Abstract
The disinfectant effects (DEs) of 10 types of chemicals, defined by their ability to destroy or inhibit oocysts and consequently prevent sporulation of Eimeria tenella field isolate, were evaluated in vitro. Correct species assignments and sample purities were confirmed by the singular internal transcribed spacer (ITS)-PCR analysis. A total of 18 treatments were performed, and the disinfection suppression levels were 75.9% for 39% benzene + 22% xylene (1:10 dilution), 85.5% for 30% cresol soup (1:1 dilution), and 91.7% for 99.9% acetic acid (1:2 dilution) group. The results indicate that acetic acid, cresol soup, and benzene+xylene are good candidates for suppression of E. tenella oocyst sporulation.
Keywords: Eimeria tenella, disinfectant, suppression, sporulation
Sanitary depopulation is a fundamental measure by which infection burden can be reduced, and thus could contribute to an effective coccidiosis control strategy. This strategy helps to reduce exogenous Eimeria spp. at different stages of development [1]. Coccidial oocysts are extremely resistant to physical and chemical stress [2,3]. Moreover, no internationally-accepted standardized method for testing antiparasitic properties of chemical disinfectants currently exists. Therefore, such a method needs to be developed in order to effectively and safely control coccidiosis in chickens [4,5,6].
Internationally there are no accepted standardized methods for testing antiparasitic properties of chemical disinfectants, although various models and procedures have been developed and are applied in many laboratories worldwide. Guimarase et al. [7] studied disinfectant effects (DEs) on Eimeria tenella, and in which 6 different disinfectants with different concentrations were used. The aim of the present study was to investigate the efficacy of various common disinfectants in reducing the viability of E. tenella oocysts specific to Korean field isolates.
Parasites were collected from infected commercial broiler chickens. After collection from broiler chickens, oocysts were purified by sedimentation method (1,300 g, 8 min). After discarding supernatants, the resultant sediment was diluted in distilled water and centrifuged once more as before. For oocyst enumeration, the MacMaster egg counting method was used. Correct species assignments and sample purities were confirmed by singular ITS-PCR. The DNA extracted from the sample was used to amplify the ITS-1 region as describe by Haug et al. [6]. E. tenella DNA was obtained with a Nucleic Acid Purification Kit (ExprepTM Plasmid DNA, GeneAll, Seoul, Korea). Primers were designed using E. tenella ITS-1 sequences obtained from GenBank (forward GTTGCGTAAATAGA-GCCCTCT and reverse GTTCCAAGCAGCATGTAACG) [6]. Initially, each primer pair was tested in pilot PCRs to define a common reaction condition optimal for all sets. Each E. tenella PCR was performed in a final volume of 20 µl, containing 10 µl of PCR Premix (EmeraldAmp™ PCR Master Mix, Takara, Otsu, Japan), 20 µM of forward primer and 20 µM of reverse primer (Standard Oligo, Bioneer, Daejeon, Korea), 3 µl of DNA (0.1-1.0 µg, diluted in TE buffer), and 3 µl of distilled water. The PCR cycling conditions consisted of an initial denaturation step at 95℃ for 10 min; 30 cycles of 98℃ for 10 sec, 52.5℃ for 30 sec, and 72℃ for 1 min; and a final extension step at 72℃ for 1 min. After these reactions, 3 µl of PCR product was visualized on a 2% agarose gel (UltraPure™ Agarose, Invitrogen, Carlsbad, California, USA) by electrophoresis.
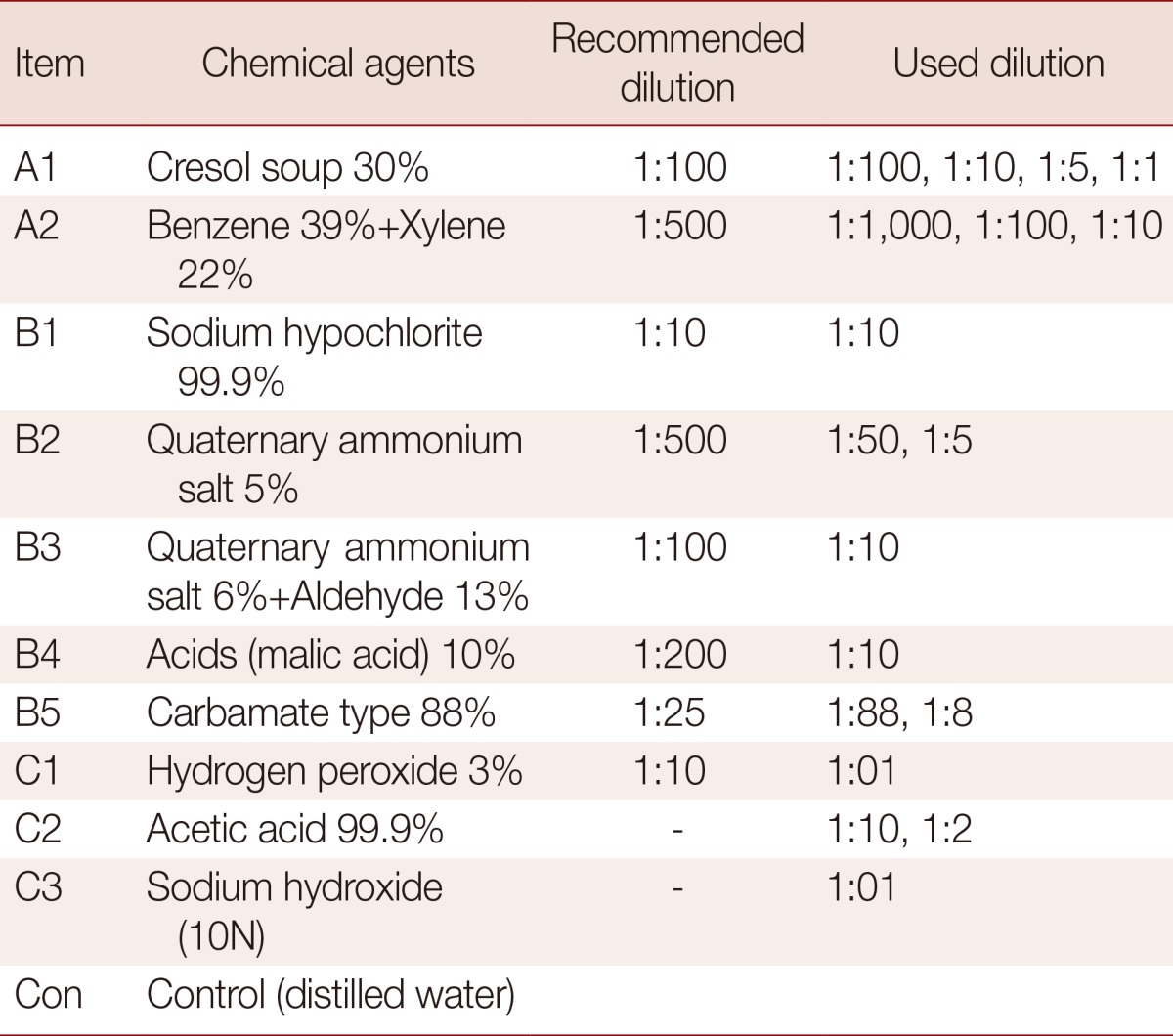
Genes were sequenced in the Jeonju Biomaterials Institute and subsequently submitted to GenBank (access no. FJ447468). E. tenella oocysts, maintained in 2% potassium dichromate, were washed and collected. Oocysts were purified and then incubated in 6-well plates containing 2% potassium dichromate solution for 6 days, with aeration, at room temperature. Oocysts were then exposed to disinfectants for 30 min. The disinfectants used are shown in Table 1. The disinfectants used can be broadly divided into the following 10 groups: A1, 30% cresol soup (Chongsol Chemical, Seoul, Korea); A2, 39% benzene (Daejin Chemical, Seoul, Korea)+22% xylene (Daejung Chemical and Metal, Seoul, Korea); B1, 99.9% sodium hypochlorite (Yuhanclorox, Seoul, Korea); B2, 5% quaternary ammonium salt (Kwangjin Pharma, Seoul, Korea); B3, 6% quaternary ammonium salt+13% aldehyde (KBNP Inc., Seoul, Korea); B4, 10% malic acid (Wako Pure Chemical Industries, Osaka, Japan); B5, 88% carbamate type (Bayer Animal Health, Tokyo, Japan); C1, hydrogen peroxide (Wako Pure Chemical Industries); C2, 99.9% acetic acid (Wako Pure Chemical Industries); and C3, sodium hydroxide (Wako Pure Chemical Industries). Either 3 or 4 independent replicates were performed for each disinfectant. DEs were calculated according to the proportion of in vitro sporulated vs unsporulated or lysed oocysts [1].
Table 1.
Chemical agents, recommended dilutions, and dilutions used
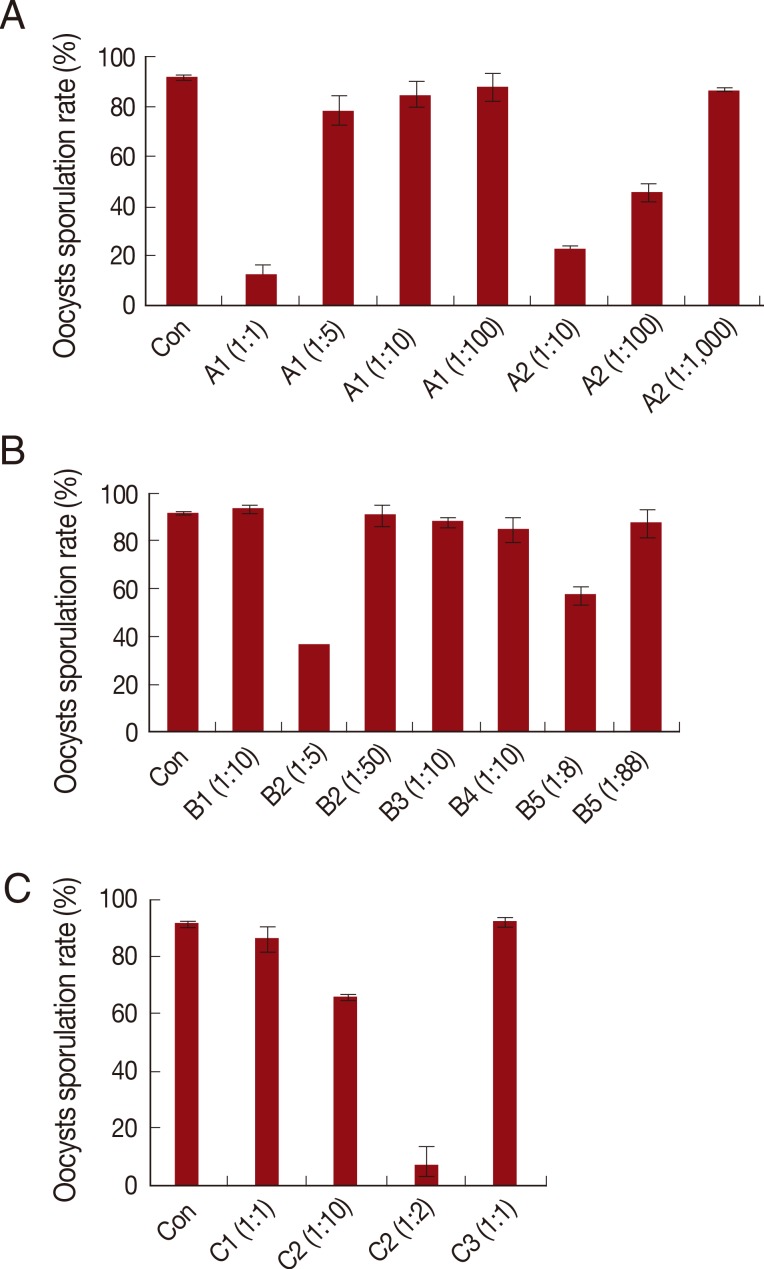
The disinfectants tested ranged considerably in their capacities to inhibit oocyst sporulation (Fig. 1A-C). Out of the 3 main groups, A1 (1:1 dilution), A2 (1:10 dilution), and C2 (1:2 dilution) showed superior disinfection action, with effect rates of 85.5%, 75.9%, and 91.7%, respectively (Fig. 2). In comparison, all other groups exhibited much lower DEs, with the exception of the quaternary ammonium compound (1:5 dilution). Multiple dilutions were tested for cresol soup, benzene+xylene, quaternary ammonium salts, carbamate and acetic acid, since these substances are commonly used as disinfectants. Among these substances, cresol soup, benzene+xylene, quaternary salts, and acetic acid all inhibited Eimeria sporulation when used at increased concentrations.
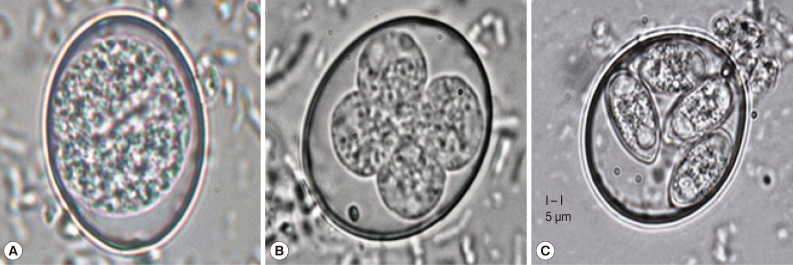
Fig. 1.
Degree of sporulation of Eimeria tenella oocysts. (A) Nonsporulated oocyst (×1,000). (B) Sporulating oocyst (×1,000). (C) Sporulated oocyst (×1,000). Bar=5 µm.
Fig. 2.
Sporulation rates of Eimeria tenella treated with various disinfectants. (A) Control, 30% cresol soup, 39% benzene+22% xylene. (B) Control, 99.9% sodium hypochlorite, 5% quaternary ammonium salt, 6% quaternary ammonium salt+13% aldehyde, acids (10% malic acid, 88% carbamate type). (C) Control, 3% hydrogen peroxide, 99.9% acetic acid, 10N sodium hydroxide. Values are presented as the mean±SD of 4 independent replicates.
DEs express the percent inhibition of oocyst sporulation [1]. Suppressive effects of each disinfectant on oocyst sporulation were determined after an in vitro incubation [3]. DEs are known to depend on the strain of parasite and on the length of time oocysts are exposed to disinfectants, with disinfection success directly related to the time that the disinfectant is in contact with the oocysts. Desirable properties of an ideal disinfectant include quick-rinsing and fast-acting, requiring only a short period of contact to achieve inhibition of oocyst sporulation. The present study treated Eimeria oocysts for 30 min.
Among the disinfectants tested, 30% cresol soup, 39% benzene+22% xylene, 5% quaternary ammonium salt, 88% carbamate type, and 99.9% acetic acid all inhibited sporulation of E. tenella oocysts in a dose-dependent manner. Furthermore, the sporulation of E. tenella oocysts treated with 60% orthodichlorobenzene+30% xylene was inhibited by 79.5%, as shown in a previous study [7]. Similar effects were found in the present study, 75.9%, with 39% benzene+22% xylene when used at a 1:5 dilution. Thus, similar results were obtained with 2 chemical agents as observed in previous studies. Oocyst sporulation was previously shown to be inhibited after incubation in 10% NaOH for 2, 10, and 30 min [8]. Our results were consistent with this previous study, since oocysts treated with NaOH (10N) at a 1:1 ratio also failed to sporulate.
The DEs of cresol-based disinfectants, such as Preventol (4%), were previously shown to range from 17% to 49% for various strains of coccidia [1]. These results are not in agreement with our findings, since it was observed that cresol effectively inhibited sporulation up to 85.5%. The cresol-based product, Neopredisan 135-1 (R) (NP), was previously shown to inactivate Isospora suis oocysts in vitro, with a concentration of 2-4% able to induce lysis of more than 95% of sporulated oocysts within a contact time of 30 min [9]. Furthermore, these conditions completely destroyed all oocysts after a contact time of 90 min or more [9]. These results were similar to the results observed for cresol. In another study, the ability of 11 disinfectants to inactivate oocysts in rodents was examined, with an efficacy greater than 95% obtained for 3.7% ammonia in a period of 5 min [10]. This result contradicts the data obtained in our study.
The anticoccidial effects of acetic acid at different concentrations in broiler chickens have also been previously evaluated and compared with those of amprolium; it was found that 3% acetic acid had the maximum anticoccidial effects [11]. This study also found that the maximum disinfecting effects were exerted by acetic acid, although this study used 99.95% at a 1:2 dilution. The antimicrobial effects of quaternary salts have also been tested, but none were shown to be effective against either the parasitic protozoan, E. tenella, or the helminth, trichostrongyle nematodes [5]. The present study found that quaternary salts only inhibited sporulation by 13%, even when used at a 10-fold greater concentration than the recommended dose. This study did not observe any disinfectant advantages of using a quaternary ammonium compound, even when it was mixed with aldehyde. Anticoccidial activity of herbal complex was also studied in broiler chickens challenged with Eimeria tenella [12]. Taken together, the present findings indicate that acetic acid, cresol soup and benzene+xylene are good candidates for suppression of E. tenella oocyst sporulation.
Footnotes
The author has no conflict of interest related to this work.
References
- 1.Daugschies A, Bose R, Marx J, Teich K, Friedhoff KT. Development and application of a standardized assay for chemical disinfection of coccidia oocysts. Vet Parasitol. 2002;103:299–308. doi: 10.1016/s0304-4017(01)00581-7. [DOI] [PubMed] [Google Scholar]
- 2.McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams RB. Laboratory tests of phenolic disinfectants as oocysticides against the chicken coccidium Eimeria tenella. Vet Rec. 1997;141:447–448. doi: 10.1136/vr.141.17.447. [DOI] [PubMed] [Google Scholar]
- 4.Rehman TU, Khan MN, Sajid MS, Abbas RZ, Arshad M, Iqbal Z. Epidemiology of Eimeria and associated risk factors in cattle of district Toba Tek Singh, Pakistan. Parasitol Res. 2011;108:1171–1177. doi: 10.1007/s00436-010-2159-5. [DOI] [PubMed] [Google Scholar]
- 5.Williams RB. Tracing the emergence of drug-resistance in coccidia (Eimeria spp.) of commercial broiler flocks medicated with decoquinate for the first time in the United Kingdom. Vet Parasitol. 2006;135:1–14. doi: 10.1016/j.vetpar.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Haug A, Thebo P, Mattsson JG. A simplified protocol for molecular identification of Eimeria species in field samples. Vet Parasitol. 2007;146:35–45. doi: 10.1016/j.vetpar.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Guimaraes JS, Jr, Bogado ALG, Da Cunha TCB, Garcia JL. In vitro evaluation of the disinfection efficacy on Eimeria tenella, unsporulated oocysts isolated from broilers. Rev Bras Parasitol Vet. 2007;16:67–71. [PubMed] [Google Scholar]
- 8.Hilbrich P. Disinfection tests on Eimeria tenella oocytes. Berl Munch Tierarztl Wochenschr. 1975;88:144–148. [PubMed] [Google Scholar]
- 9.Daugschies A, Agneessens J, Goossens L, Mengel H, Veys P. The effect of a metaphylactic treatment with diclazuril (Vecoxan®) on the oocyst excretion and growth performance of calves exposed to a natural Eimeria infection. Vet Parasitol. 2007;149:199–206. doi: 10.1016/j.vetpar.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Ayeni AO, Dingeldein E, Durr U. Studies on the inactivation of coccidian oocysts. Acta Vet Acad Sci Hung. 1972;22:111–122. [PubMed] [Google Scholar]
- 11.Grier N. Synthesis and antimicrobial evaluation of quaternary salts of 4-phenyl-1,2,3,6-tetrahydropyridine and 3,6-dimethyl-6-phenyl-tetrahydro-2H-1,3-oxazine. J Pharm Sci. 1979;68:407–411. doi: 10.1002/jps.2600680404. [DOI] [PubMed] [Google Scholar]
- 12.Zaman MA, Iqbal Z, Abbas RZ, Khan MN. Anticoccidial activity of herbal complex in broiler chickens challenged with Eimeria tenella. Parasitology. 2012;139:237–243. doi: 10.1017/S003118201100182X. [DOI] [PubMed] [Google Scholar]