Abstract
Babesiosis is an emerging tick-borne disease in humans worldwide; however, little is known about the frequency of infection or prevalence of this disease in other parts of the world, excluding North America. In this study, we aimed to investigate Babesia microti infection frequency in a human population in Mongolia. One hundred blood samples were collected from stock farmers living in Khutul city of Selenge province, Mongolia. The sera and DNA from blood samples were evaluated for the presence of B. microti infection by using indirect fluorescent antibody (IFA) tests and PCR. The positive detection rates obtained using the IFA tests and PCR assays were 7% and 3%, respectively. This study is the first to detect of B. microti infections based on antibody seroprevalence or PCR assays for the presence of B. microti DNA in a Mongolian population.
Keywords: Babesia microti, IFA test, PCR, Mongolia
Babesiosis, caused by an intraerythrocytic protozoan parasite, is a common disease in wildlife worldwide and is gaining attention as an emerging tick-borne zoonosis in humans [1,2]. Although approximately 100 Babesia species infect animals, only a few have been reported to infect humans [3,4]. Most cases of human babesiosis have been caused by Babesia divergens, Babesia microti, or Babesia duncani, with the cattle-infecting species B. divergens being the most common in Europe, and the rodent-infecting species B. microti and B. duncani being the most common in North America. Together, these species have caused a significant number of human infections [1,5]. Several cases of B. duncani and B. duncani-type organisms have been identified in patients on the Pacific coast of the United States from northern California to Washington state [6,7]. B. microti is a parasite in wild rodents, and most cases of babesiosis in humans caused by B. microti are transmitted from wild rodents through Ixodes tick bites or inadvertently during blood transfusions [8,9]. In Asia, B. microti-like parasites have caused illnesses in Japan and Taiwan [10,11], and a novel Babesia sp. (KO1) has been identified in South Korea [12].
Mongolia, situated in central Asia between Russia to the north and China to the south, is an agricultural country focused on the livestock industry. Animal husbandry of cattle, sheep, goats, and horses brings many people into contact with ticks while herding the livestock pasturing on steppes. Previous reports have confirmed the presence of Babesia gene fragments from parasitic species in field-collected Dermacentor nuttalli ticks in Mongolia [13], and babesiosis in Mongolian horses is widespread [14]. Also, B. microti DNA was reportedly detected in small mammals in Russia [16] and in ticks of Inner Mongolia [17]. Furthermore, the highest incidence of human Lyme borreliosis transmitted by tick has been reported in Selenge province [15].
Similarly, previous reports of B. microti infection in Mongolia are limited to ticks and animals, and minimal information exists regarding B. microti infection among the Mongolian population involved in the livestock industry, even though they are highly exposed in the B. microti infection. The objective of this study was to serologically and molecularly detect B. microti infection in small stock farmers in Khutul city of Selenge province in northern Mongolia, which is adjacent to neighboring regions, Russia.
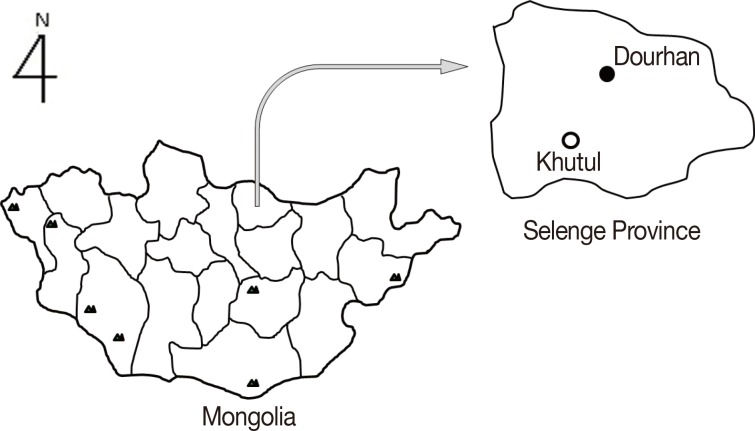
One hundred blood samples were collected in April 2011 from residential stock farmers living in Khutul, near the Russian border (Fig. 1). Genomic DNA was extracted from the blood samples using a DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions and was stored in 100 µl of Tris-EDTA at 20℃. The blood samples were separated by centrifugation at 2,000 g for 10 min, and the collected sera were stored at 20℃ until use.
Fig. 1.
The survey region for Babesia microti infections among stock farmers living in Khutul City in Selenge Province, northern Mongolia.
The DNA samples were used as templates for a nested-PCR to amplify the B. microti 18S ribosomal ribonucleic acid (18S rRNA) gene [18]. The PCR primers included the 1st forward (5'-GCCAGTAGTCATATGCTTGTGTTA-3') and reverse (5'-CTCCTTCCTY TAAGTGATAAGGTTCAC-3') for the initial reactions and 2nd forward (5'-CCATGCAT GTCTWAGTAYAARCTTTTA-3') and reverse (5'-CCTYTAAGTGATAAGGTTCACAA AACTT-3') for the subsequent reactions. Amplifications were performed in 20-µl reactions by using AccuPower PCR master mix (Bioneer, Daejeon, Korea) containing 1 µM each of the 1st forward and reverse primers, sterile water, and 1 µg of DNA sample. The second PCR was then performed using 2 µl of the 1st PCR product and the 2nd PCR primers. The thermal cycler conditions were 94℃ for 5 min; 35 cycles of 94℃ for 60 sec, 59℃ for 45 sec, and 72℃ for 45 sec; and a final extension at 72℃ for 10 min. Amplification products were resolved by electrophoresis on 1.5% agarose gels in Tris-acetate-EDTA buffer and were visualized using Safe-Pinky DNA gel staining solution (GenDepot, Houston, Texas, USA). The positive PCR products were then purified using an agarose gel DNA purification kit (Qiagen) and sequenced using an ABI PRISM 3730xl Analyzer (Applied Biosystems, Foster City, California, USA).
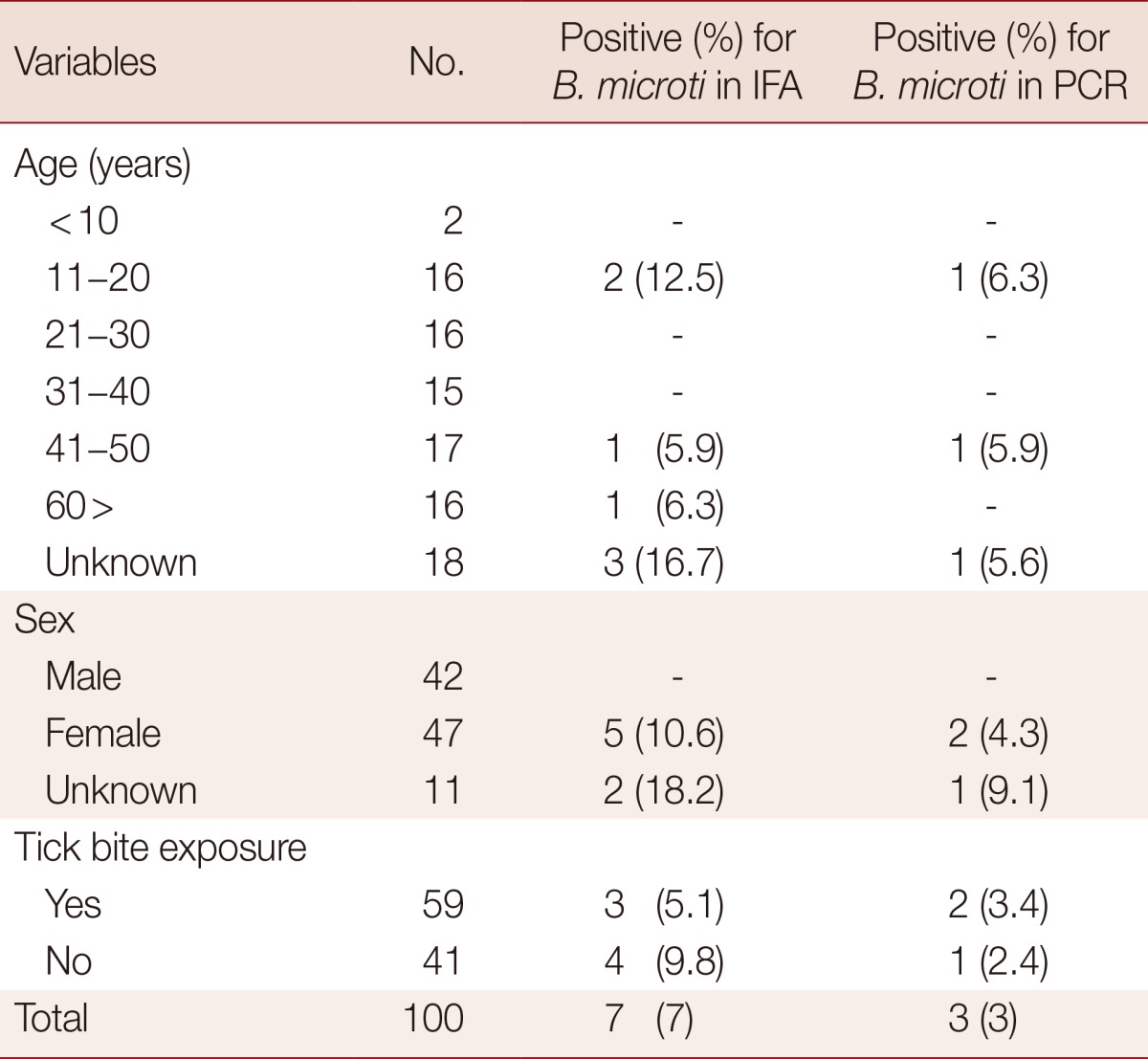
IgG antibodies to B. microti were detected in the serum samples by using an indirect fluorescent antibody (IFA) test kit (Focus Technologies, Inc., Cypress, California, USA). A cut-off value of 1:64 was used in accordance with the manufacturer's recommendations. The serum samples were diluted 64-fold in PBS, and 25 µl of each was placed on slides coated with fixed B. microti antigen derived from RBCs from B. microti-infected hamsters. Proper positive and negative controls in IFA test kit were performed in all testing. The slides were incubated in a humid environment at 37℃ for 30 min and washed 5 times with PBS. Diluted fluorescent-labeled anti-human IgG conjugate was placed on the slides before they were incubated in a humid environment at 37℃ for 30 min. The slides were washed 5 times with PBS and air-dried prior to examination by fluorescence microscopy at×400 magnification. Positive samples were consecutively diluted 2-fold to establish the endpoint titer. The IFA test detected antibodies to B. microti in 7% of the samples, with reported titers ranging between 64 and 1,024. The PCR assay detected B. microti 18S rRNA in 3% of the samples. Three of the samples tested positive in both assays, while 4 were seropositive in the IFA test but negative in the PCR assay (Table 1). The PCR-positive samples had IFA titers of both 512 and 1,024. Sequencing results showed that all PCR-positive samples were similar to B. microti. One of the 2 sequences obtained from positive samples were similar to a Clethrionomys sp. isolated from Russia (AY144693) and the other was similar to a human isolate from China (KF4110827). Five of the 7 B. microti-positive samples were from females, and 2 additional positive results were obtained for subjects of unknown sex (Table 2). No statistical differences were observed between the positive and negative results with regard to age and history of tick bites.
Table 1.
Indirect fluorescent antibody (IFA) and PCR assay results for Babesia microti infections among residents of Khutul city in Selenge province, Mongolia

Table 2.
Positive rate of B. microti in IFA and PCR according to age, gender, and tick bite exposure
Some parasitic Babesia isolates that are extensively distributed in Mongolia are considered pathogenic only in animals, such as B. equi (EMA-1) from pasture ticks and B. microti (BC48) [14,19] and B. bigemina from Mongolian cattle [20]. Different parasitic Babesia spp. can cause babesiosis in humans, resulting in distinct geographical distributions determined by the presence of the intermediate hosts. In Eurasia, babesiosis caused by B. divergens is rare, but it is more lethal to human and bovine hosts [1,21] than North American hosts, infected predominantly by B. microti [22].
Our study is the first to detect B. microti in human subjects in Mongolia by utilizing seroprevalence and molecular assays, and our results may provide insights into the diagnosis and control of this disease in Mongolia. A broader and more detailed survey for other human infections of zoonotic Babesia spp., such as B. divergens and B. duncani is warranted in other regions of Mongolia. Traditionally, the diagnosis of babesiosis in humans has been based on detection of parasites in blood smears from patients with clinical symptoms of the disease. Because the clinical symptoms of babesiosis is usually mild in humans and the infection rate of RBCs is less than 1% [23,24], it is difficult to diagnose babesiosis by microscopic examination. The absence or presence of a laboratory manifestation depends on the level of parasitemia in the host patient.
Although B. microti is widely distributed throughout the world, symptomatic infections in humans are seldom reported [25]. In the present study, none of the individuals who tested positive exhibited the associated clinical symptoms of fever or anemia. Another difficulty with diagnosing Babesia-specific antibodies in human sera has been the lack of commercially available standardized tests for use in diagnostic laboratories [26]. However, the IFA tests now available have been shown to be sensitive, specific, and reproducible methods for detecting B. microti-specific antibodies in human sera [27]. In this study, we evaluated a commercially available IFA test to detect antibodies to B. microti in the sera of individuals. In addition, we screened specifically for the B. microti 18S rRNA gene by analyzing blood samples by using a PCR assay. The results of the IFA tests and PCR assays were similar, suggesting that both methods could be utilized for diagnosing B. microti infections in humans. Poyraz et al. [28] previously reported that no statistical differences exist in the seropositivity between sexes or age groups in Turkey, the same results found in our study. Another study found that the prevalence of antibodies to B. microti or B. divergens was considerably higher in tick-exposed individuals than that found in a control group of healthy blood donors [27].
Interestingly, our study found no significant differences in the prevalence of B. microti infections between individuals who had been bitten by ticks and those who had not. This suggests the need for an investigation into what vector species carrying B. microti, including ticks, are present in Khutul, Mongolia. This study is the first to report B. microti infections among stock farmers in Khutul city of Selenge Province, Mongolia, based on antibody seroprevalence and a PCR assay for the presence of B. microti 18S rRNA in the blood of subjects. Although no correlation was found between positive tests for infection and tick bite exposure, the risk of infection from tick-borne diseases may still exist in this region. The IFA test and PCR assay proved to be useful tools for screening of B. microti infections, and further serological and molecular epidemiological surveys should be performed with larger sample sizes throughout Mongolia. These studies should be conducted in conjunction with an investigation of the prevalence of ticks and rodents in order to determine which species is the primary B. microti infection vector in Mongolia.
ACKNOWLEDGMENTS
This research was performed in collaboration with the Korea CDC and Mongolia NCCD and funded by a grant (4847-302-210-13, 2011) from the Korea National Institute of Health, Korea Centers for Disease Control and Prevention.
Footnotes
We have no conflict of interest related to this study.
References
- 1.Homer MJ, Aguilar-Delfin I, Telford SR, 3rd, Krause PJ, Persing DH. Babesiosis. Clin Microbiol Rev. 2000;13:451–469. doi: 10.1128/cmr.13.3.451-469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herwaldt BL, Caccio S, Gherlinzoni F, Aspock H, Slemenda SB, Piccaluga P, Martinelli G, Edelhofer R, Hollenstein U, Poletti G, Pampiglione S, Loschenberger K, Tura S, Pieniazek NJ. Molecular characterization of a non-Babesia divergens organism causing zoonotic babesiosis in Europe. Emerg Infect Dis. 2003;9:942–948. doi: 10.3201/eid0908.020748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spielman A. Human babesiosis on Nantucket Island: transmission by nymphal Ixodes ticks. Am J Trop Med Hyg. 1976;25:784–787. doi: 10.4269/ajtmh.1976.25.784. [DOI] [PubMed] [Google Scholar]
- 4.Vannier E, Gewurz BE, Krause PJ. Human babesiosis. Infect Dis Clin North Am. 2008;22:469–488. doi: 10.1016/j.idc.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunfeld KP, Brade V. Zoonotic Babesia: possibly emerging pathogens to be considered for tick-infested humans in Central Europe. Int J Med Microbiol. 2004;293(supp. 37):93–103. doi: 10.1016/s1433-1128(04)80014-7. [DOI] [PubMed] [Google Scholar]
- 6.Persing DH, Herwaldt BL, Glaser C, Lane RS, Thomford JW, Mathiesen D, Krause PJ, Phillip DF, Conrad PA. Infection with a Babesia-like organism in northern California. N Engl J Med. 1995;332:298–303. doi: 10.1056/NEJM199502023320504. [DOI] [PubMed] [Google Scholar]
- 7.Conrad PA, Jemtrup AM, Carreno RA, Thomford J, Wainwright K, Eberhard M, Quick R, Telford SR, 3rd, Herwaldt BL. Description of Babesia duncani n.sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int J Parasitol. 2006;36:779–789. doi: 10.1016/j.ijpara.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Leiby DA. Babesiosis and blood transfusion: flying under the radar. Vox Sang. 2006;90:157–165. doi: 10.1111/j.1423-0410.2006.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popovsky MA. Transfusion-transmitted babesiosis. Transfusion. 1991;31:296–298. doi: 10.1046/j.1537-2995.1991.31491213290.x. [DOI] [PubMed] [Google Scholar]
- 10.Shih CM, Liu LP, Chung WC, Ong SJ, Wang CC. Human babesiosis in Taiwan: asymptomatic infection with a Babesia microti-like organism in a Taiwanese woman. J Clin Microbiol. 1997;35:450–454. doi: 10.1128/jcm.35.2.450-454.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito-Ito A, Tsuji M, Wei Q, He S, Matsui T, Kohsaki M, Arai S, Kamiyama T, Hioki K, Ishihara C. Transfusion-acquired, autochthonous human babesiosis in Japan: isolation of Babesia microti-like parasites with hu-RBC-SCID mice. J Clin Microbiol. 2000;38:4511–4516. doi: 10.1128/jcm.38.12.4511-4516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JY, Cho SH, Joo HN, Tsuji M, Cho SR, Park IJ, Chung GT, Ju JW, Cheun HI, Lee HW, Lee YH, Kim TS. First case of human babesiosis in Korea: detection and characterization of a novel type of Babesia sp. (KO1) similar to ovine Babesia. J Clin Microbiol. 2007;45:2084–2087. doi: 10.1128/JCM.01334-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Battsetseg B, Xuan X, Ikadai H, Bautista JL, Byambaa B, Boldbaatar D, Battur B, Battsetseg G, Batsukh Z, Igarashi I, Nagasawa H, Mikami T, Fujisaki K. Detection of Babesia caballi and Babesia equi in Dermacentor nuttalli adult ticks. Int J Parasitol. 2001;31:384–386. doi: 10.1016/s0020-7519(01)00120-5. [DOI] [PubMed] [Google Scholar]
- 14.Battsetseg B, Lucero S, Xuan X, Claveria F, Byambaa B, Battur B, Boldbaatar D, Batsukh Z, Khaliunaa T, Battsetseg G, Igarashi I, Nagasawa H, Fujisaki K. Detection of equine Babesia spp. gene fragments in Dermacentor nuttalli Olenev 1929 infesting mongolian horses, and their amplification in egg and larval progenies. J Vet Med Sci. 2002;64:727–730. doi: 10.1292/jvms.64.727. [DOI] [PubMed] [Google Scholar]
- 15.Scholz HC, Margos G, Derschum H, Speck S, Tserennorov D, Erdenebat N, Undraa B, Enkhtuja M, Battsetseg J, Otgonchimeg C, Otgonsuren G, Nymadulam B, Römer A, Thomas A, Essbauer S, Wölfel R, Kiefer D, Zöller L, Otgonbaatar D, Fingerle V. High prevalence of genetically diverse Borrelia bavariensis-like strains in Ixodes persulcatus from Selenge Aimag, Mongolia. Ticks Tick Borne Dis. 2013;4:89–92. doi: 10.1016/j.ttbdis.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Rar VA, Epikhina TI, Livanova NN, Pukhovskaia NM, Vysochina NP, Ivanov LI. Detection of Babesia spp. DNA in small mammals and ixodic ticks on the territory of north Ural, west Siberia and far east of Russia. Mol Gen Mikrobiol Virusol. 2010;3:26–30. [PubMed] [Google Scholar]
- 17.Hao GF, Li H, Sun Y, Ge RP, Qia GQ, Li B, Tian WZ, Shi NX, Yang XY. Detection of tick and tick-borne pathogen in some ports of Inner Mongolia. Zhonghua Liu Xing Bing Xue Za Zhi. 2009;30:365–367. [PubMed] [Google Scholar]
- 18.Zamoto A, Tsuji M, Kawabuchi T, Wei Q, Asakawa M, Ishihara C. U.S.-type Babesia microti isolated from small wild mammals in Eastern Hokkaido, Japan. J Vet Med Sci. 2004;66:919–926. doi: 10.1292/jvms.66.919. [DOI] [PubMed] [Google Scholar]
- 19.Avarzed A, De Waal DT, Igarashi I, Saito A, Oyamada T, Toyoda Y, Suzuki N. Prevalence of equine piroplasmosis in Central Mongolia. Onderstepoort J Vet Res. 1997;64:141–145. [PubMed] [Google Scholar]
- 20.Sivakumar T, Altangerel K, Battsetseg B, Battur B, Aboulaila M, Munkhjargal T, Yoshinari T, Yokoyama N, Igarashi I. Genetic detection of Babesia bigemina from Mongolian cattle using apical membrane antigen-1 gene-based PCR assay. Vet Parasitol. 2012;187:17–22. doi: 10.1016/j.vetpar.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Malandrin L, L Hostis M, Chauvin A. Isolation of Babesia divergens from carrier cattle blood using in vitro culture. Vet Res. 2004;35:131–139. doi: 10.1051/vetres:2003047. [DOI] [PubMed] [Google Scholar]
- 22.Krause PJ, Telford SR, 3rd, Spielman A, Sikand V, Ryan R, Christianson D, Burke G, Brassard P, Pollack R, Peck J, Persing DH. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA. 1996;275:1657–1660. [PubMed] [Google Scholar]
- 23.Vannier E, Krause PJ. Human babesiosis. N Engl J Med. 2012;366:2397–2407. doi: 10.1056/NEJMra1202018. [DOI] [PubMed] [Google Scholar]
- 24.Welc-Faleciak R, Bajer A, Bednarska M, Paziewska A, Sinski E. Long term monitoring of Babesia microti infection in BALB/c mice using nested PCR. Ann Agric Environ Med. 2007;14:287–290. [PubMed] [Google Scholar]
- 25.Sun Y, Liu G, Yang L, Xu R, Cao W. Babesia microti-like rodent parasites isolated from Ixodes persulcatus (Acari: Ixodidae) in Heilongjiang Province, China. Vet Parasitol. 2008;156:333–339. doi: 10.1016/j.vetpar.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Hunfeld KP, Lambert A, Kampen H, Albert S, Epe C, Brade V, Tenter AM. Seroprevalence of Babesia infections in humans exposed to ticks in midwestern Germany. J Clin Microbiol. 2002;40:2431–2436. doi: 10.1128/JCM.40.7.2431-2436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krause PJ, Telford SR, 3rd, Ryan R, Conrad PA, Wilson M, Thomford JW, Spielman A. Diagnosis of babesiosis: evaluation of a serologic test for the detection of Babesia microti antibody. J Infect Dis. 1994;169:923–926. doi: 10.1093/infdis/169.4.923. [DOI] [PubMed] [Google Scholar]
- 28.Poyraz O, Gunes T. Seroprevalance of Babesia microti in humans living in rural areas of the Sinop region. Turkiye Parazitol Derg. 2010;34:81–85. [PubMed] [Google Scholar]