Abstract
The Deakin/Graeff hypothesis proposes that different subpopulations of serotonergic neurons through topographically organized projections to forebrain and brainstem structures modulate the response to acute and chronic stressors, and that dysfunction of these neurons increases vulnerability to affective and anxiety disorders, including Panic Disorder. We outline evidence supporting the existence of a serotonergic system originally discussed by Deakin/Graeff that is implicated in the inhibition of panic-like behavioral and physiological responses. Evidence supporting this panic inhibition system comes from the following observations: 1) serotonergic neurons located in the ‘ventrolateral dorsal raphe nucleus (DRVL) as well as the ventrolateral periaqueductal gray (VLPAG) inhibit dorsal periaqueductal gray-elicited panic-like responses; 2) chronic, but not acute, antidepressant treatment potentiates serotonin’s panicolytic effect; 3) contextual fear activates a central nucleus of the amygdala-DRVL/VLPAG circuit implicated in mediating freezing and inhibiting panic-like escape behaviors; 4) DRVL/VLPAG serotonergic neurons are central chemoreceptors and modulate the behavioral and cardiorespiratory response to panicogenic agents such as sodium lactate and CO2. Implications of the panic inhibition system are discussed.
Keywords: acid-sensing ion channels, amygdala, carbon dioxide, corticotropin-releasing hormone, dorsal raphe nucleus, panic attack, Panic Disorder, periaqueductal gray, serotonin, sodium lactate, stress, TASK
1. Introduction to Panic Disorder
1.1. Symptomatology and epidemiology of Panic Disorder
Panic Disorder (PD) is classified by the occurrence of recurrent unexpected panic attacks that are not explained by substance use or another medical condition, and are followed by maladaptive changes in behavior and cognition for at least one month. Following a panic attack (PA), individuals may ruminate over the consequences of future attacks (e.g., losing control, going crazy, and adverse health conditions), which may lead the individual to avoid situations or settings in which a PA previously occurred or where help may be unavailable. The PA can be expected (i.e., cued) or unexpected and is characterized as a sudden episode of intense fear or discomfort that contains four or more of the following 13 cognitive and physical symptoms: 1) palpitations, pounding heart, or accelerated heart rate, 2) sweating, 3) trembling or shaking, 4) sensations of shortness of breath or smothering, 5) feelings of choking, 6) chest pain or discomfort, 7) nausea or abdominal distress, 8) feeling dizzy, unsteady, light-headed, or faint, 9) chills or heat sensations, 10) paresthesias (numbness or tingling sensations), 11) derealization (feelings of unreality) or depersonalization (being detached from oneself), 12) fear of losing control or “going crazy”, and 13) fear of dying (American Psychiatric Association, 2013).
Epidemiological surveys across the United States and many European countries suggest a lifetime prevalence of 2-5% for PD (Angst, 1998;Goodwin et al., 2005;Kessler et al., 2006;Kessler et al., 2005a;Kessler et al., 2012), although European countries, relative to the United States, have lower prevalence. Moreover, according to the United States National Comorbidity Survey Replication findings the lifetime prevalence for having an isolated PA is a staggering 22.7% (Kessler et al., 2006). Females are more likely to suffer from PD and PAs, although the gender difference is less prominent for attacks (Gater et al., 1998;Kessler et al., 2005b). There is also evidence for considerable cultural differences in the prevalence of PD and symptomatology of PAs (Craske et al., 2010;Lewis-Fernandez et al., 2010). Individuals diagnosed with PD and PAs in general have comorbidity with other anxiety disorders (e.g., generalized anxiety disorder (GAD), separation anxiety disorder (SAD), and particularly with agoraphobia) and affective disorders, impulse control disorders, or substance abuse and dependence disorders; consequently, panic vulnerability may be a component of many anxiety, affective, and substance abuse and dependence disorders (Biederman et al., 2001;Biederman et al., 2006;Bienvenu et al., 2006;Craske et al., 2010;Kessler et al., 2005b;Klein, 1993;Noyes, Jr. et al., 1992;Noyes, Jr., 2001;Preter and Klein, 2008).
In addition to psychiatric comorbidity, PD is comorbid with a number of medical disorders such as cardiac, respiratory, and thyroid diseases (Simon and Fischmann, 2005). Because PAs can be present in all psychiatric disorders and some medical conditions, the recently released 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) now lists PAs as a specifier, i.e., a prognostic factor used to aid in the diagnosis of the severity, course, and comorbidity of all DSM-V disorders (American Psychiatric Association, 2013). Clearly, there is significant demand and significant unmet need for the treatment of panic disorder; considering the ubiquity of PAs, future research should not only provide insight into the neurobiological mechanisms of PD and PAs, but also other comorbid psychiatric and medical disorders.
1.2. Experimentally-induced panic: putative panicogenic agents
Unlike other mental disorders, PD is relatively unique as its pathognomonic feature, the PA, can be experimentally-induced in PD patients, but rarely in healthy individuals, by exposure to a rapidly expanding list of putative panicogenic agents with diverse pharmacological profiles, including sodium lactate (Pitts, Jr. and McClure, Jr., 1967), carbon dioxide (CO2; Gorman et al., 1984), cholecystokinin tetrapeptide (CCK-4; Bradwejn et al., 1991;Bradwejn et al., 1992), yohimbine (α2-receptor antagonist)(Charney et al., 1987), sodium bicarbonate (Gorman et al., 1989a), caffeine (Charney et al., 1985), isoproterenol hydrochloride (Nesse et al., 1984;Rainey, Jr. et al., 1984), meta-chlorophenylpiperazine (van der Wee et al., 2004;m-CPP, 5-HT2C agonist; van Veen et al., 2007), and fenfluramine (a serotonin-releasing agent; Targum and Marshall, 1989). At the very least, a well-validated panicogenic agent should fulfill the following criteria: 1) the agent should be safe for use in humans; 2) the agent should specifically and reliably induce PAs in PD patients, but not healthy individuals; 3) the induced PA should be transient and reversible; 4) the PA should be blocked by therapeutics known to be effective in treating naturally occurring PAs in PD (e.g., selective-serotonin reuptake inhibitors, SSRIs); and 5) the induced PA should share the symptomatology of naturally occurring PAs (Griez and Schruers, 1998;Guttmacher et al., 1983;Kellner, 2011).
Based on these criteria and the pharmacological reactivity of the various challenges, others have argued that some of these compounds may elicit a behavioral, cognitive and physiological response more akin to anticipatory or conflict anxiety, fear, stress, or pain (Griez and Schruers, 1998;Kellner, 2011;Klein, 1993). Among these candidate panicogenic agents, the sodium lactate and CO2 challenges are the most widely used and well-validated experimental methods to induce panic (Amaral et al., 2013;Griez and Schruers, 1998;Klein, 1993); the CCK-4 challenge shows promise as an experimental model of PAs, but several issues remain unresolved (Kellner, 2011).
1.3. Towards a Panic Disorder hypothesis: hyperventilation theories, exaggerated fear networks, and false suffocation alarms
A unifying feature of these experimentally-induced panic attacks and naturally occurring panic attacks is that the individual often experiences respiratory abnormalities, including dyspnea (air hunger, breathlessness), hyperventilation and increased minute ventilation (Sinha et al., 2000). These reports, taken together with evidence showing high comorbidity between PD and a number of respiratory disorders (e.g., asthma, COPD; Simon and Fischmann, 2005), led many to postulate respiratory dysfunction as having a central role in PD. Ley’s hyperventilation theory of panic (Ley, 1985) and later updated as the dyspnea/suffocation theory of panic (Ley, 1989), for example, proposed that acute hyperventilation triggered PAs (or a subtype of hyperventilatory PAs, see Ley, 1992) and that the dyspnea (air hunger, breathlessness) experienced during the PA gave rise to the intense feelings of fear often accompanying the attack. In contrast, Klein’s false suffocation alarm theory (1993) compellingly argued the hyperventilation accompanying panic and the chronic hyperventilation commonly observed in PD patients was caused by the dysfunction of a putative suffocation alarm system, which rendered the system hypersensitive to physiological changes predicting suffocation, including changes in arteriole partial pressure of CO2 (pCO2) or lactate levels, and when triggered resulted in a PA. The chronic hyperventilation was thought to be a compensatory mechanism in order to keep pCO2 levels sufficiently low so as not to trigger the hypersensitive suffocation alarm. Preter and Klein (2008) recently updated the false suffocation alarm theory to include a role for endogenous opioid dysfunction in reducing the sensitivity threshold of the suffocation alarm.
Attempting to explain clinical observations that both pharmacological intervention and cognitive behavioral therapy are successful in treating PD, Gorman and colleagues (1989b) posited a neuroanatomical hypothesis of panic consisting of a network of cortical, limbic and brainstem structures implicated in mediating the phobic avoidance, anticipatory anxiety and panic attacks observed in PD patients; the neuroanatomical hypothesis of PD was revised based on preclinical rodent models identifying the neural structures mediating fear-conditioning and avoidance behavior (Gorman et al., 2000). As Klein has repeatedly indicated (Klein, 1993;Klein, 2002;Preter and Klein, 2008), although panic and fear share many characteristics they have important physiological distinctions such as the lack of HPA-axis activation in panic (Hollander et al., 1989;Kellner and Wiedemann, 1998;Levin et al., 1987;Woods et al., 1988), which is a common neuroendocrine response to fear-related stimuli like exposure to a predator (Blanchard et al., 1998), predator odor (Masini et al., 2005;Masini et al., 2006), or conditioned fear (Cordero et al., 1998), and the presence of dyspnea in panic, which rarely occurs in natural fear responses (Klein, 1993;Preter and Klein, 2008). Although a comprehensive theory of panic disorder is still being formulated, as we shall see next, there is unequivocal neuropsychological evidence for the dissociation of fear and panic.
1.4. Bilateral focal lesions of the amygdala lead to increased vulnerability to CO2-evoked panic
Urbach-Weith disease (also called lipoid proteinosis or hyalinosis cutis et mucosae) is an extremely rare hereditary disease characterized by the infiltration of a hyaline-like material (lipoid protein) into the skin, mucous membranes, and virtually every organ, including the brain (Emsley and Paster, 1985). As the disease progresses to internal organs, an estimated 52% of cases have bilateral calcification of the medial temporal lobes (Emsley and Paster, 1985) resulting in considerable neuropsychiatric abnormalities (Thornton et al., 2008). In some of these cases, the bilateral calcification is restricted to the amygdala and even select subregions, providing unique insights into the role of the amygdala in both fear and panic processes. Human patients with focal bilateral amygdala lesions resulting from Urbach-Wiethe disease do not condition to aversive stimuli (Bechara et al., 1995), fail to recognize fearful faces (Adolphs and Tranel, 2000), and demonstrate an absence of fear during exposure to fear-provoking stimuli, including life-threatening traumatic events (Feinstein et al., 2011). However, a recent study found that these patients have increased vulnerability to CO2-evoked panic (Feinstein et al., 2013), suggesting that 1) the amygdala is not necessary for CO2-evoked panic, and 2) an intact amygdala may normally inhibit panic. Furthermore, the absence of prior spontaneous panic attacks in the lesion patients (Feinstein et al., 2013) suggests that amygdala dysfunction alone is not sufficient to cause spontaneous panic attacks or panic disorder.
1.5. Functional topography of midbrain serotonergic systems: recent advances and new opportunities
After Klein’s (1964) remarkable discovery that panic attacks were responsive to treatment with the tricyclic antidepressant, imipramine, but not low doses of benzodiazepines, which were effective at treating Generalized Anxiety Disorder (GAD), the path was paved for PD’s recognition as a distinct nosological class of anxiety in the 3rd edition of the DSM (American Psychiatric Association, 1980). Further, this led to an accumulation of clinical data illustrating the therapeutic effects on panic of a number of drugs that target the serotonergic system (Bell and Nutt, 1998b;Mochcovitch and Nardi, 2010;Nutt, 2005) and the inevitable hypothesis that dysfunctional serotonergic systems may be central to the etiology or pathophysiology of PD, or both (Bell and Nutt, 1998a;Graeff, 2004).
In 1991, Bill Deakin and Frederico Graeff first proposed the hypothesis that different subpopulations of serotonergic neurons in the dorsal raphe nucleus (DR) and median raphe nucleus (MnR), through topographically organized projections to different brain targets, had unique functions that were relevant to the pathophysiology of anxiety and affective disorders (Deakin and Graeff, 1991;Graeff et al., 1996). These different subpopulations of serotonergic neurons included 1) serotonergic neurons within the DR projecting to the dorsal periaqueductal gray (DPAG) that function to inhibit panic-/escape-like physiological and behavioral responses, 2) serotonergic neurons within the DR projecting to the amygdala, that facilitate conditioned fear and conflict anxiety-like responses, and 3) serotonergic neurons within the MnR that increase stress resilience and mediate antidepressant-like effects (Deakin and Graeff, 1991;Graeff et al., 1996).
We and others have made significant advances in testing this hypothesis, and have found evidence supporting each of the three systems originally hypothesized by Deakin and Graeff. We and others have found evidence for the following systems: 1) Panic inhibition system, a serotonergic system in the ventrolateral part of the dorsal raphe nucleus (DRVL)/ventrolateral periaqueductal gray (VLPAG), projecting to the DPAG, that a) is activated by panicogenic agents, including sodium lactate (Johnson et al., 2008) and hypercapnia (elevated atmospheric CO2; Johnson et al., 2005), b) selectively responds with increased tph2 mRNA expression following amygdala priming (Donner et al., 2012), a model of vulnerability to sodium lactate-induced panic attacks (Johnson et al., 2013;Sajdyk et al., 1999), and c) is selectively dysregulated in an animal model of vulnerability to lactate-induced panic-like responses (Johnson et al., 2004;Johnson et al., 2007). 2) Conflict anxiety facilitation system, a serotonergic system in the midline dorsal and caudal parts of the dorsal raphe nucleus (DRD/DRC), projecting to the basolateral nucleus of the amygdala (BLA; Abrams et al., 2005), that is activated by anxiogenic drugs (Abrams et al., 2005), anxiety-related neuropeptides (Staub et al., 2005;Staub et al., 2006), and anxiety-provoking stimuli (Spannuth et al., 2011); this system is sensitized following inescapable shock in a model of learned helplessness (Rozeske et al., 2011). 3) Stress resilence/antidepressant system, a serotonergic system in the interfascicular part of the dorsal raphe nucleus (DRI)/MnR, projecting to the hippocampus (Kohler and Steinbusch, 1982) and medial prefrontal cortex (Porrino and Goldman-Rakic, 1982), that is activated in association with antidepressant-like behavioral responses (Lowry et al., 2007). These advances in defining topographically organized subpopulations of serotonergic neurons provide opportunities to define, in detail, how these different serotonergic systems are controlled by afferent control mechanisms, and how these systems relate to emotional behaviors relevant to anxiety and affective disorders.
This review will focus on the panic inhibition system. Evidence for a panic inhibition system involving a circuit from the CE-DRVL/VLPAG-dorsal periaqueductal gray (DPAG) (Figure 1) comes from the following observations: 1) serotonin inhibits DPAG-evoked behavioral and sympathoexcitatory responses that mimic the symptomatology of PAs, and chronic, but not acute, antidepressants facilitate serotonergic inhibition of panic-like responses; 2) the DRVL/VLPAG 5-HT neurons are chemosensitive and form an important component of a sympathomotor command center capable of modulating the behavioral, cognitive, cardiovascular, and respiratory aspects of PD; 3) CRH neurons in the CE activate DRVL 5-HT neurons via CRHR2 receptors; and 4) panicogenic agents such as sodium lactate and CO2 activate the panic inhibition system. A major aim will be to determine how these serotonergic neurons are differentially controlled, and how they influence panic-like responses.
Figure 1.
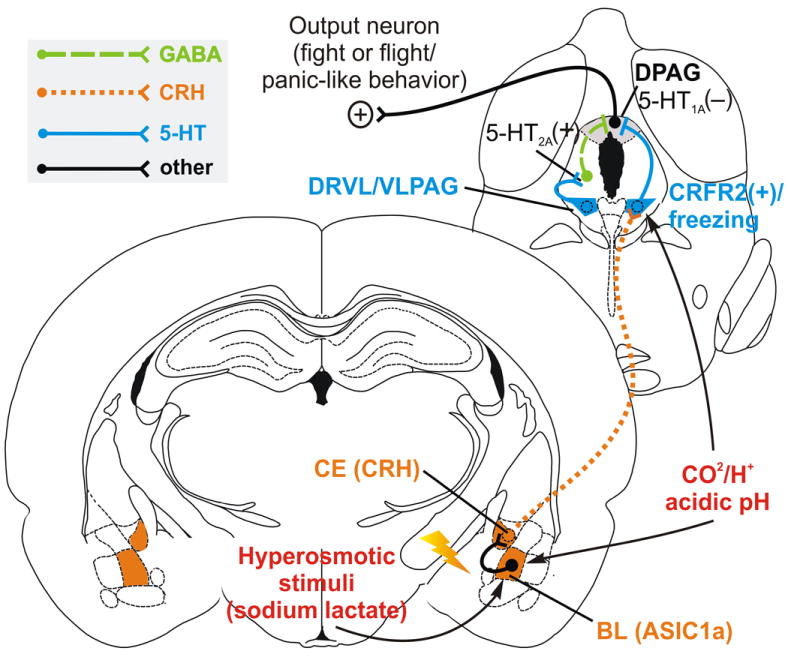
Hypothetical model illustrating amygdala inhibition of panic via activation of a serotonergic panic inhibition system in the DRVL/VLPAG. Stress-induced elevation of corticotropin-releasing factor (CRF) within the basolateral amygdala (BL) acts on CRF receptor type 1 receptors (CRFR1), leading to hyperexcitability of BL projection neurons that target the central amygdaloid nucleus (CE). Projections from the CE release CRF into the DRLV/VLPAG, activating excitatory CRFR2 in the DR, resulting in activation of DRVL/VLPAG serotonergic neurons. Increased serotonergic signaling from the DRVL/VLPAG on postsynaptic 5-HT1A and 5-HT2A receptors inhibit the dorsal PAG (DPAG), inhibiting panic/escape-like fight-or-flight responses. Panicogenic agents including CO2 and sodium lactate activate the amygdala, activating the panic inhibition system. Abbreviations: 5-HT1A, 5-Hydryoxytryptamine type 1A receptor; 5- HT2A, 5-Hydryoxytryptamine type 2A receptor; ASIC1a, acid sensing ion channel 1a; BL, basolateral amygdala; CE, central nucleus of the amygdala; CO2, carbon dioxide; CRH, corticotropin-releasing hormone; CRHR2, corticotropin-releasing hormone type 2 receptor; DPAG, dorsal periaqueductal gray; DRVL/VLPAG, ventrolateral part of the dorsal raphe nucleus/ventrolateral periaqueductal gray; GABA, γ-aminobutyric acid; H+, hydrogen ion. Coronal brain diagrams reproduced from Paxinos and Watson (1998) by permission of Elsevier.
2. A putative panic inhibition system involving the amygdala, dorsal raphe nucleus and periaqueductal gray
2.1. The DPAG mediates panic-/escape-like physiological and behavioral responses
As mentioned above, patients with focal bilateral amygdala lesions resulting from Urbach-Wiethe disease have increased vulnerability to CO2-evoked panic (Feinstein et al., 2013), suggesting that the neural mechanisms underlying CO2-evoked panic are downstream of the amygdala. Furthermore, the absence of prior spontaneous panic attacks in the lesion patients (Feinstein et al., 2013) suggests that amygdala dysfunction alone is not sufficient to cause spontaneous panic attacks or panic disorder. Identifying brain regions that play a role in induction of panic attacks remains an important objective. A key neural substrate for induction of panic-like physiological and behavioral responses is the DPAG. Studies by Fernandez de Molina and Hunsperger demonstrated that activation of the DPAG induces defensive responses in cats, such as aggressive behavior, or escape, similar to the defensive responses when confronted with predators (Fernandez de Molina and Hunsperger, 1962;Fernandez de Molina and Hunsperger, 1959). Stimulation of the DPAG in rats is aversive as they readily learn to switch off the stimulus (Schenberg et al., 2001). Subsequent studies in rodents have demonstrated that microstimulation of the DPAG induces sympathoexciatory responses (i.e., tachycardia and hypertension) (Keay and Bandler, 2001;Schenberg et al., 1993) as well as escape or flight behaviors (Beckett and Marsden, 1997;Beckett et al., 1992;Jacob et al., 2002).
In humans, electrostimulation of the DPAG induces intense emotions including anxiety, panic, terror, and feelings of imminent death (Nashold, Jr. et al., 1969). Consistent with these early stimulation studies in humans, positron emission tomographic (PET) imaging studies have demonstrated that a distributed system, including the insular cortex, amygdala and the tectum of the midbrain (DPAG and deep collicular layers) respond with increased blood flow during sodium lactate-induce panic attacks (Reiman et al., 1989). A similar network of limbic structures (e.g., anterior insula, amygdala, PAG) is activated during hypercapnia and dyspnea (i.e., air hunger) caused by repeated inhalation of 8% CO2 (Brannan et al., 2001). The similarities between DPAG-evoked defensive behaviors and panic attacks in humans have led to the hypothesis that DPAG-evoked innate responses are a valid model of human panic attacks (Deakin and Graeff, 1991;Jenck et al., 1995;Schenberg et al., 2001). Indeed, stimulation of the DPAG in rodents fails to alter stress-related HPA axis hormones such as adrenocorticotropic hormone (ACTH) (Schenberg et al., 2008), a relevant physiological finding considering the absence of HPA axis activation during CO2-and lactate-induced PAs (Hollander et al., 1989;Kellner and Wiedemann, 1998;Levin et al., 1987;Woods et al., 1988). Supporting the idea that DPAG-evoked sympathoexcitatory responses and flight behaviors may be relevant for panic, central and peripheral application of the panicogenic agent, CCK-4, enhances the DPAG-evoked sympathoexcitatory responses and flight, respectively, suggesting alternate pathways for CCK-4 modulation of DPAG-evoked tachycardia and flight (Mongeau and Marsden, 1997b). Likewise, predator-elicited flight, a DPAG mediated behavior, is increased by panicogenic agents and reduced by pharmacological agents successful in treating PD (Griebel et al., 1996a). These studies in rodents and humans are consistent with the hypothesis that the DPAG elicits many of the behavioral and physiological phenomena that are characteristic of PAs, and that DPAG-elicited responses may be relevant for studying the neurobiology of PD.
Since stimulation of the DPAG elicits the entire spectrum of defensive behaviors, e.g., aggression, flight, freezing, jumping, rearing, squealing, and vocalization (Beckett and Marsden, 1997;Beckett et al., 1992;Brandao et al., 2008;Fanselow, 1994;Sandner et al., 1987) and physiological responses, e.g., tachycardia, hypertension (Keay and Bandler, 2001;Schenberg et al., 1993), that resemble predator exposure (Blanchard et al., 1986b;Blanchard et al., 1998;Blanchard and Blanchard, 1989) and human panic attacks (Del-Ben and Graeff, 2009;Schenberg et al., 2001), it is important to note that the subdivisions of the PAG are highly interconnected and the DPAG provides substantial projections to the DRVL/VLPAG region (Cameron et al., 1995a;Cameron et al., 1995b;Jansen et al., 1998;Oka et al., 2008). This raises the possibility that the DPAG may inhibit the activity of the DRVL/VLPAG serotonergic panic inhibition system. Consistent with this notion is the hypothesis that the DPAG and DRVL/VLPAG regions have opposing functions, with the former eliciting active coping responses and the latter coordinating reactive (passive) coping strategies (Fanselow, 1994;Jansen et al., 1998;Keay and Bandler, 2001;Vianna and Brandao, 2003). Considering these coping strategies often evoke mutually exclusive behavioral and physiological responses (e.g., flight vs. freeze or tachycardia vs. bradycardia), the idea of the DPAG inhibiting the panic inhibition system merits further investigation.
2.2. Evidence for a suffocation alarm system in the PAG
Further support for the PAG as a key structure in panic-like physiological and behavioral responses comes from studies that show activation of PAG central chemoreceptors following localized administration of potassium cyanide (KCN) increases c-Fos labeling throughout the PAG (Hayward and Von Reitzenstein, 2002) and evokes behavioral arousal, cardiovascular responses, and hyperventilation (Franchini et al., 1997;Franchini and Krieger, 1993). Local application of KCN into the PAG activates PAG chemoreceptors and elicits chemoreflex responses through its inhibition of mitochondrial cytochrome c oxidase; therefore, KCN is a potent inhibitor of cellular respiration, in effect depriving the cell of utilizing oxygen (i.e., anoxia). The added benefit of using KCN, rather than hypoxic conditions, is its selectivity when applied locally because it eliminates the possiblility of activating peripheral afferents (e.g., baroreceptors), which, in turn, could activate PAG central chemoreceptors (Hayward and Von Reitzenstein, 2002). On the other hand, hypercapnia caused by exposure to CO2 appears to selectively activate a subpopulation of PAG neurons in the caudal VLPAG (Johnson et al., 2010a;Teppema et al., 1997).
In order to better delineate the role of the PAG-evoked responses to anoxia and hypercapnia, an elegant study by Schenberg and colleagues (2012) investigated the effects of administration of either CO2, or KCN, or a combination of both on spontaneous or DPAG-stimulated behavior in animals with or without prior electrolytic lesion of the DPAG. Slow infusions of low doses of KCN alone evoked spontaneous defensive behaviors and potentiated DPAG-stimulated flight behaviors (e.g., galloping), and the effects of KCN were blocked by prior DPAG lesion; conversely, CO2 alone increased behavioral arousal and attenuated DPAG-stimulated behaviors, possibly due to activation of neurons in the VLPAG and adjacent DRVL (Teppema et al., 1997) that are known to inhibit DPAG output (see section 3 in this review and also; Johnson et al., 2004;Pobbe and Zangrossi, Jr., 2005;Schimitel et al., 2012;Stezhka and Lovick, 1994). Moreover, CO2 given in combination with KCN did not block KCN-evoked behaviors, which would be predicted given the antagonistic effects of CO2 or KCN alone, but rather paradoxically, CO2 facilitates KCN-evoked behaviors (Schimitel et al., 2012). As suggested by the authors, these data provide evidence that the PAG may be the neuroanatomical substrate for Klein’s suffocation alarm system as this region contains an anoxia-sensitive alarm system that is hypersensitive to concomitant hypercapnia resulting from CO2 exposure (Schimitel et al., 2012).
2.3. Serotonin acts in the DPAG to inhibit panic-/escape-like physiological and behavioral responses
According to the Deakin/Graeff Hypothesis (Deakin and Graeff, 1991;Graeff et al., 1996), serotonergic systems arising from the DR and projecting to the amygdala facilitate conditioned fear and conflict anxiety-like behaviors, while serotonergic systems arising from the DR and projecting to the DPAG inhibit innate panic-/escape-like behaviors. Stimulation of the DR, including the DRVL/VLPAG, DRV, and DRD subregions, increases 5-HT 14-fold in the DPAG in addition to blocking escape in the elevated T-maze (Viana et al., 1997). The anti-panic effects appear to be mediated by 5-HT1A and 5-HT2A receptors as intra-DPAG microinjections of antagonists at these receptors block the effects of DR stimulation on escape (Pobbe and Zangrossi, Jr., 2005). Intra-DPAG injections of 5-HT1A and 5-HT2A receptor agonists (de Bortoli et al., 2008;de Bortoli et al., 2006;Jacob et al., 2002;Zanoveli et al., 2005;Zanoveli et al., 2007), but not 5-HT2C receptor agonists (Yamashita et al., 2011), inhibit escape behaviors, either following electrical stimulation of the DPAG, or as assessed in intact, unstimulated, rats exposed to the elevated T-maze. Intra-DPAG injections of 5-HT1A and 5-HT2A receptor antagonists in the absence of electrical stimulation of the DPAG have no effect on escape behaviors (de Paula Soares and Zangrossi, Jr., 2004;Nogueira and Graeff, 1995;Yamashita et al., 2011;Zanoveli et al., 2010), suggesting that there is little or no tonic serotonergic influence on DPAG-mediated escape behaviors. All of these studies were conducted in the elevated T-maze. Escape provoked by exposure to an ethologically relevant threat, like a predator in the mouse defense battery test, another well-validated model of panic (Griebel et al., 1996b), is also attenuated by intra-DPAG microinjection of 5-HT1A and 5-HT2A receptor agonists (Pobbe et al., 2011).
An important question from these studies is how 5-HT inhibits DPAG-mediated behaviors through 5-HT1A and 5-HT2A receptors, which have opposing receptor signaling pathways. 5-HT1A receptors couple to Gi/Go and mediate inhibitory neurotransmission, whereas 5-HT2A receptors couple preferentially to Gq/11 and mediate excitatory neurotransmission (Hoyer et al., 2002). Microinjections of 5-HT1A receptor agonists into the DPAG inhibit neuronal firing of a subpopulation of neurons, presumably local glutamatergic “on” cells or the projection neurons themselves (Brandao et al., 1991;Brandao et al., 2008). In contrast, the majority of 5-HT2A receptor expression in the DPAG is localized to GABAergic neurons (Brandao et al., 1991;Griffiths and Lovick, 2002), and the anti-escape effects of local microinjection of DOI, a 5-HT2A receptor agonist, are blocked by intra-DPAG microinjection of bicuculline, a GABAA receptor antagonist (de Oliveira et al., 2011). Together, these data suggest that serotonin acts via inhibitory 5-HT1A receptors on local glutamatergic “on” cells or projection neurons, and via stimulatory 5-HT2A receptors on inhibitory GABAergic neurons in the DPAG, to inhibit panic-/escape-like responses.
2.4. Chronic treatment with antidepressant drugs acts to potentiate the anti-panic effects of serotonin in the DPAG
Current therapies in panic disorder include antidepressants, e.g., selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors, such as phenelzine, and tricyclic antidepressants, such as imipramine (Nutt, 2005). Benzodiazepines are also used, although there are concerns about long term use due to the potential for tolerance, abuse, dependence, and withdrawal (Nutt, 2005). If the serotonergic inhibition of the DPAG plays a central role in panic disorder, anti-panic drugs should facilitate serotonergic signaling within the DPAG, and this effect should be evident in a time course, 3-4 weeks, that coincides with the time course of clinical efficacy (Nierenberg, 2000;Schneier, 1990). This is indeed the case. Chronic (21 day), but not acute, treatment with fluoxetine (de Bortoli et al., 2006), sertraline (Zanoveli et al., 2007), or imipramine (Jacob et al., 2002;Mongeau and Marsden, 1997a) increases the inhibitory effects of intra-DPAG 5-HT1A and 5-HT2A receptor agonists on escape behaviors. Treatment with buspirone, a partial 5-HT1A receptor agonist that is used for treatment of generalized anxiety disorder (Connor and Davidson, 1998) but does not have therapeutic value in the treatment of panic disorder (Nutt, 2005), has no effect on these measures (de Bortoli et al., 2006;Zanoveli et al., 2005).
Interestingly, pindolol, a β-adrenergic receptor antagonist and 5-HT1A receptor partial agonist, given in combination with paroxetine, is reported to hasten the onset of action and enhance treatment responsivity in depression and anxiety disorders like PD. Rats treated with this pindolol-paroxetine combination show increased escape latencies in the elevated T-maze, a panicolytic effect, which is blocked by pretreatment with the 5-HT1A receptor antagonist, WAY-100635 (Sela et al., 2011). Another model revealed that DPAG-evoked flight behaviors (e.g., galloping) are sensitive to the actions of panicolytic and panicogenic drugs, with these drugs reducing and facilitating DPAG-evoked behaviors, respectively (Schenberg et al., 2001). In this model, chronic (21 days), but not acute treatment with clomipramine or fluoxetine raised the threshold of DPAG-evoked flight behaviors, including galloping, indicative of a panicolytic effect of chronic antidepressant treatment (Vargas and Schenberg, 2001).
In addition to enhanced sensitivity of 5-HT1A and 5-HT2A receptors in the DPAG following chronic treatment with fluoxetine, chronic treatment with fluoxetine, but not buspirone, increases serotonin release in the DPAG and inhibits escape-like behaviors in the elevated T-maze, an anxiolytic behavioral effect that is prevented by intra-DPAG microinjection of the 5-HT1A receptor antagonist, WAY-100635 (Zanoveli et al., 2010). Together, these data suggest that both increased serotonin release within the DPAG and increased sensitivity of 5-HT1A and 5-HT2A receptors in the DPAG contribute to the anti-panic effects of chronic antidepressant treatment.
3. The DRVL/VLPAG serotonergic system, a critical node in panic physiology
3.1. DRVL/VLPAG serotonergic neurons form part of a sympathootor command center
As chronic treatment with fluoxetine inhibits escape behavior via increased serotonin release in the DPAG, and sensitizes 5-HT1A and 5-HT2A receptors in the DPAG, an important question is, where does serotonergic innervation of the DPAG originate? Studies by Zangrossi and colleagues (2005) have shown that disinhibition (resulting in increased activity) of serotonergic neurons in the DR inhibits escape behaviors in the elevated T-maze, an anti-panic effect, and this effect can be prevented by prior inhibition of 5-HT1A receptors in the DPAG. Retrograde tracing studies have shown that innervation of the DPAG originates in the DRVL/VLPAG region (Stezhka and Lovick, 1997). Serotonergic neurons in this region are anatomically positioned to modulate the fight-or-flight response; they are part of a sympathomotor command center, projecting, via multisynaptic pathways, to both the adrenal medulla, and the sympathetically deinnervated gastrocnemius muscle (Kerman et al., 2006). We have shown that this group of serotonergic neurons is strongly activated by panicogenic stimuli, such as sodium lactate (Johnson et al., 2008) and as we will discuss next, hypercapnia (Johnson et al., 2005).
3.2. DRVL/VLPAG and medullary serotonergic neurons are central chemosensory receptors
A compelling body of evidence suggests serotonergic neurons in both the midbrain and medullary raphe nuclei are critical for chemosensitive responses to small decreases in pH due to increased arteriole pCO2, and form an important component of a network of neural structures controlling the behavioral and respiratory response to hypercapic stimuli (Guyenet et al., 2013;Hodges and Richerson, 2010;Richerson, 2004). George Richerson and his colleagues have shown using perforated patch clamp recordings that serotonergic neurons in the medullary raphe (Wang et al., 2001) and DR (Severson et al., 2003) are highly CO2/pH sensitive in vitro. Confocal imaging revealed these chemosensitive medullary raphe and DR 5-HT neurons are closely associated with large branches of the basilar artery confirming these neurons are ideally positioned to sense small fluctuations in arterial pCO2 (Bradley et al., 2002;Severson et al., 2003). Since this evidence was generated using in vitro slice preparations, it is important to note that our lab and others have revealed these 5-HT neurons are chemosensitive in vivo. Inhalation of 20% CO2 for 5 min increases c-Fos expression predominantly in a subpopulation of rat DR serotonergic neurons located in the DRVL/VLPG region (Johnson et al., 2005). Likewise, extracellular single-unit recordings in freely moving cats reveal subpopulations of 5-HT neurons in the raphe obscurus (ROb), raphe pallidus nuclei (RPa) (Veasey et al., 1995) as well as the DR (Veasey et al., 1997) that increase their firing rates in response to hypercapnia. These data clearly illustrate that serotonergic neurons in the midbrain (e.g., DRVL/VLPAG) and medullary raphe nuclei respond to hypercapnia both in vitro and in vivo.
Recent studies are providing details about the contribution of these chemosensitive serotonergic systems in controlling the behavioral and ventilatory responses to hypercapnic challenge. The genetic deletion of all serotonin neurons in mice severely impairs behavioral arousal to hypercapnic challenge (e.g., 10% CO2), while arousal to hypoxia, sound, or air puff remains unaltered, thus ruling out the possibility of a general impairment in arousal (Buchanan and Richerson, 2010). Furthermore, Susan Dymecki, George Richerson and colleagues (2011) using a pharmacogenetic approach expressed the synthetic G protein-coupled receptor Di in all serotonergic neurons in order to silence 5-HT neurons prior to CO2 challenge. They report that neuronal silencing of 5-HT neurons attenuates the normal increase in ventilation caused by hypercapnia. These findings were recently corroborated in an in situ juvenile rat brainstem preparation that retained phrenic nerve output similar to the patterns observed during breathing in vivo (Corcoran et al., 2013). Exposure of the rat brainstem preparation to a mildly hypercapnic perfusate increases phrenic nerve discharge, evidence of increased ventilation as this nerve innervates the diaphragm, and this hypercapnia-induced increase in nerve discharge is attenuated by prior application of the 5-HT1A agonist, 8-OH-DPAT, and the 5-HT2A antagonist, ketanserin (Corcoran et al., 2013). These treatments have the net effect of reducing 5-HT neurotransmission via 5-HT1A receptor mediated autoinhibition or blockade of postsynaptic 5-HT2A receptor activity, suggesting that 5-HT alters phrenic nerve discharge in part through stimulation of 5-HT2A receptors. These studies, taken together, provide compelling evidence that the serotonergic neurons in the DRVL/VLPAG and medullary raphe nuclei are chemosensitive to pH/CO2 and play an important role in mediating the behavioral arousal and increased respiration in response to hypercapnia.
3.3. DRVL/VLPAG serotonergic neurons are activated by panicogenic agents and altered in rodent models of chronic anxiety states
Consistent with a role for DRVL/VLPAG serotonergic neurons in inhibition of panic-like responses, we have shown that, while infusions of sodium lactate in control rats activate these neurons, infusions of sodium lactate fail to activate DRVL/VLPAG serotonergic neurons in rats made panic-prone following infusions of the GABA synthesis inhibitor, L-allylglycine, into the dorsomedial hypothalamus (Figure 2) (Johnson et al., 2007;Johnson et al., 2008). While control rats show no cardiovascular or anxiety-like behavioral responses to sodium lactate infusions that are similar to clinical sodium lactate infusions (Liebowitz et al., 1986), as measured by mean arterial pressure (MAP), heart rate (HR), respiratory rate (RR), and behavior in the social interaction test, panic-prone rats respond with decreased social interaction (an anxiogenic effect observed with or without sodium lactate infusion) and with increased sodium lactate-induced MAP, HR, and RR (Johnson et al., 2007). We hypothesize that these panic-like physiological responses are due to a failure to activate DRVL/VLPAG serotonergic neurons, thus disinhibiting DPAG projection neurons mediating panic-like responses. It’s important to note that sodium lactate infusion had no effect on c-Fos expression in other subsets of serotonergic neurons, such as those in the conflict anxiety-related DRD.
Figure 2.
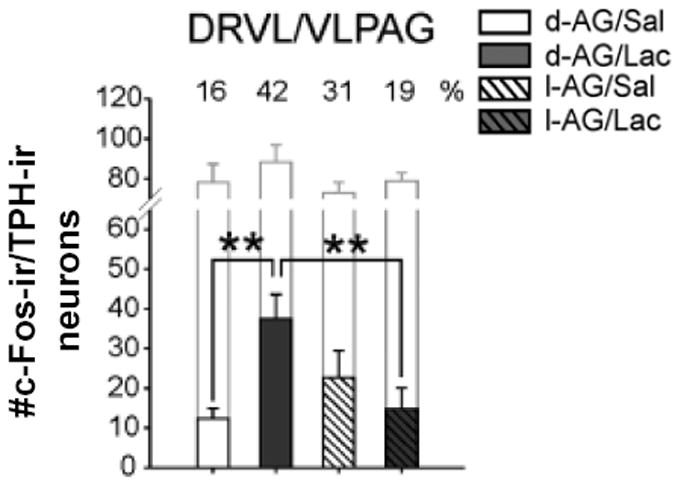
Effects of sodium lactate infusion (0.5 M sodium lactate, i.v.) or 0.9% saline over 15 min, similar to clinical lactate infusions,(Liebowitz et al., 1986) on c-Fos expression in tryptophan hydroxylase (Tph)-immunoreactive (ir) neurons in the DRVL/VLPAG in control rats (intra-DMH D-allylglycine; inactive enantiomer) or panic prone rats (intra-DMH L-allylglycine; 3.5 nmol per 0.5 μl per hour over 5 days) delivered via osmotic minipump. Whereas sodium lactate infusion increased c-Fos expression in D-allylglycine treated control rats, it had no effect in L-allylglycine-treated panic prone rats. Abbreviations: d-AG, D-allylglycine; l-AG, L-allylglycine; Lac, sodium lactate; Sal, saline. **p < 0.01, Fisher’s Protected Least Significant Difference test. Open bars, total number of TPH-ir neurons. Numbers indicate % of TPH-ir neurons that were c-Fos-ir. Reprinted from Johnson et al., (2008) by permission of SAGE.
In addition to these findings in a rat model of PD, we have demonstrated selective dysregulation of DRVL/VLPAG serotonergic neurons in 3 rat models of chronic anxiety states. First, we have shown that amygdala priming (using 5 × daily bilateral infusions of 6 fmol urocortin 1 into the BL) in male rats, which results in a chronic anxiety-like state as measured in the social interaction test, resulted in increased expression of tph2, encoding Tph2, the rate-limiting enzyme in biosynthesis of serotonin, selectively in the DRVL/VLPAG serotonergic neurons and the caudal DRV serotonergic neurons (which have also been shown to project to the DPAG (Stezhka and Lovick, 1994;Vertes, 1991)), but not in any other subdivision of the DR (Donner et al., 2012). In addition, tph2 mRNA expression was highly correlated with anxiety-like behavior in the social interaction test (r = 0.729; p = 0.001) (Donner et al., 2012). Second, we have shown that social defeat increases tph2 mRNA expression, but only in the DRVL/VLPAG serotonergic neurons, and only in rats previously exposed to maternal separation (Gardner et al., 2009). This increase in tph2 in the DRVL/VLPAG serotonergic neurons may play a role in the shift from a proactive emotional coping style to a reactive emotional coping style, which is observed following repeated defeat (Gardner et al., 2005;Paul et al., 2011). Consistent with this hypothesis, we have found that c-Fos expression in DRVL/VLPAG serotonergic neurons, but not DRD serotonergic neurons, is correlated with the duration of freezing behavior during social defeat (Paul et al., 2011). Third, we have shown that female rats, but not male rats, previously exposed to adolescent social isolation show decreased tph2 mRNA expression selectively in the DRVL/VLPAG and DRV serotonergic neurons as adults (Lukkes, Lowry et al., unpublished, and (Lukkes et al., 2013)). Decreased tph2 expression in the DRVL/VLPAG serotonergic neurons, if reflective of decreased serotonergic neurotransmission, would be predicted to result in vulnerability to panic-like responses to panic-inducing agents. Indeed, other studies have shown that female rats, but not male rats, exposed to neonatal maternal separation show an increased hypercapnic ventilator response to CO2 as adults (Dumont et al., 2011;Genest et al., 2007). Furthermore, recent studies in mice have shown that an unstable maternal environment (cross-fostering during postnatal day 2 (PD2)-PD5) results in increased sensitivity to CO2 during development and adulthood, relative to normally-reared mice (D’Amato et al., 2011). Finally, in humans, events involving childhood separation from caregivers or an unstable parental environment are associated with heightened CO2 sensitivity and increased risk for panic disorder in adulthood (Battaglia et al., 1995;Klein, 1995).
3.4. A CE/DRVL/VLPAG circuit mediating expression of contextual fear (freezing) and inhibition of panic-/escape-like physiological and behavioral responses
As mentioned above in section 1.4., patients with focal bilateral amygdala lesions resulting from Urbach-Wiethe disease do not condition to aversive stimuli (Bechara et al., 1995). However, a recent study found that these patients have increased vulnerability to CO2-evoked panic (Feinstein et al., 2013), suggesting that 1) the amygdala is not necessary for CO2-evoked panic, and 2) an intact amygdala may normally inhibit panic. In addition, panic patients have reduced amygdala gray matter volume (Asami et al., 2009;Hayano et al., 2009). These findings in humans are consistent with rodent studies showing that the amygdala, including the BL and central nucleus of the amygdala (CE), mediate expression of contextual fear (freezing behavior), probably via the amygdala-VLPAG pathway (Amaral and Price, 1984;LeDoux et al., 1988;Martinez et al., 2006;Nader et al., 2001), and mediate post-DPAG stimulation freezing (Martinez et al., 2006). Our own data support the hypothesis that a CE-DRVL/VLPAG serotonergic system mediates expression of contextual fear and, consequently, inhibits panic/escape-like responses. We found that exposing rats to the context where they received fear conditioning 24 h earlier resulted in a strong activation of DRVL/VLPAG serotonergic neurons, but not serotonergic neurons in other subregions of the DR, including the conflict anxiety-related DRD/DRC (Spannuth et al., 2011).
The CE, especially the medial (CeM) subdivision, sends direct projections to the DR, including the DRVL/VLPAG subregion (Peyron et al., 1998a;Rizvi et al., 1991). Based on chronic recording of the CE in behaving rats, Paré and colleagues have proposed that conditioned freezing depends on increased CeM responses to the CS (Duvarci, 2011). Although the functional consequences of the putative activation of a CE-DR circuit are not yet clear, electrical stimulation of the CeM, specifically, results in bradycardia, hypotension, and behavioral quiescence (Cox et al., 1987;Kapp et al., 1982), physiologic, and behavioral respsonses that are also observed following stimulation of the DRVL/VLPAG region (Keay and Bandler, 2001). Collectively, these responses are consistent with an “anti-panic-like” effect of CE/DRVL/VLPAG serotonergic stimulation, preventing panic-like physiological responses (tachycardia, hypertension) and behavioral responses (flight/escape). Consistent with a role for the amygdala in inhibition of panic-like responses, Schenberg and colleagues found that kindling of the BL attenuated DPAG-evoked escape behavior (galloping) (Tannure et al., 2009), considered to be a model of PAs (Schenberg et al., 2001). Together, these data suggest that the increased tph2 expression selectively within DRVL/VLPAG serotonergic neurons that we observed following urocortin 1 priming of the BL is likely dependent on increased activity of a CE/DRVL/VLPAG pathway. This hypothesis is consistent with findings that priming of the BL using repeated injections of CRH increases fear-potentiated startle, which is dependent on the CE (Walker et al., 2003;Walker and Davis, 1997), but does not increase light-enhanced startle (Bijlsma et al., 2011), which is dependent on the bed nucleus of the stria terminalis (Walker et al., 2003;Walker and Davis, 1997).
4. Neuropeptide modulation of the panic inhibition system
4.1. CRH projections from the CE activate DRVL/VLPAG serotonergic neurons via CRHR2
We hypothesize that the neural mechanism underlying the proposed activation of DRVL/VLPAG serotonergic neurons by the CE involves a subpopulation of CRH neurons that projects to and activates the DRVL/VLPAG via CRH type 2 receptors (CRHR2). The DR contains abundant CRH-immunoreactive (ir) fibers that display a topographical distribution. The CRH-ir fibers are preferentially distributed in the dorsolateral region caudally, which encompasses the DRVL/VLPAG subregion, and the ventromedial subdivision rostrally (Kirby et al., 2000;Lowry et al., 2000;Valentino et al., 2001). Immunohistochemical staining shows these CRH-ir fibers are in close proximity to tryptophan hydroxylase (TPH)-ir neurons, suggesting CRH has direct actions on serotonergic neurons Examination of the ultrastructural characteristics of CRH-ir terminals using electron microscopy reveals heterogeneity of CRH synapses in different subregions of the DR. In the dorsolateral regions, the CRH-ir terminals frequently associate with dendrites and the synapses are characterized as asymmetric (type1), which are excitatory; whereas in the ventromedial subdivision, the CRH-ir fibers favor contact with axon terminals and the synapses are more frequently symmetric (type 2), which are thought to be inhibitory (Valentino et al., 2001). The regional heterogeneity of CRH-ir synapses in the DR is consistent with electrophysiological reports suggesting that serotonergic neurons in the DRVL/VLPAG have increased intrinsic excitability properties that render them more susceptible to stress-induced activation (Crawford et al., 2010).
Electrophysiological recordings of putative 5-HT neurons reveal CRH has both excitatory and inhibitory effects that depend on the subregion and dose of CRH administered (Kirby et al., 2000;Lowry et al., 2000;Valentino et al., 2001). These CRH terminals can influence DR activity via both CRH type 1 receptors (CRHR1) and CRHR2, which are expressed throughout the DR (Chalmers et al., 1995;Day et al., 2004;De Souza et al., 1985;Lukkes et al., 2011;Potter et al., 1994), and, in the case of CRHR2, colocalize with serotonergic neurons (Day et al., 2004;Lukkes et al., 2011). These studies provide clear evidence that CRH fibers densely innervate the DR, including the DRVL/VLPAG subregion, and have complex actions on both serotonergic and nonserotonergic neurons via CRHR1 and CRHR2.
Neuroanatomical and tract-tracing studies suggest the CE as the origin of a portion of these CRH terminals in the DR. The vast majority of CRH-expressing neurons in the CE are located in the lateral (CeL) subdivision of the CE, with only moderate amounts expressed in the CeM subdivision (Cassell et al., 1986;Gray and Magnuson, 1992;Sakanaka et al., 1986;Veening et al., 1984;Wang et al., 2011). Although the DRVL/VLPAG subregion primarily receives input from the CeM, sparse projections from the CeL exist (Oka et al., 2008;Petrovich and Swanson, 1997;Rizvi et al., 1991). Moreover, Gray and Magnuson (1992) using a combined immunohistochemical and retrograde tract tracing approach show CRH neurons in both the CeL and CeM directly innervate the DRVL/VLPAG subregion. Since CRH neurons in the CeL also send projections to the CeM, BnST, and hypothalamus (Petrovich and Swanson, 1997;Sakanaka et al., 1986), it is possible that CeL CRH neurons alter DRVL/VLPAG serotonergic neurons through indirect pathways (see section 4.2 and 4.3).
A growing body of evidence suggests these CRH projections from the CeM and CeL are implicated in fear-related behavioral responses in part through activating the DR, including DRVL/VLPAG serotonergic neurons. Priming of the BLA, a structure critical for processing fear-related stimuli and gating output of the CE (Adolphs, 2013;LeDoux et al., 1988), by repeated urocortin 1 (UCN1) injections increases anxiety-like behavior and elevates tph2 mRNA in the DRVL/VLPAG (Donner et al., 2012). Microinjection of urocortin 2 (UCN2), a neuropeptide in the CRH family and a selective CRHR2 agonist, directly into the DR activates serotonergic neurons and increases extracellular 5-HT in DR projection regions like the BLA (Amat et al., 2004). This is consistent with electrophysiological findings revealing that UCN2 increases serotonergic neuronal firing rates via CRHR2 stimulation (Pernar et al., 2004). Direct microinjection of CRH into the DR increases freezing, which is associated with serotonin release in the CE (Forster et al., 2006), a structure innervated by the DRVL/VLPAG (Rizvi et al., 1991). We and others have demonstrated that increases in serotonin release following injections of CRH into the DR can be prevented by blockade of DR CRHR2 (Forster et al., 2008;Lukkes et al., 2008). These data suggest a hypothetical model in which CRH-expressing neurons in the CE act on CRHR2 in the DR to activate DRVL/VLPAG serotonergic neurons projecting to the DPAG, thus inhibiting panic-like responses (Figure 1).
4.2. BnST CRH neurons activate the conflict anxiety facilitation system
Although CRH neurons in the CE project directly to the DRVL/VLPAG subregion (Gray and Magnuson, 1992), many of the CRH-ir fibers in the central gray region are spared following electrolytic lesions of the CE (Sakanaka et al., 1986), suggesting other structures provide significant CRH input to the DRVL/VLPAG. Both the CeM and CeL send substantial projections to the BnST (Dong et al., 2001;Petrovich and Swanson, 1997) and these tracts terminate in regions of the BnST that contain CRH neurons (Gray and Magnuson, 1992;Moga et al., 1989). Furthermore, these BnST CRH neurons densely innervate the DRVL/VLPAG subregion, suggesting the BnST may modulate the panic inhibition system directly (Gray and Magnuson, 1992). A variety of stressors elevate CRH mRNA in the BnST (Choi et al., 2006;Funk et al., 2006;Kim et al., 2006) and this nucleus is considered to be a critical node in mediating anxiety-like behavioral responses (Davis et al., 2010;Davis and Shi, 1999;Hammack et al., 2009;Rosen and Schulkin, 1998). Sink and colleagues (2013) report that overexpression of CRH within the BnST using a lentiviral vector modulates conditioned anxiety (i.e., sustained fear-potentiated startle), but not unconditioned anxiety (i.e., elevated plus-maze), and these behavioral changes may be due to compensatory changes resulting in decreased CRHR1 receptor expression within the BnST and decreased CRHR2 receptor expression within the DRD. As we mentioned in section 1.5, the DRD is an important component of a conflict anxiety facilitation system that has reciprocal projections to forebrain limbic structures involved in eliciting anxiety-like behavior responses such as the BnST (Fox and Lowry, 2013;Hale and Lowry, 2011;Lowry et al., 2008;Paul and Lowry, 2013). Although beyond the scope of this review, it is worth mentioning that serotonergic modulation of BnST activity and anxiety-like behavior is complex and involves several BnST neuron types, multiple 5-HT receptor subtypes (e.g., 5-HT1A, 5-HT2A, 5-HT2C, and 5-HT7), as well as the relative receptor expression patterns during both normal and pathological anxiety states (Guo et al., 2009;Hammack et al., 2009).
Consistent with the notion that the BnST facilatates anxiety-like behavior, priming of the BnST by repeated stimulation of BnST CRH receptors using UCN1 produces long-lasting increases in anxiety-like behavior in the social interaction test, while sodium lactate infusion, which is a potent panicogen, fails to alter cardiovascular measures in BnST primed rats (Lee et al., 2008). Likewise, chronic disinhibition of the BnST by repeated infusion of the GABA synthesis inhibitor, L-allylglycine, increases anxiety-like behavior, while sodium lactacte-induced cardiorespiratory responses are unaltered (Sajdyk et al., 2008). These studies suggest the BnST is implicated in mediating anxiety-like behavior, reminiscent of generalized anxiety in humans, but not panic-like behavioral and physiologic responses. Future studies should elucidate the functional role, if any, that BnST CRH neurons have in modulating the activity of the panic inhibition system via their projections to the DRVL/VLPAG serotonergic neurons.
4.3. Interactions between CRH, hypocretinergic/orexinergic, and serotonergic systems: implications for anxiety and panic
4.3.1. Hypothalamic hypocretinergic/orexinergic and extended amygdala CRH neurons comprise a stress-sensitive circuit
The neuropeptides, hypocretin-1/orexin-A (hcrt-1/orx-A) and hypocretin-II/orexin-B (hcrt-2/orx- B), were discovered over 15 years ago and were initially thought to be critical for feeding behavior due to their location in the lateral hypothalamus (LH) and perifornical hypothalamus (PeF), regions long known to elicit feeding (de Lecea et al., 1998;Sakurai et al., 1998). Since then, studies have documented a myriad of functions attributed to the hypocretins/orexins, including roles in sleep and wakefulness, energy homeostasis, reward, sensory modulation, endocrine function, autonomic control, cognition, and motivated behavior (Carrive, 2013;de Lecea, 2010;Koob and Le, 2008;Li et al., 2014;Sakurai and Mieda, 2011;Sellayah and Sikder, 2013;Tsujino and Sakurai, 2013). These hcrt/orx neurons are potently activated by diverse stressors such as cold exposure (Ida et al., 2000;Sakamoto et al., 2004), peripheral inflammation (Watanabe et al., 2005), immobilization (Ida et al., 2000;Sakamoto et al., 2004), restraint (Reyes et al., 2003;Winsky-Sommerer et al., 2004), foot shock (Watanabe et al., 2005;Zhu et al., 2002), novelty stress (i.e., brightly lit novel environment; Berridge et al., 1999) and high-arousal waking (i.e., diurnal novelty-stress; Espana et al., 2003), and are thought to coordinate the behavioral, neuroendocrine, and cardiorespiratory stress response (for reviews see, Berridge et al., 2010;Carrive, 2013;Kuwaki, 2011;Winsky-Sommerer et al., 2005;Zhang et al., 2006). The hypocretinergic/orexinergic system is ideally positioned to control multiple components of the stress response through widespread projections to cortical, limbic, brainstem and spinal cord structures, including, but not limited to, the CE, BnST, rostral and caudal raphe nuclei, and PAG (for a more comprehensive list of orexin efferents see, Li et al., 2014;Nambu et al., 1999;Peyron et al., 1998b;Sakurai, 2007;Sakurai and Mieda, 2011).
Hypocretins/orexins may control the behavioral and physiologic response to stress through interactions with extrahypothalamic CRH systems located in extended amygdala structures like the CE and BnST. Application of hcrt-1/orx-A and hcrt-2/orx-B to an in vitro slice preparation excites a population of “low threshold burst“ neurons located in the CeM through activation of postsynaptic hcrt-2/orx-2 receptors (Bisetti et al., 2006). It is unclear what neurochemical phenotype these CeA neurons comprise, however, a portion of them may be CRH neurons because i.c.v. administration of hcrt-1/orx-A, relative to saline control, results in increased immunohistochemical staining of c-Fos, a marker of neuronal activity, in CE CRH-ir neurons (Sakamoto et al., 2004). In regards to the BnST, microinjection of hcrt-1/orx-A directly into the BnST evokes action potentials in a subset of BnST neurons and increases anxiety-like behavior in the elevated plus-maze and social interaction test (Lungwitz et al., 2012). These behavioral alterations may be mediated by hcrt-1/orx-A interactions with glutamate as prior treatment with the NMDA antagonist, AP5, or the AMPA receptor antagonists, CNQX and DNQX, completely or partially blocks the increase in anxiety-like behavior, respectively (Lungwitz et al., 2012). This supports the notion that the hcrt/orx system activates a subset of BnST neurons to promote anxiety-like behavioral responses, although future studies will need to determine whether hcrt/orx targets BnST CRH neurons. Consistent with this evidence of hcrt/orx interacting with CRH systems, i.c.v. microinjection of hrct-1/orx-A dose-dependently reinstates previously extinguished cocaine-seeking behavior (and food-seeking behavior), an effect that can be blocked by i.c.v. pretreatment with the nonselective CRH receptor antagonist, D-Phe-CRH (Boutrel et al., 2005). Furthermore, pretreatment with the hcrt-1/orx-1 receptor antagonist, SB-334867, abolishes stress (foot shock)-induced reinstatement of cocaine-seeking behavior, suggesting hcrt/orx may modulate the brain reward system through its actions on brain stress circuitry (Boutrel et al., 2005).
Evidence also suggests that the CRH system modulates the activity of hcrt/orx neurons. The LH/PeF contains dense CRH-ir fibers that are apposed to hcrt/orx neurons, which also express both CRHR1 and CRHR2 (Winsky-Sommerer et al., 2004;Winsky-Sommerer et al., 2005). Electrophysiological recordings suggest CRH depolarizes hcrt/orx neurons and this activation can be blocked by the CRHR1 antagonist, astressin (Winsky-Sommerer et al., 2004;Winsky-Sommerer et al., 2005). Supporting the role of CRHR1-induced activation of hcrt/orx neurons, the stress-induced activation of hcrt/orx neurons, as measured by elevated c-Fos, is severely diminished in genetically engineered mice that lack CRHR1 (Winsky-Sommerer et al., 2004;Winsky-Sommerer et al., 2005). The origin of these CRH-ir fibers that contact hcrt/orx neurons is unclear, but evidence suggests a portion of the CRH input comes from the extended amygdala. In an elegant retrograde tracing study, Sakurai and colleagues (2005) mapped the input to hcrt/orx neurons by constructing a transgenic mouse line expressing a fusion protein exclusively in hcrt/orx neurons. This protein is taken up by terminals that form synapses with hcrt/orx neurons and then retrogradely transported to neuronal cell bodies, thus allowing the visualization of GFP in neurons that project specifically to hcrt/orx neurons. This technique revealed projections from the subdivisions of the BnST (i.e., ventral lateral) and CE (CeM and CeL) that contain CRH neurons. Traditional anterograde and retrograde tract tracing studies confirm these projections from the BnST and CE (Nakamura et al., 2009;Yoshida et al., 2006) and in the case of the CE, some of these projections to the LH contain CRH (Sakanaka et al., 1986). Most of the terminals arising from the CE are immunoreactive for glutamic acid decarboxylase, an enzyme necessary for GABA synthesis (Nakamura et al., 2009), which is not surprising since CE output neurons are thought to be comprised primarily of GABAergic neurons. Taken together, these studies suggest hypocretinergic/orexinergic neurons in the LH/PeF and CRH neurons in the CE and BnST comprise a stress-sensitive circuit capable of modulating arousal, reward, and many aspects of the stress response.
4.3.2. A hypocretinergic/orexinergic-serotonergic circuit implicated in stress, anxiety and panic
The hypocretinergic/orexinergic system has extensive projections to brainstem serotonergic systems originating in both the rostral (e.g., DR and median raphe nuclei, MnR) and caudal (e.g, raphe magnus, obscurus and pallidus) raphe nuclei, positioning this network to control the behavioral and autonomic response to anxiety-, fear-, and panic-provoking stimuli (Nambu et al., 1999;Peyron et al., 1998b). Microinjection of the retrograde tracer, WGA-apo-HRP-gold (WG), into distinct subregions of the DR has revealed topographically organized projections from hypothalamic hcrt-1/orx-A neurons (Lee et al., 2005). Following WG injections into the rostral DR, double labeled hcrt-1/orx-A neurons are located in the dorsal half of the LH as well as the dorsomedial hypothalamus (DMH). Injections in the intermediate DR, an area including the DRD and DRV subdivisions, result in double-labeled hcrt-1/orx-A neurons in the ventromedial LH, PeF, and dorsal to the PeF. The DRC receives hcrt-1/orx-A projections from the PeF and the posterior hypothalamus (PH). Finally, the lateral wings of the DR, which corresponds to the DRVL/VLPAG subregion, has projections from the ventral LH and PeF, with sparser projections from the dorsal LH and area dorsal to the PeF. Relative to other DR subregions, the DRVL/VLPAG contains the highest density of hcrt/orx-ir fibers (Nambu et al., 1999;Peyron et al., 1998b). There is no difference in the contralateral or ipsilateral pattern of projections to the DRVL/VLPAG, although the regions of the hypothalamus contralateral to the injection site contain approximately 50-70% fewer labeled cells (Lee et al., 2005). These topographically organized hypocretinergic/orexinergic projections to specific subregions of the DR may have important functional implications for how stress and panicogenic stimuli modulate serotonergic systems (see below).
Consistent with diffuse hcrt/orx projections to the serotonergic raphe nuclei, both hcrt/orx-1 and -2 receptors, which are linked to Gq-proteins, are found in rostral and caudal raphe nuclei, and are especially abundant in the DR (Cluderay et al., 2002;Greco and Shiromani, 2001;Hervieu et al., 2001;Kilduff and de Lecea, 2001;Marcus et al., 2001;Trivedi et al., 1998). In the DR, in vivo and in vitro electrophysiological studies suggest hcrt/orx has predominantly direct excitatory effects on serotonergic neurons that are mediated in part by hcrt/orx-1 and -2 receptors (Brown et al., 2001;Brown et al., 2002;Kohlmeier et al., 2008;Liu et al., 2002;Takahashi et al., 2005;Wang et al., 2005), although indirect inhibitory effects on serotonergic neurons are also observed likely through GABAergic interneurons (Liu et al., 2002). This is in agreement with immunohistochemical and immunocytochemical studies revealing hcrt/orx-ir fibers associate with both serotonergic and GABAergic neurons within the DR (Liu et al., 2002;Wang et al., 2005). The relative expression pattern of hcrt/orx receptors on serotonergic and nonserotonergic neurons may explain the differential effects of hcrt/orx infusion on extracellular 5-HT in the DR and MnR. For example, infusion of hcrt-1/orx-A, which has equal affinity for both hcrt/orx-1 and-2 receptors, increases extracellular 5-HT in the DR, but has no effect in the MnR; whereas infusion of hcrt-2/orx-B, which has a 10-fold higher selectivity for the hcrt/orx-2 receptor, slightly elevates extracellular 5-HT in both the DR and MnR (Tao et al., 2006). Lastly, it is important to note that serotonergic systems innervate the hcrt/orx system, especially serotonergic neurons in the MnR, paramedian raphe nucleus, RPa, RMg, and minimal input from the DR (Sakurai et al., 2005). This serotonergic input strongly hyperpolarizes hcrt/orx neurons through postsynaptic 5-HT1A receptors, suggesting this hcrt/orx-5-HT circuit forms a negative feedback cycle (Muraki et al., 2004;Yamanaka et al., 2003). The functional purpose of a hypocretinergic/orexinergic-serotonergic circuit is unclear, but evidence suggests it is important for modulating arousal, sleep/wake states, physiologic and behavioral responses to stress, and dysfunction of this circuit may be implicated in anxiety and panic.
Recent evidence suggests the hypocretinergic/orexinergic system is implicated in the pathophysiology of panic disorder. Chronic disinhibition of the DMH/PeF using a GABA synthesis inhibitor makes rats susceptible to panicogenic agents such as sodium-lactate, which elicits panic attacks in humans with panic disorder, and behavioral and sympathetic responses that resemble a panic attack in panic-prone rats, but not control rats (Shekhar et al., 1996). In panic-prone rats (i.e., rats with chronic intra-DMH/PeF microinjection of L-allylglycine, a GABA synthesis inhibitor), administration of sodium lactate activates orexin neurons, as measured by elevated c-Fos expression, and increases physiologic and behavioral indices of panic-like responses, including increased anxiety-like behavior, arousal, heart rate, and mean arterial pressure (Johnson et al., 2010b). Moreover, these panic-like responses are attenuated by prior intra-DMH/PeF injection of a gene silencer (siRNA) that targets the gene encoding hcrt/orx or systemic pretreatment with the selective hcrt-1/orx-1 antagonist, SB334867 (Johnson et al., 2010b). The lactate-induced increase in anxiety-like behavior is likely due to hcrt-1/orx-A altering BnST activity as microinjection of SB334867 directly into the BnST blocks this effect (Johnson et al., 2010b). Consistent with a role of hcrt/orx mediating panic-like responses in panic-prone rodents, human patients with panic disorder have elevated levels of hcrt/orx in the cerebrospinal fluid (Johnson et al., 2010b). These findings taken together with evidence of dense hcrt/orx projections having both excitatory and inhibitory actions on serotonergic and GABAergic DRVL/VLPAG neurons (Lee et al., 2005;Liu et al., 2002;Nambu et al., 1999;Peyron et al., 1998b;Wang et al., 2005) as well as the failure of sodium lactate to activate serotonergic neurons in the DRVL/VLPAG panic inhibition system (Johnson et al., 2008) in panic-prone rats, raise the possibility that hcrt/orx neurons inhibit the serotonergic neurons in the DRVL/VLPAG panic inhibition system via GABAergic interneurons. Considering the role of the BnST in anxiety and its connections with the DRD (see section 4.2), the increase in anxiety-like behavior observed in panic-prone rats may involve activation of a conflict anxiety facilitation system involving hcrt/orx activation of BnST CRH neurons that project to DRD serotonergic neurons. Future studies should investigate the role of hcrt/orx neurons in orchestrating the activity of functionally distinct serotonergic systems.
5.1. Panicogenic agents activate a distributed network of anxiety-, fear- and panic-related brain regions
5.1.1. Acid-sensing ion channels in the amygdala: implications for fear and panic
The final consideration is how panicogenic agents, such as CO2 and sodium lactate, potentiate the activity of the panic inhibition system (CE/DRVL/VLPAG/DPAG pathway), in concert with activation of panic-inducing circuits. It’s highly likely that the mechanisms involved are different for different panicogens. CO2 causes acidosis (Richerson, 2004) and may interact with acid-sensing ion channels in the BL to activate the CE/DRVL/VLPAG/DPAG pathway; the absence of this panic inhibition signaling pathway in patients with bilateral lesions of the amygdala may account for the increased vulnerability to CO2-induced panic attacks in these patients (Feinstein et al., 2013). Consistent with this hypothesis, the acid sensing ion channel ASIC1a is abundantly expressed in the BL, and knockout of ASIC1a results in a loss of context-dependent fear memory, as measured by freezing (Coryell et al., 2008). Local expression of ASIC1a in the BL of ASIC1a−/− mice rescues context-dependent fear memory, but not the unconditioned fear response to predator odor (Coryell et al., 2008). Conversely, transgenic overexpression of ASIC1a enhances fear conditioning (Wemmie et al., 2004). Exposure of mice to hypercapnic acidosis (e.g. 5 or 10% CO2) results in increased CO2-evoked freezing, CO2-potentiated contextual fear (e.g., freezing), avoidance of CO2, and reduced time in the center of an open-field arena, all of which are blocked by genetic elimination of ASIC1a (Ziemann et al., 2009). Further, expressing ASIC1a in the BL of ASIC1a−/− mice using an adeno-associated virus restored the CO2-evoked freezing, highlighting the specificity of the effects. Sophisticated developments in MRI methods, including the amide proton transfer (APT) and T1 in the rotating frame (T1ρ) techniques (Jokivarsi et al., 2010;Jones et al., 2006;Makela et al., 2001;Zhou et al., 2003), now allow for measurement of small changes in brain pH by detecting the transfer of hydrogen ions (H+) between water and proteins. A recent study shows widespread alterations in mouse and human brain pH following inhalation of 5% CO2, relative to breathing room air (Magnotta et al., 2012). The method is also sensitive enough to detect pH changes related to normal function; for example, a visual task (e.g., flashing checkerboard) causes localized acidosis, as measured by brain lactate and pH, in the visual cortex (Magnotta et al., 2012). These studies, taken together with evidence that PD patients, but not healthy individuals, show an elevated visual cortex lactate response to a similar visual task (Maddock et al., 2009), and reports documenting the anxiolytic effects of ASIC inhibitors in several preclinical rodent models (Dwyer et al., 2009), highlight the importance of pH in both normal and abnormal brain function. Overall, these data are consistent with the hypothesis that ASICs in the BL detect changes in pH/pCO2 and modulate fear-related behavior accordingly, likely via descending projections to the CE/DRVL/VLPAG/DPAG pathway in order to promote contextual or cued fear (e.g., freezing) and inhibit DPAG-flight behaviors.
5.1.2. Acid-sensing ion channels are expressed throughout the panic inhibition system
Although most of the research into the role of ASICs in CO2-mediated behavioral and physiological responses emphasizes ASICs in the BL, ASIC1a is also expressed in a distributed system of structures implicated in anxiety, fear and panic, including the central and medial nuclei of the amygdala, bed nucleus of the stria terminalis (BnST), cingulate cortex, somatosensory cortex, habenula, lateral hypothalamus, and, moreover, ASIC1a is particularly enriched in the PAG, including the region encompassing serotonergic neurons in the DRVL/VLPAG (Coryell et al., 2007). Therefore, ASICs are expressed throughout the panic inhibition system. This would be predicted considering the evidence in patients with focal bilateral amygdala lesions, who display deficits in fear conditioning (Bechara et al., 1995), but increased vulnerability to CO2-evoked panic (Feinstein et al., 2013). Indeed, these data are consistent with the idea that contextual or cued fear, which is measured as an increase in freezing, is mediated in part through ASIC1a activation of BL projecting neurons that activate the CE/DRVL/VLPAG/DPAG panic inhibition system in order to facilitate freezing and inhibit escape. An important direction for future research will be to determine if serotonergic neurons in the DRVL/VLPAG express ASICs and how they contribute to the behavioral and physiological responses to panicogenic agents.
5.1.3. Sodium lactate-induced panic activates angiotensin II neurons
Sodium lactate, in stark contrast, causes alkalosis, not acidosis (Peskind et al., 1998). Clinical studies have demonstrated that it is likely that hypernatremia (increased blood sodium concentrations) mediate sodium lactate-induced panic, as equimolar sodium chloride was just as effective in inducing panic attacks as sodium lactate (Peskind et al., 1998). Together with Shekhar and colleagues, we have proposed a role for angiotensin II, possibly derived from angiogensin II-synthesizing neurons in circumventricular organs, in sodium lactate-induced panic (Johnson et al., 2013;Shekhar et al., 2006;Shekhar and Keim, 1997). Direct iontophoretic application of angiotensin II in the BL increases neuronal firing rates (Albrecht et al., 2000), and we have shown that sodium lactate-induced panic-like responses in the amygdala priming model can be prevented by intra-BL injections of the angiotensin II receptor antagonist, saralasin (Johnson et al., 2013).
5.1.4. Serotonergic neurons express two-pore domain K+ TASK channels capable of sensing fluctuations in extracellular pH
The recently discovered two-pore domain K+ TASK (TWIK-related acid sensitive K+ channel) channels are important contributors to the resting membrane potential and provide a buffer towards depolarization (Enyedi and Czirjak, 2010). Two types of TASK channels, TASK1 and TASK2, are unique in relation to other TASK channel family members because these channels are sensitive to changes in extracellular pH, both acidosis and alkalosis, within the physiological range (Enyedi and Czirjak, 2010;Talley et al., 2000). Acidosis inhibits TASK channels and consequently increases membrane excitability; alkalosis, on the other hand, excites TASK channels and resists membrane depolarization (Enyedi and Czirjak, 2010).
Serotonergic neurons of the dorsal raphe nucleus (including the DRVL/VLPAG) and medullary raphe nuclei express TASK1 and TASK3 mRNA transcripts and whole-cell voltage clamp recordings identify a K+ conductance characteristic of TASK channels that is sensitive to pH (Washburn et al., 2002). Interestingly, TASK channels, similar to other background K+ channels, are inhibited by a number of neurotransmitter systems, including the serotonergic system, in order to facilitate depolarization of the membrane potential (Talley et al., 2000). The mechanism behind 5-HT’s ability to inhibit the activity of TASK channels remains elusive; however, preliminary evidence suggests it involves coupling with 5-HT2C receptors, which are linked to Gq/11 and mediate excitatory neurotransmission (Figure 3).
Figure 3.
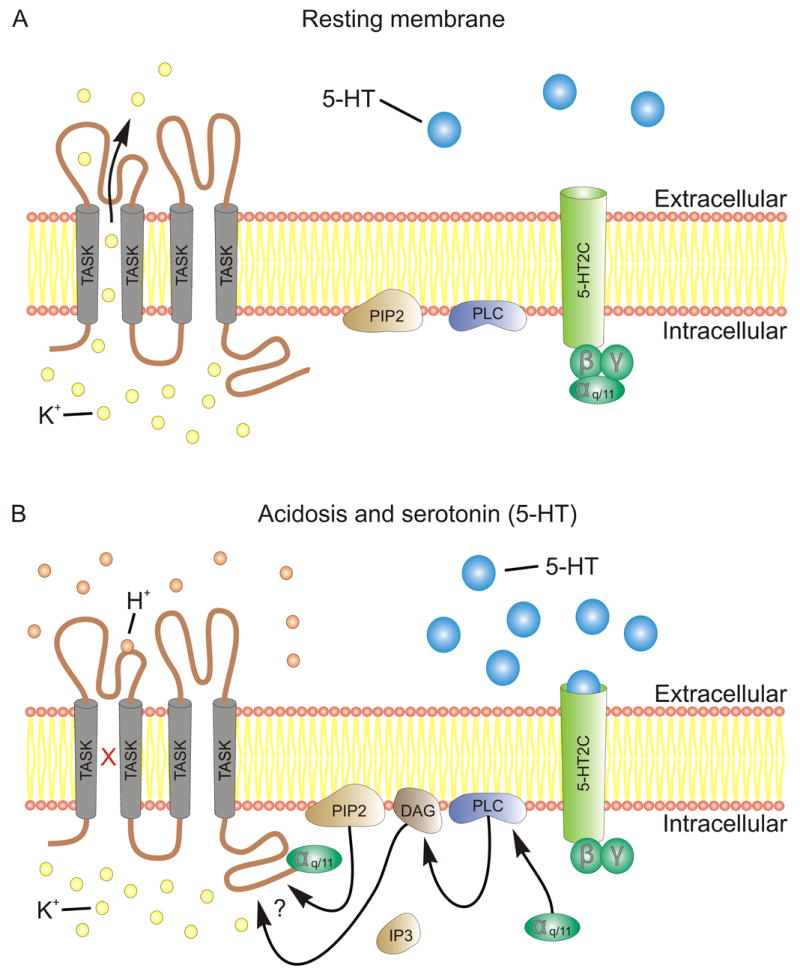
Schematic depicting pH- and serotonin-mediated inactivation of TASK channels. (A) At rest, TASK channels leak K+ ions into the extracellular space to establish the resting membrane potential and provide a countervailing force to depolarizing currents. (B) Acidosis (e.g., CO2 inhalation) increases the concentration of hydrogen ions, which bind an extracellular loop of the TASK channel to inactivate TASK, thus increasing membrane excitability. Likewise, activation of 5-HT2C receptors causes the dissociation of Gq/11 α; the G protein α-subunit (Gq/11) can inactivate the TASK channel directly by binding the C-terminus. It is unclear whether other factors in this signaling pathway inactivate TASK (Enyedi and Czirjak, 2010). Abbreviations: 5-HT, 5-Hydroxytryptamine (serotonin); 5-HT2C, 5-Hydryoxytryptamine type 2C receptor; αq/11, G-protein subunit α; β, G-protein subunit β; DAG, diacyl glycerol; γ, G-protein subunit γ; H+, hydrogen ion; IP3, inositol 1,4,5-trisphosphate; K+, Potassium ion; PIP2, phospholipid phosphatidylinositol 4,5-bisphosphate; PLC, phospholipase C; TASK, TWIK-related acid sensitive K+ channel.
Studies investigating serotonergic modulation of the mammalian startle reflex illustrate interactions between 5-HT2C and TASK channels. This reflex is well characterized and involves giant neurons located in the caudal pontine reticular formation (PnC), which receive converging sensory inputs, integrate this information, and send direct projections to spinal motor neurons involved in eliciting startle (for reviews see, Koch, 1999;Yeomans et al., 2002). There is ample evidence suggesting serotonergic systems are modulated by acoustic stimuli and in turn modulate aspects of the acoustic startle response, including serotonergic neurons in the medullary raphe (e.g., RPa and raphe magnus nucleus, RMg; for a review, see Davis, 1980), MnR (Daugherty et al., 2001;Dilts and Boadle-Biber, 1995), and the anxiety-related DRD (Spannuth et al., 2011) and DRC (Evans et al., 2009). Weber and colleagues (2008) demonstrate that PnC giant neurons express bothTASK3 channels and 5-HT2C receptors; moreover, acidosis (pH = 6.4) inactivates TASK3, and this effect can be mimicked by stimulation of 5-HT2C receptors. This suggests serotonergic neurons gate PnC giant neuron excitability via coupling of 5-HT2C receptors with TASK3, which inactivates these channels in order to permit membrane depolarization to enhance startle (Weber et al., 2008). Considering that TASK channels have a chemosensory role in the retrotrapezoid nucleus (Wang et al., 2013), a critical structure for the central chemorespiratory reflex (Guyenet et al., 2008), as well as in the carotid bodies (Buckler and Turner, 2013), the primary location of peripheral chemoreceptors, an important objective for future research will be to determine the role of TASK channels in serotonergic chemosensation and in turn how serotonergic neurotransmission modulates TASK activity.
6.1. Conclusion
Over twenty years ago Deakin and Graeff proposed that functionally distinct subpopulations of serotonergic neurons located in the DR and MnR modulate aspects of defensive behavior through topographically organized projections to forebrain limbic structures and brainstem structures involved in the fight-or-flight response. Dysfunction of these pathways results in failure to adapt to stress and vulnerability to affective and anxiety disorders, including PD (Deakin and Graeff, 1991;Graeff et al., 1996). In this review, we have outlined evidence for a putative panic inhibition system, one of the original pathways discussed by Deakin and Graeff, which consists of a circuit involving CRH neurons in the CeA that project to DRVL/VLPAG serotonergic neurons. These DRVL/VLPAG serotonergic neurons facilitate freezing behavior and restrain panic-related behaviors (e.g., flight/escape) elicited by DPAG stimulation. Several converging lines of evidence support this hypothesis, including observations that serotonin inhibits DPAG- evoked behavioral and sympathoexcitatory responses that mimic the symptomatology of PAs, and that chronic, but not acute, antidepressant treatment potentiates serotonergic inhibition of panic-like responses. Serotonergic neurons in the DRVL/VLPAG are chemosensitive and ideally positioned to modulate diverse aspects of the behavioral and cardiorespiratory responses to panicogenic agents. Lastly, developments in our understanding of the neurobiological mechanisms of conditionied fear suggest a CE/DRVL/VLPAG pathway mediates freezing, the primary behavioral index of fear in contextual and cued fear conditioning.
6.1.1. A panic-inhibition system: implications for anxiety, fear, and panic
The evidence presented here for a CE/DRVL/VLPAG/DPAG panic-inhibition system together with the observations that Urbach-Wiethe patients who have bilateral focal lesions of the amygdala show deficits in fear conditioning with a concomitant vulnerability to CO2-evoked panic (see section 1.4), suggest that the amygdala may normally inhibit panic and that fear and panic are dissociable states, rather than panic being an exaggerated fear state. The distinction between fear and panic, however, is often obfuscated, especially in rodent models of anxiety-, fear-, and panic-related behavior. The notion that contextual or cued fear stimuli activate a CE/DRVL/VLPAG/DPAG to promote freezing, while inhibiting DPAG-evoked escape/flight behaviors – in other words, fear inhibits panic – is supported by contemporary theories on the hierarchical organization of defensive behavior.
Based on ethologically relevant models of defensive behavior in mice and rats, Blanchard and colleagues (2001;1986a;1989) argue that the behavioral responses toward threatening stimuli such as a predator are organized in hierarchical levels based on the proximity and presence of threat as well as constraints imposed by the environment (e.g., lack of escape routes). In a novel environment or an environment where a predator was previously encountered rodents will typically engage in risk assessment behaviors to determine the nature of any potential threats. If a predator is detected and there is a suitable escape route than flight ensues; however, if there are no available escape routes and the predator is at a sufficiently safe distance that attack is not imminent, then the rodent proceeds to the next line of defense, termed distal threat, which involves freezing in order to avoid detection by the predator. The last level of defense, called proximal defense, is initiated when the predator comes into close contact with the prey and may strike, which results in defensive threat and attack behaviors. This framework of defensive behavior is echoed in McNaughton and Corr’s (2004) two dimensional defense system theory, which postulates that the dimensions of defensive approach and defensive avoidance interact with defensive distance (i.e., distance of threat) to dictate the selected behavioral coping strategy. The evidence outlining a putative CE/DRVL/VLPAG/DPAG panic inhibition system that facilitates conditioned fear-related behaviors like freezing, while inhibiting panic-related behaviors like escape is consistent with these models of defensive behavior. This is not surprising considering that freezing and flight are mutually exclusive behaviors located on opposite ends of the behavioral response spectrum to distal and proximal threat. A system whereby fear (i.e., freezing) reduces panic (i.e., flight) has clear adaptive value as engaging in freezing behavior while inhibiting flight would decrease the probability of being detected by a predator or engaging in potentially life-threatening physical confrontation.
Highlights.
DRVL/VLPAG serotoninergic neurons are part of a panic inhibition system
Chronic SSRIs potentiate 5-HT inhibition of DPAG-evoked panic (e.g., escape)
These 5HT neurons are chemosensitive and respond to panicogenic agents (e.g., CO2)
DRN serotonergic neurons express pH-sensitive ion channels (e.g., ASIC1a, TASK)
Amygdalar CRH activates DRVL/VLPAG 5-HT neurons to promote freezing/inhibit escape
Acknowledgments
Sources of financial support
C.A. Lowry receives grant support from the National Institute of Mental Health (R01MH065702, R01DA019921), the National Science Foundation (NSF-IOS 0921969), the Depressive and Bipolar Disorder Alternative Treatment Foundation, and is the recipient of a NARSAD, Brain & Behavior Research Foundation 2010 Young Investigator Award. He reports the following activities for the previous two years: consultant for Enlight Biosciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–997. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Adolphs R. The biology of fear. Curr Biol. 2013;23:R79–R93. doi: 10.1016/j.cub.2012.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adolphs R, Tranel D. Emotion recognition and the human amygdala. In: Aggleton JP, editor. The Amygdala. New York: Oxford University Press; 2000. pp. 587–630. [Google Scholar]
- 4.Albrecht D, Nitschke T, Von Bohlen Und HO. Various effects of angiotensin II on amygdaloid neuronal activity in normotensive control and hypertensive transgenic [TGR(mREN-2)27] rats. FASEB J. 2000;14:925–931. doi: 10.1096/fasebj.14.7.925. [DOI] [PubMed] [Google Scholar]
- 5.Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- 6.Amaral JM, Spadaro PT, Pereira VM, Silva AC, Nardi AE. The carbon dioxide challenge test in panic disorder: a systematic review of preclinical and clinical research. Rev Bras Psiquiatr. 2013;35:318–331. doi: 10.1590/1516-4446-2012-1045. [DOI] [PubMed] [Google Scholar]
- 7.Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, Watkins LR, Maier SF. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–519. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- 9.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 10.Angst J. Panic disorder: History and epidemiology. Eur Psychiatry. 1998;13(Suppl 2):51s–55s. doi: 10.1016/S0924-9338(98)80014-X. [DOI] [PubMed] [Google Scholar]
- 11.Asami T, Yamasue H, Hayano F, Nakamura M, Uehara K, Otsuka T, Roppongi T, Nihashi N, Inoue T, Hirayasu Y. Sexually dimorphic gray matter volume reduction in patients with panic disorder. Psychiatry Res. 2009;173:128–134. doi: 10.1016/j.pscychresns.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Battaglia M, Bertella S, Politi E, Bernardeschi L, Perna G, Gabriele A, Bellodi L. Age at onset of panic disorder: influence of familial liability to the disease and of childhood separation anxiety disorder. Am J Psychiatry. 1995;152:1362–1364. doi: 10.1176/ajp.152.9.1362. [DOI] [PubMed] [Google Scholar]
- 13.Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- 14.Beckett S, Marsden CA. The effect of central and systemic injection of the 5-HT1A receptor agonist 8-OHDPAT and the 5-HT1A receptor antagonist WAY100635 on periaqueductal grey-induced defence behaviour. J Psychopharmacol. 1997;11:35–40. doi: 10.1177/026988119701100111. [DOI] [PubMed] [Google Scholar]
- 15.Beckett SR, Lawrence AJ, Marsden CA, Marshall PW. Attenuation of chemically induced defence response by 5-HT1 receptor agonists administered into the periaqueductal gray. Psychopharmacology (Berl) 1992;108:110–114. doi: 10.1007/BF02245294. [DOI] [PubMed] [Google Scholar]
- 16.Bell CJ, Nutt DJ. Serotonin and panic. Br J Psychiatry. 1998a;172:465–471. doi: 10.1192/bjp.172.6.465. [DOI] [PubMed] [Google Scholar]
- 17.Bell CJ, Nutt DJ. Serotonin and panic. Br J Psychiatry. 1998b;172:465–471. doi: 10.1192/bjp.172.6.465. [DOI] [PubMed] [Google Scholar]
- 18.Berridge CW, Espana RA, Vittoz NM. Hypocretin/orexin in arousal and stress. Brain Res. 2010;1314:91–102. doi: 10.1016/j.brainres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berridge CW, Mitton E, Clark W, Roth RH. Engagement in a non-escape (displacement) behavior elicits a selective and lateralized suppression of frontal cortical dopaminergic utilization in stress. Synapse. 1999;32:187–197. doi: 10.1002/(SICI)1098-2396(19990601)32:3<187::AID-SYN5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Biederman J, Faraone SV, Hirshfeld-Becker DR, Friedman D, Robin JA, Rosenbaum JF. Patterns of psychopathology and dysfunction in high-risk children of parents with panic disorder and major depression. Am J Psychiatry. 2001;158:49–57. doi: 10.1176/appi.ajp.158.1.49. [DOI] [PubMed] [Google Scholar]
- 21.Biederman J, Petty CR, Faraone SV, Hirshfeld-Becker DR, Henin A, Brauer L, Kaufman B, Rosenbaum JF. Antecedents to panic disorder in nonreferred adults. J Clin Psychiatry. 2006;67:1179–1186. doi: 10.4088/jcp.v67n0802. [DOI] [PubMed] [Google Scholar]
- 22.Bienvenu OJ, Onyike CU, Stein MB, Chen LS, Samuels J, Nestadt G, Eaton WW. Agoraphobia in adults: incidence and longitudinal relationship with panic. Br J Psychiatry. 2006;188:432–438. doi: 10.1192/bjp.bp.105.010827. [DOI] [PubMed] [Google Scholar]
- 23.Bijlsma EY, van Leeuwen ML, Westphal KG, Olivier B, Groenink L. Local repeated corticotropin-releasing factor infusion exacerbates anxiety- and fear-related behavior: differential involvement of the basolateral amygdala and medial prefrontal cortex. Neuroscience. 2011;173:82–92. doi: 10.1016/j.neuroscience.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 24.Bisetti A, Cvetkovic V, Serafin M, Bayer L, Machard D, Jones BE, Muhlethaler M. Excitatory action of hypocretin/orexin on neurons of the central medial amygdala. Neuroscience. 2006;142:999–1004. doi: 10.1016/j.neuroscience.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Blanchard DC, Griebel G, Blanchard RJ. Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci Biobehav Rev. 2001;25:205–218. doi: 10.1016/s0149-7634(01)00009-4. [DOI] [PubMed] [Google Scholar]
- 26.Blanchard RJ, Blanchard DC. Attack and defense in rodents as ethoexperimental models for the study of emotion. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13(Suppl):S3–14. doi: 10.1016/0278-5846(89)90105-x. [DOI] [PubMed] [Google Scholar]
- 27.Blanchard RJ, Flannelly KJ, Blanchard DC. Defensive behavior of laboratory and wild Rattus norvegicus. J Comp Psychol. 1986a;100:101–107. [PubMed] [Google Scholar]
- 28.Blanchard RJ, Flannelly KJ, Blanchard DC. Defensive behavior of laboratory and wild Rattus norvegicus. J Comp Psychol. 1986b;100:101–107. [PubMed] [Google Scholar]
- 29.Blanchard RJ, Nikulina JN, Sakai RR, McKittrick C, McEwen B, Blanchard DC. Behavioral and endocrine change following chronic predatory stress. Physiol Behav. 1998;63:561–569. doi: 10.1016/s0031-9384(97)00508-8. [DOI] [PubMed] [Google Scholar]
- 30.Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de LL. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradley SR, Pieribone VA, Wang W, Severson CA, Jacobs RA, Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat Neurosci. 2002;5:401–402. doi: 10.1038/nn848. [DOI] [PubMed] [Google Scholar]
- 32.Bradwejn J, Koszycki D, Payeur R, Bourin M, Borthwick H. Replication of action of cholecystokinin tetrapeptide in panic disorder: clinical and behavioral findings. Am J Psychiatry. 1992;149:962–964. doi: 10.1176/ajp.149.7.962. [DOI] [PubMed] [Google Scholar]
- 33.Bradwejn J, Koszycki D, Shriqui C. Enhanced sensitivity to cholecystokinin tetrapeptide in panic disorder. Clinical and behavioral findings. Arch Gen Psychiatry. 1991;48:603–610. doi: 10.1001/archpsyc.1991.01810310021005. [DOI] [PubMed] [Google Scholar]
- 34.Brandao ML, Lopez-Garcia JA, Graeff FG, Roberts MH. Electrophysiological evidence for excitatory 5-HT2 and depressant 5-HT1A receptors on neurones of the rat midbrain tectum. Brain Res. 1991;556:259–266. doi: 10.1016/0006-8993(91)90313-k. [DOI] [PubMed] [Google Scholar]
- 35.Brandao ML, Zanoveli JM, Ruiz-Martinez RC, Oliveira LC, Landeira-Fernandez J. Different patterns of freezing behavior organized in the periaqueductal gray of rats: association with different types of anxiety. Behav Brain Res. 2008;188:1–13. doi: 10.1016/j.bbr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Brannan S, Liotti M, Egan G, Shade R, Madden L, Robillard R, Abplanalp B, Stofer K, Denton D, Fox PT. Neuroimaging of cerebral activations and deactivations associated with hypercapnia and hunger for air. Proc Natl Acad Sci U S A. 2001;98:2029–2034. doi: 10.1073/pnas.98.4.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown RE, Sergeeva O, Eriksson KS, Haas HL. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology. 2001;40:457–459. doi: 10.1016/s0028-3908(00)00178-7. [DOI] [PubMed] [Google Scholar]
- 38.Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline) J Neurosci. 2002;22:8850–8859. doi: 10.1523/JNEUROSCI.22-20-08850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci U S A. 2010;107:16354–16359. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buckler KJ, Turner PJ. Oxygen sensitivity of mitochondrial function in rat arterial chemoreceptor cells. J Physiol (Lond) 2013;591:3549–3563. doi: 10.1113/jphysiol.2013.257741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cameron AA, Khan IA, Westlund KN, Cliffer KD, Willis WD. The efferent projections of the periaqueductal gray in the rat: a Phaseolus vulgaris-leucoagglutinin study. I. Ascending projections. J Comp Neurol. 1995a;351:568–584. doi: 10.1002/cne.903510407. [DOI] [PubMed] [Google Scholar]
- 42.Cameron AA, Khan IA, Westlund KN, Willis WD. The efferent projections of the periaqueductal gray in the rat: a Phaseolus vulgaris-leucoagglutinin study. II. Descending projections. J Comp Neurol. 1995b;351:585–601. doi: 10.1002/cne.903510408. [DOI] [PubMed] [Google Scholar]
- 43.Carrive P. Orexin, orexin receptor antagonists and central cardiovascular control. Front Neurosci. 2013;7:257. doi: 10.3389/fnins.2013.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cassell MD, Gray TS, Kiss JZ. Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological, and immunocytochemical study. J Comp Neurol. 1986;246:478–499. doi: 10.1002/cne.902460406. [DOI] [PubMed] [Google Scholar]
- 45.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charney DS, Heninger GR, Jatlow PI. Increased anxiogenic effects of caffeine in panic disorders. Arch Gen Psychiatry. 1985;42:233–243. doi: 10.1001/archpsyc.1985.01790260027003. [DOI] [PubMed] [Google Scholar]
- 47.Charney DS, Woods SW, Goodman WK, Heninger GR. Neurobiological mechanisms of panic anxiety: biochemical and behavioral correlates of yohimbine-induced panic attacks. Am J Psychiatry. 1987;144:1030–1036. doi: 10.1176/ajp.144.8.1030. [DOI] [PubMed] [Google Scholar]
- 48.Choi DC, Nguyen MM, Tamashiro KL, Ma LY, Sakai RR, Herman JP. Chronic social stress in the visible burrow system modulates stress-related gene expression in the bed nucleus of the stria terminalis. Physiol Behav. 2006;89:301–310. doi: 10.1016/j.physbeh.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 49.Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul Pept. 2002;104:131–144. doi: 10.1016/s0167-0115(01)00357-3. [DOI] [PubMed] [Google Scholar]
- 50.Connor KM, Davidson JR. Generalized anxiety disorder: neurobiological and pharmacotherapeutic perspectives. Biol Psychiatry. 1998;44:1286–1294. doi: 10.1016/s0006-3223(98)00285-6. [DOI] [PubMed] [Google Scholar]
- 51.Corcoran AE, Richerson GB, Harris MB. Serotonergic mechanisms are necessary for central respiratory chemoresponsiveness in situ. Respir Physiol Neurobiol. 2013;186:214–220. doi: 10.1016/j.resp.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cordero MI, Merino JJ, Sandi C. Correlational relationship between shock intensity and corticosterone secretion on the establishment and subsequent expression of contextual fear conditioning. Behav Neurosci. 1998;112:885–891. doi: 10.1037//0735-7044.112.4.885. [DOI] [PubMed] [Google Scholar]
- 53.Coryell MW, Wunsch AM, Haenfler JM, Allen JE, McBride JL, Davidson BL, Wemmie JA. Restoring Acid-sensing ion channel-1a in the amygdala of knock-out mice rescues fear memory but not unconditioned fear responses. J Neurosci. 2008;28:13738–13741. doi: 10.1523/JNEUROSCI.3907-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coryell MW, Ziemann AE, Westmoreland PJ, Haenfler JM, Kurjakovic Z, Zha XM, Price M, Schnizler MK, Wemmie JA. Targeting ASIC1a reduces innate fear and alters neuronal activity in the fear circuit. Biol Psychiatry. 2007;62:1140–1148. doi: 10.1016/j.biopsych.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 55.Cox GE, Jordan D, Paton JF, Spyer KM, Wood LM. Cardiovascular and phrenic nerve responses to stimulation of the amygdala central nucleus in the anaesthetized rabbit. J Physiol. 1987;389:541–556. doi: 10.1113/jphysiol.1987.sp016671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Craske MG, Kircanski K, Epstein A, Wittchen HU, Pine DS, Lewis-Fernandez R, Hinton D. Panic disorder: a review of DSM-IV panic disorder and proposals for DSM-V. Depress Anxiety. 2010;27:93–112. doi: 10.1002/da.20654. [DOI] [PubMed] [Google Scholar]
- 57.Crawford LK, Craige CP, Beck SG. Increased intrinsic excitability of lateral wing serotonin neurons of the dorsal raphe: a mechanism for selective activation in stress circuits. J Neurophysiol. 2010;103:2652–2663. doi: 10.1152/jn.01132.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.D’Amato FR, Zanettini C, Lampis V, Coccurello R, Pascucci T, Ventura R, Puglisi-Allegra S, Spatola CA, Pesenti-Gritti P, Oddi D, Moles A, Battaglia M. Unstable maternal environment, separation anxiety, and heightened CO2 sensitivity induced by gene-by-environment interplay. PLoS ONE. 2011;6:e18637. doi: 10.1371/journal.pone.0018637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Daugherty WP, Corley KC, Phan TH, Boadle-Biber MC. Further studies on the activation of rat median raphe serotonergic neurons by inescapable sound stress. Brain Res. 2001;923:103–111. doi: 10.1016/s0006-8993(01)03198-5. [DOI] [PubMed] [Google Scholar]
- 60.Davis M. Neurochemical modulation of sensory-motor reactivity: acoustic and tactile startle reflexes. Neurosci Biobehav Rev. 1980;4:241–263. doi: 10.1016/0149-7634(80)90016-0. [DOI] [PubMed] [Google Scholar]
- 61.Davis M, Shi C. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann N Y Acad Sci. 1999;877:281–291. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- 62.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S. Differential expression of 5HT-1A, alpha(1b) adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J Comp Neurol. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Bortoli VC, Nogueira RL, Zangrossi H., Jr Alprazolam potentiates the antiaversive effect induced by the activation of 5-HT(1A) and 5-HT (2A) receptors in the rat dorsal periaqueductal gray. Psychopharmacology (Berl) 2008;198:341–349. doi: 10.1007/s00213-008-1134-7. [DOI] [PubMed] [Google Scholar]
- 65.de Bortoli VC, Nogueira RL, Zangrossi H., Jr Effects of fluoxetine and buspirone on the panicolytic-like response induced by the activation of 5-HT1A and 5-HT2A receptors in the rat dorsal periaqueductal gray. Psychopharmacology (Berl) 2006;183:422–428. doi: 10.1007/s00213-005-0189-y. [DOI] [PubMed] [Google Scholar]
- 66.de Lecea L. A decade of hypocretins: past, present and future of the neurobiology of arousal. Acta Physiol (Oxf) 2010;198:203–208. doi: 10.1111/j.1748-1716.2009.02004.x. [DOI] [PubMed] [Google Scholar]
- 67.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, Van Den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Oliveira ST, de Bortoli VC, Zangrossi H., Jr Serotonin-2A receptor regulation of panic-like behavior in the rat dorsal periaqueductal gray matter: the role of GABA. Psychopharmacology (Berl) 2011;218:725–732. doi: 10.1007/s00213-011-2369-2. [DOI] [PubMed] [Google Scholar]
- 69.de Paula Soares V, Zangrossi H., Jr Involvement of 5-HT1A and 5-HT2 receptors of the dorsal periaqueductal gray in the regulation of the defensive behaviors generated by the elevated T-maze. Brain Res Bull. 2004;64:181–188. doi: 10.1016/j.brainresbull.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 70.De Souza EB, Insel TR, Perrin MH, Rivier J, Vale WW, Kuhar MJ. Corticotropin-releasing factor receptors are widely distributed within the rat central nervous system: an autoradiographic study. J Neurosci. 1985;5:3189–3203. doi: 10.1523/JNEUROSCI.05-12-03189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deakin JFW, Graeff FG. 5-HT and mechanisms of defence. Journal of Psychopharmacology. 1991;5:305–315. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- 72.Del-Ben CM, Graeff FG. Panic disorder: is the PAG involved? Neural Plast. 2009;2009 doi: 10.1155/2009/108135. 108135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dilts RP, Boadle-Biber MC. Differential activation of the 5-hydroxytryptamine-containing neurons of the midbrain raphe of the rat in response to randomly presented inescapable sound. Neurosci Lett. 1995;199:78–80. doi: 10.1016/0304-3940(95)12027-2. [DOI] [PubMed] [Google Scholar]
- 74.Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- 75.Donner NC, Johnson PL, Fitz SD, Kellen KE, Shekhar A, Lowry CA. Elevated tph2 mRNA expression in a rat model of chronic anxiety. Depress Anxiety. 2012;29:307–319. doi: 10.1002/da.21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dumont FS, Biancardi V, Kinkead R. Hypercapnic ventilatory response of anesthetized female rats subjected to neonatal maternal separation: insight into the origins of panic attacks? Respir Physiol Neurobiol. 2011;175:288–295. doi: 10.1016/j.resp.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 77.Duvarci S. Central amygdala activity during fear conditioning. 2011 doi: 10.1523/JNEUROSCI.4985-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dwyer JM, Rizzo SJ, Neal SJ, Lin Q, Jow F, Arias RL, Rosenzweig-Lipson S, Dunlop J, Beyer CE. Acid sensing ion channel (ASIC) inhibitors exhibit anxiolytic-like activity in preclinical pharmacological models. Psychopharmacology (Berl) 2009;203:41–52. doi: 10.1007/s00213-008-1373-7. [DOI] [PubMed] [Google Scholar]
- 79.Emsley RA, Paster L. Lipoid proteinosis presenting with neuropsychiatric manifestations. J Neurol Neurosurg Psychiatry. 1985;48:1290–1292. doi: 10.1136/jnnp.48.12.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 81.Espana RA, Valentino RJ, Berridge CW. Fos immunoreactivity in hypocretin-synthesizing and hypocretin-1 receptor-expressing neurons: effects of diurnal and nocturnal spontaneous waking, stress and hypocretin-1 administration. Neuroscience. 2003;121:201–217. doi: 10.1016/s0306-4522(03)00334-8. [DOI] [PubMed] [Google Scholar]
- 82.Evans AK, Heerkens JL, Lowry CA. Acoustic stimulation in vivo and corticotropin-releasing factor in vitro increase tryptophan hydroxylase activity in the rat caudal dorsal raphe nucleus. Neurosci Lett. 2009;455:36–41. doi: 10.1016/j.neulet.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 83.Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychon Bull Rev. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- 84.Feinstein JS, Adolphs R, Damasio A, Tranel D. The human amygdala and the induction and experience of fear. Curr Biol. 2011;21:34–38. doi: 10.1016/j.cub.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Feinstein JS, Buzza C, Hurlemann R, Follmer RL, Dahdaleh NS, Coryell WH, Welsh MJ, Tranel D, Wemmie JA. Fear and panic in humans with bilateral amygdala damage. Nat Neurosci. 2013 doi: 10.1038/nn.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fernandez de Molina A, Hunsperger RW. Central representation of affective reactions in forebrain and brain stem: electrical stimulation of amygdala, stria terminalis, and adjacent structures. J Physiol (Lond) 1959;145:251–265. doi: 10.1113/jphysiol.1959.sp006140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fernandez de Molina A, Hunsperger RW. Organization of the subcortical system governing defence and flight reactions in the cat. J Physiol (Lond) 1962;160:200–213. doi: 10.1113/jphysiol.1962.sp006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Forster GL, Feng N, Watt MJ, Korzan WJ, Mouw NJ, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe elicits temporally distinct serotonergic responses in the limbic system in relation to fear behavior. Neuroscience. 2006;141:1047–1055. doi: 10.1016/j.neuroscience.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 89.Forster GL, Pringle RB, Mouw NJ, Vuong SM, Watt MJ, Burke AR, Lowry CA, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphe nucleus activity. Eur J Neurosci. 2008;28:299–310. doi: 10.1111/j.1460-9568.2008.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fox JH, Lowry CA. Corticotropin-releasing factor-related peptides, serotonergic systems, and emotional behavior. Front Neurosci. 2013;7:169. doi: 10.3389/fnins.2013.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Franchini KG, Krieger EM. Cardiovascular responses of conscious rats to carotid body chemoreceptor stimulation by intravenous KCN. J Auton Nerv Syst. 1993;42:63–69. doi: 10.1016/0165-1838(93)90342-r. [DOI] [PubMed] [Google Scholar]
- 92.Franchini KG, Oliveira VL, Krieger EM. Hemodynamics of chemoreflex activation in unanesthetized rats. Hypertension. 1997;30:699–703. doi: 10.1161/01.hyp.30.3.699. [DOI] [PubMed] [Google Scholar]
- 93.Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–243. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 94.Gardner KL, Hale MW, Oldfield S, Lightman SL, Plotsky PM, Lowry CA. Adverse experience during early life and adulthood interact to elevate tph2 mRNA expression in serotonergic neurons within the dorsal raphe nucleus. Neuroscience. 2009;163:991–1001. doi: 10.1016/j.neuroscience.2009.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA. Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience. 2005;136:181–191. doi: 10.1016/j.neuroscience.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 96.Gater R, Tansella M, Korten A, Tiemens BG, Mavreas VG, Olatawura MO. Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: report from the World Health Organization Collaborative Study on Psychological Problems in General Health Care. Arch Gen Psychiatry. 1998;55:405–413. doi: 10.1001/archpsyc.55.5.405. [DOI] [PubMed] [Google Scholar]
- 97.Genest SE, Gulemetova R, Laforest S, Drolet G, Kinkead R. Neonatal maternal separation induces sex-specific augmentation of the hypercapnic ventilatory response in awake rat. J Appl Physiol. 2007;102:1416–1421. doi: 10.1152/japplphysiol.00454.2006. [DOI] [PubMed] [Google Scholar]
- 98.Goodwin RD, Faravelli C, Rosi S, Cosci F, Truglia E, de GR, Wittchen HU. The epidemiology of panic disorder and agoraphobia in Europe. Eur Neuropsychopharmacol. 2005;15:435–443. doi: 10.1016/j.euroneuro.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 99.Gorman JM, Askanazi J, Liebowitz MR, Fyer AJ, Stein J, Kinney JM, Klein DF. Response to hyperventilation in a group of patients with panic disorder. Am J Psychiatry. 1984;141:857–861. doi: 10.1176/ajp.141.7.857. [DOI] [PubMed] [Google Scholar]
- 100.Gorman JM, Battista D, Goetz RR, Dillon DJ, Liebowitz MR, Fyer AJ, Kahn JP, Sandberg D, Klein DF. A comparison of sodium bicarbonate and sodium lactate infusion in the induction of panic attacks. Arch Gen Psychiatry. 1989a;46:145–150. doi: 10.1001/archpsyc.1989.01810020047008. [DOI] [PubMed] [Google Scholar]
- 101.Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry. 2000;157:493–505. doi: 10.1176/appi.ajp.157.4.493. [DOI] [PubMed] [Google Scholar]
- 102.Gorman JM, Liebowitz MR, Fyer AJ, Stein J. A neuroanatomical hypothesis for panic disorder. Am J Psychiatry. 1989b;146:148–161. doi: 10.1176/ajp.146.2.148. [DOI] [PubMed] [Google Scholar]
- 103.Graeff FG. Serotonin, the periaqueductal gray and panic. Neurosci Biobehav Rev. 2004;28:239–259. doi: 10.1016/j.neubiorev.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 104.Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- 105.Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13:451–460. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- 106.Greco MA, Shiromani PJ. Hypocretin receptor protein and mRNA expression in the dorsolateral pons of rats. Brain Res Mol Brain Res. 2001;88:176–182. doi: 10.1016/s0169-328x(01)00039-0. [DOI] [PubMed] [Google Scholar]
- 107.Griebel G, Blanchard DC, Blanchard RJ. Predator-elicited flight responses in Swiss-Webster mice: an experimental model of panic attacks. Prog Neuropsychopharmacol Biol Psychiatry. 1996a;20:185–205. doi: 10.1016/0278-5846(95)00305-3. [DOI] [PubMed] [Google Scholar]
- 108.Griebel G, Blanchard DC, Blanchard RJ. Predator-elicited flight responses in Swiss-Webster mice: an experimental model of panic attacks. Prog Neuropsychopharmacol Biol Psychiatry. 1996b;20:185–205. doi: 10.1016/0278-5846(95)00305-3. [DOI] [PubMed] [Google Scholar]
- 109.Griez E, Schruers K. Experimental pathophysiology of panic. J Psychosom Res. 1998;45:493–503. doi: 10.1016/s0022-3999(98)00027-0. [DOI] [PubMed] [Google Scholar]
- 110.Griffiths JL, Lovick TA. Co-localization of 5-HT 2A - receptor- and GABA-immunoreactivity in neurones in the periaqueductal grey matter of the rat. Neurosci Lett. 2002;326:151–154. doi: 10.1016/s0304-3940(02)00182-9. [DOI] [PubMed] [Google Scholar]
- 111.Guo JD, Hammack SE, Hazra R, Levita L, Rainnie DG. Bi-directional modulation of bed nucleus of stria terminalis neurons by 5-HT: molecular expression and functional properties of excitatory 5-HT receptor subtypes. Neuroscience. 2009;164:1776–1793. doi: 10.1016/j.neuroscience.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guttmacher LB, Murphy DL, Insel TR. Pharmacologic models of anxiety. Compr Psychiatry. 1983;24:312–326. doi: 10.1016/0010-440x(83)90059-7. [DOI] [PubMed] [Google Scholar]
- 113.Guyenet PG, Abbott SB, Stornetta RL. The respiratory chemoreception conundrum: light at the end of the tunnel? Brain Res. 2013;1511:126–137. doi: 10.1016/j.brainres.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol (Lond) 2008;586:2043–2048. doi: 10.1113/jphysiol.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hale MW, Lowry CA. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology (Berl) 2011;213:243–264. doi: 10.1007/s00213-010-2089-z. [DOI] [PubMed] [Google Scholar]
- 116.Hammack SE, Guo JD, Hazra R, Dabrowska J, Myers KM, Rainnie DG. The response of neurons in the bed nucleus of the stria terminalis to serotonin: implications for anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1309–1320. doi: 10.1016/j.pnpbp.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hayano F, Nakamura M, Asami T, Uehara K, Yoshida T, Roppongi T, Otsuka T, Inoue T, Hirayasu Y. Smaller amygdala is associated with anxiety in patients with panic disorder. Psychiatry Clin Neurosci. 2009;63:266–276. doi: 10.1111/j.1440-1819.2009.01960.x. [DOI] [PubMed] [Google Scholar]
- 118.Hayward LF, Von Reitzenstein M. c-Fos expression in the midbrain periaqueductal gray after chemoreceptor and baroreceptor activation. Am J Physiol Heart Circ Physiol. 2002;283:H1975–H1984. doi: 10.1152/ajpheart.00300.2002. [DOI] [PubMed] [Google Scholar]
- 119.Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103:777–797. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- 120.Hodges MR, Richerson GB. Medullary serotonin neurons and their roles in central respiratory chemoreception. Respir Physiol Neurobiol. 2010;173:256–263. doi: 10.1016/j.resp.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hollander E, Liebowitz MR, Gorman JM, Cohen B, Fyer A, Klein DF. Cortisol and sodium lactate-induced panic. Arch Gen Psychiatry. 1989;46:135–140. doi: 10.1001/archpsyc.1989.01810020037007. [DOI] [PubMed] [Google Scholar]
- 122.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 123.Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, Murakami N. Possible involvement of orexin in the stress reaction in rats. Biochem Biophys Res Commun. 2000;270:318–323. doi: 10.1006/bbrc.2000.2412. [DOI] [PubMed] [Google Scholar]
- 124.Jacob CA, Cabral AH, Almeida LP, Magierek V, Ramos PL, Zanoveli JM, Landeira-Fernandez J, Zangrossi H, Nogueira RL. Chronic imipramine enhances 5-HT(1A) and 5-HT(2) receptors-mediated inhibition of panic-like behavior in the rat dorsal periaqueductal gray. Pharmacol Biochem Behav. 2002;72:761–766. doi: 10.1016/s0091-3057(01)00785-7. [DOI] [PubMed] [Google Scholar]
- 125.Jansen ASP, Farkas E, Mac SJ, Loewy AD. Local connections between the columns of the periaqueductal gray matter: a case for intrinsic neuromodulation. Brain research. 1998;784(1998):329–336. doi: 10.1016/s0006-8993(98)00473-9. [DOI] [PubMed] [Google Scholar]; Brain Res. 797:368. [Google Scholar]
- 126.Jenck F, Moreau JL, Martin JR. Dorsal periaqueductal gray-induced aversion as a simulation of panic anxiety: elements of face and predictive validity. Psychiatry Res. 1995;57:181–191. doi: 10.1016/0165-1781(95)02673-k. [DOI] [PubMed] [Google Scholar]
- 127.Johnson PL, Fitz SD, Hollis JH, Moratalla R, Lightman SL, Shekhar A, Lowry CA. Induction of c-Fos in ‘panic/defence’-related brain circuits following brief hypercarbic gas exposure. J Psychopharmacol 2010. 2010a Jan 15; doi: 10.1177/0269881109353464. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 128.Johnson PL, Hollis JH, Moratalla R, Lightman SL, Lowry CA. Acute hypercarbic gas exposure reveals functionally distinct subpopulations of serotonergic neurons in rats. J Psychopharmacol. 2005;19:327–341. doi: 10.1177/0269881105053281. [DOI] [PubMed] [Google Scholar]
- 129.Johnson PL, Lightman SL, Lowry CA. A functional subset of serotonergic neurons in the rat ventrolateral periaqueductal gray implicated in the inhibition of sympathoexcitation and panic. Ann N Y Acad Sci. 2004;1018:58–64. doi: 10.1196/annals.1296.006. [DOI] [PubMed] [Google Scholar]
- 130.Johnson PL, Lowry CA, Truitt W, Shekhar A. Disruption of GABAergic tone in the dorsomedial hypothalamus attenuates responses in a subset of serotonergic neurons in the dorsal raphe nucleus following lactate-induced panic. J Psychopharmacol. 2008;22:642–645. doi: 10.1177/0269881107082900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Johnson PL, Sajdyk TJ, Fitz SD, Hale MW, Lowry CA, Hay-Schmidt A, Shekhar A. Angiotensin II’s role in sodium lactate-induced panic-like responses in rats with repeated urocortin 1 injections into the basolateral amygdala: Amygdalar angiotensin receptors and panic. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44C:248–256. doi: 10.1016/j.pnpbp.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Traskman-Bendz L, Goddard AW, Brundin L, Shekhar A. A key role for orexin in panic anxiety. Nat Med. 2010b;16:111–115. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Johnson PL, Truitt WA, Fitz SD, Lowry CA, Shekhar A. Neural Pathways Underlying Lactate-Induced Panic. Neuropsychopharmacology. 2007;33:2093–2107. doi: 10.1038/sj.npp.1301621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jokivarsi KT, Hiltunen Y, Grohn H, Tuunanen P, Grohn OH, Kauppinen RA. Estimation of the onset time of cerebral ischemia using T1rho and T2 MRI in rats. Stroke. 2010;41:2335–2340. doi: 10.1161/STROKEAHA.110.587394. [DOI] [PubMed] [Google Scholar]
- 135.Jones CK, Schlosser MJ, van Zijl PC, Pomper MG, Golay X, Zhou J. Amide proton transfer imaging of human brain tumors at 3T. Magn Reson Med. 2006;56:585–592. doi: 10.1002/mrm.20989. [DOI] [PubMed] [Google Scholar]
- 136.Kapp BS, Gallagher M, Underwood MD, McNall CL, Whitehorn D. Cardiovascular responses elicited by electrical stimulation of the amygdala central nucleus in the rabbit. Brain Res. 1982;234:251–262. doi: 10.1016/0006-8993(82)90866-6. [DOI] [PubMed] [Google Scholar]
- 137.Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 138.Kellner M. Experimental panic provocation in healthy man-a translational role in anti-panic drug development? Dialogues Clin Neurosci. 2011;13:485–493. doi: 10.31887/DCNS.2011.13.4/mkellner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kellner M, Wiedemann K. Nonresponse of adrenocorticotropic hormone in first-ever lactate-induced panic attacks in healthy volunteers. Arch Gen Psychiatry. 1998;55:85–86. doi: 10.1001/archpsyc.55.1.85. [DOI] [PubMed] [Google Scholar]
- 140.Kerman IA, Shabrang C, Taylor L, Akil H, Watson SJ. Relationship of presympathetic-premotor neurons to the serotonergic transmitter system in the rat brainstem. J Comp Neurol. 2006;499:882–896. doi: 10.1002/cne.21129. [DOI] [PubMed] [Google Scholar]
- 141.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005a;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 142.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005b;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kessler RC, Chiu WT, Jin R, Ruscio AM, Shear K, Walters EE. The epidemiology of panic attacks, panic disorder, and agoraphobia in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2006;63:415–424. doi: 10.1001/archpsyc.63.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kilduff TS, de Lecea L. Mapping of the mRNAs for the hypocretin/orexin and melanin-concentrating hormone receptors: networks of overlapping peptide systems. J Comp Neurol. 2001;435:1–5. doi: 10.1002/cne.1189. [DOI] [PubMed] [Google Scholar]
- 146.Kim SJ, Park SH, Choi SH, Moon BH, Lee KJ, Kang SW, Lee MS, Choi SH, Chun BG, Shin KH. Effects of repeated tianeptine treatment on CRF mRNA expression in non-stressed and chronic mild stress-exposed rats. Neuropharmacology. 2006;50:824–833. doi: 10.1016/j.neuropharm.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 147.Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- 148.Klein DF. DELINEATION OF TWO DRUG-RESPONSIVE ANXIETY SYNDROMES. Psychopharmacologia. 1964;5:397–408. doi: 10.1007/BF02193476. [DOI] [PubMed] [Google Scholar]
- 149.Klein DF. Response differences of spontaneous panic and fear. Arch Gen Psychiatry. 2002;59:567–569. doi: 10.1001/archpsyc.59.6.567. [DOI] [PubMed] [Google Scholar]
- 150.Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Arch Gen Psychiatry. 1993;50:306–317. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- 151.Klein RG. Is panic disorder associated with childhood separation anxiety disorder? Clin Neuropharmacol. 1995;18:S7–S14. [Google Scholar]
- 152.Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- 153.Kohler C, Steinbusch H. Identification of serotonin and non-serotonin-containing neurons of the mid-brain raphe projecting to the entorhinal area and the hippocampal formation. A combined immunohistochemical and fluorescent retrograde tracing study in the rat brain. Neuroscience. 1982;7:951–975. doi: 10.1016/0306-4522(82)90054-9. [DOI] [PubMed] [Google Scholar]
- 154.Kohlmeier KA, Watanabe S, Tyler CJ, Burlet S, Leonard CS. Dual orexin actions on dorsal raphe and laterodorsal tegmentum neurons: noisy cation current activation and selective enhancement of Ca2+ transients mediated by L-type calcium channels. J Neurophysiol. 2008;100:2265–2281. doi: 10.1152/jn.01388.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Koob GF, Le MM. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kuwaki T. Orexin links emotional stress to autonomic functions. Auton Neurosci. 2011;161:20–27. doi: 10.1016/j.autneu.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 157.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee HS, Park SH, Song WC, Waterhouse BD. Retrograde study of hypocretin-1 (orexin-A) projections to subdivisions of the dorsal raphe nucleus in the rat. Brain Res. 2005;1059:35–45. doi: 10.1016/j.brainres.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 159.Lee Y, Fitz S, Johnson PL, Shekhar A. Repeated stimulation of CRF receptors in the BNST of rats selectively induces social but not panic-like anxiety. Neuropsychopharmacology. 2008;33:2586–2594. doi: 10.1038/sj.npp.1301674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Levin AP, Doran AR, Liebowitz MR, Fyer AJ, Gorman JM, Klein DF, Paul SM. Pituitary adrenocortical unresponsiveness in lactate-induced panic. Psychiatry Res. 1987;21:23–32. doi: 10.1016/0165-1781(87)90058-8. [DOI] [PubMed] [Google Scholar]
- 161.Lewis-Fernandez R, Hinton DE, Laria AJ, Patterson EH, Hofmann SG, Craske MG, Stein DJ, Asnaani A, Liao B. Culture and the anxiety disorders: recommendations for DSM-V. Depress Anxiety. 2010;27:212–229. doi: 10.1002/da.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ley R. Dyspneic-fear and catastrophic cognitions in hyperventilatory panic attacks. Behav Res Ther. 1989;27:549–554. doi: 10.1016/0005-7967(89)90089-2. [DOI] [PubMed] [Google Scholar]
- 163.Ley R. Agoraphobia, the panic attack and the hyperventilation syndrome. Behav Res Ther. 1985;23:79–81. doi: 10.1016/0005-7967(85)90145-7. [DOI] [PubMed] [Google Scholar]
- 164.Ley R. The many faces of Pan: psychological and physiological differences among three types of panic attacks. Behav Res Ther. 1992;30:347–357. doi: 10.1016/0005-7967(92)90046-j. [DOI] [PubMed] [Google Scholar]
- 165.Li J, Hu Z, de Lecea L. The hypocretins/orexins: integrators of multiple physiological functions. Br J Pharmacol. 2014;171:332–350. doi: 10.1111/bph.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liebowitz MR, Gorman JM, Fyer A, Dillon D, Levitt M, Klein DF. Possible mechanisms for lactate’s induction of panic. Am J Psychiatry. 1986;143:495–502. doi: 10.1176/ajp.143.4.495. [DOI] [PubMed] [Google Scholar]
- 167.Liu RJ, Van Den Pol AN, Aghajanian GK. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci. 2002;22:9453–9464. doi: 10.1523/JNEUROSCI.22-21-09453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann N Y Acad Sci. 2008;1148:86–94. doi: 10.1196/annals.1410.004. [DOI] [PubMed] [Google Scholar]
- 169.Lowry CA, Hollis JH, de VA, Pan B, Brunet LR, Hunt JR, Paton JF, van KE, Knight DM, Evans AK, Rook GA, Lightman SL. Identification of an immune-responsive mesolimbocortical serotonergic system: Potential role in regulation of emotional behavior. Neuroscience. 2007;146:756–772. doi: 10.1016/j.neuroscience.2007.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lowry CA, Rodda JE, Lightman SL, Ingram CD. Corticotropin-releasing factor increases In vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: evidence for activation of a topographically organized mesolimbocortical serotonergic system. J Neurosci. 2000;20:7728–7736. doi: 10.1523/JNEUROSCI.20-20-07728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lukkes JL, Forster GL, Renner KJ, Summers CH. Corticotropin-releasing factor 1 and 2 receptors in the dorsal raphe differentially affect serotonin release in the nucleus accumbens. Eur J Pharmacol. 2008;578:185–193. doi: 10.1016/j.ejphar.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lukkes JL, Kopelman JM, Donner NC, Hale MW, Lowry CA. Development × environment interactions control tph2 mRNA expression. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lukkes JL, Staub DR, Dietrich A, Truitt W, Neufeld-Cohen A, Chen A, Johnson PL, Shekhar A, Lowry CA. Topographical distribution of corticotropin-releasing factor type 2 receptor-like immunoreactivity in the rat dorsal raphe nucleus: co- localization with tryptophan hydroxylase. Neuroscience. 2011;183:47–63. doi: 10.1016/j.neuroscience.2011.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lungwitz EA, Molosh A, Johnson PL, Harvey BP, Dirks RC, Dietrich A, Minick P, Shekhar A, Truitt WA. Orexin-A induces anxiety-like behavior through interactions with glutamatergic receptors in the bed nucleus of the stria terminalis of rats. Physiol Behav. 2012;107:726–732. doi: 10.1016/j.physbeh.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Maddock RJ, Buonocore MH, Copeland LE, Richards AL. Elevated brain lactate responses to neural activation in panic disorder: a dynamic 1H-MRS study. Mol Psychiatry. 2009;14:537–545. doi: 10.1038/sj.mp.4002137. [DOI] [PubMed] [Google Scholar]
- 176.Magnotta VA, Heo HY, Dlouhy BJ, Dahdaleh NS, Follmer RL, Thedens DR, Welsh MJ, Wemmie JA. Detecting activity-evoked pH changes in human brain. Proc Natl Acad Sci U S A. 2012;109:8270–8273. doi: 10.1073/pnas.1205902109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Makela HI, Grohn OH, Kettunen MI, Kauppinen RA. Proton exchange as a relaxation mechanism for T1 in the rotating frame in native and immobilized protein solutions. Biochem Biophys Res Commun. 2001;289:813–818. doi: 10.1006/bbrc.2001.6058. [DOI] [PubMed] [Google Scholar]
- 178.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 179.Martinez RC, de Oliveira AR, Brandao ML. Conditioned and unconditioned fear organized in the periaqueductal gray are differentially sensitive to injections of muscimol into amygdaloid nuclei. Neurobiol Learn Mem. 2006;85:58–65. doi: 10.1016/j.nlm.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 180.Masini CV, Sauer S, Campeau S. Ferret odor as a processive stress model in rats: neurochemical, behavioral, and endocrine evidence. Behav Neurosci. 2005;119:280–292. doi: 10.1037/0735-7044.119.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Masini CV, Sauer S, White J, Day HE, Campeau S. Non-associative defensive responses of rats to ferret odor. Physiol Behav. 2006;87:72–81. doi: 10.1016/j.physbeh.2005.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci Biobehav Rev. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 183.Mochcovitch MD, Nardi AE. Selective serotonin-reuptake inhibitors in the treatment of panic disorder: a systematic review of placebo-controlled studies. Expert Rev Neurother. 2010;10:1285–1293. doi: 10.1586/ern.10.110. [DOI] [PubMed] [Google Scholar]
- 184.Moga MM, Saper CB, Gray TS. Bed nucleus of the stria terminalis: cytoarchitecture, immunohistochemistry, and projection to the parabrachial nucleus in the rat. J Comp Neurol. 1989;283:315–332. doi: 10.1002/cne.902830302. [DOI] [PubMed] [Google Scholar]
- 185.Mongeau R, Marsden CA. Effect of central and peripheral administrations of cholecystokinin-tetrapeptide on panic-like reactions induced by stimulation of the dorsal periaqueductal grey area in the rat. Biol Psychiatry. 1997b;42:335–344. doi: 10.1016/S0006-3223(96)00407-6. [DOI] [PubMed] [Google Scholar]
- 186.Mongeau R, Marsden CA. Effect of imipramine treatments on the 5-HT1A-receptor-mediated inhibition of panic-like behaviours in rats. Psychopharmacology (Berl) 1997a;131:321–328. doi: 10.1007/s002130050299. [DOI] [PubMed] [Google Scholar]
- 187.Muraki Y, Yamanaka A, Tsujino N, Kilduff TS, Goto K, Sakurai T. Serotonergic regulation of the orexin/hypocretin neurons through the 5-HT1A receptor. J Neurosci. 2004;24:7159–7166. doi: 10.1523/JNEUROSCI.1027-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nader K, Majidishad P, Amorapanth P, LeDoux JE. Damage to the lateral and central, but not other, amygdaloid nuclei prevents the acquisition of auditory fear conditioning. Learn Mem. 2001;8:156–163. doi: 10.1101/lm.38101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nakamura S, Tsumori T, Yokota S, Oka T, Yasui Y. Amygdaloid axons innervate melanin-concentrating hormone- and orexin-containing neurons in the mouse lateral hypothalamus. Brain Res. 2009;1278:66–74. doi: 10.1016/j.brainres.2009.04.049. [DOI] [PubMed] [Google Scholar]
- 190.Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- 191.Nashold BS, Jr, Wilson WP, Slaughter DG. Sensations evoked by stimulation in the midbrain of man. J Neurosurg. 1969;30:14–24. doi: 10.3171/jns.1969.30.1.0014. [DOI] [PubMed] [Google Scholar]
- 192.Nesse RM, Cameron OG, Curtis GC, McCann DS, Huber-Smith MJ. Adrenergic function in patients with panic anxiety. Arch Gen Psychiatry. 1984;41:771–776. doi: 10.1001/archpsyc.1984.01790190045005. [DOI] [PubMed] [Google Scholar]
- 193.Nierenberg AA. Timing of onset of antidepressant response with fluoxetine treatment. 2000 doi: 10.1176/appi.ajp.157.9.1423. [DOI] [PubMed] [Google Scholar]
- 194.Nogueira RL, Graeff FG. Role of 5-HT receptor subtypes in the modulation of dorsal periaqueductal gray generated aversion. Pharmacol Biochem Behav. 1995;52:1–6. doi: 10.1016/0091-3057(94)00402-5. [DOI] [PubMed] [Google Scholar]
- 195.Noyes R., Jr Comorbidity in generalized anxiety disorder. Psychiatr Clin North Am. 2001;24:41–55. doi: 10.1016/s0193-953x(05)70205-7. [DOI] [PubMed] [Google Scholar]
- 196.Noyes R, Jr, Woodman C, Garvey MJ, Cook BL, Suelzer M, Clancy J, Anderson DJ. Generalized anxiety disorder vs. panic disorder Distinguishing characteristics and patterns of comorbidity. J Nerv Ment Dis. 1992;180:369–379. doi: 10.1097/00005053-199206000-00006. [DOI] [PubMed] [Google Scholar]
- 197.Nutt DJ. Overview of diagnosis and drug treatments of anxiety disorders. CNS Spectr. 2005;10:49–56. doi: 10.1017/s1092852900009901. [DOI] [PubMed] [Google Scholar]
- 198.Oka T, Tsumori T, Yokota S, Yasui Y. Neuroanatomical and neurochemical organization of projections from the central amygdaloid nucleus to the nucleus retroambiguus via the periaqueductal gray in the rat. Neurosci Res. 2008;62:286–298. doi: 10.1016/j.neures.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 199.Paul ED, Hale MW, Lukkes JL, Valentine MJ, Sarchet DM, Lowry CA. Repeated social defeat increases reactive emotional coping behavior and alters functional responses in serotonergic neurons in the rat dorsal raphe nucleus. Physiol Behav. 2011;104:272–282. doi: 10.1016/j.physbeh.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Paul ED, Lowry CA. Functional topography of serotonergic systems supports the Deakin/Graeff hypothesis of anxiety and affective disorders. J Psychopharmacol. 2013;27:1090–1106. doi: 10.1177/0269881113490328. [DOI] [PubMed] [Google Scholar]
- 201.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Fourth Edition. San Diego: Academic Press; 1998. [Google Scholar]
- 202.Pernar L, Curtis AL, Vale WW, Rivier JE, Valentino RJ. Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J Neurosci. 2004;24:1305–1311. doi: 10.1523/JNEUROSCI.2885-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Peskind ER, Jensen CF, Pascualy M, Tsuang D, Cowley D, Martin DC, Wilkinson CW, Raskind MA. Sodium lactate and hypertonic sodium chloride induce equivalent panic incidence, panic symptoms, and hypernatremia in panic disorder. Biol Psychiatry. 1998;44:1007–1016. doi: 10.1016/s0006-3223(98)00053-5. [DOI] [PubMed] [Google Scholar]
- 204.Petrovich GD, Swanson LW. Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Res. 1997;763:247–254. doi: 10.1016/s0006-8993(96)01361-3. [DOI] [PubMed] [Google Scholar]
- 205.Peyron C, Petit J-M, Rampon C, Jouvet M, Luppi P-H. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998a;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- 206.Peyron C, Tighe DK, Van Den Pol AN, de LL, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998b;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pitts FN, Jr, McClure JN., Jr Lactate metabolism in anxiety neurosis. N Engl J Med. 1967;277:1329–1336. doi: 10.1056/NEJM196712212772502. [DOI] [PubMed] [Google Scholar]
- 208.Pobbe RL, Zangrossi H., Jr 5-HT(1A) and 5-HT(2A) receptors in the rat dorsal periaqueductal gray mediate the antipanic-like effect induced by the stimulation of serotonergic neurons in the dorsal raphe nucleus. Psychopharmacology (Berl) 2005;183:314–321. doi: 10.1007/s00213-005-0196-z. [DOI] [PubMed] [Google Scholar]
- 209.Pobbe RL, Zangrossi H, Jr, Blanchard DC, Blanchard RJ. Involvement of dorsal raphe nucleus and dorsal periaqueductal gray 5-HT receptors in the modulation of mouse defensive behaviors. Eur Neuropsychopharmacol. 2011;21:306–315. doi: 10.1016/j.euroneuro.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Porrino LJ, Goldman-Rakic PS. Brainstem innervation of prefrontal and anterior cingulate cortex in the rhesus monkey revealed by retrograde transport of HRP. J Comp Neurol. 1982;205:63–76. doi: 10.1002/cne.902050107. [DOI] [PubMed] [Google Scholar]
- 211.Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale W. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci U S A. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Preter M, Klein DF. Panic, suffocation false alarms, separation anxiety and endogenous opioids. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:603–612. doi: 10.1016/j.pnpbp.2007.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rainey JM, Jr, Pohl RB, Williams M, Knitter E, Freedman RR, Ettedgui E. A comparison of lactate and isoproterenol anxiety states. Psychopathology. 1984;17(Suppl 1):74–82. doi: 10.1159/000284080. [DOI] [PubMed] [Google Scholar]
- 214.Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Reiman EM, Raichle ME, Robins E, Mintun MA, Fusselman MJ, Fox PT, Price JL, Hackman KA. Neuroanatomical correlates of a lactate-induced anxiety attack. Arch Gen Psychiatry. 1989;46:493–500. doi: 10.1001/archpsyc.1989.01810060013003. [DOI] [PubMed] [Google Scholar]
- 216.Reyes TM, Walker JR, DeCino C, Hogenesch JB, Sawchenko PE. Categorically distinct acute stressors elicit dissimilar transcriptional profiles in the paraventricular nucleus of the hypothalamus. J Neurosci. 2003;23:5607–5616. doi: 10.1523/JNEUROSCI.23-13-05607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- 218.Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. J Comp Neurol. 1991;303:121–131. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- 219.Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychol Rev. 1998;105:325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- 220.Rozeske RR, Evans AK, Frank MG, Watkins LR, Lowry CA, Maier SF. Uncontrollable, But Not Controllable, Stress Desensitizes 5-HT1A Receptors in the Dorsal Raphe Nucleus. J Neurosci. 2011;31:14107–14115. doi: 10.1523/JNEUROSCI.3095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sajdyk T, Johnson P, Fitz S, Shekhar A. Chronic inhibition of GABA synthesis in the bed nucleus of the stria terminalis elicits anxiety-like behavior. J Psychopharmacol. 2008;22:633–641. doi: 10.1177/0269881107082902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res. 1999;100:207–215. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- 223.Sakamoto F, Yamada S, Ueta Y. Centrally administered orexin-A activates corticotropin-releasing factor-containing neurons in the hypothalamic paraventricular nucleus and central amygdaloid nucleus of rats: possible involvement of central orexins on stress-activated central CRF neurons. Regul Pept. 2004;118:183–191. doi: 10.1016/j.regpep.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 224.Sakanaka M, Shibasaki T, Lederis K. Distribution of efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- 225.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 226.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 227.Sakurai T, Mieda M. Connectomics of orexin-producing neurons: interface of systems of emotion, energy homeostasis and arousal. Trends Pharmacol Sci. 2011;32:451–462. doi: 10.1016/j.tips.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 228.Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, Koyama Y, Shioda S, Yanagisawa M. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 229.Sandner G, Schmitt P, Karli P. Mapping of jumping, rearing, squealing and switch-off behaviors elicited by periaqueductal gray stimulation in the rat. Physiol Behav. 1987;39:333–339. doi: 10.1016/0031-9384(87)90231-9. [DOI] [PubMed] [Google Scholar]
- 230.Schenberg LC, Bittencourt AS, Sudre EC, Vargas LC. Modeling panic attacks. Neurosci Biobehav Rev. 2001;25:647–659. doi: 10.1016/s0149-7634(01)00060-4. [DOI] [PubMed] [Google Scholar]
- 231.Schenberg LC, Dos Reis AM, Ferreira Povoa RM, Tufik S, Silva SR. A panic attack-like unusual stress reaction. Horm Behav. 2008;54:584–591. doi: 10.1016/j.yhbeh.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 232.Schenberg LC, Vasquez EC, da Costa MB. Cardiac baroreflex dynamics during the defence reaction in freely moving rats. Brain Res. 1993;621:50–58. doi: 10.1016/0006-8993(93)90296-y. [DOI] [PubMed] [Google Scholar]
- 233.Schimitel FG, de Almeida GM, Pitol DN, Armini RS, Tufik S, Schenberg LC. Evidence of a suffocation alarm system within the periaqueductal gray matter of the rat. Neuroscience. 2012;200:59–73. doi: 10.1016/j.neuroscience.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 234.Schneier FR. Fluoxetine in panic disorder. 1990 doi: 10.1097/00004714-199004000-00007. [DOI] [PubMed] [Google Scholar]
- 235.Sela VR, Biesdorf C, Ramos DH, Zangrossi H, Jr, Graeff FG, Audi EA. Serotonin-1A receptors in the dorsal periaqueductal gray matter mediate the panicolytic-like effect of pindolol and paroxetine combination in the elevated T-maze. Neurosci Lett. 2011;495:63–66. doi: 10.1016/j.neulet.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 236.Sellayah D, Sikder D. Food for thought: understanding the multifaceted nature of orexins. Endocrinology. 2013;154:3990–3999. doi: 10.1210/en.2013-1488. [DOI] [PubMed] [Google Scholar]
- 237.Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci. 2003;6:1139–1140. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- 238.Shekhar A, Johnson PL, Sajdyk TJ, Fitz SD, Keim SR, Kelley PE, Gehlert DR, DiMicco JA. Angiotensin-II is a putative neurotransmitter in lactate-induced panic-like responses in rats with disruption of GABAergic inhibition in the dorsomedial hypothalamus. J Neurosci. 2006;26:9205–9215. doi: 10.1523/JNEUROSCI.2491-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Shekhar A, Keim SR. The circumventricular organs form a potential neural pathway for lactate sensitivity: implications for panic disorder. J Neurosci. 1997;17:9726–9735. doi: 10.1523/JNEUROSCI.17-24-09726.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Shekhar A, Keim SR, Simon JR, McBride WJ. Dorsomedial hypothalamic GABA dysfunction produces physiological arousal following sodium lactate infusions. Pharmacol Biochem Behav. 1996;55:249–256. doi: 10.1016/s0091-3057(96)00077-9. [DOI] [PubMed] [Google Scholar]
- 241.Simon NM, Fischmann D. The implications of medical and psychiatric comorbidity with panic disorder. J Clin Psychiatry. 2005;66(Suppl 4):8–15. [PubMed] [Google Scholar]
- 242.Sinha S, Papp LA, Gorman JM. How study of respiratory physiology aided our understanding of abnormal brain function in panic disorder. J Affect Disord. 2000;61:191–200. doi: 10.1016/s0165-0327(00)00337-2. [DOI] [PubMed] [Google Scholar]
- 243.Sink KS, Walker DL, Freeman SM, Flandreau EI, Ressler KJ, Davis M. Effects of continuously enhanced corticotropin releasing factor expression within the bed nucleus of the stria terminalis on conditioned and unconditioned anxiety. Mol Psychiatry. 2013;18:308–319. doi: 10.1038/mp.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Spannuth BM, Hale MW, Evans AK, Lukkes JL, Campeau S, Lowry CA. Investigation of a central nucleus of the amygdala/dorsal raphe nucleus serotonergic circuit implicated in fear-potentiated startle. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Staub DR, Evans AK, Lowry CA. Evidence supporting a role for corticotropin-releasing factor type 2 (CRF(2)) receptors in the regulation of subpopulations of serotonergic neurons. Brain Res. 2006;1070:77–89. doi: 10.1016/j.brainres.2005.10.096. [DOI] [PubMed] [Google Scholar]
- 246.Staub DR, Spiga F, Lowry CA. Urocortin 2 increases c-Fos expression in topographically organized subpopulations of serotonergic neurons in the rat dorsal raphe nucleus. Brain Res. 2005;1044:176–189. doi: 10.1016/j.brainres.2005.02.080. [DOI] [PubMed] [Google Scholar]
- 247.Stezhka VV, Lovick TA. Projections from dorsal raphe nucleus to the periaqueductal grey matter: studies in slices of rat midbrain maintained in vitro. Neurosci Lett. 1997;230:57–60. doi: 10.1016/s0304-3940(97)00464-3. [DOI] [PubMed] [Google Scholar]
- 248.Stezhka VV, Lovick TA. Inhibitory and excitatory projections from the dorsal raphe nucleus to neurons in the dorsolateral periaqueductal gray matter in slices of midbrain maintained in vitro. Neuroscience. 1994;62:177–187. doi: 10.1016/0306-4522(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 249.Takahashi K, Wang QP, Guan JL, Kayama Y, Shioda S, Koyama Y. State-dependent effects of orexins on the serotonergic dorsal raphe neurons in the rat. Regul Pept. 2005;126:43–47. doi: 10.1016/j.regpep.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 250.Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- 251.Tannure RM, Bittencourt AS, Schenberg LC. Short-term full kindling of the amygdala dissociates natural and periaqueductal gray-evoked flight behaviors of the rat. Behav Brain Res. 2009;199:247–256. doi: 10.1016/j.bbr.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 252.Tao R, Ma Z, McKenna JT, Thakkar MM, Winston S, Strecker RE, McCarley RW. Differential effect of orexins (hypocretins) on serotonin release in the dorsal and median raphe nuclei of freely behaving rats. Neuroscience. 2006;141:1101–1105. doi: 10.1016/j.neuroscience.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 253.Targum SD, Marshall LE. Fenfluramine provocation of anxiety in patients with panic disorder. Psychiatry Res. 1989;28:295–306. doi: 10.1016/0165-1781(89)90210-2. [DOI] [PubMed] [Google Scholar]
- 254.Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388:169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 255.Thornton HB, Nel D, Thornton D, van HJ, Baker GA, Stein DJ. The neuropsychiatry and neuropsychology of lipoid proteinosis. J Neuropsychiatry Clin Neurosci. 2008;20:86–92. doi: 10.1176/jnp.2008.20.1.86. [DOI] [PubMed] [Google Scholar]
- 256.Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- 257.Tsujino N, Sakurai T. Role of orexin in modulating arousal, feeding, and motivation. Front Behav Neurosci. 2013;7:28. doi: 10.3389/fnbeh.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Valentino RJ, Liouterman L, Van Bockstaele EJ. Evidence for regional heterogeneity in corticotropin-releasing factor interactions in the dorsal raphe nucleus. J Comp Neurol. 2001;435:450–463. doi: 10.1002/cne.1043. [DOI] [PubMed] [Google Scholar]
- 259.van der Wee NJ, Fiselier J, van Megen HJ, Westenberg HG. Behavioural effects of rapid intravenous administration of meta-chlorophenylpiperazine in patients with panic disorder and controls. Eur Neuropsychopharmacol. 2004;14:413–417. doi: 10.1016/j.euroneuro.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 260.van Veen JF, van der Wee NJ, Fiselier J, van V, I, Westenberg HG. Behavioural effects of rapid intravenous administration of meta-chlorophenylpiperazine (m-CPP) in patients with generalized social anxiety disorder, panic disorder and healthy controls. Eur Neuropsychopharmacol. 2007;17:637–642. doi: 10.1016/j.euroneuro.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 261.Vargas LC, Schenberg LC. Long-term effects of clomipramine and fluoxetine on dorsal periaqueductal grey-evoked innate defensive behaviours of the rat. Psychopharmacology (Berl) 2001;155:260–268. doi: 10.1007/s002130100698. [DOI] [PubMed] [Google Scholar]
- 262.Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic dorsal raphe neurons to specific motor challenges in freely moving cats. Neuroscience. 1997;79:161–169. doi: 10.1016/s0306-4522(96)00673-2. [DOI] [PubMed] [Google Scholar]
- 263.Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res. 1984;303:337–357. doi: 10.1016/0006-8993(84)91220-4. [DOI] [PubMed] [Google Scholar]
- 265.Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- 266.Viana MB, Graeff FG, Loschmann PA. Kainate microinjection into the dorsal raphe nucleus induces 5-HT release in the amygdala and periaqueductal gray. Pharmacol Biochem Behav. 1997;58:167–172. doi: 10.1016/s0091-3057(96)00451-0. [DOI] [PubMed] [Google Scholar]
- 267.Vianna DM, Brandao ML. Anatomical connections of the periaqueductal gray: specific neural substrates for different kinds of fear. Braz J Med Biol Res. 2003;36:557–566. doi: 10.1590/s0100-879x2003000500002. [DOI] [PubMed] [Google Scholar]
- 268.Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 270.Wang L, Goebel-Stengel M, Stengel A, Wu SV, Ohning G, Tache Y. Comparison of CRF-immunoreactive neurons distribution in mouse and rat brains and selective induction of Fos in rat hypothalamic CRF neurons by abdominal surgery. Brain Res. 2011;1415:34–46. doi: 10.1016/j.brainres.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wang QP, Koyama Y, Guan JL, Takahashi K, Kayama Y, Shioda S. The orexinergic synaptic innervation of serotonin- and orexin 1-receptor-containing neurons in the dorsal raphe nucleus. Regul Pept. 2005;126:35–42. doi: 10.1016/j.regpep.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 272.Wang S, Benamer N, Zanella S, Kumar NN, Shi Y, Bevengut M, Penton D, Guyenet PG, Lesage F, Gestreau C, Barhanin J, Bayliss DA. TASK-2 Channels Contribute to pH Sensitivity of Retrotrapezoid Nucleus Chemoreceptor Neurons. J Neurosci. 2013;33:16033–16044. doi: 10.1523/JNEUROSCI.2451-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol. 2001;85:2224–2235. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- 274.Washburn CP, Sirois JE, Talley EM, Guyenet PG, Bayliss DA. Serotonergic raphe neurons express TASK channel transcripts and a TASK-like pH- and halothane-sensitive K+ conductance. J Neurosci. 2002;22:1256–1265. doi: 10.1523/JNEUROSCI.22-04-01256.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Watanabe S, Kuwaki T, Yanagisawa M, Fukuda Y, Shimoyama M. Persistent pain and stress activate pain-inhibitory orexin pathways. Neuroreport. 2005;16:5–8. doi: 10.1097/00001756-200501190-00002. [DOI] [PubMed] [Google Scholar]
- 276.Weber M, Schmitt A, Wischmeyer E, Doring F. Excitability of pontine startle processing neurones is regulated by the two-pore-domain K+ channel TASK-3 coupled to 5-HT2C receptors. Eur J Neurosci. 2008;28:931–940. doi: 10.1111/j.1460-9568.2008.06400.x. [DOI] [PubMed] [Google Scholar]
- 277.Wemmie JA, Coryell MW, Askwith CC, Lamani E, Leonard AS, Sigmund CD, Welsh MJ. Overexpression of acid-sensing ion channel 1a in transgenic mice increases acquired fear-related behavior. Proc Natl Acad Sci U S A. 2004;101:3621–3626. doi: 10.1073/pnas.0308753101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Winsky-Sommerer R, Boutrel B, de LL. Stress and arousal: the corticotrophin-releasing factor/hypocretin circuitry. Mol Neurobiol. 2005;32:285–294. doi: 10.1385/MN:32:3:285. [DOI] [PubMed] [Google Scholar]
- 279.Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de LL. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Woods SW, Charney DS, Goodman WK, Heninger GR. Carbon dioxide-induced anxiety. Behavioral, physiologic, and biochemical effects of carbon dioxide in patients with panic disorders and healthy subjects. Arch Gen Psychiatry. 1988;45:43–52. doi: 10.1001/archpsyc.1988.01800250051007. [DOI] [PubMed] [Google Scholar]
- 281.Yamanaka A, Muraki Y, Tsujino N, Goto K, Sakurai T. Regulation of orexin neurons by the monoaminergic and cholinergic systems. Biochem Biophys Res Commun. 2003;303:120–129. doi: 10.1016/s0006-291x(03)00299-7. [DOI] [PubMed] [Google Scholar]
- 282.Yamashita PS, de Bortoli VC, Zangrossi H., Jr 5-HT2C receptor regulation of defensive responses in the rat dorsal periaqueductal gray. Neuropharmacology. 2011;60:216–222. doi: 10.1016/j.neuropharm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 283.Yeomans JS, Li L, Scott BW, Frankland PW. Tactile, acoustic and vestibular systems sum to elicit the startle reflex. Neurosci Biobehav Rev. 2002;26:1–11. doi: 10.1016/s0149-7634(01)00057-4. [DOI] [PubMed] [Google Scholar]
- 284.Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Zanoveli JM, Nogueira RL, Zangrossi H., Jr Chronic imipramine treatment sensitizes 5-HT1A and 5-HT 2 A receptors in the dorsal periaqueductal gray matter: evidence from the elevated T-maze test of anxiety. Behav Pharmacol. 2005;16:543–552. doi: 10.1097/01.fbp.0000179280.05654.5a. [DOI] [PubMed] [Google Scholar]
- 286.Zanoveli JM, Nogueira RL, Zangrossi H., Jr Enhanced reactivity of 5-HT1A receptors in the rat dorsal periaqueductal gray matter after chronic treatment with fluoxetine and sertraline: evidence from the elevated T-maze. Neuropharmacology. 2007;52:1188–1195. doi: 10.1016/j.neuropharm.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 287.Zanoveli JM, Pobbe RL, de Bortoli VC, Carvalho MC, Brandao ML, Zangrossi H., Jr Facilitation of 5-HT1A-mediated neurotransmission in dorsal periaqueductal grey matter accounts for the panicolytic-like effect of chronic fluoxetine. Int J Neuropsychopharmacol. 2010;13:1079–1088. doi: 10.1017/S146114570999099X. [DOI] [PubMed] [Google Scholar]
- 288.Zhang W, Shimoyama M, Fukuda Y, Kuwaki T. Multiple components of the defense response depend on orexin: evidence from orexin knockout mice and orexin neuron-ablated mice. Auton Neurosci. 2006;126-127:139–145. doi: 10.1016/j.autneu.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 289.Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003;9:1085–1090. doi: 10.1038/nm907. [DOI] [PubMed] [Google Scholar]
- 290.Zhu L, Onaka T, Sakurai T, Yada T. Activation of orexin neurones after noxious but not conditioned fear stimuli in rats. Neuroreport. 2002;13:1351–1353. doi: 10.1097/00001756-200207190-00027. [DOI] [PubMed] [Google Scholar]
- 291.Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, III, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]


