-Pflucker JC, Rothenberg EV, Anderson MK. The basic helix-loop-helix transcription factor HEBAlt is expressed in pro-T cells and enhances the generation of T cell precursors. J.Immunol. 2006;177:109–119. doi: 10.4049/jimmunol.177.1.109. [DOI] [PubMed] [Google Scholar]
- 47.Yagi R, Zhong C, Northrup DL, Yu F, Bouladoux N, Spencer S, Hu G, Barron L, Sharma S, Nakayama T, Belkaid Y, Zhao K, Zhu J. The transcription factor GATA3 is critical for the development of all IL-7Rα-expressing innate lymphoid cells. Immunity. 2014;40:378–388. doi: 10.1016/j.immuni.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sasaki T, Onodera A, Hosokawa H, Watanabe Y, Horiuchi S, Yamashita J, Tanaka H, Ogawa Y, Suzuki Y, Nakayama T. Genome-wide gene expression profiling revealed a critical role for GATA3 in the maintenance of the Th2 cell identity. PLoS One. 2013;8:e66468. doi: 10.1371/journal.pone.0066468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahata B, Zhang X, Kolodziejczyk AA, Proserpio V, Haim-Vilmovsky L, Taylor AE, Hebenstreit D, Dingler FA, Moignard V, Göttgens B, Arlt W, McKenzie AN, Teichmann SA. Single-cell RNA sequencing reveals T helper cells synthesizing steroids de novo to contribute to immune homeostasis. Cell Rep. 2014;7:1130–1142. doi: 10.1016/j.celrep.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ikawa T, Hirose S, Masuda K, Kakugawa K, Satoh R, Shibano-Satoh A, Kominami R, Katsura Y, Kawamoto H. An essential developmental checkpoint for production of the T cell lineage. Science. 2010;329:93–96. doi: 10.1126/science.1188995. [DOI] [PubMed] [Google Scholar]
- 51.Heinzel K, Benz C, Martins VC, Haidl ID, Bleul CC. Bone marrow-derived hemopoietic precursors commit to the T cell lineage only after arrival in the thymic microenvironment. J Immunol. 2007;178:858–868. doi: 10.4049/jimmunol.178.2.858. [DOI] [PubMed] [Google Scholar]
- 52.Rothenberg EV. T cell lineage commitment: identity and renunciation. J Immunol. 2011;186:6649–6655. doi: 10.4049/jimmunol.1003703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klein Wolterink RG, Garcia-Ojeda ME, Vosshenrich CA, Hendriks RW, Di Santo JP. The intrathymic crossroads of T and NK cell differentiation. Immunological reviews. 2010;238:126–137. doi: 10.1111/j.1600-065X.2010.00960.x. [DOI] [PubMed] [Google Scholar]
- 54.Yang Q, Jeremiah Bell J, Bhandoola A. T-cell lineage determination. Immunological reviews. 2010;238:12–22. doi: 10.1111/j.1600-065X.2010.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dontje W, Schotte R, Cupedo T, Nagasawa M, Scheeren F, Gimeno R, Spits H, Blom B. Delta-Like1-induced Notch1 signalling regulates the human plasmacytoid dendritic cell versus T cell lineage decision through control of GATA-3 and Spi-B. Blood. 2006;107:2446–2452. doi: 10.1182/blood-2005-05-2090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.