Abstract
Microscopy is considered as the gold standard for malaria diagnosis although its wide application is limited by the requirement of highly experienced microscopists. PCR and serological tests provide efficient diagnostic performance and have been applied for malaria diagnosis and research. The aim of this study was to investigate the diagnostic performance of nested PCR and a recently developed an ELISA-based new rapid diagnosis test (RDT), NovaLisa test kit, for diagnosis of malaria infection, using microscopic method as the gold standard. The performance of nested-PCR as a malaria diagnostic tool is excellent with respect to its high accuracy, sensitivity, specificity, and ability to discriminate Plasmodium species. The sensitivity and specificity of nested-PCR compared with the microscopic method for detection of Plasmodium falciparum, Plasmodium vivax, and P. falciparum/P. vivax mixed infection were 71.4 vs 100%, 100 vs 98.7%, and 100 vs 95.0%, respectively. The sensitivity and specificity of the ELISA-based NovaLisa test kit compared with the microscopic method for detection of Plasmodium genus were 89.0 vs 91.6%, respectively. NovaLisa test kit provided comparable diagnostic performance. Its relatively low cost, simplicity, and rapidity enables large scale field application.
Keywords: Plasmodium falciparum, Plasmodium vivax, nested-PCR, ELISA-based NovaLisa test kit
INTRODUCTION
Malaria is one of the major public health problems in tropical countries affecting more than 300 million people around the world with 2-3 million deaths annually. The global populations at risk are more than 40%. In Thailand, Plasmodium falciparum and Plasmodium vivax are responsible for the majority of cases [1]. Rapid and accurate diagnosis is therefore important in case detection and management. The conventional method by clinical diagnosis based on patients'signs and symptoms, including fever, headache, weakness, myalgia, chills, dizziness, abdominal pain, diarrhea, nausea, vomiting, anorexia, and pruritus are non-specific and provide variable results [2]. Microscopic examination by light microscopy is recognized as the "gold standard" for definitive malaria diagnosis, but requires experienced microscopists and implementation of good quality control and assurance system [3]. Non-microscopic techniques used in malaria diagnosis include those which detect parasite-specific antigens/products in patient blood, antibodies against malaria parasites, or parasite nucleic acids [2]. Most can differentiate different Plasmodium species. For malaria antigen detection, various immune-chromatographic test kits, the rapid diagnosis tests (RDTs), offer a useful alternative to microscopy in clinical settings where reliable microscopic diagnosis is not available. However, before these tests can be widely adopted, several issues remain to be addressed, including quality assurance of diagnostic performance and affordable cost when applied in field conditions.
Serological methods for diagnosis of malaria, e.g., ELISA and indirect immunofluorescence (IFA), are usually based on detection of antibodies against asexual blood stage malaria parasites [4]. The tests do not detect current infection but past exposure. However, they are useful when applied in epidemiological surveys for screening of potential blood donors and in providing evidence of recent infection in non-immune individuals [5]. In addition, the tests can be applied for detection of hidden parasite reservoirs (malaria carriers).
Recently, PCR-based techniques which detect parasite nucleic acids have been developed and considered as the most specific and sensitive diagnostic tool for malaria, particularly in cases with low parasitemia or mixed infection [6]. Furthermore, the techniques have widely been applied as a diagnostic tool for monitoring therapeutic response and parasite resistance following treatment with antimalarial drug regimens. One limitation of the PCR-based methods is that the parasite DNA can remain in the blood stream long after infection has been cleared and therefore differentiating an active infection from a recently cleared infection is difficult. The PCR-based methods which detect malaria antigen detection are widely applied as a diagnostic tool for rapid diagnosis and treatment. On the other hand, the ELISA-based methods which detect antibodies of all Plasmodium spp. are useful when applied as a screening tool for malaria infection in endemic areas, particularly for blood donation or transfusion, as well as for epidemiological investigation.
The objective of the study was to investigate the diagnostic performance of nested-PCR and a recently developed serological ELISA-based NovaLisa test kit for diagnosis of malaria infection in an endemic area of Thailand, using microscopic method as the gold standard.
MATERIALS AND METHODS
Study area and sample collection
The study was conducted during July 2011 and December 2012 at Mae Sot General Hospital, Mae Sot District, Tak Province, Thailand, the malaria endemic area along Thai-Myanmar border with highest annual case incidence [7]. Approval of the study protocol was obtained from the Ethics Committee of the Ministry of Public Health of Thailand. A total of 245 blood samples were included in the analysis, 94 from patients with signs and symptoms of malaria with P. falciparum and P. vivax mono-infection as well as P. falciparum/P. vivax mixed infection (79 males and 15 females, aged 12-65 years, admission parasitemia 45-17,000 ring-stage parasites/µl blood), 60 from patients with fever related to other infections (32 males and 28 females, aged 16-90 years), and 91 healthy subjects (69 males and 22 females, aged 19-53 years). Written informed consents for study participation were obtained from all participants prior to the study. The exclusion criteria for the group with malarial infection were those with previous antimalarial treatment or presence of clinical signs and symptoms of severe malaria. Blood sample was collected from each subject through venipuncture, into a glass tube containing citrate (0.5 ml blood for microscopic examination), EDTA (2 ml blood for NovaLisa test kit), and filter paper (Whatman No. 3: 200-300 µl blood spot for nested-PCR). For the diagnosis by NovaLisa test kit, plasma sample was prepared from each blood sample through centrifugation at 3,000 g for 10 min.
Malaria diagnosis
Microscopic examination: Thick blood smears were prepared for all blood samples and stained with 10% Giemsa. The malaria parasite was detected under light microscope for both number and species identification. The number of parasites was counted against 200 leucocytes and parasite density was estimated by assuming 8,000 leucocytes/µl blood. Sample was considered negative when no parasite was detected after examining 100 microscopic fields. The malaria microscopic examination was performed by 2 independent experienced microscopists from Mae Sot General Hospital and Chulabhorn International College of Medicine, Thammasat University, Thailand. Each blood slide was blinded, and the result was masked to both microscopists. In order to check for inter-observer variability, a double blinded cross reading of a random sampling of 100 blood slides was carried out by the senior microscopist.
Diagnosis by nested-PCR: Genomic DNA was extracted from each dried blood spot sample using QIAamp DNA extraction mini-kit (QIAGEN, Valencia, California, USA) and stored at -20℃ until used as template for PCR amplification. Primers and amplification conditions used for genotyping were according to the previously described methods with modification [8,9,10]. All primers were obtained from Fermentas Co. Ltd. (Maryland, USA). The rPLU1 and rPLU5 primers were used for amplification of the Plasmodium genomic DNA. PCR cycling condition was as follows: denaturation at 94℃ for 4 min, followed by 35 cycles of 94℃ for 30 sec, 55℃ for 1 min, 72℃ for 1 min, and then 72℃ for 4 min. For detection of P. falciparum, the PCR products of the first reaction were further amplified using rFAL1 and rFAL2 primers. PCR cycling condition was as follows: denaturation at 94℃ for 4 min, followed by 35 cycles of 94℃ for 30 sec, 55℃ for 1 min, 72℃ for 1 min, and then 72℃ for 4 min.
For detection of P. vivax, the PCR products of the first reaction were further amplified using rVIV1 and rVIV2 primers. PCR cycling conditions was as follows: denaturation at 94℃ for 4 min, followed by 35 cycles of 94℃ for 30 sec, 55℃ for 1 min, 72℃ for 1 min, and then 72℃ for 4 min. For detection of P. malariae, the PCR products of the first reaction were further amplified using rMAL1 and rMAL2 primers. PCR cycling condition was as follows: denaturation at 94℃ for 4 min, followed by 35 cycles of 94℃ for 30 sec, 55℃ for 1 min, 72℃ for 1 min, and then 72℃ for 4 min. For detection of P. ovale, the PCR products of the first reaction were further amplified using rOVA1 and rOVA2 primers. PCR cycling condition was as follows: denaturation at 94℃ for 4 min, followed by 35 cycles of 94℃ for 30 sec, 55℃ for 1 min, 72℃ for 1 min, and then 72℃ for 4 min. The amplified PCR products from all steps were separated on 1% agarose gel electrophoresis.
Diagnosis by ELISA-based NovaLisa test kit: Malaria diagnosis by NovaLisa test kit (NovaTec Immundiagnostica GmbH, Dietzenbach, Germany) was performed in all plasma samples using the method described by the manufacturer (http://www.novatec-id.com). Briefly, control and test blood samples (100 µl each) were added in a 96-well plate coated with malarial-specific antigens (MSP1, CSP, and a chimeric multi-epitope antigen from P. falciparum and P. vivax). These antigens were used for antibody detection of Plasmodium spp. from clinical samples. The formed immune complex was visualized as a blue color after incubation with histidine-rich protein (HRP) and TMB-substrate solution. The enzymatic reaction was stopped by adding sulphuric acid. Color change was detected with a varioskan flash plate reader (Thermo Fisher Scientific Inc., Massachusetts, USA) at the wavelengths of 450/620 nm.
Data analysis
NovaLisa test kit were evaluated using microscopic method as the gold standard. Performance parameters included sensitivity, specificity, positive prediction value (PPV), negative prediction value (NPV), false positive rate, and false negative rate. Sensitivity of the test was calculated as the number of true positives/(no. of true positives+no. of false negatives) ×100. The specificity of the test was calculated as the number of true negatives/(no. of true negatives+no. of false positives) ×100. PPV and NPV were determined from the number of true positive/(no. of true positive+no. of false positive) ×100, and the number of true negative/(no. of true negative+no. of false negative) ×100, respectively. False positive and false negative rates were determined from 1-(specificity) and 1-(sensitivity), respectively. The detection limit was calculated from the sample with the lowest parasitemia with the true positive result. Kappa statistic was applied to investigate the consistency of results between nested-PCR, ELISA-based NovaLisa test kit, and microscopic examination. The strength of agreement was categorized based on the kappa values as follows: poorly correlated (<0), slightly correlated (0-0.20), fairly correlated (0.21-0.40), moderately correlated (0.41-0.60), substantially correlated (0.61-0.80), and perfectly correlated (0.81-1.0).
RESULTS
Diagnostic performance of nested-PCR and ELISA-based NovaLisa test kit in comparison with the microscopic method
The diagnostic performance of nested-PCR and ELISA-based NovaLisa test kit in the 3 groups of 245 blood samples are summarized in Table 1. The sensitivity, specificity, PPV, NPV, false positive, and false negative rates and agreement between tests (kappa values) of nested-PCR and ELISA-based NovaLisa test kit compared with the microscopic method are shown in Table 2. Out of 94 malaria-infected blood samples (35, 51, and 8 samples with P. falciparum, P. vivax, and P. falciparum/P. vivax mixed infection, respectively), nested-PCR provided positive results for P. falciparum, P. vivax, and P. falciparum/P. vivax mixed infection in 25, 53, and 16 samples, respectively. ELISA-based NovaLisa test kit which detects only Plasmodium genus without species discrimination provided positive results in 97 samples. For control samples from healthy subjects and patients with other fever-related infections, nested-PCR provided negative results in 91 (100%) and 60 (100%), respectively, ELISA-based NovaLisa test kit provided negative results in 93 (102.2%) and 55 (91.7%), respectively.
Table 1.
Detection of P. falciparum and P. vivax by nested-PCR and a new ELISA-based NovaLisa test kita in comparison with the microscopic method
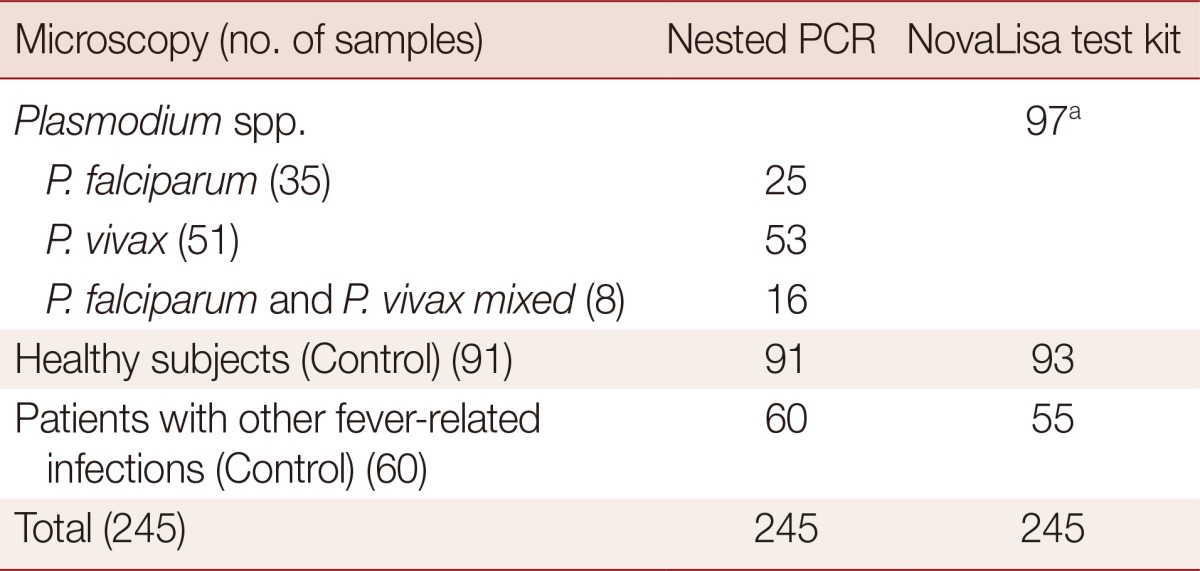
aELISA-based NovaLisa test detects only the Plasmodium genus.
Table 2.
The test performance of nested-PCR and a new ELISA-based NovaLisa test kit for detection of P. falciparum and P. vivax in comparison with the microscopic method
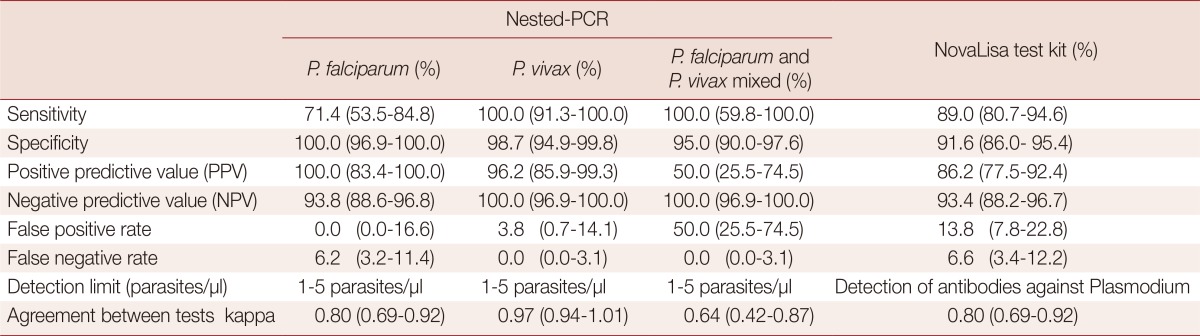
Data are presented as percentage (95% confidence interval; CI) for all parameters except the detection limit (parasites/µl).
The sensitivity and specificity of nested PCR compared with the microscopic method for detection of P. falciparum, P. vivax, and P. falciparum/P. vivax mixed infection were 71.4 vs 100%, 100 vs 98.7%, and 100 vs 95.0%, respectively. The sensitivity and specificity of ELISA-based NovaLisa test kit compared with the microscopic method for detection of the Plasmodium genus were 89.0% and 91.6%, respectively. The detection limit of all methods for detecting the Plasmodium genus, either for each species, mixed infection/all species (for NovaLisa test kit) was 1-5 parasites/µl. The strength of agreement between nested-PCR and microscopic method for detection of P. vivax was categorized as almost perfect correlation with a kappa value of 0.97. The strength of agreement between both methods for detection of P. falciparum and P. falciparum/P. vivax mixed infection was categorized as substantial correlation with kappa values of 0.80 and 0.64, respectively. For ELISA-based NovaLisa test kit, agreement with microscopic results in detecting the Plasmodium genus was also categorized as substantial correlation with a kappa value of 0.80.
DISCUSSION
P. falciparum and P. vivax are the 2 major malarial species in Thailand with the ratio of approximately equal [11]. Co-infection with both malarial species and infections with other species (P. ovale and P. malariae) account for only 8.0 [12] and less than 1%, respectively [13]. Results of the present study revealed excellent diagnostic efficiency of nested-PCR for detection of the 2 major malaria species in Thailand with sensitivity and specificity of approaching 100%. The diagnostic efficiency of the ELISA-based diagnostic test NovaLisa test kit in detecting all Plasmodium species was relatively high with regard to sensitivity (89.0%) and specificity (91.6%). ELISA methods have been reported to provide false negative results in detecting malaria parasite in some cases with acute infection due to the immunological window between infection and antibody production, as well as the high variability of Plasmodium blood-stage antigens [14].
The diagnostic performance of nested-PCR and ELISA-based NovaLisa test kit observed in the present study was in agreement with that reported in previous studies [10,14]. The sensitivity of DiaMed ELISA test kit in detecting the Plasmodium genus was shown by Doderer et al. [14] to be 84.2%. PCR and the ELISA-based RDTs including the Malaria Antigen Pf/Pan™, Malaria Ag-Pf™, Malaria Ag-Pv™ CareStart™, and Malaria HRP2/pLDH COMBO test kits have previously been shown to provide results with very good correlation with the microscopic method in detecting certain species of malaria (P<0.001) [10,15]. It was noted for the observation of a relatively better agreement between nested PCR and microscopic results for detecting P. vivax (kappa value=0.97) compared with P. falciparum mono-infection (kappa value=0.80) and P. falciparum/P. vivax mixed infection (kappa value=0.64). The diagnostic efficiency of nested PCR in detecting the mixed infection thus appeared to be relatively lower than NovaLisa test (kappa value=0.80). False positive tests can occur as a result of cross reactivity with antibodies against Yersinia, Schistosoma and Entamoeba which are contaminated in urine or feces. On the other hand, false negative results can also be found due to bacterial contamination as a result of the blockage of antibodies and immune-complex formation or repeated freeze-thaw cycles of the specimens.
In conclusion, the performance of nested PCR as a malaria diagnostic tool is excellent with respect to its high accuracy, sensitivity, specificity, and ability to discriminate Plasmodium species. However, the test is time-consuming and relatively expensive, which limits the application in large scale routine malarial diagnosis [16]. The ELISA-based NovaLisa test kit provided comparable diagnosis efficiency with nested PCR, but its relatively low cost, simplicity and rapidity enable the large scale application in other medical laboratories including blood bank screening to exclude Plasmodium-infected blood sample. In addition, the test can be applied in epidemiological surveys for providing evidence of recent infection in non-immune individuals as well as in detecting and eliminating hidden parasite reservoirs (malaria carriers) [17].
ACKNOWLEDGMENTS
The study was supported by The Commission on Higher Education, Ministry of Education of Thailand, The National Research University Project of Thailand (NRU), Office of Higher Education Commission, Thammasat University (Center of Excellence in Pharmacology and Molecular Biology of Malaria and Cholangiocarcinoma), The Royal Golden Jubilee Ph.D. Programme, Thailand Research Fund, Thammasat University Joint Fund, and Graduated Student Grant to P. Thongdee (Grant no. PHD/0365/2552), Thammasat University. We thank Dr. Andreas Latz, NovaTec Immundiagnostica GmbH Technologie and Waldpark Company for providing the ELISA-based NovaLisa test kits for this study.
Footnotes
We have no conflict of interest related to this study.
References
- 1.Na-Bangchang K, Ruengweerayut R, Mahamad P, Ruengweerayut K, Chaijaroenkul W. Declining in efficacy of a three-day combination regimen of mefloquine-artesunate in a multi-drug resistance area along the Thai-Myanmar border. Malar J. 2010;9:273. doi: 10.1186/1475-2875-9-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tangpukdee N, Duangdee C, Wilairatana P, Krudsood S. Malaria diagnosis: a brief review. Korean J Parasitol. 2009;47:93–102. doi: 10.3347/kjp.2009.47.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yatsushiro S, Yamamura S, Yamaguchi Y, Shinohara Y, Tamiya E, Horii T, Baba Y, Kataoka M. Rapid and highly sensitive detection of malaria-infected erythrocytes using a cell microarray chip. PLoS One. 2010;5:e13179. doi: 10.1371/journal.pone.0013179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.She RC, Rawlins ML, Mohl R, Perkins SL, Hill HR, Litwin CM. Comparison of immunofluorescence antibody testing and two enzyme immunoassays in the serologic diagnosis of malaria. J Travel Med. 2007;14:105–111. doi: 10.1111/j.1708-8305.2006.00087.x. [DOI] [PubMed] [Google Scholar]
- 5.Reesink HW. European strategies against the parasite transfusion risk. Transfus Clin Biol. 2005;12:1–4. doi: 10.1016/j.tracli.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Kamau E, Tolbert LS, Kortepeter L, Pratt M, Nyakoe N, Muringo L, Ogutu B, Waitumbi JN, Ockenhouse CF. Development of a highly sensitive genus-specific quantitative reverse transcriptase real-time PCR assay for detection and quantitation of plasmodium by amplifying RNA and DNA of the 18S rRNA genes. J Clin Microbiol. 2011;49:2946–2953. doi: 10.1128/JCM.00276-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bureau of Epidemiology, Department of Disease Control, Ministry of Public Health, Thailand. Annual epidemiological surveillance report 2011. [Accessed 8 July 2013]. Available from. http://www.boe.moph.go.th/Annual/AESR2011/index.html.
- 8.Wooden J, Kyes S, Sibley CH. PCR and strain identification in Plasmodium falciparum. Parasitol Today. 1993;9:303–305. doi: 10.1016/0169-4758(93)90131-x. [DOI] [PubMed] [Google Scholar]
- 9.Singh B, Bobogare A, Cox-Singh J, Snounou G, Abdullah MS, Rahman HA. A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am J Trop Med Hyg. 1999;60:687–692. doi: 10.4269/ajtmh.1999.60.687. [DOI] [PubMed] [Google Scholar]
- 10.Chaijaroenkul W, Wongchai T, Ruangweerayut R, Na-Bangchang K. Evaluation of rapid diagnostics for Plasmodium falciparum and P. vivax in Mae Sot malaria endemic area, Thailand. Korean J Parasitol. 2011;49:33–38. doi: 10.3347/kjp.2011.49.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phimpraphi W, Paul RE, Yimsamran S, Puangsa-art S, Thanyavanich N, Maneeboonyang W, Prommongkol S, Sornklom S, Chaimungkun W, Chavez IF, Blanc H, Looareesuwan S, Sakuntabhai A, Singhasivanon P. Longitudinal study of Plasmodium falciparum and Plasmodium vivax in a Karen population in Thailand. Malar J. 2008;7:99. doi: 10.1186/1475-2875-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinko B, Oguike MC, Larbi JA, Bousema T, Sutherland CJ. Persistent detection of Plasmodium falciparum, P. malariae, P. ovale curtisi and P. ovale wallikeri after ACT treatment of asymptomatic Ghanaian school-children. Int J Parasitol Drugs Drug Resist. 2013;3:45–50. doi: 10.1016/j.ijpddr.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Na-Bangchang K, Congpuong K. Current malaria status and distribution of drug resistance in East and Southeast Asia with special focus to Thailand. Tohoku J Exp Med. 2007;211:99–113. doi: 10.1620/tjem.211.99. [DOI] [PubMed] [Google Scholar]
- 14.Doderer C, Heschung A, Guntz P, Cazenave JP, Hansmann Y, Senegas A, Pfaff AW, Abdelrahman T, Candolfi E. A new ELISA kit which uses a combination of Plasmodium falciparum extract and recombinant Plasmodium vivax antigens as an alternative to IFAT for detection of malaria antibodies. Malar J. 2007;6:19. doi: 10.1186/1475-2875-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moges B, Amare B, Belyhun Y, Tekeste Z, Gizachew M, Workineh M, Gebrehiwot A, Woldeyohannes D, Mulu A, Kassu A. Comparison of CareStart HRP2/pLDH COMBO rapid malaria test with light microscopy in north-west Ethiopia. Malar J. 2012;11:234. doi: 10.1186/1475-2875-11-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noedl H, Yingyuen K, Laoboonchai A, Fukuda M, Sirichaisinthop J, Miller RS. Sensitivity and specificity of an antigen detection ELISA for malaria diagnosis. Am J Trop Med Hyg. 2006;75:1205–1208. [PubMed] [Google Scholar]
- 17.Choudhury N, Jolly JG, Mahajan RC, Ganguly NK, Dubey ML, Agnihotri SK. Malaria screening to prevent transmission by transfusion: an evaluation of techniques. Med Lab Sci. 1991;48:206–211. [PubMed] [Google Scholar]


